Abstract
The rise of antibiotic-resistant Mycobacterium tuberculosis and non- tuberculous mycobacterial infections has placed ever-increasing importance on discovering new antibiotics to treat these diseases. Recently, a new penem, T405, was discovered to have strong antimicrobial activity against M. tuberculosis and Mycobacteroides abscessus. Here, a penem library of C2 side-chain variants was synthesized, and their antimicrobial activities were evaluated against M. tuberculosis H37Rv and M. abscessus ATCC 19977. Several new penems with antimicrobial activity stronger than the standard-of-care carbapenem antibiotics were identified with some candidates improving on the activity of the lead compound, T405. Moreover, many candidates showed little or no increase in the minimum inhibitory concentration in the presence of serum compared to the highly protein-bound T405. The penems with the strongest activity identified in this study were then biochemically characterized by reaction with the representative L,D-transpeptidase LdtMt2 and the representative penicillin-binding protein D,D-carboxypeptidase DacB2.
Keywords: M. tuberculosis, M. abscessus, β-lactam, antibiotic, penem, structure–activity relationship
Graphical Abstract
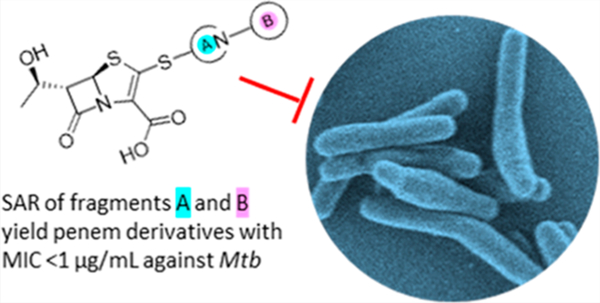
Tuberculosis (TB), the disease state of Mycobacterium tuberculosis (Mtb) infection, is one of the deadliest infectious diseases. Each year, an estimated 10 million people fall ill to TB and 1.5 million die from the disease.1 The standard-of-care treatment for drug-susceptible TB involves the administration of four antibiotics for 2 months, followed by two antibiotics for another 4 months. Additionally, the emergence of multidrug-resistant (MDR), extensively drug- resistant (XDR), and totally drug-resistant (TDR) strains of Mtb have placed ever-growing importance on developing new treatments for TB.2 Moreover, non-TB mycobacteria (NTM) show strong intrinsic resistance to a broad spectrum of antibiotics. One of the most difficult-to-treat NTM diseases is the one caused by Mycobacteroides abscessus (Mab), which is associated with a cure rate as low as 30–50%.3,4 Mab pulmonary infections require multidrug therapy that lasts for 12–18 months, but treatment is often cut short due to drug toxicity. As there are no FDA-approved antibiotics to treat Mab disease, current treatment recommendations rely on antibiotics approved for other indications. The recommended regimes include an induction phase of at least 2 months with three to four antibiotics, typically including amikacin and a β- lactam, imipenem, or cefoxitin.5 The limited success of such treatments, despite their duration, demonstrates the pressing need for more effective treatments to fight against Mtb and NTM infections.
The β-lactam class of antibiotics has been hugely successful in treating a broad spectrum of bacterial diseases, making up more than 50% of all prescribed antibiotics.6 Historically, β- lactams were considered ineffective against mycobacteria,7,8 but more recently there has been renewed interest in their use to treat mycobacterial infections.9 Of the five subclasses of β- lactam antibiotics, carbapenems and penems stand out as the most potent against mycobacteria.10–12 Unsurprisingly, these two classes are very similar with the only structural difference between the pharmacophores being the presence of a sulfur in the penem at position 1 of the bicyclic ring instead of a methylene in carbapenems (Scheme 1). Carbapenems have been heavily explored with multiple drugs on the market, while penems are only represented commercially by faropenem. Meropenem, a member of the carbapenem subclass, in combination with clavulanate, a β-lactamase inhibitor, has shown unmistakable bactericidal activity in the sputum of TB patients and has been used effectively in the clinic for treating MDR TB infections.12–15 These results have spurred interest in developing new carbapenems against Mtb.16,17 The recent development of atypical carbapenems has shown promising progress against Mtb and Mab.18 Additionally, faropenem and the newly developed penem T405 have displayed potent activity against Mtb and Mab.10,11 While carbapenems have been the main focus in β-lactam development against TB, penems remain relatively unexplored.
Scheme 1. Structural Comparison between Carbapenems and Penems Highlighted with the Red Arrowa.
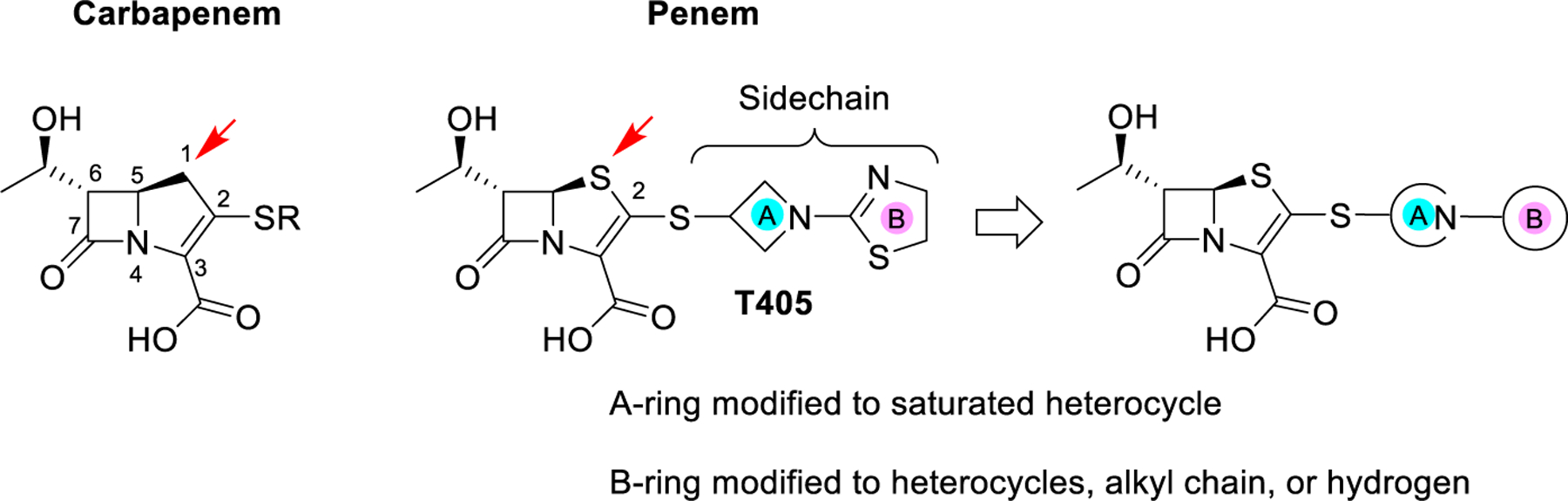
aThe design strategy for the new penem library generation based upon the T405 side-chain structure.
The mechanism of action of β-lactam antibiotics takes place through the inhibition of essential enzymes in the biosynthesis of peptidoglycan, the exoskeleton of bacterial cells.19 As a result, mounting turgor pressure ruptures the cell wall, leading to cell lysis and death. The bacterial peptidoglycan building block is composed of a β(1 → 4)-linked N-acetylglucosamine (GlcNAc)-N-acetylmuramic acid (MurNAc) sugar backbone with a MurNAc-linked-L-Ala-D-γGlu-meso-DAP-D-Ala-D-Ala peptide stem (diaminopimelate = DAP). In most bacteria, the majority of peptide stems are cross-linked between D-Ala4 of one stem and meso-DAP3 of an adjacent stem. These cross- linkages are commonly known as 4 → 3 linkages. Mtb and NTMs possess atypical peptidoglycan as the majority of peptide stems are cross-linked between meso-DAP3 of one stem and meso-DAP3 of an adjacent stem, known as 3 → 3 linkages.20–22 Thus, there are two classes of enzymes in mycobacteria that β-lactams must inhibit to be successful antibiotics, the penicillin-binding proteins (PBPs) and the L,D- transpeptidases (Ldts). The PBP enzyme class contains the D,D-transpeptidases, which are responsible for the formation of the classical 4 → 3 peptidoglycan cross-linkages, and the homologous D,D-carboxypeptidases, which are responsible for the generation of the tetrapeptide substrates of Ldts. This class of enzymes utilizes an active site serine nucleophile to attack the C-terminal D-Ala-D-Ala peptide bond, generating an acyl–enzyme intermediate while releasing a D-Ala. The acyl–enzyme intermediate is then either accepted by an incoming peptide strand, in the case of D,D-transpeptidases, or hydrolyzed to give the truncated four-amino acid strand, in the case of D,D-carboxypeptidases. The β-lactams utilize this native reactivity by lending their strained amide to be attacked, in turn forming a stable ester-bound intermediate that inhibits the PBP from further reaction.23 The evolutionarily distinct Ldt class utilizes a cysteine nucleophile to attack the meso-Dap-D-Ala peptide bond, yielding a thioester acyl–enzyme intermediate. This intermediate is then accepted by the incoming peptide to form the 3 → 3 cross-link.24 When reacted with a β-lactam, the thioester linkage generated is more labile compared to the oxyester linkage of PBPs. This difference results in an intrinsic β-lactam resistance of the Ldt enzyme class.20,21 Carbapenems and penems are the β-lactams that most effectively inhibit both classes of these enzymes.25–31
Undoubtedly, the largest contributors to Mtb resistance against β-lactam antibiotics are the presence of the highly active class A Ambler β-lactamase, BlaC, and select PBPs .32,33 BlaC efficiently hydrolyzes nearly all classes of β-lactams.34 Notably, however, some carbapenems are turned over only slowly by BlaC, resulting in an inherent advantage compared to other β-lactams.12,34 In combination with clavulanate, a well- established β-lactamase inhibitor, many β-lactams regain activity against Mtb.12
A new penem, T405, was identified from an antibiotic screen against Mtb and showed strong antimicrobial activity against laboratory strains of Mtb and Mab, as well as a panel of 20 clinical isolates of Mab.11 Moreover, T405 was found efficacious in treating Mab pulmonary infection in an in vivo model.35 While T405 utilizes the penem pharmacophore, the side-chain branching from C2 gives the drug its unique properties (Scheme 1). The C2 side chain of T405 is the same as the carbapenem tebipenem and is composed of the azetidine A-ring and the dihydrothiazole B-ring (Scheme 1). Addition- ally, it was discovered that T405 has high plasma protein binding (PPB), 98% bound, as shown in a rapid equilibrium dialysis assessment.11 More moderate PPB could enable daily dosing intervals in the clinic while also lowering the dose required to achieve the desired free-drug levels in plasma. To better understand the influence of the C2 side chain on the activity of T405 and in the hope of generating more potent antibiotics against Mtb and Mab with reduced PPB, modifications to the C2 side-chain rings were designed and synthesized. These rings were separately modified to generate a library to probe the influence of each ring on antibiotic activity and PPB. The structure–activity relationship (SAR) of these newly generated penems was assessed by in vitro antimicrobial activity assays. Moreover, the most potent compounds were further examined in in vitro inhibition assays with the D,D- carboxypeptidase and PBP representative DacB2 and the Ldt representative LdtMt2. Lastly, a subgroup of the most active penems was tested in a minimum inhibition concentration (MIC) serum shift assay to identify highly active penems that have a lower PPB than T405.
RESULTS
Chemistry.
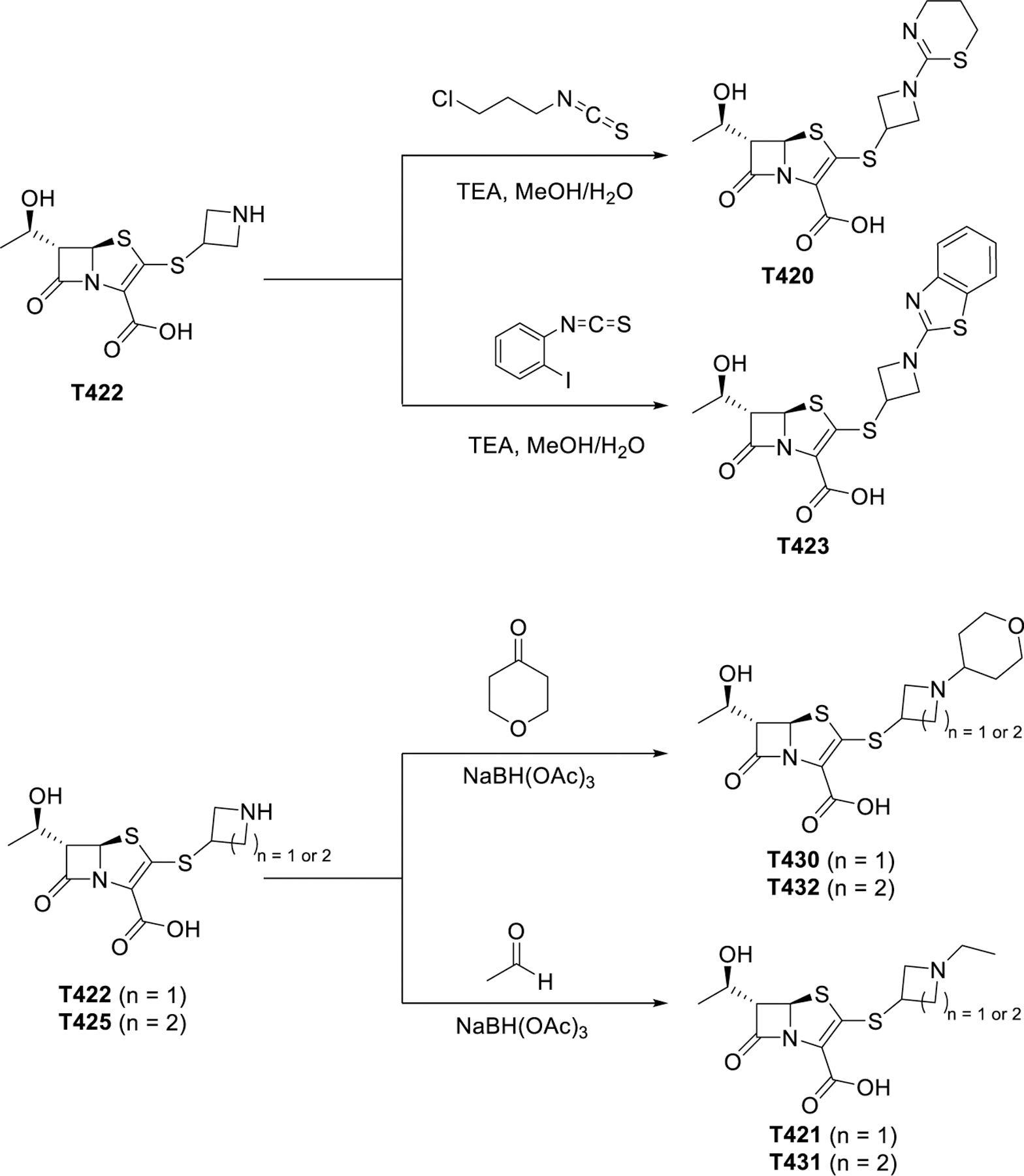
To elucidate the effect of the C2 side chain on T405 antimicrobial activity, a variety of penems with different C2 side chains was synthesized. The C2 side chains varied in two sections denoted as the A-ring and the B-ring (Scheme 1). The C2 side chains were synthesized starting from an amino alcohol. The nitrogen of heterocycle A was first either protected as its allyl carbamate (alloc) via reaction with allyl chloroformate or coupled with 2-chloro benzimidazole (denoted by X in Scheme 2). Once the reactive nitrogen was masked, the alcohol was activated as methanesulfonate, 2, by reaction with methanesulfonyl chloride. The protected thiol was then installed through substitution of the activated alcohol with potassium thioacetate to yield 3. Lastly, the thioacetate was reacted with sodium methoxide to reveal deprotected C2 side chain thiol, 4.
Scheme 2. Synthesis of Penem Library.
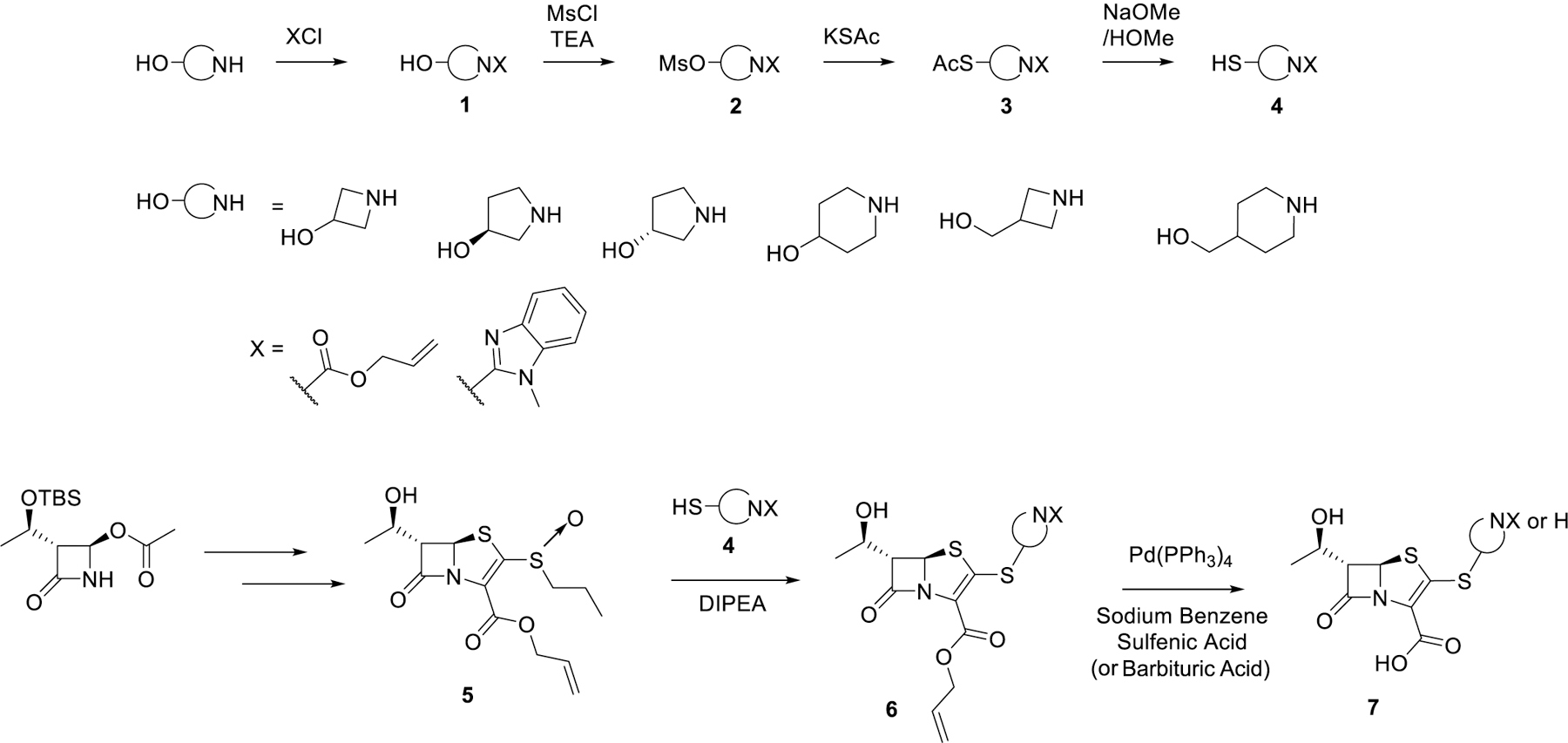
The penem core was prepared as previously described to give access to compound 5.11 By employing a convergent strategy to the synthesis, the C2 side chains could be incorporated at a late stage into the oxidized penem core, 5, through a β-addition/elimination reaction. Once the thioether C2 side chain was installed, global allyl deprotection efficiently afforded the deprotected carboxylic acid (T418) and the secondary nitrogen if applicable (T422, T425, T426, T427, T428, and T429; for structures refer to Table 1). The secondary nitrogen could then be further derivatized through reductive amination (T421, T430, T431, and T432) or by the reaction with thioisocyanates followed by in situ intermolecular cyclization (T423 and T420) (Scheme 3).
Table 1.
MIC (μg/mL) (μM) of Penem Library against Mtb H 37 Rv, with and without Clavulanate (5 μg/mL), and against Mab ATCC 19977 a
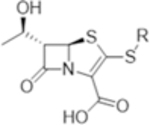
|
MIC-μg/ml (μM) |
|||
|---|---|---|---|---|
| Compound | R: | Mtb H37Rv | Mtb H37Rv with Clavulanate (5 μg/ml) | Mab ATCC 19977 |
| T418 |
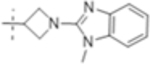
|
2 (4.6) | 0.5 (1.2) | 64 (150) |
| T423 |
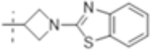
|
8 (18) | 2(4.6) | 64 (150) |
| T430 |
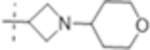
|
4 (10) | ND | 4 (10) |
| T420 |
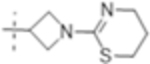
|
2 (5.0) | 0.5 (1.3) | 16 (40) |
| T405 |
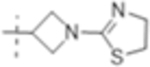
|
0.5 (1.3) | 0.5 (1.3) | 2(5.2) |
| T421 |
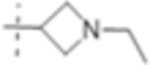
|
1 (3.0) | 0.5 (1.5) | 4 (12) |
| T422 |

|
0.5 (1.7) | 0.5 (1.7) | 4 (13) |
| T432 |
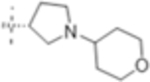
|
4 (10) | ND | 4 (10) |
| T431 |
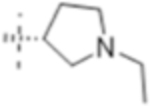
|
4 (12) | ND | 4 (12) |
| T425 |
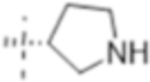
|
0.5 (1.6) | 0.25 (0.79) | 2 (6.3) |
| T426 |
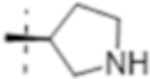
|
0.5 (1.6) | 0.25 (0.79) | 2(6.3) |
| T427 |
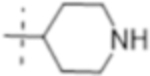
|
1 (3.0) | 0.5 (1.5) | 4 (12) |
| T428 |
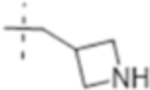
|
0.5 (1.6) | 0.25 (0.79) | 8 (25) |
| T429 |
|
0.5 (1.5) | 0.25 (0.73) | 4 (12) |
| lmipenem | 4 (13) | ND | 8 (27) | |
| Meropenem | 8 (21) | 0.5 (1.3) | 8 (21) | |
| Tebipenem | 1 (2.6) | 0.25 (0.65) | 64 (170) | |
Scheme 3. Penem Generation Using Late-Stage Modification of Secondary Nitrogen.
Activity against Mtb.
The MIC was determined against Mtb H37Rv with and without the addition of the β-lactamase inhibitor clavulanate (Table 1). Initial SAR investigation explored modifications of the B-ring of T405 while maintaining the azetidine A-ring. While increasing the B-ring from five members to six in T420 increased the MIC by two dilutions, activity was recovered in the presence of clavulanate. The N-methyl benzimidazole B-ring of T418, increased the MIC by fourfold although it showed the strongest activity of the compounds containing an aromatic ring. In a continuing trend, the benzothiazole of T423 increased the MIC 16-fold.
Truncation of the B-ring of T405 to yield just the terminal azetidine T422 showed the same activity as T405. To further explore the role of the A-ring on the antimicrobial activity, rings of increasing size were synthesized while maintaining the secondary nitrogen of the truncated B-ring. Increasing the A- ring size to five members in T425 and T426 produced the same MIC as T405. Moreover, the addition of clavulanate lowered the MIC of both compounds twofold to 0.25 μg/mL. There was no stereochemical preference in activity between the two diastereomers T425 and T426. Upon increasing the A- ring to six members in T427, the MIC increased twofold. By extending the six-membered ring from the penem core by a methylene in T429; however, the activity was recovered. Lastly, by extending T422 by a methylene, T428, the MIC in the presence of clavulanate improved by twofold as well. Adding an ethyl or adding a tetrahydropyran to T422 to form T421 or T430, as well as to T425 to from T431 and T432, respectively, only decreased the antimicrobial activity com- pared to the secondary amine.
Activity against Mab.
The in vitro antibacterial activity of the penem library was tested against the Mab strain ATCC 19977. It was previously shown that T405 possesses strong antimicrobial activity against this laboratory strain as well as 20 clinical isolates of the Mab complex.11 Much like in Mtb, increasing the size of the B-ring of T405 to a six-membered ring in T420 had a negative effect on the activity against Mab. Moreover, introduction of any aromatic ring at this position had a detrimental effect on the activity against Mab as shown by T418 and T423. Removal of the B-ring of T405 resulted in 2-fold weaker activity for T422. However, when the size of the A-ring was changed to five-membered, the MIC was restored to that of T405, 2.0 μg/mL. When the size of the A-ring was increased again and/or spaced from the penem core by a methylene, the activity was reduced again by twofold or more.
Penem Reactions with LdtMt2.
A group of penems, T405, T422, T426, and T428, which had the greatest antimicrobial activity was reacted with LdtMt2. The resulting covalent drug adduct was measured by intact-protein ultra-performance liquid chromatography (UPLC)–high-resolution mass spec- trometry (HRMS) analysis. All tested penems formed a +86 Da LdtMt2 adduct (Figure 1), owing to the characteristic scission of the C5–C6 bond, a process that has been reported previously for the penem, faropenem,28,37 and C5-substituted carbapenems.18 Notably, fragmentation to the +86 Da adduct is irreversible, unlike the related reversible carbapenem adducts of LdtMt2,38 and its hydrolytic stability has been previously characterized.10
Figure 1.
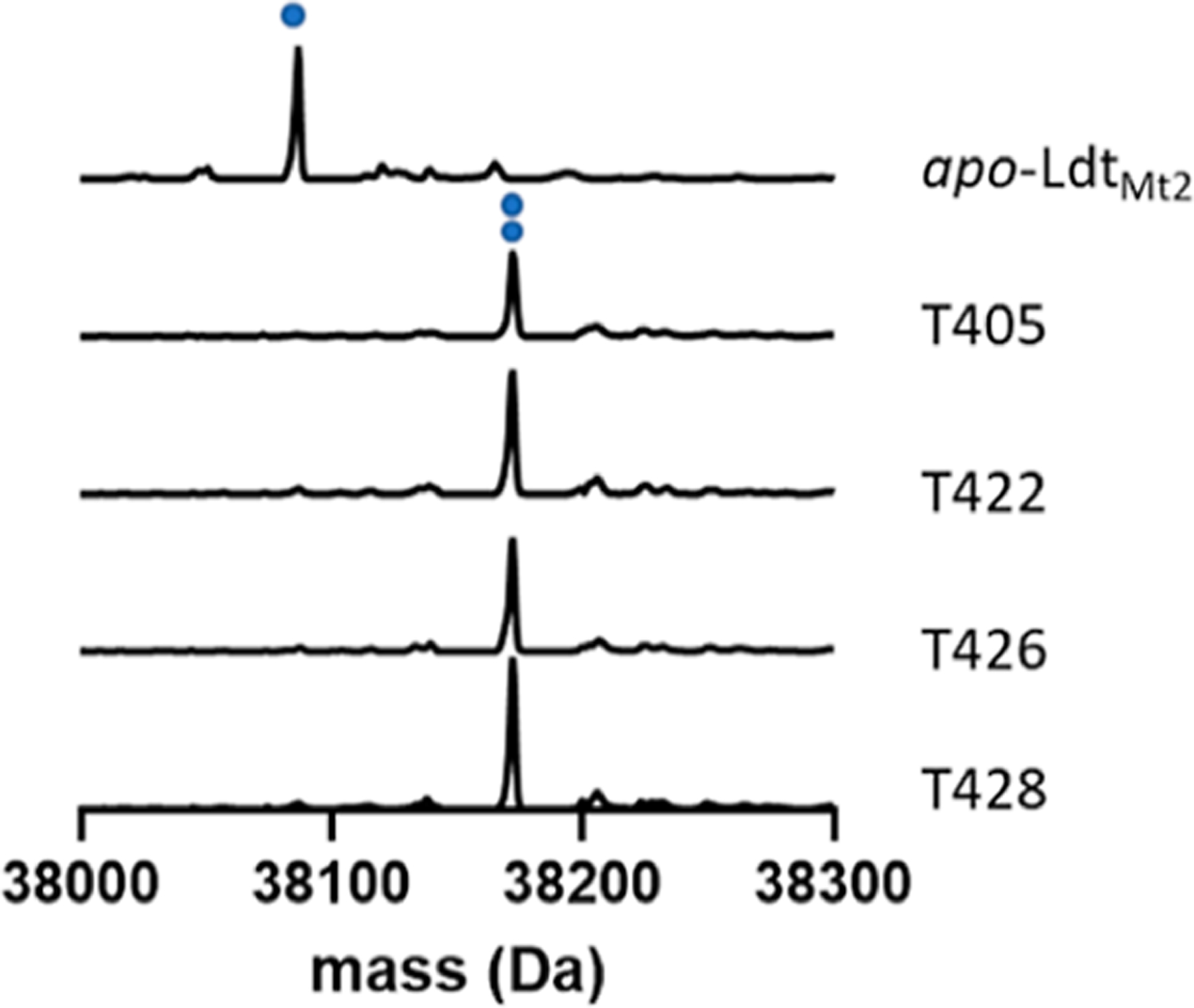
LdtMt2 is acylated by all experimental penems tested, with each forming identical M+86 fragments. The single blue dot represents apo-LdtMt2, m/z = 38,086 Da, and two blue dots represent the LdtMt2–penem fragment adduct, 38,172 Da.
Penem Reactions with DacB2.
The D,D-carboxypeptidase DacB2 is a well-studied exemplar of the broader PBP enzyme class, and its activity is required to precede the function of Ldts.39 We thus analyzed covalent inhibition of DacB2 by select penems to gain insights into the best inhibitor(s) of DacB2 and potential inhibition efficiency for PBPs in general. The adduct off-rate, or adduct stability, directly relates to enzyme occupancy. For slow inhibitors, such as carbapenems, the adduct stability has been shown to be more correlated with in vivo activity than the adduct on-rate.40–42 To measure the adduct stability, DacB2 and each penem were reacted, the resultant DacB2–penem adduct was washed free of drug, and extent of residual binding was measured by the appearance of apo-DacB2 (Figure 2A) after an overnight incubation. As a point of comparison, the same experiment was performed with the clinically used carbapenem, meropenem (Figure 2B). We found that DacB2–T405 and DacB2–T428 exhibited some loss of adduct over time, retaining 76% and 86% of inhibitor, respectively, after 24 h while DacB2–T422, DacB2–T426, as well as DacB2–meropenem remained completely bound over this period of time (Figure 2C,D).
Figure 2.
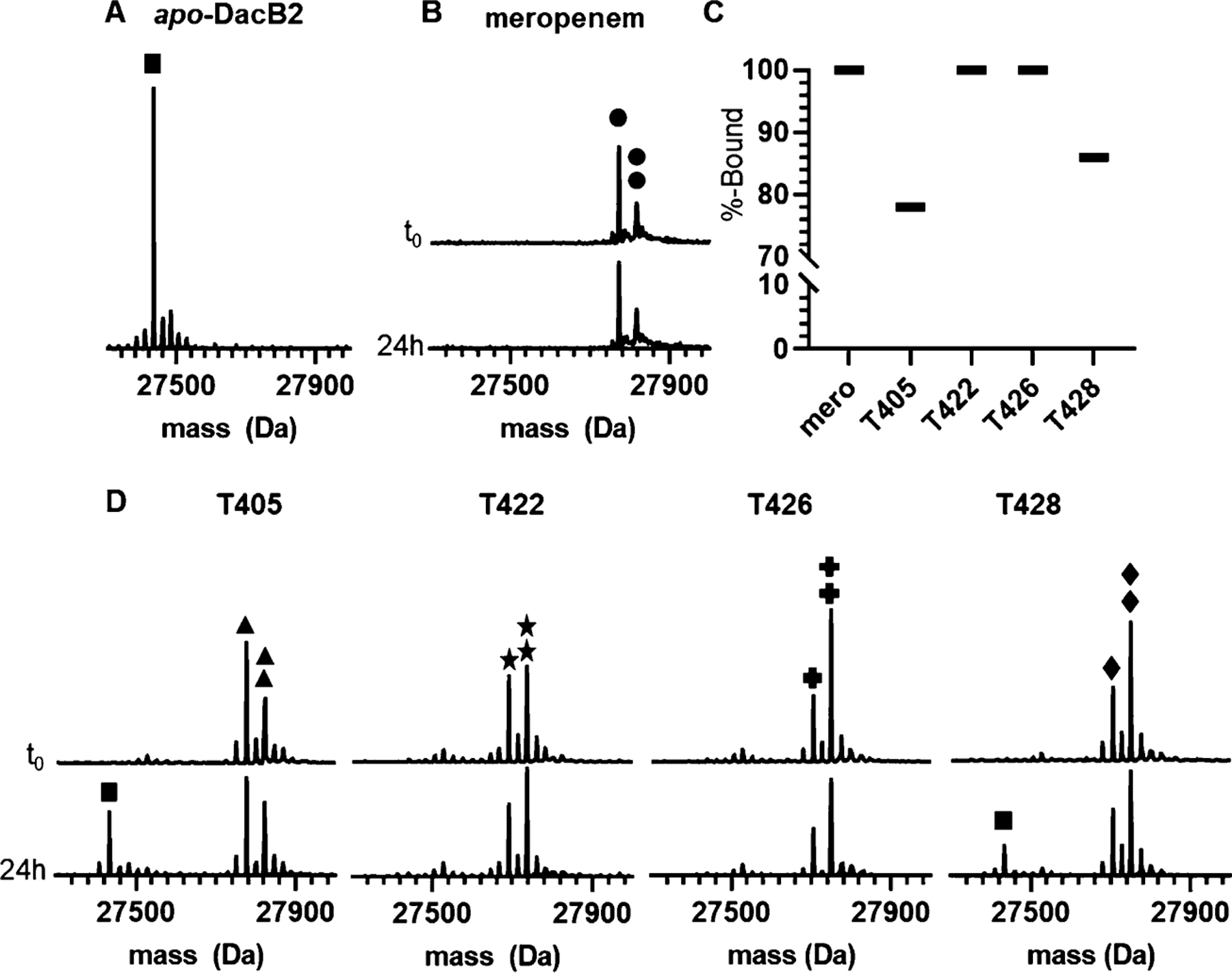
Intact-protein UPLC–HRMS analysis demonstrates differential stability of select DacB2–penem adducts after washing away the free drug and incubating for 24 h. (A) UPLC–HRMS analysis of apo-DacB2 (one square, 27,435 Da). (B) Meropenem fully reacts with DacB2, and the DacB2–meropenem adduct (decarboxylated, one circle, 27,774 Da; intact, two circles, 27,818 Da) is stable after the removal of drug for 24 h. (C) Like meropenem, T422 and T426 form adducts that are stable for at least 24 h after drug removal, while T405 and T428 exhibit the loss of 24% and 14% of drug, respectively. (D) Mass spectra of T405 (decarboxylated, one triangle, 27,778 Da; intact, two triangles 27,822 Da), T422 (decarboxylated, one star, 28,033 Da; intact, two stars, 28,077 Da), T426 (decarboxylated, one cross, 27,708 Da; intact, two crosses, 27,752 Da), and T428 (decarboxylated, one diamonds, 27,708 Da; intact, two diamonds, 27,752 Da) adducts of DacB2 before and after washout. One black square represents apo-DacB2 (27,435 Da).
Effect of Serum on MIC.
T405 previously exhibited 98% PPB by rapid equilibrium dialysis. Among the newly synthesized penems, we sought similar or better antimyco- bacterial activity compared to T405, with lower PPB to be advanced for in vivo analysis. To estimate the impact of PPB in vivo, an MIC serum shift assay was performed on the most active penems. MICs were measured against Mtb H37Rv in a normal 7H9 medium, 7H9 with 25% human serum, or 7H9 with 25% mice serum (Table 2). The shift of the MIC from the normal 7H9 medium to that with the serum added is reported as a MIC fold increase. The fold increase indicates the degree to which the drug is bound to the serum and not free to interact with cellular targets in Mtb.
Table 2.
MIC (μg/mL) (μM) of Penem Derivatives against Mtb H37Rv in Regular 7H9 Medium, 7H9 +25% Human Serum, and 7H9 +25% Mouse Serum
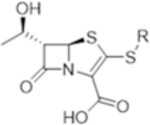 R: |
MIC-μg/ml (μM) against Mtb H37Rv |
|||
|---|---|---|---|---|
| Compound | Regular 7H9 | 25% Human Serum | 25% Mouse Serum | |
| T405 |
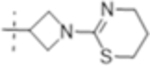
|
0.5 (1.3) | 8 (21) | 8 (21) |
| T420 |
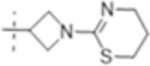
|
2 (5.0) | 8 (20) | 4 (10) |
| T430 |
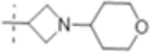
|
4 (10) | ND | 16 (41) |
| T421 |
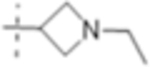
|
2 (6.1) | 4 (12) | 4 (12) |
| T422 |
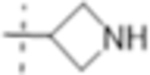
|
1 (3.3) | 2 (6.6) | 2 (6.6) |
| T432 |
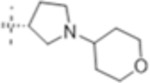
|
4 (10) | ND | 16 (40) |
| T431 |
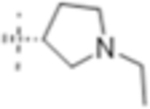
|
4 (12) | ND | 16 (46) |
| T426 |
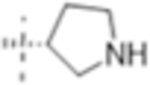
|
0.5 (1.6) | 4 (13) | 1(3.2) |
| T425 |
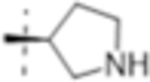
|
0.5 (1.6) | 2 (6.3) | 1 (3.2) |
| T427 |
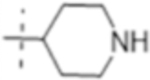
|
1(3.0) | 8 (24) | 2 (6.1) |
| T428 |
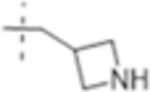
|
1 1(3.2) | 2 (6.3) | 2 (6.3) |
| T429 |
|
0.5 (1.5) | 8 (23) | 2 (5.8) |
| Meropenem | 8 (21) | 16 (42) | 8 (21) | |
From the MIC serum shift measurements, it was observed that T405 had the largest shift with a 16-fold increase in MIC in the presence of human serum and mouse serum. This increase is consistent with its high PPB, confirming the MIC shift as an effective assay to estimate PPB of the compound. Many of the compounds with alkyl or heterocycle modification on the nitrogen also showed an increase in MIC with the addition of serum (T420, T421, T430, T432, and T431). While all compounds showed some extent of MIC shift, many with a secondary nitrogen did not have more than a twofold increase. However, this observation is not universally true as T429 and T427 break this pattern with human serum.
DISCUSSION
An SAR database of penems was prepared and used to investigate the influence of the T405 C2 side chain on antimicrobial activity against Mtb H37Rv and Mab ATCC 19977. To examine this question, the C2 side chain was varied at two sites, the A-ring and the B-ring (Scheme 1). This SAR study resulted in five compounds having activities comparable to T405 against Mtb and 15 compounds with better or equivalent activity compared to meropenem, the carbapenem recommended for use against MDR-TB. Clavulanate is commonly administered with carbapenems to inhibit BlaC. This supplementation has a large effect whereby the MIC of meropenem shifts from 8 μg/mL without clavulanate to 0.5 μg/mL with it.12 In contrast, several penem antibiotics that already possessed low MICs did not show such a large response, if any, to the addition of the β-lactamase inhibitor. This difference would indicate that either the penems are not significantly hydrolyzed by BlaC or that the limit of the MICs is not dependent on the rate of their hydrolysis. Therefore, this group of compounds has potential for use as a sole agent treatment as opposed to requiring combination therapy with, for example, clavulanate. In our previous study, we determined that the inclusion of the β-lactamase inhibitor avibactam did not significantly alter the MIC of our penems against Mab.11 Based on these data, we hypothesized that these penems are more resistant to the β-lactamase activity present in Mab. Owing to this precedent, a β-lactamase inhibitor was deemed unnecessary for MIC determinations of our penems against Mab and therefore not included.
The penem library was also tested against Mab ATCC 19977 that is intrinsically resistant to several antibiotics. Many of the same trends in the SAR data were borne out between the two mycobacteria. Most notably, any aromatic ring in the C2 side chain drastically reduced the activity. Additionally, several side chains produced good activity against Mab, with MICs averaging around 4 μg/mL. Only the five-membered ring penems, T425 and T426, had activity equal to T405 against Mab (MIC of 1 μg/mL). By way of comparison, the carbapenem imipenem, the first-line drug in the treatment of Mab infection has an MIC of 8 μg/mL. Eleven penems from the library showed equal or lower MIC compared to imipenem.
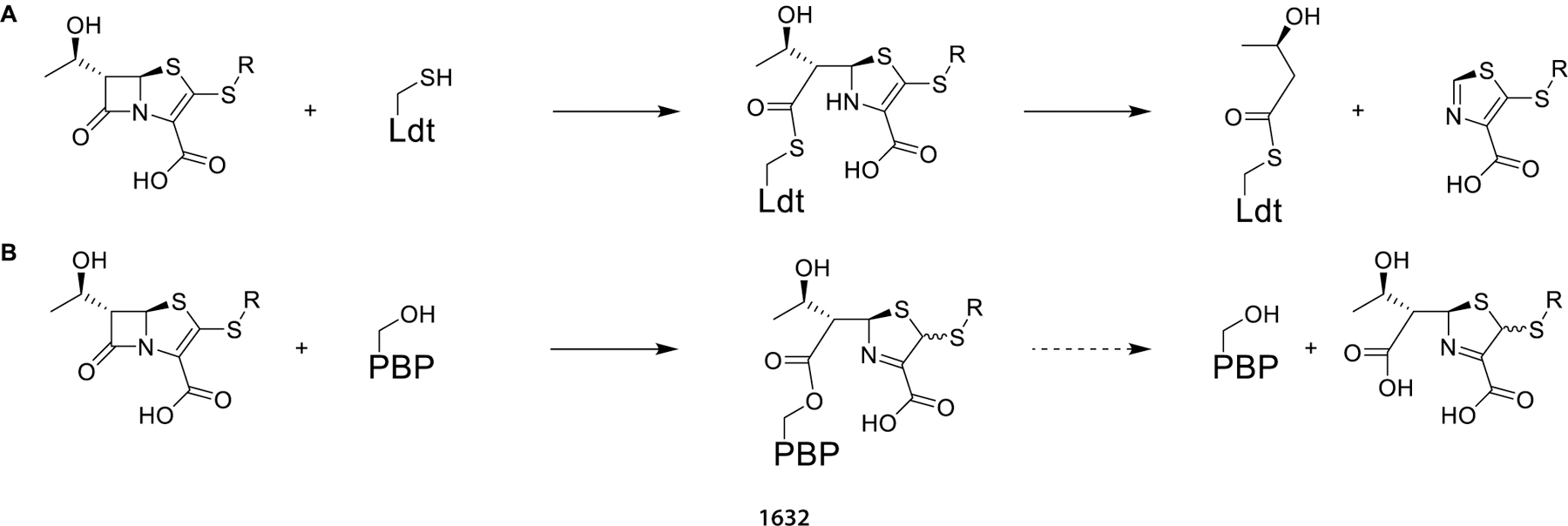
Of the three classes of β-lactam targets known to confer antibacterial activity, namely, Ldts, PBPs, and β-lactamases, representative Ldts and PBPs were selected for target engagement assays with select penems. As the β-lactamase inhibitor clavulanate has minimal effect on the MIC of these penems, BlaC is likely not responsible for differential activity and was not separately evaluated in enzyme inhibition assays. The adduct formation and stability of the most active penems, T422, T426, and T428, was measured against LdtMt2 and compared to the penem T405. When adduct formation was monitored with LdtMt2, in-solution fragmentation to a M+86 Da 3-hydroxybutyryl group ensued for each penem (Scheme 4A). This observation agrees with previous studies of the faropenem adduct with Ldts.28,37 Moreover, the data show that the penem breakdown on LdtMt2 is not dependent on the identity of the side chain. When these results are combined with the observation that faropenem rapidly forms the same M +86 Da fragment after initial thioester adduct formation to active-site cysteine variants of β-lactamases,37 it is clear that fragmentation of penem thioester adducts is facile and generalizable. When this group of penems was tested against the PBP representative DacB2, each penem formed intact adducts (Scheme 4B). When monitored for 24 h T422 and T426 were fully bound, as seen for meropenem, while T405 and T428 were partially hydrolyzed. This behavior would indicate that T422 and T426 are better inhibitors of DacB2 because of their longevity bound to the protein. It has been hypothesized that antibiotics with long-lasting enzyme inhibition are particularly desirable to be effective against slow-growing bacterial species such as Mtb that divide approximately every 24 h, and as precedented by D-cycloserine inhibition of Mtb alanine racemase.40 Due to DacB2 stability in vitro, adduct lifetimes were measured at 20 °C as opposed to a physiological temperature of 37 °C, where chemical processes are faster and drug lifetimes would be correspondingly shorter.
Scheme 4. (A) Representation of the Penem Reaction with Ldt (B) Representation of the Penem Reaction with PBP.
The MIC serum shift measurements were used as a functional measure of PPB for the penem library. The measurements showed a high MIC serum shift with the highly PPB T405 and little or no shift for meropenem, which has negligible PPB, confirming the utility of the MIC serum shift assay. These results implied that the addition of a heterocycle or alkyl chain to the A-ring of the side chain increases the PPB. Moreover, the C2 side chains with just the secondary nitrogen showed the lowest MIC serum shift in general. These trends agree with the loosely correlated observation that more non- polar groups in a molecule correlate with higher PPB, while polar groups, such as amines, lower PPB.43 From this study, T422, T425, T426, and T428 were all indicated as compounds with low MICs against Mtb H37Rv and low PPB.
Several penem antibiotics developed in this study showed activity against Mtb and Mab that was more potent than the respective carbapenem clinical comparators. MIC data from the library show the importance of the A-ring to the activity of the candidates while modifications to the B-ring were shown to be either negative or neutral. Moreover, the in vitro analysis showed that the side chain has no effect on the adduct stability with Ldts but does affect the adduct stability when reacted with PBPs. This observation suggests the difference in the side- chain activity is more dependent on the resulting inhibition of PBPs. The database generated here sheds light on the role the side chain plays on the activity of penems against mycobacteria and could have broader applications to other β-lactam molecules. Furthermore, the MIC serum shift assay revealed alternative penems that display lower PPB and, therefore, more potent activity in the presence of serum than T405. These candidates are to be advanced for future in vivo pharmaco- kinetic and efficacy studies.
MATERIALS AND METHODS
Chemical Compounds.
Penems were synthesized as described in the Supporting Information Imipenem and meropenem were purchased from Carbosynth (San Diego, CA). Clavulanate was purchased from Sigma-Aldrich (St. Louis, MO).
MIC Assay.
MICs were determined as previously described using the broth microdilution assay in Middlebrook 7H9 media supplemented with 10% oleic acid, albumin, dextrose, and catalase but without Tween 80.44,45 Powdered drug stocks were reconstituted in dimethyl sulfoxide and twofold serial dilutions were prepared in Middlebrook 7H9 broth to obtain final drug concentrations in 96-well microtiter plates. Approximately, 105 colony forming units (cfu)/mL of bacteria from an exponentially growing culture were added to each well. Mab and Mtb cultured without drugs in Middlebrook 7H9 broth alone were included in each plate as negative controls. Imipenem and meropenem were used as positive controls for Mab and Mtb, respectively, in accordance with their clinical use as the preferred carbapenem for each infection. Plates were incubated at 30 °C for 72 h and at 37 °C for 7 d for MIC determination against Mab and Mtb, respectively, in accordance to Clinical and Laboratory Standards Institute (CLSI) guidelines.46 Growth or lack thereof was assessed by visual inspection and an MIC for each drug was defined as the lowest concentration that prevented visible growth. MIC assays against Mtb were performed two or three times for each penem, except that T418, T423, T430, T431, and T432 were only assayed once due to material constraints. The modal MICs are presented in Table 1. Replicates did not differ from the modal MIC by more than one dilution. Likewise, MIC assays against Mab were performed two times. If MICs of two biological replicates differed, then the assay was repeated for the third time. The MIC presented in Table 1 is the modal MIC.
Serum Shift MIC Assay.
MIC measurements were performed as described above with the exception that parallel wells used Middlebrook 7H9 media alone or supplemented with either 25 wt % mouse serum or 25 wt % human serum. Mouse serum was isolated from uninfected, untreated adult female BALB/c mice, and filtered through an 0.22 μm filter. Human serum was obtained from a healthy volunteer and filtered through an 0.22 μm filter.
Protein Expression and Purification.
LdtMt2 (ΔN55) and DacB2(ΔN27) were expressed and purified as previously described.47 Enzyme concentrations were determined using the Beer–Lambert Law by measuring A280 by ultraviolet–visible (UV–vis) spectroscopy in 7 M guanidinium chloride and calculated extinction coefficient for DacB2,28 and 84,000 M−1 cm−1 for LdtMt2, previously determined by amino acid analysis.38
UPLC–HRMS Analysis.
UPLC–HRMS experiments were analyzed on a Waters ACQUITY H-Class UPLC system equipped with a multiwavelength UV–vis diode array detector in conjunction with a Waters ACQUITY BEH-300 μL UPLC column packed with a C4 stationary phase (2.1 × 50 mm; 1.7 μm) to analyze intact proteins in tandem with HRMS analysis by a Waters Xevo-G2 quadrupole-time of flight electrospray ionization MS.
Intact Protein UPLC–HRMS.
Enzyme samples were separated at 60 °C to enhance peak resolution with a flow rate of 0.3 mL/min and the following mobile phase: 0 to 1 min 90% water, 10% ACN, 0.1% formic acid (FA); 1 to 7.5 min gradient up to 20% water, 80% ACN, 0.1% FA; 7.5 to 8.4 min 20% water, 80% ACN, 0.1% FA; 8.4 to 8.5 min linear gradient up to 90% water, 10% ACN, 0.1% FA; and 8.5 to 10 min 90% water + 10% ACN, 0.1% FA. The first minute of eluate was discarded to remove salts and buffer online. Samples were analyzed in the positive mode and deconvoluted from m/z distributions into neutral masses using the Maxent1 algorithm within MassLynx. Data were then normalized to the sum of intensities and plotted within Prism 9.3.0.
LdtMt2 Adduct Formation.
Each penem (20 μM) was incubated separately with LdtMt2 (2 μM) for 1 h in 25 mM HEPES pH 7.0 buffer at 20 °C. Samples were then subjected to intact protein UPLC–HRMS analysis for determination of adduct formation.
DacB2 Adduct Stability Comparison.
Penems and meropenem (20 μM) were incubated individually with DacB2 (2 μM) for 1 h in 25 mM HEPES pH 7.0 buffer at 20 °C. Complete adduct formation was confirmed by intact protein UPLC–HRMS analysis at which point excess (carba)- penem was removed by two sequential buffer exchanges with Thermo-Scientific Zeba 7 kDa spin desalting columns, which had been pre-equilibrated with 25 mM HEPES pH 7.0 buffer. After 24 h, DacB2 was again subjected to intact protein UPLC–HRMS analysis. Percent bound was determined in BiopharmaLynx 1.3.2 by summing ion counts of intact and decarboxylated forms of DacB2–adducts and dividing by the sum of apo and bound form ion counts, as described previously.38
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the NIH award R01 AI137329. E.C.M. was supported by the NIH award F31 HL147392 and T.A.Z. by T32 GM135131. We gratefully acknowledge Drs. I. P. Mortimer and J. Catazaro for their help with ESI-MS and NMR measurements, respectively.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Hunter R. Batchelder, Department of Chemistry, Johns Hopkins University, Baltimore, Maryland 21218, United States
Trevor A. Zandi, T. C. Jenkins Department of Biophysics, Johns Hopkins University, Baltimore, Maryland 21218, United States
Amit Kaushik, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, United States.
Akul Naik, Department of Chemistry, Johns Hopkins University, Baltimore, Maryland 21218, United States.
Elizabeth Story-Roller, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, United States.
Emily C. Maggioncalda, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, United States
Gyanu Lamichhane, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, United States.
Eric L. Nuermberger, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287, United States
Craig A. Townsend, Department of Chemistry, Johns Hopkins University, Baltimore, Maryland 21218, United States
REFERENCES
- (1).Tuberculosis. https://www.who.int/health-topics/tuberculosis#tab=tab_1 (accessed Jul 26, 2021).
- (2).Migliori GB; Tiberi S; Zumla A; Petersen E; Chakaya JM; Wejse C; Muñoz Torrico MM,Duarte R; Alffenaar JW; Schaaf HS; Marais BJ; Cirillo DM; Alagna R; Rendon A; Pontali E; Piubello A; Figueroa J; Ferlazzo G; García-Basteiro A; Centis R; Visca D; D’Ambrosio L; Sotgiu G; Arkub T; Akkerman OW; Aleksa A; Belilovski E; Bernal E; Blanc FX; Boeree M; Borisov S; Bruchfeld J; Cadiñanos Loidi J;, Caminero JA; Carvalho AC; Cebrian Gallardo JJ; Charalampos M; Danila E; Davies Forsman L; Denholm J; Dheda K; Diel R; Diktanas S; Dobler C; Enwerem M; Esposito S; Escobar Salinas N; Filippov A; Formenti B; García García JM; Goletti D; Gomez Rosso R; Gualano G; Isaakidis P; Kaluzhenina A; Koirala S; Kuksa L; Kunst H; Li Y; Magis-Escurra C; Manfrin V; Manga S; Manika K; Marchese V; Martínez Robles E; Maryandyshev A; Matteelli A; Mariani A; Mazza-Stalder J; Mello F; Mendoza L; Mesi A; Miliauskas S; Mustafa Hamdan H; Ndjeka N; Nieto Marcos M; Ottenhoff THM; Palmero DJ; Palmieri F; Papavasileiou A; Payen MC; Pontarelli A; Pretti Dalcolmo M; Quirós Fernandez S; Romero R; Rossato Silva D; Santos AP; Seaworth B; Sinitsyn M; Skrahina A; Solovic I; Spanevello A; Tadolini M; Torres C; Udwadia Z; van den Boom M; Volchenkov GV; Yedilbayev A; Zaleskis R; Zellweger JP MDR/XDR-TB Management of Patients and Contacts: Challenges Facing the New Decade. The 2020 Clinical Update by the Global Tuberculosis Network. Int. J. Infect. Dis 2020, 92, S15–S25. [DOI] [PubMed] [Google Scholar]
- (3).Diel R; Ringshausen F; Richter E; Welker L; Schmitz J; Nienhaus A Microbiological and Clinical Outcomes of Treating Non- Mycobacterium Avium Complex Nontuberculous Mycobacte- rial Pulmonary Disease. Chest 2017, 152, 120–142. [DOI] [PubMed] [Google Scholar]
- (4).Jarand J; Levin A; Zhang L; Huitt G; Mitchell JD; Daley CL Clinical and Microbiologic Outcomes in Patients Receiving Treatment for Mycobacterium abscessus Pulmonary Disease. Clin. Infect. Dis 2011, 52, 565–571. [DOI] [PubMed] [Google Scholar]
- (5).Daley CL; Iaccarino JM; Lange C; Cambau E; Wallace RJ; Andrejak C; Böttger EC; Brozek J; Griffith DE; Guglielmetti L; Huitt GA; Knight SL; Leitman P; Marras TK; Olivier KN; Santin M; Stout JE; Tortoli E; van Ingen J; Wagner D; Winthrop KL Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis 2020, 71, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hamad B The Antibiotics Market. Nat. Rev. Drug Discovery 2010, 9, 675–676. [DOI] [PubMed] [Google Scholar]
- (7).Robinson HJ Toxciity and Efficacy of Penicillin. J. Pharmacol. Exp. Ther 1943, 77, 70. [Google Scholar]
- (8).Smith MI; Emmart EW The Action of Penicillium Extracts in Experimental Tuberculosis. Public Health Rep 1944, 59, 417.19315967 [Google Scholar]
- (9).Story-Roller E; Lamichhane G Have We Realized the Full Potential of β-Lactams for Treating Drug-Resistant TB? IUBMB Life 2018, 70, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Dhar N; Dubée V; Ballell L; Cuinet G; Hugonnet JE; Signorino-Gelo F; Barros D; Arthur M; McKinney JD Rapid Cytolysis of Mycobacterium Tuberculosis by Faropenem, an Orally Bioavailable β-Lactam Antibiotic. Antimicrob. Agents Chemother 2015, 59, 1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Batchelder HR; Story-Roller E; Lloyd EP; Kaushik A; Bigelow KM; Maggioncalda EC; Nuermberger EL; Lamichhane G; Townsend CA Development of a Penem Antibiotic against Mycobacteroides abscessus. Commun. Biol 2020, 3, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hugonnet JE; Tremblay LW; Boshoff HI; Barry CE; Blanchard JS Meropenem-Clavulanate Is Effective against Extensively Drug-Resistant Mycobacterium Tuberculosis. Sciencee 2009, 323, 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tiberi S; Payen MC; Sotgiu G; D’Ambrosio L; Alarcon Guizado VA; Alffenaar JW; Abdo Arbex MA; Caminero JA; Centis R; De Lorenzo S; Gaga M; Gualano G; Roby Arias AJR; Scardigli A; Skrahina A; Solovic I; Sulis G; Tadolini M; Akkerman OW; Alarcon Arrascue EA; Aleska A; Avchinko V; Bonini EH; Chong Marín FAC; Collahuazo López LC; de Vries G; Dore S; Kunst H; Matteelli A; Moschos C; Palmieri F; Papavasileiou A; Spanevello A; Vargas Vasquez DV; Viggiani P; White V; Zumla A; Migliori GB Effectiveness and Safety of Meropenem/Clavulanate-Containing Regimens in the Treatment of MDR- and XDR-TB. Eur. Respir. J 2016, 47, 1235–1243. [DOI] [PubMed] [Google Scholar]
- (14).Diacon AH; van der Merwe L; Barnard M; von Groote-Bidlingmaier F; Lange C; García-Basteiro AL; Sevene E; Ballell L; Barros-Aguirre D β-Lactams against Tuberculosis — New Trick for an Old Dog? N. Engl. J. Med 2016, 375, 393–394. [DOI] [PubMed] [Google Scholar]
- (15).De Jager V; Gupte N; Nunes S; Barnes GL; van Wijk RC; Mostert J; Dorman SE; Abulfathi AA; Upton CM; Faraj A; Nuermberger EL; Lamichhane G; Svensson EM; Simonsson U; Diacon AH; Dooley KE Early Bactericidal Activity of Meropenem Plus Clavulanate (+/−Rifampin) For TB: The COMRADE Randomized, Phase 2 Trial. Am. J. Respir. Crit. Care Med 2022, 205, 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Iannazzo L; Soroka D; Triboulet S; Fonvielle M; Compain F; Dubée V; Mainardi JL; Hugonnet JE; Braud E; Arthur M; Etheve-Quelquejeu M Routes of Synthesis of Carbapenems for Optimizing Both the Inactivation of L,D-Transpeptidase LdtMt1 of Mycobacterium Tuberculosis and the Stability toward Hydrolysis by β-Lactamase BlaC. J. Med. Chem 2016, 59, 3427–3438. [DOI] [PubMed] [Google Scholar]
- (17).Saidjalolov S; Edoo Z; Fonvielle M; Mayer L; Iannazzo L; Arthur M; Etheve-Quelquejeu M; Braud E Synthesis of Carbapenems Containing Peptidoglycan Mimetics and Inhibition of the Cross-Linking Activity of a Transpeptidase of L,D Specificity. Chem.—Eur. J 2021, 27, 3542–3551. [DOI] [PubMed] [Google Scholar]
- (18).Gupta R; Al-Kharji NMSA; Alqurafi MA; Nguyen TQ; Chai W; Quan P; Malhotra R; Simcox BS; Mortimer P; Brammer Basta LA; Rohde KH; Buynak JD Atypically Modified Carbapenem Antibiotics Display Improved Antimycobacte- rial Activity in the Absence of β-Lactamase Inhibitors. ACS Infect. Dis 2021, 7, 2425–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kitano K; Tomasz A Triggering of Autolytic Cell Wall Degradation in Escherichia Coli by Beta-Lactam Antibiotics. Antimicrob. Agents Chemother 1979, 16, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lavollay M; Arthur M; Fourgeaud M; Dubost L; Marie A; Veziris N; Blanot D; Gutmann L; Mainardi JL The Peptidoglycan of Stationary-Phase Mycobacterium Tuberculosis Predominantly Contains Cross-Links Generated by L,D-Trans- peptidation. J. Bacteriol 2008, 190, 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lavollay M; Fourgeaud M; Herrmann JL; Dubost L; Marie A; Gutmann L; Arthur M; Mainardi JL The Peptidoglycan of Mycobacterium Abscessus Is Predominantly Cross- Linked by L,D-Transpeptidases. J. Bacteriol 2011, 193, 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gupta R; Lavollay M; Mainardi JL; Arthur M; Bishai WR; Lamichhane G The Mycobacterium Tuberculosis Protein Ldt Mt2 Is a Nonclassical Transpeptidase Required for Virulence and Resistance to Amoxicillin. Nat. Med 2010, 16, 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Blumberg PM; Strominger JL Interaction of Penicillin with the Bacterial Cell: Penicillin-Binding Proteins and Penicillin-Sensitive Enzymes. Bacteriol. Rev 1974, 38, 291–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Erdemli SB; Gupta R; Bishai WR; Lamichhane G; Amzel LM; Bianchet MA Targeting the Cell Wall of Mycobacterium Tuberculosis: Structure and Mechanism of L,D- Transpeptidase 2. Structure 2012, 20, 2103–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kim HS; Kim J; Im HN; Yoon JY; An DR; Yoon HJ; Kim JY; Min HK; Kim SJ; Lee JY; Han BW; Suh SW Structural Basis for the Inhibition of Mycobacterium Tuberculosis L,D-Transpeptidase by Meropenem, a Drug Effective against Extensively Drug-Resistant Strains. Acta Crystallogr., Sect. D: Biol. Crystallogr 2013, 69, 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Dubée V; Triboulet S; Mainardi JL; Etheve-Quelquejeu M; Gutmann L; Marie A; Dubost L; Hugonnet JE; Arthur M Inactivation of Mycobacterium Tuberculosis L,D-Transpeptidase LdtMt1 by Carbapenems and Cephalosporins. Antimicrob. Agents Chemother 2012, 56, 4189–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cordillot M; Dubée V; Triboulet S; Dubost L; Marie A; Hugonnet JE; Arthur M; Mainardi JL In Vitro Cross-Linking of Mycobacterium tuberculosis Peptidoglycan by l , d -Transpeptidases and Inactivation of These Enzymes by Carbapenems. Antimicrob. Agents Chemother 2013, 57, 5940–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kumar P; Kaushik A; Lloyd EP; Li S-G; Mattoo R; Ammerman NC; Bell DT; Perryman AL; Zandi TA; Ekins S; Ginell SL; Townsend CA; Freundlich JS; Lamichhane G Non-Classical Transpeptidases Yield Insight into New Antibacterials. Nat. Chem. Biol 2017, 13, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Steiner EM; Schneider G; Schnell R Binding and Processing of β-Lactam Antibiotics by the Transpeptidase LdtMt2 from Mycobacterium tuberculosis. FEBS J 2017, 284, 725–741. [DOI] [PubMed] [Google Scholar]
- (30).Kumar P; Chauhan V; Silva JRA; Lameira J; d’Andrea FB; Li SG; Ginell SL; Freundlich JS; Alves CN; Bailey S; Cohen KA; Lamichhane G Mycobacterium abscessus L,D-Trans- peptidases Are Susceptible to Inactivation by Carbapenems and Cephalosporins but Not Penicillins. Antimicrob. Agents Chemother 2017, 61, No. e00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bianchet MA; Pan YH; Basta LAB; Saavedra H; Lloyd EP; Kumar P; Mattoo R; Townsend CA; Lamichhane G Structural Insight into the Inactivation of Mycobacterium Tuber- culosis Non-Classical Transpeptidase LdtMt2 by Biapenem and Tebipenem. BMC Biochem 2017, 18, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wang F; Cassidy C; Sacchettini JC Crystal Structure and Activity Studies of the Mycobacterium Tuberculosis β-Lactamase Reveal Its Critical Role in Resistance to β-Lactam Antibiotics. Antimicrob. Agents Chemother 2006, 50, 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Kumar G; Galanis C; Batchelder HR; Townsend CA; Lamichhane G Penicillin Binding Proteins and β-Lactamases of Mycobacterium Tuberculosis: Reexamination of the Historical Paradigm. mSphere 2022, 7, No. e0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Tremblay LW; Fan F; Blanchard JS Biochemical and Structural Characterization of Mycobacterium Tuberculosis β- Lactamase with the Carbapenems Ertapenem and Doripenem. Biochemistry 2010, 49, 3766–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rimal B; Batchelder HR; Story-Roller E; Panthi CM; Tabor C; Nuermberger EL; Townsend CA; Lamichhane G T405, a New Penem, Exhibits In Vivo Efficacy against M. Abscessus and Synergy with β-Lactams Imipenem and Cefditoren. Antimicrob. Agents Chemother 2022, 66, No. e0053622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kaushik A; Makkar N; Pandey P; Parrish N; Singh U; Lamichhane G Carbapenems and Rifampin Exhibit Synergy against Mycobacterium Tuberculosis and Mycobacterium Abscessus. Anti- microb. Agents Chemother 2015, 59, 6561–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lohans CT; Chan HTH; Malla TR; Kumar K; Kamps JJAG; McArdle DJB; van Groesen E; de Munnik M; Tooke CL; Spencer J; Paton RS; Brem J; Schofield CJ Non- Hydrolytic β-Lactam Antibiotic Fragmentation by L,D-Transpepti- dases and Serine β-Lactamase Cysteine Variants. Angew. Chem., Int. Ed 2019, 58, 1990–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zandi TA; Townsend CA Competing Off-Loading Mechanisms of Meropenem from an L,D-Transpeptidase Reduce Antibiotic Effectiveness. Proc. Natl. Acad. Sci. U.S.A 2021, 118, No. e2008610118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kumar P; Arora K; Lloyd JR; Lee IY; Nair V; Fischer E; Boshoff HIM; Barry CE Meropenem Inhibits D,D-Carboxypeptidase Activity in Mycobacterium Tuberculosis. Mol. Microbiol 2012, 86, 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Walkup GK; You Z; Ross PL; Allen EKH; Daryaee F; Hale MR; O’Donnell J; Ehmann DE; Schuck VJA; Buurman ET; Choy AL; Hajec L; Murphy-Benenato K; Marone V; Patey SA; Grosser LA; Johnstone M; Walker SG; Tonge PJ; Fisher SL Translating Slow-Binding Inhibition Kinetics into Cellular and in Vivo Effects. Nat. Chem. Biol 2015, 11, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Copeland RA; Pompliano DL; Meek TD Drug-Target Residence Time and Its Implications for Lead Optimization. Nat. Rev. Drug Discovery 2006, 5, 730–739. [DOI] [PubMed] [Google Scholar]
- (42).Lu H; Tonge PJ Drug-Target Residence Time: Critical Information for Lead Optimization. Curr. Opin. Chem. Biol 2010, 14, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hall LM; Hall LH; Kier LB QSAR Modeling of β-Lactam Binding to Human Serum Proteins. J. Comput.-Aided Mol. Des 2003, 17, 103–118. [DOI] [PubMed] [Google Scholar]
- (44).Kaushik A; Ammerman NC; Tasneen R; Story-Roller E; Dooley KE; Dorman SE; Nuermberger EL; Lamichhane G In vitro and in vivo activity of biapenem against drug-susceptible and rifampicin-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother 2017, 72, 2320–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kaushik A; Ammerman NC; Lee J; Martins O; Kreiswirth BN; Lamichhane G; Parrish NM; Nuermberger EL In Vitro Activity of the New β-Lactamase Inhibitors Relebactam and Vaborbactam in Combination with β-Lactams against Mycobacterium abscessus Complex Clinical Isolates. Antimicrob. Agents Chemother 2019, 63, No. e02623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Clinical and Laboratory Standards Institute. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes; Woods GL,Ed.; Clinical and Laboratory Standards Institute, 2018; Supplement M62. [PubMed] [Google Scholar]
- (47).Brammer Basta LAB; Ghosh A; Pan Y; Jakoncic J; Lloyd EP; Townsend CA; Lamichhane G; Bianchet MA Loss of a Functionally and Structurally Distinct LD-Transpeptidase, LdtMt5, Compromises Cell Wall Integrity in Mycobacterium Tuberculosis. J. Biol. Chem 2015, 290, 25670–25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


