Abstract
Introduction:
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and encompasses Crohn’s disease and ulcerative colitis. Women appear to have more severe and recurring symptoms of IBD compared to men, most likely due to hormonal fluctuations. Studies have shown that mitochondrial dysfunction plays a role in the development of inflammation and there is evidence of colon mitochondrial alterations in IBD models and patients. In this study we have identified the presence of sex-specific colon mitochondrial dysfunction in a rat model of IBD.
Methods:
Eight-week-old male and female rats were treated with indomethacin to induce IBD and mitoTEMPO was administered daily either after or before induction of IBD and until euthanasia. Colons were collected for histology and mitochondrial experiments. Intact mitochondrial respiration, reactive oxygen species (mtROS), the activities of the individual electron transport complexes and the activities of the antioxidant enzymes were measured to assess mitochondrial function.
Results:
IBD male rats showed a decrease in citrate synthase activity, cardiolipin levels, catalase activity and an increase in mtROS production. IBD females show a decrease in intact colon mitochondrial respiration, colon mitochondria respiratory control ratio (RCR), complex I activity, complex IV activity, and an increase in mtROS. Interestingly, control females showed a significantly higher rate of complex I and II-driven intact mitochondrial respiration, MCFA oxidation, complex II activity, complex III activity, and complex IV activity compared to control males. The use of a mitochondrial-targeted therapy, mitoTEMPO, improved the disease and colon mitochondrial function in female IBD rats. However, in the males there was no observed improvement, likely due to the decrease in catalase activity.
Conclusions:
Our study provides a better understanding of the role mitochondria in the development of IBD and highlights sex differences in colon mitochondrial function. It also opens an avenue for the development of strategies to re-establish normal mitochondrial function that could provide more options for preventive and therapeutic interventions for IBD.
Keywords: Inflammatory bowel disease, mitochondria, oxidative phosphorylation, reactive oxygen species, mitochondrial-targeted therapies, MitoTEMPO
Introduction
In the US, approximately 3 million people suffer from inflammatory bowel disease (IBD), with the highest prevalence in the non-Hispanic white population1. IBD is an umbrella term for two chronic inflammatory conditions, Crohn’s Disease (CD) and Ulcerative Colitis (UC)2. Both of these conditions are characterized by chronic inflammation of the gastrointestinal (GI) track2. CD can affect any part of the GI tract from the mouth to the anus, but most often, it affects the portion of the small intestines before the large intestine/colon. The damaged areas usually appear as inflamed patches that are next to areas of healthy tissue. Inflammation may reach through the multiple layers of the GI tract that can result in blockages of the GI track requiring surgical removal1. UC occurs only in the large intestines and rectum. Inflammation is continuous usually starting in the rectum and spreading further into the colon. Inflammation is present only in the innermost layer of the lining of the colon. Chronic inflammation of the colon leads to a higher risk for colorectal cancer (CRC). Currently, there is no known cure for IBD with treatments mainly focusing on reducing inflammation and symptoms2.
To study IBD, many rodent models are available and all have advantages and disadvantages3. In particular, indomethacin-induced IBD in rats is a well-established model. Indomethacin is an easily administered, widely available, and clinically relevant compound that induces distinct acute and chronic phases of IBD that involve both the small and large intestine3. This model also has an association with extraintestinal lesions as well as differences in genetic susceptibility among inbred rat strains. This model has been very useful in identifying the role of various factors involved in the pathogenesis of intestinal inflammation and should provide a reliable and noninvasive model for drug screening3.
GI inflammation is a result of decreased intestinal functionality associated with epithelial barrier dysfunction4,5. The epithelial barrier refers to the property of the intestinal mucosa that ensures adequate containment of undesirable luminal contents within the intestine while preserving the ability to absorb nutrients. Its role is to protect the gut mucosal tissues and circulatory system from exposure to proinflammatory molecules, such as microorganisms, toxins, and antigens that are vital for the maintenance of health and well-being4. Mitochondria play an essential role in the maintenance of the epithelial barrier4,5. Mitochondria are the organelles responsible for energy production within the cell. Mitochondrial dysfunction, which results in an increase in mitochondrial reactive oxygen species (mtROS), is associated with intestinal disease and the development of colorectal cancer6–16. A proposed mechanism is that mtROS increases in the GI epithelial cells, this will target the commensal bacteria leading to gut dysbiosis, decrease substrate availability for the epithelial cells, decreased ATP production, and a decrease in the mucosal layer. These changes induce leakage of pathogen-associated molecular patterns (PAMPs) that trigger inflammation4. Many studies show evidence of mitochondrial dysfunction in IBD models and human samples6–18. There has also been evidence mitochondrial complex IV is altered in UC and can lead to the development of CRC7. However, further investigation into the mechanism(s) by which mitochondria function induces the development of intestinal diseases is needed.
Sex differences are observed in IBD. Both CD and UC show sex differences in disease characteristics19. For example, both the incidence and severity of CD are reportedly increased in female patients compared to males, and female gender is a risk factor for relapse in UC1,2,19. Female patients experience dichotomous changes in disease symptoms during puberty, pregnancy and menopause19. Genome linkage studies have revealed high frequency haplotypes in chromosome X associated with IBD in females, although the specific genetic loci that confer this bias remain elusive20. Interestingly, mitochondria are also regulated by sex hormones via the estrogen and androgen receptors, which can lead to differences in mitochondrial function between males and females21,22. However, most studies investigating IBD rat models use only one sex. Therefore, mechanistic investigations into the role of sex in the development of IBD are limited. Understanding the link between sex, mitochondrial function, and the development of IBD will provide additional information for the development of therapeutic options.
Currently there are various mitochondrial-targeted therapies that could prove useful. One in particular, mitoTEMPO, a superoxide dismutase (SOD) mimetic, has been shown to improve epithelial barrier function, to reduce bacteria internalization and transcytosis, to reduce colitis in the dextran sulfate sodium (DSS) mouse model of UC, and to reduce DSS-induced proinflammatory cytokine9. MitoTEMPOL, another SOD mimetic but with a slightly different structure, combined with a biocompatible B-cyclodextrin-derived material has been shown to improve the disease in both acute and chronic mouse models of IBD23. Therefore, mitochondrial-targeted therapy proves to be a potential treatment option for IBD patients.
The goals of the present study were (1) to investigate if colon mitochondrial dysfunction and increased mitochondrial ROS (mtROS) is present in the indomethacin-induced model of IBD; (2) to determine if any sex differences are observed in the mitochondrial dysfunction; and (3) to determine if treatment with the mitochondrial-targeted therapy, mitoTEMPO, would improve the disease activity.
Materials And Methods
5.1. Animals
Animals were purchased from Harlan Laboratory at 7 weeks of age and allowed to acclimate for at least 1 week before use. All animals were housed in the Center for Comparative Research animal facilities of the University of Mississippi Medical Center (UMMC). Animals were kept on a 12 h light/12 h dark cycle and fed a standard laboratory rodent diet (Teklad 8640). All procedures were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
5.2. Indomethacin-induced inflammatory bowel disease
Male and female Sprague Dawley rats at 8 weeks of age were randomly assigned to either control or the indomethacin group. Indomethacin (7.5 mg/kg) was administered to the indomethacin group by subcutaneous injection once a day for two days, exactly 24 hours apart3. Control rats received saline/ethanol. Indomethacin is known to cause small intestinal and colonic ulcerations3. The peak for the disease is reported to be 2–3 days after indomethacin treatment with a duration up to 7 days. However, it has also be reported that the elevation of inflammatory markers can be observed for up to 90 days24. Therefore, animals were euthanized on day 2, day 3, day 7, and day 30 for experiments to determine the peak of the disease. Animal body weight along with food intake was measured daily. For mitoTEMPO treatment groups, rats were randomly assigned into control, control + mitoTEMPO, indomethacin, and indomethacin + mitoTEMPO groups. MitoTEMPO (1mg/kg) was injected intraperitoneal daily either at the start of indomethacin treatment (prophylaxis protocol) or after the indomethacin injections (treatment protocol). Total disease activity score was determined using the sum of the score for weight loss (0 = ≤ 2%; 1 = 3 to 6%; 2 = 7 to 12%; 3 = > 12%), stool consistency (0 = Normal Stool; 1 = Semi-solid Stool; 2 = Loose to Pasty Stool; 3 = Diarrhea), and color of stool (0 = brown; 1 = dark brown; 2 = black; 3 = bloody). Colon lengths and weights were also measured upon removal as another indicator of disease.
5.3. Histology
A section of the colon was fixed in 10% formalin for 24–48 hours. After fixation the tissue was stored in 70% ethanol. The tissues were embedded in paraffin, and serially cut into 4-μm-thick sections and stained with hematoxylin and eosin at the Histology Core located within the Department of Physiology and Biophysics at the University of Mississippi Medical Center. Microscopic images were taken at 10x using the Zeiss Axio Imager.M2.
5.4. Colon Tissue Homogenate
The remaining colon tissue was weighed and placed in isolation buffer (200 mM mannitol, 50 mM sucrose, 5 mM MOPS, pH 7.15, 0.1% BSA) for further cleaning to ensure removal of feces. The colon was then incubated with1 mL of Bacterial Proteinase XXIV (Sigma, 2 mg/ml) for 5 min. The colon was removed from proteinase and incubated with homogenization buffer (200 mM mannitol, 50 mM sucrose, 5 mM MOPS, pH 7.15, 20 mg/ml BSA, 1 mM EDTA, 0.2 mM PMSF). The colon was then cut into small pieces and homogenized gently for 5 strokes in a Potter-Elvehjem homogenizer where the pestle was shaved to reduce the diameter to 1 mm. The tissue homogenate was used immediately for intact mitochondrial experiments and mtROS experiments. After finishing the assay, colon tissue homogenates were fast-frozen in liquid nitrogen and used for further mitochondrial enzyme experiments.
2.5. Intact mitochondria assays
Intact mitochondria respiration was measured using the Oroboros O2k FluoRespirometer at 37°C by previously published methods25,26. Briefly, each chamber of the O2k was calibrated for the O2 concentration in nano-pured water. For complex I-driven and complex II-driven respiration, reaction mixture included buffer containing 10 mM KPi, 5 mM MgCl2, 30 mM KCl, 1 mM EDTA, and 75 mM Tris, pH 7.5 with additions of 7.3 μM cytochrome c and either 20 mM glutamate and 5 mM malate or 20 mM succinate. The total volume of the assay mixture was 2.1 ml. State 2 respiration were initiated by injecting 100 μL fresh colon tissue homogenate. State 3 respiration (ATP synthesis) is achieved by adding 2 mM ADP. The respiratory control ratio (RCR) was calculated by the ratio of state 3 to state 2 respiration. For fatty acid oxidation, the reaction was performed in the same assay buffer as above with 0.5 mM malate, 2 mM ATP, 1 mM NAD+, 25 μM cytochrome c, 0.1 mM coenzyme A, and either 0.5 mM oleate (long-chain fatty acid, LCFA) or 0.5 mM octanoate (medium-chain fatty acid, MCFA). The reaction was started by injecting 100 μL of fresh colon tissue homogenate. For LCFA oxidation, 2 mM carnitine was added to support the transport of LCFA through the inner mitochondrial membrane.
5.6. Mitochondrial ROS measurement
Mitochondrial ROS (mtROS) was measured using the Amplex Red assay, which measures the production of H2O226. Amplex Red (10 μM) is converted to resorufin (highly fluorescent) by the reaction of H2O2 and horseradish peroxidase (HRP, 1U/mL). Superoxide dismutase (SOD, 5U/ml) was also added to convert superoxide (SO) to H2O2. The rate of H2O2 production was obtained by calibrating the fluorescence response of known concentrations of H2O2 in the Oroboros O2k FluoRespirometer. By stoichiometry, the rate of SO production is twice the rate of H2O2 production. Therefore, we calculated the parameter of ‘percent electron leak’, defined as the percentage of electrons from NADH or succinate that are shunted to SO, as opposed to owing to O2 in Complex IV. Expression of ROS production as ‘percent electron leak’ enhances the comparisons of mtROS production induced in different animals.
5.7. Mitochondrial content
Citrate synthase activity and cardiolipin are reported as the best markers for mitochondrial content27. Citrate synthase activity was measured via production of coenzyme A (CoASH) from oxaloacetate. In the presence of 5,5′-dithio-bis- (2-nitobenzoic acid) (DTNB) and free sulfhydryl groups of coenzyme A react to form free 5-thio-2-nitrobenzoate anions which were measured at 412 nm (ε = 13.6 mM−1cm−1) using plate reader SpectraMax M5. The reaction mixture included 1 mM DNTB, 0.3 mM acetyl CoA, 1% sodium cholate, 200 μL of colon tissue homogenates. The reaction was initiated by adding 0.5 mM oxaloacetate. The rates were normalized to gram of tissue used to prepare the homogenate. The citrate synthase rates were used to normalize all mitochondrial rates to mitochondrial content. Cardiolipin was measured using the Abcam Cardiolipin Assay Kit (ab241036). Colon tissue homogenates were prepared and the assay was performed as outlined in the kit manual.
5.8. Individual mitochondrial enzymes
Complexes I, II, and III activities were measured using a Jasco UV-Vis spectrophotometer at 25°C following previously published methods25,26. Briefly, complex I activity was measured as the time-dependent oxidation of NADH at 340 nm using a measured extinction coefficient of 6.22 mM−1cm−1. The reaction mixture in a regular quartz cuvette contained 10 mM KPi, 5 mM MgCl2, 30 mM KCl, 1 mM EDTA, 75 mM Tris, pH 7.5, 100 μM Coenzyme Q1 (CoQ1), 5 μM antimycin A, and 100 μL colon tissue homogenates in a final volume of 2 mL. The reaction was initiated by adding 100 μM NADH and the fastest rates were obtained after NADH was added. Complex II activity was measured as the time-dependent oxidation of 2,6-dichloroindophenol sodium salt hydrate (DCPIP) at 600 nm using a measured extinction coefficient of 19.1 mM−1cm−1. The reaction mixture in a micro-cuvette contained 10 mM KPi, 5 mM MgCl2, 30 mM KCl, 1 mM EDTA, 75 mM Tris, pH 7.5, 20 mM succinate, 80 μM DCPIP, 1 mM KCN, 5 μM Antimycin A, 4 μM rotenone, and 20 μL of colon tissue homogenates in a final volume of 200 μL. Reactions were initiated by adding 50 μM decylubiquinone and the linear rates were obtained. Complex III activity was measured as the time-dependent reduction of cytochrome c at 550 nm using a measured extinction coefficient of 18.7 mM−1cm−1. The reaction mixture in a micro-cuvette contained 10 mM KPi, 5 mM MgCl2, 30 mM KCl, 1 mM EDTA, 75 mM Tris, pH 7.5, 3 mM KCN, 4 μM rotenone, 60 μM cytochrome c (LeeBio, cat # 192-10-1), 20 μL of colon tissue homogenates in a final volume of 200 μL. Reactions were initiated with 100 μM reduced decylubiquinone (Fisher Scientific, cat # NC1291121). The rate was linear for 1–2 min. Complex IV activity was measured at 37°C as the rate of oxygen consumption using an Oroboros FluoRespirometer. Ascorbate and N,N,N’,N’-Tetramethyl-p-phenylenediamine dihydrochloride (TMPD) were used to donate electrons to soluble cytochrome c. Reaction mixture contained 50 mM Tris, 8 mM KCl, 1mM EDTA, 16 μg/μl catalase, pH 7.4, 3 mM ascorbate, 0.3 mM TMPD, 25 μM cytochrome c. Reactions were initiated by addition of 100 μL of thawed colon tissue homogenates in a final volume of 2 mL. For each enzyme, the inhibited non-enzymatic background rates were subtracted from the enzymatic rates.
5.9. Antioxidant Measurements
The activity of the antioxidant enzymes glutathione peroxidase (GPx), glutathione S-transferase (GST), Glutathione reductase (GR), catalase (CAT), superoxide dismutase (SOD) and glutathione (GLT) levels were determined using colorimetric assay kits from Cayman Chemical following the manufacturer’s protocols.
5.10. Statistics
Data are expressed as mean ± SE. Comparisons between two groups were performed using unpaired Student t test. Comparisons between multiple experimental groups were made by a one-way ANOVA analysis. Statistical significance is indicated as a p value ≤ 0.05. All studies had an n ≥ 4. All analyses were performed with GraphPad Prism version 9.0.
Results
6.1. Indomethacin-induced IBD model.
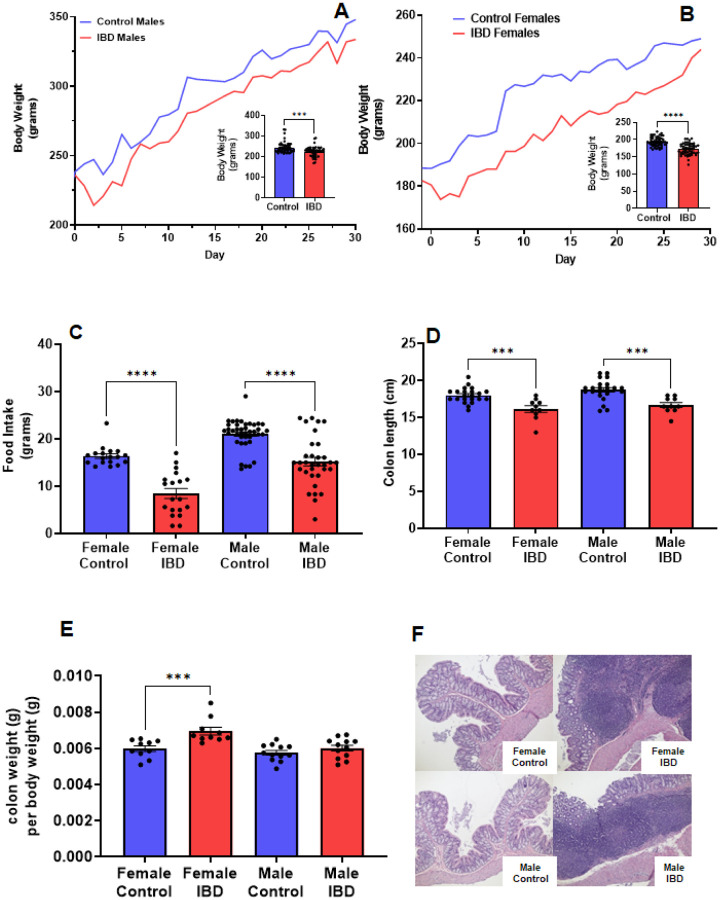
One of the key indicators of the induction of IBD by indomethacin is the decline of body weight at the peak of the disease, which is reported to be 2–3 days following treatment3. In our animals, we observed a significant decline of body weight on day 2 and day 3 (Fig. 1A&B) following injection with their body weight recovering over time. Males showed an 8% loss in body weight at the peak of the disease (Fig. 1A). Females showed a more significant loss of body weight (11%, Fig. 1B). This loss in body weight could be attributed to the significant decrease in food intake observed at the peak of the disease (Fig. 1C). Another indicator of indomethacin induced IBD is the decrease in colon lengths. In Fig. 1D, both male and female IBD rats showed a significant decrease in colon length at the peak of the disease. This decrease was returned to normal at day 7 and day 30 (Supplemental Fig. 1). Only the females showed a significant increase in colon weight to body weight ratio, indicating hypertrophy (Fig. 1E). Another indicator of indomethacin induced IBD is the increase in GI inflammation. In our animals, we observed a significant increase of inflammatory cell infiltration in the colons at the peak of the disease with females being higher (Fig. 1F). Taken together these results indicate that indomethacin induced an acute GI inflammatory response that peaked at day 2–3 following treatment.
Figure 1.
Indomethacin-induced IBD decrease body weight, food intake, and colon length while showing an increase in colon inflammation at day 2–3 following injections. The body weight of male (A) and female (B) rats was monitored daily for 30 days. The peak of the disease is at day 2–3. The inserts show a significant decrease in body weight at the peak of the disease for both males (p≤0.001) and females (p≤0.0001). (C) The food intake of these animals was also significantly decreased at the peak of the disease (p≤0.0001). (D) Colon length was decreased in both male and female IBD rats. (E) Colon weight divided by the body weight was used as an indicator for hypertrophy. (F) Histology showed an increase in inflammatory cell infiltration in males and females.
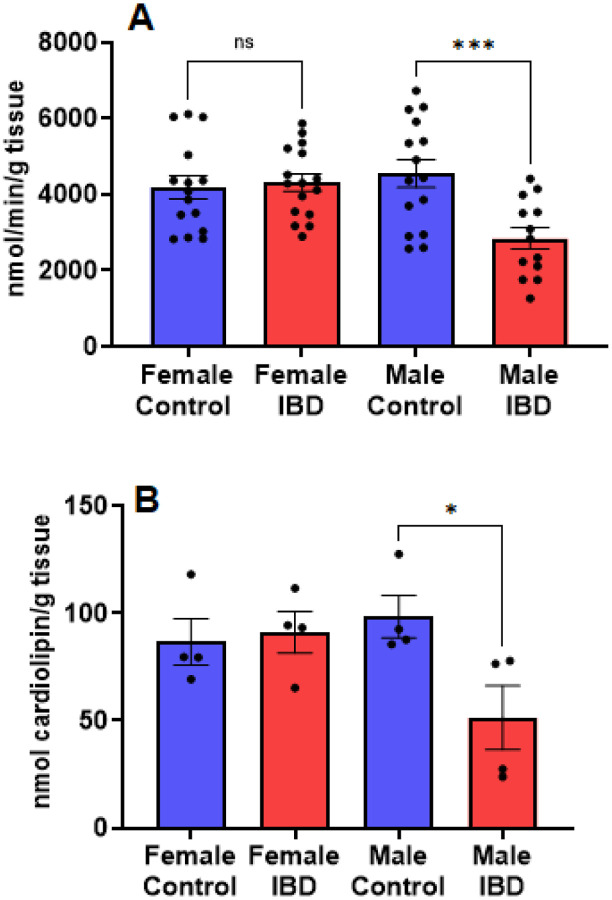
6.2. Mitochondrial content decreases in IBD male rats
Citrate synthase activity and cardiolipin levels were used as markers for mitochondrial content in the tissue homogenate samples27. Indomethacin-induced IBD resulted in a significant loss of citrate synthase activity in male colon samples but not females (Fig. 2A). A similar trend was observed when measuring cardiolipin levels (Fig. 2B). Therefore, we relied on citrate synthase activity to normalize mitochondrial activities to mitochondrial content. This normalization allows us to conclude that any observed differences in activity are not due to changes in the number of mitochondria in the colon.
Figure 2.
(A) Citrate synthase activity is a reliable marker of mitochondrial content. In our colon tissue homogenate samples, we saw a significant decrease in citrate synthase activity in males (p≤0.001), but not females. (B) Cardiolipin levels, another marker for mitochondrial content, showed a similar result (p≤0.05). Mitochondrial respiration was normalized to mitochondrial content using citrate synthase activity.
6.3. Intact mitochondrial respiration decreases in IBD female rats.
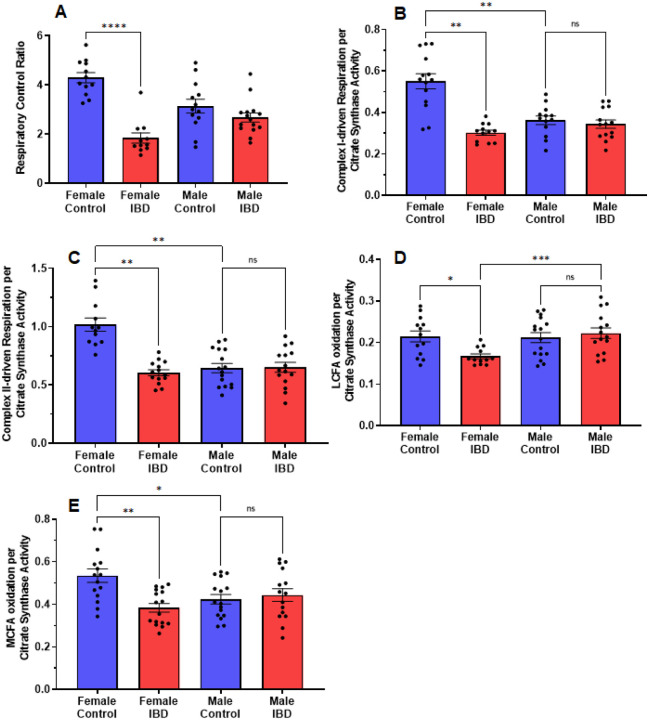
Our initial exploration showed the mitochondrial function was significantly decreased in females at the peak of the disease, but full activity returned at day 7 and 30 (Supplemental Fig. 2A) with no change on any day with the males (Supplemental Fig. 2B). Therefore, for the remaining experiments rats were used on day 2 or 3 following treatment. The quality of intact mitochondria is determined by measuring the respiratory control ratio (RCR) as described in the methods. The higher the ratio the more intact the mitochondria resulting in better production of ATP. For colon mitochondria, controls had an average RCR of 4 (Fig. 3A). IBD females showed a significant decrease in RCR to an average of 2 with no significant difference seen in IBD males (Fig. 3A).
Figure 3.
(A) The respiratory control ratio (RCR is an indicator of the quality of mitochondria. This was determined as outlined in methods. A significant decrease in RCR was seen only in females (p≤0.001), which suggests poorly coupled mitochondria. (B) Complex I-driven mitochondrial respiration (20 mM glutamate and 10 mM malate plus 2mM ADP) shows a significant decrease in females with IBD compared to controls (p≤0.01). No significant difference was observed in males with IBD compared to controls. Male controls were significantly lower compared to females (p≤0.05). (C) Complex II-driven mitochondrial respiration (20 mM succinate plus 2 mM ADP) shows a significant decrease in activity in females with IBD compared to controls (p≤ 0.01). No significant difference was observed in males with IBD compared to controls. Male controls were significantly lower compared to females (p≤ 0.05). (D) LCFA oxidation (0.5 mM Oleate plus 2 mM carnitine) shows a significant decrease in activity in females compared to controls (p≤0.05). No significant difference was observed in males. (E) MCFA oxidation (0.5 mM octanoate) shows a significant shows a significant decrease in activity in females with IBD compared to controls (p ≤ 0.01). No significant difference was observed in males. Male controls were significantly lower compared to females (p≤0.05).
When examining the rates of respiration, the colon mitochondria from female rats with IBD showed a significant decrease in complex I-driven respiration (Fig. 3B), complex II-driven respiration (Fig. 3C), LCFA oxidation (Fig. 3D), and MCFA oxidation (Fig. 3E) when normalized to mitochondrial content. However, the males showed no significant change when normalized to mitochondrial content. Interestingly, control females had significantly higher complex I and complex II-driven colonic mitochondrial respiration as well as MCFA oxidation compared to control males (Fig. 3B, C, and E).
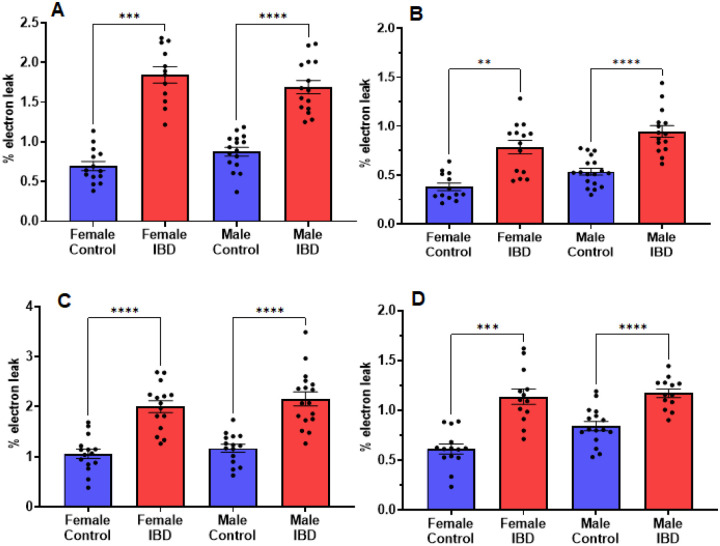
6.4. mtROS production increases in IBD rats
Mitochondrial ROS (mtROS) production was measured simultaneously with mitochondrial respiration. In both male and female IBD rats, mtROS was increased (Fig. 4). During complex I-driven respiration (Fig. 4A), complex II-driven respiration (Fig. 4B), and LCFA oxidation (Fig. 4C), mtROS was increased by roughly 2-fold for both IBD males and females. Using MCFA, females showed roughly a 2-fold increase in mtROS while males showed a 1.4-fold increase (Fig. 4D).
Figure 4.
Mitochondrial reactive oxygen species (mtROS) was measured simultaneously with the respiration rate presented in Figure 3. Amplex Red was used to assess the rate of mtROS production. During A. Complex I-driven respiration; B. Complex II-driven respiration; C. LCFA oxidation; and D. MCFA oxidation, mtROS production was significantly increased in both males and females with IBD compared to controls. (** = p ≤ 0.01; *** = p ≤ 0.001; **** = p≤ 0.0001).
6.5. IBD rats show alterations in individual mitochondrial enzymes
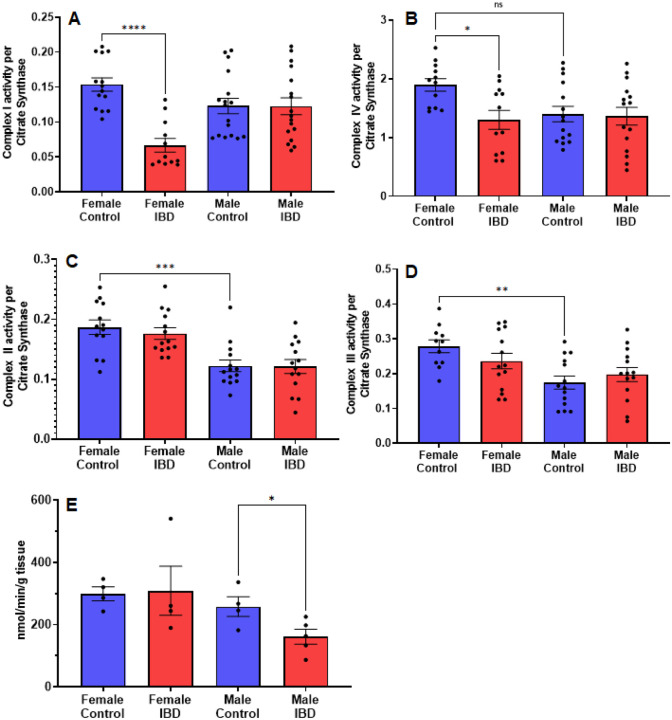
To determine what is causing the changes in colon mitochondrial function in our IBD rats, we explored the activity of the individual mitochondrial complexes of the electron transport chain and antioxidant enzymes. Colon mitochondrial complex I (Fig. 5A) and complex IV (Fig. 5B) activities were significantly decreased in females compared to controls with no change in complex II (Fig. 5C) or III activity (5D). The IBD males showed no significant changes for any of the complexes compared to controls (Fig. 5). Interestingly, female controls showed a significantly higher activity compared to males for complexes II, III, and IV (Fig. 5).
Figure 5.
Individual mitochondrial enzymes activities were measure to determine the location of mitochondrial dysfunction. (A) Complex I activity was significantly decreased in IBD females (p≤ 0.05) compared to controls, but not in males. (B) Complex IV activity showed a significant decrease in IBD females (p≤ 0.05) compared to controls. Again, males showed a lower control activity than females (p≤ 0.05). (C) Complex III activity showed a similar trend with no change in the IBD animals but with the control activity in males being significantly lower than females (p≤ 0.05). (D) Complex II activity showed no significant differences in IBD animals. However, males showed a significantly lower control activity than females (p≤ 0.05). (E). When measuring the antioxidant enzyme activities, IBD males showed a significant decrease in catalase activity (p≤ 0.05) compared to controls.
To determine why the IBD males have an increase in mtROS but no change in electron transport enzymes, we explored mtROS defense mechanisms. We measured superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR) and glutathione (GLT) levels, which are common components that are altered in disease states28. We saw no significant change in GR, GST, GPx, GLT, and SOD in both males and females (Supplemental Fig. 3). However, IBD males showed a significant decrease in CAT activity (Fig. 5E).
6.6. MitoTEMPO improves disease activity and mitochondrial function in IBD females
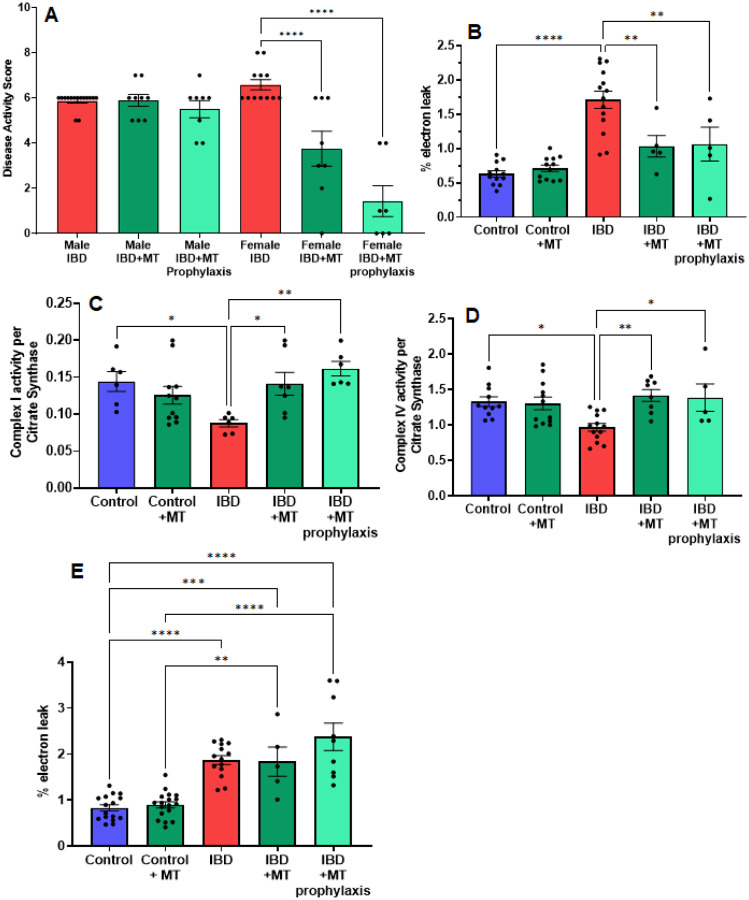
With the mitochondrial dysfunction mentioned above, targeting mitochondria to improve their activity may be a potential therapeutic option. We chose to begin our exploration of mitochondrial-targeted therapies by administering mitoTEMPO as described in methods. MitoTEMPO is a mitochondrial targeted superoxide mimetic. In IBD female rats, we found that the disease activity decreased with mitoTEMPO treatment (Fig. 6A). The prophylaxis protocol was the best at improving the disease. MitoTEMPO also lowered the colon mtROS production in female IBD rats (Fig. 6B) and improved complex I (Fig. 6C) and complex IV activity (Fig. 6D). However, mitoTEMPO did not improve the disease in male rats (Fig. 6A). In Fig. 6E, there is no change in colon mtROS with mitoTEMPO with the IBD male rats.
Figure 6.
(A) The disease activity score was calculated as outlined in the methods. IBD animals received the mitochondrial-targeted therapy, MitoTEMPO (MT), daily follow indomethacin injects until the end of the experiment (MT treatment) or daily before indomethacin injections until the end of the experiment (MT prophylaxis treatment). Males showed no significant change when treated with MT. However, the females saw a decrease in disease activity with MT treatment (p≤ 0.05) and MT prophylaxis treatment (p≤0.0001), with the prophylaxis treatment being more significant. (B) IBD females that received MT treatment (p≤ 0.05) and MT prophylaxis treatment (p ≤ 0.01) showed a significant decrease in mtROS production compared to IBD females without. (C) Complex I activity was increased in IBD females that received MT treatment (p≤ 0.05) (D) Complex IV activity was increased in IBD females that received MT treatment (p ≤ 0.01) and MT prophylaxis treatment (p≤ 0.05). (E). MitoTEMPO treatment does not improve mtROS production in IBD male rats.
Discussion
Clinical studies have shown sex differences in onset, severity, and extra intestinal manifestation in IBD1,2,19. This is likely due to the hormonal change and studies have shown at puberty there is an increase in IBD diagnosis in females than males. We chose to use rats at the age of puberty to explore this effect in the indomethacin induced IBD rat model. We found that two injections 24 hours apart induced acute GI inflammation resulting in weight loss two days after injections in both male and female rats. IBD female rats showed a more significant loss in body weight, a significant increase in colon hypertrophy, and slightly higher disease activity score compared to IBD male rats. These results indicate that there are slight differences in the severity of the disease between males and females, which is similar to the observed clinical variations between males and females.
Mitochondrial function is important to maintaining epithelial barrier function11. To begin our exploration into mitochondrial function in our IBD model, we measured mitochondrial content as either citrate synthase activity or cardiolipin levels. Cardiolipin has been reported as the most reliable measure of mitochondrial content followed by citrate synthase27. What we have demonstrated here is that both measures show the same trend. Due to these similarities, we used citrate synthase activity to normalize to mitochondrial content. IBD male rats have a significant decrease in colon mitochondrial content compared to controls while females showed no change in mitochondrial content in the IBD rats. These results indicate that the males likely have improved mitophagy mechanisms compared to females.
Mitochondrial oxidative phosphorylation (OXPHOS) is important for generating ATP. ATP production is essential to maintaining epithelial barrier function. Electrons enter the electron transport chain through two different pathways. One pathway is by the oxidation of NADH by complex I. The other is the oxidation of succinate to fumarate by complex II. Both complex I and II will transfer electrons to a membrane bound electron acceptor ubiquinone to generate ubiquionol. Electrons are then transferred through complex III to cytochrome c. Cytochrome c will bind and transfer an electron to complex IV, which ultimately transfers electrons to oxygen to generate water. Complex I, III, and IV are proton pumps which pump protons from the matrix to the inner membrane space to generate a proton motive force that is utilized by ATP synthase to convert ADP to ATP. Defects in OXPHOS have been implicated in various diseases, especially those related to inflammation29. Respiratory control ratios (RCRs) are an indication of how efficiently the mitochondria are generating ATP. Female IBD rats showed a significant decrease in colonic mitochondrial RCR values. When normalized to citrate synthase, only the female IBD rats showed a decrease in intact colonic mitochondrial respiration. These results indicate that protons are leaking through the inner mitochondrial membrane by a pathway other than ATP synthase, resulting in lower ATP production and lower OXPHOS capacity. The loss of ATP in IBD female colon mitochondria will impair the ability of epithelial cells to maintain barrier function resulting in GI inflammation4.
When mitochondrial oxidative phosphorylation is decreased, mitochondrial ROS (mtROS) production is increased. This occurs through the production of superoxide, which will contribute to cellular oxidative stress and is linked to diseases and epithelial barrier dysfunction4,30. Both male and female IBD rats showed a significant increase in the production of mtROS compared to control regardless of substrate. This increase in mtROS will contribute to the gut dysbiosis, lower ATP production by the epithelial cells, and epithelial barrier dysfunction resulting in GI inflammation4. There are two significant locations for mtROS production from OXPHOS, complex I and complex III. Complex I will produce mtROS when the FMN site is reduced to FMNH2 leading to a high NADH/NAD+ ration, which increases the likelihood that electrons will be transferred to O2 instead of through the iron-sulfur centers31. Complex I and III will produce mtROS if there is an increase in the highly reactive semiquionone, which will quickly react with O2 to produce mtROS31. Any inhibition in the electron transport chain will result in an increase in the reduction of these intermediates that will increase the probability of mtROS production. In the IBD female colon mitochondria, we found that complex I and IV activities are decreased, which would lead to an increase in both FMH2 and the semiquinone, ultimately leading to the increase in mtROS.
Although mtROS may be produced there is a balance between antioxidants and mtROS. However, when the balance between antioxidants and ROS species is disrupted, oxidative stress can occur28. Assessment of the antioxidant status is correlated to the extent of oxidative stress. Redox homeostasis is regulated either enzymatically or non-enzymatically. Superoxide dismutase, catalase, glutathione peroxidase, glutathione S-transferase, glutathione reductase and glutathione levels are common components that are altered in disease states28. When we explored these antioxidant mechanisms, the only one that showed a significant difference was catalase. Catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen32. In colons from male IBD rats, we found that catalase was significantly decreased. The amplex red assay measures the presence of H2O2. Therefore, since the male IBD rats have a decrease in catalase activity there will be an increase in H2O2.
Based on these results, using a mitochondrial-targeted therapy is a feasible option for treatment. MitoTEMPO is a mitochondria-targeted superoxide dismutase mimetic that possesses superoxide and alkyl radical scavenging properties33.1 This compound combines the antioxidant piperidine nitroxide TEMPO with the lipophilic cation triphenylphosphonium, which allows it to pass through lipid bilayers and accumulate in mitochondria33. Mitochondrial targeting of superoxide scavenging via mitoTEMPO has been examined for potential therapeutic benefit to a variety of mitochondrial dysfunctions arising from excessive reactive oxygen species34–36. IBD disease activity was improved with mitoTEMPO treatment in female rats but not males. As expected mitoTEMPO decreased mtROS in the colon of IBD females rats and it also improved the activity of complex I and IV. However, mitoTEMPO increased mtROS in males. This is likely due to mitoTEMPO converting superoxide to H2O2. However, the males have lower catalase activity leading to an increase in the levels of H2O2. A mitochondrial-targeted catalase mimetic will potentially be a better treatment option for the males but creating a catalase mimetic can be challenging. However, a study in 2016 designed a nanosystem based on Tempol and a biocompatible B-cyclodextrin-derived material23. The Tempol serves as a SOD mimetic, while the nanocontainer is mainly composed of a hydrogen peroxide-eliminating material that functions as a catalase-mimicker23. A treatment like this may work better for our male IBD colon mitochondrial function and will be explored in the future. Another feasible option is the supplementation with MCFAs. MCFAs have been shown to lower mtROS in other disease models26. In this study, the males produce less mtROS with MCFA than other mitochondrial substrates. We will also explore other mitochondrial-targeted therapies in the future.
Interestingly, our findings also highlight sex differences in colon mitochondrial function. There have been several studies exploring the effect of sex hormones on mitochondrial function37. Estrogen acts through the estrogen receptor β (ERβ) to increase mitochondrial DNA (mtDNA)–encoded transcription of cytochrome oxidase subunits I, III, and IV; increase respiration capacity; elevate antioxidant activity; and inhibit apoptosis22. Studies have shown that an increase in the androgen receptor (AR) negatively regulates the expression, assembly, integrity, and function of individual mitochondrial complexes resulting in decreased mitochondrial OXPHOS21. The animals used in this study are at the age of puberty for rats. Therefore, sex hormones are on the rise, which will increase the chances of observing sex differences. When comparing control males and females, we observed differences in colon mitochondrial function. Control males showed a significant decrease in complex I-driven respiration, complex II-driven respiration and MCFA oxidation compared to control females. Control males also showed a significant decrease in complex II, III, and IV activity compared to control females. This highlights the importance of identifying sex differences in mitochondrial function in disease models. In future studies, we will alter the sex hormone status in our IBD animals to further determine the effects of sex hormones on mitochondrial function.
In conclusion, we have shown that female SD rats have an increase in disease severity compared to males. We have also demonstrated that there are differences in colon mitochondrial function between male and female SD rats. This may contribute to the differences in disease severity. While both male and female rats have increases in mtROS production, the cause for the increase is different between males and females. In females, complex I and IV have decreased activity resulting in the potential for increased mtROS. In males, catalase activity is decrease which will result in an increase of H2O2. Targeting mitochondria with the superoxide mimetic (mitoTEMPO) improves the disease activity in females, decreases mtROS, and improves complex I and IV activity. However, it did not prove to be a viable treatment option for males.
Perspectives And Significance.
Our findings have an important clinical relevance. Our study highlights the need for studying sex differences in disease models, mitochondrial function studies, and drug treatment. Our study provides a better understanding of the role mitochondria in the development of IBD but more work is needed using other IBD models. Our study opens an avenue for future studies to understand the role of sex hormones in the regulation of mitochondrial function and to investigate strategies to re-establish normal mitochondrial function that could provide options for preventive and therapeutic interventions for IBD.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM104357 and the National Heart, Lung and Blood Institute under Award Number P01HL51971. Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM121334. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- IBD
inflammatory bowel disease
- GI
gastrointestinal
- CD
Crohn’s disease
- UC
ulcerative colitis
- CRC
colorectal cancer
- DSS
dextran sulfate sodium
- PAMPs
pathogen-associated molecular patterns
- mtROS
mitochondrial reactive oxygen species
- RCR
respiratory control ratio
- SOD
superoxide dismutase
- SO
superoxide
- MCFA
medium-chain fatty acid
- LCFA
long-chain fatty acid
- GPx
glutathione peroxidase
- GST
glutathione s-transferase
- GR
glutathione reductase
- CAT
catalase
- OXPHOS
oxidative phosphorylation
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental protocols were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 8th Edition, 2011, and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Files
Contributor Information
Ngoc Hoang, The University of Mississippi Medical Center.
Karen Brooks, The University of Mississippi Medical Center.
Kristin Edwards, The University of Mississippi Medical Center.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its additional information les.
References
- 1.Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-Risk Behaviors and Chronic Conditions Among Adults with Inflammatory Bowel Disease — United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep. 2018;67:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M’Koma AE. Inflammatory bowel disease: An expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Med Hyg (Geneve). 2001;59:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard JWO, Towarnicki SG. Mitochondria, the gut microbiome and ROS. Cell Signal. 2020;75:109737. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes. 2020;11:285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sifroni KG, et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol Cell Biochem. 2010;342:111–5. [DOI] [PubMed] [Google Scholar]
- 7.Ussakli CH, et al. Mitochondria and tumor progression in ulcerative colitis. J Natl Cancer Inst. 2013;105:1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak EA, Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol. 2015;3:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol. 2014;184:2516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaloian S, et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut. 2020;69:1939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger E et al. Mitochondrial function controls intestinal epithelial stemness and proliferation.Nat. Commun. 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santhanam S, et al. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2158–68. [DOI] [PubMed] [Google Scholar]
- 13.Smith SA et al. Mitochondrial dysfunction in inflammatory bowel disease alters intestinal epithelial metabolism of hepatic acylcarnitines.J. Clin. Invest. 131, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolli VK, Natarajan K, Isaac B, Selvakumar D, Abraham P. Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol. 2014;33:1051–65. [DOI] [PubMed] [Google Scholar]
- 15.Santhanam S, et al. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2158–68. [DOI] [PubMed] [Google Scholar]
- 16.Restivo NL, Srivastava MD, Schafer IA, Hoppel CL. Mitochondrial dysfunction in a patient with crohn disease: Possible role in pathogenesis. J Pediatr Gastroenterol Nutr. 2004;38:534–8. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DN, et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut. 2020;69:1928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heller S, et al. Reduced mitochondrial activity in colonocytes facilitates AMPKα2-dependent inflammation. FASEB J. 2017;31:2013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendy A, Goodman IPE. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masayuki Saruta MD, Stephan R, Targan MD, Ling Mei MD, Andrew F, Ippoliti MD, Kent D, Taylor MD, Jerome I, Rotter M. High Frequency Haplotypes in the X Chromosome Locus TLR8 are Associated with Both CD and UC in Females. Inflamm Bowel Dis. 2009;15:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajpai P, Koc E, Sonpavde G, Singh R, Singh KK. Mitochondrial localization, import, and mitochondrial function of the androgen receptor. J Biol Chem. 2019;294:6621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH. Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann N Y Acad Sci. 2015;1350:52–60. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials. 2016;105:206–21. [DOI] [PubMed] [Google Scholar]
- 24.Porras M, Martín MT, Soler M, Vergara P. Intestinal motor disorders associated with cyclical bacterial overgrowth in a rat model of enteritis. Am J Physiol - Gastrointest Liver Physiol. 2004;287:58–64. [DOI] [PubMed] [Google Scholar]
- 25.Shirey K, Stover KR, Cleary J, Hoang N, Hosler J. Membrane-Anchored Cyclic Peptides as Effectors of Mitochondrial Oxidative Phosphorylation. Biochemistry. 2016;55:2100–11. [DOI] [PubMed] [Google Scholar]
- 26.Edwards KS, et al. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res Cardiol. 2018;113:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen S, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell. Longev. 2019, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Missiroli S et al. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders.J. Clin. Med. 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimmich GA. Preparation and Properties of Mucosal Epithelial Cells Isolated from Small Intestine of the Chicken. Biochemistry. 1970;9:3659–68. [DOI] [PubMed] [Google Scholar]
- 31.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma I, Ahmad P, Catalase. A Versatile Antioxidant in Plants. Oxidative Damage to Plants Antioxid Networks Signal. 2014;131–48. 10.1016/B978-0-12-799963-0.00004-6. [DOI] [Google Scholar]
- 33.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalova AE, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang HL, Sedlic F, Bosnjak Z, Nilakantan V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic Biol Med. 2010;49:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang HL, et al. Partial attenuation of cytotoxicity and apoptosis by SOD1 in ischemic renal epithelial cells. Apoptosis. 2009;14:1176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultanova RF, Schibalski R, Yankelevich IA, Stadler K, Ilatovskaya DV. Sex differences in renal mitochondrial function: A hormone-gous opportunity for research. Am J Physiol - Ren Physiol. 2020;319:F1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional information les.