Abstract
Objective
Transdiagnostic psychotherapies have been proposed as an effective means for addressing the needs of patients with multiple, comorbid disorders. Yet, it remains unknown whether transdiagnostic approaches empirically outperform disorder-specific psychotherapies for patients with comorbid disorders. Thus, this study tested whether comorbidity moderated the efficacy of transdiagnostic behavior therapy (TBT) and behavioral activation (BA) for patients with various affective disorders.
Methods
Data derived from a randomized controlled trial in which 93 treatment-seeking veterans received 12 sessions of TBT (n = 46) or BA (n = 47). Baseline comorbidity was assessed with a diagnostic interview. Patients rated their symptoms and functioning throughout treatment, and therapists recorded premature treatment discontinuation.
Results
Multilevel models revealed significant interactive effects on changes in symptoms and functioning, but not on the posttreatment levels of these outcomes; whereas patients with more comorbidity experienced greater reductions in distress and symptom interference in TBT compared to BA, those with one disorder had better outcomes in BA. Similarly, whereas patients with more comorbidity were less likely to prematurely discontinue TBT compared to BA, those with one disorder were less likely to prematurely discontinue BA.
Conclusions
The results lend empirical support to previously untested hypotheses for potential benefits of transdiagnostic psychotherapies.
Trial registration:
ClinicalTrials.gov identifier: NCT01947647.
Keywords: aptitude-treatment interaction research, comorbidity, transdiagnostic behavior therapy, behavioral activation, anxiety disorders, mood disorders, dropout
Introduction
Affective disorders, which include depressive, anxiety, trauma- and stressor-related, and obsessive and compulsive and related disorders in the Diagnostic and Statistical Manual for the Mental Disorders fifth edition (DSM-5; American Psychiatric Association, 2013), are among the most prevalent and burdensome mental health conditions (Haslam et al., 2005; Kessler et al., 2005). Moreover, these disorders are highly comorbid (e.g., Brown et al., 2001; Kessler et al., 2003); a meta-analysis revealed that, for example, most patients with depression also meet criteria for one or more anxiety disorder (s) (Saha et al., 2021). Yet, although many empirically supported psychotherapies exist for these conditions, the vast majority are highly differentiated, disorder-specific treatments (DSTs) that rarely focus on diagnostic comorbidities (Barlow et al., 2004). This discrepancy between typical clinical presentations and the focus of DSTs creates dilemmas for clinicians (e.g., deciding which condition is primary, delivering multiple DSTs sequentially).
Compounding this problem, some research suggests that common comorbidities may limit the efficacy of DSTs for affective disorders. For example, when delivering DSTs for depression, patients with comorbid anxiety disorders experienced less improvement (e.g., Assmann et al., 2018; Vittengl et al., 2019; Young et al., 2017) and required longer treatment courses (Andreescu et al., 2007). Similarly, in the context of DSTs for posttraumatic stress disorder (PTSD), a recent meta-analysis revealed that these treatments were less effective for patients with more severe comorbid depressive symptoms (Kline et al., 2021). Moreover, in the context of DSTs for anxiety disorders, some studies have found that patients with comorbid major depressive disorder (MDD) had poorer outcomes (e.g., Fracalanza et al., 2014; Keefe et al., 2019). However, it is worth noting that other studies have found that overall diagnostic comorbidity does not hamper the efficacy of DSTs for anxiety disorders (for a review see Olantunji et al., 2010). Taken together, although the specific impact of comorbidity on patient outcomes may vary depending on contextual factors, these results suggest that, in at least some cases, DSTs for affective disorders may not adequately address diagnostic comorbidity.
One commonly proposed solution for this problem is the development and use of transdiagnostic treatments, or those that implement consistent principles or strategies across disorders to target core underlying symptoms (Barlow et al., 2004; Gros et al., 2016; McEvoy et al., 2009). Perhaps owing to their efficacy and overlapping treatment components across disorders, most transdiagnostic approaches to date have been cognitive behavioral therapies (CBTs; Andersen et al., 2016). Overall, the results have been promising; a meta-analysis that included 8 randomized controlled trials (RCTs) revealed that transdiagnostic CBTs promoted moderate-to-large improvements in anxiety, depression, and general mental health symptoms (Andersen et al., 2016). Moreover, transdiagnostic CBTs appear to be at least comparable in efficacy to DSTs in both individual (Barlow et al., 2017; Gros & Allan, 2019) and group formats (Gros et al., 2019; Norton & Barrera, 2012). However, among the affective disorders, most of the controlled research to date on transdiagnostic CBTs has focused on the treatment of anxiety disorders (Andersen et al., 2016; Barlow et al., 2017; Norton & Barrera, 2012). Therefore, although the general efficacy of these approaches is well-established, less is known about the extent to which transdiagnostic CBTs address some of the most common and potentially difficult to treat diagnostic comorbidities, such as MDD and PTSD (e.g., Kline et al., 2021).
One promising exception to this is transdiagnostic behavior therapy (TBT; Gros, 2014), which was designed to also include all affective disorders, including MDD and PTSD. To accomplish this broader focus, TBT targets transdiagnostic avoidance that is common across these disorders through different types of exposure practices. To date, TBT pilot studies and dissemination efforts demonstrated large within-group effect sizes across affective disorders (Gros, 2014; Gros et al., 2017). Moreover, an open trial comparing group TBT to three group DSTs, revealed comparable between-group efficacy and large within-group effect sizes for the included treatments (Gros et al., 2019). Finally, in an RCT of individual TBT and behavioral activation (BA), comparable improvements were observed on several outcomes, but with slightly greater reductions in anxiety (d = .17) and depression (d = .18) in the TBT condition (Gros & Allan, 2019).
Despite these encouraging results, it remains largely unknown whether transdiagnostic CBTs, such as TBT, represent more effective treatment options than DSTs for patients with multiple comorbid affective disorders. In fact, we are aware of only one study that at least partially tested this longstanding notion. Specifically, in a study that used benchmarking to compare the clinically significant improvement rates from 3 trials of group transdiagnostic CBT (pooled N = 79) to the results of 7 published DST trials, the authors found that 66.7% of treatment completers in transdiagnostic CBT displayed clinically significant improvement on comorbid disorders, whereas only 48.5% of completers achieved this type of improvement in DSTs (Norton et al., 2013). Although these results are promising, given that benchmarking comparisons have well-documented limitations (e.g., lack of random assignment, differences between samples), the results of this comparison should be interpreted with caution and replication is needed in RCTs that specifically compare transdiagnostic approaches to DSTs.
Thus, drawing on the aforementioned RCT of TBT and BA (Gros & Allan, 2019), the present study investigated whether patient diagnostic comorbidity moderated the differential efficacy of these two treatments. Given the transdiagnostic nature of this question, this study focused on outcomes that would be relevant across disorders: global distress, transdiagnostic symptom interference, and premature discontinuation/dropout. Moreover, we selected measures of both symptoms (global distress) and functional impairment (transdiagnostic symptom interference) in order to more fully capture the recommended elements of a core psychotherapy assessment battery (Horowitz et al., 1997). Across these outcomes, we hypothesized that TBT would outperform BA for patients with a greater number of comorbid disorders, whereas we expected these two treatments to be comparably efficacious for patients with less comorbidity.
Importantly, although BA was originally developed to treat depression, it also has well-established efficacy across many of the affective disorders (e.g., Stein et al., 2020), including in the present RCT in which TBT and BA achieved comparable outcomes even when controlling for patient diagnoses (Gros & Allan, 2019). Therefore, for the present study, BA represented an appropriate comparison condition in that it is disorder-specific in focus (i.e., it targets core depressive symptomatology by aiming to increase pleasant, reinforcing activities and reducing unpleasant events), while also possessing demonstrated effectiveness across the affective disorders. Thus, it seems likely that any differences between TBT and BA for patients with higher levels of comorbidity would stem from the beneficial impact of TBT’s transdiagnostic focus rather than from BA representing a poorer fit for nondepressed patients. However, to more definitively test this alternative explanation for any differential treatment effects, we also replicated our analyses controlling for any treatment by depressive disorder interactive effects.
Materials and Methods
Study Design
Data derived from the RCT that compared the efficacy of TBT (n = 46) to BA (n = 47) for veterans with affective disorders (Gros & Allan, 2019; ClinicalTrials.gov #NCT01947647).1
Participants
Patients were treatment-seeking veterans who requested an empirically supported psychotherapy for depressive and/or anxiety symptoms at primary care and mental health clinics within a large South-eastern Veterans Affairs Medical Center (VAMC). Patients were veterans between 18 and 80 years of age who meet criteria for a DSM-5 principal affective disorder diagnosis, including panic disorder and/or agoraphobia (PD/AG), social anxiety disorder, specific phobia, generalized anxiety disorder (GAD), obsessive compulsive disorder, PTSD, MDD, or persistent depressive disorder. In brief, study exclusion criteria were: a) a recent psychiatric hospitalization or suicide attempt; b) a current substance use disorder; c) an acute, severe illness or medical condition that could interfere with study procedures; d) recently initiated a new psychiatric medication; or e) a diagnosis of psychotic symptoms, schizophrenia, personality disorder, and/or bipolar disorder. The patient demographic and diagnostic characteristics are presented in Table 1.
Table 1.
Descriptive Statistics for Baseline Demographics and Clinical Characteristics by Condition.
| TBT (n = 46) | BA (n = 47) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| DASS | 29.77 | 15.76 | 27.51 | 11.43 |
| IIRS | 51.91 | 17.55 | 52.47 | 16.64 |
| Diagnostic comorbidity | 2.26 | 1.08 | 2.36 | 0.97 |
| Age | 43.46 | 11.55 | 42.60 | 12.91 |
| Percentage | Percentage | |||
| Disorders | ||||
| Major depressive | 41.3% | 40.4% | ||
| Generalized anxiety | 30.4% | 29.8% | ||
| Panic | 26.1% | 29.8% | ||
| Social anxiety | 28.3% | 23.4% | ||
| Posttraumatic stress | 32.6% | 40.4% | ||
| Obsessive-compulsive | 8.7% | 4.3% | ||
| Sex | ||||
| Male | 80.4% | 72.3% | ||
| Female | 19.6% | 27.7% | ||
| Race/ethnicity | ||||
| White | 37.0% | 57.4% | ||
| Black | 54.3% | 34.0% | ||
| Latino/a | 4.3% | 6.4% | ||
| Native American | 2.2% | 2.1% | ||
| Other | 2.2% | 0% | ||
| Education | ||||
| High school or less | 19.5% | 27.7% | ||
| Some college | 56.5% | 46.8% | ||
| Four-year college degree | 10.9% | 8.5% | ||
| Graduate work | 8.7% | 10.6% | ||
| Missing | 4.3% | 6.4% | ||
| Relationship status | ||||
| Single/never married | 17.4% | 31.9% | ||
| Single/previously married | 32.6% | 23.4% | ||
| Married | 45.7% | 38.3% | ||
| Missing | 4.3% | 6.4% | ||
Note. TBT = Transdiagnostic Behavior Therapy; BA = Behavioral Activation; SD = standard deviation; DASS = Depression, Anxiety, Stress Scale; IIRS = Illness Intrusiveness Rating Scale.
Treatments
Both treatments consisted of 12 (45–60 min) weekly sessions of individual psychotherapy delivered by one masters-level therapist and one doctoral-level therapist who were both crossed over conditions. These therapists received extensive training in both TBT and BA through workshops, pre-trial practice cases, and ongoing supervision on the treatments throughout the RCT. To assess adherence, a clinician trained in both treatments rated 20% of session recordings on session-specific 5-point fidelity rating scales; results indicated that TBT (M = 4.8; SD = 0.5) and BA (M = 4.6; SD = 0.6) were delivered with high fidelity (Gros & Allan, 2019).
Transdiagnostic behavior therapy (TBT).
TBT targets transdiagnostic avoidance through education, practice, and mastery of four different types of exposure for negative emotions: situational/in-vivo, physical/interoceptive, thought/imaginal, and (positive) emotional/behavioral activation. More specifically, although TBT can focus on all of the aforementioned types of exposure, TBT is designed to flexibly target the most personally relevant types of avoidance for each individual patient. Therefore, it is likely that different types of exposure will be weighted more or less heavily for individual patients. For example, a TBT therapist might focus on physical and situational exposures for a patient who primarily avoids internal sensations and the situations that could trigger them (e.g., panic disorder), but who does not typically avoid thoughts or positive emotions. As noted, TBT has received initial support both in an individual (Gros, 2014; Gros et al., 2017) and group format (Gros et al., 2019). Treatment includes: psychoeducation on symptoms (session 1), identification of treatment motivators and goals (session 2), psychoeducation on avoidance and exposure (session 3), getting started with exposures (session 4), exposure implementation (session 5), exposure maintenance and problem-solving (session 6), maintenance of exposure practices and incorporation of optional modules (sessions 7–11), and relapse prevention (session 12).
Behavioral activation (BA).
BA focuses on teaching patients to monitor their mood and daily activities with the goal of increasing pleasant, reinforcing activities and reducing unpleasant events (Hopko et al., 2003; Lejuez et al., 2010). BA has demonstrated reliable effectiveness for depression (Stein et al., 2020). Behavioral activation practices also have been shown to be effective in the treatment of PTSD and other related affective disorders (Jakupcak et al., 2006; Lejuez et al., 2010; Stein et al., 2020). In this trial, the BA condition followed a published manual (Lejuez et al., 2010). Although there was some overlap between BA and TBT, the primary exposure component and multi-disorder focus of TBT renders it distinct from BA. Moreover, whereas TBT is designed to target the most personally relevant forms of avoidance for each patient, BA had a uniform focus on increasing pleasant, reinforcing activities (positive emotional exposures) across all patients to specifically target symptoms most common to depression.
Measures
Anxiety disorder interview schedule 5 (ADIS-5).
Diagnostic comorbidity, or the total number of distinct affective disorder diagnoses, was measured with the ADIS-5, which is a well-established, semi-structured interview that assesses a wide range of psychiatric disorders (Brown, 2014). The ADIS-5 assesses current and past DSM diagnoses, severity scores, and lists of feared and avoided situations for the anxiety disorders. It has demonstrated excellent inter-rater reliability and validity for assessing emotional disorder diagnoses. In this trial, an independent rater scored 20% of interviews; results indicated excellent inter-rater agreement for the most common diagnoses of MDD (85.0%), PD/AG (100%), and PTSD (100%; Gros & Allan, 2019).2
Depression anxiety stress scales, 21-item version (DASS).
The DASS (Lovibond & Lovibond, 1995) is a 21-item measure with three subscales designed to assess dysphoric mood (depression subscale; DASS-D), fear and autonomic arousal (anxiety subscale; DASS-A), and tension and agitation (stress subscale; DASS-S). Although the DASS-21 subscales can be analyzed separately, they can also be summed to create a total score that captures global psychological distress (Campbell-Sills & Brown, 2010; Henry & Crawford, 2005). The DASS-21 total score has demonstrated good reliability and validity (Antony et al., 1998; Lovibond & Lovibond, 1995). In this study, the DASS total score demonstrated good internal consistency across all assessments (αs > .90).
Illness intrusiveness ratings scale (IIRS).
The IIRS (Devins et al., 1983) is a 13-item transdiagnostic questionnaire that assesses the extent to which psychiatric symptoms interfere with important domains of life (e.g., health, diet, work). The IIRS has demonstrated strong psychometric properties in participants with physical and/or emotional health problems (Devins, 2010; Devins et al., 2001). In this study, the IIRS demonstrated good internal consistency across all assessments (αs > .88).
Post-treatment therapist review (PTTR).
The study-specific, therapist-rated PTTR was created to capture a variety of outcomes at posttreatment, including session and study completion, reasons for treatment noncompletion, homework completion/engagement, and therapist-perceived improvement/progress (Gros & Allan, 2019). This study used the item assessing treatment completion (yes/no) to capture premature discontinuation.
Procedures
Relevant to this study, interested patients were assessed for eligibility during a baseline assessment that included consent documents, the ADIS-5, and self-report measures (including the DASS and IIRS). Eligible patients were then randomly assigned to TBT or BA (see Gros & Allan, 2019). Patients completed the DASS and IIRS at baseline, after every odd-numbered session, and at posttreatment (i.e., one week after session 12). Therapists completed the PTTR for each patient at posttreatment. All study procedures were approved by the local VAMC Research and Development committee and the institutional review board at an affiliated university.
Data Analytic Plan
Regarding preliminary analyses, we tested for between-group differences on baseline variables. If chance differences between the treatments existed on these variables, we included the relevant variable (s) as a covariate in our primary analyses. We also tested whether there were chance differences between the conditions on the study variables at baseline (i.e., DASS, IIRS, and diagnostic comorbidity). Finally, we also examined the correlation between our two continuous outcome variables (DASS, IIRS) to ensure that they were distinct enough to be analyzed separately.
To test our research question for the two continuous outcomes (DASS, IIRS), we used multilevel modeling (MLM) as facilitated by the Mplus 8.4 software (Muthén & Muthén, 1998). This analytic approach accounts for dependency in the data due to repeated outcome assessments over time and addresses missing data using maximum likelihood estimation; this approach retained all participants who completed at least one outcome assessment (n = 90). Although missing data was addressed using maximum likelihood estimation, it is worth noting that the amount of missing data was relatively high, though within acceptable limits (< 50% missing at any given timepoint). For example, ~31% of participants were missing outcomes data at midtreatment (session 5), and ~46% of participants were missing outcomes data at posttreatment.
More specifically, we fit 2-level, random slopes models with within-person change over time at level 1 and between-person differences at level 2. In these models, time was coded in weeks and centered at posttreatment (one week after session 12), so that the model intercept represented each patients’ final level of the relevant outcome. Although outcome change in psychotherapy is often non-linear, we selected a linear change model for two primary reasons. First, given this study’s relatively small sample size, modeling more complex change trajectories (e.g., quadratic change) would have resulted in a model that fell below the recommended number of participants per parameter (e.g., Kline, 2016). Second, preliminary analyses suggested that a linear change model appeared to roughly capture the average shape of change in this sample; that is, unconditional quadratic models for our two continuous outcomes revealed that there was no significant, average quadratic change pattern (ps > .05). Moreover, a visual inspection of data plots for each patient suggested that any curvature appeared slight for most participants. Therefore, for these reasons, we retained the more parsimonious linear change model for all primary analyses. In these models, treatment condition (TBT = 0, BA = 1), diagnostic comorbidity, and the treatment × comorbidity interaction were included as level 2 (between-patient) predictors of patients’ weekly linear change rate (slope) and posttreatment outcome level (intercept). Diagnostic comorbidity was grand-mean centered prior to the creation of the interaction term to enhance the interpretability of the intercepts.
Additionally, given that interactive effects are often not normally distributed (e.g., Hayes, 2018), we used the Bayesian estimator in Mplus, which does not assume normality (Muthén & Asparouhov, 2012). Moreover, we also selected this approach, because there is some evidence that the Bayesian estimator provides more accurate multilevel parameter estimates for samples with smaller cluster sizes (Muthén & Asparouhov, 2012). This approach determines statistical significance with 95% credible intervals (CIs), which indicate that there is a 95% chance that the true effect falls within that interval. Therefore, effects are considered statistically significant when the 95% CI does not contain zero. Finally, to measure effect size, we calculated a version of Cohen’s d. Specifically, for the treatment × comorbidity interactions, we calculated the number of SDs on the outcome variable by which the two treatment groups were expected to differ for every additional comorbid diagnosis.
For our binary premature discontinuation outcome, we used the SPSS version 23.0 software to fit a logistic regression with treatment, diagnostic comorbidity, and the treatment × comorbidity interaction included as predictors. To enhance interpretability, effect sizes were represented in both an odds ratio and a probability metric. Given our interest in differential discontinuation by treatment condition as a function of comorbidity, we focused our analyses on the patients who received at least one session of the relevant intervention (n = 80).
Finally, because BA was primarily designed to treat depression, we conducted several supplementary analyses to at least partially rule out alternative explanations for any significant treatment by comorbidity interactions. Specifically, because MDD was the most common diagnosis in the TBT and BA RCT (Gros & Allan, 2019), it seemed possible that our total diagnostic comorbidity variable could serve as an unintentional proxy for whether patients met criteria for one or more additional disorders beyond MDD. If this was indeed the case for a sizeable subgroup of patients, then any significant comorbidity interactive effects favoring TBT over BA could potentially stem from BA being a poorer fit for non-depressed patients rather than from the impact of total diagnostic comorbidity. Therefore, to rule out this possibility, we replicated our primary analyses controlling for any treatment × MDD interactive effects (i.e., any differential treatment effectiveness as a function of whether patients presented with depression).
Results
There were no statistically significant baseline differences between the two treatments on any study outcomes, demographics, or clinical diagnoses (all ps > .05). Total diagnostic comorbidity also did not differ significantly between conditions, χ2(4) = 3.43, p = .49. Across both groups, patients met DSM-5 criteria for an average of 2.31 (SD = 1.02) affective disorders (range = 1–5). Of the 20% of patients who only met criteria for one disorder, the most common diagnoses were MDD (n = 10; 53%), PD/AG (n = 4; 21%), and PTSD (n = 3; 16%). Of the 80% of patients who met criteria for 2 or more disorders, the most common comorbidities were PTSD and MDD (n = 21; 28%), MDD and PD/AG (n = 17; 23%), and MDD and GAD (n = 16; 22%). As is typical in psychotherapy research, our symptom distress (DASS) and functioning (IIRS) outcomes were only moderately correlated across all timepoints (r = .57, p < .001), suggesting that they capture related, but distinct, aspects of treatment outcome.
Prior to conducting our primary treatment × comorbidity interaction models, we first fit MLMs examining average treatment effects on the global distress and symptom interference outcomes in order to provide context for any interactions. Results indicated that there were no significant treatment (TBT = 0; BA = 1) effects on the rate of weekly distress reduction during treatment (γ11 = 0.20, SD = 0.25, 95% CI −0.21, 0.72) or on posttreatment levels of distress (γ01 = −0.61, SD = 2.60, 95% CI −6.44, 4.04). Similarly, results also indicated that there were no significant treatment effects on the rate of weekly symptom interference reduction during treatment (γ11 = 0.01, SD = 0.41, 95% CI −0.71, 0.85) or on posttreatment levels of symptom interference (γ01 = −1.96, SD = 5.55, 95% CI −12.31, 9.47).
The full MLM results for the treatment × comorbidity interactive effect on the global distress outcome are presented in Model 1 of Table 2. Results indicated that although there was no significant treatment × comorbidity interactive effect on posttreatment distress (γ03 = 2.99, SD = 3.12, 95% CI −3.01, 8.88), there was a significant treatment × comorbidity interactive effect on distress reduction (γ13 = 0.48, SD = 0.27, 95% CI 0.05, 1.02). As depicted in Panel A of Figure 1, whereas patients with high diagnostic comorbidity (+1.5 SDs) experienced significantly greater distress reduction in TBT vs. BA, those with low diagnostic comorbidity (−1.5 SDs) experienced greater distress reduction in BA vs. TBT. Expressed as an effect size, for every additional comorbid diagnosis, TBT patients experienced 0.47 SDs more improvement across the entire treatment period compared to BA patients (or a small-to-moderate standardized effect)
Table 2.
Comorbidity as a Moderator of the Differential Effect of TBT vs. BA on Global Distress and Transdiagnostic Symptom Interference (N = 90).
| DASS Total Score | IIRS Total Score | |||
|---|---|---|---|---|
| Fixed effects | Coefficient (SD) | 95% CI | Coefficient (SD) | 95% CI |
| Posttreatment Outcome (intercept), γ00 | 15.86* (2.03) | [11.53, 19.50] | 38.68* (3.85) | [31.05, 46.59] |
| Treatment, γ01 | −0.66 (3.42) | [−6.63, 6.06] | −2.93 (6.31) | [−14.46, 9.00] |
| Comorbidity, γ02 | 1.31 (1.86) | [−2.84, 4.34] | 1.21 (3.33) | [−6.04, 7.45] |
| Treatment × Comorbidity, γ03 | 2.99 (3.12) | [−3.01, 8.88] | 6.45 (5.40) | [−4.00, 17.48] |
| Linear Outcome Change (slope), γ10 | −1.12* (0.17) | [−1.48, −0.80] | −1.34* (0.28) | [−1.87, −0.83] |
| Treatment, γ11 | 0.25 (0.29) | [−0.39, 0.78] | −0.0004 (0.45) | [−0.98, 0.91] |
| Comorbidity, γ12 | −0.47* (0.15) | [−0.79, −0.21] | −0.47 (0.24) | [−0.97, 0.004] |
| Treatment × Comorbidity, γ13 | 0.48* (0.27) | [0.05, 1.02] | 0.63* (0.41) | [0.05, 1.51] |
Note. DASS = Depression Anxiety Stress Scales; IIRS = Illness Intrusiveness Ratings Scale; SD = standard deviation; CI = credible interval; ES = effect size; Treatment = Transdiagnostic Behavior Therapy coded as 0, Behavioral Activation coded as 1.
Indicates that the 95% CI does not include zero.
Figure 1.
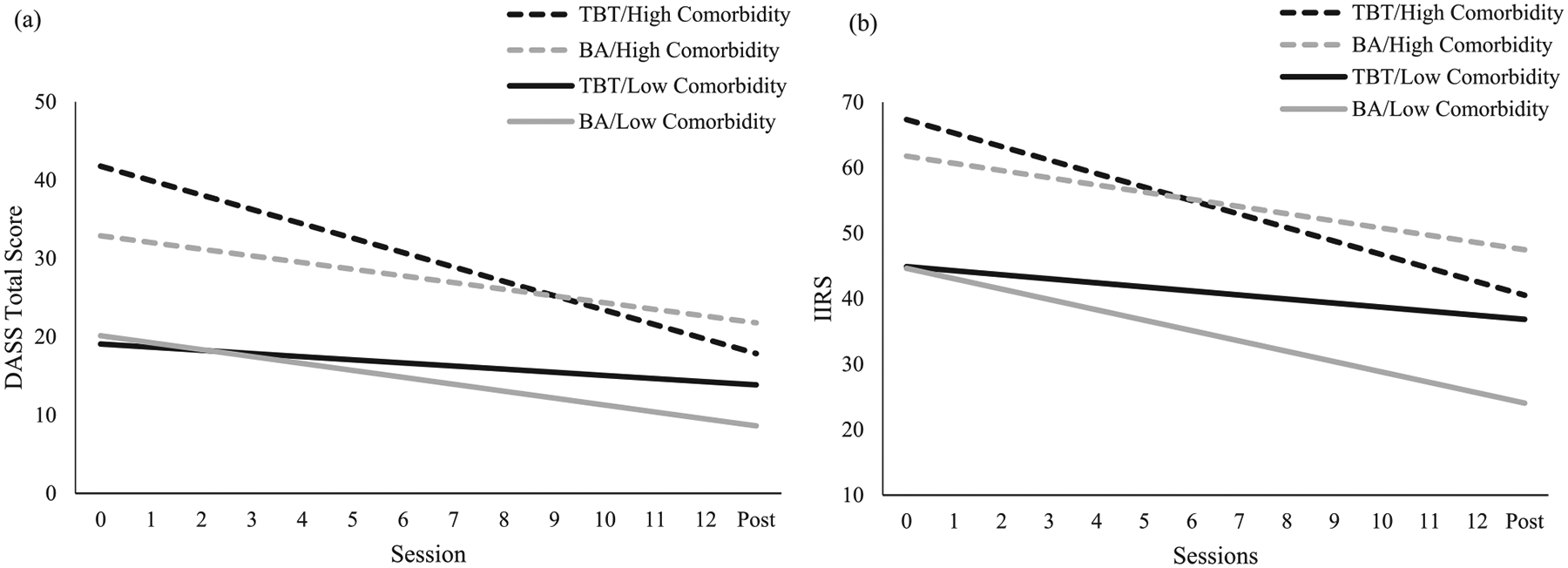
Panel a depicts global distress trajectories for BA (grey lines) vs. TBT (black lines) patients who have high (dashed lines) vs. low (solid lines) levels of clinician-rated diagnostic comorbidity. Panel b depicts transdiagnostic symptom interference trajectories for BA (grey lines) vs. TBT (black lines) patients who have high (dashed lines) vs. low (solid lines) levels of clinician-rated diagnostic comorbidity. High and low levels of comorbidity were represented as 1.5 SDs above and below the mean, respectively.
Note. DASS = Depression Anxiety Stress Scales; TBT = transdiagnostic behavior therapy; BA = behavioral activation; IIRS = Illness Intrusiveness Ratings Scale.
The full MLM results for the treatment × comorbidity interactive effect on the symptom interference outcome are presented in Model 2 of Table 2. Results indicated that although there was no significant treatment × comorbidity interactive effect on posttreatment symptom interference (γ03 = 6.45, SD = 5.40, 95% CI −4.00, 17.48), there was a significant treatment × comorbidity interactive effect on reductions in symptom interference (γ13 = 0.63, SD = 0.41, 95% CI 0.05, 1.51). As depicted in Panel B of Figure 1, whereas patients with high diagnostic comorbidity (+1.5 SDs) experienced significantly greater distress reduction in TBT vs. BA, those with low diagnostic comorbidity (−1.5 SDs) experienced greater distress reduction in BA vs. TBT. Expressed as an effect size, for every additional comorbid diagnosis, TBT patients experienced 0.43 SDs more improvement across the entire treatment period compared to BA patients (or a small-to-moderate standardized effect).3
The logistic regression results for premature discontinuation are presented in Table 3. Descriptively, of the 80 patients who initiated treatment, 41% of BA patients and 24% of TBT patients prematurely discontinued treatment. There was a significant treatment × comorbidity interactive effect such that patients with higher diagnostic comorbidity were less likely to prematurely discontinue TBT vs. BA, whereas those with lower diagnostic comorbidity were less likely to discontinue BA vs. TBT, B = 1.15, SE = 0.57, p = .04. Expressed as an odds ratio, for every additional diagnosis, patients in BA vs. TBT have a 3.14 times greater chance of prematurely discontinuing treatment. Expressed as conditional probabilities, high comorbidity patients (+1.5 SDs) had an 8% chance of discontinuing TBT compared to a 54% chance in BA. In contrast, low comorbidity patients (−1.5 SDs) had a 26% chance of discontinuing BA compared to a 46% chance in TBT.
Table 3.
Comorbidity as a Moderator of the Differential Effect of TBT vs. BA on Dropout (N = 80).
| Variables | B | SE | Wald | p | OR | OR 95% CI |
|---|---|---|---|---|---|---|
| Constant | −1.29 | 0.42 | 9.44 | .002 | 0.28 | – |
| Treatment | 0.85 | 0.54 | 2.48 | .12 | 2.34 | [0.81, 6.72] |
| Comorbidity | −0.74 | 0.45 | 2.68 | .10 | 0.48 | [0.20, 1.16] |
| Treatment × Comorbidity | 1.15 | 0.57 | 4.08 | .04 | 3.14 | [1.03, 9.55] |
Note. SE = standard error; OR = odds ratio; CI = confidence interval; Treatment = Transdiagnostic Behavior Therapy coded as 0, Behavioral Activation coded as 1.
Finally, as noted, we conducted supplemental analyses to investigate the possibility that the present results might be less about the impact of total diagnostic comorbidity and more about our use of BA as a comparison treatment. Results indicated that when controlling for any treatment × MDD interactive effects, the previously reported treatment × comorbidity interactive effects on reductions in distress (γ13 = 0.60, SD = 0.27, 95% CI 0.05, 1.16) and symptom interference (γ13 = 0.96, SD = 0.41, 95% CI 0.13, 1.76) remained statistically significant and similarly sized. Additionally, there were no significant treatment by MDD status interactive effects on any of the distress or symptom interference outcomes (all ps > .14). Finally, the results were similar for the premature termination outcome; specifically, the previously reported treatment × comorbidity interactive effect remained statistically significant and similarly sized (B = 1.13, SE = 0.57, p = .047), and the treatment × MDD interactive effect was unrelated to premature termination (B = −0.34, SE = 1.21, p = .78). Therefore, the results suggest that the significant treatment × comorbidity interactive effects were not driven by differential treatment effectiveness for patients with and without depression.
Discussion
The present study tested whether patient diagnostic comorbidity moderated the differential efficacy of TBT and BA in a sample of racially diverse veterans with affective disorders. As hypothesized, compared to BA, TBT promoted greater reductions in distress and symptom interference and lower rates of premature discontinuation for patients with more diagnostic comorbidity. However, unexpectedly, despite promoting greater reductions in distress and symptom interference, there were no significant treatment by comorbidity interactive effects on the level of these outcomes at posttreatment. Also counter to our expectations, BA promoted greater reductions in distress, symptom interference, and lower rates of premature discontinuation than TBT for patients with low diagnostic comorbidity. Together, these initial findings are some of the first to lend empirical support to the longstanding theoretical notion that transdiagnostic treatments may be more effective than DSTs for addressing comorbidity.
There are several potential explanations for these findings that require further investigation. For example, it seems plausible that, when contrasted with DSTs, transdiagnostic approaches may better address the common mechanisms that contribute to the symptomatic/functional impairment across the affective disorders. This notion is one of the primary hypotheses supporting the development and implementation of transdiagnostic CBTs (Barlow et al., 2004; Gros et al., 2016; McEvoy et al., 2009). Applied to the present context, TBT’s focus on addressing different forms of avoidance through engagement in the most personally relevant types of exposure could represent a particularly efficient means for clinicians to address core transdiagnostic symptoms without creating a diffuse focus that could dilute treatment efficacy (Gros & Oglesby, 2019). In contrast, BA’s primary focus on increasing engagement in pleasant, values-based activities (positive emotional exposures in TBT terminology) could be somewhat insufficient for the patients with multiple comorbid problems, leaving significant symptoms unaddressed. Although preliminary and requiring replication, these findings may suggest that when working with patients who have multiple affective disorders, a focus on all relevant forms of avoidance could be a key factor in promoting more robust positive outcomes.
An alternative or complementary explanation for these findings could be that patients with multiple comorbid problems may find the rationale for TBT more credible and hope-inspiring than the rationale for BA. This explanation fits with experimental research suggesting that patients report more positive outcome expectations and credibility perceptions when treatment rationales emphasize a broad (rather than specific) focus on core cognitive, affective, and behavioral processes (Ahmed & Westra, 2009; Ametrano et al., 2017). And, one might expect such a broad focus (as is emphasized in TBT) to be especially appealing to patients with multiple problems. In other words, TBT’s broad, transdiagnostic focus on addressing the core processes maintaining all of a person’s problems might be seen by patients with high levels of comorbidity as a particularly good fit, whereas BA’s sole focus on increasing pleasant, reinforcing activities and reducing unpleasant events may be seen by these patients as somewhat inadequate for addressing their concerns. These more positive treatment beliefs could lead to better treatment engagement (including in the form of better attendance/lower rates of premature discontinuation), which could, in turn, lead to greater symptomatic/functional improvement (Constantino, Coyne et al., 2018; Constantino, Vîslă et al., 2018). However, these notions require direct investigation in future transdiagnostic research.
Regarding the somewhat unexpected result that BA was more effective for patients with less diagnostic comorbidity across all of our outcomes (symptomatic and functional improvement and premature termination), this finding could fit with some authors’ speculations that transdiagnostic approaches may dilute the focus and efficacy of the most-needed treatment components (Craske et al., 2007). This may be especially likely for patients with a single disorder. For example, if a patient presented with MDD only (the most common single disorder diagnosis in the present study), it could be most beneficial for therapists to focus all their efforts on reducing depressive symptoms through behavioral activation. However, these findings were unexpected and therefore require more direct testing in future studies before firm conclusions can be drawn.
Also counter to our hypotheses, although the two treatments promoted different amounts of improvement in distress and symptom interference for patients with high vs. low levels of comorbidity, this interactive effect did not result in significant differences in patients’ posttreatment outcome levels. Although unexpected, there are several possible explanations for these results. First, despite the fact that there were no statistically significant baseline differences in severity between the two treatments, it is possible that small differences in initial severity could explain this pattern of results. This hypothesis was only partially supported by our supplemental analyses that revealed interactive effects that approached significance in the expected direction when we controlled for baseline severity (one-tailed ps = .05 and .06 for the posttreatment distress and symptom interference outcomes, respectively). Alternatively, the nonsignificant treatment by comorbidity interactive effects at posttreatment could indicate that the between-group differences in symptomatic and functional improvement were rather small and would therefore require longer than 12 weeks of treatment to result in significant differences in outcome level. Finally, it is also possible that the relatively high levels of missing data at posttreatment resulted in less reliable estimates, which could have resulted in lower statistical power to detect interactive effects. However, it is worth reiterating that these explanations are speculative, and it will be important for future research to replicate our analyses, especially in larger samples that will be better powered to detect moderation.
This study also has several preliminary clinical implications. If replicated, the present findings could suggest a possible means for using information many clinicians already routinely collect to make measurement-based treatment selection decisions (Kraemer et al., 2002). Namely, clinicians could consider gathering diagnostic information from their patients and then implementing transdiagnostic approaches, like TBT, for patients with multiple affective disorders, and DSTs, like BA, for patients with just one affective disorder. However, it is worth reiterating that in both the existing literature and the present study, comorbidity seems to be the rule rather than the exception (e.g., Saha et al., 2020). Therefore, given finite training resources and time constraints, it could be important to focus training and dissemination efforts on transdiagnostic approaches, as they may represent the best fit for the largest number of patients.
The present study had several limitations. First, although this sample had a relatively high level of racial/ethnic diversity, it also consisted of mostly male veterans, which could limit generalizability to women and civilian populations. Second, given that TBT explicitly targets a broader range of disorders (e.g., PTSD, depressive disorders) than some other transdiagnostic approaches (e.g., Norton & Barrera, 2012), it is unknown whether the present results would generalize to other transdiagnostic treatments. Similarly, it is unknown whether the results would generalize to other DSTs beyond BA. Relatedly, the use of BA as a comparison group across different patient diagnoses (rather than matching patients with different primary disorders to different diagnosis-specific DSTs) could have impacted our results. Third, the comorbidity index used in this study was based on categorical diagnoses, which could miss some nuance in terms of symptom presentations. Future research, especially in larger samples, could also examine the impact of specific types of comorbidity (e.g., PTSD and MDD). Fourth, because the Gros and Allan (2019) trial did not include a therapist identification variable, we were unable to test for possible therapist effects on the study outcomes. Fifth, given that the Gros and Allan (2019) trial excluded patients with personality disorders, the generalizability of our results to this population is limited and future work will need to examine the impact of comorbid personality pathology on the differential efficacy of transdiagnostic and disorder-specific approaches. Sixth, the relatively small sample size for this study prevented us from examining more complex change patterns (e.g., quadratic outcome change), which could have impacted our results. Finally, this study was a secondary analysis of existing trial data. Therefore, to increase confidence in these results, future work would need to test the possible benefits of prospectively matching patients to treatments based on their level of diagnostic comorbidity.
Limitations notwithstanding, the present results provide preliminary support for the longstanding notion that transdiagnostic treatments may be more effective than DSTs for patients with multiple disorders (Norton et al., 2013). Given the rates of comorbidity (Brown et al., 2001) and overlap across disorders and their matching DSTs (Barlow et al., 2004), transdiagnostic treatments have often been hypothesized to represent a better fit to patients with affective disorders. These findings provide initial support for these notions. Additionally, the present results add to the growing literature supporting the value of focusing training and implementation efforts on transdiagnostic approaches to maximize treatment benefits for the largest number of patients (Ametaj et al., 2021; Gros et al., 2017).
Clinical or methodological significance of this article:
The present study provides some of the first empirical evidence that transdiagnostic psychotherapies may be more effective than disorder-specific approaches for patients with multiple, comorbid conditions. Given high rates of diagnostic comorbidity across affective disorders, the present results support the value of focusing training and implementation efforts on transdiagnostic approaches to maximize treatment benefits for the largest number of patients.
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by Department of Veteran Affairs Clinical Sciences Research and Development Career Development Award: [grant number CX000845].
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, 105, no copyright protection is available for such works under U.S. Law.
See Gros and Allan (2019) for details on the flow of participants through the trial (i.e., CONSORT).
Given that inter-rater agreement was calculated for only 20% of the interviews, it was not possible to calculate inter-rater agreement for some of the less common diagnoses.
Because we unexpectedly found significant treatment × comorbidity interactive effects for reductions in distress and symptom interference but not for patients’ final posttreatment levels of these outcomes, we conducted a post-hoc analysis examining whether this finding could be due to baseline differences in severity between the groups. Results indicated that when controlling for baseline severity these interactive effects remained nonsignificant, though the effects were slightly larger in size and approached significance for posttreatment distress (γ03 = 4.22, SD = 2.71, one-tailed p = .052, 95% CI −0.79, 9.84) and symptom interference (γ03 = 6.84, SD = 4.60, one-tailed p = .055, 95% CI −1.35, 16.41).
Disclosure Statement
No potential conflict of interest was reported by the author(s).
References
- Ahmed M, & Westra HA (2009). Impact of a treatment rationale on expectancy and engagement in cognitive behavioral therapy for social anxiety. Cognitive Therapy and Research, 33 (3), 314–322. 10.1007/s10608-008-9182-1 [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Publishing. [Google Scholar]
- Ametaj AA, Tirpak JW, Cassiello-Robbins C, Snow R, Rassaby MM, Beer K, & Sauer-Zavala S (2021). A preliminary investigation of provider attitudes toward a transdiagnostic treatment: Outcomes from training workshops with the unified protocol. Administration and Policy in Mental Health and Mental Health Services Research, 1–15. 10.1007/s10488-020-01101-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametrano RM, Constantino MJ, & Nalven T (2017). The influence of expectancy persuasion techniques on socially anxious analogue patients’ treatment beliefs and therapeutic actions. International Journal of Cognitive Therapy, 10(3), 187–205. 10.1521/ijct.2017.10.3.187 [DOI] [Google Scholar]
- Andersen P, Toner P, Bland M, & McMillan D (2016). Effectiveness of transdiagnostic cognitive behaviour therapy for anxiety and depression in adults: A systematic review and meta-analysis. Behavioural and Cognitive Psychotherapy, 44(6), 673–690. 10.1017/S1352465816000229 [DOI] [PubMed] [Google Scholar]
- Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, & Reynolds CF (2007). Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: Controlled study. British Journal of Psychiatry, 190(4), 344–349. 10.1192/bjp.bp.106.027169 [DOI] [PubMed] [Google Scholar]
- Antony MM, Bieling PJ, Cox BJ, Enns MW, & Swinson RP (1998). Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment, 10 (2), 176–181. 10.1037/1040-3590.10.2.176 [DOI] [Google Scholar]
- Assmann N, Schramm E, Kriston L, Hautzinger M, Härter M, Schweiger U, & Klein JP (2018). Moderating effect of comorbid anxiety disorders on treatment outcome in a randomized controlled psychotherapy trial in early-onset persistently depressed outpatients. Depression and Anxiety, 35(10), 1001–1008. 10.1002/da.22839 [DOI] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, & Choate ML (2004). Toward a unified treatment for emotional disorders. Behavior Therapy, 35 (2), 205–230. 10.1016/S0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Bullis JR, Gallagher MW, Murray-Latin H, Sauer-Zavala S, Bentley KH, Thompson-Hollands J, Conklin LR, Boswell JF, Ametaj A, Carl JR, Boettcher HT, & Cassiello-Robbins C (2017). The unified protocol for transdiagnostic treatment of emotional disorders compared with diagnosis-specific protocols for anxiety disorders. JAMA Psychiatry, 74 (9), 875–884. 10.1001/jamapsychiatry.2017.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA (2014). Anxiety and related disorders interview Schedule for DSM-5RG (ADIS-5)-adult and lifetime version: Clinician manual. Oxford University Press. [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, & Mancill RB (2001). Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology, 110(4), 585. 10.1037/0021-843X.110.4.585 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, & Brown TA (2010). Generalized anxiety disorder. In Antony MM, & Barlow DH (Eds.), Handbook of assessment and treatment planning for psychological disorders (pp. 224–266). Guilford Press. [Google Scholar]
- Constantino MJ, Coyne AE, Boswell JF, Iles BR, & Vîslă A (2018). A meta-analysis of the association between patients’ early perception of treatment credibility and their posttreatment outcomes. Psychotherapy, 55(4), 486–495. 10.1037/pst0000168 [DOI] [PubMed] [Google Scholar]
- Constantino MJ, Vîslă A, Coyne AE, & Boswell JF (2018). A meta-analysis of the association between patients’ early treatment outcome expectation and their posttreatment outcomes. Psychotherapy, 55(4), 473–485. 10.1037/pst0000169 [DOI] [PubMed] [Google Scholar]
- Craske MG, Farchione TJ, Allen LB, Barrios V, Stoyanova M, & Rose R (2007). Cognitive behavioral therapy for panic disorder and comorbidity: More of the same or less of more? Behaviour Research and Therapy, 45(6), 1095–1109. 10.1016/j.brat.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Devins GM (2010). Using the Illness Intrusiveness Ratings scale to understand health-related quality of life in chronic disease. Journal of Psychosomatic Research, 68(6), 591–602. 10.1016/j.jpsychores.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Devins GM, Binik YM, Hutchinson TA, Hollomby DJ, Barré PE, & Guttmann RD (1983). The emotional impact of end-stage renal disease: Importance of patients’ perceptions of intrusiveness and control. The International Journal of Psychiatry in Medicine, 13(4), 327–343. 10.2190/5DCP-25BV-U1G9-9G7C [DOI] [PubMed] [Google Scholar]
- Devins GM, Dion R, Pelletier LG, Shapiro CM, Abbey S, Raiz LR, Binik YM, McGowan P, Kutner NG, Beanlands H, & Edworthy SM (2001). Structure of lifestyle disruptions in chronic disease. Medical Care, 39(10), 1097–1104. 10.1097/00005650-200110000-00007 [DOI] [PubMed] [Google Scholar]
- Fracalanza K, McCabe RE, Taylor VH, & Antony MM (2014). The effect of comorbid major depressive disorder or bipolar disorder on cognitive behavioral therapy for social anxiety disorder. Journal of Affective Disorders, 162, 61–66. 10.1016/j.jad.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Gros DF (2014). Development and initial evaluation of transdiagnostic behavior therapy (TBT) for veterans with affective disorders. Psychiatry Research, 220(1–2), 275–282. 10.1016/j.psychres.2014.08.018 [DOI] [PubMed] [Google Scholar]
- Gros DF, & Allan NP (2019). A randomized controlled trial comparing transdiagnostic behavior therapy (TBT) and behavioral activation in veterans with affective disorders. Psychiatry Research, 281), 10.1016/j.psychres.2019.112541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros DF, Allan NP, & Szafranski DD (2016). Movement towards transdiagnostic psychotherapeutic practices for the affective disorders. Evidence Based Mental Health, 19(3), e10–e12. 10.1136/eb-2015-102286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros DF, Merrifield C, Rowa K, Szafranski DD, Young L, & McCabe RM (2019). A naturalistic comparison of group transdiagnostic behaviour therapy (TBT) and disorder-specific cognitive behavioural therapy groups for the affective disorders. Behavioural and Cognitive Psychotherapy, 47(1), 39–51. 10.1017/S1352465818000309 [DOI] [PubMed] [Google Scholar]
- Gros DF, & Oglesby ME (2019). A new transdiagnostic psychotherapy for veterans with affective disorders: Transdiagnostic behavior therapy (TBT). Psychiatry, 82(1), 83–84. 10.1080/00332747.2018.1520021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros DF, Szafranski DD, & Shead SD (2017). A real world dissemination and implementation of transdiagnostic behavior therapy (TBT) for veterans with affective disorders. Journal of Anxiety Disorders, 46, 72–77. 10.1016/j.janxdis.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Haslam C, Atkinson S, Brown SS, & Haslam RA (2005). Anxiety and depression in the workplace: Effects on the individual and organisation (a focus group investigation). Journal of Affective Disorders, 88(2), 209–215. 10.1016/j.jad.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 2nd edition. Guilford Press. [Google Scholar]
- Henry JD, & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, & Eifert GH (2003). Contemporary behavioral activation treatments for depression: Procedures, principles and progress. Clinical Psychology Review, 23(5), 699–717. 10.1016/S0272-7358(03)00070-9 [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Strupp HH, Lambert MJ, & Elkin I (1997). Overview and summary of the Core Battery Conference. In Strupp HH, Horowitz LM, & Lambert MJ (Eds.), Measuring patient changes in mood, anxiety, and personality disorders: Toward a core battery (pp. 11–54). American Psychological Association. 10.1037/10232-001. [DOI] [Google Scholar]
- Jakupcak M, Roberts LJ, Martell C, Mulick P, Michael S, Reed R, Balsam KF, Yoshimoto D, & McFall M (2006). A pilot study of behavioral activation for veterans with posttraumatic stress disorder. Journal of Traumatic Stress, 19(3), 387–391. 10.1002/jts.20125 [DOI] [PubMed] [Google Scholar]
- Keefe JR, Chambless DL, Barber JP, & Milrod BL (2019). Treatment of anxiety and mood comorbidities in cognitive-behavioral and psychodynamic therapies for panic disorder. Journal of Psychiatric Research, 114, 34–40. 10.1016/j.jpsychires.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, & Wang PS (2003). The epidemiology of major depressive disorder. JAMA, 289(23), 3095–3105. 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication, Archives of General Psychiatry 617-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AC, Cooper AA, Rytwinski NK, & Feeny NC (2021). The effect of concurrent depression on PTSD outcomes in trauma-focused psychotherapy: A meta-analysis of randomized controlled trials. Behavior Therapy, 52(1), 250–266. 10.1016/j.beth.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2016). Principles and practice of structural equation modeling (4th ed). Guilford Press. [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59(10), 877–883. 10.1001/archpsyc.59.10.877 [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Hopko DR, Acierno R, Daughters SB, & Pagoto SL (2010). Ten year revision of the brief behavioral activation treatment for depression: Revised treatment manual. Behavior Modification, 35(2), 111–161. 10.1177/0145445510390929 [DOI] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- McEvoy PM, Nathan P, & Norton PJ (2009). Efficacy of transdiagnostic treatments: A review of published outcome studies and future research directions. Journal of Cognitive Psychotherapy, 23(1), 20–33. 10.1891/0889-8391.23.1.20 [DOI] [Google Scholar]
- Muthén B, & Asparouhov T (2012). Bayesian structural equation modeling: A more flexible representation of substantive theory. Psychological Methods, 17(3), 313–335. 10.1037/a0026802 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998). 1998–2017 Mplus user’s guide (8th ed.). Author. [Google Scholar]
- Norton PJ, & Barrera TL (2012). Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: A preliminary randomized controlled noninferiority trial. Depression and Anxiety, 29(10), 874–882. 10.1002/da.21974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton PJ, Barrera TL, Mathew AR, Chamberlain LD, Szafranski DD, Reddy R, & Smith AH (2013). Effect of transdiagnostic CBT for anxiety disorders on comorbid diagnoses. Depression and Anxiety, 30(2), 168–173. 10.1002/da.22018 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, & Tolin DF (2010). A meta-analysis of the influence of comorbidity on treatment outcome in the anxiety disorders. Clinical Psychology Review, 30(6), 642–654. 10.1016/j.cpr.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Saha S, Lim CCW, Cannon DL, Burton L, Bremner M, Cosgrove P, Huo Y, & McGrath J (2021). Comorbidity between mood and anxiety disorders: A systematic review and meta-analysis. Depression and Anxiety, 38(3), 286–306. 10.1002/da.v38.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Carl E, Cuijpers P, Karyotaki E, & Smits JAJ (2020). Looking beyond depression: A meta-analysis of the effect of behavioral activation on depression, anxiety, and activation. Psychological Medicine, 10.1017/S0033291720000239 [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Smits JAJ, Thase ME, & Jarrett RB (2019). Do comorbid social and other anxiety disorders predict outcomes during and after cognitive therapy for depression? Journal of Affective Disorders, 242, 150–158. 10.1016/j.jad.2018.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L, & Davies M (2017). Impact of comorbid anxiety in an effectiveness study of interpersonal psychotherapy for depressed adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 45(8), 904–912. 10.1097/01.chi.0000222791.23927.5f [DOI] [PubMed] [Google Scholar]


