Abstract
Millions of children sustain a concussion annually. Concussion disrupts cellular signaling and neural pathways within the brain but the resulting metabolic disruptions are not well characterized. Magnetic resonance spectroscopy (MRS) can examine key brain metabolites (e.g., N‐acetyl Aspartate (tNAA), glutamate (Glx), creatine (tCr), choline (tCho), and myo‐Inositol (mI)) to better understand these disruptions. In this study, we used MRS to examine differences in brain metabolites between children and adolescents with concussion versus orthopedic injury. Children and adolescents with concussion (n = 361) or orthopedic injury (OI) (n = 184) aged 8 to 17 years were recruited from five emergency departments across Canada. MRS data were collected from the left dorsolateral prefrontal cortex (L‐DLPFC) using point resolved spectroscopy (PRESS) at 3 T at a mean of 12 days post‐injury (median 10 days post‐injury, range 2–33 days). Univariate analyses for each metabolite found no statistically significant metabolite differences between groups. Within each analysis, several covariates were statistically significant. Follow‐up analyses designed to account for possible confounding factors including age, site, scanner, vendor, time since injury, and tissue type (and interactions as appropriate) did not find any metabolite group differences. In the largest sample of pediatric concussion studied with MRS to date, we found no metabolite differences between concussion and OI groups in the L‐DLPFC. We suggest that at 2 weeks post‐injury in a general pediatric concussion population, brain metabolites in the L‐DLPFC are not specifically affected by brain injury.
Keywords: brain metabolites, concussion, magnetic resonance spectroscopy, multi‐site, multi‐vendor, orthopedic injury control, pediatric mTBI
In the largest pediatric concussion in MRS data set to date, we found no differences in brain metabolites between concussion and orthopedic injury in the first two weeks of injury in the L‐DLPFC. This indicates that in a general pediatric concussion population, brain metabolites are either unaffected or recovered.
1. INTRODUCTION
Millions of children in North America sustain concussions annually (Faul et al., 2010). Unlike more severe cases of traumatic brain injury, concussion is not typically associated with any overt structural damage on diagnostic neuroimaging (e.g., CT and MRI), but nonetheless may be associated with underlying metabolic damage to the brain. Concussion is characterized in the acute phase by an ionic flux and an indiscriminate release of glutamate, potassium efflux, and sodium and calcium influx. This results in the characteristic spreading depression‐like excitatory cascade leading to disruptions in cellular signaling and inflammation within the brain (Corwin et al., 2017; Gardner et al., 2014; Giza & Hovda, 2014; Honce et al., 2016; Moffett et al., 2013). Characterization of these disruptions using neuroimaging biomarkers has been limited in pediatrics (Chamard & Lichtenstein, 2018).
Magnetic resonance spectroscopy (MRS) is the only in vivo, noninvasive method to study brain chemistry. The primary metabolites reported from proton MRS are total N‐acetyl Aspartate (tNAA: combination of N‐acetyl aspartate (NAA) and N‐acetyl aspartyl glutamate (NAAG)), glutamate (often reported as Glx; the combination of glutamate and its precursor glutamine), total creatine (tCr; the combination of creatine and phosphocreatine), total choline (tCho; the combination of choline, glycerophosphocholine, and phosphocholine), and myo‐Inositol (mI; the combination of myo‐inositol and glycine). MRS has the potential to reveal alterations in these metabolites following concussion as a biomarker of pathophysiology.
In adult concussion research, tNAA is typically decreased following concussion at multiple timepoints (Govindaraju et al., 2004; Henry et al., 2010; Johnson et al., 2012; Signoretti et al., 2010; Sivák et al., 2014; Vagnozzi et al., 2010; Vagnozzi et al., 2013; Veeramuthu et al., 2018), and the decrease has been suggested to indicate neuronal damage or dysfunction associated with brain injury. Conversely, Glx, tCr, tCho, and mI have been shown to increase following concussion in some studies (Chamard et al., 2014; Croall et al., 2015; Gasparovic et al., 2009; Govindaraju et al., 2004; Johnson et al., 2012; Kierans et al., 2014; Lin et al., 2012; Yeo et al., 2011) taken to indicate glutamatergic excitotoxic responses (Chamard et al., 2014), an upregulation of membrane pumps and cellular repair processes (Gasparovic et al., 2009; Yeo et al., 2011), cellular damage (Govindaraju et al., 2004; Rae, 2014), and offsetting ionic imbalances (Churchill et al., 2020; Henry et al., 2011; Meyer et al., 2019; Vagnozzi et al., 2013) that occur with the characteristic shearing forces and resulting water imbalances in the brain (Giza & Hovda, 2014) respectively. The current literature, however, is less convincing for Glx, tCr, tCho, and mI changes (Joyce et al., 2022).
There are a limited number of studies examining pediatric concussion using MRS (Bartnik‐Olson et al., 2014; MacMaster et al., 2019; Manning et al., 2017; Maugans et al., 2012; Menshchikov et al., 2020; Meyer et al., 2019). Similar to adult research, generally tNAA is lower following a single concussion, with data collected within 72 h (Menshchikov et al., 2020), at 1‐year (Bartnik‐Olson et al., 2014), and greater than 1‐year post‐injury (MacMaster et al., 2019). However, caution in drawing conclusions about a decrease in tNAA is required; one study found decreased tNAA only in the right dorsal lateral prefrontal cortex (R‐DLPFC) and not the left, L‐DLPFC and this decrease was only in children with one prior concussion, not those with multiple concussions (MacMaster et al., 2019). Additionally, two studies of pediatric concussion reported no differences in tNAA in multiple regions (Maugans et al., 2012; Meyer et al., 2019). In terms of other metabolites, a longitudinal study of pediatric concussion reported increased Glx at 2 weeks post‐injury, a difference that persisted for up to 1 year, compared with healthy controls (Meyer et al., 2019). However, two other studies did not report Glx differences (MacMaster et al., 2019; Menshchikov et al., 2020). Two studies report alterations in mI, one of which showed an increase within 72 h from injury (Menshchikov et al., 2020), and the other showed decreases >1 month post‐concussion (Meyer et al., 2019).
Given that brain metabolites change during development (Blüml & Panigrahy, 2013), it is perhaps unsurprising that there are inconsistencies between adult and pediatric concussion results. Furthermore, small sample sizes limit the ability of previous work to fully consider known metabolite changes with development in the study of pediatric concussion. Currently, most pediatric studies compare concussion participants to healthy controls (Bartnik‐Olson et al., 2014; Manning et al., 2017; Maugans et al., 2012; Menshchikov et al., 2020; Meyer et al., 2019). This does not allow for study of metabolites specific to concussion. This study uses orthopedic injury as a comparison group to control for exposure to injury. Additionally, many previous concussion studies are in specific injury populations (sport‐related concussion), whereas a goal of the current study was to study all concussions. Finally, integrating results from small sample studies across multiple regions with data collected at different timepoints complicates an already heterogeneous injury. While limited to a single region, the current study aims to resolve some of these challenges in the current literature within the context of developmental changes.
The goal of this study was to examine differences in MRS‐measured metabolites (tNAA, tCr, tCho, Glx, and mI) between children with concussion and those with an orthopedic injury (OI) at ~1–2 weeks post‐injury in the L‐DLPFC. The functions of the DLPFC are associated with many concussion symptoms and is consistent with the recommended voxel location by the ENIGMA MRS working group in traumatic brain injury (Bartnik‐Olson et al., 2021). This study includes the largest sample of pediatric concussion to be studied with MRS to date.
2. MATERIALS AND METHODS
2.1. Study design and procedure
The data for this study were acquired as a part of the Advancing Concussion Assessment in Pediatrics (A‐CAP) Study (Yeates et al., 2017). The study recruited children aged 8 to <17 years that presented within 48 h of either concussion or OI injury to the emergency department (ED) of one of five hospitals that are part of the Pediatric Emergency Research Canada (PERC) network (Bialy et al., 2018): Alberta Children's Hospital (Calgary), Stollery Children's Hospital (Edmonton), British Columbia Children's Hospital (Vancouver), Children's Hospital of Eastern Ontario (Ottawa), and Centre Hospitalier Universitaire (CHU) Sainte‐Justine (Montreal). Children returned for an assessment that included MRS 1–2 weeks post‐injury. Due to the large recruitment goal of the parent study (700 concussion and 300 OI), there was no matching of OI to concussion participants as to maximize recruitment. The large study size enables sufficient power to account for the site statistically. All sites scanned both OI and concussion participants. The study was approved by the research ethics board at each participating site and informed consent and assent were obtained from the parents/guardians and the youth participants, respectively. For detailed information on the larger study protocol, including the inclusion/exclusion criteria, and other acquired data not analyzed in this study please refer to Yeates et al. (2017).
2.2. Participants
The concussion group was defined as children who had sustained a blunt head trauma and presented with at least one of the following three findings: an observed loss of consciousness, a Glasgow Coma Scale (GCS) score of 13 or 14, or at least one acute sign or symptom of concussion. Acute signs and symptoms of concussion include post‐traumatic amnesia, focal neurological deficits, skull fracture, post‐traumatic seizure, vomiting, headache, dizziness, or other mental status abnormalities. Children who showed delayed neurological deterioration (GCS < 13) or required neurosurgical intervention were excluded. Participants were also excluded if they had a loss of consciousness of greater than 30 min, post‐traumatic amnesia of greater than 24 h, or any associated injuries with scores of greater than 4 on the Abbreviated Injury Scale (American Association for Automotive Medicine, 1990).
The OI group was defined as children who had sustained either an upper or lower extremity fracture, sprain, or strain due to blunt force/physical trauma resulting in an Abbreviated Injury Scale (American Association for Automotive Medicine, 1990) score of 4 or less. Youths were excluded from the OI group if they had any head trauma or suspicion of concussion and concussion‐related symptoms at the time of recruitment, or an injury requiring surgical intervention or procedural sedation. Youths were excluded from both groups if they sustained a concussion within 3 months of the study.
2.3. Magnetic resonance imaging
All imaging was done at 3 T (General Electric MR750w in Calgary; General Electric MR750 in Montreal and Vancouver; Siemens Prisma in Edmonton and Montreal; Siemens Skyra in Ottawa).
2.4. T1‐weighted acquisition
Sites using a Siemens scanner acquired 3D T1‐weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with TR/TE/TI =1880 ms/2.9 ms/948 ms and a field of view of 25.6 cm2. Sites using a GE scanner acquired 3D T1‐weighted fast spoiled gradient echo brain volume (FSPGR BRAVO) sequence with a TR/TE/TI = 8.25 ms/3.16 ms/600 ms with a field of view of 24 cm2. Both acquisitions used 192 slices, a flip angle of 10° and had a voxel size of 0.8 × 0.8 × 0.8 mm3.
2.5. Magnetic resonance spectroscopy
A short‐echo PRESS MRS acquisition was performed using the following parameters: TE/TR = 30 ms/2000 ms, 96 water‐suppressed averages, 8 unsuppressed water averages, spectral width of 5000 Hz (GE) or 2000 Hz (Siemens), and number of points 4096 (GE) or 2048 (Siemens). The PRESS sequence was chosen because it was readily available across all sites and is consistent with the recommendations in the ENIGMA MRS working group in traumatic brain injury (Bartnik‐Olson et al., 2021). The 2 × 2 × 2 cm3 voxel was placed in the left dorsolateral prefrontal cortex (L‐DLPFC) (Figure 1). The DLPFC was chosen as it has functional relevance in concussion symptoms, is involved with executive functions such as working memory (Badre & Wagner, 2004; Gaines & Soper, 2018; Hertrich et al., 2021), and has been studied in previous pediatric concussion studies (MacMaster et al., 2019; Menshchikov et al., 2020). Each site was provided with several reference images to ensure standardized voxel placement.
FIGURE 1.
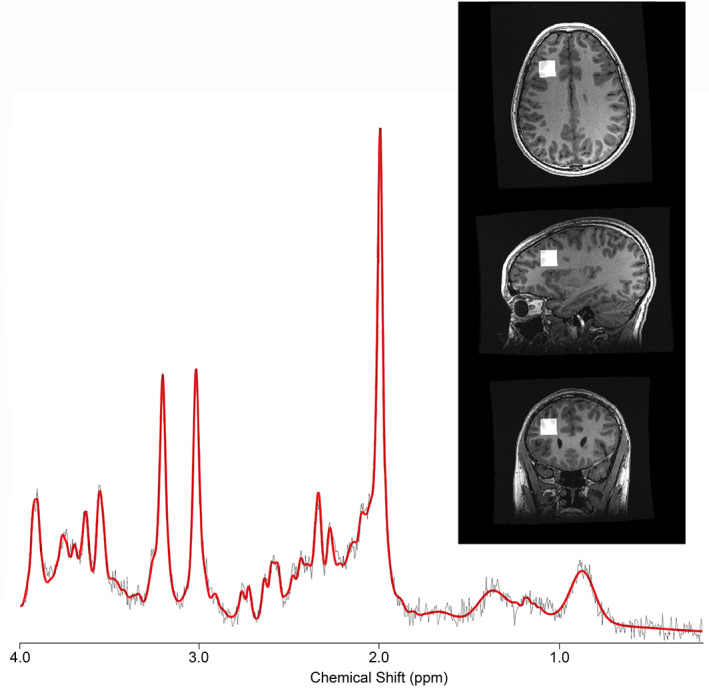
Representative spectrum from a single participant. The black line indicates the raw data, and the red line indicates the overall fit from LCModel after processing. The inset shows the voxel location in the left dorsolateral prefrontal cortex.
2.6. Data processing
For GE data, because the individual averages were available, data were pre‐processed using FID‐A (Simpson et al., 2017). The pre‐processing pipeline included the following: the combination of receiver channels, removal of bad averages, retrospective shot‐by‐shot frequency and phase correction, left shifting, and zero‐order phase correction. The Siemens platform only had the overall average spectrum available. Therefore, the only pre‐processing performed on the Siemens data was by the vendor software prior to export of the data.
The pre‐processed data were analyzed with LCModel version 6.3‐1J (Provencher, 2001), which includes eddy‐current correction and water scaling. Customized basis sets for each vendor were generated in FID‐A using specific pulse shapes and relevant parameters (e.g., spectral width and number of points) and included the following metabolites: alanine, aspartate, 𝛽‐hydroxybutarate, choline, citrate, creatine, ethanol, gamma‐aminobutyric acid, glucose, glutamine, glutamate, glycine, glycerophosphocholine, glutathione, myo‐Inositol, lactate, N‐acetyl‐aspartate, N‐acetyl‐aspartyl‐glutamate, phosphocholine, phosphocreatine, phosphoenolamine, Scyllo‐inositol, taurine. The LCModel default macromolecular and lipid basis sets were also included.
Results from LCModel were then corrected using literature values for T1 and T2 relaxation times and proton density for metabolite quantification (see Tables A1 and A2 in the Appendix A for relaxation values used in correction). Equations specified in Gasparovic et al. (2006), Gasparovic et al. (2018), and in the expert's consensus of MRS in Near et al. (2020) were used. The co‐registration and segmentation function from Gannet (Version 3.1) (Edden et al., 2014; Harris et al., 2015) were used to register the MRS voxel to the corresponding anatomical image and segment it into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) fractions. Cr‐referenced metabolite results are also reported for consistency with previous literature.
2.7. Quality control
Spectral quality was first assessed with visual inspection by a single analyzer (PLL), with more challenging cases, input from a second expert analyzer was sought (ADH). Additional thresholds for quality were an SNR of at least 45 and a CRLB for each metabolite of less than 20%. Additionally, high linewidth (the full‐width half‐maximum, FWHM) or low SNR (but still above the threshold) were used to perform a confirmatory visual assessment of data quality. Data quality is quantified and reported by SNR, FWHM and CRLB. The linewidth and SNR were calculated based off the water peak and the NAA peak respectively in FID‐A for all data (GE and Siemens).
2.8. Statistical analysis
All statistical analyses were completed using IBM SPSS 26 (IBM Corp. Released 2019. IBM SPSS Statistics for MacOS, Version 26.0. Armonk, NY: IBM Corp). In the primary analysis, univariate ANCOVAs were conducted for each metabolite separately (tNAA, tCr, tCho, Glx, and mI) to examine group differences between the concussion and OI groups. Each model included the following covariates: age, sex, site (five different sites), and vendor (GE and Siemens scanners).
Follow‐up analyses were conducted to investigate additional factors of interest. First, given profound developmental changes in the brain across the age range studied, a group by age at injury interaction term was included to determine whether metabolic responses to concussion and OI vary as a function of age. Given tissue type is associated with underlying metabolite content and suggestions that metabolites in WM and GM respond differently to concussion (Menshchikov et al., 2020), a second model that included the covariates voxel tissue fraction of white matter (fWM) and group by fWM interaction was tested. A third model including age by fWM interaction term was included to control for the potential of changing tissue fractions by age. Because imaging data were collected across a range of time post‐injury, a fourth model was tested including covariates of time since injury (in hours) and group by time since injury interaction. Analyses for individual site and vendor were completed to ensure metabolite effects were uniform across these parameters. Finally, tCr‐referenced metabolites were also analyzed as a confirmatory analysis to account for the potential of a changing water signal.
3. RESULTS
In the A‐CAP parent study, a total of 967 participants were recruited from the ED, and of these, 827 participants returned for an assessment at mean 12 days (median 10 days, range 2–33 days) post‐injury. Of these, 596 participants completed the MRS scan, and 545 MRS data sets passed quality assurance. SNR had a similar range across injury groups but was higher in the concussion compared to the OI group (p = .009, Table 1). Additionally, the mean SNR(tNAA) of GE/Siemens data was 82.8/185 and the FWHM was 10.6 Hz/8.34 Hz. Data included in all analyses met quality requirements outlined in Section 2.7.
TABLE 1.
Participant demographics and magnetic resonance spectroscopic (MRS) data characteristics of all participants included in the analysis across concussion and OI groups
| Concussion | OI | p‐value | |||||
|---|---|---|---|---|---|---|---|
| N = 361 | N = 184 | ||||||
| Demographics | Mean | SD | Range | Mean | SD | Range | |
| Sex (% male) | 62 | ‐ | ‐ | 55 | ‐ | ‐ | .142 |
| Age (years) | 12.31 | 2.46 | 8–16.97 | 12.57 | 2.19 | 8.05–16.85 | .201 |
| Time since injury (hours) | 277.40 | 135.0 | 37.17–790.17 | 281.73 | 133.24 | 62.5–659.47 | .738 |
| Time since injury (days) | 11.6 | 5.6 | 1.5–32.9 | 11.7 | 5.6 | 2.6–27.5 | ‐ |
| MRS characteristics | |||||||
| FWHM (Hz) | 9.34 | 4.31 | 3.59–38.2 | 9.83 | 3.75 | 3.79–21.53 | .196 |
| SNR (tNAA) | 135.85 | 59.24 | 45.13–284.48 | 121.76 | 58.86 | 45.19–263.44 | .009 |
| fWM | 0.578 | 0.093 | 0.16–0.9 | 0.579 | 0.099 | 0.25–0.87 | .909 |
Note: Independent sample t tests and Chi‐square tests were used where appropriate to test for group differences. p‐values in bold are statistically significant at p < .05.
Abbreviations: FWHM, full‐width half‐maximum; fWM, fraction white matter; OI, orthopedic injury; SNR(tNAA), signal‐to‐noise ratio obtained from the tNAA peak.
3.1. Demographics
The total number of MRS data sets that passed quality control was 545 with 361 concussion participants and 184 OI participants (Table 1). The time since concussion was 11.6 ± 5.6 days. The time since OI was 11.7 ± 5.6 days. Participant demographics and MRS characteristics can be found in Table 1. The groups did not differ in age, sex, or time since injury.
3.2. Primary analysis: Metabolite comparisons between concussion and OI
Figure 2 illustrates metabolite levels across the two groups. Means and standard deviations can be found in Table B1 (Appendix B). The ANCOVA models demonstrate that groups did not differ statistically significantly for any metabolite (Table 2) and with very small effect sizes (ηp 2 = <0.003). Several covariates were statistically significantly related to metabolite levels. First, age was a statistically significant covariate for tNAA and Glx models (positive association for tNAA and negative association for Glx with metabolites). Site was a statistically significant covariate for tNAA, tCho, and Glx, and vendor was a statistically significant covariate for all metabolites. As SNR was statistically significantly different between groups, it was also included in follow‐up analyses as a covariate but did not affect the primary outcomes.
FIGURE 2.
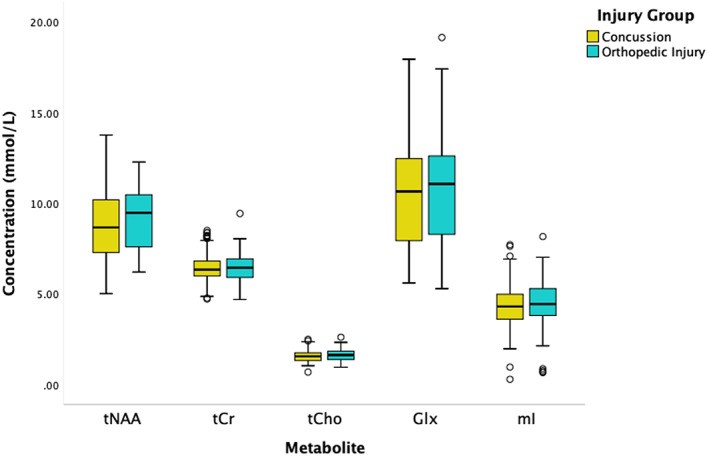
Concentrations of the five metabolites of interest in mmol/L separated by group (concussion in yellow and Orthopedic Injury (OI) in teal). The black lines in the boxes represent the median and the box represents the interquartile range, with the 1.5 times the interquartile range values represented by the whiskers. The circles represent outliers (at least greater than 1.5 times the interquartile range from the median). No statistically significant group differences were found between concussion and OI for any metabolite. See Table 2 for full statistical results.
TABLE 2.
Summary of the independent univariate ANCOVA models for each metabolite to investigate group differences (concussion vs. orthopedic injury)
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| ANCOVA model | 0.008 | .928 | 0.226 | .635 | 1.487 | .223 | 0.297 | .586 | 0.03 | .863 |
| Covariates | ||||||||||
| Group | 0.008 | .928 | 0.226 | .635 | 1.487 | .223 | 0.297 | .586 | 0.03 | .863 |
| Age | 21.56 | .0001 | 0.247 | .619 | 0.894 | .345 | 11.19 | .001 | 0.001 | .978 |
| Sex | 0.017 | .896 | 1.166 | .281 | 0.022 | .882 | 3.726 | .054 | 0.015 | .902 |
| Site | 7.789 | .005 | 2.975 | .085 | 14.20 | .0001 | 5.509 | .019 | 0.567 | .452 |
| Vendor | 1589 | .0001 | 155.3 | .0001 | 370.6 | .0001 | 554.7 | .0001 | 497.7 | .0001 |
Note: The model significance is shown as well as the contributions of each covariate within the model. p‐values in bold were considered statistically significant at p < .05.
3.3. Follow‐up analyses
3.3.1. Age
Since age had a statistically significant effect on tNAA and Glx models, in this first follow‐up analysis, an age‐by‐group interaction term was included for all metabolite ANCOVA models to determine whether age influenced group differences. No age‐by‐group interactions were statistically significant for any metabolite. Full model results are in Table B2.
3.3.2. Tissue effects
Since metabolites are present in different concentrations in WM and GM (Hetherington et al., 1994; Menshchikov et al., 2020; Pouwels & Frahm, 1998), and the voxel composition was mixed, we investigated whether voxel‐tissue content affected the results by including fWM as a covariate in the next series of univariate ANCOVA models. Group differences remained non‐statistically significant for all metabolites, although fWM was a statistically significant covariate for the models of tNAA, tCr, Glx, and mI (p < .0001) in which tNAA, Glx, and mI had positive associations while, tCr had a negative association with fWM. Full model results are in Table B3. As the fWM was related to tNAA, tCr, Glx, and mI levels, a group by fWM interaction term was then included, but was not statistically significant in any model. Full model results are in Table B4. There were no significant effects of the fWM by age interaction term (Table B5).
3.3.3. Time since injury
Since MRS data were acquired across a range of time following injury (11.6 ± 5.6 days, range 1.5–32.9 days), a final series of ANCOVA models included time since injury (in hours) as a covariate. Time since injury was statistically significantly associated with tCr and mI levels, no other metabolite showed an effect of time since injury, and all group differences remained non‐statistically significant. The inclusion of an interaction term of group by time since injury did not change these results. Full model results are in Tables B6 and B7.
3.3.4. Site and vendor
Given the effects of site and vendor in the primary analysis, univariate ANCOVAs were run for each site individually (Calgary, Edmonton, Vancouver, Ottawa) to examine group differences at each site. The data from Montreal was omitted because of the small and disproportionate sample (<10 OI and > 25 Concussion participants). The metabolites did not differ between groups at any site (full results in Table B8). Similarly, univariate ANCOVAs were run for each vendor individually (GE and Siemens). Once again, metabolites were not statistically significantly different between groups for either vendor (full results in Table B9). Vendor‐related effects are visualized in Figure 3.
FIGURE 3.
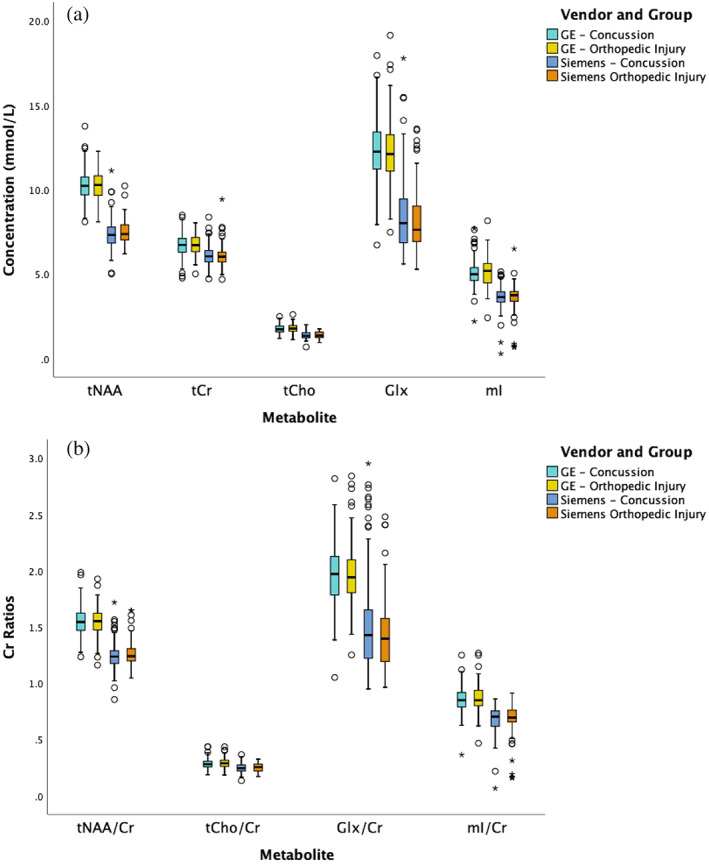
(a) Absolute and (b) tCr‐referenced concentrations of all metabolites separated by the vendor and injury group. The black lines in the boxes represent the median and the box represents the interquartile range, with the 1.5 times the interquartile range values represented by the whiskers. The circles represent outliers (at least greater than 1.5 times the interquartile range from the median) and the asterisks represent statistically significant outliers (at least three times the interquartile range from the median); however, all data met quality control requirements and visual inspection did not suggest the data should be excluded. For all metabolites, there were no statistically significant differences between concussion and OI; however, for all metabolites there were statistically significant differences between vendors.
3.3.5. Creatine reference
The primary statistical models to detect group differences between concussion and OI and the division of analysis of data by vendor were repeated using tCr‐referencing. Figure 4 shows that the tCr‐referenced results were consistent with the primary results in which there were no statistically significant group differences. Metabolite levels were statistically significantly associated with vendor (p < .0001), consistent with the absolute water‐referenced data. Similar to the absolute metabolite quantification, vendor‐related effects are seen in tCr‐referenced metabolites (Figure 3b).
FIGURE 4.
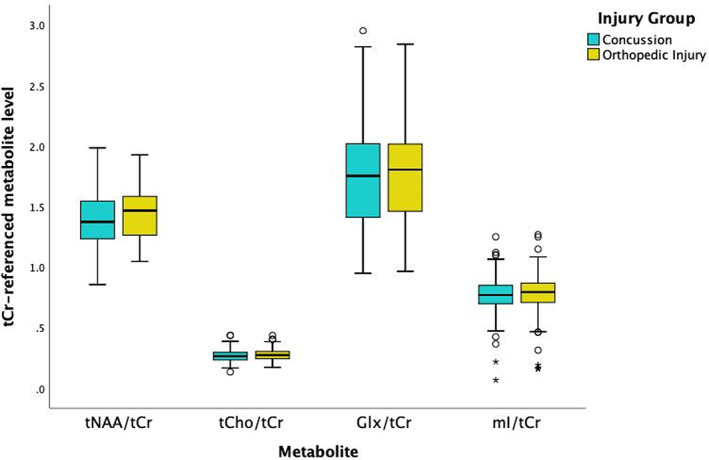
Metabolite levels referenced to tCr separated by group. The black lines in the boxes represent the median and the box represents the interquartile range, with the maximum and minimum values represented by the whiskers on either end of the box plot. The circles represent outliers (at least 1.5 times the interquartile range from the median) outside the maximum and minimum and the asterisk represent statistically significant outliers (at least three times the interquartile range from the median). All outliers passed quality control. No statistically significant group differences between concussion and OI were found for any Cr‐referenced metabolite.
4. DISCUSSION
In this MRS study of pediatric concussion, no differences in metabolite levels (tNAA, tCr, tCho, Glx, and mI) were detected between concussion and OI at a mean of 12 days post‐concussion. This is the first pediatric concussion MRS study of its size (NConcussion = 361). Prior pediatric MRS studies had concussion groups composed of 35 participants or fewer.
4.1. Primary analysis: Metabolite comparisons between concussion and OI
Our study shows no differences in metabolites following injury; however, metabolite changes may occur at timepoints post‐injury outside of the timeframe studied here (mean of 12 days). For example, previous work has shown metabolic disturbances within 72 h after injury (Menshchikov et al., 2020). Combined with our findings, this suggests metabolite alterations may resolve shortly within days of injury. Metabolite differences found greater than 3 months post‐injury (Bartnik‐Olson et al., 2014; MacMaster et al., 2019; Manning et al., 2017) suggest emergent alterations may develop long after injury or in concussion sub‐populations. Our findings of no statistically significant differences in tNAA, tCr, tCho, and mI are in agreement with two previous pediatric studies with frontal voxels (Maugans et al., 2012; Meyer et al., 2019).
Our findings contrast with the more extensive adult literature that more consistently demonstrates group differences between concussion and controls. tNAA in adults is typically decreased following concussion at multiple timepoints in the frontal lobe (Henry et al., 2010; Sarmento et al., 2009; Sivák et al., 2014; Vagnozzi et al., 2008; Vagnozzi et al., 2010; Vagnozzi et al., 2013; Veeramuthu et al., 2018). Conversely in adults, Glx, tCr, tCho, and mI have been shown to increase following concussion in some studies (Chamard et al., 2014; Sarmento et al., 2009; Vagnozzi et al., 2013), while some others have shown decreases or no changes in levels of these metabolites (Henry et al., 2010; Schranz et al., 2018) in the frontal lobe. A recent meta‐analysis of MRS in TBI shows the effect of different brain region on MRS results (Joyce et al., 2022), suggesting studies of multiple brain regions are needed to fully understand metabolite changes in pediatric concussion.
Differences between the extensive adult literature and the limited pediatric literature, in combination with the results of the current study indicates that results in adults cannot necessarily be extrapolated to children and adolescents, motivating further exploration into the effects of age.
4.2. Follow‐up analyses
4.2.1. Age
Age was positively associated with tNAA and negatively associated with Glx, consistent with the literature (Blüml & Panigrahy, 2013; Degnan et al., 2014; Holmes et al., 2017; Kreis et al., 1993). However, we did not find any statistically significant interactions indicating that age moderated group differences in metabolite levels. Across pediatric and adult research, there is some indication that tNAA shows larger reductions with concussion in older populations (Joyce et al., 2022), suggesting age effects may become pronounced later in life. This may indicate metabolite differences in children and youth normalize faster, possibly due to higher brain plasticity (Dennis et al., 2013; Kolb & Gibb, 2011).
4.2.2. Tissue effects
Some metabolites have different concentrations in WM and GM (Goryawala et al., 2016; Govindaraju et al., 2000; Hetherington et al., 1994; Menshchikov et al., 2020; Pouwels & Frahm, 1998; Rae, 2014; Zhang & Shen, 2015). Therefore, we modeled tissue‐related effects by including fWM (tissue fraction of white matter) as a covariate and an interaction term in a follow‐up analysis. fWM was statistically significantly associated with tNAA, tCr, Glx, and mI levels, suggesting concentrations of these metabolites differs between WM and GM, consistent with existing literature (Goryawala et al., 2016; Govindaraju et al., 2000; Hetherington et al., 1994; Menshchikov et al., 2020; Pouwels & Frahm, 1998; Rae, 2014; Zhang & Shen, 2015). However, the interaction between group and fWM was not statistically significant for any metabolite, indicating voxel‐tissue content does not moderate group differences in metabolite levels.
A challenge in assessing voxel‐tissue content is the use of combined metabolite signals, which is common in MRS (e.g., tNAA is the sum of NAA and NAAG). For example, the concentration of NAAG is lower in GM compared with WM (Menshchikov et al., 2020), whereas the opposite is true for NAA (Hetherington et al., 1994; Pouwels & Frahm, 1998; Wang & Li, 1998). A recent study that used advanced methods to differentiate NAA and NAAG suggests the decrease in tNAA in concussion is driven by decreased NAAG (Menshchikov et al., 2020). This may explain why tNAA changes are seen predominantly in WM. Similar challenges exist for the other metabolites where different tissue types (WM and GM) may have different metabolite concentrations (Gasparovic et al., 2009; Kirov et al., 2013; Menshchikov et al., 2020; Meyer et al., 2019; Yeo et al., 2011). Currently, no pediatric studies have shown changes in tCr (Bartnik‐Olson et al., 2014; Maugans et al., 2012; Meyer et al., 2019) and only one study showed changes in tCho (Pouwels & Frahm, 1998). The adult literature showing changes in these metabolites, however, have been predominantly conducted in WM voxel focused studies (Gasparovic et al., 2009; Kirov et al., 2013; Yeo et al., 2011), rather than a voxel of mixed tissue centered on the DLPFC. Thus, although we accounted for voxel‐tissue fraction in the current study, the mixed tissue in the voxel and the combined signals measure (tNAA, tCr, tCho, Glx, mI) may have limited the ability to detect tissue‐related changes.
4.2.3. Time since injury
Previous pediatric concussion studies have examined different time periods post‐injury and have yielded different results. In the current study, the large range of times post‐injury that data were collected motivated examining time since injury. No effect of time since injury was detected for any metabolite. However, conclusions are not definitive from this cross‐sectional data or that in the literature. Longitudinal analyses are required to investigate these changes over longer periods of time.
4.2.4. Site and vendor
Site and vendor were statistically significantly related to metabolite levels, as was expected given known vendor differences between scanners and MRS pulse sequence implementations. A study of healthy controls across 26 sites confirmed vendor and site impacts metabolite levels (Považan et al., 2020). To mitigate the effect of vendor, customized basis sets using the specific vendor pulses were used in the current study, but this does not completely remove the effects of the vendor, consistent with previous work (Považan et al., 2020). Given that the results did not change even with the analysis of group differences by separate vendors and sites (and the sample sizes of these analyses were still large compared to the literature), we do not believe that site or vendor effects obscured any potential metabolite differences. Additionally, chemical shift displacement artifact is known to be present in PRESS data and the direction differs by vendors. However, by default, there is consistency within each vendor and within the site procedures. Given the secondary analyses by vendor and by site are consistent with the primary analysis, we do not think chemical shift displacement artifact is influencing the overall result.
Currently, established approaches to account for the variance introduced via different sites/vendors are standard but are not perfect. An alternative is harmonization of MRS data across sites/vendors. However, there is only one current study using ComBat harmonization of healthy participants with MRS data (Bell et al., 2022). ComBat harmonization for MRS is not yet established in clinical cohorts and pediatrics.
4.2.5. Creatine reference
Many studies report metabolites in reference to Cr as they do not collect water signal. Furthermore, water signal can differ by injury group and MRI vendor. All statistical analyses in the present study were repeated with Cr‐referenced metabolites as a confirmatory analysis of the absolute quantified data. No inconsistencies between the absolute and the Cr‐referenced data were found.
4.3. Strengths, limitations, and future directions
This study has several strengths. First, the study used a comparison group of OI to control for exposure to traumatic injury and thus specifically investigate metabolic changes due to concussion. A diffusion study of children comparing concussion, OI, and non‐injured controls showed the structural connectome of the concussion and OI groups were not statistically significantly different from each other, and both were different from the non‐injured controls. This suggests the injury experience itself results in diffusion differences, rather than head injury specifically (Ware et al., 2022). It is yet to be studied whether this finding extends to metabolites. Second, we applied rigorous pre‐processing of MRS data when possible. Pre‐processing, which is increasing in the field, was completed for the GE data according to recent recommendations (Near et al., 2020), but has not always been the convention and may have impacted previous studies. As MRS results are known to be influenced by many factors (Joyce et al., 2022), an additional strength is that through follow‐up analyses many factors were thoroughly examined to validate the primary result. Finally, this was a multi‐site and multi‐vendor study with general inclusion criteria, as a result, the sample studied is more reflective of a general concussion population.
The study has several limitations. First, SNR was higher in the concussion group compared to the OI group; however, the ranges were highly overlapping and indeed all included data exceeded quality thresholds. Moreover, while not statistically significant, metabolite levels were lower in the concussion group, despite having higher SNR. The increased SNR in the concussion group could be a spurious finding; the effect size of the SNR between groups is low (ηp 2 = 0.013). Alternatively, the higher SNR in the concussion group may reflect increased compliance and less motion in the concussion group due to greater personal investment in study outcomes resulting in better quality data. When SNR was included as a covariate in the ANCOVA models, all models remained non‐statistically significant. Second, this study was limited to a single DLPFC voxel with mixed WM and GM tissue content. Studies that include multiple voxels or use multi‐voxel MRS (magnetic resonance spectroscopic imaging [MRSI]) may provide additional insight into the effects of region and tissue type (Bartnik‐Olson et al., 2014; Maugans et al., 2012; Meyer et al., 2019). Additionally, common to all single voxel studies, the voxel is large compared to the thickness of the cortical grey matter. We aimed to center on the DLPFC, but this was not always possible as it was important to avoid macromolecule and lipid contamination from outside the brain. Regardless, the frontal voxel location is still associated with higher‐order cognitive functions that are related to concussion symptoms. The greater A‐CAP study (Yeates et al., 2017) aimed to study pediatric concussion in general, thus did not constrain factors that may affect metabolite results. Where possible, we have performed additional analyses to examine the influence of additional factors such as time since injury or tissue fraction. However, additional factors may also impact whether metabolites are changed following concussion, such as time beyond the 2‐week window, voxel location, mechanism of injury, or sub‐groups (e.g., sport‐related concussion). Lastly, the symptom status of participants was not considered in the current study. A lack of metabolite changes may not be surprising if participants are no longer experiencing symptoms. While unlikely that all participants have fully recovered from symptoms in this study, future studies could explore the relationships between symptom status and the degree of metabolite changes.
5. CONCLUSION
This is the largest study to date using MRS to examine tNAA, tCr, tCho, Glx, and mI levels in pediatric concussion. Concussion and OI groups were compared in both a primary analysis to examine group differences as well as several follow‐up analyses exploring covariates that may affect metabolite differences between groups. No statistically significant group differences between concussion and OI were detected for any metabolite, and the magnitude of group differences was consistently small. Overall, at 12 days post‐injury, brain metabolites in the L‐DLPFC in youth with concussion appear to not be impacted specifically by brain injury.
FUNDING INFORMATION
This project received funding from Alberta Children's Hospital Research Institute, the Hotchkiss Brain Institute, the Canadian Institutes of Health Research Foundation Grant (FDN143304), and the Canadian Foundation for Innovation and John Evans Leaders Fund (35763). Ashley D. Harris holds a Canada Research Chair in Magnetic Resonance Spectroscopy in Brain Injury. Funders had no role in study design, data collection, analysis, interpretation, writing, or decision to submit.
CONFLICT OF INTEREST STATEMENT
No authors have any conflicts of interest to disclose.
ETHICS STATEMENT
The study was approved by the research ethics board at each participating site.
PATIENT CONSENT STATEMENT
Informed consent and assent were obtained from the parents/guardians and the youth participants, respectively.
ACKNOWLEDGMENTS
Canada Researach Chair (ADH) and Ronald and Irene Ward Chair in Pediatric Brain Injury (KOY).
APPENDIX A. METHODS
TABLE A1.
GM, WM, and CSF relaxation times for T1 and T2 for water.
| GM | WM | CSF | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| Water | 1.304 | 0.11 | 0.83 | 0.08 | 4 | 2.55 |
| Water Density | 0.78 | 0.65 | 0.97 | |||
TABLE A2.
T1 and T2 relaxation times of various metabolites used in the correction of relaxation effects.
| T1 | T2 | |
|---|---|---|
| NAA | 1.515 | 0.274 |
| Cr | 1.365 | 0.17 |
| Cho | 1.23 | 0.222 |
| Glx | 1.23 | 0.2 |
| mI | 1.04 | 0.2 |
Note: Values of GM and WM were averaged from Posse et al. (2007).
APPENDIX B. RESULTS
TABLE B1.
Mean and standard deviation (SD) of metabolite concentrations in mmol/l for total N‐acetyl Aspartate (tNAA), total Creatine (tCr), total Choline (tCho), Glx (Glu + Gln), and mI (Glycine+Inositol) in each group (Concussion and OI).
| Concussion | OI | |
|---|---|---|
| tNAA (mmol/L ± SD) | 8.77 ± 1.67 | 9.13 ± 1.58 |
| tCr (mmol/L ± SD) | 6.41 ± 0.692 | 6.48 ± 0.732 |
| tCho (mmol/L ± SD) | 1.55 ± 0.296 | 1.62 ± 0.309 |
| mI (mmol/L ± SD) | 4.33 ± 0.980 | 4.50 ± 1.13 |
| Glx (mmol/L ± SD) | 10.35 ± 2.76 | 10.7 ± 2.77 |
Abbreviation: OI, orthopedic injury.
TABLE B2.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, Glx, and mI) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.018, p = .895 | F = 0.16, p = .69 | F = 1.511, p = .22 | F = 0.254, p = .614 | F = 0.025, p = .874 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.940 | .333 | 2.711 | .100 | 0.237 | .627 | 0.671 | .413 | 0.086 | .77 |
| Age | 14.5 | .0001 | 0.082 | .774 | 0.944 | .332 | 11.74 | .001 | 0.026 | .871 |
| Sex | 0.006 | .940 | 0.964 | .327 | 0.018 | .894 | 3.917 | .048 | 0.011 | .917 |
| Vendor | 1589 | .0001 | 156.3 | .0001 | 370 | .0001 | 554.9 | .0001 | 497 | .0001 |
| Site | 7.528 | .006 | 3.264 | .071 | 14.05 | .0001 | 5.298 | .022 | 0.587 | .444 |
| Group*age | 0.939 | .333 | 3.102 | .079 | 0.073 | 0.786 | 0.870 | .351 | 0.108 | .742 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. This model includes the Group*Age interaction term as a covariate. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B3.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, Glx, and mI) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.007, p = .932 | F = 0.326, p = .568 | F = 1.493, p = .222 | F = 0.347, p = .556 | F = 0.034, p = .853 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.007 | .932 | 0.326 | .568 | 1.493 | .222 | 0.347 | .556 | 0.034 | .853 |
| fWM | 123.9 | .0001 | 188.5 | .0001 | 1.647 | .200 | 67.46 | .000 | 31.27 | .0001 |
| Age | 46.3 | .0001 | 7.251 | .007 | 1.284 | .258 | 4.983 | .026 | 0.701 | .403 |
| Sex | 0.011 | .916 | 0.893 | .345 | 0.032 | .859 | 4.973 | .026 | 0.0001 | .999 |
| Vendor | 1972 | .0001 | 218.2 | .0001 | 369.7 | .000 | 632 | .000 | 531.2 | .0001 |
| Site | 14.32 | .0001 | 1.294 | .256 | 13.56 | .000 | 8.985 | .003 | 0.178 | .674 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. The covariates included within the univariate ANCOVAs were age, sex, site, and vendor. This model includes fraction white matter (fWM) as a covariate. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B4.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, Glx, and mI) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.008, p = .93 | F = 0.318, p = .573 | F = 1.482, p = .224 | F = 0.336, p = .562 | F = 0.035, p = .851 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.200 | .655 | 1.172 | .279 | 0.207 | .649 | 2.751 | .098 | 0.166 | .683 |
| Age | 46.35 | .0001 | 7.404 | .007 | 1.318 | .252 | 4.816 | .029 | 0.684 | .408 |
| Sex | 0.014 | .906 | 0.827 | .363 | 0.039 | .844 | 5.226 | .023 | 0.0001 | .999 |
| Site | 14.48 | .0001 | 1.064 | .303 | 13.03 | .0001 | 9.892 | .002 | 0.205 | .651 |
| Vendor | 1970 | .0001 | 219 | .0001 | 368.7 | .0001 | 636.1 | .0001 | 529.8 | .0001 |
| fWM | 116.8 | .0001 | 183 | .0001 | 2.010 | .157 | 70.59 | .0001 | 27.69 | .0001 |
| Group*fWM | 0.193 | .660 | 1.427 | .233 | 0.446 | .505 | 3.174 | .075 | 0.146 | .702 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. The covariates included within the univariate ANCOVAs were age, sex, site, and vendor. This model includes fraction white matter (fWM) and the interaction term Group*fWM as a covariate. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B5.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, Glx, and mI) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.008, p = .93 | F = 0.334, p = .563 | F = 1.427, p = .233 | F = 0.317, p = .574 | F = 0.033, p = .857 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.008 | .929 | 0.334 | .563 | 1.427 | .233 | 0.317 | .574 | 0.033 | .857 |
| Age | 1.321 | .251 | 0.016 | .900 | 1.26 | .262 | 0.324 | .570 | 0.085 | .771 |
| Sex | 0.011 | .916 | 0.890 | .346 | 0.03 | .862 | 4.958 | .026 | 0.0001 | .999 |
| Site | 14.29 | .0001 | 1.289 | .257 | 13.6 | .0001 | 8.952 | .003 | 0.178 | .673 |
| Vendor | 1954 | .0001 | 215 | .0001 | 363.3 | .0001 | 630.9 | .0001 | 526.6 | .0001 |
| fWM | 3.67 | .056 | 7.776 | .005 | 0.506 | .477 | 0.359 | .550 | 0.736 | .391 |
| Age*fWM | 0.014 | .905 | 0.081 | .776 | 0.925 | .337 | 0.843 | .359 | 0.028 | .868 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. The covariates included within the univariate ANCOVAs were age, sex, site, and vendor. This model includes fraction white matter (fWM) and the interaction term age*fWM as a covariate. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B6.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, Glx, and mI) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.063, p = .801 | F = 0.124, p = .725 | F = 1.377, p = .241 | F = 0.690, p = .407 | F = 0.120, p = .729 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.063 | .801 | 0.124 | .725 | 1.377 | .241 | 0.690 | .407 | 0.120 | .729 |
| Age | 21.58 | .0001 | 0.171 | .679 | 0.654 | .419 | 9.585 | .002 | 0.009 | .923 |
| Sex | 0.213 | .645 | 0.204 | .652 | 0.136 | .712 | 4.629 | .032 | 0.021 | .884 |
| Vendor | 1403 | .0001 | 147.3 | .0001 | 305.7 | .0001 | 426.3 | .0001 | 442.5 | .0001 |
| Site | 14.88 | .0001 | 0.232 | .630 | 11.02 | .001 | 1.666 | .197 | 0.098 | .755 |
| Time since injury | 3.332 | .069 | 8.277 | .004 | 2.950 | .087 | 0.808 | .369 | 13.94 | .0001 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. The covariates included within the univariate ANCOVAs were age, sex, site, and vendor. This model includes the time since injury (in hours) term as a covariate. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B7.
Results from the univariate ANCOVAs from each metabolite (tNAA, tCr, tCho, and Glx) between groups (Concussion vs. OI).
| tNAA | tCr | tCho | Glx | mI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA model | F = 0.067, p = .795 | F = 0.123, p = .726 | F = 1.389, p = .239 | F = 0.671, p = .413 | F = 0.118, p = .731 | |||||
| F | p | F | p | F | p | F | p | F | p | |
| Covariates | ||||||||||
| Group | 0.601 | .438 | 0.010 | .920 | 1.009 | .316 | 0.305 | .581 | 0.002 | .967 |
| Age | 21.54 | .0001 | 0.171 | .680 | 0.655 | .419 | 9.599 | .002 | 0.009 | .923 |
| Sex | 0.219 | .640 | 0.203 | .653 | 0.140 | .708 | 4.671 | .031 | 0.022 | .882 |
| Site | 15.23 | .0001 | 0.225 | .635 | 11.23 | .001 | 1.871 | .172 | 0.108 | .743 |
| Vendor | 1398 | .0001 | 146.2 | .0001 | 305 | .0001 | 427 | .0001 | 439.8 | .0001 |
| Time since injury | 2.339 | .127 | 7.465 | .007 | 2.192 | .139 | 0.319 | .572 | 12.29 | .0001 |
| Group*time since injury | 0.547 | .460 | 0.003 | .955 | 0.304 | .582 | 1.021 | .313 | 0.045 | .833 |
Note: The significance of the model is included as well as the contributions of each covariate within the model. The covariates included within the univariate ANCOVAs were age, sex, site, and vendor. This model includes the time since injury (in hours) term as well as the group*time since injury interaction term as covariates. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B8.
Results from the univariate ANCOVAs for each metabolite (tNAA, tCr, tCho, Glx, mI) from each site.
| tNAA | tCr | tCho | Glx | mI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury group comparison | Site | F | p | F | p | F | p | F | p | F | p |
| Calgary | 0.2 | .655 | 0.47 | .494 | 0.32 | .573 | 0.072 | .789 | 1.332 | .251 | |
| Edmonton | 0.41 | .523 | 0.823 | .366 | 0.117 | .733 | 0.076 | .783 | 0.535 | .466 | |
| Ottawa | 1.49 | .225 | 2.61 | .109 | 0.194 | .661 | 0.235 | .629 | 0.218 | .642 | |
| Vancouver | 0.363 | .548 | 0.006 | .938 | 1.108 | .295 | 0.198 | .657 | 0.033 | .855 | |
Note: Described here are the F‐statistic and the significance of injury group (Concussion vs. OI) for each metabolite. The data from Montreal was omitted due to insufficient and disproportionate sample (<10 OI and > 25 Concussion participants). p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
TABLE B9.
Results from the univariate ANCOVAs for each metabolite (tNAA, tCr, tCho, Glx, mI) from each vendor.
| tNAA | tCr | tCho | Glx | mI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury group comparison | Vendor | F | p | F | p | F | p | F | p | F | p |
| GE | 0.251 | .617 | 0.508 | .477 | 2.714 | .101 | 0.247 | .620 | 0.038 | .846 | |
| Siemens | 1.66 | .20 | 0.644 | .423 | 0.226 | .635 | 0.101 | .751 | 0.06 | .82 | |
Note: Described here are the F‐statistic and the significance of injury group (Concussion vs. OI) for each metabolite. p‐values in bold were considered statistically significant at p < .05.
Abbreviation: OI, orthopedic injury.
La, P. L. , Joyce, J. M. , Bell, T. K. , Mauthner, M. , Craig, W. , Doan, Q. , Beauchamp, M. H. , Zemek, R. , Yeates, K. O. , & Harris, A. D. (2023). Brain metabolites measured with magnetic resonance spectroscopy in pediatric concussion and orthopedic injury: An Advancing Concussion Assessment in Pediatrics (A‐CAP) study. Human Brain Mapping, 44(6), 2493–2508. 10.1002/hbm.26226
Funding information Alberta Children's Hospital Research Institute; Canada Research Chairs; Canadian Foundation for Innovation and John Evans Leaders Fund, Grant/Award Number: 35763; Canadian Institutes of Health Research, Grant/Award Number: FDN143304; Hotchkiss Brain Institute, University of Calgary
DATA AVAILABILITY STATEMENT
A data set with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator, subject to a signed data access agreement.
REFERENCES
- American Association for Automotive Medicine . (1990). The abbreviated injury scale (AIS)‐1990 revision. Des Plaines American Association for Automotive Medicine. [Google Scholar]
- Badre, D. , & Wagner, A. D. (2004). Selection, integration, and conflict monitoring. Neuron, 41, 473–487. 10.1016/s0896-6273(03)00851-1 [DOI] [PubMed] [Google Scholar]
- Bartnik‐Olson, B. L. , Alger, J. R. , Babikian, T. , Harris, A. D. , Holshouser, B. , Kirov, I. I. , Maudsley, A. A. , Thompson, P. M. , Dennis, E. L. , Tate, D. F. , Wilde, E. A. , & Lin, A. (2021). The clinical utility of proton magnetic resonance spectroscopy in traumatic brain injury: Recommendations from the ENIGMA MRS working group. Brain Imaging and Behavior, 15, 504–525. 10.1007/s11682-020-00330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik‐Olson, B. L. , Holshouser, B. , Wang, H. , Grube, M. , Tong, K. , Wong, V. , & Ashwal, S. (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. Journal of Neurotrauma, 31, 1497–1506. 10.1089/neu.2013.3213 [DOI] [PubMed] [Google Scholar]
- Bell, T. K. , Godfrey, K. J. , Ware, A. L. , Yeates, K. O. , & Harris, A. D. (2022). Harmonization of multi‐site MRS data with ComBat. NeuroImage, 257, 257. 10.1016/j.neuroimage.2022.119330 [DOI] [PubMed] [Google Scholar]
- Bialy, L. , Plint, A. , Zemek, R. , Johnson, D. , Klassen, T. , Osmond, M. , & Freedman, S. B. (2018). Pediatric emergency research Canada. Pediatric Emergency Care, 34, 138–144. 10.1097/PEC.0000000000001360 [DOI] [PubMed] [Google Scholar]
- Blüml, S. , & Panigrahy, A. (2013). MR spectroscopy of pediatric brain disorders. Springer. 10.1007/978-1-4419-5864-8 [DOI] [Google Scholar]
- Chamard, E. , Henry, L. , Boulanger, Y. , Lassonde, M. , & Théoret, H. (2014). A follow‐up study of neurometabolic alterations in female concussed athletes. Journal of Neurotrauma, 31, 339–345. 10.1089/neu.2013.3083 [DOI] [PubMed] [Google Scholar]
- Chamard, E. , & Lichtenstein, J. D. (2018). A systematic review of neuroimaging findings in children and adolescents with sports‐related concussion. Brain Injury, 32, 816–831. 10.1080/02699052.2018.1463106 [DOI] [PubMed] [Google Scholar]
- Churchill, N. W. , Hutchison, M. G. , Graham, S. J. , & Schweizer, T. A. (2020). Neurometabolites and sport‐related concussion: From acute injury to one year after medical clearance. NeuroImage: Clinical, 27, 102258. 10.1016/j.nicl.2020.102258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin, D. J. , Grady, M. F. , Joffe, M. D. , & Zonfrillo, M. R. (2017). Pediatric mild traumatic brain injury in the acute setting. Pediatric Emergency Care, 33, 643–649. 10.1097/PEC.0000000000001252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croall, I. , Smith, F. E. , & Blamire, A. M. (2015). Magnetic resonance spectroscopy for traumatic brain injury. Topics in Magnetic Resonance Imaging, 24, 267–274. 10.1097/RMR.0000000000000063 [DOI] [PubMed] [Google Scholar]
- Degnan, A. J. , Ceschin, R. , Lee, V. , Schmithorst, V. J. , Blüml, S. , & Panigrahy, A. (2014). Early metabolic development of posteromedial cortex and thalamus in humans analyzed via in vivo quantitative magnetic resonance spectroscopy. The Journal of Comparative Neurology, 522, 3717–3732. 10.1002/cne.23634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, M. , Spiegler, B. J. , Juranek, J. J. , Bigler, E. D. , Snead, O. C. , & Fletcher, J. M. (2013). Age, plasticity, and homeostasis in childhood brain disorders. Neuroscience and Biobehavioral Reviews, 37, 2760–2773. 10.1016/j.neubiorev.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Puts, N. A. J. , Harris, A. D. , Barker, P. B. , & Evans, C. J. (2014). Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid–edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging, 40, 1445–1452. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, M. , Xu, L. , Wald, M. M. , & Coronado, V. G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002‐2006. Centers for Disease Control and Prevention National Center for Injury Prevention and Control. [Google Scholar]
- Gaines, K. D. , & Soper, H. V. (2018). Neuropsychological assessment of executive functions following pediatric traumatic brain injury. Applied Neuropsychology: Child, 7, 31–43. 10.1080/21622965.2016.1229406 [DOI] [PubMed] [Google Scholar]
- Gardner, A. , Iverson, G. L. , & Stanwell, P. (2014). A systematic review of proton magnetic resonance spectroscopy findings in sport‐related concussion. Journal of Neurotrauma, 31, 1–18. 10.1089/neu.2013.3079 [DOI] [PubMed] [Google Scholar]
- Gasparovic, C. , Chen, H. , & Mullins, P. G. (2018). Errors in 1H‐MRS estimates of brain metabolite concentrations caused by failing to take into account tissue‐specific signal relaxation. NMR in Biomedicine, 31, 1–9. 10.1002/nbm.3914 [DOI] [PubMed] [Google Scholar]
- Gasparovic, C. , Song, T. , Devier, D. , Bockholt, H. J. , Caprihan, A. , Mullins, P. G. , Posse, S. , Jung, R. E. , & Morrison, L. A. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 55, 1219–1226. 10.1002/mrm.20901 [DOI] [PubMed] [Google Scholar]
- Gasparovic, C. , Yeo, R. , Mannell, M. , Ling, J. , Elgie, R. , Phillips, J. , Doezema, D. , & Mayer, A. R. (2009). Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An 1H‐magnetic resonance spectroscopy study. Journal of Neurotrauma, 26, 1635–1643. 10.1089/neu.2009.0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza, C. C. , & Hovda, D. A. (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75, S24–S33. 10.1227/NEU.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryawala, M. Z. , Sheriff, S. , & Maudsley, A. A. (2016). Regional distributions of brain glutamate and glutamine in normal subjects. NMR in Biomedicine, 29, 1108–1116. 10.1002/nbm.3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju, V. , Gauger, G. E. , Manley, G. T. , Ebel, A. , Meeker, M. , & Maudsley, A. A. (2004). Volumetric proton spectroscopic imaging of mild traumatic brain injury. American Journal of Neuroradiology, 25, 730–737. [PMC free article] [PubMed] [Google Scholar]
- Govindaraju, V. , Young, K. , & Maudsley, A. A. (2000). Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine, 13, 129–153. [DOI] [PubMed] [Google Scholar]
- Harris, A. D., Puts, N. A. J., & Edden, R. A. E. (2015), Tissue correction for GABA‐edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging, 42, 1431–1440. https://doi.org.ezproxy.lib.ucalgary.ca/10.1002/jmri.24903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, L. C. , Tremblay, S. , Leclerc, S. , Khiat, A. , Boulanger, Y. , Ellemberg, D. , & Lassonde, M. (2011). Metabolic changes in concussed American football players during the acute and chronic post‐injury phases. BMC Neurology, 11, 1–10. 10.1186/1471-2377-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, L. C. , Tremblay, S. , Yvan, B. , Ellemberg, D. , & Maryse, L. (2010). Neurometabolic changes in the acute phase after sports concussion correlate with symptom severity. Journal of Neurotrauma, 76, 65–76. [DOI] [PubMed] [Google Scholar]
- Hertrich, I. , Dietrich, S. , Blum, C. , & Ackermann, H. (2021). The role of the dorsolateral prefrontal cortex for speech and language processing. Frontiers in Human Neuroscience, 15, 1–16. 10.3389/fnhum.2021.645209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington, H. P. , Mason, G. F. , Pan, J. W. , Ponder, S. L. , Vaughan, J. T. , Twieg, D. B. , & Pohost, G. M. (1994). Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4.1T. Magnetic Resonance in Medicine, 32, 565–571. 10.1002/mrm.1910320504 [DOI] [PubMed] [Google Scholar]
- Holmes, M. J. , Robertson, F. C. , Little, F. , Randall, S. R. , Cotton, M. F. , Van Der Kouwe, A. J. W. , Laughton, B. , & Meintjes, E. M. (2017). Longitudinal increases of brain metabolite levels in 5‐10 year old children. PLoS One, 12, 1–14. 10.1371/journal.pone.0180973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honce, J. M. , Nyberg, E. , Jones, I. , & Nagae, L. (2016). Neuroimaging of concussion. Physical Medicine and Rehabilitation Clinics of North America, 27, 411–428. 10.1016/j.pmr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Johnson, B. , Zhang, K. , Gay, M. , Neuberger, T. , Horovitz, S. , Hallett, M. , Sebastianelli, W. , & Slobounov, S. (2012). Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: An 1H‐MRS study. Neuroscience Letters, 509, 5–8. 10.1016/j.neulet.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, J. M. , La, P. L. , Walker, R. , & Harris, A. D. (2022). Magnetic resonance spectroscopy of traumatic brain injury and subconcussive hits: A systematic review and meta‐analysis. Journal of Neurotrauma, 39, 1455–1476. 10.1089/neu.2022.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans, A. S. , Kirov, I. I. , Gonen, O. , Haemer, G. , Nisenbaum, E. , Babb, J. S. , Grossman, R. I. , & Lui, Y. W. (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology, 82, 521–528. 10.1212/WNL.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov, I. I. , Tal, A. , Babb, J. S. , Lui, Y. W. , Grossman, R. I. , & Gonen, O. (2013). Diffuse axonal injury in mild traumatic brain injury: A 3D multivoxel proton MR spectroscopy study Ivan. Journal of Neurology, 260, 242–252. 10.1007/s00415-012-6626-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, B. , & Gibb, R. (2011). Brain plasticity and behaviour in the developing brain. Journal of Canadian Academy of Child and Adolescent Psychiatry, 20, 265–276. [PMC free article] [PubMed] [Google Scholar]
- Kreis, R. , Ernst, T. , & Ross, B. D. (1993). Development of the human brain: In vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 30, 424–437. 10.1002/mrm.1910300405 [DOI] [PubMed] [Google Scholar]
- Lin, A. P. , Liao, H. J. , Merugumala, S. K. , Prabhu, S. P. , Meehan, W. P. , & Ross, B. D. (2012). Metabolic imaging of mild traumatic brain injury. Brain Imaging and Behavior, 6, 208–223. 10.1007/s11682-012-9181-4 [DOI] [PubMed] [Google Scholar]
- MacMaster, F. P. , McLellan, Q. , Harris, A. D. , Virani, S. , Barlow, K. M. , Langevin, L. M. , Yeates, K. O. , & Brooks, B. L. (2019). N‐acetyl‐aspartate in the dorsolateral prefrontal cortex Long after concussion in youth. The Journal of Head Trauma Rehabilitation, 35, E127–E135. 10.1097/HTR.0000000000000535 [DOI] [PubMed] [Google Scholar]
- Manning, K. Y. , Schranz, A. , Bartha, R. , Dekaban, G. A. , Barreira, C. , Brown, A. , Fischer, L. , Asem, K. , Doherty, T. J. , Fraser, D. D. , Holmes, J. , & Menon, R. S. (2017). Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology, 89, 2157–2166. 10.1212/WNL.0000000000004669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugans, T. A. , Farley, C. , Altaye, M. , Leach, J. , & Cecil, K. M. (2012). Pediatric sports‐related concussion produces cerebral blood flow alterations. Pediatrics, 129, 28–37. 10.1542/peds.2011-2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshchikov, P. , Ivantsova, A. , Manzhurtsev, A. , Ublinskiy, M. , Yakovlev, A. , Melnikov, I. , Kupriyanov, D. , Akhadov, T. , & Semenova, N. (2020). Separate N‐acetyl aspartyl glutamate, N‐acetyl aspartate, aspartate, and glutamate quantification after pediatric mild traumatic brain injury in the acute phase. Magnetic Resonance in Medicine, 84, 2918–2931. 10.1002/mrm.28332 [DOI] [PubMed] [Google Scholar]
- Meyer, E. J. , Stout, J. N. , Chung, A. W. , Grant, P. E. , Mannix, R. , & Gagoski, B. (2019). Longitudinal changes in magnetic resonance spectroscopy in pediatric concussion: A pilot study. Frontiers in Neurology, 10, 1–7. 10.3389/fneur.2019.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett, J. R. , Arun, P. , Ariyannur, P. S. , & Namboodiri, A. M. A. (2013). N‐Acetylaspartate reductions in brain injury: Impact on post‐injury neuroenergetics, lipid synthesis, and protein acetylation. Frontiers in Neuroenergetics, 5, 1–19. 10.3389/fnene.2013.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near, J. , Harris, A. D. , Juchem, C. , Kreis, R. , Marjańska, M. , Öz, G. , Slotboom, J. , Wilson, M. , & Gasparovic, C. (2020). Preprocessing, analysis and quantification in single‐voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR in Biomedicine, 34, 1–23. 10.1002/nbm.4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse, S. , Otazo, R. , Caprihan, A. , Bustillo, J. , Chen, H. , Henry, P. G. , Marjanska, M. , Gasparovic, C. , Zuo, C. , Magnotta, V. , Mueller, B. , Mullins, P. , Renshaw, P. , Ugurbil, K. , Lim, K. O. , & Alger, J. R. (2007). Proton echo‐planar spectroscopic imaging of J‐coupled resonances in human brain at 3 and 4 tesla. Magnetic Resonance in Medicine, 58, 236–244. 10.1002/mrm.21287 [DOI] [PubMed] [Google Scholar]
- Pouwels, P. J. W. , & Frahm, J. (1998). Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magnetic Resonance in Medicine, 39, 53–60. 10.1002/mrm.1910390110 [DOI] [PubMed] [Google Scholar]
- Považan, M. , Mikkelsen, M. , Berrington, A. , Bhattacharyya, P. K. , Brix, M. K. , Buur, P. F. , Cecil, K. M. , Chan, K. L. , Chen, D. Y. T. , Craven, A. R. , Cuypers, K. , Dacko, M. , Duncan, N. W. , Dydak, U. , Edmondson, D. A. , Ende, G. , Ersland, L. , Forbes, M. A. , Gao, F. , … Barker, P. B. (2020). Comparison of multivendor single‐voxel MR spectroscopy data acquired in healthy brain at 26 sites. Radiology, 295, 171–180. 10.1148/radiol.2020191037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher, S. W. (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 14, 260–264. 10.1002/nbm.698 [DOI] [PubMed] [Google Scholar]
- Rae, C. D. (2014). A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochemical Research, 39, 1–36. 10.1007/s11064-013-1199-5 [DOI] [PubMed] [Google Scholar]
- Sarmento, E. , Moreira, P. , Brito, C. , Souza, J. , Jevoux, C. , & Bigal, M. (2009). Proton spectroscopy in patients with post‐traumatic headache attributed to mild head injury. Headache, 49, 1345–1352. 10.1111/j.1526-4610.2009.01494.x [DOI] [PubMed] [Google Scholar]
- Schranz, A. L. , Manning, K. Y. , Dekaban, G. A. , Fischer, L. , Jevremovic, T. , Blackney, K. , Barreira, C. , Doherty, T. J. , Fraser, D. D. , Brown, A. , Holmes, J. , Menon, R. S. , & Bartha, R. (2018). Reduced brain glutamine in female varsity rugby athletes after concussion and in non‐concussed athletes after a season of play. Human Brain Mapping, 39, 1489–1499. 10.1002/hbm.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti, S. , Vagnozzi, R. , Tavazzi, B. , & Lazzarino, G. (2010). Biochemical and neurochemical sequelae following mild traumatic brain injury: Summary of experimental data and clinical implications. Neurosurgical Focus, 29, 1–12. 10.3171/2010.9.FOCUS10183 [DOI] [PubMed] [Google Scholar]
- Simpson, R. , Devenyi, G. A. , Jezzard, P. , Hennessy, T. J. , & Near, J. (2017). Advanced processing and simulation of MRS data using the FID appliance (FID‐A)—An open source, MATLAB‐based toolkit. Magnetic Resonance in Medicine, 77, 23–33. 10.1002/mrm.26091 [DOI] [PubMed] [Google Scholar]
- Sivák, S. , Bittšanský, M. , Grossmann, J. , Nosál’, V. , Kantorová, E. , Siváková, J. , Demková, A. , Hnilicová, P. , Dobrota, D. , & Kurča, E. (2014). Clinical correlations of proton magnetic resonance spectroscopy findings in acute phase after mild traumatic brain injury. Brain Injury, 28, 341–346. 10.3109/02699052.2013.865270 [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Signoretti, S. , Cristofori, L. , Alessandrini, F. , Floris, R. , Isgró, E. , Ria, A. , Marziali, S. , Zoccatelli, G. , Tavazzi, B. , Del Bolgia, F. , Sorge, R. , Broglio, S. P. , McIntosh, T. K. , & Lazzarino, G. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain, 133, 3232–3242. 10.1093/brain/awq200 [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Signoretti, S. , Floris, R. , Marziali, S. , Manara, M. , Amorini, A. M. , Belli, A. , di Pietro, V. , D'Urso, S. , Pastore, F. S. , Lazzarino, G. , & Tavazzi, B. (2013). Decrease in N‐acetylaspartate following concussion may be coupled to decrease in creatine. The Journal of Head Trauma Rehabilitation, 28, 284–292. 10.1097/HTR.0b013e3182795045 [DOI] [PubMed] [Google Scholar]
- Vagnozzi, R. , Signoretti, S. , Tavazzi, B. , Floris, R. , Ludovici, A. , Marziali, S. , Tarascio, G. , Amorini, A. M. , Di Pietro, V. , & Roberto Delfini, G. L. (2008). Temporalwindow of metabolic brain vulnerability to concussion: A pilot 1H‐magnetic resonance spectroscopic study in concussed athletes—Part III. Neurosurgery, 62, 1296. 10.1227/01.NEU.0000316421.58568.AD [DOI] [PubMed] [Google Scholar]
- Veeramuthu, V. , Seow, P. , Narayanan, V. , Wong, J. H. D. , Tan, L. K. , Hernowo, A. T. , & Ramli, N. (2018). Neurometabolites alteration in the acute phase of mild traumatic brain injury (mTBI): An in vivo proton magnetic resonance spectroscopy (1H‐MRS) study. Academic Radiology, 25, 1167–1177. 10.1016/j.acra.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , & Li, S. J. (1998). Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 39, 28–33. 10.1002/mrm.1910390107 [DOI] [PubMed] [Google Scholar]
- Ware, A. L. , Yeates, K. O. , Geeraert, B. , Long, X. , Beauchamp, M. H. , Craig, W. , Doan, Q. , Freedman, S. B. , Goodyear, B. G. , Zemek, R. , Lebel, C. , & Pediatric Emergency Research Canada A‐CAP Study Team . (2022). Structural connectome differences in pediatric mild traumatic brain and orthopedic injury. Human Brain Mapping, 43, 1032–1046. 10.1002/hbm.25705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates, K. O. , Beauchamp, M. , Craig, W. , Doan, Q. , Zemek, R. , Bjornson, B. , Gravel, J. , Mikrogianakis, A. , Goodyear, B. , Abdeen, N. , Beaulieu, C. , Dehaes, M. , Deschenes, S. , Harris, A. , Lebel, C. , Lamont, R. , Williamson, T. , Barlow, K. M. , Bernier, F. , … Pediatric Emergency Research Canada (PERC) . (2017). Advancing concussion assessment in pediatrics (A‐CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Protocol study. BMJ Open, 7, 1–14. 10.1136/bmjopen-2017-017012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, R. A. , Gasparovic, C. , Merideth, F. , Ruhl, D. , Doezema, D. , & Mayer, A. R. (2011). A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. Journal of Neurotrauma, 28, 1–11. 10.1089/neu.2010.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , & Shen, J. (2015). Regional and tissue‐specific differences in brain glutamate concentration measured by in vivo single voxel MRS. Journal of Neuroscience Methods, 239, 94–99. 10.1016/j.jneumeth.2014.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data set with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator, subject to a signed data access agreement.


