Abstract
Theta burst stimulation (TBS) has been widely used in the treatment of mental disorders, but the cerebral functional difference between intermittent TBS (iTBS) and continuous TBS (cTBS) after one single session of stimulation is not clear. Here we applied resting‐state functional magnetic resonance imaging (RS‐FMRI) to evaluate the alterations in intrinsic brain activity after iTBS and cTBS in the precuneus. We recruited 32 healthy young adults and performed a single session each of iTBS and cTBS at a 1‐week interval. RS‐fMRI was collected at baseline before and immediately after the stimulation. Parameters for regional brain activity (ALFF/fALFF/ReHo) and functional connectivity (FC) with the stimulated site of the precuneus after iTBS and cTBS were calculated and compared between each stimulation using a paired t‐test. Correlation analysis among those parameters was calculated to explore whether changes in functional connectivity were associated with local spontaneous activity. After iTBS stimulation, fALFF increased in the bilateral precuneus, while fALFF decreased in the bilateral middle temporal gyrus. Reductions in precuneus FC were found in the bilateral cuneus, superior occipital gyrus, superior temporal gyrus, precentral gyrus, and postcentral gyrus, which correlated with regional activity. After cTBS, fALFF decreased in the bilateral insula, and precuneus FC was decreased in the bilateral inferior occipital gyrus and increased in the thalamus. In the current study, we observed that one session of iTBS or cTBS could cause inhibitory effects in remote brain regions, but only iTBS caused significant local activation in the target region.
Keywords: fMRI, functional connectivity, precuneus, spontaneous activity, theta burst stimulation
We explored the brain activity after one session of theta burst stimulation (TBS) in precuneus. The spontaneous activity increased in the right precuneus after iTBS but not cTBS. Reduced functional connectivity (FC) of the precuneus with other cortex after TBS were related to regional activity
1. INTRODUCTION
Theta burst stimulation (TBS) is a transcranial magnetic stimulation (TMS) protocol in which 50 Hz triplets are repeated at 5 Hz (Huang et al., 2005; Wischnewski & Schutter, 2015), so TBS has the advantage of delivering more pulses in a shorter period of time than the conventional mode (Barker et al., 1985). Intermittent TBS (iTBS) was conducted with trains of 2 s repeated every 10 s, and MEP studies reported that iTBS had a localized cortical activation effect (Gedankien et al., 2017), and it has been used for the treatment of depression (Cole et al., 2020a). In addition, continuous TBS (cTBS), which is conducted without intervals, has a local cortical inhibition effect (Chung et al., 2017), and it has been used in the treatment of anxiety disorders (Lefaucheur et al., 2020). Functional magnetic resonance imaging (fMRI) scans conducted after TBS have been used to examine the immediate response of the brain to TBS (Bergmann et al., 2021). However, how a session of TBS is regulated at the whole‐brain level remains unclear.
The precuneus is a key hub in the default mode network (DMN) (Luo et al., 2020; Zhang & Li, 2012), participating in autobiographical memory retrieval (Müller et al., 2018), emotional stimulus processing (Cavanna & Trimble, 2006), and self‐related processing (Cabanis et al., 2013). The precuneus is not only engaged in internal attention, but also interacts with the left frontal–parietal network to participate in external attention tasks (Fransson & Marrelec, 2008), Previous studies have found that the precuneus and left frontoparietal network (lFPN) display state‐dependent interactions (Utevsky et al., 2014), and damage to the precuneus/posterior cingulate cortex can lead to dysfunction of intentional activities which result in schizophrenia, autism, depression, and attention deficit hyperactivity disorder (Leech & Sharp, 2014; Leech & Smallwood, 2019). Previous EEG studies suggested that high‐frequency stimulation of the precuneus can improve episodic memory in patients with Alzheimer disease (Koch et al., 2018), but the whole‐brain mechanism of the regulatory effect of this target is not clear.
To better understand the changes in overall brain function after iTBS and cTBS precuneus stimulation, we assessed each change in brain activity using resting functional magnetic resonance imaging (RS‐FMRI), iTBS, and cTBS precuneus functional after stimulation. To characterize the regional effect of TBS, we calculated the fractional amplitude of low‐frequency fluctuations (ALFF), fractional ALFF (fALFF), and regional homogeneity (ReHo) (Zang et al., 2004; Zou et al., 2008), and compared the changes in whole‐brain spontaneous activity before and after iTBS and cTBS. Then, we investigated the changes in the functional connectivity between the stimulated site and the whole brain after iTBS and cTBS to explore the regulatory role of the precuneus in brain networks as the key node of the DMN.
2. MATERIAL AND METHODS
2.1. Study design, participants, and TMS protocol
Thirty‐two 18‐ to 26‐year‐old college students (15 women, 17 men, mean age 22.31 years, SD 2.16 years) were recruited to participate in this experiment. The inclusion criteria were self‐reported absence of physical and mental illness. Coffee and tea were banned for 12 h before the experiment. Individuals who were taking regular medication were excluded.
After baseline MRIscans, we used magnetic resonance image navigation (Junjianwanfeng, NT‐1000, China) to target TMS based on the subjects' structural MRI. As Figure 1 shows, the stimulation target was the right precuneus, for which the MNI coordinates were x = 6, y = −70, z = 44 (Koch et al., 2018; Kwok et al., 2012; Ye et al., 2019). Then the individual's target was obtained by reverse registration of MNI coordinates. The effect range of the actual coils was closer to the superior parietal lobule cortex. A second MRI scan was performed immediately after iTBS. To eliminate the interference between different modes of stimulation at the same target, cTBS stimulation was performed 1 week after the interval. A third MRI scan was performed immediately after the stimulation.
FIGURE 1.
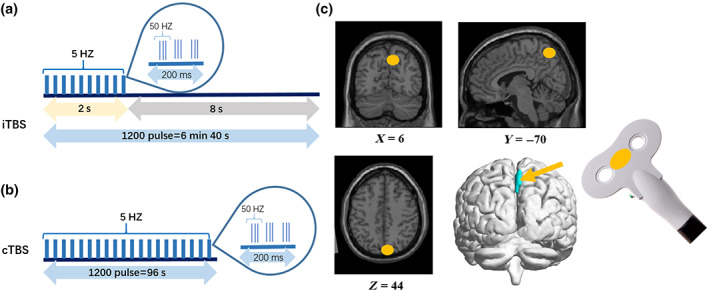
Transcranial magnetic stimulation protocol and target. (a) iTBS protocol and cTBS protocol; (b) TMS target placed in the right precuneus (MNI: X = 6, Y = −70, Z = 44). cTBS, continuous theta burst stimulation; iTBS, intermittent theta burst stimulation; TMS, transcranial magnetic stimulation.
All participants underwent TMS using the Pulse Magnetic Stimulator (Junjianwanfeng, RT‐100, China). Prior to stimulation, the M1 region of the right motor cortex was located with the help of transcranial magnetic stimulation (TMS) navigation, followed by motor threshold (MT) detection. The motor threshold is based on the minimum strength required to activate the abductor hallucis brevis and the strength required to activate the muscle contraction 5 out of 10 times. The stimulus intensity was 100% of the MT. iTBS was standard mode, 5 Hz nested 50 Hz mode, stimulation interval of 2 s every 8 s, 1200 pulses, a total of 6 min and 40 s. cTBS had no stimulation interval and delivered 1200 pulses for 48 s.
2.2. Magnetic resonance scanning parameters
Since the structural MRI was scanned first, the average time for functional imaging to begin scanning was 10 min after stimulation. The average time for spectral scanning to begin scanning was 20 min after stimulation. MRI data acquisition was carried out by using a 3.0 T UNITED IMAGING MRI scanner at West China Hospital of Sichuan University, Chengdu, China. All scans were performed on a 12‐channel phased‐array head coil with foam pads to restrict head motion. All participants stayed in a supine position, remained awake with their eyes closed, and relaxed without concentrating on anything in particular. Foam pads were employed to reduce head motion. To attenuate the scanner noise, the participants wore earplugs during scanning. The MRI scanning sequence parameters were as follows:
The 3D T1‐weighted magnetization‐prepared rapid acquisition gradient‐echo sequence: repetition time (TR) = 6.7 ms; slices = 220; inversion time (TI) = 950 ms; flip angle (FA) = 8°; voxel size = 1 × 1 × 1 mm3; and field of view (FOV) = 256 mm × 256 mm.
fMRI uses a T2‐weighted echo‐planar imaging (EPI) sequence: time points = 240; slice thickness = 3.5 mm; slices = 34 mm; interslice gap = 20 mm; echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; FA = 90°; matrix = 64 × 64; voxel size = 3.5 × 3.5 × 3.5 mm3; and FOV = 224 mm × 224 mm.
2.3. Imaging preprocessing
The 3D T1 and T2 weighted images of each subject were examined manually for anatomic abnormalities. Both 3D T1 and RS‐fMRI data were preprocessed using the DPABI toolbox (http://rfmri.org/dpabi, version 4.3) (Yan et al., 2016), which utilizes Statistical Parametric Mapping software. Prior to image preprocessing, the radiologist performed a manual examination and excluded data with significant anatomical abnormalities before image preprocessing. The first 10 time points were discarded to avoid any nonequilibrium effects of tissue magnetization. Then, we conducted slice time correction, excluded images with a maximum head motion >1.0 mm, and performed head movement correction with six motion parameters. Then, we registered the fMRI images to 3D‐T1 anatomical images and transformed them to the Montreal Neurological Institute (MNI) template with 3 mm × 3 mm × 3 mm resolution. The global mean signal, cerebrospinal fluid (CSF), and white matter signals were corrected as nuisance covariates to reduce nonneuronal contributions to BOLD signals.
2.4. Regional spontaneous activity calculation
The spectral data were processed using a synthetic data processing platform, and the integral under the curve was calculated to represent the concentration of each transmitter. To characterize the local activity and its consistency in resting brain regions (Zang et al., 2004; Zou et al., 2008), the regional homogeneity (ReHo) was calculated by measuring the similarity of a given voxel to its 26 surrounding voxels. Then, the images were spatially smoothed by using an 8 mm full‐width‐half‐maximum Gaussian kernel. The amplitude of low‐frequency fluctuations (ALFF) and fractional ALFF (fALFF) was calculated before smoothing and bandpass filtering (0.01–0.1).
2.5. Seed‐based region of interest of functional connectivity
The functional connectivity was calculated after smoothing. The peak coordinate of the right precuneus (X = 6, Y = −70, Z = 44) was used as the center of the circle, and a small ball was drawn with a radius of 8 mm to construct the region of interest (ROI). The seed point was used to calculate the functional linkage map between all voxels in the whole brain and the region of interest.
2.6. Statistical analysis
We conducted a paired t‐test of images based on statistical parametric mapping to calculate the brain regional activity with the instantaneous effect of theta burst stimulation. Gaussian random field (GRF) correction was used with voxel p < .005 and cluster p < .005 (Shunkai et al., 2021). Furthermore, we conducted conjunction analysis by using the ImCalc function in Statistical Parametric Mapping (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/) to extract the common regions among ALFF/fALFF and ReHo changes after TBS. If there is a brain region where all the local spontaneous activities increase or decrease at the same time after the same stimulation, this brain region will be extracted as the ROI mask, and the functional connectivity changes between this seed and the whole brain will be calculated.
The changes in the functional connectivity of the whole brain with the stimulus site (the right precuneus) after iTBS and cTBS were also calculated using paired t‐test with the same threshold of GRF correction. Then, we used the brain regions with significant changes in functional connectivity with the right precuneus after TBS stimulation (Z > 4) as the mask of the brain regions of interest and obtained six ROIs after iTBSas follows: right sensorimotor cortex (R‐SMC), left sensorimotor cortex (L‐SMC), right superior temporal gyrus (R‐STG), left superior temporal gyrus (L‐STG), right insula (R‐insula), and b‐occipital cortex (B‐OCC); and three ROIs after cTBS as follows: insula, right lingual, and thalamus. Functional connectivity signals, ALFF/fALFF and ReHo values were extracted from the brain region by using those masks above, and correlation analysis was performed between the functional connectivity in each ROI with its local spontaneous activity and FC in the same region. And Granger causality test (SPAAAU software online) was used to explore whether the functional connectivity changes of each ROI had a causal relationship with the ALFF/fALFF and ReHo changes in the same region.
3. RESULTS
3.1. Regional effect of iTBS and cTBS on the precuneus
After iTBS, ALFF activity increased in the bilateral precuneus, posterior cingulate cortex, cerebellum posterior lobe and cerebellum crus (GRF corrected: voxel p < .005 and cluster p < .005), and ALFF activity decreased in the bilateral superior temporal gyrus, medial superior occipital gyrus (msOccG), caudal cuneus gyrus cuneus (cCunG), rostral lingual gyrus (rLinG), precentral gyrus, and postcentral gyrus (as Figure 2a and Table 1 show). After iTBS stimulation, fALFF activity increased in the bilateral precuneus and decreased in the bilateral middle temporal gyrus and fusiform gyrus (as Figure 2b and Table 2 show). After iTBS stimulation, ReHo activity increased in the left parietal lobe, posterior cingulate gyrus (PCG), and cerebellum posterior lobe. After iTBS stimulation, ReHo activity decreased in the bilateral medial superior occipital gyrus (msOccG), caudal cuneus gyrus cuneus (cCunG), rostral lingual gyrus (rLinG), precentral gyrus, postcentral gyrus, and right medial frontal gyrus (as Figure 2a and Table 3 show).
FIGURE 2.
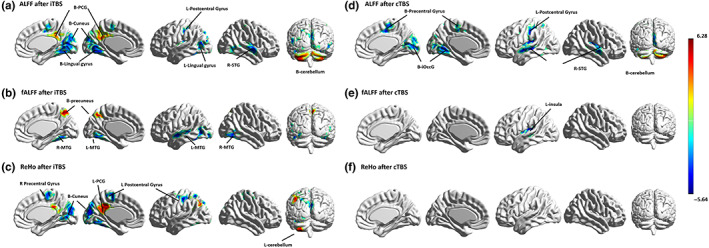
Brain regional activity after iTBS and cTBS on right precuneus. ALFF, amplitude of low‐frequency fluctuations; B, bilateral; cTBS, continuous theta burst stimulation; fALFF, fractional amplitude of low‐frequency fluctuations; iOccG, inferior occipital gyrus; iTBS, intermittent theta burst stimulation; L, left; PCG, posterior cingulate gyrus; MTG, middle temporal gyrus; R, right; ReHo, regional homogeneity; STG, superior temporal gyrus.
TABLE 1.
ALFF change after TBS for right precuneus
| Cluster peak region | Peak MNI coordinate | Peak intensity | Voxels | Structure |
|---|---|---|---|---|
| iTBS effect | ||||
| Left cerebellum | −24 −69 −36 | 6.2777 | 1358 | Cerebellum posterior lobe |
| 309 | Cerebelum_Crus2_L | |||
| 252 | Cerebelum_Crus2_R | |||
| 246 | Cerebelum_Crus1_L (aal) | |||
| 131 | Cerebelum_Crus1_R (aal) | |||
| Cingulate gyrus | 0 −48 33 | 4.3262 | 172 | Cingulate Gyrus |
| 113 | Precuneus_L | |||
| 104 | Precuneus_R | |||
| Sub‐lobar | 36 −9 6 | −4.9906 | 290 | Temporal_Sup_R |
| 137 | Sub‐lobar | |||
| Medial superior occipital gyrus (msOccG) | −21 −81 39 | −5.3782 | 517 | Cuneus |
| 451 | Lingual gyrus | |||
| 340 | Temporal lobe | |||
| 281 | Calcarine_R (aal) | |||
| 256 | Lingual_L (aal) | |||
| 250 | Lingual_R (aal) | |||
| Postcentral gyrus | −42 −3 15 | −5.6353 | 164 | Precentral gyrus |
| 155 | Postcentral gyrus | |||
| 144 | Sub‐gyral | |||
| 138 | Postcentral_L (aal) | |||
| 116 | Medial frontal gyrus | |||
| cTBS effect | ||||
| Cerebellum posterior lobe | −27 −72 −51 | 5.5281 | 376 | Cerebellum posterior lobe |
| 138 | Cerebelum_Crus1_L | |||
| Cerebelum_Crus2_R | 18 −81 −48 | 5.6955 | 565 | Right cerebellum |
| 217 | Cerebelum_Crus2_R | |||
| 161 | Cerebelum_Crus1_R | |||
| Inferior occipital gyrus (iOccG) | 27 −63 18 | −4.09 | 313 | Cuneus |
| 238 | Lingual gyrus | |||
| 223 | Lingual_L | |||
| 107 | Fusiform_L | |||
| Temporal_Sup_R | 60 −12 0 | −5.1525 | 141 | Temporal_Sup_R (aal) |
| 129 | Sub‐lobar | |||
| 110 | Superior temporal gyrus | |||
| Sub‐gyral | −24 −15 48 | −6.7399 | 363 | Sub‐gyral |
| 281 | Temporal lobe | |||
| 248 | Postcentral_L (aal) | |||
| 246 | Sub‐lobar | |||
| 244 | Postcentral gyrus | |||
| 216 | Precentral gyrus | |||
| 180 | Medial frontal gyrus | |||
| 176 | Insula | |||
| 131 | Temporal_Mid_L (aal) | |||
| 126 | Temporal_Sup_L (aal) |
Note: GRF corrected voxel p < .005; cluster p < .005.
Abbreviations: ALFF, amplitude of low‐frequency fluctuations; cTBS, continuous theta burst stimulation; iTBS, intermittent theta burst stimulation; L, left; Mid, middle; PCG, posterior cingulate gyrus; R, right; Sup, superior.
TABLE 2.
fALFF change after TBS for right precuneus
| Cluster peak region | Peak MNI coordinate | Peak intensity | Voxels | Structure |
|---|---|---|---|---|
| iTBS effect | ||||
| Right middle temporal gyrus | 39 −54 12 | −4.233 | 222 | Right fusiform gyrus |
| 120 | Right middle temporal gyrus | |||
| Left middle temporal gyrus | −45 −54 −21 | −5.191 | 370 | Left middle temporal gyrus |
| 247 | Left fusiform gyrus | |||
| Bilateral precuneus | 6 −72 54 | 4.9912 | 238 | Right precuneus |
| 74 | Left precuneus | |||
| cTBS effect | ||||
| Left insula | −33 −24 15 | −5.2212 | 170 | Left sub‐lobar |
| 119 | Left insula |
Note: GRF corrected voxel p < .005; cluster p < .005.
Abbreviations: cTBS, continuous theta burst stimulation; fALFF, fractional amplitude of low‐frequency fluctuations; iTBS, intermittent theta burst stimulation.
TABLE 3.
ReHo change after TBS for right precuneus
| Cluster peak region | Peak MNI coordinate | Peak intensity | Voxels | Structure |
|---|---|---|---|---|
| iTBS effect | ||||
| Left cerebellum | −27 −75 −51 | 5.7648 | 243 | Cerebellum posterior lobe |
| Cuneus | −6 −87 18 | −4.7818 | 626 | Left cuneus |
| 334 | Left lingual gyrus | |||
| 273 | Left fusiform gyrus | |||
| 135 | Right cuneus | |||
| Left parietal–occipital junction | −24 −39 36 | 4.5277 | 258 | Left parietal lobe |
| 203 | Left posterior cingulate gyrus | |||
| 164 | Left inferior occipital gyrus (iOccG) | |||
| 132 | Left lateroventral fusiform gyrus (A37lv) | |||
| 103 | Left angular gyrus | |||
| Left parietal lobe | −30 −54 60 | −4.5272 | 182 | Left postcentral gyrus |
| 136 | Left precentral gyrus | |||
| Right precentral gyrus | 36 −30 60 | −3.896 | 159 | Right precentral gyrus |
| 147 | Right medial frontal gyrus | |||
| cTBS effect | ||||
| — | — | — | — | — |
Note: GRF corrected voxel p < .005; cluster p < .005.
Abbreviations: cTBS, continuous theta burst stimulation; iTBS, intermittent theta burst stimulation; ReHo, regional homogeneity.
After cTBS (Figure 2d), ALFF activity increased in the bilateral cerebellum crus (GRF corrected: voxel p < .005 and cluster p < .005) and decreased in the bilateral superior temporal gyrus, left middle temporal gyrus, lingual gyrus, fusiform, medial frontal gyrus, precentral, and postcentral gyrus. fALFF decreased in the left sublobar and insula after cTBS. There were no changes in ReHo after cTBS.
3.2. Connectivity effect of iTBS and cTBS on the precuneus
Figure 3 and Table 4 show reduced functional connectivity in the right precuneus with the bilateral cuneus, lingual gyrus, superior occipital gyrus, superior temporal gyrus, precentral gyrus, postcentral gyrus, cerebellum anterior lobe, and right insula after iTBS stimulation (GRF corrected: voxel p < .005 and cluster p < .005). Figure 3 shows that functional connectivity increased between the right precuneus and bilateral thalamus and decreased between the right precuneus and bilateral inferior occipital gyrus (iOccG) after cTBS stimulation (GRF corrected: voxel p < .005 and cluster p < .005).
FIGURE 3.
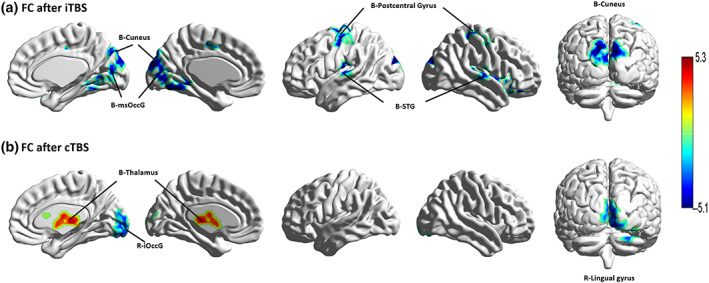
Functional connectivity after iTBS and cTBS on right precuneus. B, bilateral; cTBS, continuous theta burst stimulation; FC, functional connectivity; iOccG, inferior occipital gyrus; iTBS, intermittent theta burst stimulation; L, left; msOccG, medial superior occipital gyrus; R, right; STG, superior temporal gyrus.
TABLE 4.
FC of right precuneus after TBS
| Cluster peak region | Peak MNI coordinate | Peak intensity | Voxels | Structure |
|---|---|---|---|---|
| iTBS effect | ||||
| Occipital lobe | 12 −90 27 | −6.4555 | 672 | Caudal cuneus gyrus cuneus (cCunG) |
| 191 | Lingual_L | |||
| 184 | Rostral lingual gyrus (rLinG) | |||
| 300 | Medial superior occipital gyrus (msOccG) | |||
| 149 | Cuneus_L | |||
| 134 | Cuneus_R | |||
| 126 | Fusiform_L | |||
| 124 | Cerebellum anterior lobe | |||
| Temporal_Sup_R | 39 −33 9 | −5.7876 | 293 | Temporal_Sup_R |
| 110 | Sub‐lobar | |||
| Temporal_Sup_L | −60 −21 9 | −6.0189 | 200 | Temporal_Sup_L |
| Precentral_R | 39 −3 24 | −6.2062 | 205 | Precentral_R |
| 151 | Postcentral_R | |||
| Precentral_L | −24 −15 69 | −5.8008 | 200 | Precentral_L |
| 123 | Postcentral_L | |||
| Insula | 45 18 −12 | −5.518 | 196 | Insula_R |
| cTBS effect | ||||
| Occipital lobe | 24 −87 −24 | −5.0989 | 348 | Inferior occipital gyrus (iOccG) |
| 138 | Lingual gyrus | |||
| 109 | Cuneus | |||
| Sub‐lobar | 21 −6 18 | 5.2971 | 550 | Sub‐lobar |
| 129 | Thalamus |
Note: GRF corrected voxel p < .005; cluster p < .005.
Abbreviations: cTBS, continuous theta burst stimulation; FC, functional connectivity; iTBS, intermittent theta burst stimulation; L, left; PCG, posterior cingulate gyrus; R, right; Sup, superior.
3.3. Alteration of FC after iTBS
After iTBS (as Figure 4 show), the decreased functional connectivity with the right precuneus of the bilateral cuneus, medial superior occipital gyrus, superior temporal gyrus, right postcentral gyrus, and left precentral gyrus was significantly positively correlated with the ALFF/fALFF and ReHo values of the same regions (p < .05). After cTBS, the decreased functional connectivity with the right precuneus of the inferior occipital gyrus was significantly positively correlated with the ALFF and ReHo values of the same regions (p < .05). FC with the right precuneus of the inferior occipital gyrus was significantly positively correlated with FC between the right precuneus and left insula (r = .6, p < .001), and ALFF in the inferior occipital gyrus was positively correlated with ALFF in the left insula (r = .575, p = .001). However, the increased functional connectivity between the right precuneus and bilateral thalamus had no significant correlation with the ALFF/fALFF and ReHo values of the bilateral thalamus and insula after cTBS (p > .05). Moreover, we found that only FC between right precuneus and bilateral inferior superior gyrus has causal relationship with ReHo in the same region by Granger causality test (F = 3.522, p = .045). There are no functional connectivity changes of each ROI that had a causal relationship with the ALFF/fALFF and ReHo changes in the same region.
FIGURE 4.
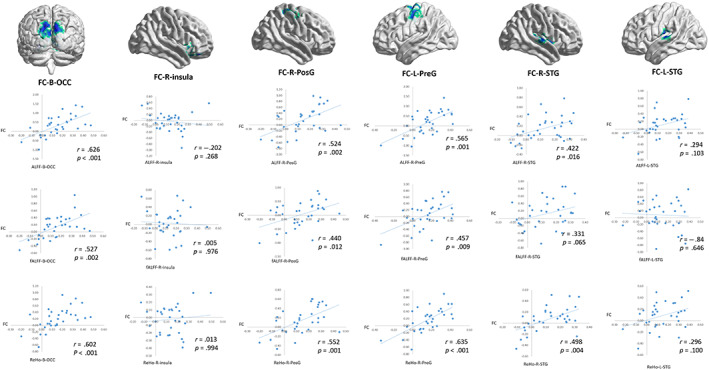
Regional correlation of FC within the right precuneus after iTBS. ALFF, amplitude of low‐frequency fluctuations; B, bilateral; cTBS, continuous theta burst stimulation; fALFF, fractional amplitude of low‐frequency fluctuations; FC, functional connectivity; iTBS, intermittent theta burst stimulation; L, left; OCC, occipital cortex; PosG, postcentral gyrus; PreG, postcentral gyrus; R, right; STG, superior temporal gyrus.
3.4. Common region which ALFF/fALFF and ReHo change
After iTBS, ALFF/fALFF and ReHo all decreased in the left lingual gyrus and fusiform gyrus (peak MNI −21 −67 −9; Number of voxels: 119) is the common region which (as Figure S1 show). And we found that only FC between bilateral precuneus (peak MNI −21 −67 −9; Number of voxels: 119) and left lingual gyrus decreased after iTBS (as Figure S2 show). In addition, there was no overlap of spontaneous activity changes in any brain regions after cTBS.
4. DISCUSSION
In the current study, we found that spontaneous neural activity increased in the precuneus and posterior cingulate cortex after a single session of iTBS on the precuneus. Further analysis showed that the functional connectivity between the precuneus and sensorimotor and the visual cortex was reduced, and the decrease in functional connectivity was correlated with the decrease in local spontaneous activity in the same regions. On the other hand, after cTBS stimulation, fALFF in the posterior insula decreased, and functional connectivity between the right precuneus and bilateral thalamus increased. This study revealed the local activation and global effect of TBS, extending the results of previous studies.
4.1. Regional effect of iTBS
Our study found that the fALFF increased in the right precuneus after iTBS. This finding reveals the local activation effect of iTBS stimulating brain sites from multiple dimensions of spontaneous activity and spectrum. This is consistent with previous meta‐analyses of EEG studies reporting that iTBS significantly increased cortical excitability from 3 to 60 min after stimulation (Wischnewski & Schutter, 2015). TBS was found to prolong cortical excitability for 10–50 min (Chung et al., 2016; Corp et al., 2020). Notably, stimulation of the right precuneus also induced fALFF to increase in the contralateral precuneus, so we speculated that there is a synergistic effect of functional connectivity in the bilateral precuneus, which will lead to increased activity of the contralateral precuneus when stimulating one side of the precuneus. Considering that the direct stimulation range of the type 8 coil was 2–2.5 cm (Deng et al., 2020), the direct effect of magnetic stimulation cannot be ignored.
After iTBS stimulation, ALFF increased in the bilateral posterior cingulate gyrus (PCG), and ReHo activity increased in the left cingulate gyrus (PCG). The PCG is located in the subcortical region near the lower part of the precuneus (Leech & Smallwood, 2019). Both the PCG and precuneus are engaged in the retrieval of episodic memory (Greicius et al., 2003), which contributes to internally directed cognition (Leech & Sharp, 2014) and plays a key role in the DMN. A low‐frequency amplitude represents changes in local spontaneous brain activity, and local consistency represents local functional connectivity (Jiang & Zuo, 2016). Our results showed that iTBS in the right precuneus caused an increase in spontaneous activity in the posterior cingulate gyrus adjacent to the stimulation site, as well as changes in local functional connectivities in the contralateral posterior cingulate gyrus.
4.2. Connectivity effect of iTBS
After right precuneus iTBS, ReHo activity decreased in the bilateral precentral gyrus and left postcentral gyrus, and the functional connectivity between the right precuneus and bilateral precentral gyrus and left postcentral gyrus was decreased compared with baseline. Both the precentral and postcentral gyrus belong to the sensorimotor network, and the interaction between the sensorimotor network and DMN contributes to cognitive processing such as motor learning, emotion recognition, and expression. Our study extends the interaction between these two networks; in other words, as an important node of the DMN, the precuneus can inhibit the sensorimotor network when receiving local excitatory objective stimuli.
Notably, the reduction in functional connectivity of the stimulation target was positively associated with the reduction in ALFF and ReHo in the same region after iTBS. This finding revealed the indirect effects of TMS on the distal brain areas of the target region through functional connectivity (Cole et al., 2020b; Fox et al., 2012), and this effect depends on the functional network of the target and its interaction with other networks (Cocchi et al., 2015). Although iTBS has a regional effect on cortical excitation, it does not excite the whole brain cortical region. It only regulates the brain network to which the stimulation site belongs through functional connections, thus affecting the activity of the distal brain region. In other words, the activation effect of iTBS is limited to the local brain region of the stimulation target, and its effect on the whole brain depends on the location of the stimulation target in the whole brain functional network and the interactions between other networks.
After iTBS stimulation, ALFF activity decreased in the bilateral superior temporal gyrus, fALFF activity decreased in the bilateral middle temporal gyrus and fusiform gyrus, and ReHo activity decreased in the left fusiform gyrus. The functional connection between the right precuneus and superior temporal gyrus was weakened after iTBS stimulation. Although both the precuneus and MTG are involved in social functions, such as reflecting the mental states of the self and others (Rolls et al., 2020), they are in different subsystems as part of the DMN. MTG is mainly involved in complex thinking functions such as self‐construction and belief (Boccadoro et al., 2019). The precuneus is not only involved in self‐referential cognitive activities such as rumination (Zhou et al., 2020). It also regulates the interaction between the middle temporal gyrus and other default network brain regions (Andrews‐Hanna et al., 2010). Our findings are consistent with temporal gyrus activity being regulated by the precuneus. However, whether this functional linkage has a structural basis is unclear and requires further study.
4.3. Regional and connectivity effect difference between iTBS and cTBS
Notably, functional connectivity decreased between the right precuneus and bilateral medial superior occipital gyrus (msOccG), caudal cuneus gyrus (cCunG), and rostral lingual gyrus (rLinG) after iTBS, while functional connectivity decreased between the right precuneus and bilateral inferior occipital gyrus (iOccG) after cTBS. Importantly, the left lingual gyrus is the common region which ALFF/fALFF and ReHo all decreased after iTBS, and we found that only FC between bilateral precuneus and left lingual gyrus decreased after iTBS. That is, after stimulation with different modes of TBS, the functional connectivity decreased between the stimulation site (right precuneus) and the occipital lobe with different locations. The mechanism of this phenomenon may be that the precuneus triggers different changes in brain network activity in response to different stimuli.
Decreased ALFF and ReHo were found in the bilateral msOccG and cuneus after iTBS, and the reduction in precuneus FC in the bilateral cuneus and msOccG was correlated with regional activity. Moreover, Granger causality analysis showed that only FC between right precuneus and bilateral occipital gyrus has causal relationship with ReHo in the same region, which implied that iTBS of the precuneus may inhibit spontaneous activity in the msOccG and cuneus. Both the msOccG and cCunG belong to visual networks and are involved in visual signal integration (Bello et al., 2020), and the visual network then transmits these signals to the DMN, which translates them into abstract visual perception representations such as aesthetics (Vessel et al., 2019). Therefore, the inhibitory effect on the msOccG and cuneus is due to the excitatory effect of the default network by precuneus iTBS.
On the other hand, the functional connectivity between the precuneus and the inferior occipital gyrus after cTBS is positively associated with spontaneous activity in this location. The iOccG receives visual signals from the retina and processes the original visual information and then participates in spatial cognition (Li et al., 2019). In other words, the spontaneous activity of the inferior occipital gyrus inhibits the reception and recognition of incoming signals from the retina. In particular, after cTBS, the decrease in ALFF in the inferior occipital gyrus was positively correlated with the decrease in ALFF in the left insula, and the FC between the right precuneus and left insula was positively correlated with the FC between the right precuneus and inferior occipital gyrus. The inferior fronto‐occipital tract arises in part from the inferior occipital gyrus, and the precuneus passes through the insular white matter to the frontal lobe (De Benedictis et al., 2021). Both the insula and precuneus are involved in self‐information processing and belong to midline cortical regions (Cabanis et al., 2013), and the insula's function is to selectively pay attention to external and internal stimuli (Menon & Uddin, 2010). Therefore, we speculate that the insula changed its attentional choices to inhibit the primary visual cortex after precuneus cTBS. Even though there is no causal relationship between the FC of right precuneus with left insula and ALFF/fALFF and ReHo changes in the same region, the change in spontaneous activity in the left insula may still be due to the remote impact of cTBS on the whole brain, which is regulated via functional connectivity.
The functional connectivity between the right precuneus and bilateral thalamus increased compared with baseline. Our findings indicated that cTBS has an excitatory effect on the deep areas of the brain, while there was no change in the regional effect at the cortical stimulation site after cTBS. A previous study also reported no local fMRI signals in the stimulated area after low‐frequency TMS (Castrillon et al., 2020). This result is inconsistent with the cortical inhibitory effect of cTBS reported by an EEG study (Chung et al., 2017). One possible explanation is that EEG is more sensitive to local cortical activity (Bestmann & Feredoes, 2013). Furthermore, we speculated that the increase in the functional connectivity between the precuneus and the thalamus changed the excitability of the thalamus after cTBS, and the thalamus, as the hub of signal filtering, affected the neural activity of the brain region where the signal was projected, such as the insula. However, the effect of cTBS is more complex and needs to be verified by more experiments.
In addition, spontaneous activity increased in the cerebellum after both iTBS and cTBS, but the scope of the cerebellum after cTBS was smaller than that after iTBS. The posterior cerebellum is involved in social reasoning and mentalization (van Overwalle et al., 2019; van Overwalle & Mariën, 2016), and the functional connection between the posterior cerebellum and the precuneus has been demonstrated in previous studies (van Overwalle et al., 2020). With iTBS stimulation, more brain regions experienced changes in local consistency. It is believed that this is not only a direct effect of the activity of brain regions connected with fALFF function but also the result of the interaction between nodes of the brain functional connection network.
4.4. Limitations
First, the sample size of this study was small, with only 32 participants. Future studies with larger samples are needed to obtain more generalizable results. Second, we only included young people, and this age group is relatively limited, and the findings need to be confirmed by samples with a larger age span in the future. In addition, we did not compare brain activity differences in objective stimuli between diseased and health states, which needs to be explored in the future. In addition, magnetic resonance imaging data before cTBS stimulation were lacking in this study. Although previous studies have shown that individual TMS effects could be washed out after a single stimulation for 1 week, it is difficult to completely exclude the influence of iTBS in the previous week on the brain state before cTBS stimulation.
5. CONCLUSION
Overall, our study reveals differences in the effects of different TBS stimulation modes at the same site, and the local stimulation effect spread widely to associated functional brain networks. In contrast to iTBS, which showed significant local activation in the target region and inhibitory effects in remote brain regions, cTBS showed an activation effect in deep brain regions, while there was no significant difference in the fMRI signal of the local cortex. Notably, the remote effects of stimulation depend on the stimulation site and its interaction with distal brain regions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1. Common region which ALFF/fALFF and ReHo all decreased after iTBS
Figure S2. Functional connectivity of left lingual gyrus after iTBS
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (Grant Number 82027808); CAMS clinical and translational research fund (Grant Number 2021‐I2M‐C&T‐B‐097); CAMS Innovation Fund for Medical Sciences (Grant Number: 2019‐I2M‐5‐012); Sichuan Science and Technology Program (Grant Number: 2022YFS0048); Science Specialty Program of Sichuan University (Grant/Award Number: 2020SCUNL210).
Xu, X. , Li, X. , Qi, X. , Jiang, X. , Xing, H. , Huang, X. , & Gong, Q. (2023). Effect of regional intrinsic activity following two kinds of theta burst stimulation on precuneus. Human Brain Mapping, 44(6), 2254–2265. 10.1002/hbm.26207
Funding information CAMS Innovation Fund for Medical Sciences, Grant/Award Number: 2019‐I2M‐5‐012; CAMS Clinical and Translational Research Fund, Grant/Award Number: 2021‐I2M‐C&T‐B‐097; National Natural Science Foundation of China, Grant/Award Number: 82027808; Science Specialty Program of Sichuan University, Grant/Award Number: 2020SCUNL210; Sichuan Science and Technology Program, Grant/Award Number: 2022YFS0048
Contributor Information
Haoyang Xing, Email: xhy@scu.edu.cn.
Xiaoqi Huang, Email: julianahuang@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andrews‐Hanna, J. R. , Reidler, J. S. , Sepulcre, J. , Poulin, R. , & Buckner, R. L. (2010). Functional‐anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, A. T. , Jalinous, R. , & Freeston, I. L. (1985). Non‐invasive magnetic stimulation of human motor cortex. Lancet (London, England), 1(8437), 1106–1107. 10.1016/s0140-6736(85)92413-4 [DOI] [PubMed] [Google Scholar]
- Bello, U. M. , Kranz, G. S. , Winser, S. J. , & Chan, C. C. H. (2020). Neural processes underlying mirror‐induced visual illusion: An activation likelihood estimation meta‐analysis. Frontiers in Human Neuroscience, 14, 276. 10.3389/fnhum.2020.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, T. O. , Varatheeswaran, R. , Hanlon, C. A. , Madsen, K. H. , Thielscher, A. , & Siebner, H. R. (2021). Concurrent TMS‐fMRI for causal network perturbation and proof of target engagement. NeuroImage, 237, 118093. 10.1016/j.neuroimage.2021.118093 [DOI] [PubMed] [Google Scholar]
- Bestmann, S. , & Feredoes, E. (2013). Combined neurostimulation and neuroimaging in cognitive neuroscience: Past, present, and future. Annals of the New York Academy of Sciences, 1296, 11–30. 10.1111/nyas.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccadoro, S. , Cracco, E. , Hudson, A. R. , Bardi, L. , Nijhof, A. D. , Wiersema, J. R. , Brass, M. , & Mueller, S. C. (2019). Defining the neural correlates of spontaneous theory of mind (ToM): An fMRI multi‐study investigation. NeuroImage, 203, 116193. 10.1016/j.neuroimage.2019.116193 [DOI] [PubMed] [Google Scholar]
- Cabanis, M. , Pyka, M. , Mehl, S. , Müller, B. W. , Loos‐Jankowiak, S. , Winterer, G. , Wölwer, W. , Musso, F. , Klingberg, S. , Rapp, A. M. , Langohr, K. , Wiedemann, G. , Herrlich, J. , Walter, H. , Wagner, M. , Schnell, K. , Vogeley, K. , Kockler, H. , Shah, N. J. , … Kircher, T. (2013). The precuneus and the insula in self‐attributional processes. Cognitive, Affective, & Behavioral Neuroscience, 13(2), 330–345. 10.3758/s13415-012-0143-5 [DOI] [PubMed] [Google Scholar]
- Castrillon, G. , Sollmann, N. , Kurcyus, K. , Razi, A. , Krieg, S. M. , & Riedl, V. (2020). The physiological effects of noninvasive brain stimulation fundamentally differ across the human cortex. Science Advances, 6(5), eaay2739. 10.1126/sciadv.aay2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chung, S. W. , Hill, A. T. , Rogasch, N. C. , Hoy, K. E. , & Fitzgerald, P. B. (2016). Use of theta‐burst stimulation in changing excitability of motor cortex: A systematic review and meta‐analysis. Neuroscience & Biobehavioral Reviews, 63, 43–64. 10.1016/j.neubiorev.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Chung, S. W. , Lewis, B. P. , Rogasch, N. C. , Saeki, T. , Thomson, R. H. , Hoy, K. E. , Bailey, N. W. , & Fitzgerald, P. B. (2017). Demonstration of short‐term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: A TMS‐EEG study. Clinical Neurophysiology, 128(7), 1117–1126. 10.1016/j.clinph.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Cocchi, L. , Sale, M. V. , Lord, A. , Zalesky, A. , Breakspear, M. , & Mattingley, J. B. (2015). Dissociable effects of local inhibitory and excitatory theta‐burst stimulation on large‐scale brain dynamics. Journal of Neurophysiology, 113(9), 3375–3385. 10.1152/jn.00850.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, E. J. , Stimpson, K. H. , Bentzley, B. S. , Gulser, M. , Cherian, K. , Tischler, C. , Nejad, R. , Pankow, H. , Choi, E. , Aaron, H. , Espil, F. M. , Pannu, J. , Xiao, X. , Duvio, D. , Solvason, H. B. , Hawkins, J. , Guerra, A. , Jo, B. , Raj, K. S. , … Williams, N. R. (2020a). Stanford accelerated intelligent neuromodulation therapy for treatment‐resistant depression. American Journal of Psychiatry, 177(8), 716–726. 10.1176/appi.ajp.2019.19070720 [DOI] [PubMed] [Google Scholar]
- Corp, D. T. , Bereznicki, H. G. K. , Clark, G. M. , Youssef, G. J. , Fried, P. J. , Jannati, A. , Davies, C. B. , Gomes‐Osman, J. , Stamm, J. , Chung, S. W. , Bowe, S. J. , Rogasch, N. C. , Fitzgerald, P. B. , Koch, G. , Di Lazzaro, V. , Pascual‐Leone, A. , Enticott, P. G. , & Big TMS Data Collaboration . (2020). Large‐scale analysis of interindividual variability in theta‐burst stimulation data: Results from the ‘big TMS data collaboration’. Brain Stimulation, 13(5), 1476–1488. 10.1016/j.brs.2020.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis, A. , Marras, C. E. , Petit, L. , & Sarubbo, S. (2021). The inferior fronto‐occipital fascicle: A century of controversies from anatomy theaters to operative neurosurgery. Journal of Neurosurgical Sciences, 65(6), 605–615. 10.23736/S0390-5616.21.05360-1 [DOI] [PubMed] [Google Scholar]
- Deng, Z.‐D. , Luber, B. , Balderston, N. L. , Velez Afanador, M. , Noh, M. M. , Thomas, J. , Altekruse, W. C. , Exley, S. L. , Awasthi, S. , & Lisanby, S. H. (2020). Device‐based modulation of neurocircuits as a therapeutic for psychiatric disorders. Annual Review of Pharmacology and Toxicology, 60, 591–614. 10.1146/annurev-pharmtox-010919-023253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Buckner, R. L. , White, M. P. , Greicius, M. D. , & Pascual‐Leone, A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72(7), 595–603. 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson, P. , & Marrelec, G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage, 42(3), 1178–1184. 10.1016/j.neuroimage.2008.05.059 [DOI] [PubMed] [Google Scholar]
- Gedankien, T. , Fried, P. J. , Pascual‐Leone, A. , & Shafi, M. M. (2017). Intermittent theta‐burst stimulation induces correlated changes in cortical and corticospinal excitability in healthy older subjects. Clinical Neurophysiology, 128(12), 2419–2427. 10.1016/j.clinph.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M. D. , Krasnow, B. , Reiss, A. L. , & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y.‐Z. , Edwards, M. J. , Rounis, E. , Bhatia, K. P. , & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Jiang, L. , & Zuo, X.‐N. (2016). Regional homogeneity: A multimodal, multiscale neuroimaging marker of the human connectome. The Neuroscientist, 22(5), 486–505. 10.1177/1073858415595004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, G. , Bonnì, S. , Pellicciari, M. C. , Casula, E. P. , Mancini, M. , Esposito, R. , Ponzo, V. , Picazio, S. , Di Lorenzo, F. , Serra, L. , Motta, C. , Maiella, M. , Marra, C. , Cercignani, M. , Martorana, A. , Caltagirone, C. , & Bozzali, M. (2018). Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. NeuroImage, 169, 302–311. 10.1016/j.neuroimage.2017.12.048 [DOI] [PubMed] [Google Scholar]
- Kwok, S. C. , Shallice, T. , & Macaluso, E. (2012). Functional anatomy of temporal organisation and domain‐specificity of episodic memory retrieval. Neuropsychologia, 50(12), 2943–2955. 10.1016/j.neuropsychologia.2012.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, R. , & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, R. , & Smallwood, J. (2019). The posterior cingulate cortex: Insights from structure and function. Handbook of Clinical Neurology, 166, 73–85. 10.1016/B978-0-444-64196-0.00005-4 [DOI] [PubMed] [Google Scholar]
- Lefaucheur, J.‐P. , Aleman, A. , Baeken, C. , Benninger, D. H. , Brunelin, J. , Di Lazzaro, V. , Filipović, S. R. , Grefkes, C. , Hasan, A. , Hummel, F. C. , Jääskeläinen, S. K. , Langguth, B. , Leocani, L. , Londero, A. , Nardone, R. , Nguyen, J. P. , Nyffeler, T. , Oliveira‐Maia, A. J. , Oliviero, A. , … Ziemann, U. (2020). Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014‐2018). Clinical Neurophysiology, 131(2), 474–528. 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Kong, F. , Ji, M. , Luo, Y. , Lan, J. , & You, X. (2019). Shared and distinct neural bases of large‐ and small‐scale spatial ability: A coordinate‐based activation likelihood estimation meta‐analysis. Frontiers in Neuroscience, 12, 1021. 10.3389/fnins.2018.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Z. , Zeng, L.‐L. , Qin, J. , Hou, C. , Shen, H. , & Hu, D. (2020). Functional parcellation of human brain precuneus using density‐based clustering. Cerebral Cortex, 30(1), 269–282. 10.1093/cercor/bhz086 [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5‐6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, N. G. , Riemer, M. , Brandt, L. , & Wolbers, T. (2018). Repetitive transcranial magnetic stimulation reveals a causal role of the human precuneus in spatial updating. Scientific Reports, 8(1), 10171. 10.1038/s41598-018-28487-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls, E. T. , Zhou, Y. , Cheng, W. , Gilson, M. , Deco, G. , & Feng, J. (2020). Effective connectivity in autism. Autism Research, 13(1), 32–44. 10.1002/aur.2235 [DOI] [PubMed] [Google Scholar]
- Shunkai, L. , Su, T. , Zhong, S. , Chen, G. , Zhang, Y. , Zhao, H. , Chen, P. , Tang, G. , Qi, Z. , He, J. , Zhu, Y. , Lv, S. , Song, Z. , Miao, H. , Hu, Y. , Jia, Y. , & Wang, Y. (2021). Abnormal dynamic functional connectivity of hippocampal subregions associated with working memory impairment in melancholic depression. Psychological Medicine, 1, 1–13. 10.1017/S0033291721004906 [DOI] [PubMed] [Google Scholar]
- Utevsky, A. V. , Smith, D. V. , & Huettel, S. A. (2014). Precuneus is a functional core of the default‐mode network. The Journal of Neuroscience, 34(3), 932–940. 10.1523/JNEUROSCI.4227-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overwalle, F. , de Coninck, S. , Heleven, E. , Perrotta, G. , Taib, N. O. B. , Manto, M. , & Mariën, P. (2019). The role of the cerebellum in reconstructing social action sequences: A pilot study. Social Cognitive and Affective Neuroscience, 14(5), 549–558. 10.1093/scan/nsz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overwalle, F. , & Mariën, P. (2016). Functional connectivity between the cerebrum and cerebellum in social cognition: A multi‐study analysis. NeuroImage, 124, 248–255. 10.1016/j.neuroimage.2015.09.001 [DOI] [PubMed] [Google Scholar]
- van Overwalle, F. , van de Steen, F. , van Dun, K. , & Heleven, E. (2020). Connectivity between the cerebrum and cerebellum during social and non‐social sequencing using dynamic causal modelling. NeuroImage, 206, 116326. 10.1016/j.neuroimage.2019.116326 [DOI] [PubMed] [Google Scholar]
- Vessel, E. A. , Isik, A. I. , Belfi, A. M. , Stahl, J. L. , & Starr, G. G. (2019). The default‐mode network represents aesthetic appeal that generalizes across visual domains. Proceedings of the National Academy of Sciences of the United States of America, 116(38), 19155–19164. 10.1073/pnas.1902650116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski, M. , & Schutter, D. J. L. G. (2015). Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulation, 8(4), 685–692. 10.1016/j.brs.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Yan, C.‐G. , Wang, X.‐D. , Zuo, X.‐N. , & Zang, Y.‐F. (2016). DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Ye, Q. , Zou, F. , Dayan, M. , Lau, H. , Hu, Y. , & Kwok, S. C. (2019). Individual susceptibility to TMS affirms the precuneal role in meta‐memory upon recollection. Brain Structure & Function, 224(7), 2407–2419. 10.1007/s00429-019-01909-6 [DOI] [PubMed] [Google Scholar]
- Zang, Y. F. , Jiang, T. Z. , Lu, Y. L. , He, Y. , & Tian, L. X. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22(1), 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , & Li, C. R. (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59(4), 3548–3562. 10.1016/j.neuroimage.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H.‐X. , Chen, X. , Shen, Y.‐Q. , Li, L. , Chen, N.‐X. , Zhu, Z.‐C. , Castellanos, F. X. , & Yan, C.‐G. (2020). Rumination and the default mode network: Meta‐analysis of brain imaging studies and implications for depression. NeuroImage, 206, 116287. 10.1016/j.neuroimage.2019.116287 [DOI] [PubMed] [Google Scholar]
- Zou, Q.‐H. , Zhu, C.‐Z. , Yang, Y. , Zuo, X.‐N. , Long, X.‐Y. , Cao, Q.‐J. , Wang, Y. F. , & Zang, Y.‐F. (2008). An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: Fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–141. 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Common region which ALFF/fALFF and ReHo all decreased after iTBS
Figure S2. Functional connectivity of left lingual gyrus after iTBS
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


