Abstract
The choroid plexus (ChP) is part of the blood‐cerebrospinal fluid barrier, regulating brain homeostasis and the brain's response to peripheral events. Its upregulation and enlargement are considered essential in psychosis. However, the timing of the ChP enlargement has not been established. This study introduces a novel magnetic resonance imaging‐based segmentation method to examine ChP volumes in two cohorts of individuals with psychosis. The first sample consists of 41 individuals with early course psychosis (mean duration of illness = 1.78 years) and 30 healthy individuals. The second sample consists of 30 individuals with chronic psychosis (mean duration of illness = 7.96 years) and 34 healthy individuals. We utilized manual segmentation to measure ChP volumes. We applied ANCOVAs to compare normalized ChP volumes between groups and partial correlations to investigate the relationship between ChP, LV volumes, and clinical characteristics. Our segmentation demonstrated good reliability (.87). We further showed a significant ChP volume increase in early psychosis (left: p < .00010, right: p < .00010) and a significant positive correlation between higher ChP and higher LV volumes in chronic psychosis (left: r = .54, p = .0030, right: r = .68; p < .0010). Our study suggests that ChP enlargement may be a marker of acute response around disease onset. It might also play a modulatory role in the chronic enlargement of lateral ventricles, often reported in psychosis. Future longitudinal studies should investigate the dynamics of ChP enlargement as a promising marker for novel therapeutic strategies.
Keywords: cerebrospinal fluid, manual segmentation, neuroinflammation, perivascular space, schizophrenia, ventricle
The choroid plexus (ChP) plays an essential role in psychosis but is challenging to study in vivo. Here, we develop a novel magnetic resonance imaging‐based segmentation method to examine ChP volumes in individuals with psychosis. Our findings suggest that ChP enlargements may be a marker of acute response around disease onset and might play a modulatory role in the chronic enlargement of lateral ventricles.
1. INTRODUCTION
Psychosis constitutes a group of severe psychiatric disorders that affect ~5% of the world population and can significantly impact afflicted individuals, families, and society (Gerlinger et al., 2013; van Os et al., 2009). Thus, an important focus of research is understanding the pathophysiology of psychosis in order to develop novel diagnostic, prognostic, and treatment strategies.
While the importance of the choroid plexus (ChP) for the pathophysiology of psychosis has been suggested since the 1970s (Rudin, 1979, 1980), it has only recently attracted the attention of clinical neuroscientists as a potential marker for diagnosis, prognosis, and treatment. The ChP represents the vital part of the blood‐cerebrospinal fluid (CSF) barrier, formed by an epithelial cell monolayer and a stromal component located in the lateral, third, and fourth ventricles (Demeestere et al., 2015). The ChP plays a crucial role in maintaining brain homeostasis, given its barrier and secretory functions. It provides structural support for the brain, produces most of the CSF, and secrets various endocrine products, including serotonin, vasopressin, transthyretin, growth factors, carriers, and chemokines (Alshehri et al., 2015; Brown et al., 2004; Szmydynger‐Chodobska et al., 2009; Turner et al., 2014). As the blood‐CSF barrier constitutes the main interface between the periphery and central nervous system, the ChP is also critical for brain development. Furthermore, it assists in eliminating toxins, provides protection against oxidative stress, and regulates the immunological crosstalk between peripheral and central inflammation (Marques et al., 2009). Specifically, the ChP responds to peripheral inflammatory signals by producing central pro‐inflammatory cytokines and modulating immune cells' transmigration into the brain (Demeestere et al., 2015; Mortazavi et al., 2014).
Significantly, structural and functional disruptions of the ChP have been related to abnormal brain development, chronic stress, and aging in animals (Imura & Sato, 2008; Lowe & Wyrobek, 2012; Murthy et al., 2019; Sathyanesan et al., 2012), as well as several disorders in humans, including neuroinflammatory and septic conditions (Goldim et al., 2019; Rudin, 1980), neurodevelopmental and mood disorders (Devorak et al., 2015; McCann et al., 2021; Murck et al., 2021; Ricigliano et al., 2021), and psychosis (Demeestere et al., 2015; Marinescu et al., 2013). Indeed, for individuals with psychosis, post‐mortem studies report increased pro‐inflammatory gene expression in the ChP (Kim et al., 2016). Additionally, two case reports describe an association between a tumorous increase of the ChP and psychotic symptoms, with symptom remission following tumor resection (Arasappa et al., 2013; Carson et al., 1997).
While lateral ventricle (LV) enlargements are a hallmark finding in psychosis (Fannon et al., 2000; Sanfilipo et al., 2000; Shenton et al., 2001; Silverman et al., 1998), computed tomography (CT) studies identified an association between the presence and size of ChP calcification in the LV and symptom severity in psychosis (Bersani et al., 1999; Marinescu et al., 2013; Sandyk, 1993). Furthermore, two magnetic resonance imaging (MRI) studies reported enlarged ChP volume in individuals with psychosis compared with healthy individuals (Lizano et al., 2019; Zhou et al., 2020). The first study compared individuals across the psychosis spectrum with first‐degree relatives and healthy individuals and described enlarged ChP volumes in individuals with psychosis related to higher peripheral pro‐inflammatory interleukin‐6 (Lizano et al., 2019). The second study focused on individuals with a first‐episode diagnosis of schizophrenia and found enlarged ChP volumes linked to increased allostatic load. In line with earlier theories on the pathophysiology of psychosis (Feigenson et al., 2014; Weinberger, 1995), both studies concluded that enlarged ChP volume might be a marker of inflammatory brain response related to psychosis. Of note, blood, clinical, and imaging studies found evidence for peripheral inflammation, as well as an acute, potentially neuroinflammatory brain response around the onset of psychosis (Bustan et al., 2018; Fillman et al., 2013; Lyall et al., 2018; Pasternak et al., 2012; Pasternak et al., 2015; Petrikis et al., 2015; van Berckel et al., 2008).
Here, we aim to extend previous work on the role of ChP volumes in psychosis. Given its size, shape, and location, it is difficult to identify the ChP volume utilizing automatic methods (Tadayon et al., 2020). We, therefore, developed and validated a manual segmentation method for the ChP. We then applied this method to two independent cohorts: (1) 41 individuals with early course psychosis (mean duration of illness = 1.78 years) and 30 matched healthy individuals, and (2) 30 individuals with chronic psychosis (mean duration of illness n = 7.96 years) and 34 matched healthy individuals. We tested whether or not we could replicate ChP enlargements in psychosis using more accurate manual segmentation. We also investigated how ChP volumes relate to disease chronicity and associate with LV volumes in early versus chronic psychosis. Based on previous studies that suggested neuroinflammation around psychosis onset (Feigenson et al., 2014; Najjar et al., 2013; Najjar & Pearlman, 2015; Pasternak et al., 2012; Upthegrove et al., 2014) and the notion that the ChP enlargements might indicate neuroinflammation, we hypothesized that ChP enlargement would be more prominent in early course psychosis compared with more chronic stages.
2. MATERIALS AND METHODS
We utilized two independent data sets for the study, and data processing was performed in parallel using the same methods and processing pipelines. We opted for this approach to examine individuals from different studies at different stages of the disorder. Data set #1 comprised 41 individuals with early course psychosis and 30 matched healthy individuals (HC) recruited by the Human Connectome Project at the Brigham and Women's Hospital. Please note that while the Human Connectome Project is a much larger, multisite study, we limited analyses to individuals that were collected at Brigham and Women's Hospital for the present study. Data set #2 comprised 30 individuals with chronic psychosis and 34 HC, recruited at the Department of Psychiatry at University Hospital Brno, Brno, Czech Republic. All participants provided informed written consent, and the review boards of Harvard Medical School and the University Hospital Brno approved the study.
2.1. Data collection
While all individuals with psychosis in data set #2 were diagnosed with schizophrenia, data set #1 included individuals diagnosed with any DSM‐5 non‐affective or affective psychosis. We, therefore, opted to use the term “individuals with psychosis” throughout the manuscript. However, in an additional analysis, we only included individuals diagnosed with schizophrenia from data set # 1.
2.1.1. Data set #1: Early course psychosis
Individuals with psychosis were diagnosed with a DSM‐5 non‐affective or affective psychosis defined by the Structured Clinical Interview for DSM‐5‐Research version (SCID‐5‐RV) or DSM‐5‐RV interview (First, 2015). Duration of illness was determined via the SCID interview, and duration of illness longer than 5 years was considered an exclusion criterion. The Positive and Negative Syndrome Scale (PANSS) was administered to determine symptom severity (Kay et al., 1987). We calculated chlorpromazine equivalent dosages (CPZ) at the scan date for all individuals with psychosis with complete medication information following previously established norms (Gardner et al., 2010).
Exclusion criteria for all individuals were an IQ less than 70 based on medical history/WASI‐II (McCrimmon & Smith, 2013), contraindication to MRI scan, DSM‐5 (Diagnostic and statistical manual of mental disorders: DSM‐5™, 2013) diagnosis of substance‐induced psychosis, or psychotic disorder due to medical condition based on SCID interview (First, 2015), and known organic brain damage.
2.1.2. Data set #2: Chronic psychosis
Individuals with psychosis in this data set were also diagnosed utilizing the SCID‐5‐Research version criteria (First, 2015), and the duration of illness was determined via the SCID interview. The PANSS was administered to determine symptom severity (Kay et al., 1987), and we calculated CPZ (on the day of scanning) for all individuals with psychosis.
Exclusion criteria for all individuals included contraindication to an MRI scan or a history of brain disorder, neurological injury, or substance abuse. Additional exclusion criteria for HC were a history of psychiatric illness themselves or in first or second‐degree relatives, assessed with the Mini‐International Neuropsychiatric Interview (Sheehan et al., 1998).
2.2. Image acquisition
2.2.1. Data set #1: Early course psychosis
A 3 T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) with 32 channel head coils was used to scan the participants. A whole‐brain, high‐resolution three‐dimensional T1‐weighted magnetization prepared rapid gradient echo (MPRAGE) was used to acquire 240 sagittal slices with a field of view = 256 × 240 × 166 mm3, 0.8 mm isotropic voxels, TR = 2400 ms, TE = 2.22 ms, flip angle = 8°.
2.2.2. Data set #2: Chronic psychosis
A 3 T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) with 64 channel head–neck coils was used to collect the structural MRI data. A whole brain, high‐resolution three‐dimensional T1‐weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was used to acquire 240 sagittal slices with a field of view = 224 × 224 mm2, 1 mm3 isotropic voxel, TR = 2300 ms, TE = 2.33 ms, flip angle = 8°.
2.3. Preprocessing
Both data sets were processed employing the same processing pipeline (https://github.com/pnlbwh/pnlpipe). Structural T1 images underwent visual quality control, were realigned to the AC‐PC line and were centered. Subsequently, brain masks were generated using 3D Slicer (software version 4.5; www.slicer.org) and edited manually. Images were then parcellated into 176 gray and white matter regions using FreeSurfer version 6® (Fischl, 2012). We derived intracranial volume, excluding the ventricle volume (ICV) and LV volumes, from this FreeSurfer segmentation for statistical analyses.
2.4. Segmentation of the ChP
While FreeSurfer® is a widely accepted tool for segmentation and parcellation of gray matter, previous studies reported overestimating and misidentifying structures surrounded by high‐intensity voxels (Cherbuin et al., 2009; Tae et al., 2008) (see Figure S1 demonstrating errors in FreeSurfer segmentation of ChP when compared with manual segmentation). We, therefore, opted to segment the ChP manually.
Based on the guidance of our neuroanatomy team (Drs. Jarrett Rushmore, Nikos Makris, and Edward Yeterian), we focused our segmentation on the trigonum ventriculi in the atrium of the lateral ventricle. The trigonum ventriculi is a triangular cavity of the lateral ventricles at the transition between the body of the lateral ventricles and the occipital and temporal horns, which contains the most reliably identifiable and sizable portion of the ChP, distinct from the surrounding structures (Figure 1). After identifying the trigonum ventriculi, we primarily utilized the coronal view to segment the ChP. Next, editing of the segmentation was conducted in axial and sagittal views, making sure not to include voxels from white or gray matter structures bordering the ventricle (e.g., the corpus callosum body superiorly, the fornix, hippocampus, thalamus, the splenium of the corpus callosum medially, the vertical portion of the caudate laterally, and the cerebral white matter inferiorly). Finally, the 3D view was used to complete the manual segmentation process (Figure 1). Last, we extracted the ChP volume measurements from the ChP segmentation for both hemispheres.
FIGURE 1.
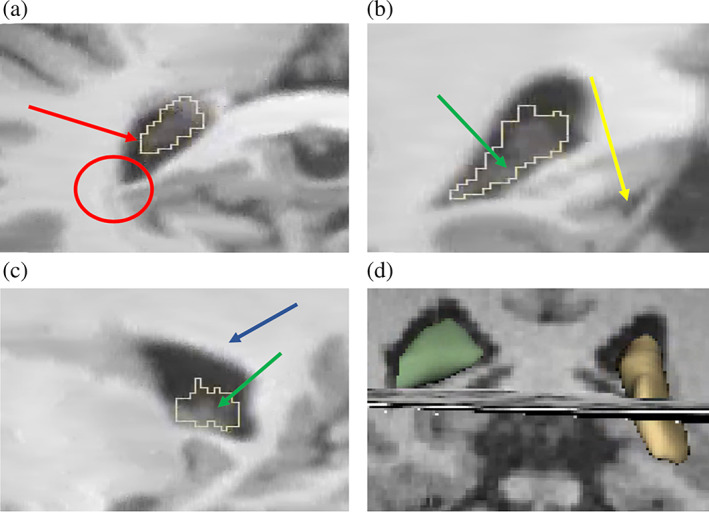
Manual segmentation of the Choroid Plexus using Slicer® (a) Axial view (b) Coronal view (c) Sagittal view. The coronal view was foremost used to guide the manual segmentation (b). ChP voxels were defined as hyperintense voxels within the ventricles. Voxels surrounded by clearly identified ChP tissue and located immediately next to a hypointense non‐ChP voxel were included. In the case of two adjacent voxels with the same density at the boundary, the inner one was included (red arrow), and the outer one was excluded (red circle). After the segmentation in the coronal view, editing was conducted in the axial and sagittal view (a, c), making sure not to include grey or white matter, especially at the boundaries of the lateral ventricles (green arrow), thalamus (yellow arrow), and corpus callosum (blue arrow). Further, a simultaneous 3D image (d) served to check for holes, gaps, and islands of (non‐) ChP tissue, which we then eliminated manually.
2.5. Intra‐ and inter‐rater reliability
OS and MS carried out all segmentations. To test intra‐rater reliability, 10 randomized individuals were segmented twice by the same rater, revealing an intraclass correlation coefficient of .87, considered good to excellent reliability (Cicchetti & Nelson, 1994; Koo & Li, 2016). Ten randomized individuals were also manually segmented by both raters, revealing an intraclass correlation coefficient of .93. Given that the inter‐rater reliability cannot be higher than the intra‐rater reliability, we can assume an intraclass correlation coefficient of .87, which is considered good to excellent reliability (Cicchetti & Nelson, 1994; Koo & Li, 2016).
2.6. Statistical analyses
We performed all statistical analyses using SPSS version 27 and GraphPad Prism 9. Normalized (by ICV, as derived from FreeSurfer) left and right ChP and LV volumes were utilized for all analyses. Again, we conducted the same statistical analyses for data sets #1 and #2 in parallel. We opted for this approach (instead of combining the data sets) because the two data sets were collected as part of two independent cohorts at two different sites.
2.6.1. Group comparisons
ChP volume
To test for ChP enlargement, we calculated two ANCOVAs (for left and right hemispheres separately) with normalized ChP volume as the dependent variable, group (individuals with psychosis versus HC) as the independent variable, and sex and age as covariates. A p‐value < .025 was considered significant (Bonferroni correction for two tests).
LV volume
Given the functional and structural connection between the ChP and LV, we decided to explore the role of LV volumes for our analyses. Therefore, we calculated two additional ANCOVAs to compare the normalized LV volumes between groups. Normalized LV volumes were included as the dependent variable, the group (individuals with psychosis versus HC) was the independent variable, and sex and age were covariates. A p‐value < .025 was considered significant (Bonferroni correction for two tests).
Sensitivity analyses
As a sensitivity analysis, we repeated the group comparisons for data set # 1, only including individuals with schizophrenia (n = 21) and healthy individuals (n = 30).
2.6.2. Correlation analyses in individuals with psychosis
ChP and LV volume
To further elucidate the relationship between ChP and LV volumes, we computed additional partial correlation analyses (corrected for sex and age). We calculated the association between normalized ChP volumes and normalized LV volumes separately for each hemisphere in individuals with psychosis. A p‐value < .025 was considered significant (Bonferroni correction for two tests).
ChP and clinical variables
Last, we calculated partial correlations (corrected for age and sex) between normalized ChP volumes and symptom severity (defined by PANSS total, positive, and negative scores), CPZ, and duration of illness in individuals with psychosis. A p‐value < .005 was considered significant (Bonferroni correction for 10 tests).
3. RESULTS
3.1. Demographics
Data set #1 comprised 41 individuals with psychosis and 30 HC. Data set #2 comprised 30 individuals with psychosis and 34 sex and age‐matched HC. Because of age and sex differences in data sets #1 and 2 (see Table 1), we opted to include these variables as covariates in all analyses. For more demographical and clinical information, please see also Table 1.
TABLE 1.
Demographical variables.
| Healthy controls | Individuals with psychosis | Test statistic | |
|---|---|---|---|
| Data set #1: Early course psychosis | |||
| n (%) | 30 (42.30%) | 41 (57.70%) | |
| Age in years (mean ± SD) | 25.03 ± 4.52 | 21.44 ± 4.01 | t (69) = 3.54, p < .0010 |
| Sex (n/%) |
F: 12/40% M: 18/60% |
F: 15/36.60% M: 26/63.40% |
χ2 = .086, p = .77 |
| Education in years (mean ± SD) | 15.77 ± 2.09 | 13.09 ± 1.59 | t (53) = 5.40, p < .0010 |
| Duration of illness in years (mean ± SD) | 1.78 ± 1.17 | ||
| PANSS total (mean ± SD) | 47.38 ± 9.81 | ||
| PANSS negative symptoms (mean ± SD) | 12.61 ± 4.79 | ||
| PANSS positive symptoms (mean ± SD) | 13.27 ± 4.16 | ||
| Data set #2: Chronic psychosis | |||
| n (%) | 34 (53.13%) | 30 (46.87%) | |
| Age in years (mean ± SD) | 32.50 ± 9.10 | 33.07 ± 9.48 | t (62) = −.24, p = .81 |
| Sex (n/%) |
F: 13/38.24% M: 21/61.76% |
F: 11/36.67% M: 19/63.33% |
χ2 = .017, p = .90 |
| Education in years (mean ± SD) | 14.91 ± 2.71 | 13.93 ± 2.95 | t (62) = 1.38, p = .17 |
| Duration of illness in years (mean ± SD) | 7.96 ± 6.45 | ||
| PANSS total (mean ± SD) | 57.13 ± 15.38 | ||
| PANSS negative symptoms (mean ± SD) | 15.60 ± 6.31 | ||
| PANSS positive symptoms (mean ± SD) | 11.80 ± 4.77 | ||
Abbreviations: PANSS, positive and negative symptom scale; SD, standard deviation (Kay et al., 1987).
3.2. Data set #1: Early course psychosis
3.2.1. Group comparisons
ChP volume
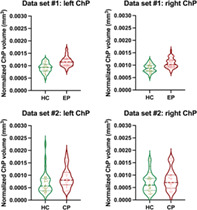
Individuals with early course psychosis presented with significantly higher left and right normalized ChP volumes than HC (left: F [1, 67] = 22.23, p < .00010, partial η2 = .25; right: F [1, 67] = 20.30, p < .00010, partial η2 = .23) (Table 2, Figure 2). The differences remained significant when controlling for normalized LV volumes as an additional covariate (left: F [1, 66] = 26.57, p < .00010, partial η2 = .29; right: F [1, 66] = 21.03, p < .00010, partial η2 = .24).
TABLE 2.
Group comparison for ChP volumes.
| Healthy controls | Individuals with psychosis | Test statistic (ANCOVA, corrected for age and sex) | Test statistic (ANCOVA, corrected for age, sex, and normalized LV volume) | |
|---|---|---|---|---|
| Data set #1: Early course psychosis | ||||
| Normalized ChP volume left hemisphere in mm3 (mean ± SD) | .00092 ± .00019 | .0012 ± .00020 | F (1, 67) = 22.23, p < .00010*, η2 = .25 | F (1, 66) = 26.57, p < .00010*, η2 = .29 |
| Normalized ChP volume right hemisphere in mm3 (mean ± SD) | .00087 ± .00016 | .0011 ± .00019 | F (1, 67) = 20.30, p < .00010*, η2 = .23 | F (1, 66) = 21.03, p < .00010*, η2 = .24 |
| Data set #2: Chronic psychosis | ||||
|
Normalized ChP volume left hemisphere in mm3 (mean ± SD) |
.00069 ± .00043 | .00089 ± .00040 | F (1, 60) = 3.45, p = .068, η2 = .054 | F (1, 59) = .30, p = .58, η2 = .0050 |
| Normalized ChP volume right hemisphere in mm3 (mean ± SD) | .00068 ± .00038 | .00081 ± .00042 | F (1, 60) = 1.72, p = .20, η2 = .028 | F (1, 59) = .005, p = .94, η2 < .00010 |
Abbreviations: ChP = choroid plexus; SD, standard deviation.
p < .025 (after Bonferroni correction for two tests).
FIGURE 2.
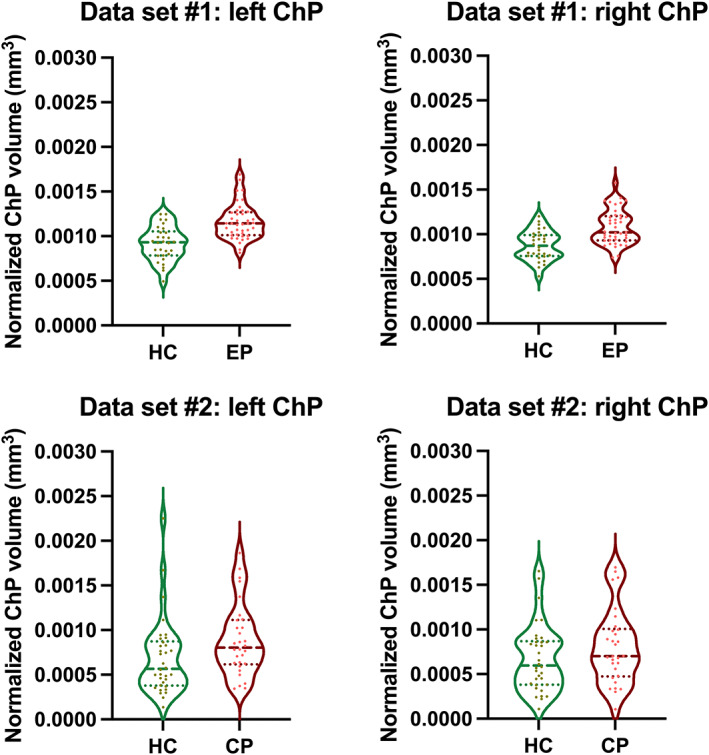
Choroid Plexus volume. Violin plots for Choroid Plexus (ChP) volume in (a) early course psychosis (ECP) individuals and healthy controls (HC) and (b) chronic psychosis (CP) individuals and (HC). Broken lines: median; Dotted lines: quartiles.
LV volume
Individuals with early course psychosis demonstrated increased left and right LV volumes, which reached the significance threshold only for the right LV (left: F [1, 67] = 2.89, p = .094, partial η2 = .041, right: F [1, 67] = 6.10, p = .016, partial η2 = .083) (Table 3).
TABLE 3.
Group comparison for LV volume.
| Healthy controls | Individuals with psychosis | Test statistic (ANCOVA, corrected for age and sex) | |
|---|---|---|---|
|
Data set #1: Early course psychosis |
|||
| Normalized LV volume left hemisphere in mm3 (mean ± SD) | .0057 ± .0021 | .0065 ± .0026 | F (1, 67) = 2.89, p = .094, η2 = .041 |
| Normalized LV volume right hemisphere in mm3 (mean ± SD) | .0048 ± .0017 | .0061 ± .0022 | F (1, 67) = 6.10, p = .016*, η2 = .083 |
| Data set #2: Chronic psychosis | |||
| Normalized LV volume left hemisphere in mm3 (mean ± SD) | .0067 ± .0035 | .0091 ± .0051 | F (1, 60) = 5.12, p = .027, η2 = .079 |
| Normalized LV volume right hemisphere in mm3 (mean ± SD) | .0063 ± .0028 | .0083 ± .0051 | F (1, 60) = 3.85, p = .054, η2 = .060 |
Abbreviations: LV, lateral ventricle; SD, standard deviation.
p < .025 (after Bonferroni correction for two tests).
Sensitivity analyses
We repeated the group comparisons, including individuals with schizophrenia (n = 21) and healthy individuals (n = 30). We again observed a difference in ChP volumes (left: F [1, 47] = 14.89, p < .00010, partial η2 = .24; right: F [1, 47] = 15.03, p < .00010, partial η2 = .24). Group differences for the LV were only significant for the right hemisphere (left: F [1, 47] = 2.82, p = .10, partial η2 = .057, right: F [1, 47] = 6.35, p = .015, partial η2 = .12).
3.2.2. Correlation analyses in individuals with psychosis
ChP and LV volume
We did not observe a significant correlation between the left normalized ChP volume and the left normalized LV volume (r = −.12; p = .46). We also did not see a significant correlation between the right normalized ChP volume and right normalized LV volume (r = .012, p = .94) (Table S1).
ChP and clinical variables
Partial correlations (corrected for age and sex) did not show significant correlations between normalized ChP volumes and total, positive, or negative symptom severity or duration of illness. A positive correlation between CPZ and ChP volumes (left: r = .43, p = .031; right: r = .36, p = .079) did not reach the Bonferroni‐corrected significance threshold (Table S2).
3.3. Data set #2: Chronic psychosis
3.3.1. Group comparisons
ChP volume
Individuals with chronic psychosis presented with higher left and right normalized ChP volumes, which did not reach the significance threshold (left: F [1, 60] = 3.45, p = .068, partial η2 = .054; right: F [1, 60] = 1.72, p = .20, partial η2 = .028) (Figure 2, Table 2). The differences were also not significant when controlling for normalized LV volume as an additional covariate (left: F [1, 59] = .30, p = .58, partial η2 = .0050; right: F [1, 59] = .0050, p = .94, partial η2 < .00010).
LV volume
Individuals with chronic psychosis exhibited higher normalized left and right LV volumes which did not reach the significance threshold (left: F [1, 60] = 5.12, p = .027, partial η2 = .079; right: F [1, 60] = 3.85, p = .054, η2 = .060) (Table 3).
3.3.2. Correlation analyses in individuals with psychosis
ChP and LV volume
Partial correlations (corrected for age and sex) demonstrated that larger normalized ChP volumes were significantly associated with larger normalized LV volumes (left: r = .54, p = .0030; right: r = .68; p < .0010) in individuals with psychosis (Table S1).
ChP and clinical variables
Partial correlations (corrected for age and sex) showed no significant correlations between normalized left or right ChP volume and total, positive, and negative symptom severity, current CPZ, or duration of illness (Table S2).
4. DISCUSSION
The present study is the first to investigate ChP volumes in individuals with psychosis utilizing a manual segmentation method. As hypothesized, we report higher ChP volumes in individuals with psychosis than in healthy individuals. Interestingly, group differences were significant when comparing early course psychosis and healthy individuals but did not reach the significance threshold when comparing individuals with chronic psychosis and healthy individuals. While these findings might suggest that ChP enlargements are more pronounced around disease onset, some caution is needed, since several other factors (e.g., diagnosis, medication, IQ, smoking, substance use), which were not fully explored here, can also have an impact on the results, they might also suggest that ChP enlargements are more pronounced around disease onset. Further, more extensive studies are needed to follow up on this finding and the association between the ChP and LV.
4.1. ChP volume
Aligning with two other MRI studies of the ChP volume in psychosis (Lizano et al., 2019; Zhou et al., 2020), we observe higher left and right ChP volumes in individuals with psychosis. However, comparisons were statistically significant only for early course psychosis. First, we would like to acknowledge that our sample size is relatively small, and our results are, therefore, preliminary.
As highlighted above, two previous studies examined ChP volume in psychosis. The first included individuals with chronic psychosis, and the second focused on treatment‐naïve first‐episode psychosis. Contrary to our findings, the first reported significant ChP enlargements and an association between increased ChP volume and elevated peripheral pro‐inflammatory IL‐6 (Lizano et al., 2019). The second MRI study in psychosis demonstrated a correlation between ChP enlargement and “allostatic load,” calculated based on cardiovascular, metabolic, neuroendocrine, and immune‐inflammatory markers. The authors suggested that this correlation indicates that the ChP can be considered a structural biomarker for the interaction between the central nervous system and peripheral processes (e.g., inflammation) in the early stage of psychosis (Zhou et al., 2020). Here, we found ChP enlargements in psychosis in both data sets. However, they only reached the significant threshold for the early‐course psychosis data set. While this finding might be related to the relatively small sample, we would also like to highlight that we used a more accurate manual segmentation method than previous studies.
Aligning with the idea that ChP enlargements might be related to neuroinflammation are post‐mortem studies that revealed an increased pro‐inflammatory gene expression in the ChP in individuals with psychosis (Kim et al., 2016). Supporting evidence comes from preclinical studies that characterize the ChP as a central mediator between peripheral and central inflammation (Goldim et al., 2019; Hubert, Dumot, et al., 2019; Mortazavi et al., 2014; Szmydynger‐Chodobska et al., 2009). Moreover, imaging studies have described ChP enlargements related to several neuroinflammatory disorders, such as sepsis, multiple sclerosis, and lupus erythematosus (Dixon & Pérez, 2020; Engelhardt & Sorokin, 2009; Hubert, Chauveau, et al., 2019; Ricigliano et al., 2021; Rudin, 1981).
While studies examining the ChP in psychosis are still sparse, a plethora of evidence supports the role of inflammation in the pathophysiology of psychosis. Several studies report elevated levels of peripheral inflammatory markers in psychosis (Schlaaff et al., 2020), extensive blood–brain barrier leakage in a subset of individuals with psychosis (Kamintsky et al., 2020), and increased immune cell transmigration into the brain in those with psychosis (Cai et al., 2020). While positron emission tomography (PET) studies of activated glial cells in individuals with psychosis remain inconclusive (Marques et al., 2019; Plaven‐Sigray et al., 2018), diffusion MRI studies have shown that individuals with psychosis present with increased free‐water levels during the early stages of the disorders that might be associated with an acute immunological response of the brain (Lyall et al., 2018; Pasternak et al., 2012). Indeed, a large, multisite study in psychosis supports this claim by showing a correlation between increased extracellular free‐water and peripheral pro‐inflammatory markers (Di Biase et al., 2020).
In light of these previous findings, the interpretation that ChP volumes might reflect an inflammatory brain response in the early rather than later stages of psychosis is compelling. However, as detailed in the introduction, the ChP plays a crucial role in maintaining brain homeostasis in general (Alshehri et al., 2015; Brown et al., 2004; Szmydynger‐Chodobska et al., 2009; Turner et al., 2014) and constitutes a main interface between the periphery and central nervous system. In particular, the ChP forms the blood‐CSF barrier and, together with the blood–brain barrier and the glymphatic system, controls the internal brain milieu's homeostasis, removes toxic waste, and provides the clean environment the brain requires to function optimally (Achariyar et al., 2016; Jessen et al., 2015; Johanson et al., 2011; Shetty & Zanirati, 2020). A failure of this clearance system has been associated with several neurological and psychiatric disorders (Reddy & van der Werf, 2020; Segawa et al., 2021; Yan et al., 2021). While studying the brain's clearance system in‐vivo is challenging, previous studies have suggested that increased free‐water in early course psychosis might be a proxy for the glymphatic system performance (Demiral et al., 2019). Thus, future multi‐modal studies, including blood and CSF markers and different types of imaging (e.g., PET), are needed to parse the role of the ChP for inflammation and the clearance system in psychosis.
4.2. Association between ChP volume and clinical variables
We did not observe significant correlations between ChP volumes and total symptom severity for individuals with chronic psychosis, although the left ChP volume had a positive correlation with total symptom severity close to the significance threshold. It is important to note that in both data sets, individuals with psychosis were relatively stable clinically, and this small range of PANSS scores might contribute to the limited findings relating ChP to clinical symptomatology. Therefore, while previous MRI studies also reported no correlation between ChP volume and symptom severity (Lizano et al., 2019), more extensive and better‐powered studies are needed to fully understand the relationship between ChP volume increases and the pathophysiology of psychosis.
Medication dosage on the scan day was significantly higher in data set #2 compared with data set #1. This fact is of particular interest given previous evidence of the interaction between antipsychotic medication and inflammation in psychosis (Pandurangi & Buckley, 2020). However, we did not observe a significant relationship between medication dosage on the day of the scan and increased ChP volumes in either data set. Furthermore, preclinical studies demonstrated a relationship between the dopamine and serotonin system and the ChP blood flow, not the ChP volume. Nonetheless, future research is needed to characterize the relationship between the ChP volumes and brain levels of neurotransmitters to determine whether the ChP might be a target for antipsychotic drugs (Castellani et al., 2019; Nakayama et al., 2007). Additionally, future studies should examine the influence of lifetime antipsychotic medication, different types of antipsychotic medication, and other medications, such as lithium, which might have anti‐inflammatory properties.
4.3. Association between ChP volume and lateral ventricles
Our tests for enlarged LV reach significance levels only for the right LV in early psychosis, although increased LV volumes are widely recognized as a hallmark of psychosis (Alliey‐Rodriguez et al., 2019; Del Re et al., 2016; Johnstone et al., 1976). We believe that our failure to replicate these findings (van Erp et al., 2016) is most likely related to our relatively small sample and the exploratory nature of our study.
Notably, we observe a positive correlation between ChP and LV volumes in chronic psychosis, suggesting that ChP enlargements might contribute to LV abnormalities. Indeed, previous studies demonstrate that ChP pathological growth could cause ventricular enlargement in normal pressure communicating hydrocephalus (Maurizi, 1987). Additionally, previous CT studies report correlations between ChP calcifications and LV enlargement in psychosis (Kay et al., 1991). These findings may suggest that LV enlargement is a long‐term consequence of ChP‐related pathology, and thus, the finding that this relationship is observed predominantly in chronic psychosis is not surprising. However, larger longitudinal studies are needed to understand the relationship between ChP, LV, and CSF in psychosis.
4.4. Limitations and future directions
We acknowledge several limitations in the present study. As already highlighted, our sample size was relatively small, and our results are preliminary. Additionally, while we investigate the ChP in two distinct cohorts (one early course and one chronic) utilizing the same processing and analysis pipelines, longitudinal multi‐modal studies are needed to characterize the role of the ChP over illness trajectory, and both cohorts in this study had baseline data only. In addition, we would like to acknowledge that the different imaging resolutions between data sets might influence our findings, where a higher resolution may provide a more accurate volumetric characterization of the ChP and LV volumes.
Furthermore, we believe that using manual parcellation is one of the strengths of our analysis, given its novelty and its excellent intra‐ and inter‐rater reliability. However, future studies are needed to further validate the manual segmentation method. Also, since our method is very time‐consuming, developing better‐automated segmentation methods is critical when examining the ChP in large‐scale studies. We believe that our manual segmentation method may be used to train automated machine learning‐based approaches in the future.
Further, while we examined the association between ChP volume and symptom severity/medication/duration of illness and corrected our analyses for age and sex, we were not able to investigate the influence on ChP volume of other variables, such as markers of peripheral inflammation, more defined diagnosis and medication information, smoking, substance abuse, race, or cognitive functioning (Devorak et al., 2015; Lee et al., 2018; Lizano et al., 2019; Nixon, 2008; Rudin, 1980; Serot et al., 2003).
Of particular note, the ChP is involved in many processes. Generally, the ChP is responsible for CSF production and preserving brain homeostasis (Szmydynger‐Chodobska et al., 2009). Furthermore, it may be part of the neurogenic system (Nogueira et al., 2014) and thus engaged in guarding the limbic system (Rudin, 1980) and modulating the neurogenesis in the hippocampus (Demeestere et al., 2015). Structural and functional abnormalities of the limbic system and the hippocampus are among the most consistent findings in individuals with psychosis (Baglivo et al., 2018; Del Arco & Mora, 2009; Francis et al., 2013; Haukvik et al., 2015; Haukvik et al., 2018; Suzuki et al., 2005; Wood et al., 2001; Zhong et al., 2016). Thus, future and more extensive studies are needed to characterize the relation between ChP abnormalities and the pathophysiology of psychosis.
Lastly, it is critical to note that the enlargement of the ChP may be associated with peripheral inflammation. Previous studies have demonstrated peripheral inflammation in individuals with psychosis (Potvin et al., 2008; Upthegrove et al., 2014), and one post‐mortem study showed that the ChP responds to peripheral inflammatory signals by upregulating pro‐inflammatory genes in psychosis (Kim et al., 2016). Furthermore, an increase in the ChP volume has been demonstrated in reaction to the complex regional pain syndrome‐ a disorder with peripheral and central components (Hubert, Chauveau, et al., 2019). Thus, more extensive longitudinal studies are required to characterize the ChP over the illness trajectory, its interaction with peripheral and central markers, and its relationship with the clinical presentation of the disorder.
5. CONCLUSION
The present study is the first to utilize manual segmentation of the ChP to study its role in psychosis. In summary, we demonstrated significant ChP enlargement in individuals with early‐course psychosis and a positive association between ChP and LV volumes in chronic psychosis. We speculate that ChP enlargement might reflect a neuroinflammatory response around disease onset. Thus, ChP volume may serve as a promising marker for monitoring response to novel diagnostic and therapeutic strategies. However, more extensive longitudinal studies that further examine the influence of, for example, medication, symptom severity, duration of illness, substance use, and peripheral inflammation are needed to follow up on our findings.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
The authors would like to thank all individuals for participating in this study. This work was supported by the National Institutes of Health (NIH) (grant numbers R01MH102377) (PI: Dr. Marek Kubicki), K24MH110807 (PI: Dr. Marek Kubicki), 5U01MH109977‐04 (PIs: Drs. Martha E. Shenton and Alan Breier, as well as Drs. Daphne Holt, Kathryn E. Lewandowski, Matcheri S. Keshavan, and Dost Öngür). We acknowledge the core facility MAFIL supported by the Czech‐BioImaging large RI project (LM2018129 funded by MEYS CR) to obtain scientific data presented in this article. We further acknowledge support by the Harvard Medical School Livingston Fellowship Award (PI: Dr. Johanna Seitz‐Holland), the Young Investigator Grant sponsored by Mary and John Osterhaus and the Brain & Behavior Research Foundation (PI: Dr. Johanna Seitz‐Holland), and the Evangelische Studienstiftung Villigst (PI: Carina Heller).
Senay, O. , Seethaler, M. , Makris, N. , Yeterian, E. , Rushmore, J. , Cho, K. I. K. , Rizzoni, E. , Heller, C. , Pasternak, O. , Szczepankiewicz, F. , Westin, C.‐F. , Losak, J. , Ustohal, L. , Tomandl, J. , Vojtisek, L. , Kudlicka, P. , Kikinis, Z. , Holt, D. , Lewandowski, K. E. , … Kubicki, M. (2023). A preliminary choroid plexus volumetric study in individuals with psychosis. Human Brain Mapping, 44(6), 2465–2478. 10.1002/hbm.26224
Olcay Senay and Magdalena Seethaler contributed equally.
Johanna Seitz‐Holland and Marek Kubicki contributed equally.
Funding information National Institutes of Health (NIH), Grant/Award Numbers: 5U01MH109977‐04, K24MH110807, R01MH102377; core facility MAFIL supported by the Czech‐BioImaging large RI project, Grant/Award Number: LM2018129; Harvard Medical School Livingston Fellowship Award; Young Investigator Grant sponsored by Mary and John Osterhaus and the Brain & Behavior Research Foundation; Evangelische Studienstiftung Villigst
DATA AVAILABILITY STATEMENT
The data are currently not available but part of the data will be available publicly (Human Connectome Project).
REFERENCES
- Achariyar, T. M. , Li, B. , Peng, W. , Verghese, P. B. , Shi, Y. , McConnell, E. , Benraiss, A. , Kasper, T. , Song, W. , Takano, T. , Holtzman, D. M. , Nedergaard, M. , & Deane, R. (2016). Glymphatic distribution of CSF‐derived apoE into brain is isoform specific and suppressed during sleep deprivation. Molecular Neurodegeneration, 11(1), 74. 10.1186/s13024-016-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliey‐Rodriguez, N. , Grey, T. A. , Shafee, R. , Asif, H. , Lutz, O. , Bolo, N. R. , Padmanabhan, J. , Tandon, N. , Klinger, M. , Reis, K. , Spring, J. , Coppes, L. , Zeng, V. , Hegde, R. R. , Hoang, D. T. , Bannai, D. , Nawaz, U. , Henson, P. , Liu, S. , … Gershon, E. S. (2019). NRXN1 is associated with enlargement of the temporal horns of the lateral ventricles in psychosis. Translational Psychiatry, 9(1), 230. 10.1038/s41398-019-0564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri, B. , D'Souza, D. G. , Lee, J. Y. , Petratos, S. , & Richardson, S. J. (2015). The diversity of mechanisms influenced by transthyretin in neurobiology: Development, disease and endocrine disruption. Journal of Neuroendocrinology, 27(5), 303–323. 10.1111/jne.12271 [DOI] [PubMed] [Google Scholar]
- Arasappa, R. , Danivas, V. , & Venkatasubramanian, G. (2013). Choroid plexus papilloma presenting as schizophrenia: A case report. The Journal of Neuropsychiatry and Clinical Neurosciences, 25(1), E26–E27. 10.1176/appi.neuropsych.12010017 [DOI] [PubMed] [Google Scholar]
- Baglivo, V. , Cao, B. , Mwangi, B. , Bellani, M. , Perlini, C. , Lasalvia, A. , Dusi, N. , Bonetto, C. , Cristofalo, D. , Alessandrini, F. , Zoccatelli, G. , Ciceri, E. , Dario, L. , Enrico, C. , Francesca, P. , Mazzi, F. , Paolo, S. , Balestrieri, M. , Soares, J. C. , … Brambilla, P. (2018). Hippocampal subfield volumes in patients with first‐episode psychosis. Schizophrenia Bulletin, 44(3), 552–559. 10.1093/schbul/sbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani, G. , Garavini, A. , Taddei, I. , Tanfani, G. , & Pancheri, P. (1999). Choroid plexus calcification as a possible clue of serotonin implication in schizophrenia. Neuroscience Letters, 259(3), 169–172. 10.1016/s0304-3940(98)00935-5 [DOI] [PubMed] [Google Scholar]
- Brown, P. D. , Davies, S. L. , Speake, T. , & Millar, I. D. (2004). Molecular mechanisms of cerebrospinal fluid production. Neuroscience, 129(4), 957–970. 10.1016/j.neuroscience.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustan, Y. , Drapisz, A. , Ben Dor, D. H. , Avrahami, M. , Schwartz‐Lifshitz, M. , Weizman, A. , & Barzilay, R. (2018). Elevated neutrophil to lymphocyte ratio in non‐affective psychotic adolescent inpatients: Evidence for early association between inflammation and psychosis. Psychiatry Research, 262, 149–153. 10.1016/j.psychres.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Cai, H. Q. , Catts, V. S. , Webster, M. J. , Galletly, C. , Liu, D. , O'Donnell, M. , Weickert, T. W. , & Weickert, C. S. (2020). Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Molecular Psychiatry, 25(4), 761–775. 10.1038/s41380-018-0235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, B. S. , Weingart, J. D. , Guarnieri, M. , & Fisher, P. G. (1997). Third ventricular choroid plexus papilloma with psychosis. Case report. Journal of Neurosurgery, 87(1), 103–105. 10.3171/jns.1997.87.1.0103 [DOI] [PubMed] [Google Scholar]
- Castellani, G. , Contarini, G. , Mereu, M. , Albanesi, E. , Devroye, C. , D'Amore, C. , Ferretti, V. , De Martin, S. , & Papaleo, F. (2019). Dopamine‐mediated immunomodulation affects choroid plexus function. Brain, Behavior, and Immunity, 81, 138–150. 10.1016/j.bbi.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Cherbuin, N. , Anstey, K. J. , Réglade‐Meslin, C. , & Sachdev, P. S. (2009). In vivo hippocampal measurement and memory: A comparison of manual tracing and automated segmentation in a large community‐based sample. PLoS One, 4(4), e5265. 10.1371/journal.pone.0005265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti, D. V. , & Nelson, L. D. (1994). Re‐examining threats to the reliability and validity of putative brain‐behavior relationships: New guidelines for assessing the effect of patients lost to follow‐up. Journal of Clinical and Experimental Neuropsychology, 16(3), 339–343. 10.1080/01688639408402644 [DOI] [PubMed] [Google Scholar]
- Del Arco, A. , & Mora, F. (2009). Neurotransmitters and prefrontal cortex‐limbic system interactions: Implications for plasticity and psychiatric disorders. Journal of Neural Transmission (Vienna), 116(8), 941–952. 10.1007/s00702-009-0243-8 [DOI] [PubMed] [Google Scholar]
- Del Re, E. C. , Konishi, J. , Bouix, S. , Blokland, G. A. , Mesholam‐Gately, R. I. , Goldstein, J. , Kubicki, M. , Wojcik, J. , Pasternak, O. , Seidman, L. J. , Petryshen, T. , Hirayasu, Y. , Niznikiewicz, M. , Shenton, M. E. , & McCarley, R. W. (2016). Enlarged lateral ventricles inversely correlate with reduced corpus callosum central volume in first episode schizophrenia: Association with functional measures. Brain Imaging and Behavior, 10(4), 1264–1273. 10.1007/s11682-015-9493-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeestere, D. , Libert, C. , & Vandenbroucke, R. E. (2015). Therapeutic implications of the choroid plexus‐cerebrospinal fluid interface in neuropsychiatric disorders. Brain, Behavior, and Immunity, 50, 1–13. 10.1016/j.bbi.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Demiral, Ş. B. , Tomasi, D. , Sarlls, J. , Lee, H. , Wiers, C. E. , Zehra, A. , Srivastava, T. , Ke, K. , Shokri‐Kojori, E. , Freeman, C. R. , Lindgren, E. , Ramirez, V. , Miller, G. , Bandettini, P. , Horovitz, S. , Wang, G. J. , Benveniste, H. , & Volkow, N. D. (2019). Apparent diffusion coefficient changes in human brain during sleep ‐ does it inform on the existence of a glymphatic system? NeuroImage, 185, 263–273. 10.1016/j.neuroimage.2018.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devorak, J. , Torres‐Platas, S. G. , Davoli, M. A. , Prud'homme, J. , Turecki, G. , & Mechawar, N. (2015). Cellular and molecular inflammatory profile of the choroid plexus in depression and suicide. Frontiers in Psychiatry, 6, 138. 10.3389/fpsyt.2015.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase, M. A. , Zalesky, A. , Cetin‐Karayumak, S. , Rathi, Y. , Lv, J. , Boerrigter, D. , North, H. , Tooney, P. , Pantelis, C. , Pasternak, O. , Shannon Weickert, C. , & Cropley, V. L. (2020). Large‐scale evidence for an association between peripheral inflammation and white matter free water in schizophrenia and healthy individuals. Schizophrenia Bulletin, 47, 542–551. 10.1093/schbul/sbaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- . (2013). Diagnostic and statistical manual of mental disorders: DSM‐5™ (5th ed., p. xliv‐947). American Psychiatric Publishing, Inc. [Google Scholar]
- Dixon, G. A. , & Pérez, C. A. (2020). Multiple sclerosis and the choroid plexus: Emerging concepts of disease immunopathophysiology. Pediatric Neurology, 103, 65–75. 10.1016/j.pediatrneurol.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Engelhardt, B. , & Sorokin, L. (2009). The blood‐brain and the blood‐cerebrospinal fluid barriers: Function and dysfunction. Seminars in Immunopathology, 31(4), 497–511. 10.1007/s00281-009-0177-0 [DOI] [PubMed] [Google Scholar]
- Fannon, D. , Tennakoon, L. , Sumich, A. , O'Ceallaigh, S. , Doku, V. , Chitnis, X. , Lowe, J. , Soni, W. , & Sharma, T. (2000). Third ventricle enlargement and developmental delay in first‐episode psychosis: Preliminary findings. The British Journal of Psychiatry, 177, 354–359. 10.1192/bjp.177.4.354 [DOI] [PubMed] [Google Scholar]
- Feigenson, K. A. , Kusnecov, A. W. , & Silverstein, S. M. (2014). Inflammation and the two‐hit hypothesis of schizophrenia. Neuroscience and Biobehavioral Reviews, 38, 72–93. 10.1016/j.neubiorev.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman, S. G. , Cloonan, N. , Catts, V. S. , Miller, L. C. , Wong, J. , McCrossin, T. , Cairns, M. , & Weickert, C. S. (2013). Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Molecular Psychiatry, 18(2), 206–214. 10.1038/mp.2012.110 [DOI] [PubMed] [Google Scholar]
- First, M. B. (2015). Structured clinical interview for the DSM (SCID). In The encyclopedia of clinical psychology (pp. 1–6). John Wiley & Sons. 10.1002/9781118625392.wbecp351 [DOI] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, A. N. , Seidman, L. J. , Tandon, N. , Shenton, M. E. , Thermenos, H. W. , Mesholam‐Gately, R. I. , van Elst, L. T. , Tuschen‐Caffier, B. , DeLisi, L. E. , & Keshavan, M. S. (2013). Reduced subicular subdivisions of the hippocampal formation and verbal declarative memory impairments in young relatives at risk for schizophrenia. Schizophrenia Research, 151(1–3), 154–157. 10.1016/j.schres.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Gardner, D. M. , Murphy, A. L. , O'Donnell, H. , Centorrino, F. , & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686–693. 10.1176/appi.ajp.2009.09060802 [DOI] [PubMed] [Google Scholar]
- Gerlinger, G. , Hauser, M. , De Hert, M. , Lacluyse, K. , Wampers, M. , & Correll, C. U. (2013). Personal stigma in schizophrenia spectrum disorders: A systematic review of prevalence rates, correlates, impact and interventions. World Psychiatry, 12(2), 155–164. 10.1002/wps.20040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldim, M. P. , Danielski, L. G. , Rodrigues, J. F. , Joaquim, L. , Garbossa, L. , de Oliveira Junior, A. N. , Metzker, K. L. L. , Giustina, A. D. , Cardoso, T. , Barichello, T. , & Petronilho, F. (2019). Oxidative stress in the choroid plexus contributes to blood‐cerebrospinal fluid barrier disruption during sepsis development. Microvascular Research, 123, 19–24. 10.1016/j.mvr.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K. , Tamnes, C. K. , Soderman, E. , & Agartz, I. (2018). Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: A systematic review and meta‐analysis. Journal of Psychiatric Research, 104, 217–226. 10.1016/j.jpsychires.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Haukvik, U. K. , Westlye, L. T. , Morch‐Johnsen, L. , Jorgensen, K. N. , Lange, E. H. , Dale, A. M. , Melle, I. , Andreassen, O. A. , & Agartz, I. (2015). In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biological Psychiatry, 77(6), 581–588. 10.1016/j.biopsych.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Hubert, V. , Chauveau, F. , Dumot, C. , Ong, E. , Berner, L. P. , Canet‐Soulas, E. , Ghersi‐Egea, J. F. , & Wiart, M. (2019). Clinical imaging of choroid plexus in health and in brain disorders: A mini‐review. Frontiers in Molecular Neuroscience, 12, 34. 10.3389/fnmol.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert, V. , Dumot, C. , Ong, E. , Amaz, C. , Canet‐Soulas, E. , Chauveau, F. , & Wiart, M. (2019). MRI coupled with clinically‐applicable iron oxide nanoparticles reveals choroid plexus involvement in a murine model of neuroinflammation. Scientific Reports, 9(1), 10046. 10.1038/s41598-019-46566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura, K. , & Sato, I. (2008). Novel localization of tenascin‐X in adult mouse leptomeninges and choroid plexus. Annals of Anatomy, 190(4), 324–328. 10.1016/j.aanat.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Jessen, N. A. , Munk, A. S. , Lundgaard, I. , & Nedergaard, M. (2015). The glymphatic system: A Beginner's guide. Neurochemical Research, 40(12), 2583–2599. 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson, C. , Stopa, E. , McMillan, P. , Roth, D. , Funk, J. , & Krinke, G. (2011). The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: Toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicologic Pathology, 39(1), 186–212. 10.1177/0192623310394214 [DOI] [PubMed] [Google Scholar]
- Johnstone, E. C. , Crow, T. J. , Frith, C. D. , Husband, J. , & Kreel, L. (1976). Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet, 2(7992), 924–926. [DOI] [PubMed] [Google Scholar]
- Kamintsky, L. , Cairns, K. A. , Veksler, R. , Bowen, C. , Beyea, S. D. , Friedman, A. , & Calkin, C. (2020). Blood‐brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clinical, 26, 102049. 10.1016/j.nicl.2019.102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, S. R. , Fiszbein, A. , & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. [DOI] [PubMed] [Google Scholar]
- Kay, S. R. , Sandyk, R. , & Merriam, A. E. (1991). Neuroradiological facets of cognitive abnormality in schizophrenia. The International Journal of Neuroscience, 58(1–2), 83–93. 10.3109/00207459108987185 [DOI] [PubMed] [Google Scholar]
- Kim, S. , Hwang, Y. , Lee, D. , & Webster, M. J. (2016). Transcriptome sequencing of the choroid plexus in schizophrenia. Translational Psychiatry, 6(11), e964. 10.1038/tp.2016.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. M. , Jacobs, H. I. L. , Marquié, M. , Becker, J. A. , Andrea, N. V. , Jin, D. S. , Schultz, A. P. , Frosch, M. P. , Gómez‐Isla, T. , Sperling, R. A. , & Johnson, K. A. (2018). 18F‐flortaucipir binding in choroid plexus: Related to race and hippocampus signal. Journal of Alzheimer's Disease, 62(4), 1691–1702. 10.3233/jad-170840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano, P. , Lutz, O. , Ling, G. , Lee, A. M. , Eum, S. , Bishop, J. R. , Kelly, S. , Pasternak, O. , Clementz, B. , Pearlson, G. , Sweeney, J. A. , Gershon, E. , Tamminga, C. , & Keshavan, M. (2019). Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis Spectrum. The American Journal of Psychiatry, 176(7), 564–572. 10.1176/appi.ajp.2019.18070825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, X. , & Wyrobek, A. (2012). Characterization of the early CNS stress biomarkers and profiles associated with neuropsychiatric diseases. Current Genomics, 13(6), 489–497. 10.2174/138920212802510448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall, A. E. , Pasternak, O. , Robinson, D. G. , Newell, D. , Trampush, J. W. , Gallego, J. A. , Fava, M. , Malhotra, A. K. , Karlsgodt, K. H. , Kubicki, M. , & Szeszko, P. R. (2018). Greater extracellular free‐water in first‐episode psychosis predicts better neurocognitive functioning. Molecular Psychiatry, 23(3), 701–707. 10.1038/mp.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinescu, I. , Udristoiu, I. , & Marinescu, D. (2013). Choroid plexus calcification: Clinical, neuroimaging and histopathological correlations in schizophrenia. Romanian Journal of Morphology and Embryology, 54(2), 365–369. [PubMed] [Google Scholar]
- Marques, F. , Sousa, J. C. , Coppola, G. , Falcao, A. M. , Rodrigues, A. J. , Geschwind, D. H. , Sousa, N. , Correia‐Neves, M. , & Palha, J. A. (2009). Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. Journal of Cerebral Blood Flow and Metabolism, 29(5), 921–932. 10.1038/jcbfm.2009.15 [DOI] [PubMed] [Google Scholar]
- Marques, T. R. , Ashok, A. H. , Pillinger, T. , Veronese, M. , Turkheimer, F. E. , Dazzan, P. , Sommer, I. E. C. , & Howes, O. D. (2019). Neuroinflammation in schizophrenia: Meta‐analysis of in vivo microglial imaging studies. Psychological Medicine, 49(13), 2186–2196. 10.1017/S0033291718003057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi, C. P. (1987). The pathophysiology of enlarged ventricles in normal pressure communicating hydrocephalus and schizophrenia: A possible therapeutic role for melatonin. Medical Hypotheses, 23(1), 61–66. 10.1016/0306-9877(87)90182-4 [DOI] [PubMed] [Google Scholar]
- McCann, B. , Levman, J. , Baumer, N. , Lam, M. Y. , Shiohama, T. , Cogger, L. , MacDonald, A. , Ijner, P. , & Takahashi, E. (2021). Structural magnetic resonance imaging demonstrates volumetric brain abnormalities in down syndrome: Newborns to young adults. Neuroimage Clinical, 32, 102815. 10.1016/j.nicl.2021.102815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon, A. W. , & Smith, A. D. (2013). Review of the Wechsler abbreviated scale of intelligence, second edition (WASI‐II). Journal of Psychoeducational Assessment, 31(3), 337–341. 10.1177/0734282912467756 [DOI] [Google Scholar]
- Mortazavi, M. M. , Griessenauer, C. J. , Adeeb, N. , Deep, A. , Bavarsad Shahripour, R. , Loukas, M. , Tubbs, R. I. , & Tubbs, R. S. (2014). The choroid plexus: A comprehensive review of its history, anatomy, function, histology, embryology, and surgical considerations. Child's Nervous System, 30(2), 205–214. 10.1007/s00381-013-2326-y [DOI] [PubMed] [Google Scholar]
- Murck, H. , Luerweg, B. , Hahn, J. , Braunisch, M. , Jezova, D. , Zavorotnyy, M. , Konrad, C. , Jansen, A. , & Kircher, T. (2021). Ventricular volume, white matter alterations and outcome of major depression and their relationship to endocrine parameters ‐ A pilot study. The World Journal of Biological Psychiatry, 22(2), 104–118. 10.1080/15622975.2020.1757754 [DOI] [PubMed] [Google Scholar]
- Murthy, S. , Kane, G. A. , Katchur, N. J. , Lara Mejia, P. S. , Obiofuma, G. , Buschman, T. J. , McEwen, B. S. , & Gould, E. (2019). Perineuronal nets, inhibitory interneurons, and anxiety‐related ventral hippocampal neuronal oscillations are altered by early life adversity. Biological Psychiatry, 85(12), 1011–1020. 10.1016/j.biopsych.2019.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar, S. , & Pearlman, D. M. (2015). Neuroinflammation and white matter pathology in schizophrenia: Systematic review. Schizophrenia Research, 161(1), 102–112. 10.1016/j.schres.2014.04.041 [DOI] [PubMed] [Google Scholar]
- Najjar, S. , Pearlman, D. M. , Alper, K. , Najjar, A. , & Devinsky, O. (2013). Neuroinflammation and psychiatric illness. Journal of Neuroinflammation, 10, 43. 10.1186/1742-2094-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, H. , Kitaichi, K. , Ito, Y. , Hashimoto, K. , Takagi, K. , Yokoi, T. , Takagi, K. , Ozaki, N. , Yamamoto, T. , & Hasegawa, T. (2007). The role of organic cation transporter‐3 in methamphetamine disposition and its behavioral response in rats. Brain Research, 1184, 260–269. 10.1016/j.brainres.2007.09.072 [DOI] [PubMed] [Google Scholar]
- Nixon, P. F. (2008). Glutamate export at the choroid plexus in health, thiamin deficiency, and ethanol intoxication: Review and hypothesis. Alcoholism, Clinical and Experimental Research, 32(8), 1339–1349. 10.1111/j.1530-0277.2008.00727.x [DOI] [PubMed] [Google Scholar]
- Nogueira, A. B. , Sogayar, M. C. , Colquhoun, A. , Siqueira, S. A. , Nogueira, A. B. , Marchiori, P. E. , & Teixeira, M. J. (2014). Existence of a potential neurogenic system in the adult human brain. Journal of Translational Medicine, 12, 75. 10.1186/1479-5876-12-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandurangi, A. K. , & Buckley, P. F. (2020). Inflammation, antipsychotic drugs, and evidence for effectiveness of anti‐inflammatory agents in schizophrenia. Current Topics in Behavioral Neurosciences, 44, 227–244. 10.1007/7854_2019_91 [DOI] [PubMed] [Google Scholar]
- Pasternak, O. , Westin, C. F. , Bouix, S. , Seidman, L. J. , Goldstein, J. M. , Woo, T. U. , Petryshen, T. L. , Mesholam‐Gately, R. I. , McCarley, R. W. , Kikinis, R. , Shenton, M. E. , & Kubicki, M. (2012). Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. The Journal of Neuroscience, 32(48), 17365–17372. 10.1523/jneurosci.2904-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak, O. , Westin, C. F. , Dahlben, B. , Bouix, S. , & Kubicki, M. (2015). The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophrenia Research, 161(1), 113–118. 10.1016/j.schres.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikis, P. , Voulgari, P. V. , Tzallas, A. T. , Archimandriti, D. T. , Skapinakis, P. , & Mavreas, V. (2015). Cytokine profile in drug‐naïve, first episode patients with psychosis. Journal of Psychosomatic Research, 79(4), 324–327. 10.1016/j.jpsychores.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Plaven‐Sigray, P. , Matheson, G. J. , Collste, K. , Ashok, A. H. , Coughlin, J. M. , Howes, O. D. , Mizrahi, R. , Pomper, M. G. , Rusjan, P. , Veronese, M. , Wang, Y. , & Cervenka, S. (2018). Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: A meta‐analysis using individual participant data. Biological Psychiatry, 84(6), 433–442. 10.1016/j.biopsych.2018.02.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin, S. , Stip, E. , Sepehry, A. A. , Gendron, A. , Bah, R. , & Kouassi, E. (2008). Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological Psychiatry, 63, 801–808. [DOI] [PubMed] [Google Scholar]
- Reddy, O. C. , & van der Werf, Y. D. (2020). The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sciences, 10(11), 868. 10.3390/brainsci10110868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricigliano, V. A. G. , Morena, E. , Colombi, A. , Tonietto, M. , Hamzaoui, M. , Poirion, E. , Bottlaender, M. , Gervais, P. , Louapre, C. , Bodini, B. , & Stankoff, B. (2021). Choroid plexus enlargement in inflammatory multiple sclerosis: 3.0‐T MRI and translocator protein PET evaluation. Radiology, 301(1), 166–177. 10.1148/radiol.2021204426 [DOI] [PubMed] [Google Scholar]
- Rudin, D. O. (1979). Covert transport dysfunction in the choroid plexus as a possible cause of schizophrenia. Schizophrenia Bulletin, 5(4), 623–626. 10.1093/schbul/5.4.623 [DOI] [PubMed] [Google Scholar]
- Rudin, D. O. (1980). The choroid plexus and system disease in mental illness. I. a new brain attack mechanism via the second blood‐‐brain barrier. Biological Psychiatry, 15(4), 517–539. [PubMed] [Google Scholar]
- Rudin, D. O. (1981). The choroid plexus and system disease in mental illness. II. Systemic lupus erythematosus: A combined transport dysfunction model for schizophrenia. Biological Psychiatry, 16(4), 373–397. [PubMed] [Google Scholar]
- Sandyk, R. (1993). Choroid plexus calcification as a possible marker of hallucinations in schizophrenia. The International Journal of Neuroscience, 71(1–4), 87–92. 10.3109/00207459309000595 [DOI] [PubMed] [Google Scholar]
- Sanfilipo, M. , Lafargue, T. , Arena, L. , Rusinek, H. , Kushner, K. , Lautin, A. , Loneragan, C. , Vaid, G. , Rotrosen, J. , & Wolkin, A. (2000). Fine volumetric analysis of the cerebral ventricular system in schizophrenia: Further evidence for multifocal mild to moderate enlargement. Schizophrenia Bulletin, 26(1), 201–216. 10.1093/oxfordjournals.schbul.a033440 [DOI] [PubMed] [Google Scholar]
- Sathyanesan, M. , Girgenti, M. J. , Banasr, M. , Stone, K. , Bruce, C. , Guilchicek, E. , Wilczak‐Havill, K. , Nairn, A. , Williams, K. , Sass, S. , Duman, J. G. , & Newton, S. S. (2012). A molecular characterization of the choroid plexus and stress‐induced gene regulation. Translational Psychiatry, 2, e139. 10.1038/tp.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaaff, K. , Dobrowolny, H. , Frodl, T. , Mawrin, C. , Gos, T. , Steiner, J. , & Bogerts, B. (2020). Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain, Behavior, and Immunity, 88, 497–506. 10.1016/j.bbi.2020.04.021 [DOI] [PubMed] [Google Scholar]
- Segawa, K. , Blumenthal, Y. , Yamawaki, Y. , & Ohtsuki, G. (2021). A destruction model of the vascular and lymphatic systems in the emergence of psychiatric symptoms. Biology (Basel), 10(1), 10034. 10.3390/biology10010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serot, J. M. , Béné, M. C. , & Faure, G. C. (2003). Choroid plexus, aging of the brain, and Alzheimer's disease. Frontiers in Bioscience, 8, s515–s521. 10.2741/1085 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , Hergueta, T. , Baker, R. , & Dunbar, G. C. (1998). The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59 Suppl 20, 22‐33;quiz 34‐57. [PubMed] [Google Scholar]
- Shenton, M. E. , Dickey, C. C. , Frumin, M. , & McCarley, R. W. (2001). A review of MRI findings in schizophrenia. Schizophrenia Research, 49(1–2), 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty, A. K. , & Zanirati, G. (2020). The interstitial system of the brain in health and disease. Aging and Disease, 11(1), 200–211. 10.14336/ad.2020.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, J. M. , Smith, C. J. , Guo, S. L. , Mohs, R. C. , Siever, L. J. , & Davis, K. L. (1998). Lateral ventricular enlargement in schizophrenic probands and their siblings with schizophrenia‐related disorders. Biological Psychiatry, 43(2), 97–106. 10.1016/s0006-3223(97)00247-3 [DOI] [PubMed] [Google Scholar]
- Suzuki, M. , Zhou, S. Y. , Takahashi, T. , Hagino, H. , Kawasaki, Y. , Niu, L. , Matsui, M. , Seto, H. , & Kurachi, M. (2005). Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain, 128(Pt 9), 2109–2122. 10.1093/brain/awh554 [DOI] [PubMed] [Google Scholar]
- Szmydynger‐Chodobska, J. , Strazielle, N. , Zink, B. J. , Ghersi‐Egea, J. F. , & Chodobski, A. (2009). The role of the choroid plexus in neutrophil invasion after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 29(9), 1503–1516. 10.1038/jcbfm.2009.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayon, E. , Moret, B. , Sprugnoli, G. , Monti, L. , Pascual‐Leone, A. , & Santarnecchi, E. (2020). Improving choroid plexus segmentation in the healthy and diseased brain: Relevance for tau‐PET imaging in dementia. Journal of Alzheimer's Disease, 74(4), 1057–1068. 10.3233/jad-190706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae, W. S. , Kim, S. S. , Lee, K. U. , Nam, E. C. , & Kim, K. W. (2008). Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology, 50(7), 569–581. 10.1007/s00234-008-0383-9 [DOI] [PubMed] [Google Scholar]
- Turner, C. A. , Thompson, R. C. , Bunney, W. E. , Schatzberg, A. F. , Barchas, J. D. , Myers, R. M. , Akil, H. , & Watson, S. J. (2014). Altered choroid plexus gene expression in major depressive disorder. Frontiers in Human Neuroscience, 8, 238. 10.3389/fnhum.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove, R. , Manzanares‐Teson, N. , & Barnes, N. M. (2014). Cytokine function in medication‐naive first episode psychosis: A systematic review and meta‐analysis. Schizophrenia Research, 155(1–3), 101–108. 10.1016/j.schres.2014.03.005 [DOI] [PubMed] [Google Scholar]
- van Berckel, B. N. , Bossong, M. G. , Boellaard, R. , Kloet, R. , Schuitemaker, A. , Caspers, E. , Luurtsema, G. , Windhorst, A. D. , Cahn, W. , Lammertsma, A. A. , & Kahn, R. S. (2008). Microglia activation in recent‐onset schizophrenia: A quantitative (R)‐[11C]PK11195 positron emission tomography study. Biological Psychiatry, 64(9), 820–822. 10.1016/j.biopsych.2008.04.025 [DOI] [PubMed] [Google Scholar]
- van Erp, T. G. , Hibar, D. P. , Rasmussen, J. M. , Glahn, D. C. , Pearlson, G. D. , Andreassen, O. A. , Agartz, I. , Westlye, L. T. , Haukvik, U. K. , Dale, A. M. , Melle, I. , Hartberg, C. B. , Gruber, O. , Kraemer, B. , Zilles, D. , Donohoe, G. , Kelly, S. , McDonald, C. , Morris, D. W. , … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 585. 10.1038/mp.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os, J. , Linscott, R. J. , Myin‐Germeys, I. , Delespaul, P. , & Krabbendam, L. (2009). A systematic review and meta‐analysis of the psychosis continuum: Evidence for a psychosis proneness‐persistence‐impairment model of psychotic disorder. Psychological Medicine, 39(2), 179–195. 10.1017/s0033291708003814 [DOI] [PubMed] [Google Scholar]
- Weinberger, D. R. (1995). From neuropathology to neurodevelopment. Lancet, 346(8974), 552–557. 10.1016/s0140-6736(95)91386-6 [DOI] [PubMed] [Google Scholar]
- Wood, S. J. , Velakoulis, D. , Smith, D. J. , Bond, D. , Stuart, G. W. , McGorry, P. D. , Brewer, W. J. , Bridle, N. , Eritaia, J. , Desmond, P. , Singh, B. , Copolov, D. , & Pantelis, C. (2001). A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophrenia Research, 52(1–2), 37–46. 10.1016/s0920-9964(01)00175-x [DOI] [PubMed] [Google Scholar]
- Yan, T. , Qiu, Y. , Yu, X. , & Yang, L. (2021). Glymphatic dysfunction: A bridge between sleep disturbance and mood disorders. Frontiers in Psychiatry, 12, 658340. 10.3389/fpsyt.2021.658340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X. , Pu, W. , & Yao, S. (2016). Functional alterations of fronto‐limbic circuit and default mode network systems in first‐episode, drug‐naïve patients with major depressive disorder: A meta‐analysis of resting‐state fMRI data. Journal of Affective Disorders, 206, 280–286. 10.1016/j.jad.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Zhou, Y. F. , Huang, J. C. , Zhang, P. , Fan, F. M. , Chen, S. , Fan, H. Z. , Cui, Y. M. , Luo, X. G. , Tan, S. P. , Wang, Z. R. , Feng, W. , Yuan, Y. , Yang, F. D. , Savransky, A. , Ryan, M. , Goldwaser, E. , Chiappelli, J. , Rowland, L. M. , Kochunov, P. , … Hong, L. E. (2020). Choroid plexus enlargement and allostatic load in schizophrenia. Schizophrenia Bulletin, 46(3), 722–731. 10.1093/schbul/sbz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The data are currently not available but part of the data will be available publicly (Human Connectome Project).


