Abstract
Objectives:
To estimate the association between safety perception on vaccine acceptance and adoptions of risk mitigation strategies among dental health care workers (DHCWs).
Methods:
A survey was emailed to DHCWs in the New Jersey area from December 2020 to January 2021. Perceived safety from regular SARS-CoV-2 testing of self, coworkers, and patients and its association with vaccine hesitancy and risk mitigation were ascertained. Risk Mitigation Strategy (RiMS) scores were computed from groupings of office measures: 1) physical distancing (reduced occupancy, traffic flow, donning of masks, minimal room crowding), 2) personal protective equipment (fitted for N95; donning N95 masks; use of face shields; coverings for head, body, and feet), and 3) environmental disinfection (suction, air filtration, ultraviolet, surface wiping).
Results:
SARS-CoV-2 testing of dental professionals, coworkers, and patients were perceived to provide safety at 49%, 55%, and 68%, respectively. While dentists were least likely to feel safe with regular self-testing for SARS-CoV-2 (P < 0.001) as compared with hygienists and assistants, they were more willing than hygienists (P = 0.004; odds ratio, 1.79 [95% CI, 1.21 to 2.66]) and assistants (P < 0.001; odds ratio, 3.32 [95% CI, 1.93 to 5.71]) to receive the vaccine. RiMS scores ranged from 0 to 19 for 467 participants (mean [SD], 10.9 [2.9]). RiMS scores did not significantly differ among groups of DHCWs; however, mean RiMS scores were higher among those who received or planned to receive the COVID-19 vaccine than those with who did not (P = 0.004). DHCWs who felt safer with regular testing had greater RiMS scores than those who did not (11.0 vs. 10.3, P = 0.01).
Conclusions:
Understanding DHCWs’ perception of risk and safety is crucial, as it likely influences attitudes toward testing and implementation of office risk mitigation policies. Clinical studies that correlate risk perception and RiMS with SARS-CoV-2 testing are needed to demonstrate the effectiveness of RiMS in dental care settings.
Knowledge Transfer Statement:
Educators, clinicians, and policy makers can use the results of this study when improving attitudes toward testing and implementation of risk mitigation policies within dental offices, for current and future pandemics.
Keywords: COVID-19, dental offices, safety, infection control, vaccination hesitancy, Personal Protective Equipment
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to a worldwide health crisis with considerable morbidity and mortality coupled with a significant impact on global safety and social and economic stability (Bilinski and Emanuel 2020; Pak et al. 2020; Coker et al. 2021). In the field of dentistry and oral health care delivery, this pandemic has highlighted the risk of viral transmission and acquisition with a direct consequence on dental patients and dental health care workers (DHCWs; Meng et al. 2020). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for COVID-19, is transmitted primarily from person to person (Wang et al. 2020). According to the Centers for Disease Control and Prevention (CDC), SARS-CoV-2 is transmitted by exposure to infectious respiratory fluids from 1) inhalation of very fine respiratory droplets and aerosol particles; 2) deposition of respiratory droplets and particles on exposed mucous membranes in the mouth, nose, or eye by direct splashes and sprays; and 3) touching mucous membranes with hands that have been soiled either directly by virus-containing respiratory fluids or indirectly by touching surfaces with virus on them. These contact routes are of particular concern to DHCWs as many dental procedures require close contact and require the use of aerosol-generating equipment. Recent studies show that SARS-CoV-2 colonizes saliva (Huang et al. 2021), periodontal tissues and plaque (Gomes et al. 2021), as well as gingiva-crevicular fluid (Gupta et al. 2021) at high viral load levels. Furthermore, the extent to which dentally generated aerosols linger within operatories and its impact on infectivity are unknown (Gallagher et al. 2020; Ge et al. 2020; Koletsi et al. 2020; Peng et al. 2020; Sommerstein et al. 2020; Xu et al. 2020). It should also be considered that the oral cavity is a preferred infection target, since the oral mucosa is a site of high expression of ACE2 (angiotensin-converting enzyme 2), which is the SARS-CoV-2 receptor on human cells (Sakaguchi et al. 2020; Xu et al. 2020; Yan et al. 2020). The increasing incidence of viral variants, which may spread more rapidly than the original strain (CDC 2021b; Paul et al. 2021), compounds potential risks and/or uncertainties regarding SARS-CoV-2 infection rates to the dental practice environment.
Studies suggest that clinical DHCWs (dentists, dental assistants, and hygienists) have similar or lower risk (Estrich et al. 2020; Hughes et al. 2020; Al-Kuwari et al. 2021; Araujo et al. 2021; Estrich et al. 2021; Jungo et al. 2021) or heightened risk (Sarapultseva et al. 2021) of acquiring or transmitting SARS-CoV-2 as compared with other health care workers. While large seroepidemiologic studies designed to comparatively quantify the risk of SARS-CoV-2 infection among DHCWs are lacking, it is clear that dental patients can present with unrecognized COVID-19 (Conway et al. 2021; Lamberghini and Testai 2021; Palla and Callahan 2021). As clinical presentation of COVID-19 can include minimal or no symptoms (Day 2020), modeling suggests that weekly testing of asymptomatic health care workers reduced onward transmission (Cui et al. 2021; Kim and Koo 2021). Despite current CDC (2020) guidelines for the dental management of patients and the strategies recommended to make dentists safe via personal protective equipment and other mitigation strategies, the risk presented in dental care settings presents clear challenges to dental care provision worldwide. Vaccine acceptance and continued implementation of effective risk mitigation practices are fundamental to establishing dental practices that are safe for DHCWs and patients alike.
Recent surveys conducted to evaluate safety perception among health care workers show that about 35% agree or strongly agree that their workplace units are at higher risk of infection (Lee et al. 2021). Primary prevention of infection has thus been a priority within medical and dental practices, by diagnosis (regular testing) and prevention (vaccine and physical safety measures). There has been significant scale-up of SARS-CoV-2 virus and antibody testing worldwide, and several practices implement regular testing of DHCWs and patients (CDC 2021a; Greenwall et al. 2021; Shirazi et al. 2021). Similarly, due to global efforts, significant developments have been made with regard to vaccine discovery (with 3 Food and Drug Administration–approved US-based vaccines as of May 2021). Despite this progress, vaccine availability and rollout have been slow as anecdotal and small numbers of severe side effects (e.g., blood clots) to SARS-CoV-2 vaccine have contributed to hesitancy among medical staff (Gharpure 2020; Lazarus et al. 2020).
As DHCWs were among the first subgroups to gain access to vaccines, it is important to assess the attitudes toward the current rollout and implementation of risk mitigation practices and CDC-recommended guidelines. Understanding risks associated with transmission during dental care delivery, identifying factors associated with vaccine uptake, and implementing mitigation strategies are critical to improving patient and provider safety and future pandemic preparedness. Specifically, a comprehensive understanding of DHCWs’ safety perception (with frequent testing) and its impact on adoption of biologic and physical protective practices is a key component of improving patient safety and access to ongoing oral health care in current and future pandemics. Therefore, with data from a survey to dental professionals in the New Jersey metropolitan area, the objective of this study was to identify risk factors (including safety perception) that are associated with DHCWs’ vaccine acceptance and implementation of risk mitigation strategies (RiMS) during the COVID-19 outbreak. We hypothesized that feeling safe with regular testing will be positively associated with vaccine acceptance and implementing mitigation practices.
Methods
Design and Setting
We conducted a cross-sectional survey to assess the influence of DHCWs’ perception on behavior with respect to COVID-19 vaccine hesitancy and implementation of RiMS within the dental office. An online questionnaire was created with electronic data capture tools hosted at Rutgers University (REDCap). This study was approved by the Rutgers University Institutional Review Board (Pro2020003012). Participants provided informed consent prior to completing the survey. Data were collected anonymously, and no personal identifying information was collected.
Sampling
A convenience snowball sampling design was implemented. The survey tool was distributed via email to dental office groups in the New Jersey area (including neighboring metropolitan areas of New York, Pennsylvania, and Connecticut) based on a convenience sample of dentists, dental hygienists, assistants, and other personnel who receive continuing education program materials from the Rutgers School of Dental Medicine. Participants were not excluded if they did not live in New Jersey. Multiple personnel from the same office were allowed to complete the survey. Data were collected between December 2020 and January 2021.
Participants
All adults (>18 y of age) working in a dental clinic regardless of patient care contact and role in health care settings were eligible to participate in the study. We, however, excluded all administrative staff from these analyses.
Measures
All variables were based on self-report via survey responses. DHCWs were classified into 3 major groups based on their role in the clinic: dentists, hygienists, or assistants. Demographic information included age, sex, race, and type of dental practice (private, corporate, hospital, or academic institution). Participants were asked if they, their family members, friends, or coworkers were ever tested for SARS-CoV-2 or ever diagnosed as being positive.
To assess perception of safety from regular testing, DHCWs were asked to provide their perception of safety from regular testing of self, coworkers, or patients. Perceptions were collected via a rating scale that has been validated in many environments (Aljabri et al. 2020), and adopted by the Worker Safety Perception Survey (Hayes et al. 1998). Specifically, perceived safety from regular testing was gauged by the question “Would you feel safer if you had weekly COVID-19 testing?” with a yes, no, or not sure response. Similar questions were also asked with regard to feeling safe with testing of coworkers or patients. Perceived safety with regular SARS-CoV-2 testing of self, coworker, or patient was therefore ascertained.
Outcomes
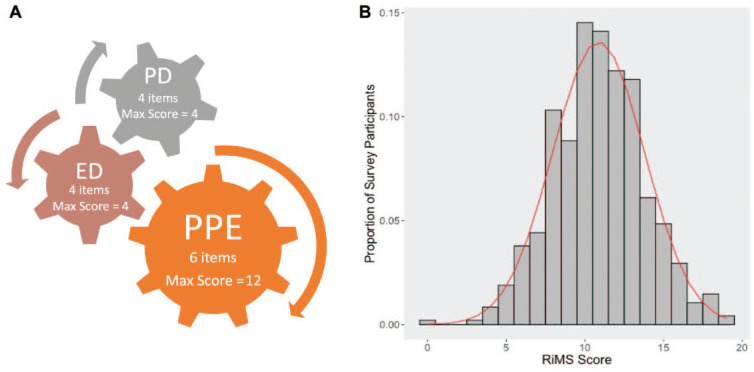
The primary outcomes of this survey were implementation of safety measures, be it biologic or physical/nonbiologic. Acceptance of biologic protection by COVID-19 vaccine was assessed by the following question: “Have you received or do you plan receive the COVID-19 vaccine?” Responses (yes, no, or not sure) were used to determine whether DHCWs were willing to take the COVID-19 vaccine or not. Implementation of physical measures of protections was assessed via a computed RiMS score. This score was derived from 3 domains of physical strategies implemented by offices to mitigate risk of transmission or acquisition (Figure). Responses to survey questions based on specific RiMS scores were divided into 3 domains, and item checklists and scale were calculated as a sum of grouped checklist items in each domain. The domains are as follows:
Figure.
Components and distribution of the risk mitigation strategy (RiMS) score. (A) Components of the RiMS score include the following: 1) physical protective equipment (PPE) that is protective to the patient and the dental health care worker (fitted for N95, use of N95, face shields, head covering, gowns, shoe covering—all or most of the time; maximum score, 12), 2) physical distancing (PD; reduced occupancy, directional flow of traffic, donning of masks, minimal waiting room crowding; maximum score, 4); and 3) environmental disinfection (ED; suction, air filtration, surface wiping, ultraviolet; maximum score, 4). (B) Distribution of RiMS scores based on survey data from 475 dental health care workers. Data follow a near-normal distribution based on the 3 domains of the RiMS score (mean [SD], 10.9 [2.9]; range, 0-19).
Physical protective equipment: being fitted for N95 masks, donning N95 masks, and using face shields and coverings for head, body, and feet—all or most of the time (maximum score, 12; 2 per unit)
Physical distancing: implementation of reduced occupancy and traffic flow, patients’ donning of masks, and minimal waiting room crowding practices within the clinic—all or most of the time (maximum score, 4; 1 per unit)
Environmental disinfection: use of suction, air filtration, surface wiping, and ultraviolet light—all or most of the time (maximum score, 4; 1 per unit).
To gain additional insight into these outcomes, we evaluated the relationship between vaccination acceptance and implementation of nonbiologic RiMSs. The association between DHCWs’ demographics and categories of safety perception and safety measures was also assessed. Self-perceived safety and its association with RiMS score and vaccine uptake/acceptance were evaluated via linear and logistic regression models, respectively. Sensitivity analyses based on sex subgroups were conducted to evaluate any sex-specific relationships.
R programming language within R Studio (version 3.6.3; R Foundation for Statistical Computing) was used to perform all univariate analyses and multivariable modeling. The statistical significance threshold was P < 0.05.
Results
We received 604 responses from all DHCWs. After administrative, clerical, and managerial staff were excluded, 475 dentists, hygienists, and assistants with partially complete or complete responses were included in the analysis: 257 (54%) dentists, 165 (35%) dental hygienists, and 53 (11%) dental assistants. The majority of survey participants were non-Hispanic White (n = 396, 83%) and from private practices (n = 415, 87%). Table 1 highlights the demographic characteristics of survey participants by DHCW category. As compared with other DHCWs, survey respondents who were dentists were significantly older and more likely to be male and of Asian race. Dental assistants who participated in this survey were more likely to be Hispanic and less likely to be involved in private practice. With the exception of 2 respondents, all hygienists and dental assistants were female.
Table 1.
Demographic Characteristics of Survey Participants.
| Participants, n (%) | P Valuea | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (N = 475) | Dentists (n = 257) | Hygienists (n = 165) | Assistants (n = 53) | P Valueb | Dentists vs. Hygienists | Dentist vs. Assistants | Hygienists vs. Assistants |
| Age, y, mean (SD) | 53.6 (11) | 56.8 (11) | 50.1 (12) | 49.8 (9) | <0.001 | <0.001 | <0.001 | 0.86 |
| Sex | 0.0005 | <0.001 | <0.001 | 0.18 | ||||
| Male | 183 (38) | 182 (71) | 1 (1) | 0 (0) | ||||
| Female | 289 (61) | 73 (28) | 164 (99) | 52 (98) | ||||
| Race | 0.01 | 0.03 | 0.13 | 0.22 | ||||
| Caucasian/White | 396 (83) | 206 (80) | 147 (89) | 43 (81) | ||||
| African American | 10 (2) | 6 (2) | 4 (2) | 0 (0) | ||||
| Asian | 29 (6) | 24 (9) | 3 (2) | 2 (4) | ||||
| Other | 38 (8) | 21 (8) | 10 (6) | 7 (16) | ||||
| Ethnicity | 0.01 | 0.34 | 0.004 | 0.03 | ||||
| Hispanic | 35 (11) | 13 (5) | 12 (7) | 10 (19) | ||||
| Non-Hispanic | 425 (89) | 234 (91) | 150 (91) | 41 (77) | ||||
| Type of practice | 0.07 | 0.54 | 0.007 | 0.30 | ||||
| Private practice | 415 (87) | 230 (90) | 143 (87) | 42 (79) | ||||
| Corporate practice | 25 (5) | 12 (5) | 10 (6) | 3 (6) | ||||
| Academic institution | 15 (3) | 10 (4) | 4 (2) | 1 (2) | ||||
| Other | 20 (4) | 5 (2) | 8 (4) | 7 (13) | ||||
| Residential community | 0.78 | 0.75 | 0.47 | 0.68 | ||||
| Urban | 37 (8) | 23 (9) | 12 (7) | 2 (4) | ||||
| Semiurban | 397 (84) | 215 (84) | 137 (83) | 45 (85) | ||||
| Rural | 37 (8) | 17 (7) | 15 (9) | 5 (9) | ||||
| Ever tested for SARS-CoV-2 | 327 (69) | 156 (61) | 131 (79) | 40 (76) | 0.003 | 0.0001 | 0.05 | 0.55 |
| Ever diagnosed with COVID-19 | 25 (5) | 7 (3) | 11 (7) | 8 (15) | 0.001 | 0.06 | 0.0006 | 0.07 |
| Safety perception with regular testing | ||||||||
| Self | 229 (48) | 96 (37) | 100 (61) | 33 (65) | <0.001 | <0.001 | 0.001 | 0.96 |
| Coworker | 259 (55) | 120 (47) | 103 (62) | 36 (71) | <0.001 | 0.002 | 0.008 | 0.57 |
| Patients at start of procedure | 325 (68) | 159 (62) | 122 (74) | 44 (83) | 0.002 | 0.02 | 0.006 | 0.24 |
| Any testing | 349 (74) | 171 (67) | 131 (79) | 47 (88) | <0.001 | 0.007 | 0.003 | 0.19 |
| All testing | 205 (43) | 87 (34) | 90 (55) | 28 (53) | <0.001 | <0.001 | 0.01 | 0.95 |
Bold indicates P < 0.05.
Pairwise comparison.
Overall comparison.
Prevalence of SARS-CoV-2 Testing Differed by DHCW Role
Dentists were least likely to test for SARS-CoV-2 (Table 1), as compared with dental hygienists and assistants. Regardless of age and sex, dentists had lower odds for testing than hygienists (P < 0.001; odds ratio [OR], 1.79 [95% CI, 1.21 to 2.66]) and dental assistants (P < 0.001; OR, 3.32 [95% CI, 1.93 to 5.71]). The lowest likelihood of SARS-CoV-2 testing observed among dentists could also explain their lower COVID-19 prevalence in our study, particularly when compared with assistants (P < 0.001).
Safety Perception with Regular Testing
Regular SARS-CoV-2 testing of self, coworker, and patient resulted in perceptions of safety in 49%, 55%, and 68%, respectively. Safety perception with regular testing of self, coworker, or patient differed across DHCWs. Specifically, dentists were least likely to feel safe with regularly self-testing for SARS-CoV-2 as compared with dental hygienists (P < 0.001) and assistants (P < 0.001). There was moderate evidence of association even in adjusted analyses with sex and age: dentists versus hygienists (OR, 0.95 [95% CI, 0.33 to 1.08]; P = 0.09) and dentists versus assistants (OR, 0.46 [95% CI, 0.20 to 0.99]; P = 0.05). There were no perception differences between hygienists and assistants. In general, males had lower odds of feeling safe with regular testing (OR, 0.56 [95% CI, 0.30 to 1.05]; P = 0.06).
Factors Associated with Vaccine Acceptance
Three-quarters of DHCWs (76%) expressed vaccine acceptance, with dentists reporting the highest acceptance rates as compared with other DHCWs. Factors associated with vaccine acceptance are presented in Table 2. Data showed that 87% of dentists were likely to take the vaccine, while only 66% of hygienists and 52% of dental assistants reported willingness to take the vaccine. This difference was significantly different, with dentists more willing than hygienists (P = 0.004; OR, 1.79 [95% CI, 1.21 to 2.66]) and dental assistants (P < 0.001; OR, 3.32 [95% CI, 1.93 to 5.71]). In conclusion, safety perception with regular testing was not associated with vaccine acceptance, although age was positively associated with vaccine acceptance.
Table 2.
Logistic Regression Analyses: Factors Associated with Vaccine Uptake among Survey Participants.
| Vaccine Acceptance, n (%) | Unadjusted Analyses | Adjusted Analysesa | |||||
|---|---|---|---|---|---|---|---|
| Factor | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Model 1 | |||||||
| DHCWs | |||||||
| Dentists | 225 (87) | 6.43 | 3.31 to 12.58 | <0.001 | 7.74 | 3.01 to 21.70 | <0.001 |
| Hygienists | 109 (66) | 1.67 | 0.88 to 3.16 | 0.11 | 1.71 | 0.86 to 3.37 | 0.12 |
| Assistants | 28 (53) | Reference | Reference | ||||
| Age | — | 1.06 | 1.04 to 1.08 | <0.001 | 1.06 | 1.04 to 1.09 | <0.001 |
| Sex | |||||||
| Male | 161 (88) | 3.45 | 2.09 to 5.92 | <0.001 | 6.47 | 0.24 to 1.62 | 0.37 |
| Female | 198 (69) | Reference | |||||
| Safety perception with regular testingb | |||||||
| Yes | 266 (74) | 1.00 | 0.61 to 1.62 | 0.99 | 1.38 | 0.77 to 2.47 | 0.27 |
| No | 80 (73) | Reference | |||||
| RiMS score | — | 1.11 | 1.03 to 1.20 | 0.005 | 1.14 | 1.05 to 1.25 | 0.003 |
| Model 2 | |||||||
| DHCWs | |||||||
| Dentists | 225 (87) | 3.85 | 2.35 to 6.41 | <0.001 | 4.54 | 2.02 to 11.35 | <0.001 |
| Assistants | 28 (53) | 0.60 | 0.32 to 1.13 | 0.11 | 0.59 | 0.30 to 1.16 | 0.12 |
| Hygienists | 109 (66) | Reference | Reference | ||||
Bold indicates P < 0.05.
DHCW, dental health care worker; OR, odd ratio; RiMS, risk mitigation strategy.
Adjusted analyses included sex, age, and safety perception with regular testing.
Of self, coworkers, or patients.
Factors Associated with Implementation of RiMS
RiMS scores had a near-normal distribution (Fig. B) and ranged from 0 to 19 (mean [SD], 10.9 [2.9]). Physical distancing was the most prevalent domain (having ≥80 participants responding to performing half of the units in the physical distancing domain “all or most of the time”). Physical protective equipment was the least compliant domain, with 35% reporting being fit-tested for N95 and 43% using N95 all or most of the time. Regardless of role, age, and sex, DHCWs who felt safer with regular testing of coworkers had greater RiMS scores than those who did not feel safer (11.0 vs. 10.3, P = 0.004). RiMS scores did not significantly differ among groups of DHCWs; however, for those who received or planned to receive the COVID-19 vaccine, RiMS scores were significantly higher than those with who did not (P = 0.004). The relationships between RiMS scores and safety perception (P = 0.02) and vaccine acceptance (P = 0.002) persisted even after adjusting for role, age, and sex (Table 3).
Table 3.
Linear Regression Analyses: Factors Associated with RiMS Scores among Survey Participants.
| Unadjusted Analyses | Adjusted Analysesa | ||||||
|---|---|---|---|---|---|---|---|
| Factor | RiMS Scoreb | Beta | SE | P Value | Beta | SE | P Value |
| Model 1 | |||||||
| DHCWs | |||||||
| Dentists | 11.00 (2.9; 4 to 19) | 0.16 | 0.44 | 0.72 | 0.28 | 0.55 | 0.62 |
| Hygienists | 10.70 (3.0; 0 to 18) | −0.13 | 0.46 | 0.78 | −0.11 | 0.47 | 0.82 |
| Assistants | 10.83 (2.8; 5 to 17) | Reference | |||||
| Vaccine acceptance | |||||||
| Yes | 11.08 (2.8; 0 to 19) | 0.91 | 0.32 | 0.004 | 1.01 | 0.36 | 0.005 |
| No | 10.17 (3.0; 3 to 18) | Reference | |||||
| Safety perception with regular testingc | |||||||
| Yes | 11.13 (2.9; 0 to 19) | 0.96 | 0.30 | 0.002 | 0.89 | 0.32 | 0.006 |
| No | 10.16 (3.0; 4 to 18) | Reference | |||||
| Model 2 | |||||||
| DHCWs | |||||||
| Dentists | 11.00 (2.9; 4 to 19) | 0.29 | 0.29 | 0.33 | 0.48 | 0.43 | 0.27 |
| Assistants | 10.70 (3.0; 0 to 18) | 0.13 | 0.46 | 0.78 | 0.03 | 0.47 | 0.95 |
| Hygienists | 10.83 (2.8; 5 to 17) | Reference | |||||
Bold indicates P < 0.05.
DHCW, dental health care worker; OR, odd ratio; RiMS, risk mitigation strategy.
Adjusted analyses included sex, age, vaccine uptake, and safety perception with regular testing.
Mean (SD; range)
Of self, coworkers, or patients.
Given that assistants and hygienists were mostly female, we ran a sensitivity analysis for females only. The aforementioned results held true when restricted to females.
Discussion
This cross-sectional survey of practicing DHCWs in and around New Jersey revealed that dentists felt less safe with regular SARS-CoV-2 testing and had a higher vaccine uptake/acceptance in the middle of the COVID-19 pandemic when compared with dental assistants and hygienists. We observed that the DHCW role was not significantly associated with adoption of RiMS. Furthermore, even though safety perception (with regular testing) was not associated with vaccine uptake, it was positively associated with RiMS scores; that is, those who felt safe with regular testing were more likely to implement physical (nonbiologic) protective measures. We also observed that DHCWs with vaccination plans had higher RiMS scores, suggesting consistent efforts to utilize all available means of protection.
When compared with hygienist and assistants, dentists were less likely to test for or be tested or diagnosed with COVID-19. While this finding does not suggest a reduced risk among dentists, as reported by others (Estrich et al. 2020), it sheds light on possible structural barriers to testing that need to be overcome in the field of dentistry. This might be attributable to the fact that as a model, testing is not a commonly used strategy in the dental setting. In contrast, universal barrier protection is a widely established concept that has been well adopted as a model for infection control. However, it is essential to understand and overcome this hesitancy in being tested, especially when asymptomatic and presymptomatic transmission of SARS-CoV-2 is a significant concern in the pandemic (Furukawa et al. 2020).
Several studies on dentists and dental hygienists have been performed, but currently no conclusive evidence has been reached to suggest that one group of DHCWs is at higher risk than another of acquiring or transmitting the virus. It was, however, interesting to observe that in the present study, dentists were less likely to feel safe with regular testing of self but were slightly more comfortable with regular testing of coworkers or patients. Furthermore, our study suggests that most DHCWs, particularly dentists, rely more on biologic protection (vaccine uptake) and physical protection (RiMS) than regular screening or testing. While rapid testing for SARS-CoV-2 in dental offices has been shown effective for early identification of infected and asymptomatic patients and staff (Ren et al. 2020), more data, training, and education are required to effect behavior change.
Beyond screening and testing, the safety measures available to us are via biologic protection (vaccination) and physical protection to prevent transmission and minimize risk of strain transmission (RiMS). DHCWs are a heterogeneous population, but most (74%) appear willing to get the vaccine. The higher vaccine uptake observed among dentists (77%) is in keeping with previous studies in the United States and China (Shekhar et al. 2021; Sun et al. 2021). A study of French health care workers showed that physicians and pharmacists were most likely to get vaccinated as compared with other hospital workers (Gagneux-Brunon et al. 2021). A recent study reported a lower acceptance of the vaccines among dental students versus medical students (Kelekar et al. 2021), highlighting need for profession-specific curricula about the vaccines and vaccine counseling skills. Nevertheless, this should not be a major challenge given the successful advocacy for and early acceptance of hepatitis B vaccination among dentists, serving as a paradigm for professional adoption of occupational health guidelines (Cooley and Lubow 1982; Cleveland et al. 1994). While the present study reflects a different study population from the survey of students, it is possible that there is a perception among nondentist DHCWs that the regular screening and infection control procedures put in place were sufficient to protect them from acquiring the virus from a patient (Cui et al. 2021). Education and socioeconomic factors associated with vaccine acceptance could also explain our findings, as those with higher education are more likely to accept newly developed vaccines (Reiter et al. 2020; Cascini et al. 2021; Echoru 2021).
As systemically delivered vaccines may not provide mucosal immunity (Donlan and Petri 2020), maintaining physical protection via the RiMS scoring domains is a critical component of preventing acquisition and spread of SARS-CoV-2. N95 respirators, gloves, full-face shields, eye protection goggles with side shields, isolation gowns, and head covers were recommended for aerosol-generating procedures by the CDC and American Dental Association (2020). DHCWs are well trained in infection control and have been using universal precautions for the past 3 decades, particularly during the HIV/AIDS epidemic (Kohn et al. 2003). It is therefore safe to say that the vaccine and RiMS are a product of training and learned behavior.
Several reviews of cross-sectional and clinical trials published within the last 12 mo support the detection of SARS-CoV-2 RNA in saliva (Butler-Laporte et al. 2021; Ibrahimi et al. 2021; Mishra et al. 2021). Advantages include self-collection that is noninvasive, safe, and painless. These analyses conclude that salivary testing can serve as a supplemental method to the gold standard nasopharyngeal swab, especially in ambulatory settings (Butler-Laporte et al. 2021; Caixeta et al. 2021). Notably, results from a meta-analytic study (Butler-Laporte et al. 2021) suggest that saliva diagnostic accuracy is similar to that of the nasopharyngeal swab assessment in the ambulatory testing environment. Furthermore, oral/nasal-induced IgA for mucosal protection could provide reduced viral transmission in infected but disease-protected individuals. The utility of IgA is critical in the asymptomatic and mild states of the infection (Russell et al. 2020).
One of the major strengths of this study was the timing of this survey. Our ability to survey at the cusp of the vaccine rollout (when first-dose vaccine dosages were made available) is extremely important as more DHCWs become more accepting at later dates. A survey conducted later would have masked a true picture of vaccine hesitancy among DHCWs. Nevertheless, this work lays the foundation for future work to assess changes in vaccine or risk mitigation uptake among DHCWs over time. While recent public mandates from governmental agencies have enforced biologic and physical preventative measures, understanding the drivers of vaccine uptake among DHCWs is significant in informing enhancements of dental continuing education curricula. Another core strength is the novel development and introduction of RiMS scores as a scoring algorithm for important protective and preventative practices. In preparing for future pandemics, nimble tools and measures are required to quickly assess the impact of policies, strategies, and practices within dental clinics. While our study participants were mostly Caucasians (83%) serving in private practices (87%), survey respondents were fairly representative of DHCWs in a diverse and multicultural region in the United States such as New Jersey with respect to race (Solana 2021) and sex (Versaci 2021). Our study is not without limitations. We were not able to cluster participants based on dental offices in the event that multiple personnel from the same office (with comparable or identical office policies) participated in the survey. Sampling and response survey bias in the range of responses was possible, although we do not expect this bias to be differential. Based on the characteristics of study participants, we believe that the dentists who responded to the survey were highly representative. However, we acknowledge that the sample population of dental hygienists and assistants (i.e., those who responded to this survey) might not have been representative of hygienists and assistants in the New Jersey metropolitan area. In addition, surveys were administered on an online platform (REDCap), so we were not able to validate or ensure accurate classification of study exposures and outcomes.
Conclusions
Investigations regarding factors influencing risk perception are necessary to guide interventions and improve dental health service responses to the continuing pandemic. DHCWs’ perception of risk and safety is crucial as it likely influences attitudes toward testing and implementation of office risk mitigation policies. Future prospective studies that correlate risk perception and mitigation strategies with SARS-CoV-2 viral and antibody testing are needed to demonstrate the effectiveness of RiMS in dental care settings. Furthermore, understanding how to overcome barriers and enhance willingness to implement protective measures remains an essential component for pandemic preparedness for the dental community.
Author Contributions
M.O. Coker, G. Subramanian, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Davidow, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; J. Fredericks-Younger, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; M.L. Gennaro, D.H. Fine, contributed to conception, design, and data interpretation, critically revised the manuscript; C.A. Feldman, contributed to conception, design, data acquisition, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank all dental health care workers who participated by completing the online survey.
Footnotes
The authors declare that there are no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors acknowledge receipt of funding for this research, authorship, and/or publication of this article. This work was supported by the NIH/NIDCR awards X01 DE030407 (Pragmatic Return to Effective Dental Infection Control through Triage and Testing) and National Dental PBRN infrastructure awards, U19 DE028717 and U01 DE02872.
References
- Al-Kuwari MG, AbdulMalik MA, Al-Nuaimi AA, Abdulmajeed J, Al-Romaihi HE, Semaan S, Kandy M. 2021. Epidemiology characteristics of COVID-19 infection amongst primary health care workers in Qatar: March–October 2020. Front Public Health. 9:679254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljabri D, Vaughn A, Austin M, White L, Li Z, Naessens J, Spaulding A. 2020. An investigation of healthcare worker perception of their workplace safety and incidence of injury. Workplace Health Saf. 68(5):214–225. [DOI] [PubMed] [Google Scholar]
- American Dental Association. 2020. Return to work interim guidance toolkit [accessed 2021 Dec 17]. https://pages.ada.org/return-to-work-toolkit-american-dental-association
- Araujo MWB, Estrich CG, Mikkelsen M, Morrissey R, Harrison B, Geisinger ML, Ioannidou E, Vujicic M. 2021. COVID-2019 among dentists in the United States: a 6-month longitudinal report of accumulative prevalence and incidence. J Am Dent Assoc. 152(6):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski A, Emanuel EJ. 2020. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 324(20):2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Laporte G, Lawandi A, Schiller I, Yao M, Dendukuri N, McDonald EG, Lee TC. 2021. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 181(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixeta DC, Oliveira SW, Cardoso-Sousa L, Cunha TM, Goulart LR, Martins MM, Marin LM, Jardim ACG, Siqueira WL, Sabino-Silva R. 2021. One-year update on salivary diagnostic of COVID-19. Front Public Health. 9:589564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascini F, Pantovic A, Al-Ajlouni Y, Failla G, Ricciardi W. 2021. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: a systematic review. EClinicalMedicine. 40:101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2020. CDC guidance for dental settings—interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic [updated 2020 Dec 4]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html
- Centers for Disease Control and Prevention. 2021. a. Guidance for healthcare workers about COVID-19 (SARS-CoV-2) testing [accessed 2021 Dec 17]. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/testing.html
- Centers for Disease Control and Prevention. 2021. b. Scientific brief: emerging SARS-CoV-2 variants [accessed 2021 Oct 21]. https://www.cdc.gov/coronavirus/2019-nCoV/more/science-and-research/scientific-brief-emerging-variants.html [PubMed]
- Cleveland JL, Siew C, Lockwood SA, Gruninger SE, Chang SB, Neidle EA, Russell CM. 1994. Factors associated with hepatitis B vaccine response among dentists. J Dent Res. 73(5):1029–1035. [DOI] [PubMed] [Google Scholar]
- Coker M, Folayan MO, Michelow IC, Oladokun RE, Torbunde N, Sam-Agudu NA. 2021. Things must not fall apart: the ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr Res. 89(5):1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DI, Culshaw S, Edwards M, Clark C, Watling C, Robertson C, Braid R, O’Keefe E, McGoldrick N, Burns J, et al. 2021. SARS-CoV-2 positivity in asymptomatic-screened dental patients. J Dent Res. 100(6):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley RL, Lubow RM. 1982. Hepatitis B vaccine: implications for dental personnel. J Am Dent Assoc. 105(1):47–49. [DOI] [PubMed] [Google Scholar]
- Cui Y, Ni S, Shen S. 2021. A network-based model to explore the role of testing in the epidemiological control of the COVID-19 pandemic. BMC Infect Dis. 21(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M. 2020. COVID-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 368:m1165. [DOI] [PubMed] [Google Scholar]
- Donlan AN, Petri WA., Jr. 2020. Mucosal immunity and the eradication of polio. Science. 368(6489):362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echoru I, Ajambo PD, Keirania E, Bukenya EEM. 2021. Sociodemographic factors associated with acceptance of COVID-19 vaccine and clinical trials in Uganda: a cross-sectional study in western Uganda. BMC Public Health. 21(1):1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrich CG, Gurenlian JR, Battrell A, Bessner SK, Lynch A, Mikkelsen M, Morrissey R, Araujo MWB, Vujicic M. 2021. COVID-19 prevalence and related practices among dental hygienists in the United States. J Dent Hyg. 95(1):6–16. [PubMed] [Google Scholar]
- Estrich CG, Mikkelsen M, Morrissey R, Geisinger ML, Ioannidou E, Vujicic M, Araujo MWB. 2020. Estimating COVID-19 prevalence and infection control practices among US dentists. J Am Dent Assoc. 151(11):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa NW, Brooks JT, Sobel J. 2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 26(7):e201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux-Brunon A, Detoc M, Bruel S, Tardy B, Rozaire O, Frappe P, Botelho-Nevers E. 2021. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. 108:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JE, Sukriti KC, Johnson IG, Al-Yaseen W, Jones R, McGregor S, Robertson M, Harris R, Innes N, Wade WG. 2020. A systematic review of contamination (aerosol, splatter and droplet generation) associated with oral surgery and its relevance to COVID-19. BDJ Open. 6(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZY, Yang LM, Xia JJ, Fu XH, Zhang YZ. 2020. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 21(5):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure R, Guo A, Bishnoi CK, Patel U, Gifford D, Tippins A, Jaffe A, Shulman E, Stone N, Mungai E, et al. 2020. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care program—United States, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 70(5):178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SC, Fachin S, da Fonseca JG, Angst PDM, Lamers ML, da Silva ISB, Nunes LN. 2021. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J Clin Periodontol. 48(7):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwall L, Cebula M, Greenwall Cohen J, Effenberger S. 2021. COVID-19 testing in a UK dental practice—results of a pilot study. Br Dent J [epub ahead of print 21 Apr 2021] in press. doi: 10.1038/s41415-021-2849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Mohindra R, Chauhan PK, Singla V, Goyal K, Sahni V, Gaur R, Verma DK, Ghosh A, Soni RK, et al. 2021. SARS-CoV-2 detection in gingival crevicular fluid. J Dent Res. 100(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes BE, Perander J, Smecko T, Trask J. 1998. Measuring perceptions of workplace safety: development and validation of the work safety scale. J Safety Res. 29(3):145–161. [Google Scholar]
- Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. 2021. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27(5):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MM, Groenewold MR, Lessem SE, Xu K, Ussery EN, Wiegand RE, Qin X, Do T, Thomas D, Tsai S, et al. 2020. Update: characteristics of health care personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb Mortal Wkly Rep. 69(38):1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi N, Delaunay-Moisan A, Hill C, Le Teuff G, Rupprecht JF, Thuret JY, Chaltiel D, Potier MC. 2021. Screening for SARS-CoV-2 by RT-PCR: saliva or nasopharyngeal swab? Rapid review and meta-analysis. PLoS One. 16(6):e0253007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungo S, Moreau N, Mazevet ME, Ejeil AL, Biosse Duplan M, Salmon B, Smail-Faugeron V. 2021. Prevalence and risk indicators of first-wave COVID-19 among oral health-care workers: a French epidemiological survey. PLoS One. 16(2):e0246586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar AK, Lucia VC, Afonso NM, Mascarenhas AK. 2021. COVID-19 vaccine acceptance and hesitancy among dental and medical students. J Am Dent Assoc. 152(8):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Koo PH. 2021. Effectiveness of testing and contact-tracing to counter COVID-19 pandemic: designed experiments of agent-based simulation. Healthcare (Basel). 9(6):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM, Centers for Disease Control and Prevention. 2003. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 52(RR-17):1–61. [PubMed] [Google Scholar]
- Koletsi D, Belibasakis GN, Eliades T. 2020. Interventions to reduce aerosolized microbes in dental practice: a systematic review with network meta-analysis of randomized controlled trials. J Dent Res. 99(11):1228–1238. [DOI] [PubMed] [Google Scholar]
- Lamberghini F, Testai FD. 2021. COVID-2019 fundamentals. J Am Dent Assoc. 152(5):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. 2020. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 27(2):225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee HJ, Hong Y, Shin YW, Chung S, Park J. 2021. Risk perception, unhealthy behavior, and anxiety due to viral epidemic among healthcare workers: the relationships with depressive and insomnia symptoms during COVID-19. Front Psychiatry. 12:615387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Hua F, Bian Z. 2020. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 99(5):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra C, Meena S, Meena JK, Tiwari S, Mathur P. 2021. Detection of three pandemic causing coronaviruses from non-respiratory samples: systematic review and meta-analysis. Sci Rep. 11(1):16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak A, Adegboye OA, Adekunle AI, Rahman KM, McBryde ES, Eisen DP. 2020. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front Public Health. 8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla B, Callahan N. 2021. What is the rate of COVID-19 infection in a population seeking oral health care? J Am Dent Assoc. 152(6):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, France AM, Aoki Y, Batra D, Biggerstaff M, Dugan V, Galloway S, Hall AJ, Johansson MA, Kondor RJ, et al. 2021. Genomic surveillance for SARS-CoV-2 variants circulating in the United States, December 2020–May 2021. MMWR Morb Mortal Wkly Rep. 70(23):846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. 2020. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter PL, Pennell ML, Katz ML. 2020. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 38(42):6500–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YF, Rasubala L, Malmstrom H, Eliav E. 2020. Dental care and oral health under the clouds of COVID-19. JDR Clin Trans Res. 5(3):202–210. [DOI] [PubMed] [Google Scholar]
- Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. 2020. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 11:611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi W, Kubota N, Shimizu T, Saruta J, Fuchida S, Kawata A, Yamamoto Y, Sugimoto M, Yakeishi M, Tsukinoki K. 2020. Existence of SARS-CoV-2 entry molecules in the oral cavity. Int J Mol Sci. 21(17):6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapultseva M, Hu D, Sarapultsev A. 2021. SARS-CoV-2 seropositivity among dental staff and the role of aspirating systems. JDR Clin Trans Res. 6(2):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. 2021. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines (Basel). 9(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi S, Stanford CM, Cooper LF. 2021. Testing for COVID-19 in dental offices: mechanism of action, application, and interpretation of laboratory and point-of-care screening tests. J Am Dent Assoc. 152(7):514-525.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana K. 2021. HPI publishes findings into racial disparities in oral health [accessed 2021 Dec 17]. https://www.ada.org/publications/ada-news/2021/april/hpi-publishes-findings-into-racial-disparities-in-oral-health
- Sommerstein R, Fux CA, Vuichard-Gysin D, Abbas M, Marschall J, Balmelli C, Troillet N, Harbarth S, Schlegel M, Widmer A, et al. 2020. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 9(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chen X, Cao M, Xiang T, Zhang J, Wang P, Dai H. 2021. Will healthcare workers accept a COVID-19 vaccine when it becomes available? A cross-sectional study in China. Front Public Health. 9:664905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaci MB. 2021. HPI: women make up growing percentage of dental workforce [accessed 2021 Jul 17]. https://www.ada.org/en/publications/ada-news/2021-archive/march/women-make-up-growing-percentage-of-dental-workforce
- Wang L, Didelot X, Yang J, Wong G, Shi Y, Liu W, Gao GF, Bi Y. 2020. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phase. Nat Commun. 11(1):5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. 2020. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. 2020. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 367(6485):1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]