Abstract
Axon initial segment (AIS) cell surface proteins mediate key biological processes in neurons including action potential initiation and axo-axonic synapse formation. However, few AIS cell surface proteins have been identified. Here, we used antibody-directed proximity biotinylation to define the cell surface proteins in close proximity to the AIS cell adhesion molecule Neurofascin. To determine the distributions of the identified proteins, we used CRISPR-mediated genome editing for insertion of epitope tags in the endogenous proteins. We found Contactin-1 (Cntn1) among the previously unknown AIS proteins we identified. Cntn1 is enriched at the AIS through interactions with Neurofascin and NrCAM. We further show that Cntn1 contributes to assembly of the AIS-extracellular matrix, and is required for AIS axo-axonic innervation by inhibitory basket cells in the cerebellum and inhibitory chandelier cells in the cortex.
The axon initial segment (AIS) is essential for proper neuronal and brain circuit function. AIS integrate synaptic inputs, generate and modulate axonal action potentials, and regulate the trafficking of proteins, vesicles, and organelles to maintain neuronal polarity. These functions depend on a tightly regulated network of scaffolding and cytoskeletal proteins that serve as an organizing platform for ion channels and cell adhesion molecules (CAMs) 1,2. However, the AIS proteins that have been described likely represent only a small fraction of the overall AIS proteome since the molecular mechanisms involved in many AIS-associated processes remain poorly defined.
Recently, proximity-dependent biotinylation (PDB) approaches have emerged as robust experimental strategies to define the molecular composition of organelles and subcellular domains 3. PDB is particularly attractive to identify AIS proteomes since the AIS is very detergent insoluble and refractory to more traditional proteomic approaches like immunoprecipitation (IP) mass-spectrometry. Streptavidin pulldown of biotinylated AIS proteins allows for the use of much stronger solubilizing detergents. We previously used one PDB approach (BioID) to discover new AIS proteins 4; we targeted the biotin ligase BirA* to the AIS by fusing it to a variety of known AIS cytoskeleton-associated proteins. These experiments identified known and some new cytoplasmic AIS proteins, including Mical3 and Septins. However, our experiments were strongly biased towards cytoplasmic proteins and recovered very few membrane and cell surface proteins. Some PDB approaches have successfully captured cell surface proteins. For example, Li et al. (2020) 5 used an extracellular, membrane tethered horseradish peroxidase (HRP) to identify cell surface proteins that function as regulators of neuronal wiring; their transgenic approach revealed the cell surface proteome of Drosophila olfactory projection neurons. Shuster et al. 6 used the same approach in mice, but restricted the expression of the membrane tethered HRP to Purkinje neurons to reveal their cell surface proteome. However, neither of these approaches was designed to interrogate subcellular domains. As an alternative approach, Takano et al. (2020) 7 used a split PDB strategy (Split-TurboID) to elucidate the cell surface proteome of astrocyte-neuron synapses. Their experiments revealed transcellular interactions between neuronal NrCAM and astrocytic NrCAM that stabilize the structure and function of inhibitory synapses.
To overcome some of the limitations of intracellular PDB for identification of AIS cell surface proteins, we used Selective Proteomic Proximity Labeling Assay Using Tyramide (SPPLAT) 8,9; the approach has also been called Biotinylation by Antibody Recognition (BAR) 10. Our application of this strategy uses highly specific primary antibodies against the extracellular domain of the AIS-enriched CAM Neurofascin (Nfasc) to direct HRP conjugated secondary antibodies to the AIS. Addition of biotin-tyramide and hydrogen peroxide generates biotin phenoxyl radicals that biotinylate membrane proteins within a range of ~250 nanometers of the peroxidase 11. We performed this labeling at multiple timepoints throughout neuronal development in vitro on live neurons. We identified all previously reported AIS extracellular, and membrane cell adhesion and recognition molecules. In addition, we found many novel membrane proteins that were reproducibly in proximity to Nfasc, with different temporal enrichment profiles. We further investigated a subset of these using CRISPR-mediated endogenous gene tagging. Among these, we identified Contactin-1 (Cntn1) as a new, bona fide AIS CAM recruited to the AIS through interaction with the AIS CAMs Nfasc and NrCAM. Remarkably, loss of Cntn1 severely impaired inhibitory axo-axonic innervation of the AIS in both cerebellar Purkinje neurons and cortical pyramidal neurons. Thus, using antibody directed extracellular proximity biotinylation, we identified Cntn1 as a new AIS protein that regulates axo-axonic innervation of the AIS.
Proximity Biotinylation at the AIS Membrane
We reasoned the Nfasc proximity proteome could be used to help define the AIS cell surface proteome since Nfasc is highly enriched at the AIS. Therefore, we adapted the SPPLAT/BAR method 8,10 for use with live, unpermeabilized neurons; we avoided fixation to maximize protein recovery and subsequent mass spectrometry. We labeled rat hippocampal neurons in culture with highly specific and validated chicken primary antibodies against the ectodomain of Nfasc 12, since its 186 kDa isoform (NF186) is highly enriched at the AIS (Fig. 1a, b), with lower concentrations along the distal axon, at growth cones, and in the soma 13. After live labeling with the anti-Nfasc primary antibody, HRP-conjugated anti-chicken secondary antibodies were used to label the anti-Nfasc primary antibody. The Nfasc-localized HRP generates the reactive biotin phenoxyl from biotin tyramide (biotin phenol), resulting in the addition of tyrosine residues to proteins in proximity to Nfasc with a range of several hundred nm 9,11. As with other PDB methods, nonspecifically and endogenously biotinylated proteins, as well as non-specific protein background adsorbing to solid phase surfaces during the enrichment steps and prior to the mass spectrometry analysis, must be excluded. The omission of the primary antibody serves as a simple and straightforward negative control. Without fixation or detergents, the membrane-impermeability of biotin-phenoxyl restricts the biotinylation reaction to the extracellular surface. Hereafter, we refer to this method as Nfasc-BAR.
Figure 1. Proximity-dependent biotinylation using Nfasc antibodies.
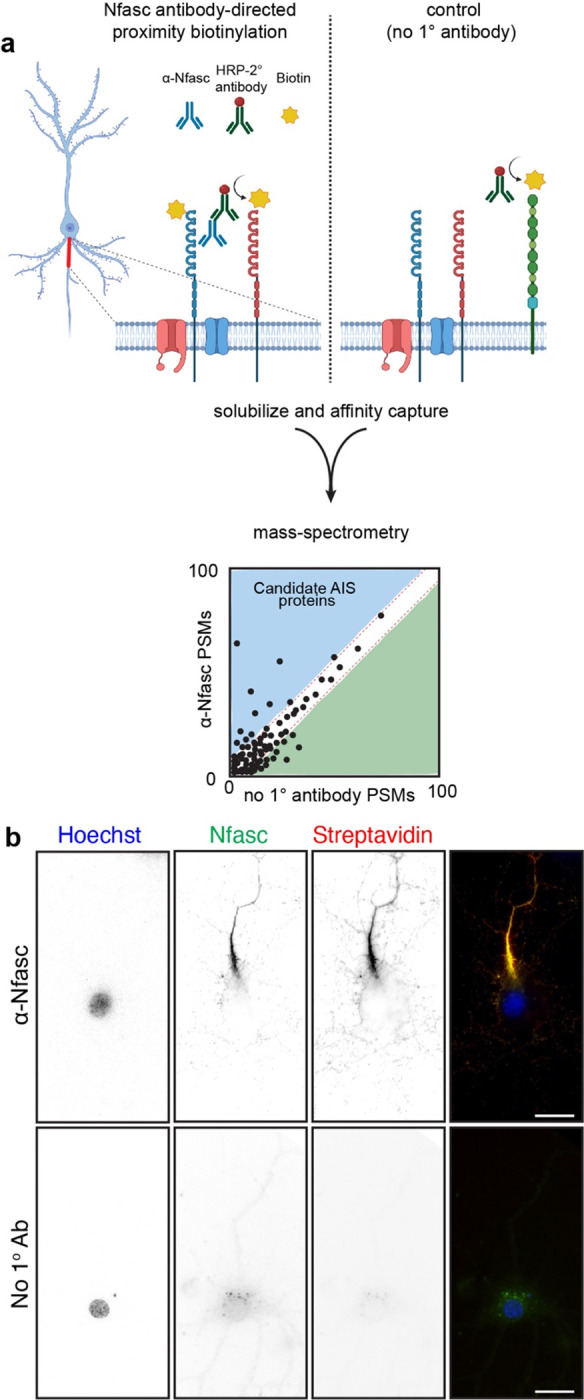
a, Illustration of the antibody-directed proximity biotinylation strategy. Anti-Nfasc antibodies bind to Nfasc, while HRP-conjugated secondary antibodies bind to the Nfasc antibodies. Addition of biotin phenol (biotin tyramide) in an H2O2 containing diluent results HRP-mediated conversion of the biotin phenol to an active radical biotin phenoxyl that covalently adds the tyramide biotin to extracellular tyrosine residues. Omission of the primary anti-Nfasc serves as a control. After stringent solubilization and affinity capture by streptavidin-conjugated magnetic beads. Biotinylated proteins are then identified by mass spectrometry. b, Fluorescence imaging of DIV14 rat hippocampal neurons labeled by Nfasc-BAR or a control condition (no primary Ab). Nfasc fluorescence (green) enrichment defines the AIS. Biotinylated proteins were detected using Alexa594-conjugated streptavidin. Scale bars, 20 μm.
We found that Nfasc-BAR resulted in AIS-enriched streptavidin labeling that colocalized with NF186 (Fig. 1b); this pattern was not seen when the anti-Nfasc antibody was omitted. The amount of biotinylation also depends on the duration of the labeling reaction (Fig. S1a). For the experiments described below, we used a reaction time of 5 minutes (Fig. S1b). To identify biotinylated AIS proteins, we then solubilized neuronal membranes using a strong solubilization buffer, purified biotinylated proteins using streptavidin-conjugated magnetic beads, and finally identified the biotinylated proteins using mass spectrometry (Fig. 1a). To confirm the reproducibility and robustness of our approach we performed surface proximity labeling in parallel using rabbit polyclonal antibodies targeting the ectodomain of NrCAM (Fig. S1c), another AnkG-binding CAM found at the AIS. Proximity biotinylation directed by NrCAM antibodies strongly labeled the AIS (Fig. S1c). Importantly, the resulting mass spectrometry datasets confirmed the robustness of the strategy since Nfasc-BAR and NrCAM-BAR proximity proteomes were highly concordant (Fig. S1d; supplemental Table 1).
NF186 proximity proteomes across neuronal development
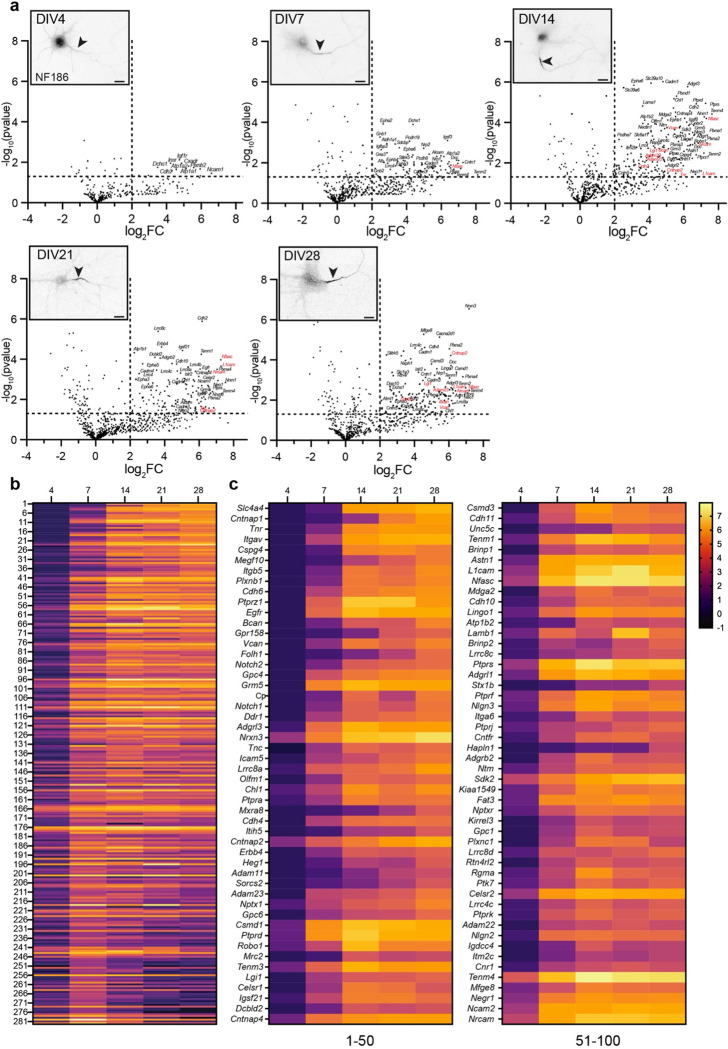
The maturation of axons includes the enrichment of proteins that mediate key functions or developmental mechanisms. For example, toward the end of the first week in vitro, the scaffolding protein AnkyrinG (AnkG) localizes to the proximal axon; this enrichment precedes and is necessary for the subsequent recruitment of Nfasc, and voltage-gated Na+ (Nav) and K+ (Kv) channels to the AIS 14,15. NF186 enrichment at the AIS and along distal axons also increases during development (Fig. 2a). To determine the extracellular Nfasc proximity proteome and how it changes during development (both before and after AIS formation), we performed Nfasc-BAR on primary hippocampal neurons at five different timepoints (supplemental Table 2): from day in vitro 4 (DIV4; prior to AIS formation) to DIV28 after establishment of the AIS-associated ECM (Fig. S2); all experiments were performed three times independently for each developmental timepoint with 2 million neurons per experiment. To compare Nfasc cell surface proximity proteomes from cultures of different ages, we normalized peptide spectral match (PSM) counts to total spectral counts in the set of endogenously biotinylated carboxylases detected (Fig. S2), as a correction factor for differences in total protein amount used in individual pulldowns (see methods). We found that as neurons, the AIS, and axons develop, the cell surface Nfasc proximity-proteome changes, with an increasing number of proteins displaying significant changes in fold enrichment (Fig. 2, supplemental Table 2). Consistent with a developing and maturing AIS and increasing levels of overall Nfasc, volcano plots (Fig. 2a and supplemental table 3) show the enrichment of proteins identified using Nfasc-BAR compared to controls at the various developmental time points. We used a cutoff of log2(Nfasc PSMs/Ctrl PSMs) or log2[fold change (FC)] >2 (vertical dotted line) with a significance cutoff of p<0.05 (horizontal dotted line).
Figure 2. NF186 proximity proteomes across neuronal development.
a, Volcano plots showing the log2-fold changes of proteins versus the statistical significance −log10(pvalue) identified using Nfasc-directed proximity biotinylation (N=3). p<0.05 was used as a cutoff for significance (horizontal dashed line). Some identified proteins are indicated (corresponding gene names listed), with those previously reported as AIS cell surface proteins in red, respectively. Inset images show immunofluorescence labeling of NF186 at different timepoints of hippocampal neuron development in vitro. Lower panels: magnified images show the Nfasc-labeled AIS at each time point. Scale bars, 20 μm. b, Heatmaps showing log2-fold changes at each timepoint for all 285 proteins that satisfied two filtering criteria [(1) normalized PSMs > 10; (2) log2FC (Nfasc/Ctrl) > 2] for at least one of five timepoints, rank-ordered by the slope of the linear regression of their log2 fold enrichment over time. c, Expanded heat map showing gene names for the proteins (1–50 and 51–100) with the largest rate of increase in PSM count (B). Data shown are from N = 3 replicates for each timepoint (see Figure S2).
To visualize the increase in proteins identified using Nfasc-BAR across development and to select candidates to focus on, we identified 285 proteins that satisfied two filtering criteria for at least one of the five timepoints: (1) normalized PSMs > 10 and (2) log2(Nfasc PSMs/Ctrl PSMs) or log2(FC) > 2 (Fig. S2; Supplemental Table 2). Among these 285 proteins there were a variety of protein expression profiles (Fig. 2b). Although present, relatively few proteins showed a reduction in the cell surface Nfasc proximity proteome. Most proteins identified in the Nfasc proximity proteome increased in abundance (Fig. 2c; only the 100 candidates with the largest increase across all time points are shown). We also plotted the log2FC at each time point for the 100 proteins with the largest fold change (DIV 4 only had 63 proteins with log2FC >2) (Fig. S3). The results for each protein were highly reproducible at each time point and consistently revealed similar sets of cell surface proteins. Cytoplasmic AIS proteins such as AnkG, β4 spectrin, and TRIM46 were conspicuously absent consistent with our experimental design to restrict the biotinylation to cell surface proteins.
The Nfasc-BAR proximity proteome includes AIS enriched proteins
Among the cell surface proteins that passed our selection criteria (Fig. S2), we found all known AIS membrane proteins and AIS enriched extracellular matrix molecules with the notable exception of ion channels (Fig. 3a). Why might that be the case since ion channels are known to be highly enriched at the AIS? The number of PSMs recovered for any protein depends on: 1) the number of available extracellular tyrosine residues (Nfasc-BAR-mediated biotinylation occurs on tyrosine residues); 2) the amount and local membrane density of the protein; and 3) the proximity of the protein to the biotinylation source. Since ion channels are highly enriched at the AIS and are in close proximity to Nfasc, their absence from our data set likely reflects the small number of extracellular tyrosine residues found in ion channels and a topology that has extracellular residues very close to the membrane. For example, the AIS-enriched K+ channel subunit KCNQ3 has four extracellular regions comprising 45 amino acids, with two of those being tyrosine (Fig. 3b); we did not detect any KCNQ3 peptides in our experiments. Similarly, Nav1.2 (encoded by Scn2a), the main voltage-gated Na+ channel expressed at AIS in DIV14 hippocampal neurons, has an average of one tyrosine per extracellular domain and many are very close to or immediately adjacent to the membrane. In contrast to ion channels, cell adhesion molecules like Nfasc and Contactin-1 (Cntn1) have large extracellular domains with many tyrosine residues (Fig. 3b). Thus, the low number of extracellular tyrosines found in ion channels and the proximity of these residues to the membrane may make them difficult to biotinylate using Nfasc-BAR; other membrane or cell surface proteins with few or inaccessible tyrosine residues may also be poorly represented in our data set.
Figure 3. Nfasc-BAR identifies known AIS membrane and membrane-associated proteins.
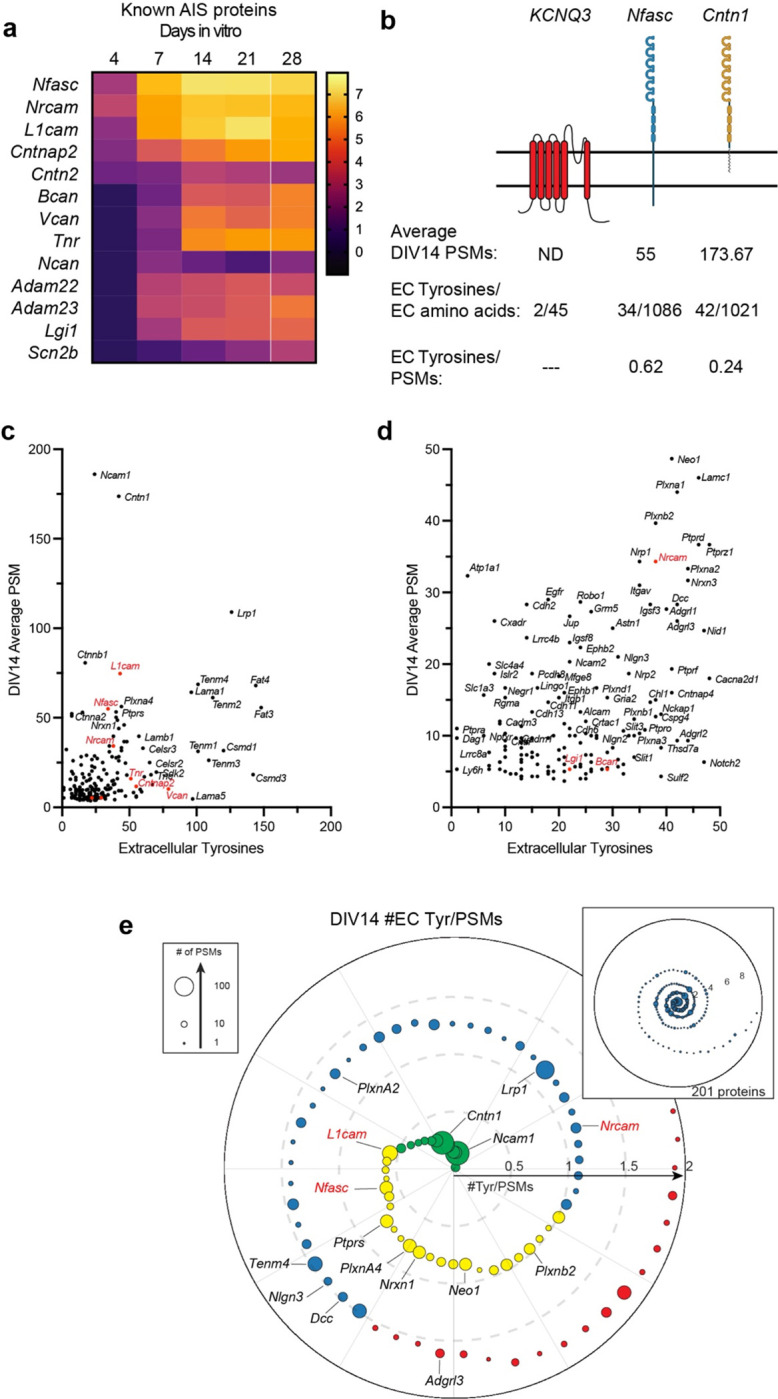
a, Known AIS membrane and membrane associated proteins and their log2FC (Nfasc/Ctrl). b, Illustration of membrane topology, average PSMs (at DIV14), and the number of extracellular tyrosine residues for three different AIS and membrane proteins. Figure generated using Biorender. c, d, Scatter plot of the number of peptide spectral matches (PSMs) for each biotinylated protein identified by mass spectrometry as compared to the number of tyrosine residues present in each protein’s extracellular domain, shown at different scales. Proteins in red were previously reported at the AIS. e, Proximity plot showing biotinylated proteins (at DIV14) ordered by extracellular (EC) tyrosine/PSM ratio. The plot is an estimate of abundance and proximity to the HRP secondary antibody bound to the Nfasc primary antibody. Each protein is represented by a circle with size proportional to the number of PSMs identified for that protein. Proteins analzyed in subsequent experiments are indicated by their gene names. EC Tyrosine/PSM ratio: 0–0.5 green, 0.5–1.0 yellow, 1.0–1.5 blue, 1.5–2.0 red.
In contrast to ion channels, an analysis of the 201 proteins identified at DIV14 showed no correlation between the number of PSMs for a protein and the number of extracellular tyrosines (Figs. 3c, d). This suggests a much stronger dependence of PSM number on proximity and protein abundance. As an estimate for both proximity and abundance, we calculated the ratio of extracellular tyrosines to PSM count for all 201 proteins identified at DIV14. Thus, a lower ratio suggests greater abundance of protein and closer proximity to the HRP-dependent biotinylation source (Fig. 3e). This analysis shows many candidates with low extracellular tyrosine/PSM ratios that were also previously reported to be directly or indirectly linked to Nfasc, including PlxnA4, Ncam1, L1CAM, and NrCAM (Fig. 3e) 16,17.
Tagging of endogenous membrane proteins
Our results across 5 developmental timepoints yielded an NF186 proximity proteome (Fig. 2a); filtering based on fold-enrichment and number of PSMs recovered resulted in 285 candidate cell surface proteins in close proximity to NF186 (Fig. S2). Among these, Nfasc-BAR successfully identified the 13 previously reported AIS-enriched cell surface proteins (excluding ion channels; Fig. 3a). However, it is unlikely that the remaining 272 proteins are also enriched at the AIS since NF186 is present in lower densities in the soma, axons, and at growth cones 13, some of the proteins were identified before the AIS forms or is mature (e.g., DIV4), and the range of SPPLAT/BAR is ~200–300 nm 9,11. Thus, proteins identified by Nfasc-BAR may be in proximity to NF186 but not enriched at the AIS. We previously used antibodies to validate the AIS proteomes we identified using BioID 4. However, antibodies are frequently non-specific and for reasons that are unclear, many antibodies that label AIS are not against their claimed targets 18–20. Therefore, to circumvent some of the challenges associated with antibodies and to look for AIS- and axon-enriched proteins, we performed CRISPR-mediated epitope tagging of endogenous proteins 19,21–23. We selected 23 different candidates (Fig. 4a) identified using Nfasc-BAR based on 1) the high fold-enrichment compared to control BAR, 2) the high number of PSMs recovered, and 3) the estimate of proximity to the biotin source (Figs. 2 and 3). Included in these 23 candidates were four cell adhesion molecules previously reported at the AIS: Nfasc, NrCAM, L1CAM, and Cntn2 24–26. To endogenously label these 23 cell surface proteins, we generated two adeno-associated viruses (AAV) to transduce cultured DIV 0 rat hippocampal neurons with 1) Cas9 and 2) a gene specific single guide RNA (sgRNA), a sgRNA that recognizes donor recognition sites (DRS) flanking spaghetti monster fluorescent protein with V5 tags (smFP-V5), and smFP-V5 (Fig. 4b). The gene specific sgRNAs were targeted to the last exon of each gene of interest allowing for the insertion of smFP-V5 in the last exon. 2 weeks after transduction, neurons were fixed and immunostained for β4 spectrin to label the AIS, and V5 to detect the endogenously tagged cell surface protein. Since we targeted the last exon (C-terminus) of each protein resulting in premature termination of the protein, it is possible the addition of the smFP-V5 disrupted the normal localization of the cell surface protein. However, C-terminal tagging of endogenous Nfasc, NrCAM, L1CAM, and Cntn2 all resulted in AIS labeling as previously reported 25–27 (Figs. 4c-f). The candidates we tested labeled AIS, axons, and dendrites (Figs. 4 and S4). For example, endogenous tagging of Ncam1 revealed uniform surface labeling in somatodendritic, AIS, and axonal domains (Fig. 4g), while Ptprs and Tenm4 showed preferential labeling of AIS and axons (Figs. 4h, i); endogenous labeling of Adgrl3 strongly labeled dendrites and spines (Fig. 4j). Among all the candidates we tested that had not previously been reported at the AIS, we found that endogenous tagging of Cntn1 showed the strongest labeling at the AIS (Fig. 4k).
Figure 4. Tagging of endogenous membrane proteins.
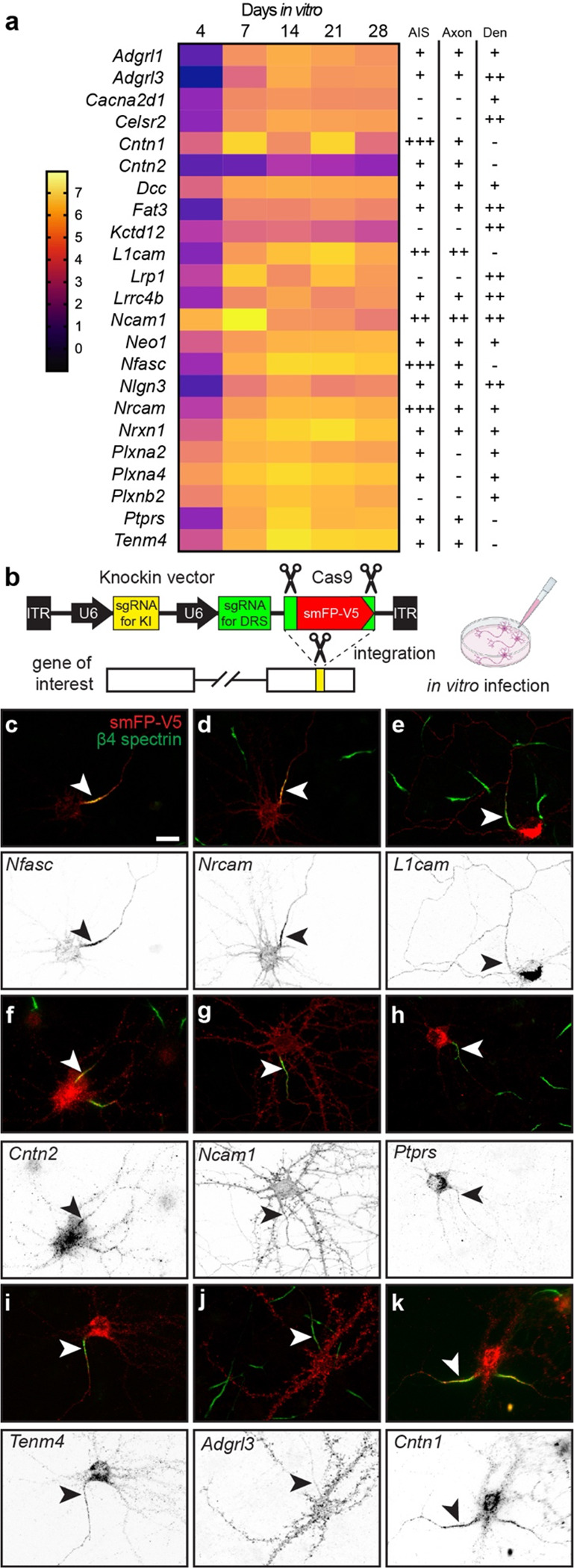
a, Proteins whose distribution was tested using endogenous protein tagging. The heatmap shows the increase in expression level is shown as a function of days in vitro (see also Figure 2c). The presence of the tagged protein in AIS, axon, and dendrite is indicated. b, Schematic of the knock-in vector for in vitro CRISPR-mediated endogenous gene tagging. DRS, donor recognition sites. c-k, Examples of smFP-V5 tagged proteins (red) enriched at the AIS (c, d, f, k), the axon (e, g), dendrites (j), or in multiple domains (e, g, h, i). AIS are labeled for β4 spectrin (green) and are indicated by an arrowhead. Scale bar, 20 μm.
Cntn1 is a bona fide AIS cell surface protein
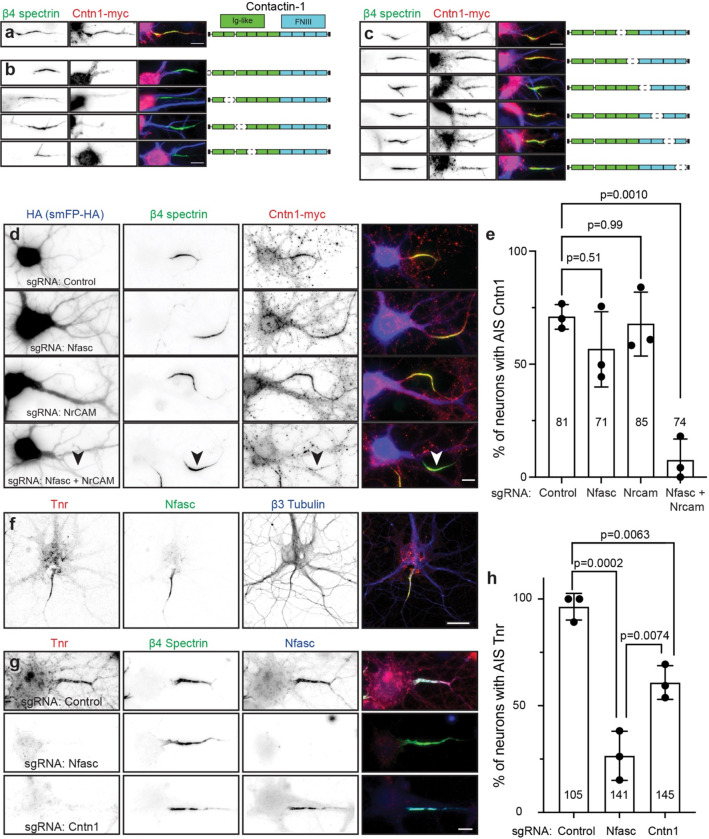
Cntn1 is a glycosylphosphatidyl inositol (GPI)-anchored cell adhesion molecule widely expressed throughout the nervous system in both neurons and glia 28. It has essential roles in forming the axoglial junctions flanking nodes of Ranvier where it forms a complex together with axonal Caspr and the glial 155 KDa splice variant of Nfasc (NF155) 29,30. Cntn1-null mice die in the 3rd postnatal week, emphasizing the importance of Cntn1 to normal function. Cntn1 was also reported at nodes of Ranvier, although its function there is unknown 31; detection of nodal or paranodal Cntn1 requires different fixation and treatment conditions 32, suggesting that in some subcellular domains Cntn1 may engage in protein-protein interactions that preclude immunostaining. With this in mind, our efforts to immunolabel Cntn1 at AIS in control mouse brain failed. Nevertheless, we performed immunostaining in vitro using a goat-polyclonal anti-Cntn1 antibody in control and Cntn1-deficient neurons. We disrupted endogenous Cntn1 expression in cultured hippocampal neurons using AAV to express Cas9 and three control or three Cntn1 specific sgRNAs (Fig. 5a). Whereas neurons transduced with Cas9 and the control sgRNAs had Cntn1 and AnkG immunolabeling at the AIS (Fig. 5b, arrowheads), neurons transduced with the Cntn1 sgRNAs lost both the perisomatic and AIS Cntn1 immunoreactivity (Fig. 5c, arrowhead). Thus, immunostaining of cultured hippocampal neurons reveals AIS Cntn1. These results also demonstrate the specificity of the Cntn1 antibody. However, loss of AIS Cntn1 had no effect on AIS AnkG (Fig. 5c, arrowhead), or clustering of Nfasc or Na+ channels (data not shown).
Figure 5. Cntn1 is a bona fide AIS protein.
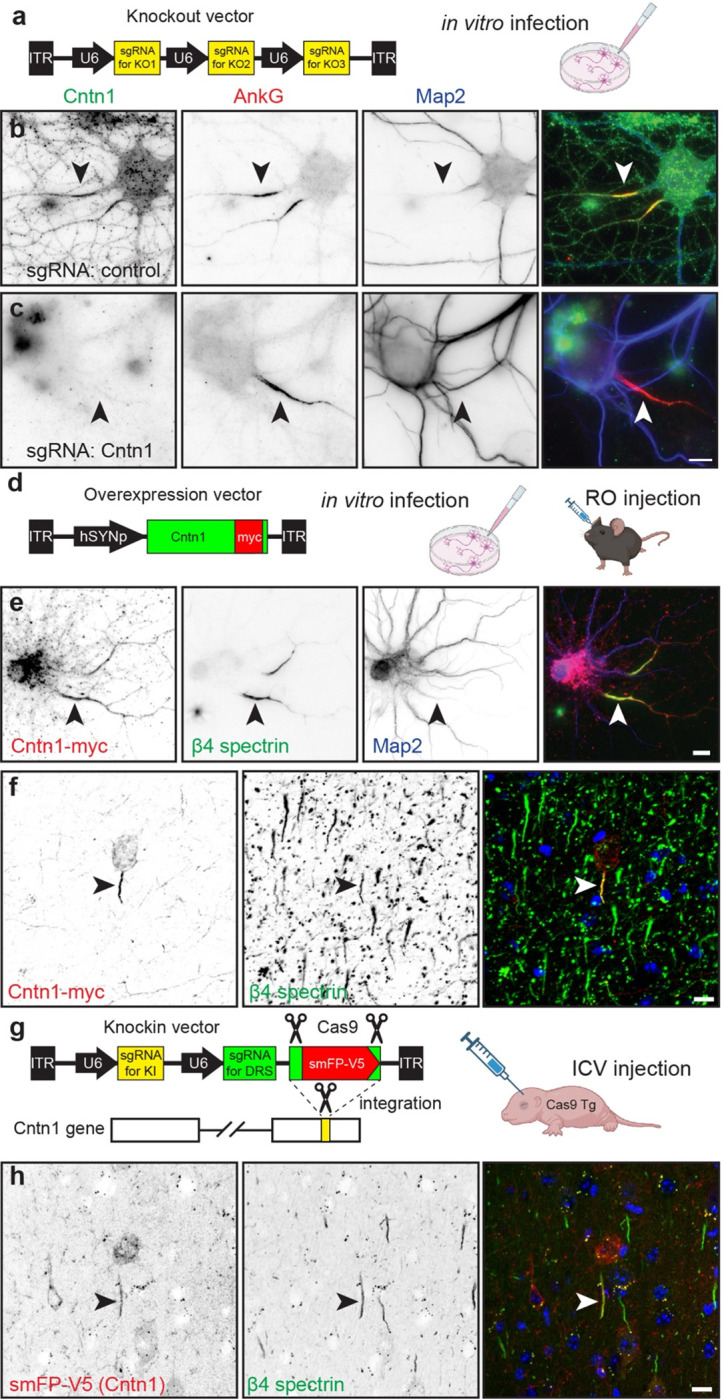
a, Schematic of the knockout vector including 3 sgRNAs targeting the gene of interesting. The AAV generated using this vector were used for in vitro transduction of neurons. b, c, Immunostaining for Cntn1 (green), AnkG (red) and Map2 (blue) after transduction with AAV to Cas9 and control (b) or Cntn1 (c) sgRNAs. Neurons transduced with the Cntn1 sgRNAs lacked AIS Cntn1, but retained robust AnkG at the AIS. AIS are indicated by the arrowheads. Scale bar, 10 μm. d, Schematic of the Cntn1-myc overexpression vector used for in vitro and in vivo infection of neurons. e, Transduction of cultured hippocampal neurons using AAV to express Myc-tagged Cntn1. Cntn1-myc (red) is enriched at the AIS (arrowhead) where it colocalizes with β4 spectrin (green). The somatodendritic domain is identified using antibodies against Map2 (blue). Scale bar, 10 μm. f, In vivo transduction of cortical neurons using AAV to express Myc-tagged Cntn1. Cntn1-myc (red) is enriched at the AIS (arrowhead) where it colocalizes with β4 spectrin (green). Nuclei are labeled using Hoechst dye (blue). Scale bar, 10 μm. g, Schematic of the knock-in vector for in vivo CRISPR-mediated endogenous tagging of Cntn1. DRS, donor recognition sites. AAV were delivered by intracerebroventricular (ICV) injection at P0. h, In vivo transduction of cortical neurons for CRISPR-dependent genome editing to tag endogenous Cntn1 using smFP-V5 (red). The smFP-V5 tagged Cntn1 colocalizes with β4 spectrin (green) at the AIS (arrowhead). Nuclei are labeled using Hoechst dye (blue). Scale bar, 10 μm.
Transduction of DIV10 cultured hippocampal neurons using AAV to express myc-tagged Cntn1 (Fig. 5d, e) also showed Cntn1-myc enriched at the AIS that colocalized with β4 spectrin at DIV14 (Fig. 5e, arrowheads). Similarly, retro-orbital injection of AAV Cntn1-myc in 13-week old mice, showed strong AIS enrichment of Cntn1-myc in transduced cortical neurons four weeks after injection (Fig. 5d, f, arrowheads). Finally, we performed in vivo AAV-dependent and CRISPR-mediated tagging of endogenous Cntn1 using smFP-V5 (Fig. 5g). As with cultured neurons (Fig. 4k), we found the sgRNA targeting Cntn1 resulted in V5 labeling of cortical neuron AISs (Fig. 5h, arrowheads). Together, these results show that Cntn1 is a bona fide AIS cell surface protein.
Cntn1 is localized at the AIS through binding to L1-family cell adhesion molecules
Since Cntn1 is a GPI-anchored cell surface protein, we reasoned that it must be recruited to the AIS through interactions with a co-receptor or some other AIS transmembrane protein. Cntn1 has 6 N-terminal immunoglobulin-like (Ig-like) and 4 C-terminal fibronectin III (FNIII) domains (Fig. 6a). To determine how Cntn1 is clustered at the AIS, we generated myc-tagged Cntn1 with various internal deletions of these domains. We found the N-terminus and first four Ig-like domains of Cntn1 are required for its AIS clustering (Fig. 6b). In contrast, deletion of the last two Ig domains or any of the FNIII domains did not affect recruitment of Cntn1 to the AIS (Fig. 6c).
Figure 6. Cntn1 is recruited to the AIS through interactions with AnkG-binding L1-family cell adhesion molecules.
a,Cntn1-myc (red) is targeted to the AIS and colocalizes with β4 spectrin (green). Cntn1 consists of 6 N-terminal Immunoglobulin (Ig)-like domains and 4 C-terminal Fibronectin type III (FNIII) domains. Scale bar, 10 μm. b, Cntn1-myc with N-terminal and internal deletions of the first 4 Ig-like domains fail to localize at the AIS. Scale bar, 10 μm. c, Cntn1-myc localization to the AIS does not depend on the last 2 Ig-like domains or any FNIII domain. Scale bar, 10 μm. d, Cultured hippocampal neurons transduced with AAV to express Cas9 and control, Nfasc, NrCAM, or Nfasc+NrCAM gRNAs. Neurons were labeled using antibodies against HA as a transduction marker (to label the spaghetti monster fluorescent protein tagged with HA, smFP-HA; blue), β4 spectrin (green), and Cntn1-myc (red). Scale bar, 10 μm. e, Quantification of the percentage of transduced neurons with AIS Cntn1-myc. N= 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Error bars, ±SEM. The total number of neurons analyzed is also indicated. f, Immunostaining of cultured hippocampal neurons using antibodies against Tenascin R (Tnr; red), β4 spectrin (green), and β3 Tubulin (blue). Scale bar, 25 μm. g, Cultured hippocampal neurons transduced with AAV to express Cas9 and control, Nfasc, or Cntn1 gRNAs. Neurons were labeled using antibodies against Tnr (red), β4 spectrin (green), and Nfasc (blue). Scale bar, 10 μm. h, Quantification of the percentage of transduced neurons with AIS Tnr. N= 3 independent experiments. Ordinary one-way ANOVA with Tukey’s multiple comparisons test. Error bars, ±SEM. The total number of neurons analyzed is also indicated.
What membrane protein recruits Cntn1 to the AIS? Biochemical and cell biological studies suggest that Cntn1 interacts with members of the AnkG-binding L1 family of cell adhesion molecules including Nfasc, L1CAM, and NrCAM 33,34. Although all three of these CAMs are enriched at the AIS, L1CAM is also found at high levels along the distal axon (Figs. 4c-e). To determine if the AIS-enriched Nfasc or NrCAM recruits Cntn1 to the AIS, we generated sgRNAs vectors (Fig. 5a) to delete Nfasc and NrCAM from neurons. Surprisingly, removal of Nfasc or NrCAM alone had no effect on the clustering of Cntn1 at the AIS (Figs. 6d, e). However, simultaneous deletion of both Nfasc and NrCAM blocked the AIS clustering of Cntn1 (Figs. 6d, arrowhead; e). These results suggest that the AIS enriched CAMs Nfasc and NrCAM redundantly recruit Cntn1 to the AIS through its first four Ig-like domains.
Cntn1 helps assemble the AIS extracellular matrix
We found Tenascin-R (Tnr) in our Nfasc-BAR proximity proteome (Fig. 3a). Tnr is an extracellular matrix molecule and a known Cntn1 interactor 33. Immunostaining of cultured hippocampal neurons using antibodies against Tnr showed that it strongly labeled the AIS and colocalizes with Nfasc (Fig. 6f). Previous studies showed that Nfasc regulates the AIS recruitment of the extracellular matrix molecule Brevican 15. To determine if Nfasc or Cntn1 also regulate the AIS clustering of extracellular Tnr, we disrupted expression of Nfasc and Cntn1; sgRNA vectors (Fig. 5a) targeting Nfasc and Cntn1 were highly efficient (Figs. 5c and 6g). Although control sgRNAs had no effect on AIS Tnr, loss of both Nfasc and Cntn1 significantly reduced Tnr’s AIS enrichment (Fig. 6g, h). Thus, Nfasc and Cntn1 both contribute to the assembly or stabilization of the Tnr-containing AIS extracellular matrix.
Cntn1 regulates the assembly of pinceau synapses in the cerebellum.
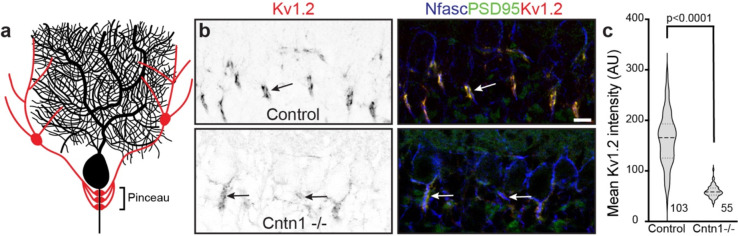
Purkinje neuron AISs are innervated by inhibitory basket cell interneurons that powerfully modulate neuronal excitability 35. Basket cells form a stereotypical ‘pinceau’ synapse (Fig. 7a), with presynaptic terminals at the AIS highly enriched in Kv1 K+ channels and PSD-95, among other proteins 36. The loss of the AIS scaffolding protein AnkG disrupts pinceau synapses and the AIS clustering of Nfasc, suggesting that their assembly requires AnkG-dependent clustering of CAMs like Nfasc and NrCAM 37. Since AIS enrichment of Cntn1 also depends on these CAMs, we wondered if Cntn1 plays important roles in cerebellar pinceau synapse assembly. Therefore, we examined pinceau synapse formation in P18 Cntn1 −/− mice; Cntn1 −/− are very sick and typically die before 3 weeks of age 30. Immunostaining of Purkinje neurons using antibodies against Nfasc, Kv1.2, and PSD95 showed stereotypical enrichment and clustering of these proteins at the AIS of control heterozygote Cntn1 −/+ mice, but Cntn1 −/− mice had profoundly disrupted pinceau synapse formation (Fig. 7b, arrows) with significantly reduced Kv1.2 and PSD95 intensity at the pinceau synapse (Fig. 7c). Thus, Cntn1 is required for proper assembly of pinceau synapses at the AIS of cerebellar Purkinje neurons.
Figure 7. Cntn1 is required for axo-axonic innervation of Purkinje neuron AIS.
a, Illustration of a Purkinje neuron (black) with a basket cell (red) forming the cerebellar pinceau on the AIS. b, Immunostaining of P17 cerebellar pinceau in control and Cntn1 −/− mouse brain using antibodies against Kv1.2 (red) and PSD95 (green) to label the pinceau, and Nfasc (blue) to label the Purkinje neuron AIS. Scale bar, 10 μm. c, Violin plot of the mean Kv1.2 intensity of the cerebellar pinceau in control and Cntn1 −/− mice. N=3 control and 2 Cntn1 −/− mice. The number of pinceau analyzed is indicated.
Cntn1 regulates axo-axonic innervation of pyramidal neurons by chandelier cells
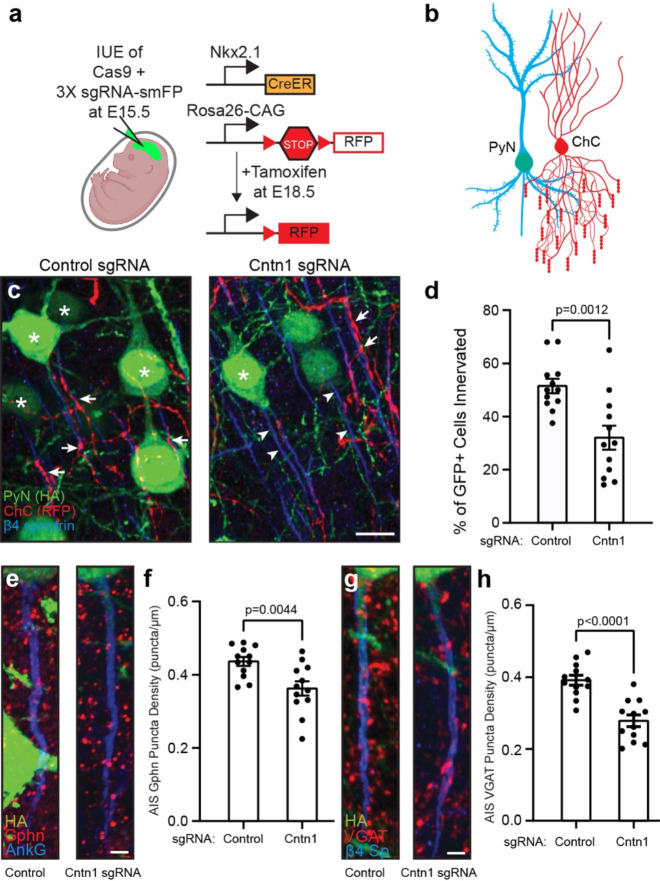
In the cortex and hippocampus, axo-axonic synapses are formed between chandelier cells (ChCs, also known as axo-axonic cells) and the AIS of pyramidal neurons (PyNs). ChCs are derived from progenitors in the ventral region of the medial ganglionic eminence. They have a unique axonal arbor consisting of multiple arrays of short, vertically oriented terminals of presynaptic boutons called cartridges, and each of these cartridges selectively innervates neighboring PyN AISs. These ChCs powerfully reduce PyN output by inhibiting AIS excitability; this inhibition can subsequently modulate brain circuit function and behavior 38–40. However, the mechanisms that control the precise innervation of PyN AISs by ChCs remains incompletely understood, with ankyrin-interacting L1CAM so far being the only CAM known to be required for ChC/PyN AIS innervation 41; since L1CAM is found throughout the axon and not just at the AIS, additional mechanisms must exist to allow for precise AIS innervation. To determine if Cntn1 regulates ChC/PyN AIS innervation, we performed in utero electroporation (IUE) using control or Cntn1-targeting sgRNA- and smFP-HA-expressing plasmids in Nkx2.1-CreER;Rosa26-loxpSTOPloxp-tdTomato (Ai9) pregnant mice at embryonic day 15.5 (E15.5) (Fig. 8a). This timing of IUE results in disruption of the Cntn1 gene in layer II/III PyNs. At E18.5 tamoxifen was administered to the pregnant mother to induce expression of tdTomato red fluorescence protein (RFP) in a sparse group of layer II ChCs (Fig. 8b). We collected brains from P17 mice and analyzed the innervation and assembly of inhibitory synapses on PyN AISs by immunostaining for AnkG or β4 spectrin to label AISs, and antibodies to gephyrin and VGAT to label post- and pre-synaptic compartments of GABAergic synapses, respectively. We found that transfection with Cntn1 sgRNA significantly reduced the percentage of PyN AISs innervated by single RFP-positive ChCs in layer II/III of the somatosensory cortex, as compared to control sgRNA (Figs. 8c, arrowheads, and d). We found that Cntn1-deficient neurons also had significantly fewer inhibitory synapses along their AIS as indicated by gephyrin (Figs. 8e and f) and VGAT puncta (Figs. 8g and h). Together, these results show that Cntn1 is required for efficient ChC/PyN AIS innervation and consequently, the proper assembly of AIS axo-axonic inhibitory synapses.
Figure 8. Pyramidal neuron Cntn1 is important for AIS synaptic innervation by ChCs.
a, Illustration of the knockout and labeling strategy for PyN and ChCs. PyNs are electroporated at E15.5 using plasmids to express Cas9 and 3X sgRNA-smFP (HA tag) to delete expression on Cntn1. ChCs are labeled by expression of red fluorescent protein (RFP) using inducible Cre (CreER) in Nkx2.1-CreER mice at E18.5. b, Illustration of ChC (red) innervation of PyN (blue/green) AIS. c, Representative images of PyNs innervated at their AIS by ChC cartridges (red) in layer II of the Ogawa, Lim et al. somatosensory cortex from Nkx2.1-CreER;Ai9 mice co-electroporated at E15.5 with a plasmid expressing Cas9 and a plasmid expressing smFP-HA and a control sgRNA or Cntn1 sgRNA; mice were sacrificed at P17. AISs and PyNs are visualized by immunostaining for β4 spectrin (blue) and HA (green), respectively. Stars in C indicate HA+ PyNs and arrows indicate ChC innervation of PyN AISs. Arrowheads in C indicate AIS of transfected with Cntn1 sgRNA and that lack innervation by ChC cartridges. Scale bar, 10 μm. d, Quantification of the percentage of HA+ PyNs innervated by single RFP+ ChCs at P17. 12 ChCs and 15–66 HA+ PyNs per ChC from 3 animals were analyzed for each condition. Data are mean ± SEM. e, f, Representative images of HA+ PyN AISs from Nkx2.1-CreER;Ai9 mice electroporated at E15.5 with plasmids indicated in (a) and sacrificed at P17. Inhibitory synapses are visualized by immunostaining for the GABAergic postsynaptic marker gephyrin (Gphn; red; e) or the GABAergic presynaptic marker VGAT (red; g). AISs (blue) are visualized by immunostaining for AnkG in e and β4 spectrin in g. Scale bars, 2 μm. f, h, Quantification of the average number of gephyrin (f) or VGAT (h) puncta per μm of HA+ PyN AIS at P17. 23–40 AISs from 4 fields of view from 3 animals were analyzed for each condition. Data are mean ± SEM.
DISCUSSION
AIS properties essential for brain function include: 1) high densities of ion channels, 2) mechanisms to regulate neuronal polarity, and 3) precise innervation by inhibitory interneurons. The molecular mechanisms regulating these properties all converge on the AIS scaffolding protein AnkG 37,41–43. However, the distinct proximal mechanisms regulating these AIS properties remain poorly understood, highlighting the need to define the composition of the AIS in much greater detail. BioID-dependent cytoplasmic proximity biotinylation and differential mass spectrometry have partially elucidated AIS proteomes 4,44, but they are clearly deficient in proteins involved in transient interactions, posttranslational modifications, and cell surface proteins mediating extra- and intercellular interactions. Thus, experimental approaches that address these deficiencies are desperately needed.
We aimed to use Nfasc-BAR to identify AIS cell surface proteins. The results were highly reproducible at each developmental time point in vitro, but showed changing profiles of cell surface proteins during development. As expected, our data sets included known AIS cell surface proteins, and consisted almost exclusively of cell surface proteins whose expression levels increased with neuronal maturation. The strategy is highly flexible and can be used with other AIS specific antibodies including those targeting cytoplasmic epitopes after detergent solubilization; the strategy can also be applied to other neuronal compartments including synapses, dendrites, and growth cones so long as highly specific and validated antibodies are used.
Since Nfasc is highly enriched at the AIS, we expected AIS-enriched membrane proteins to be over-represented in our data set. However, Nfasc is also found at lower densities in somatodendritic and distal axonal domains, and their total membrane area exceeds that of the AIS. Thus, despite the high density of AIS Nfasc, the total pool of Nfasc in non-AIS membrane is likely much greater. HRP-mediated biotinylation is very efficient and has a range ~25 times greater than BioID or APEX 11. Thus, the proteins we recovered are more accurately described as an Nfasc surface proximity proteome, with a subset of those proteins also being found at the AIS.
Given the promiscuity of Nfasc-BAR, the biggest challenge in our experiments was to determine which proteins to focus on and how to validate their presence or enrichment at the AIS. To this end, we narrowed our analysis using stringent filtering criteria including fold-enrichment, significance of that enrichment, and a minimum number of PSMs recovered. We also estimated the relative proximity to Nfasc based on the ratio of extracellular tyrosine residues to the PSMs recovered. Nfasc-BAR and the filtering criteria used here may underestimate proximity or miss AIS proteins that were not well biotinylated (e.g. ion channels). Nevertheless, among the proteins recovered and that satisfied the filtering criteria, our use of endogenous gene tagging revealed several that were present or even enriched at the AIS, but that had not been previously described as AIS proteins. In particular, we found that Cntn1 is highly enriched at the AIS. We validated its enrichment there by endogenous gene tagging in vitro and in vivo, by expression of exogenous epitope-tagged Cntn1 at the AIS in vitro and in vivo, and by immunostaining of cultured hippocampal neurons using Cntn1 antibodies whose specificity was confirmed using CRISPR-mediated gene disruption. Other candidate AIS proteins (e.g. Tenm4 and Ptprs) will require additional studies to further validate and confirm they are bona fide AIS proteins. Additional endogenous gene tagging may reveal more AIS membrane proteins since we only tested a small subset of the Nfasc proximity proteome.
The use of numerous methods to confirm that Cntn1 is a bona fide AIS protein is important since relying on antibody staining alone can lead to incorrect assignment of a protein being enriched at the AIS 18–20. Methods allowing CRISPR-mediated endogenous gene tagging are a significant advance to validate protein localization without the confound of off-target antibodies or mislocalization due to over-expression 21–23. However, the method of tag insertion we used also disrupts coding regions in the last exon of the proteins analyzed, and some proteins that depend on their C-terminal amino acids may be mislocalized. Thus, failure of a protein to localize to the AIS after endogenous gene tagging should not be considered a definitive criterion for exclusion as an AIS protein.
Cntn1 is a GPI-anchored cell adhesion molecule that has been studied in the nervous system mainly in the context of its role in axon-glia interactions as an essential component of the paranodal axoglial junction formed between axons and myelinating glia 30. There, Cntn1 participates in cis interactions in the axon with Caspr1 (Contactin ASsociated PRotein 1) and trans interactions with the glial 155 kD form of Nfasc (NF155) 29,45. Cntn1 can engage in diverse interactions with many cell adhesion and extracellular matrix molecules including Caspr1, members of the L1 family of cell adhesion molecules (Nfasc, NrCAM and L1CAM), Tnr, Tnc, and receptor tyrosine phosphatase β 28,46,47. Our experiments show that Cntn1’s AIS localization requires its first four Ig-like domains and redundant interactions with either Nfasc or NrCAM (Fig. S5a), since only simultaneous deletion of both disrupts Cntn1’s AIS localization. In addition, Cntn1 helps assemble the AIS extracellular matrix since its loss affects Tnr recruitment. Future experiments may also reveal roles for Cntn1 in association with other AIS extracellular matrix molecules or membrane proteins including Tnc and Na+ channel β subunits 48. The importance of Cntn1 in humans is highlighted by the observation that a pathogenic variant of CNTN1 caused lethal severe fetal akinesia syndrome 49.
ChCs and basket cells precisely innervate cortical PyNs and Purkinje neurons, respectively, to regulate AIS excitability. For example, Dudok et al. 38 and Schneider-Mizell et al. 39 showed that a variety of behaviors including pupil dilation, locomotion, and whisking can synchronously activate populations of ChCs to inhibit PyNs through GABAergic synapses. Together, these observations highlight the central role played by ChCs in modulating brain states and behavior. Similarly, pinceau synapses provide strong inhibitory control over Purkinje neuron output, but this is due to ephaptic inhibition rather than chemical inhibition 50. Despite their importance, the molecular mechanisms responsible for the precise innervation and maintenance of AIS axo-axonic synapses are incompletely understood.
Loss of AnkG from Purkinje neurons disrupts pinceau synapse formation and has been attributed to mislocalization of AIS NF186; however, loss of ankyrin-binding NrCAM, L1CAM, or CHL1 did not affect pinceau synapse assembly 37. These observations are consistent with our findings that Cntn1 also regulates pinceau synapse assembly (Fig. S5b), since loss of AnkG affects both NF186 and NrCAM localization 51. We report here that Cntn1’s AIS localization can be independently directed by both of these CAMs (Fig. 6d). This result is consistent with the observation that the specific deletion of NF186 alone in adults does not disrupt pinceau synapse maintenance 52, suggesting that Cntn1 can also partner with other AIS CAMs (e.g., NrCAM) for synapse maintenance. Since Cntn1 −/− mice die by P21, we cannot rule out the possibility that pinceau synapses fail to develop due to widespread developmental defects. Future studies utilizing Purkinje neuron specific deletion of Cntn1 will be required to more precisely define Cntn1’s role in pinceau synapse assembly and maintenance.
Previously, Tai et al. 41 reported a small RNAi screen of 14 candidate cell adhesion molecules to identify regulators of PyN AIS innervation by ChCs. The candidates screened included Nfasc, NrCAM, and all previously reported AIS CAMs (e.g. Cntn2). Loss of Nfasc, NrCAM, or Cntn2 alone had no effect on ChC/PyN AIS innervation; but the impact of simultaneous loss/depletion of Nfasc and NrCAM remains to be tested. Importantly, among the candidates screened, only loss of L1CAM significantly reduced PyN AIS synaptic innervation by ChCs, despite the fact that L1CAM is found not only at the AIS, but along the entire axon 25. This suggests that L1CAM may cooperate in cis with other membrane or adhesion molecules like Cntn1 for precise innervation of the AIS by ChCs (Fig. S5c). In addition to L1CAM, Hayano et al. 53 reported the cell adhesion molecule Igsf11 functions both pre- and post-synaptically in layer 2/3 of cortex to regulate assembly of PyN/ChC axo-axonic synapses. However, we did not find Igsf11 in our Nfasc-BAR results at any time point; although this may reflect Igsf11 having relatively few extracellular tyrosines. Alternatively, there may be differences between neurons in vitro and in vivo, or even between types of neurons.
One limitation of this study is that the proximity biotinylation experiments were performed on cultured neurons. AIS cell surface proteins whose localization depends on the native brain environment may not be represented. For example, AIS GABAergic synapses may form in vitro, but they are rare, making it difficult to identify presynaptic receptor(s) for Cntn1 and L1CAM using Nfasc-BAR. Future experiments using extracellular split TurboID 54, where PyNs and ChCs each express one half of TurboID may help to identify the pre-synaptic receptor found on ChCs. Alternatively, developing in situ Nfasc-BAR for use with brain tissue may reveal additional AIS cell surface proteins and their receptors.
In summary, we used extracellular proximity biotinylation to identify Cntn1 as a new AIS adhesion molecule. Cntn1 is restricted to the AIS through its binding to AnkG-localized Nfasc or NrCAM (Fig. S5a). In Purkinje neurons, Cntn1 is required for AIS innervation by basket cells (Fig. S5b). In PyNs, Cntn1 functions together with L1CAM to regulate the AIS-specific targeting and developmental assembly of PyN/ChC axo-axonic synapses (Fig. S5c). Thus, our results suggest a model where axo-axonic innervation of diverse neuron types converges on AIS-enriched Cntn1.
METHODS
Animals
Timed pregnant Sprague-Dawley rats were obtained from Charles River Laboratories. Rats were euthanized for embryo collection at E18. Brains were collected from Cntn1 −/− and +/− mice at P17 (catalog #Jax:034216, RRID:IMSR_JAX:034216). The Cntn1 mice were maintained at the Weizmann Institute of Science, Rehovot, Israel. P0 ICR mice were used for intraventricular injection of AAV for overexpression of Cntn1-Myc. Transgenic Cas9 mice (catalog #Jax: 027650, RRID: IMSR_JAX:027650) were used for intraventricular injection of AAV to perform tagging of endogenous Cntn1. Nkx2–1tm1.1(Cre/ERT2)Zjh/J and B6;129S6-Gt(Rosa)26Sortm9(CAG-tdTomato)Hze/J were a gift from Dr. Z.J. Huang 55. Swiss Webster mice were purchased from Charles River (Cat# CRL:24; RRID: IMSR_CRL:24). All experiments were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Baylor College of Medicine, the Cold Spring Harbor, and the Weizmann Institute of Sciences Institutional Animal Care and Use Committees.
Cell Culture
Primary cultures of hippocampal neurons were obtained from E18 Sprague-Dawley rat embryos. Hippocampi were dissected and dissociated. For imaging, neurons were plated onto Poly-D-Lysine (Sigma) and laminin-coated glass coverslips (Life Technologies) at a density of ~1.25 × 104 cells/cm2. For mass spectrometry, neurons were plated onto Poly-D-Lysine and laminin-coated 10 cm dishes at a density of ~2.5 × 104 cells/cm2. Hippocampal neurons were maintained in Neurobasal medium (Life Technologies) containing 1% Glutamax (Life Technologies), 1% penicillin and streptomycin (Life Technologies), and 2% B27 supplement (Life Technologies) in an incubator at 37°C with 5% CO2.
Biotinylation by Antibody Recognition
Cultured rat primary neurons (~2 × 106 primary hippocampal neurons for each condition) were biotinylated at each of 5 different timepoints. Cells were live labeled by incubating with primary antibodies diluted in culture media for 1 hr at 37°C, then washed with neurobasal media and incubated in culture media alone for 1 hr at 37°C. Cells were incubated with horseradish peroxidase (HRP)-labeled secondary antibodies diluted in culture media for 30 min at 37°C, then washed with PBS. Biotin tyramide (Perkin Elmer Cat# NEL749A001KT) was diluted 1:500 in a dilution buffer containing H2O2, and applied to cells for 5 min at 4°C. Cells were washed with PBS and lysed in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, and 1% NP-40). Biotinylated proteins were isolated using streptavidin magnetic sepharose beads (GE Healthcare Cat# 28–9857-38) overnight at 4°C and then washed seven times in RIPA buffer. Control cells were labeled and processed using the same steps except for the omission of primary antibodies.
Mass Spectrometry.
Sample-incubated streptavidin magnetic sepharose beads were resuspended in 5 mM DTT in 100 mM NH4HCO3 and incubated for 30 min at room temperature. After this, iodoacetamide was added to a final concentration of 7.5 mM and samples incubated for 30 additional minutes. In all, 0.5 μg of sequencing grade trypsin (Promega) was added to each sample and incubated at 37°C overnight. Supernatants of the beads were recovered, and beads digested again using 0.5 μg trypsin in 100 mM NH4HCO3 for 2 h. Peptides from both consecutive digestions were recovered by solid phase extraction using C18 ZipTips (Millipore, Cat# ZTC18S096), and resuspended in 0.1% formic acid for analysis by liquid chromatography-mass spectrometry (LC-MS/MS). Peptides resulting from trypsinization were analyzed on a QExactive Plus (Thermo Scientific), connected to a NanoAcquity™ Ultra Performance UPLC system (Waters). A 15-cm EasySpray C18 column (Thermo Scientific) was used to resolve peptides (90-min gradient with 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B). MS was operated in data-dependent mode to automatically switch between MS and MS/MS. The top ten precursor ions with a charge state of 2+ or higher were fragmented by high-energy collisional dissociation. Peak lists were generated using PAVA software 56. All generated peak lists were searched against the rat subset of the UniProt database (UniprotKB 2017.11.01) using Protein Prospector 57. The database search was performed with the following parameters: a mass tolerance of 20 ppm for pre- cursor masses; 30 ppm for MS/MS, cysteine carbamidomethylation as a fixed modification and acetylation of the N terminus of the protein, pyroglutamate formation from N-terminal glutamine, and oxidation of methionine as variable modifications. All spectra identified as matches to peptides of a given protein were reported, and the number of spectra (Peptide Spectral Matches, PSMs) used for label free quantitation of protein abundance in the samples.
Antibodies
The following antibodies were used for in vitro biotinylation (dilutions for each antibody are indicated in parentheses): chicken polyclonal antibody anti-neurofascin (1:1000; R&D Systems Cat# AF3235, RRID:AB_10890736), rabbit polyclonal anti-NrCAM (1:1000; Abcam Cat# ab24344, RRID:AB_448024), anti-chicken HRP-labeled secondary antibody (1:2000; Aves Labs Cat# H-1004, RRID:AB_2313517), anti-rabbit HRP-secondary antibody (1:2000; Jackson ImmunoResearch Labs Cat# 111–035-003, RRID:AB_2313567).
The following antibodies were used for immunofluorescence studies (dilutions for each antibody are indicated in parentheses): chicken polyclonal anti-neurofascin (1:500; R&D Systems Cat# AF3235, RRID:AB_10890736), chicken polyclonal anti-MAP2 (1:1000; EnCor Biotechnology Cat# CPCA-MAP2, RRID:AB_2138173), mouse monoclonal anti-Ankyrin-G (1:500; NeuroMab N106/36, RRID:AB_10673030), mouse monoclonal anti-Tenascin-R (1:250; R&D Systems Cat# MAB1624, RRID:AB_2207001), rabbit polyclonal anti-NrCAM (1:250; Abcam Cat# ab24344, RRID:AB_448024), rabbit polyclonal anti-β4 spectrin (1:500, Rasband lab, RRID:AB_2315634), rabbit polyclonal anti-Kv1.2 (1:250, James Trimmer, University of California, Davis, RRID:AB_2756300), mouse monoclonal anti-PSD-95 (1:250; Antibodies Incorporated Cat# 75–028, RRID:AB_2292909), mouse monoclonal anti-Tuj1 (1:700; BioLegend Cat# 801202, RRID:AB_10063408), goat polyclonal anti-Cntn1 (1:500; R&D Systems Cat# AF904, RRID:AB_2292070), mouse monoclonal anti-Myc (1:2000; MBL International Corporation Cat# M192, PRID: AB_11160947), rat monoclonal anti-HA (1:500; Millipore Sigma Cat# 11867423001, RRID: AB_390918), mouse monoclonal anti-V5 (1:500; Invitrogen Cat# R960CUS, RRID: AB_159298). Anti-RFP (guinea pig pAb, 1:1000, Synaptic systems 390 005), anti-gephyrin (mouse mAb IgG1, 1:500, Synaptic Systems 147 011), and anti-VGAT (guinea pig pAb, 1:500, Synaptic Systems 131 004).
The following secondary antibodies were used: Alexa Fluor 555 goat anti-rat (1:1000, Thermo Fisher Scientific A-11006), Alexa Fluor Plus 555 goat anti-rabbit (1:1000, Thermo Fisher Scientific A32732), Alexa Fluor 647 goat anti-rabbit (1:1000, Thermo Fisher Scientific A-21244), Alexa Fluor 555 goat anti-guinea pig (1:1000, Thermo Fisher Scientific A-21435), Alexa Fluor 647 goat anti-guinea pig (1:1000, Thermo Fisher Scientific A-21450), Alexa Fluor 647 goat anti-mouse IgG1 (1:1000, Thermo Fisher Scientific A-21240), Alexa Fluor 488 goat anti-mouse IgG2a (1:1000, Thermo Fisher Scientific A-21131), Aminomethylcoumarin (AMCA) anti-chicken IgY (1:1000 Jackson Immunoresearch labs 103–155-155), Alexa Fluor 488 anti-chicken IgY, (1:1000 Jackson Immunoresearch labs 103–545-155), Alexa Fluor 488 anti-mouse IgG (1:1000 Thermo Fisher Scientific A11029), Alexa Fluor 594 anti-rabbit (1:1000 Thermo Fisher Scientific A11034), Alexa Fluor 594 anti-mouse IgG (1:1000 Thermo Fisher Scientific A32742). Streptavidin Alexa Fluor 594 conjugates were purchased from Thermo Fisher Scientific (1:5000; S11227). Hoechst fluorescent reagent (1:100,000; Thermo Fisher Scientific Cat# H3569, RRID:AB_2651133) was used to label nuclei.
In Utero Electroporation and Tamoxifen Induction
To manipulate Cntn1 expression in pyramidal neurons (PyNs) and sparsely label chandelier cells (ChCs) in the same neocortical layer, ventricular zone-directed in utero electroporation targeting neocortical PyN progenitors was performed in Nkx2.1-CreER;Rosa26-loxpSTOPloxp-tdTomato (Ai9) embryos. Specifically, Swiss Webster females were bred with Nkx2.1-CreER+/−;Ai9+/+ males. Pregnant females at 15.5 days of gestation were anesthetized, the uterine horns were exposed, and approximately 1 μl of plasmid solution (0.75 μg/μl pCAG-1BPNLS-Cas9–1BPNLS + 1.5 μg/μl pAAV-3x-sgRNA-smFP (with control or target specific sgRNAs; see STAR Methods) was injected manually into the lateral ventricle of the embryos using a beveled glass micropipette (Drummond Scientific). After injection, five square 50 ms pulses of 45 V with 950 ms intervals were delivered across the uterus with two 5 mm electrode paddles (BTX, 45–0489) positioned on either side of the head (BTX, ECM830). After electroporation, the uterine horns were placed back in the abdominal cavity of the pregnant dam and the wound was surgically sutured. Tamoxifen (3 mg/30 g of body weight) was administered to the pregnant dam by oral gavage at 18.5 days of gestation to induce CreER activity and excision of the STOP cassette, resulting in tdTomato red fluorescent protein expression in a sparse population of nascent neocortical ChCs in their offspring. Pups were euthanized at postnatal day 17.
Immunofluorescence labeling
Cultured rat primary hippocampal neurons were fixed in 4% paraformaldehyde (PFA, pH 7.2) for 15 minutes at 4°C. Acutely dissected brains were drop fixed in 4% paraformaldehyde (PFA, pH 7.2) for 60 minutes at 4°C. Brains were then equilibrated overnight in 20% and 30% sucrose in 0.1 M PB overnight at 4°C. Brains were then sectioned at 12–25 μm and mounted on coverslips. Fixed neurons and brain sections were permeabilized and blocked with 10% normal goat serum in 0.1 M PM with 0.3% Triton X-100 (PBTGS) for one hour. Cells and sections were then incubated in primary antibodies diluted in PBTGS overnight at room temperature or 4°C. Tissues and cells were then washed three times using PBTGS for 5 min. each. Fluorescent secondary antibodies were then diluted in PBTGS and added to cells and tissues for one hour. Coverslips were then washed once using PBTGS, 0.1 M PB, and finally 0.05 M PB for 5 min. each. Coverslips were then mounted using Vectashield plus (vector labs) anti-fade mounting media.
For immunostaining of electroporated neonatal brains, P17 mice were deeply anesthetized with isoflurane and perfused transcardially with PBS and 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. Brains were post-fixed in 4% PFA in 0.1 M phosphate buffer overnight at 4°C and then cryoprotected with 30% sucrose in PBS. 50 μm thick coronal sections were subsequently generated using a Vibratome (Leica VT1000S). For gephyrin and VGAT immunostaining, brain slices were subjected to mild antigen retrieval with 10 mM citrate buffer for 30 minutes at 60°C. Subsequently, brain slices were blocked and permeabilized with 10% normal goat serum (NGS) and 0.3% Triton X-100 in PBS at RT for 30 minutes and then incubated with primary antibodies diluted in 2% NGS and 0.3% Triton X-100 in PBS overnight at 4°C. Fluorescent secondary antibodies diluted in 2% NGS and 0.3% Triton X-100 in PBS were applied for 2 h at RT the following day. Sections were then washed three times with PBS for 20 min per wash and mounted with Fluoromount-G (Southern Biotech).
Plasmid construction
The sgRNAs and the homology-independent donor templates were generated following strategies similar to those described previously 19,22,23,58. Briefly, the U6 promoter and scaffold sequences were PCR amplified from pMJ117 and pMJ179 (gifts from Jonathan Weissman, Addgene plasmid #85997 and #85996). The smFP-V5 (a gift from Loren Looger, Addgene plasmid #59758) was used as a knock-in donor.
The knockout constructs expressing three independent sgRNAs and a smFP-HA marker (pAAV-3x-gRNA-smFP) were generated as follows: the U6 promoter and scaffold sequences were PCR amplified from pMJ114, pMJ117, and pMJ179 (gifts from Jonathan Weissman, Addgene plasmid # 85995, #85997, and #85996). Human Synapsin1 promoter and smFP-HA were PCR amplified from pAAV-hSyn-EGFP (a gift from Bryan Roth, Addgene Plasmid #50465) and pCAG_smFP-HA (a gift from Loren Looger, Addgene plasmid #59759), respectively. The plasmid PX552 (a gift from Feng Zhang, Addgene plasmid #60958) was digested with a NotI restriction enzyme (NEB) and used as a plasmid backbone. DNA fragments were ligated together using an In-Fusion Snap Assembly Master Mix (Takara). The sgRNA sequences for knock-in and knockout are listed in the supplemental materials table. The AAV-SpCas9 plasmid (a gift from Feng Zhang, Addgene plasmid #60957) was modified by removing the HA tag.
Cntn1 constructs were generated in both pcDNA3 and AAV backbones. pcDNA3 was digested with EcoRI restriction enzyme (NEB) and pAAV-hSyn-EGFP (a gift from Bryan Roth, Addgene Plasmid #50465) was digested with BamHI and XhoI restriction enzymes (NEB) and used as plasmid backbones. Full-length and truncated Cntn1 was PCR amplified from rat contactin-myc 34 and ligated together using an In-Fusion Snap Assembly Master Mix (Takara). All DNA constructs were verified by sequencing (Genewiz and plasmidsaurus).
Adeno-associated virus (AAV) production
Small scale AAV cell-lysates were produced using the AAVpro Purification Kit (All Serotypes) (Takara) with slight modifications. Briefly, HEK293T cells were triple-transfected with AAV plasmid, helper plasmid (Agilent Technologies, Cat # 240071), and serotype PHP.S or PHP.eB plasmids (a gift from Viviana Gradinaru, Addgene plasmids #103002 and #103005) with PEI Max (Polysciences, Cat # 24765). The medium was changed the next day of transfection and cells were incubated for 3 days after transfection. HEK cells were then collected and lysed with the AAV Extraction Solution A plus. The extracted solution was centrifuged at 10,000 × g for 10 min to remove debris and mixed with Extraction Solution B. This small scale AAV solution was stored at 4°C and used for neuronal transduction into cultured neurons. AAV vectors for in vivo transduction were produced by the Baylor College of Medicine Neuroconnectivity Core or in our lab following the strategies described previously 59.
Viral transduction of neurons
For viral transduction of cultured neurons, 10 μl of AAV-Cas9 and 10 μl of AAV-sgRNA and donor, or AAV-3x-sgRNA-smFP, were added into a well of a 12-well plate at 0–1 DIV. The medium was replaced 2 days after infection. For viral transduction of neurons in vivo, AAV vectors were injected into the lateral ventricles of neonatal mice as described previously 60. Briefly, P0 to P2 pups were anesthetized on ice and 1–2 μl of AAV vectors were bilaterally injected. The pups were placed in a heated cage until the animals recovered and then returned to their mother. For transduction of neurons in adult, viruses were injected retro-orbitally in 13 week-old C57Bl/6J mice. Tissues were collected 4 weeks after infection.
Confocal Image Acquisition and Analysis of ChC/PyN AIS Innervation and GABAergic Synapse Density at PyN AISs
For analysis of ChC/PyN AIS percent innervation, images of coronal brain slices (50 μm thick) were acquired using an LSM 800 confocal laser-scanning microscope (Zeiss) with a 63x oil-immersion objective and sequential acquisition settings applied at a resolution of 1024×1024 pixels. 200 μm × 200 μm images of single RFP+ ChCs and neighboring GFP+ electroporated PyNs in layer II of the somatosensory cortex were collected using a z series of 30–36 images with a depth interval of 1 μm. ChC/PyN AIS percent innervation was calculated by dividing the number of GFP+ PyNs innervated at their AIS by a single RFP+ ChC by the total number of GFP+ PyNs in the entire 200 μm × 200 μm image z stack. Representative maximum projection images were generated from 10 z-series images with a depth interval of 1 μm. To quantify the average density of gephyrin or VGAT puncta per μm on the AIS of PyNs, 90 μm × 90 μm images were acquired at a resolution of 1280×1280 pixels using a z-series of 40–60 images with a depth interval of 0.37 μm. The number of gephyrin or VGAT puncta overlapping with AnkG+ or β4-spectrin+ PyN AISs in individual z-plane images was manually counted and AIS lengths were measured using Zeiss Zen (Blue Edition) imaging software. Gephyrin or VGAT puncta density at the AIS was then calculated by dividing the number of PyN AIS gephyrin or VGAT puncta by the length of the AIS. Representative maximum projection images of PyN AIS GABAergic synapses visualized via gephyrin or VGAT immunostaining were generated using a z-series of 10 images with a depth interval of 0.37 μm.
Image Acquisition
Images of immunofluorescence were captured using an Axio-imager Z2 microscope fitted with an apotome attachment for structured illumination (Carl Zeiss MicroImaging) and a Nikon Eclipse Ni2. 20X (0.8 NA), 40X (0.95 NA), and 63X (1.4 NA) objectives were used. Images were taken using Zen 3.2 (Zeiss) or NIS-Elements (Nikon). For measurements of AIS streptavidin fluorescence intensity, 20 neurons per timepoint per replicate were imaged and line scans were drawn using Zen 3.2 software. Images were exported to Fiji, Adobe Photoshop, and Adobe illustrator for figure presentation. Some figures were generated using Biorender.
Statistical Analysis and Quantification
Unpaired, two-tailed Student’s t-test was used for all statistical analyses unless otherwise indicated. Data were analyzed using Microsoft Excel and GraphPad Prism. All error bars are ±SEM unless otherwise indicated. PSMs were normalized using the formula PSMNorm = PSMDIVX * CsumMax / CsumDIVX. PSMDIVX is the raw PSM count for a candidate protein for a particular replicate at the specified timepoint; CsumMax is the maximum sum of seven endogenously biotinylated carboxylases Acaca, Acacb, Pc, Pcca, Pccb, Mccc1, Mccc2 for an individual replicate across all 15 replicates; CsumDIVX is the sum of seven endogenously biotinylated carboxylases Acaca, Acacb, Pc, Pcca, Pccb, Mccc1, Mccc2 for an individual replicate at the specified timepoint DIVX. Heatmaps were generated using GraphPad Prism. Candidates were rank-ordered by the slope of the linear regression of their log2 fold enrichment over time. The number of extracellular tyrosines for proteins whose log2 fold change was > 2 and that had at least ten PSMs were counted using a script that extracted protein topology from the Uniprot database (www.uniprot.org). The tyrosine-PSM proximity plot was generated using Python (www.python.org). Colors were added in Adobe Illustrator.
Extended materials
A detailed list of all materials including all gRNA sequences, antibodies, plasmids, sources, etc. is provided in the supplemental extended materials file.
Supplementary Material
ACKNOWLEDGEMENTS
The work reported here was supported by NIH research grants NS122073 (M.N.R.), MH119819 (L.V.A.) and NS116897 (L.V.A.), and by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (A.L.B., E.P., and M.N.R.). We thank Ayano Ogawa for illustrations.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
DATA AVAILABILITY STATEMENT
All mass spectrometry data sets from experiments included here are deposited at PRIDE (Proteomics Identifications Database).
REFERENCES
- 1.Leterrier C. The Axon Initial Segment: An Updated Viewpoint. J Neurosci 38, 2135–2145, doi: 10.1523/JNEUROSCI.1922-17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasband M. N. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11, 552–562 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Samavarchi-Tehrani P., Samson R. & Gingras A. C. Proximity Dependent Biotinylation: Key Enzymes and Adaptation to Proteomics Approaches. Molecular & cellular proteomics : MCP 19, 757–773, doi: 10.1074/mcp.R120.001941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdan H. et al. Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nature communications 11, 100, doi: 10.1038/s41467-019-13658-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J. et al. Cell-Surface Proteomic Profiling in the Fly Brain Uncovers Wiring Regulators. Cell 180, 373–386 e315, doi: 10.1016/j.cell.2019.12.029 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuster S. A. et al. In situ cell-type-specific cell-surface proteomic profiling in mice. Neuron, doi: 10.1016/j.neuron.2022.09.025 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takano T. et al. Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 588, 296–302, doi: 10.1038/s41586-020-2926-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees J. S., Li X. W., Perrett S., Lilley K. S. & Jackson A. P. Selective Proteomic Proximity Labeling Assay Using Tyramide (SPPLAT): A Quantitative Method for the Proteomic Analysis of Localized Membrane-Bound Protein Clusters. Current protocols in protein science / editorial board, John E. Coligan ... [et al.] 88, 19.27.11–19.27.18, doi: 10.1002/cpps.27 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Kotani N. et al. Biochemical visualization of cell surface molecular clustering in living cells. Proc Natl Acad Sci U S A 105, 7405–7409, doi: 10.1073/pnas.0710346105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar D. Z. et al. Biotinylation by antibody recognition-a method for proximity labeling. Nature methods 15, 127–133, doi: 10.1038/nmeth.4533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley J. V. et al. Radius measurement via super-resolution microscopy enables the development of a variable radii proximity labeling platform. Proc Natl Acad Sci U S A 119, e2203027119, doi: 10.1073/pnas.2203027119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amor V. et al. The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier. eLife 6, doi: 10.7554/eLife.21392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh A., Malavasi E. L., Sherman D. L. & Brophy P. J. Neurofascin and Kv7.3 are delivered to somatic and axon terminal surface membranes en route to the axon initial segment. eLife 9, doi: 10.7554/eLife.60619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y., Ogawa Y., Hedstrom K. L. & Rasband M. N. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol 176, 509–519 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedstrom K. L. et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol 178, 875–886, doi: 10.1083/jcb.200705119 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens S. R. et al. Ankyrin-R regulates fast-spiking interneuron excitability through perineuronal nets and Kv3.1b K(+) channels. eLife 10, doi: 10.7554/eLife.66491 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollerberg G. E., Thelen K., Theiss M. O. & Hochlehnert B. C. The role of cell adhesion molecules for navigating axons: density matters. Mech Dev 130, 359–372, doi: 10.1016/j.mod.2012.11.002 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Buffington S. A., Sobotzik J. M., Schultz C. & Rasband M. N. IkappaBalpha is not required for axon initial segment assembly. Mol Cell Neurosci 50, 1–9, doi: 10.1016/j.mcn.2012.03.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa Y. & Rasband M. N. Endogenously expressed Ranbp2 is not at the axon initial segment. J Cell Sci 134, doi: 10.1242/jcs.256180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K. J., Susuki K., Dours-Zimmermann M. T., Zimmermann D. R. & Rasband M. N. Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly. J Neurosci 30, 14476–14481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K. et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149, doi: 10.1038/nature20565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y. et al. Plug-and-Play Protein Modification Using Homology-Independent Universal Genome Engineering. Neuron, doi: 10.1016/j.neuron.2019.05.047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willems J. et al. ORANGE: A CRISPR/Cas9-based genome editing toolbox for epitope tagging of endogenous proteins in neurons. PLoS Biol 18, e3000665, doi: 10.1371/journal.pbio.3000665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis J. Q. & Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem 269, 27163–27166 (1994). [PubMed] [Google Scholar]
- 25.Boiko T. et al. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci 27, 590–603 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinatel D. et al. The Kv1-associated molecules TAG-1 and Caspr2 are selectively targeted to the axon initial segment in hippocampal neurons. J Cell Sci 130, 2209–2220, doi: 10.1242/jcs.202267 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Davis J. Q., Lambert S. & Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J Cell Biol 135, 1355–1367 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoda Y. & Watanabe K. Contactins: emerging key roles in the development and function of the nervous system. Cell Adh Migr 3, 64–70, doi: 10.4161/cam.3.1.7764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charles P. et al. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol 12, 217–220. (2002). [DOI] [PubMed] [Google Scholar]
- 30.Boyle M. E. et al. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 30, 385–397. (2001). [DOI] [PubMed] [Google Scholar]
- 31.Rios J. C. et al. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci 20, 8354–8364. (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colakoglu G., Bergstrom-Tyrberg U., Berglund E. O. & Ranscht B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci U S A 111, E394–403, doi: 10.1073/pnas.1313769110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkmer H., Zacharias U., Norenberg U. & Rathjen F. G. Dissection of complex molecular interactions of neurofascin with axonin-1, F11, and tenascin-R, which promote attachment and neurite formation of tectal cells. J Cell Biol 142, 1083–1093 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gollan L., Salomon D., Salzer J. L. & Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol 163, 1213–1218 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kole M. J. et al. Selective Loss of Presynaptic Potassium Channel Clusters at the Cerebellar Basket Cell Terminal Pinceau in Adam11 Mutants Reveals Their Role in Ephaptic Control of Purkinje Cell Firing. J Neurosci 35, 11433–11444, doi: 10.1523/JNEUROSCI.1346-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laube G. et al. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Res Mol Brain Res 42, 51–61 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Ango F. et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119, 257–272 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Dudok B. et al. Recruitment and inhibitory action of hippocampal axo-axonic cells during behavior. Neuron 109, 3838–3850 e3838, doi: 10.1016/j.neuron.2021.09.033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider-Mizell C. M. et al. Structure and function of axo-axonic inhibition. eLife 10, doi: 10.7554/eLife.73783 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallo N. B., Paul A. & Van Aelst L. Shedding Light on Chandelier Cell Development, Connectivity, and Contribution to Neural Disorders. Trends Neurosci 43, 565–580, doi: 10.1016/j.tins.2020.05.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai Y., Gallo N. B., Wang M., Yu J. R. & Van Aelst L. Axo-axonic Innervation of Neocortical Pyramidal Neurons by GABAergic Chandelier Cells Requires AnkyrinG-Associated L1CAM. Neuron, doi: 10.1016/j.neuron.2019.02.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D. et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143, 1295–1304 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hedstrom K. L., Ogawa Y. & Rasband M. N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183, 635–640, doi: 10.1083/jcb.200806112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torii T. et al. NuMA1 promotes axon initial segment assembly through inhibition of endocytosis. J Cell Biol 219, doi: 10.1083/jcb.201907048 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peles E. et al. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J 16, 978–988 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zisch A. H. et al. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J Cell Biol 119, 203–213, doi: 10.1083/jcb.119.1.203 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peles E. et al. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell 82, 251–260 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Kazarinova-Noyes K. et al. Contactin associates with Na+ channels and increases their functional expression. J Neurosci 21, 7517–7525. (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reischer T., Liebmann-Reindl S., Bettelheim D., Balendran-Braun S. & Streubel B. Genetic diagnosis and clinical evaluation of severe fetal akinesia syndrome. Prenat Diagn 40, 1532–1539, doi: 10.1002/pd.5809 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blot A. & Barbour B. Ultra-rapid axon-axon ephaptic inhibition of cerebellar Purkinje cells by the pinceau. Nat Neurosci 17, 289–295, doi: 10.1038/nn.3624 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Jenkins S. M. & Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155, 739–746. (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zonta B. et al. A Critical Role for Neurofascin in Regulating Action Potential Initiation through Maintenance of the Axon Initial Segment. Neuron 69, 945–956 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayano Y. et al. IgSF11 homophilic adhesion proteins promote layer-specific synaptic assembly of the cortical interneuron subtype. Sci Adv 7, doi: 10.1126/sciadv.abf1600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho K. F. et al. Split-TurboID enables contact-dependent proximity labeling in cells. Proc Natl Acad Sci U S A 117, 12143–12154, doi: 10.1073/pnas.1919528117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taniguchi H., Lu J. & Huang Z. J. The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339, 70–74, doi: 10.1126/science.1227622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan S., Price J. C., Prusiner S. B., Ghaemmaghami S. & Burlingame A. L. A data processing pipeline for mammalian proteome dynamics studies using stable isotope metabolic labeling. Molecular & cellular proteomics : MCP 10, M111 010728, doi: 10.1074/mcp.M111.010728 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clauser K. R., Baker P. & Burlingame A. L. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem 71, 2871–2882 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Suzuki K. & Izpisua Belmonte J. C. In vivo genome editing via the HITI method as a tool for gene therapy. Journal of human genetics 63, 157–164, doi: 10.1038/s10038-017-0352-4 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Konno A. & Hirai H. Efficient whole brain transduction by systemic infusion of minimally purified AAV-PHP.eB. J Neurosci Methods 346, 108914, doi: 10.1016/j.jneumeth.2020.108914 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Kim J. Y., Grunke S. D., Levites Y., Golde T. E. & Jankowsky J. L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J Vis Exp, 51863, doi: 10.3791/51863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All mass spectrometry data sets from experiments included here are deposited at PRIDE (Proteomics Identifications Database).