Abstract
Primary progressive aphasias (PPAs) are a group of neurodegenerative diseases mainly characterized by language impairment, and with variably presence of dysexecutive syndrome, behavioural disturbances and parkinsonism. Detailed knowledge of neurotransmitters impairment and its association with clinical features hold the potential to develop new tailored therapeutic approaches. In the present study, we applied JuSpace toolbox, which allowed for cross‐modal correlation of magnetic resonance imaging (MRI)‐based measures with nuclear imaging derived estimates covering various neurotransmitter systems including dopaminergic, serotonergic, noradrenergic, GABAergic and glutamatergic neurotransmission. We included 103 PPA patients and 80 age‐matched healthy controls (HC). We tested if the spatial patterns of grey matter volume (GMV) alterations in PPA patients (relative to HC) are correlated with specific neurotransmitter systems. As compared to HC, voxel‐based brain changes in PPA were significantly associated with spatial distribution of serotonin, dopamine, and glutamatergic pathways (p < .05, False Discovery Rate corrected‐corrected). Disease severity was negatively correlated with the strength of GMV colocalization of D1 receptors (p = .035) and serotonin transporter (p = .020). Moreover, we observed a significant negative correlation between positive behavioural symptoms, as measured with Frontal Behavioural Inventory, and GMV colocalization of D1 receptors (p = .007) and serotonin transporter (p < .001). This pilot study suggests that JuSpace is a helpful tool to indirectly assess neurotransmitter deficits in neurodegenerative dementias and may provide novel insight into disease mechanisms and associated clinical features.
Keywords: behavioural disturbances, magnetic resonance imaging, neurotransmitters, positron emission tomography, primary progressive aphasia
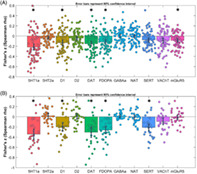
Results of spatial correlation analyses for PPA patients. Fisher's Z‐transformed correlation coefficients with respective neurotransmitter maps are displayed. Error bars represent the parametric 95% confidence interval of the mean. DAT, dopamine transporter; FDOPA, FluoroDOPA; GABAa, γ‐aminobutyric acid type A; NAT, noradrenaline; SERT, serotonin transporter. Panel A: Results of spatial correlation analyses in patients with nonfluent variant of primary progressive aphasia (avPPA); Panel B: Results of spatial correlation analyses in patients with semantic variant of primary progressive aphasia (svPPA). *p‐values < .004 (corrected for multiple comparisons).
1. INTRODUCTION
Primary progressive aphasias (PPAs) are a group of neurodegenerative diseases presenting with insidious and relentless language impairment (Gorno‐Tempini et al., 2011; Rosen et al., 2006; Van Langenhove et al., 2016). Two main PPA variants have been described within the spectrum of frontotemporal lobar degeneration: the nonfluent/agrammatic variant (avPPA), presenting with slow, effortful, hesitant and distorted speech, and the semantic variant (svPPA), which begins as difficulty finding words, particularly nouns, and single words comprehension deficits (Gorno‐Tempini et al., 2011; Marshall et al., 2018). As disease progresses, PPA patients variably present prominent dysexecutive syndrome, behavioural disturbances and parkinsonism (Butts et al., 2015; Grossman et al., 1996; Kertesz et al., 2000; Tee & Gorno‐Tempini, 2019).
avPPA is characterized by atrophy of inferior frontal gyrus and insula cortex in the dominant hemisphere, while svPPA shows asymmetric, focal cerebral atrophy chiefly involving the dominant anteroinferior and mesial temporal lobe (Gorno‐Tempini et al., 2011).
Up to now, PPA interventions rely mainly on speech training (Pagnoni et al., 2021), and on promising approaches with noninvasive brain stimulation techniques such as transcranial Direct Current Stimulation (tDCS) or repetitive Transcranial Magnetic Stimulation (Benussi, Dell'Era, Cosseddu, et al., 2020; Cotelli et al., 2014; Pytel et al., 2021; Tsapkini et al., 2018) to counteract language deficits. However, it remains important to advance symptomatic treatment, to reduce disease burden and improve patients' and carers' quality of life (Murley & Rowe, 2018).
In this view, restoring neurotransmitters deficits hold the potential to improve associated behavioural, cognitive and motor symptoms in PPAs, as a number of studies, mainly performed in autopsy case series, have reported consistent impairment of dopaminergic, serotoninergic, GABAergic and glutamatergic pathways (Murley & Rowe, 2018). Despite these clear‐cut results, clinical trials have not shown consistent benefits from the modulation of neurotransmitters on behavioural disturbances (Panza et al., 2020). Indeed, this may be due to weaknesses in research methodology, with small studies in unstratified populations, and by a poor knowledge of neurotransmitter deficits in patients diagnosed as having PPA and their correlation with clinical symptoms.
Recent advancements in positron emission tomography (PET) or single photon computed emission tomography (SPECT) tracer development resulted in a variety of novel tracers that can reliably measure the availability of specific receptors. In line with that, Dukart and colleagues have shown that drug‐induced spatial alteration patterns in resting state functional activity as measured using magnetic resonance imaging (rsfMRI) are associated with the distribution of specific receptors systems targeted by respective compounds (Dukart et al., 2018). Based on this approach, Dukart et al have recently developed JuSpace, a toolbox aimed at testing the associations between MRI‐based measures and a list of included PET and SPECT maps covering various neurotransmitter systems (Dukart et al., 2021). More in detail, JuSpace creates a spatial pattern of brain alterations based on MRI measures, comparing two different groups (e.g. patients versus healthy controls). After that, it performs a correlation between these alterations and each receptor/transporter map included in the toolbox. JuSpace therefore aims to assess if the spatial patterns of brain changes observed in the disease of interest are related to the distribution of specific neurotransmitters systems, as derived from independent healthy volunteer populations (Dukart et al., 2021).
These premises prompted the present study, aimed at applying JuSpace tool in a large sample of subjects with PPA, with the aim to evaluate the pattern of neurotransmitters deficits in avPPA and svPPA, and the correlation between neurotransmitter deficits and clinical symptoms.
2. METHODS
2.1. Subjects
Patients fulfilling current clinical criteria for probable PPA (Gorno‐Tempini et al., 2011) were consecutively recruited from the Center for Neurodegenerative Disorders, Department of Clinical and Experimental Sciences, University of Brescia, and from the IRCCS Istituto Centro San Giovanni di Dio, Italy, between January 2010 and July 2021.
For all patients, the diagnostic evaluation included a review of the full medical history, a complete neurological and neuropsychological/behavioural assessment and a MRI brain scan. Moreover, each patient underwent Mini‐Mental State Examination (MMSE), phonemic and semantic fluency, Token test, Rey complex figure copy and recall, Short Story, Trail Making test A and B, as previously published (Borroni et al., 2010; Premi et al., 2016). Behavioural disturbances were assessed with Frontal Behavioural Inventory (Borroni et al., 2010; Premi et al., 2016). Basic activities of daily living (BADL) and instrumental activities of daily living (IADL) were also assessed, and disease severity was measured by CDR Dementia Staging Instrument plus behaviour and language domains from the National Alzheimer's Coordinating Center and Frontotemporal lobar degeneration modules—sum of boxes (CDR plus NACC FTLD—SOB) (Miyagawa et al., 2020).
Diagnosis was accomplished by amyloid biomarkers in 39.8% of patients further ruling out AD (n = 38 underwent cerebrospinal fluid determinations of tau and Aβ42, n = 3 PET amyloid imaging). Moreover, definitive PPA diagnosis was ascertained by genetic analysis in 19.4% of patients (n = 18 patients carried Granulin mutations and n = 2 C9orf72 expansions all avPPA).
A healthy control (HC) group was recruited for comparisons. Each HC underwent a neurological examination and a brief neuropsychological examination to ensure cognitive performances within normal range (MMSE> = 27/30).
Full written informed consent was obtained from all participants according to the Declaration of Helsinki. The study protocol was approved by the local ethics committee of Brescia Hospital and of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli.
2.2. MRI acquisition, preprocessing and analyses
Brain structural images (three‐dimensional T1‐weighted Magnetization Prepared—RApid Gradient Echo [MPRAGE] MRI) for patients and HC were collected using three different scanners at University of Brescia: (i) 1.5‐T Siemens Simphony, (ii) 1.5‐T Siemens Avanto and (iii) 3‐T Siemens Skyra. T1‐weighted images were then processed and analysed with the voxel‐based morphometry (VBM) pipeline implemented in the Computational Anatomy Toolbox (CAT12 v.1742) (www.neuro.uni-jena.de/cat) for SPM12 (SPM12 v.7219) (www.fil.ion.ucl.ac.uk/spm/software/spm12) running on MATLAB R2019b (the MathWorks, Inc., Natick, MA, USA). The VBM pipeline consists of several stages (tissue segmentation, spatial normalization to a standard Montreal National Institute [MNI] template, modulation and smoothing), as previously described (Kurth et al., 2015). CAT12 potentially provides more robust and accurate performances compared to other VBM pipelines (Farokhian et al., 2017). The normalized and modulated grey matter images were then smoothed with 10‐mm full width at half‐maximum Gaussian kernel.
To test for group differences (avPPA vs. HC and svPPA vs. HC) in grey matter volume (GMV) a general linear model using SPM12 was implemented, considering age, gender and scanner as nuisance variables. The statistical threshold was set to p < .05 corrected for multiple comparisons (whole‐brain family‐wise error—FWE).
2.3. Spatial correlation with neurotransmitter density maps
We used the JuSpace toolbox (version 1.4) to test if the spatial patterns of GMV alterations in avPPA or svPPA patients (relative to HC) are correlated with specific neurotransmitter systems (Dukart et al., 2021). Confounding effects of age, gender and scanner type were regressed out from all images prior to these analyses (Dukart et al., 2021). We tested if the spatial structure of GMV maps in patients relative to HC was similar to the distribution of average nuclear imaging derived neurotransmitter maps, derived from independent healthy volunteer populations included in the toolbox. We considered the 5‐hydroxytryptamine 1a (5‐HT1a) receptor, the 5‐HT2a receptor, the serotonin transporter SERT, the D1 receptor, the D2 receptor, the dopamine transporter (DAT), the FluoroDOPA, the GABAa receptors, the vesicular acetylcholine transporter (VAChT), the metabotropic glutamate receptor type 5 (mGLUR5), and the noradrenaline transporter (NAT), to assess serotonin, dopamine, GABAergic and glutamatergic systems. Each map included in Juspace toolbox was available from the literature, as reported in Table S1. Using JuSpace toolbox, native normalized, modulated and smoothed grey matter images were parcelled in regions of interest using the Neuromorphometrics Atlas (MICCAI 2012 Grand Challenge and Workshop on Multi‐Atlas Labeling (www.masi.vuse.vanderbilt.edu/workshop2012/index.php/Challenge_Details). Mean regional values of GMV were extracted for all PPA patients and HC. Spearman correlation coefficients (Fisher's Z transformed) were calculated between these Z‐transformed GMV maps of the patients and the spatial distribution of the respective neurotransmitter maps included in JuSpace toolbox. Exact permutation‐based p‐values as included in JuSpace (10,000 permutations randomly assigning group labels using orthogonal permutations) were computed to check if the distribution of the observed Fisher's Z‐transformed individual correlation coefficients were significantly different from zero. All analyses were FDR corrected for the number of tests (the number of neurotransmitter maps). Spearman correlation coefficients (Fisher's Z transformed, performed to ensure a normal distribution of the obtained correlation coefficients and to allow subsequent parametric testing) were calculated between these Z‐transformed GMV maps and the spatial distribution of the respective neurotransmitter maps. A negative correlation (and resulting negative Fisher's Z) means that GMV decline in patients is strongest in regions with an initial (healthy) high availability of the studied receptor (as derived from healthy independent populations), pointing to an increased disease vulnerability of the respective regions.
Exact permutation‐based p‐values as implemented in JuSpace (10,000 permutations randomly assigning group labels using orthogonal permutations) were computed to test if the observed correlation coefficients across patients deviate from a null distribution.
2.4. Statistical analysis
Comparisons of demographic and clinical characteristics were performed by the Student's t‐test for continuous variables and the χ 2 test for categorical variables.
Spearman correlation was used to assess the relationship between each neurotransmitter output obtained with Juspace (i.e., the GMV‐neurotransmitters correlation, Fisher's Z transformed) and clinical or behavioural data. To assess the contributions of neurotransmitters output to behavioural disturbances, we computed binary logistic regression models considering GMV‐neurotransmitters correlations as independent variables throughout different behavioural groups. Statistical significance was set at p < .05, corrected for multiple comparisons (False Discovery Rate‐FDR) (SPSS Statistics 22.0, Chicago, IL, USA).
2.5. Data availability
All study data, including raw and analysed data, and materials will be available from the corresponding author, B.B., upon reasonable request. The software applied is publicly available at https://github.com/juryxy/JuSpace.
3. RESULTS
3.1. Participants
In the present study, we considered 103 patients fulfilling current clinical criteria for PPA, namely 73 with the avPPA (mean age = 66.1 ± 8.5, female = 59%) and 30 with the svPPA (mean age = 63.6 ± 8.4, female = 57%), and 80 HC (mean age = 63.1 ± 7.9, female = 75%). Clinical characteristics of PPAs groups are reported in Table 1.
TABLE 1.
Demographic and clinical characteristics of PPAs groups
| Variable | PPA | avPPA | svPPA | p value* |
|---|---|---|---|---|
| Number | 103 | 73 | 30 | ‐ |
| Age at evaluation, years | 65.4 ± 8.5 | 66.1 ± 8.5 | 63.6 ± 8.4 | .17 |
| Sex, female% | 58.3 | 59.0 | 57.0 | .83** |
| Age at onset, years | 62.9 ± 8.7 | 63.6 ± 8.5 | 61.1 ± 8.9 | .18 |
| Education, years | 9.7 ± 4.3 | 9.5 ± 4.3 | 10.3 ± 4.4 | .41 |
| Scanner type, % (A/B/C) | 23.3/24.3/52.4 | 19.2/26.0/54.8 | 33.3/20.0/46.7 | .30** |
| Clinical assessment at evaluation | ||||
| CDR plus NACC SOB | 6.0 ± 4.3 | 6.2 ± 4.5 | 5.6 ± 3.9 | .57 |
| MMSE | 17.8 ± 8.3 | 16.8 ± 8.4 | 20.3 ± 7.4 | .05 |
| Short story | 5.4 ± 4.4 | 5.4 ± 4.7 | 5.4 ± 4.0 | 1.00 |
| Fluency, letter | 14.7 ± 10.5 | 13.1 ± 9.9 | 18.3 ± 11.0 | .03 |
| Fluency, semantic | 17.8 ± 11.4 | 18.2 ± 11.5 | 17.1 ± 11.2 | .69 |
| Token test | 22.8 ± 9.3 | 21.6 ± 10.2 | 25.3 ± 6.8 | .12 |
| Rey complex figure, copy | 23.4 ± 11.8 | 20.9 ± 12.3 | 28.9 ± 8.1 | .001 |
| Rey complex figure, recall | 9.4 ± 6.6 | 9.5 ± 6.6 | 9.1 ± 6.8 | .79 |
| Trail making test, part A | 151.1 ± 144.3 | 173.6 ± 146.0 | 97.0 ± 126.8 | .02 |
| Trail making test, part B | 374.1 ± 161.1 | 411.1 ± 144.5 | 282.9 ± 166.3 | .001 |
| FBI, A | 11.4 ± 6.7 | 11.3 ± 6.8 | 11.5 ± 6.5 | .93 |
| FBI, B | 2.9 ± 3.2 | 2.1 ± 2.5 | 4.9 ± 3.8 | .001 |
| FBI, AB | 14.3 ± 8.5 | 13.4 ± 8.1 | 16.4 ± 9.0 | .11 |
Note: Neuropsychological scores are corrected for age and education, according to Italian normative data. Scanner type: A = 1.5 T Symphony, B = 1.5 T Avanto, C = 3 T Skyra. Bold values indicate p < 0.05.
Abbreviations: avPPA, nonfluent variant PPA; CDR plus NACC FTLD SOB, CDR dementia staging instrument plus behaviour and language domains from the National Alzheimer's Coordinating Center and Frontotemporal lobar degeneration modules—sum of boxes; FBI, frontal behavioural Inventory; MMSE, Mini‐Mental State Examination; PPA, primary progressive aphasia; svPPA, semantic variant PPA.
Student‐t test, unless otherwise specified.
Chi‐square test.
Standard voxel‐wise analyses of GMV demonstrated the typical pattern of brain atrophy in avPPA, with main involvement of left anterior perisylvian region, and in svPPA, affecting ventral and lateral regions of the left anterior temporal lobe, as compared to HC (see Figure 1).
FIGURE 1.
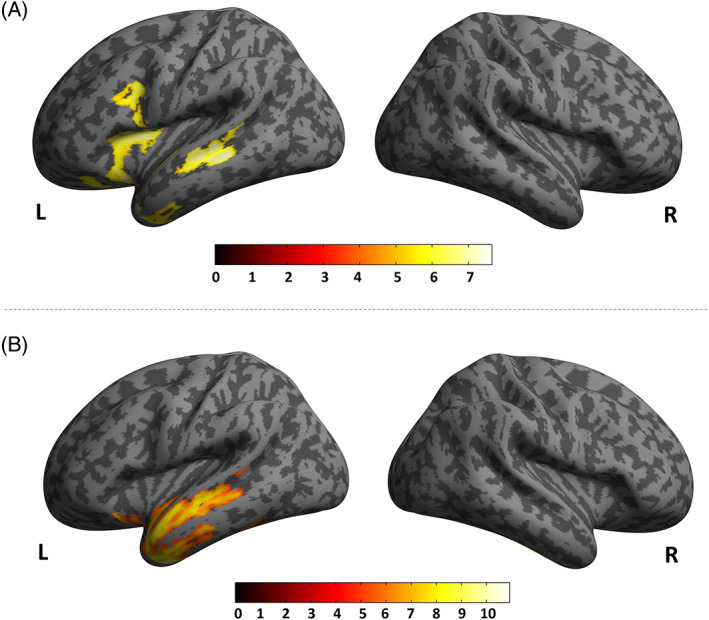
Voxel‐wise analyses in avPPA and in svPPA as compared to HC. Panel A: avPPA < HC. Panel B: svPPA < HC. Inverse comparisons (avPPA > HC and svPPA > HC) did not show any clusters above the pre‐established statistical threshold. Only clusters surviving correction for multiple comparisons (p < .05 familywise error [FEW] whole‐brain) were shown. Significant clusters were superimposed on a standardized MRI T1 3D template. avPPA, nonfluent variant of primary progressive aphasia; svPPA, semantic variant of primary progressive aphasia HC, healthy controls
3.2. Neurotransmitters deficits in avPPA and svPPA
As compared to HC, voxel‐based brain changes in avPPA were significantly associated with spatial distribution of 5‐HT1a receptors (r = −.216, p < .01 FDR‐corrected), D1 receptors (r = −.131, p < .01 FDR‐corrected), dopamine transporter DAT (r = −.152, p < .01 FDR‐corrected), FluoroDOPA (r = −.116, p < .01 FDR‐corrected), mGLUR5 (r = −.09, p < .01 FDR‐corrected). There was no significant difference in spatial distribution in regard to 5‐HT2a receptors, SERT, D2 receptors, VAChT, GABAa receptors, and NAT (all p > .05) (Figure 2, panel A).
FIGURE 2.
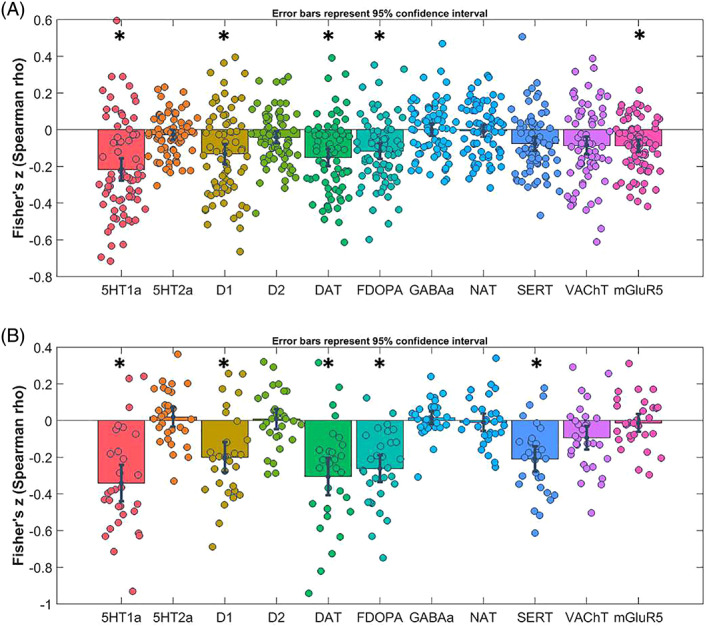
Results of spatial correlation analyses for PPA patients. Fisher's Z‐transformed correlation coefficients with respective neurotransmitter maps are displayed. Error bars represent the parametric 95% confidence interval of the mean. DAT, dopamine transporter; FDOPA, FluoroDOPA; GABAa, γ‐aminobutyric acid type A; NAT, noradrenaline; SERT, serotonin transporter. Panel A: Results of spatial correlation analyses in patients with nonfluent variant of primary progressive aphasia (avPPA); Panel B: Results of spatial correlation analyses in patients with semantic variant of primary progressive aphasia (svPPA). *p‐values < .004 (corrected for multiple comparisons)
svPPA patients showed comparable spatial distribution as compared to HC of 5‐HT1a receptors (r = −.341, p < .01 FDR‐corrected), D1 receptors (r = −.200, p < .01 FDR‐corrected), dopamine transporter DAT (r = −.301, p < .01 FDR‐corrected), FluoroDOPA (r = −.262, p < .01 FDR‐corrected), with extra involvement of serotonin transporter SERT (r = −.210, p < .01 FDR‐corrected) and with no significant differences for 5HT2a, D2, mGLUR5, VAChT, GABAa, and NAT (Figure 2, panel B).
For both groups, the negative correlation coefficients indicate GMV reduction in patients as compared to HC in areas with high neurotransmitters density.
3.3. Neurotransmitter impairment and clinical symptoms in PPA
Next, we assessed the relationship between GMV‐neurotransmitters correlation coefficients and clinical or behavioural data in PPA group. We considered only GMV‐neurotransmitters correlation coefficients significantly impaired in PPA and we excluded those highly correlated to each other (Spearman correlations coefficients > .80), namely DAT and FluoroDOPA.
Thus, we included in the present analyses 5‐HT1a receptors, SERT, D1 receptors, and mGLUR5.
Disease severity as measured by CDR plus NACC SOB was negatively correlated with the strength of GMV colocalization of D1 receptors (r = −.211, p = .035) and SERT (r = −.233, p = .020), with greater disease severity being associated with lower GMV‐neurotransmitters correlation coefficients (see Figure 3).
FIGURE 3.
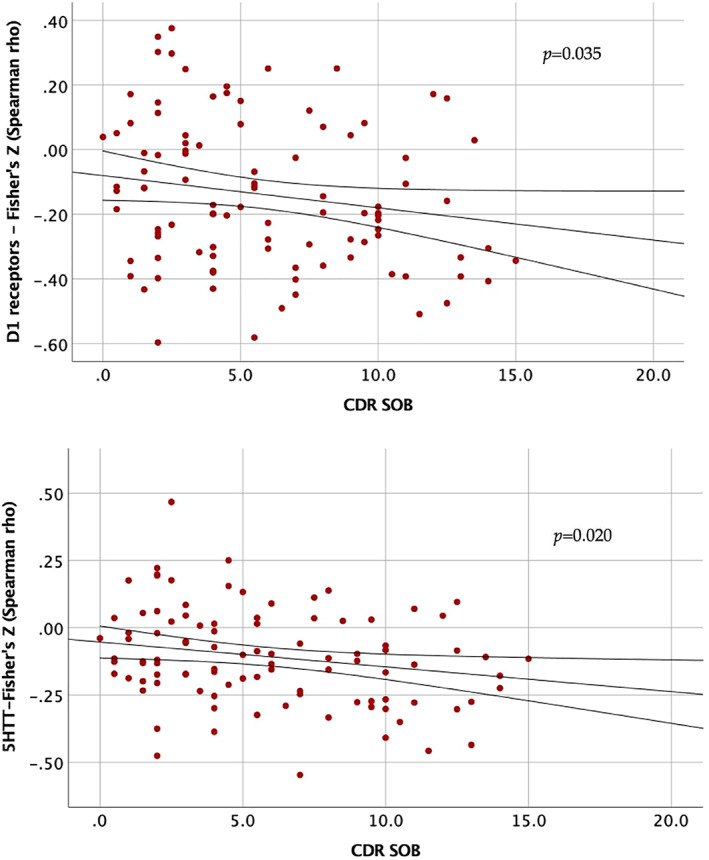
Association between disease severity and GMV‐neurotransmitter correlation coefficients. CDR SOB, CDR dementia staging instrument plus behaviour and language domains from the National Alzheimer's Coordinating Center and Frontotemporal lobar degeneration modules sum of boxes. Correlation coefficients (Fisher's Z − Spearman rho) for each neurotransmitter on y‐axis
Similarly, we observed a significant negative correlation between FBI‐B, that is, positive behavioural symptoms, and GMV colocalization of D1 receptors (r = −.277, p = .007) and SERT (r = −.358, p < .001), with more severe behavioural symptoms being associated with lower GMV‐neurotransmitters correlation coefficients; no significant correlation was detected between FBI‐A, that is, negative behavioural symptoms, and any GMV co‐localization of neurotransmitters in PPA.
When FBI‐B items were considered in binary logistic regression, presence of impulsivity was negatively associated with GMV co‐localization with D1 receptors (β = −1.1, p < .001), 5HT1a (β = −3.3, p = .01), and SERT (β = −5.1, p = .003), and binge eating with D1 receptors (β = −5.1, p = .004) and SERT (β = −5.6, p = .004).
No significant correlations between neuropsychological tests and GMV neurotransmitters co‐localization at pre‐established statistical threshold were reported.
4. DISCUSSION
The knowledge of neurotransmitters impairment in PPAs and its association with clinical features may be helpful for future tailored therapeutic approaches (Johnson et al., 2010; Kertesz et al., 2008). Restoring these deficits, individually or in combination, has the potential to improve clinical symptoms and quality of life in both patients and their carriers.
In the present work, we assessed if the spatial patterns of grey matter atrophy observed in different subtypes of PPA are related to the distribution of specific neurotransmitters systems as derived from independent healthy volunteer populations (Dukart et al., 2021). These data have been obtained by JuSpace toolbox, an integrated system for the comparison of PET and SPECT derived neurotransmitter maps with other imaging modalities such as MRI data (Dukart et al., 2021).
We found that grey matter volume alterations in patients with PPA significantly co‐localized with dopaminergic, serotoninergic and glutamatergic circuits. These findings corroborated previous literature autopsy data (Murley & Rowe, 2018), with a new in vivo imaging approach, and also allowed us to infer on correlations between neurotransmitter pathways and clinical presentation in a relative large sample of patients.
Indeed, we also demonstrated that disease severity was associated with progressive worsening of dopamine and serotonin circuits, and more interestingly, impulsivity and binge eating disturbances were strongly associated with grey matter volume co‐localization to these neurotransmitter systems.
Indeed, these findings suggest that PPA may be associated with increased vulnerability of dopamine and serotonin systems to atrophy inducing disease pathology. In this view, targeting selective neurotransmitters circuits may be considered in future clinical trials to counteract disease course.
Moreover, the major idea behind this approach is that pharmacological manipulation of specific neurotransmitters in PPA may be beneficial only considering associated clinical and behavioural features, thus reducing the cost and health burden of the disease. The core feature of PPA is the language deficit, but behavioural disturbances are frequently detected over disease course (Banks & Weintraub, 2009; de la Sablonnière et al., 2021; Fatemi et al., 2011; Gómez‐Tortosa et al., 2016; Van Langenhove et al., 2016).
Most studies evaluating pharmacological approaches in frontotemporal lobar degeneration have not reported clear‐cut results (Panza et al., 2020). Herein, we suggested that dopamine and serotonin pathways, and in particular D1 receptors and SERT, may be key in the onset of impulsivity and binge eating. Indeed, our findings confirm and extend previous literature data, which have already linked these behavioural disturbances to both dopamine and serotonin abnormalities (da Cunha‐Bang & Knudsen, 2021; Li et al., 2021; Majuri et al., 2017). Taken together, the present results, even though preliminary, provide a framework for additional studies to examine symptom‐specific pharmacological treatment approaches.
As compared to data obtained in previous neurophysiological studies (Benussi et al., 2017; Benussi, Dell'Era, Cantoni, et al., 2020; Benussi, Grassi, Palluzzi, et al., 2020), we failed to confirm a co‐localization of grey matter alteration and the GABAergic system, and we found significant glutamatergic impairment only in avPPA, even though we analysed metabotropic receptors and we did not consider NMDA glutamatergic receptors (Benussi et al., 2017; Benussi, Dell'Era, Cantoni, et al., 2020; Benussi, Grassi, Palluzzi, et al., 2020).
We acknowledge that this study entails some limitations. First, future implemented neurotransmitters maps in JuSpace may further refine the present findings. Moreover, the maps available have been recently obtained, and present some limitations that might to be addressed, for example, the variability in the number of HC cases in each map and receptor density assessment is not necessarily related to neurotransmitter density. In spite of this, JuSpace appears a promising tool to explore in vivo neurotransmitter impairment (Dukart et al., 2021) as already explored in Parkinson's disease with regard to dopaminergic and serotoninergic systems. In light with this, our results are in line with neurotransmitter impairment in FTD reported in post mortem studies (Murley & Rowe, 2018).
Second, we do not have pathological confirmation in sporadic PPA cases, and we cannot exclude misdiagnoses; however, comprehensive diagnostic workup was carried out, including markers aimed at excluding AD and genetic screening. Moreover, GRN mutations, which present specific imaging features, are overrepresented in this sample (e.g., 18 cases out of 73 avPPA) (Premi et al., 2014). Third, considering frontotemporal lobar degeneration phenotypes with prominent behavioural disturbances, such as behavioural variant frontotemporal dementia, may be of further interest.
5. CONCLUSIONS
This pilot study suggests that JuSpace is a helpful tool to indirectly assess neurotransmitter deficits in neurodegenerative dementias and may provide novel insight into disease mechanisms and associated clinical features. This approach may be useful to design and test new targets for pharmacological intervention trials.
AUTHOR CONTRIBUTIONS
Enrico Premi and Barbara Borroni contributed to study design, data analyses and writing the draft of the manuscript; Juergen Dukart, Irene Mattioli, Marta Pengo contributed to statistical analyses; Ilenia Libri, Yasmine Gadola, Maria Cotelli, Rosa Manenti, Giuliano Binetti, Stefano Gazzina, Antonella Alberici, Mauro Magoni, Giacomo Koch, Alessandro Padovani contributed to patients selection and assessment; Roberto Gasparotti contributed to imaging scan acquisition and assessment.
FUNDING INFORMATION
This study was supported by grants from Italian Ministry of Health (ACROSS Study, GR‐2018‐12365105 and Ricerca Corrente).
CONFLICT OF INTEREST
No competing interests were disclosed.
ETHICS STATEMENT
The project has been approved by the local ethics committee of Brescia Hospital and of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Italy. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
INFORMED CONSENT
Each patient signed consent form to participate to the study, and freely given, informed consent to participate in the study was obtained from participants.
Supporting information
Table S1. Receptor/transporters considered in the present study.
ACKNOWLEDGEMENT
The authors thank patients and their families for entering the study.
Premi, E. , Dukart, J. , Mattioli, I. , Libri, I. , Pengo, M. , Gadola, Y. , Cotelli, M. , Manenti, R. , Binetti, G. , Gazzina, S. , Alberici, A. , Magoni, M. , Koch, G. , Gasparotti, R. , Padovani, A. , & Borroni, B. (2023). Unravelling neurotransmitters impairment in primary progressive aphasias. Human Brain Mapping, 44(6), 2245–2253. 10.1002/hbm.26206
Funding information Italian Ministry of Health, Grant/Award Number: GR‐2018‐12365105
DATA AVAILABILITY STATEMENT
All study data, including raw and analysed data, and materials will be available from the corresponding author, B.B., upon reasonable request. The software applied is publicly available at https://github.com/juryxy/JuSpace
REFERENCES
- Banks, S. J. , & Weintraub, S. (2009). Generalized and symptom‐specific insight in behavioral variant frontotemporal dementia and primary progressive aphasia. The Journal of Neuropsychiatry and Clinical Neurosciences, 21, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi, A. , Dell'Era, V. , Cantoni, V. , Cotelli, M. S. , Cosseddu, M. , Spallazzi, M. , Micheli, A. , Turrone, R. , Alberici, A. , & Borroni, B. (2020). TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimulation, 13, 386–392. [DOI] [PubMed] [Google Scholar]
- Benussi, A. , Dell'Era, V. , Cosseddu, M. , Cantoni, V. , Cotelli, M. S. , Cotelli, M. , Manenti, R. , Benussi, L. , Brattini, C. , Alberici, A. , & Borroni, B. (2020). Transcranial stimulation in frontotemporal dementia: A randomized, double‐blind, sham‐controlled trial. Alzheimer's Dement (New York, NY), 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi, A. , Di Lorenzo, F. , Dell'Era, V. , Cosseddu, M. , Alberici, A. , Caratozzolo, S. , Cotelli, M. S. , Micheli, A. , Rozzini, L. , Depari, A. , Flammini, A. , Ponzo, V. , Martorana, A. , Caltagirone, C. , Padovani, A. , Koch, G. , & Borroni, B. (2017). Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology, 89, 665–672. [DOI] [PubMed] [Google Scholar]
- Benussi, A. , Grassi, M. , Palluzzi, F. , Koch, G. , Di Lazzaro, V. , Nardone, R. , Cantoni, V. , Dell'Era, V. , Premi, E. , Martorana, A. , di Lorenzo, F. , Bonnì, S. , Ranieri, F. , Capone, F. , Musumeci, G. , Cotelli, M. S. , Padovani, A. , & Borroni, B. (2020). Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Annals of Neurology, 87, 394–404. [DOI] [PubMed] [Google Scholar]
- Borroni, B. , Grassi, M. , Agosti, C. , Premi, E. , Archetti, S. , Alberici, A. , Bellelli, G. , Caimi, L. , Di Luca, M. , & Padovani, A. (2010). Establishing short‐term prognosis in frontotemporal lobar degeneration spectrum: Role of genetic background and clinical phenotype. Neurobiology of Aging, 31, 270–279. [DOI] [PubMed] [Google Scholar]
- Butts, A. M. , Machulda, M. M. , Duffy, J. R. , Strand, E. A. , Whitwell, J. L. , & Josephs, K. A. (2015). Neuropsychological profiles differ among the three variants of primary progressive aphasia. Journal of the International Neuropsychological Society, 21, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli, M. , Manenti, R. , Petesi, M. , Brambilla, M. , Cosseddu, M. , Zanetti, O. , Miniussi, C. , Padovani, A. , & Borroni, B. (2014). Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer's Disease, 39, 799–808. [DOI] [PubMed] [Google Scholar]
- da Cunha‐Bang, S. , & Knudsen, G. M. (2021). The modulatory role of serotonin on human impulsive aggression. Biological Psychiatry, 90, 447–457. [DOI] [PubMed] [Google Scholar]
- de la Sablonnière, J. L. , Tastevin, M. , Lavoie, M. , & Laforce, R. (2021). Longitudinal changes in cognition, Behaviours, and functional abilities in the three Main variants of primary progressive aphasia: A literature review. Brain Sciences, 11, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart, J. , Holiga, Š. , Chatham, C. , Hawkins, P. , Forsyth, A. , McMillan, R. , Myers, J. , Lingford‐Hughes, A. R. , Nutt, D. J. , Merlo‐Pich, E. , Risterucci, C. , Boak, L. , Umbricht, D. , Schobel, S. , Liu, T. , Mehta, M. A. , Zelaya, F. O. , Williams, S. C. , Brown, G. , … Sambataro, F. (2018). Cerebral blood flow predicts differential neurotransmitter activity. Scientific Reports, 8, 4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart, J. , Holiga, S. , Rullmann, M. , Lanzenberger, R. , Hawkins, P. C. T. , Mehta, M. A. , Hesse, S. , Barthel, H. , Sabri, O. , Jech, R. , & Eickhoff, S. B. (2021). JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Human Brain Mapping, 42, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhian, F. , Beheshti, I. , Sone, D. , & Matsuda, H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Frontiers in Neurology, 8, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi, Y. , Boeve, B. F. , Duffy, J. , Petersen, R. C. , Knopman, D. S. , Cejka, V. , Smith, G. E. , & Geda, Y. E. (2011). Neuropsychiatric aspects of primary progressive aphasia. The Journal of Neuropsychiatry and Clinical Neurosciences, 23, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Tortosa, E. , Rigual, R. , Prieto‐Jurczynska, C. , Mahillo‐Fernández, I. , Guerrero‐López, R. , Pérez‐Pérez, J. , & Sainz, M. J. (2016). Behavioral evolution of progressive semantic aphasia in comparison with nonfluent aphasia. Dementia and Geriatric Cognitive Disorders, 41, 1–8. [DOI] [PubMed] [Google Scholar]
- Gorno‐Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, M. , Mickanin, J. , Onishi, K. , Hughes, E. , D'Esposito, M. , Ding, X. S. , Alavi, A. , & Reivich, M. (1996). Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer's disease. Journal of Cognitive Neuroscience, 8, 135–154. [DOI] [PubMed] [Google Scholar]
- Johnson, N. A. , Rademaker, A. , Weintraub, S. , Gitelman, D. , Wienecke, C. , & Mesulam, M. (2010). Pilot trial of memantine in primary progressive aphasia. Alzheimer Disease and Associated Disorders, 24, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, A. , Martinez‐Lage, P. , Davidson, W. , & Munoz, D. G. (2000). The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology, 55, 1368–1375. [DOI] [PubMed] [Google Scholar]
- Kertesz, A. , Morlog, D. , Light, M. , Blair, M. , Davidson, W. , Jesso, S. , & Brashear, R. (2008). Galantamine in frontotemporal dementia and primary progressive aphasia. Dementia and Geriatric Cognitive Disorders, 25, 178–185. [DOI] [PubMed] [Google Scholar]
- Kurth, F. , Gaser, C. , & Luders, E. (2015). A 12‐step user guide for analyzing voxel‐wise gray matter asymmetries in statistical parametric mapping (SPM). Nature Protocols, 10, 293–304. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Simmler, L. D. , Van Zessen, R. , Flakowski, J. , Wan, J. X. , Deng, F. , Li, Y. L. , Nautiyal, K. M. , Pascoli, V. , & Lüscher, C. (2021). Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science, 373, 1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majuri, J. , Joutsa, J. , Johansson, J. , Voon, V. , Parkkola, R. , Alho, H. , Arponen, E. , & Kaasinen, V. (2017). Serotonin transporter density in binge eating disorder and pathological gambling: A PET study with [ 11 C]MADAM. European Neuropsychopharmacology, 27, 1281–1288. [DOI] [PubMed] [Google Scholar]
- Marshall, C. R. , Hardy, C. J. D. , Volkmer, A. , Russell, L. L. , Bond, R. L. , Fletcher, P. D. , Clark, C. N. , Mummery, C. J. , Schott, J. M. , Rossor, M. N. , Fox, N. C. , Crutch, S. J. , Rohrer, J. D. , & Warren, J. D. (2018). Primary progressive aphasia: A clinical approach. Journal of Neurology, 265, 1474–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa, T. , Brushaber, D. , Syrjanen, J. , Kremers, W. , Fields, J. , Forsberg, L. K. , Heuer, H. W. , Knopman, D. , Kornak, J. , Boxer, A. , Rosen, H. J. , Boeve, B. F. , Appleby, B. , Bordelon, Y. , Bove, J. , Brannelly, P. , Caso, C. , Coppola, G. , Dever, R. , … Wszolek, Z. (2020). Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: Data from the ARTFL/LEFFTDS consortium. Alzheimers Dement, 16, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley, A. G. , & Rowe, J. B. (2018). Neurotransmitter deficits from frontotemporal lobar degeneration. Brain, 141, 1263–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni, I. , Gobbi, E. , Premi, E. , Borroni, B. , Binetti, G. , Cotelli, M. , & Manenti, R. (2021). Language training for oral and written naming impairment in primary progressive aphasia: A review. Translational Neurodegeneration, 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza, F. , Lozupone, M. , Seripa, D. , Daniele, A. , Watling, M. , Giannelli, G. , & Imbimbo, B. P. (2020). Development of disease‐modifying drugs for frontotemporal dementia spectrum disorders. Nature Reviews. Neurology, 16, 213–228. [DOI] [PubMed] [Google Scholar]
- Premi, E. , Cauda F, Gasparotti R, Diano M, Archetti S, & Padovani A, Borroni, B. (2014). Multimodal fMRI resting‐state functional connectivity in Granulin mutations: The case of fronto‐parietal dementia. PLoS One, 9(9), e106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi, E. , Gualeni, V. , Costa, P. , Cosseddu, M. , Gasparotti, R. , Padovani, A. , & Borroni, B. (2016). Looking for measures of disease severity in the frontotemporal dementia continuum. Journal of Alzheimer's Disease, 52, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Pytel, V. , Cabrera‐Martín, M. N. , Delgado‐Álvarez, A. , Ayala, J. L. , Balugo, P. , Delgado‐Alonso, C. , Yus, M. , Carreras, M. T. , Carreras, J. L. , Matías‐Guiu, J. , & Matías‐Guiu, J. A. (2021). Personalized repetitive transcranial magnetic stimulation for primary progressive aphasia. Journal of Alzheimer's Disease, 84, 151–167. [DOI] [PubMed] [Google Scholar]
- Rosen, H. J. , Allison, S. C. , Ogar, J. M. , Amici, S. , Rose, K. , Dronkers, N. , Miller, B. L. , & Gorno‐Tempini, M. L. (2006). Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology, 67, 1752–1756. [DOI] [PubMed] [Google Scholar]
- Tee, B. L. , & Gorno‐Tempini, M. L. (2019). Primary progressive aphasia: A model for neurodegenerative disease. Current Opinion in Neurology, 32, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini, K. , Webster, K. T. , Ficek, B. N. , Desmond, J. E. , Onyike, C. U. , Rapp, B. , Frangakis, C. E. , & Hillis, A. E. (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer's Dement (New York, NY), 4, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove, T. , Leyton, C. E. , Piguet, O. , & Hodges, J. R. (2016). Comparing longitudinal behavior changes in the primary progressive aphasias. Journal of Alzheimer's Disease, 53, 1033–1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Receptor/transporters considered in the present study.
Data Availability Statement
All study data, including raw and analysed data, and materials will be available from the corresponding author, B.B., upon reasonable request. The software applied is publicly available at https://github.com/juryxy/JuSpace


