Abstract
In recent years, tumor immunotherapy has made significant progress. However, tumor immunotherapy, particularly immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors), benefits only a tiny proportion of patients in solid cancers. The tumor microenvironment (TME) acts a significant role in tumor immunotherapy. Studies reported that tumor-associated macrophages (TAMs), as one of the main components of TME, seriously affected the therapeutic effect of PD-1/PD-L1 inhibitors. In this review, we analyzed TAMs from epigenetic and single-cell perspectives and introduced the role and mechanisms of TAMs in anti-programmed death protein 1(anti-PD-1) therapy. In addition, we summarized combination regimens that enhance the efficacy of tumor PD-1/PD-L1 inhibitors and elaborated on the role of the TAMs in different solid cancers. Eventually, the clinical value of TAMs by influencing the therapeutic effect of tumor PD-1/PD-L1 inhibitors was discussed. These above are beneficial to elucidate poor therapeutic effect of PD-1/PD-L1 inhibitors in solid tumors from the point of view of TAMs and explore the strategies to improve its objective remission rate of solid cancers.
Keywords: Tumor-associated macrophages, Immune checkpoint inhibitors, Cancer, Combined therapy
Background
Since the US Food and Drug Administration approved ipilimumab (anti-CTLA-4) for the treatment of metastatic melanoma in 2011 [1], several checkpoint-blocking therapies targeting the PD-1/PD-L1 axis have been approved for the treatment of multiple tumor types. A good understanding of the impact of the tumor microenvironment(TME) on tumor immunotherapy is essential for effectively integrating immunotherapy with chemotherapy, targeted therapy, and other immunotherapies. Studies have shown that tumor-associated macrophages (TAMs) act a crucial role in tumor immunotherapy [2]. TAMs affect the therapeutic effect of PD-1/PD-L1 inhibitors through various mechanisms, including regulating the expression of PD-L1 in tumor cells and secreting a variety of cytokines to produce tumor-promoting TME [3–5]. Chemotherapy, targeted therapy, and radiotherapy effectively remodel TME, especially TAMs, and transform them from pro-tumor to antitumor [6–8].
In this review, we summarized the currently approved immune checkpoint inhibitors (ICIs) (Table 1) and the role of TAMs in anti-PD-1/PD-L1 treatment for solid cancers [9–16]. We described the synergistic effects of anti-PD-1/PD-L1 therapy in combination with targeted therapy, chemotherapy, radiotherapy, and other immunotherapies. The mechanism of the impact of TAMs on immunotherapy in different solid cancers was also concluded. Furthermore, the clinical application value of TAMs for the solid cancer treatment of PD-1/PD-L1 inhibitors is proposed based on the vital role of TAMs in immunotherapy.
Table 1.
The approved immune checkpoint inhibitors in the globe
| Target | Active Ingredients | First approval time | Company | Application(approved in the globe) |
|---|---|---|---|---|
| PD-1 | Nivolumab | 2014 | Bristol Myers Squibb | Melanoma, NSCLC, MPM, RCC, HL HNSCC, UC, CRC, HCC, Esophageal Cancer, GC, GEJC, EA |
| Pembrolizumab | 2014 | Merck Sharp Dohme | Melanoma, NSCLC, HNSCC, HL, PMBCL, UC, CRC, GC, Esophageal cancer, CC, HCC, MCC, RCC, Endometrial carcinoma, CSCC, TNBC | |
| Cemiplimab | 2018 | Regeneron Pharmaceuticals | CSCC, BCC, NSCLC | |
| Toripalimab | 2018 | Shanghai Junshi Biosciences Co., Ltd. | Melanoma, UC, NPC, ESCC | |
| Sintilimab | 2018 | Innovent Biologics, Inc | NSCLC, HL, HCC, ESCC, GC, GEJC | |
| Camrelizumab | 2019 | Jiangsu Hengrui Medicine Co.,Ltd. | NSCLC, HL, HCC, ESCC, NPC | |
| Tislelizumab | 2019 | Beigene, Ltd. | NSCLC, HL, UC, HCC, NPC, ESCC, CRC | |
| Zimberelimab | 2021 | Guangzhou Gloria Biosciences Co., Ltd. | HL | |
| Prolgolimab | 2020 | Biocad. | Melanoma, SC | |
| Dostarlimab | 2021 | GSK Plc | Endometrial carcinoma | |
| PD-L1 | Atezolizumab | 2016 | Genetech Inc | Melanoma, NSCLC, SCLC, HCC, ASPS |
| Durvalumab | 2017 | AstraZeneca | NSCLC, ES-SCLC, BTC, HCC | |
| Avelumab | 2017 | EMD Serono Inc | UC, MCC, RCC | |
| CTLA-4 | Ipilimumab | 2011 | Bristol Myers Squibb | Melanoma, RCC, CRC, HCC, NSCLC, MPM, Esophageal cancer |
Abbreviations: ASPS Alveolar soft part sarcoma, BCC Basal cell carcinoma, BTC Biliary tract cancer, CC Cervical cancer, CRC Colorectal cancer, CSCC Cutaneous squamous cell carcinoma, EA Esophageal adenocarcinoma, ESCC Esophageal squamous cell carcinoma, ES-SCLC Extensive-stage small cell lung cancer, GC Gastric cancer, GEJC Gastroesophageal junction cancer, HCC Hepatocellular carcinoma, HL Hodgkin lymphoma, HNSCC Head and neck squamous cell cancer, MCC Merkel cell carcinoma, MPM Malignant pleural mesothelioma, NPC Nasopharyngeal carcinoma, NSCLC Non-small cell lung cancer, PMBL Primary mediastinal B cell lymphoma, RCC Renal cell carcinoma, SC Skin cancer, SCLC Small cell lung cancer, TNBC Triple-negative breast cancer, UC Urothelial carcinoma
TAMs and TME
TME contains not only tumor cells but also innate and adaptive immune cells, fibroblasts, endothelial cells, pericytes, and non-cellular components such as extracellular matrix and soluble signals that infiltrate the tumor [17, 18]. TME is reportedly deeply associated with tumor tissue formation, survival, and metastasis [19, 20]. TAMs are the most plasticity and the highest proportion of immune cells in the TME [21]. TAMs were generally classified into two main phenotypes: classical activation (M1-like macrophages) and alternating activation (M2-like macrophages) [22]. M1-like macrophages are low in mannose receptor (CD206) and high expression of MHCII. M1-like macrophages are characterized by increased expression of inducible nitric oxide synthase (iNOS), tumor necrosis factor-α(TNF-α), and co-stimulatory molecules such as CD40, CD86, and various pro-inflammatory cytokines such as IL6, IL1b, IL12a, IL12b. M1-like macrophages induce antitumor immune responses through their T cell stimulating activity [23, 24]. TAMs were polarized to the M2 type under the induction of a variety of mediators, including IL-4, IL-10, transforming growth factor-β (TGF-β), and macrophage colony-stimulating factor (M-CSF) [24, 25]. Unlike M1, CD163+ is characteristic of M2-like macrophages, which express high mannose receptors and low levels of MHC II and release immunosuppressive cytokines such as vascular endothelial growth factor (VEGF) and arginase 1 (Arg-1), IL-10, TGF-β, indolamine 2,3-dioxygenase (IDO). In terms of cell function, M2-like macrophages promote tumor immune evasion, angiogenesis, tumor growth and metastasis [26–32].
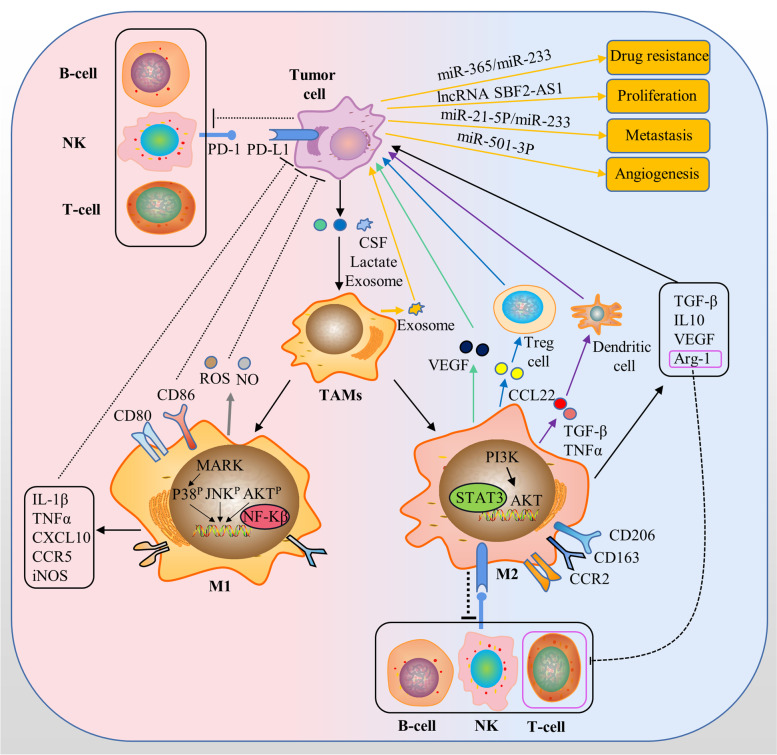
Increasing evidences suggest that TAMs play a significant role in tumor development. TAMs directly communicate with tumor cells. On the one hand, TAMs affect tumor cells through exosome metastasis of substances like some non-coding RNAs(ncRNAs) [33]. TAMs-derived miR-223 is vital for breast cancer progression. Similarly, both miR-21-5p and miR-155-5p act essential roles in the migration and invasion processes of colon cancer cells [34]. In addition, lncRNA SBF2-AS1 absorbed by pancreatic cancer cells also promotes tumor proliferation, invasion, and migration [35]. Parallelly, the TAMs-derived VEGF and miR-501-3p directly mediate the angiogenesis in the tumor tissues [36, 37]. Furthermore, the miR-365 exosomes inhibit the effects of gemcitabine by upregulating pyrimidine metabolism and increasing nucleotide triphosphate levels in cancer cells [38]. On the other hand, TAMs secrete various cytokines that act on tumor cells. For example, TNF, IL-6, and IFN- γ upregulate PD-L1 expression in tumor cells. Indirect effects of TAMs on tumor cells are achieved by influencing other immune cells to regulate the TME. TAMs directly inhibit CD8+ T-cell proliferation through the metabolism of L-arginine via Arg-1, iNOS, and oxygen radicals [39, 40]. TAMs also induce T cell inhibition by the immune checkpoint through upregulation of PD-L1 expression. Moreover, TAMs recruit Tregs through CCL22 to further inhibit the antitumor immune response of T cells [41]. M2-polarized TAMs release a variety of anti-inflammatory cytokines (e. g., TGFB1) and chemokines (e. g., CCL22) that inhibit dendritic cell maturation and thus limit antigen presentation [41].
Evidently, TAMs create a TME suitable for tumor growth by suppressing the antitumor activity of immune cells. Inversely, when TAMs are polarized to M1, they directly mediate cytotoxicity to kill tumor cells. In this case, macrophages release tumor-killer molecules, such as reactive oxygen species (ROS) and NO, which have cytotoxic effects on tumor cells [42]. The other is antibody-dependent cell-mediated cytotoxicity killing of tumor cells that requires the involvement of antitumor antibodies [43]. At the same time, the effect of TAMs on cancer cells is not unidirectional. Tumor cells also regulate TAMs to exert an immunosuppressive function through multiple mechanisms. For example, colony-stimulating factor 1 (CSF1) secreted by tumor cells favors the recruitment of monocyte-derived macrophages to the TME and polarizes them to the M2-like manner [44]. Moreover, lactate produced due to the high metabolism of tumor cells promotes M2 polarization of TAMs [45]. Consequently, TAMs build a complex immune regulatory network through various signaling mechanisms and other cells in the TME (Fig. 1).
Fig. 1.
The role of TAMs in TME. Exosomes derived from TAMs deliver various molecules into tumor cells, which contributes to tumor development. Exosomal lncRNA SBF2-AS1 facilitates tumor cell proliferation. Exosomal miR-223 and miR-21-5p promote the metastasis of tumor cells from the primary tumor to the distal organs. Exosomal miR-501-3p promotes the angiogenesis of tumors. Exosomal miR-223 and miR-365 help tumor cells develop resistance to chemotherapy. TAMs express ligand receptors for PD-1 and CTLA-4, inhibiting the cytotoxic function of T cells, NK cells, and NK cells upon activation. TAMs express chemokine CCL22, etc., to recruit Treg cells. TAMs secrete VEGF to promote angiogenesis in TME. TAMs release a variety of anti-inflammatory cytokines to inhibit dendritic cell maturation, thereby limiting antigen presentation. In addition, tumor cells affect TAMs polarization by releasing exosomes, cytokines and their metabolites
ICIs of cancer therapy
In the 90s of the twentieth century, immune checkpoint molecules were discovered, and two representative checkpoint pathways were cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and PD-1 [46, 47]. Under physiological conditions, immune checkpoints inhibit the overactivation of T cells and prevent autoimmune responses, which is an essential mechanism for maintaining the immune balance in the body. Among them, CTLA-4 is induced to be expressed on activated T cells, competing with CD28 to inhibit CD80/CD86-mediated synergistic stimulation signals. In addition, antigen-stimulated T cells upregulate the expression of PD-1 to bind to their ligand PD-L1 or programmed death ligand 2 (PD-L2), inhibiting T cell overactivation [48–50]. However, the mechanism of maintaining immune balance has become an accomplice to the tumor in the tumor state. The reason of the above is that some cancer cells strongly express ligands of immune checkpoint molecules, such as PD-L1 and PD-L2. These ligands bind to PD-1 in activated T cells, specific B cells, natural killer cells (NKs), dendritic cells (DCs), and macrophages. The interaction between PD-1 and PD-L1 significantly inhibits the antitumor immunity of cytotoxic T cells, producing immunosuppressive effects and thus causing the immune escape phenomenon of tumors [51–53]. By contrast, PD-1 is expressed more widely in various immune cells than CTLA-4, which is limited to T cells, meaning that PD-1 may play a broader role in immunomodulation.
ICIs eliminate the suppressive signals of T cell activation, consequently enabling tumor-reactive T cells to overcome regulatory mechanisms and producing a potent antitumor response [54]. To date, all approved ICIs are monoclonal antibodies (Table 1) that block CTLA-4, PD-1, or PD-L1, essential drugs for activating T cells to promote their immune function [54]. However, the efficacy of ICIs is often limited and transient, reflecting the complexity of antitumor immunity [55, 56]. For example, ICIs are less effective in treating cancers of microsatellite stability of wild gene abnormalities which don’t induce cancer antigen-specific T cells. Furthermore, immunosuppressive TME also affects the clinical effect of ICIs. Meanwhile, the effectiveness of immunotherapy is affected by various resistance mechanisms, including immune rejection, immunoediting, antigen presentation reduction, and immunosuppression of soluble cellular [57, 58]. Notably, TAMs are key to these mechanisms. Because TAMs are an important inducing factor for inhibitory TME and a fundamental reason for the resistance to ICIs. Specifically, TAMs capture anti-PD-1/PD-L1 through Fcg receptors present on the cell surface [59]. In addition, TAMs upregulate PD-L1 expression in tumor cells and other immunosuppressive cells by secreting cytokines and metabolites [60, 61]. Furthermore, TAMs directly express PD-L1 under the influence of TME state, and induce CD8+ T cell inactivation and apoptosis through PD-1 binding on the surface of CD8+ T cells [62]. In this case, treatment with anti-PD-1/PD-L1 will be less effective. In this state, if TAMs can be prevented from expressing PD-L1, it will be beneficial to the therapeutic effect of ICIs.
Analysis of TAMs from epigenetic perspectives
Epigenetics refers to heritable changes in gene function without altering the DNA sequence of a gene. Its mechanisms of action include, but are not limited to, DNA methylation, histone modification and ncRNAs [63]. Studies have shown that the epigenetic regulation of TAMs is essential for their differentiation and functional programming [64, 65].
Effect of DNA methylation
DNA methylation refers to silencing gene transcription and is characterized by the transfer of methyl groups to the cytosine ring of DNA (forming 5-methylcytosine) by DNA methyltransferases (DNMTs) [66]. DNA methylation is removed by another set of enzymes known as ten-eleven translocation proteins [67]. Studies have shown that DNMT3b knockdown induces M2 polarization and increases the expression of M2-like macrophages markers, such as Arg-1 and mannose receptor type C. Moreover, overexpression of DNMT3b inhibits the expression of IL-4-induced Arg-1 in macrophages. This suggests that DNMT3b is a vital factor in inhibiting macrophage polarization to M2 [68].
Effect of histone modification
Common histone modifications include histone acetylation-regulated histone acetylation and histone methyltransferase-mediated histone methylation [69].
Histone acetylation promotes gene transcription, which is mediated by histone acetyltransferase (HATs) and removed by histone deacetylase (HDACs). Both of them play an integral role in gene expression regulation. For example, autocrine IFN-β–Jak–STAT loops induced by Toll-like receptor (TLR) ligands and TNF are crucial links in M1 activation. The response of molecules downstream of IFN is strongly dependent on HDAC3 [25, 70]. A similar situation occurs in the mechanism of action of CCCTC-binding factor (CTCF). CTCF is a crucial transcription factor in TAMs. CTCF forms a complex with PACERR (an antisense LncRNA) to recruit HAT to the promoter region of its downstream gene PTGS2 (a tumor-promoting M2 gene). HAT induces histone acetylation and chromatin accessibility, promoting their expression and ultimately affecting M2 differentiation. Meanwhile, HAT enhances the pro-tumor metastasis effect of M2-like macrophages [71]. Similarly, HDAC is a negative regulator of M2 polarization, and HDAC9 deletion leads to the downregulation of inflammatory genes and M2 polarization [72]. Furthermore, HDAC6 intervention reduces the anti-inflammatory phenotype of TAMs. M2 polarization was inhibited after the inhibition of HDAC6 enzyme activity with the drug and increased M1 polarization [73].
Histone methylation is facilitated by histone methyl transferases and removed by histone demethylase. H3K27 methylation is a mark for transcription repression. After IL-4 treatment, H3K27 me2/3 was significantly reduced at the promoter of the M2 marker gene (i.e., Arg-1). Meanwhile, H3K27me2/3-specific demethylase Jmjd3 was significantly elevated under IL-4 induction. Jmjd3 helps keep the M2 marker gene in active state [74].
Effect of ncRNAs
NcRNAs play a significant role in the post-transcriptional control of gene expression [75]. The epigenetic remodeling by ncRNAs regulates macrophage activation and functional programming. Among them, ncRNAs that play a key role are mainly divided into three categories: microRNA (miRNA), circular RNA (circRNA), and long noncoding RNA (lncRNA). Firstly, miRNAs are small regulatory RNA molecules that modulate the expression of their target genes [76]. MiRNAs play a huge regulatory role in the gene expression and polarization processes of macrophages. Some of them induce an antitumor immune microenvironment. For example, miR-98 regulates macrophage polarization from M2-like macrophages to M1-like macrophages in hepatocellular cancer (HCC) by targeting IL-10 and induces elevated expression levels of M1-like macrophages marker cytokines, such as TNF-α, IL-1β, and IL-12 [77]. Similarly, miR-101 directly targets DUSP1 to regulate MAPKs activation and subsequent pro-inflammatory cytokines production [78]. In addition, miR-17a and miR-20a also induce M1 polarization and activate M1-like macrophages [79]. In addition, some miRNAs inhibit M2 polarization through various pathways, such as miR-155, miR-720, MiR-23a, and miR-127etc [80–83]. Moreover, unlike the above miRNAs, some miRNAs induce immunosuppressive microenvironments. For example, miR-146a facilitates M2-like macrophages marker genes expression and restricts M1-like macrophages marker gene expression [84].
Secondly, circular RNAs (circRNAs) are a class of ncRNAs that do not contain a 5′ end cap and a 3′ end poly tail [83]. It is widely involved in the regulation of tumor cell proliferation, differentiation, invasion, migration, and the formation of TME [85, 86]. For example, circCdyl promotes M1 polarization by inhibiting interferon regulatory factor 4 entry into the nucleus [87]. In addition, circPPM1F promotes lipopolysaccharide (LPS)-induced M1-like macrophages activation by enhancing the NF-κB signaling pathway [88]. It is different from the above two circRNAs, overexpression of hsa_circ_0005567 inhibited M1 polarization and promoted M2 polarization via the miR-492/SOCS2 axis [89].
Finally, long noncoding RNAs (lncRNAs) are a new class of RNA that is longer than 200 nucleotides and does not have protein-coding capabilities [90]. Some lncRNAs are involved in tumorigenesis and progression by regulating the TME. LncRNA-cox-2 inhibits tumor growth and immune evasion of HCC cells by inhibiting M2 polarization and promoting M1 polarization in macrophages [91]. LncRNA-CASC2c inhibits M2 polarization and tumor growth by inhibiting the expression and secretion of coagulation factor X [92]. LncRNA-TUC339 is highly expressed in M2-like macrophages and less expressed in M1-like macrophages. It is involved in the polarization of M2-like macrophages [93]. The epigenetic mechanisms that control macrophage polarization are complex. Enzymes and ncRNAs which play an essential role in gene modification, are expected to become new tumor markers and potential targets, providing new directions for tumor diagnosis and targeted therapy.
Analysis of TAMs at the single-cell level
As mentioned earlier, TAMs have a wide range of plasticity and heterogeneity. However, traditional sequencing methods often mix a group of cells together for sequencing, making it difficult to capture possible heterogeneity between cells. Individual cell mutations in tumor progression cannot be accurately tracked [94]. To a large extent, the single-cell RNA sequencing (scRNA-seq) technology can solve this problem.
Introduction of scRNA-seq
ScRNA-seq is a single-cell transcriptome analysis technique. The workflow typically includes sample collection, cell dissociation, single-cell capture, reverse transcription, cDNA amplification, library preparation, and sequencing and analysis [95]. ScRNA-seq enables quantitative analysis of gene expression profiles of different types of cells at the single-cell level, enabling unprecedented detail to characterize cell diversity and heterogeneous phenotypic status [96, 97]. This technique overcomes the shortcomings of traditional sequencing technology that cannot detect cell-cell heterogeneity and is an effective tool for studying gene expression patterns.
Research advance of scRNA-seq technology for TAMs
The use of scRNA-seq for TAMs research mainly focuses on the following aspects: Firstly, identification of different macrophage subsets; Secondly, construction of the TME maps; Thirdly, identification of potential prognostic markers; Fourthly, analysis of intercellular interactions in TME; Finally, Interpretation the mechanisms of TAMs in tumor treatment and drug resistance (Table 2).
Table 2.
Application of scRNA-seq in tumor-associated macrophages (TAMs)
| Research Field | Cancer | Findings | References |
|---|---|---|---|
| Identification of different macrophage subsets | Small cell lung cancer | This study identified a profibrotic, immunosuppressive monocytes/macrophage population that is particularly associated with a PLCG2 high small cell lung cancer subpopulation. | [98] |
| Breast cancer | ScRNA-seq reveals two subsets of APOE+ macrophages: the TREM2+ macrophages and the FOLR2+ macrophages. FOLR2+ macrophages are tissue-resident cells. | [99] | |
| Renal tumor | The study found a novel, tumor-specific sub-population of macrophages and differentially expressed genes (i.e., C1QA-C, APOE, and TREM2). | [100] | |
| Construction of the tumor microenvironment maps | Gallbladder cancer | M2-like macrophages, epithelial cells, and Treg were predominant in ErbB pathway mutation tumors. | [101] |
| Breast cancer | Most of the non-cancer cells are immune cells, with three distinct clusters of T lymphocytes, B lymphocytes and macrophages. Macrophages have an M2 phenotype that expresses many M2 genes, such as CD163, MS4A6A, and TGFBI, as well as genes known to promote tumor progression and angiogenesis, such as PLAUR13 and IL-8, exhibit immunosuppressive signatures. | [102] | |
| Glioma | Microglia-derived TAMs dominate in newly diagnosed tumors. However, they are overtaken by monocytes-derived TAMs after tumor recurrence, particularly in hypoxic tumor settings. | [103] | |
| Identification of potential prognostic markers | Glioma | Sex-specific gene expression in glioma-activated microglia (e.g., genes encoding MHCII complexes) may be associated with morbidity and outcomes in patients with gliomas. | [104] |
| Breast cancer | FOLR2+ macrophages positively correlate with better prognosis. | [99] | |
| Renal tumor | TREM2/APOE/C1Q+ macrophages infiltration is a potential prognostic biomarker for clear cell renal carcinoma recurrence. | [100] | |
| Analysis of intercellular interactions in TME | Gallbladder cancer | High levels of midkine, expressed by the ErbB pathway mutation tumor cells, interact with the receptor LRP1 of tumor-infiltrating macrophages and promote immunosuppressive macrophage differentiation. The crosstalk between CXCL10 secreted by macrophage and its receptor CXCR3 on Tregs was induced in gallbladder cancer with ErbB pathway mutations. | [101] |
| Breast cancer | FOLR2+ macrophages interact with tumor-infiltrating CD8+ T cells. | [99] | |
| Metastatic ovarian cancer | Macrophages in stress-high samples exhibited significantly higher expression of immunosuppressive features (C1QA, C1QB, C1QC, APOE, and TREM2), wherein TREM2 is functionally associated with T cell exhaustion. | [105] | |
| Head and neck tumors | The main contributors of PD-L1 in the TME were dendritic cells and macrophages. PD-1-PD-L1 interactions appeared to be primarily mediated by macrophages. PD-L1+ macrophages spatially associate with CD8+ T cells in the head and neck squamous cell carcinoma microenvironment. | [106] | |
| Gastric cancer | This study uncovered macrophages may contribute to HLA-E-KLRC1/KLRC2 interaction with cytotoxic CD8+ T cells and natural killer cells. | [107] | |
| Colon cancer | In tumors, TAMs and dendritic cells, as the core of the predictive network harbor the most connections with other cell types. | [108] | |
| Explore the mechanisms of drug intervention | Colon cancer | Two distinct TAMs subsets show differential sensitivity to CSF-1R blockade treatment with anti-CSF-1R preferentially depletes macrophage populations with an inflammatory signature but spare macrophage subset that expresses proangiogenic and tumorigenic genes. | [108] |
| Pan-cancer | Anti-PD-1 therapy decreases the number of Arg-1+ TAMs while increasing Arg-1-TAMs. On a local scale, a new cell subpopulation rich in chemotaxis and interferon response genes is formed. | [109] | |
| Pancreatic cancer | Anti-CD47 treatment led to changes in TME with increased pro-inflammatory macrophages, while reduced anti-inflammatory macrophages. | [110] | |
| Metastatic lung cancer | Macrophages demonstrated an inversion in relative abundance during tumor response and resistance to treatment. | [111] | |
| Explore the mechanisms of drug resistance | Metastatic ovarian cancer | Stress-associated cancer cells strongly associate with a shift toward immunocompromised states within macrophages and CD8+ T cells. This stress-associated state provides cancer cells with adaptation, promoting chemoresistance. | [105] |
| Pan-cancer | Tumor vessel co-option is a resistance mechanism against anti-angiogenic therapy. Matrix-remodeling macrophages might assist invasive cancer cells to co-opt vessels. An M1-like macrophage subtype may keep vascular cells quiescent. | [112] | |
| Explore the mechanisms of non-drug interventions | Pancreatic cancer | After radiofrequency ablation, the percentage of Mac_s5 lacking mature markers decreased significantly; The proportion of Mac_s1 with anti-inflammatory gene expression profiles was also significantly reduced, and the proportion of Mac_s2 and Mac_s3 cells with anti-tumor functions increased. | [113] |
Abbreviations: TAMs Tumor-associated macrophages
ScRNA-seq technology is used to identify different macrophage subsets and construct TME maps. In the analysis of macrophages in various tumors using scRNA-seq, macrophages are found to rely on different activation stimuli to obtain heterogeneous phenotypes. There are significant changes in gene expression in macrophages in TME compared to normal tissue [114–116]. For example, in a study of single-cell sequencing and protein activity of monocytic macrophages in kidney cancer tissues and adjacent tissues, a population of CD11C+ /CD163+ macrophages was identified to be higher than normal tissues in tumor tissues [100]. Compared to other cell populations and non-tumor macrophages, TAMs have unique differentially expressed genes (C1QA-C, APOE, and TREM1) and differentially active proteins (LILRB5, APOE, and TREM2) [117, 118]. Furthermore, scRNA-seq technology is used to construct TME maps. Single-cell transcriptome analysis of tumor tissue allows the characterization of heterogeneous tumor cells, adjacent stromal cells, and immune cells [102]. For example, M2-like macrophages, epithelial cells, and Treg were predominant in ErbB pathway mutation tumors [101]. In addition, the composition of TME varies at different stages of the tumor. The study found that microglia-derived TAMs dominate in newly diagnosed tumors. However, they are overtaken by monocytes-derived TAMs after tumor recurrence [103]. A good understanding of the TME maps of different tumor subtypes helps develop effective treatment strategies.
ScRNA-seq technology is used to identify potential prognostic markers. Different subpopulations of TAMs have unique marker genes that are sometimes linked to a patient’s prognosis [119]. Studies have reported that sex-specific gene expression in glioma-activated microglia (e.g., genes encoding MHCII complexes) may be associated with morbidity and outcomes in patients with gliomas [104]. Reliable prognostic markers guide physicians to understand disease trends and make rational clinical decisions.
ScRNA-seq technology is used to analyze cell-cell interactions in TME. As the central node of the cell-cell interaction network, TAMs play a vital role in the signal communication of TME. When tumors progress, FOLR2+ TAMs acquire the ability to activate naïve CD8+ T cells. FOLR2+ TRMs prime naive CD8+ T cells into polyfunctional effectors [99]. Understanding cell-cell communication networks helps us to use appropriate strategies to reshape TME.
ScRNA-seq technology is used to interpret the mechanisms of TAMs in tumor treatment and drug resistance. TAMs are one of the effector cells in various tumor treatment options. One of the mechanisms of action of many drugs to treat tumors is to alter TME by influencing TAMs. For example, after anti-CD47 treatment, the proportion of macrophages decreased while the proportion of lymphocytes increased, significantly reducing tumor growth [110]. TAMs also play an essential role in non-drug antineoplastic therapy. Radiofrequency ablation (RFA) is an effective local therapy approach for treating solitary tumors [120]. RFA treatment reduced the proportion of immunosuppressive cells, including TAMs, while increasing the percentage of functional T cells in distant non-RFA tumors [113]. Furthermore, TAMs also act a huge role in tumor drug resistance. Tumor vessel co-option is a resistance mechanism against anti-angiogenic therapy. Studies have shown that Matrix-remodeling macrophages might assist invasive cancer cells to co-opt vessels [112]. Clarifying the mechanism of action and resistance of drugs help guide the rational use of drugs in clinical practice and maximize the benefits for patients.
Interaction effect of TAMs and PD-1/PD-L1 inhibitors in TME
Effects of TAMs on PD-1/PD-L1 expression and TME
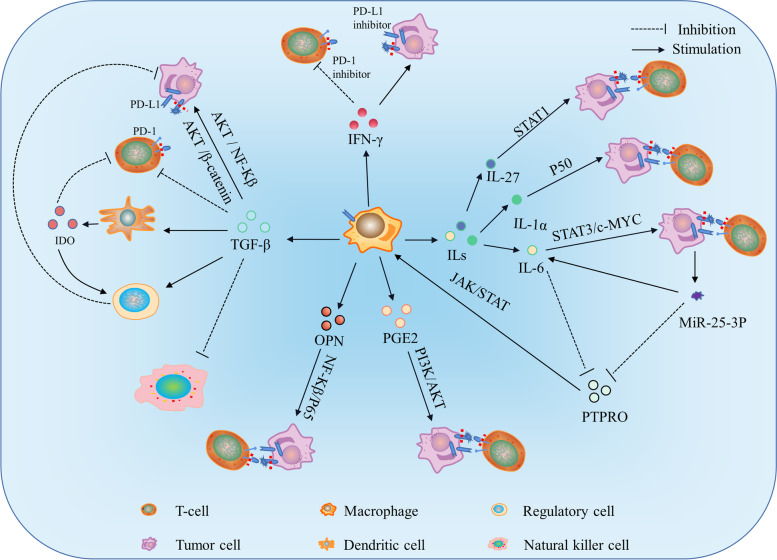
As mentioned earlier, macrophages affect PD-L1 expression through various signaling pathways mediated by multiple cytokines. For example, TGF-β upregulates tumor cell and TAMs PD-L1 expression through the AKT/NF-kB or AKT/β-catenin (β-catenin plays a critical role in polarizing macrophages to TAMs, resulting in epithelial-mesenchymal transition and tumor progression) pathway after binding to its receptor [121, 122]. IFN-γ, a factor that promotes the transcription of new PD-L1 mRNAs by activating the transcription factor STAT1, facilitates PD-L1 transcription and translation rather than shifting PD-L1 stored intracellularly to the cell surface [123]. Under the action of pro-inflammatory factors such as LPS, IL-1β, and TNFα, TAMs synthesize large amounts of PGE2 [124]. PGE2 inhibits T cell activation and function by increasing PD-L1 expression. As a downstream of cyclooxygenase 2 (COX-2), PGE2 levels in TAMs are regulated by the expression of COX-2 and microsomal PGE2 synthase 1 [125]. In ovarian cancer cells, PGE2 upregulates the PD-L1 expression of tumor cells by activating the PI3K/ Akt/ mTOR pathway [126]. Osteopontin (OPN) is expressed in both tumor cells and TAMs. OPN-expressing macrophages upregulate PD-L1 expression via regulating the NF-kB/p65 pathway and aggravate tumor progression [127]. Interleukins of TAMs, such as IL-1a, IL-10, IL-27, IL-6, etc., also significantly influence the expression of PD-L1. Among them, IL-1a and IL-27 induce the transcription of new PD-L1 mRNA, thereby increasing the expression of PD-L1. The combined application of IL-1a/ IL-10 and IFN-γ enhances the expression of PL-L1, indicating synergy between different signaling pathways. There is no combined enhancement effect for IL-27 [128]. IL-1a signaling drives PD-L1 protein expression through p65, while IL-27 signaling drives PD-L1 protein expression through STAT1. IL-6 promotes PD-L1 expression in macrophages by regulating protein tyrosine phosphatase, receptor type O (PTPRO), either directly or indirectly through an IFN- γ -dependent mechanism. PTPRO is a negative regulator of the JAK2/STAT3 signaling pathway that induces immunosuppression. MiR-25-3p reduces transcription and protein expression by targeting the 3’UTR of PTPRO in macrophages. IL-6 upregulates miR-253p in tumor cells by STAT3/c-MYC signaling [129]. Furthermore, previous studies have shown that miR-25-3p secreted by tumor cells promotes IL-6 secretion in TAMs through exosomes [129] (Fig. 2).
Fig. 2.
The release of multiple cytokines through TAMs affects PD-L1 expression. TGF-β upregulates tumor cell and PD-L1 expression of TAMs through the AKT/NF-kB or AKT/β-catenin pathway. IFN-γ upregulates PD-L1 expression by activating the transcription factor STAT1. PGE2 upregulates tumor cell PD-L1 expression by activating the PI3K/Akt/mTOR pathway. ILs upregulate PD-L1 expression in tumor cells through different pathways
Effect of PD-1/PD-L1 on macrophages
Plenty of stimuli upregulate PD-1 expression. The upregulated PD-1 inhibits the Janus N-terminal-linked kinase signaling pathway and PI3K/Akt pathway by re-recruiting SHP-2. And then, PD-1 affects the function of macrophages and downregulates the expression of co-stimulatory molecules, such as CD86, MHC I, and II proteins [130]. Studies have reported that PD-1+ TAMs have a reduced phagocytic capacity compared to PD-1- TAMs [131]. Moreover, the PD-L1 exosomes secreted by tumor cells have a positive feedback effect on the expression of PD-L1 in macrophages, which leads to M2 polarization of TAMs [132]. In addition, PD-1 has been shown to be associated with the apoptosis of macrophages. The expression of PD-1 in macrophages after hydrogen peroxide treatment is increased. Moreover, PD-1 negatively regulates the activation of the survival-promoting AKT pathway in macrophages through the PD-1-SHP-2 signaling axis, ultimately leading to increased macrophage apoptosis [133].
Effect of anti-PD-1/PD-L1 therapy on macrophages
Studies have shown that anti-PD-1/PD-L1 therapy promotes macrophage maturation. After anti-PD-L1 treatment, the number of cell subsets lacking classic macrophage maturity markers such as Mertk in tumors decreases, while the number of Mertk-expressing cell subsets increases significantly. This increase is reflected in subpopulations and the overall number of macrophages [134, 135]. Furthermore, anti-PD-L1 therapy activates macrophages by upregulating the expression of the co-stimulatory molecules CD86 and MHC II. In addition, anti-PD-L1 treatment reduces the level of M2-like macrophages markers, such as Arg-1, on TAMs by promoting the production of IFN-γ in CD8 T cells. Meanwhile, anti-PD-L1 therapy increases the levels of M1-like macrophages markers such as iNOS, MHC II, and CD40 and promotes polarization of macrophages towards the pro-inflammatory phenotype. In addition, anti-PD-L1 therapy enhances the phagocytic ability of macrophages and the ability of macrophages to promote T cell activation and proliferation, increasing tumor clearance [136]. Meanwhile, it inhibits the polarization of macrophages to anti-inflammatory and immunosuppressive macrophages that promote tumor growth. The effect of this complex polarization is manifested both in changes of the function and molecular markers and in changes of genome-wide expression levels. Changes in genome-wide expression levels are mainly reflected in increased gene and protein expression of antigen presentation mechanisms. These include a variety of gene sets consisting of MHC molecules and phagocytosis-related Fcγ receptors, downstream IFN-γ, pro-inflammatory signaling, chemokine expression, TLR/NF-kB, and autophagy pathway upregulation [136].
Effect of PD-1/PD-L1 inhibitor therapy combining with targeted agents/chemotherapy agents in solid cancers
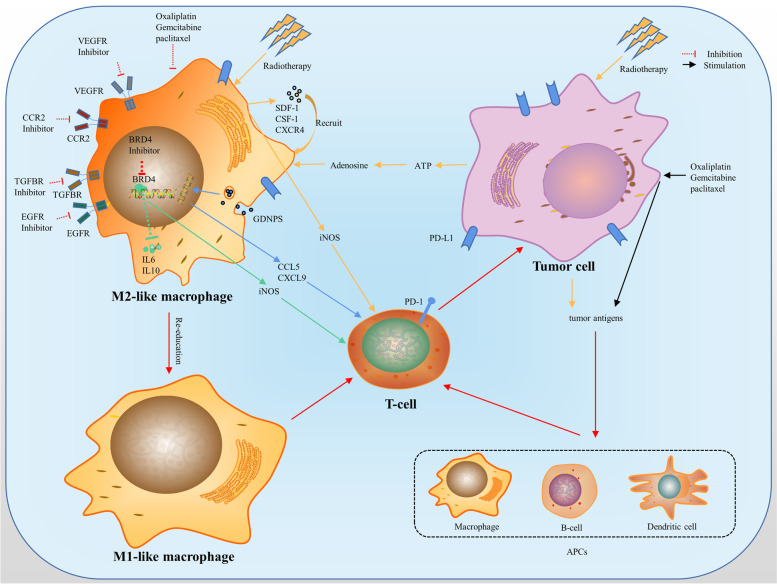
As mentioned earlier, immune checkpoints induce immunosuppression. In addition to abnormal angiogenesis, immunosuppressive immune cells or cytokines, cancer-associated fat cells, and overactive cancer-associated fibroblasts modulate cancer immunity and promote immune tolerance [137–139]. On the one hand, studies have shown that removing the pre-existing immunosuppressive environment of TME enhances the efficacy of anti-PD-1/PD-L1 and helps to overcome primary drug resistance in cancer patients [140, 141] (Fig. 3). On the other hand, enhancing positive factors also improve the anti-PD-1 effect of PD-L1 therapy, such as immunogenic cancer cell death, immune support cytokines, and specialized antigen-presenting cells [142]. ICIs suppress the immune checkpoints and improve immunosuppression, while sometimes, a single ICI does not effectively activate the immune response. Traditional treatment options such as small molecule targeted drugs, chemotherapy, and radiation therapy improve immunosuppressive TME. Therefore, combining PD-1/PD-L1 inhibitors with these conventional therapies may improve the sensitivity to activate the antitumor immune response and the response rate of patients.
Fig. 3.
Combination therapy reverses M2-like macrophages into M1-like macrophages and activates T cells, promoting antitumor effects. Radiation therapy and chemotherapy induce tumor cell death and the release of tumor-associated antigens. Tumor-associated antigens are administered to T cells and activate T cells through antigen-presenting cells. Macrophages are re-recruited by tumor-associated antigens and radiation-induced cytokines. M1 polarization induced by chemotherapy drugs, radiation therapy, and targeted agents enhances immunotherapy sensitivity
Combining with targeted therapy
Targeted agents are mainly divided into antiangiogenic agents (bevacizumab, ramucirumab, or aflibercept) or anti-epidermal growth factor receptor (anti-EGFR) agents (cetuximab or panitumumab) according to different targets and other agents [143].
Antiangiogenic agents
Angiogenesis abnormalities and immunosuppression in TME are two significant barriers to effective cancer immunotherapy [144, 145]. VEGF promotes the growth and survival of vascular endothelial cells. However, excessive angiogenesis may affect the maturation process of neovascularization and promote the formation of immunosuppressive TME [137, 138, 146]. In addition, cancer cells and stromal cells are reported to produce VEGF. VEGF upregulates the expression levels of PD-1 and other inhibitory checkpoints involved in CD8+ T cell failure and leads to non-response to anti-PD-1 therapy [147, 148]. Anti-VEGF receptor (anti-VEGFR) therapy relieves intraneoplastic hypoxia and immunosuppression by modulating abnormal tumor vessels. These above suggests that immunotherapy in combination with antiangiogenic agents may improve therapeutic outcomes [6, 149]. Lenvatinib is a multi-receptor tyrosine kinase inhibitor that suppresses its immunomodulatory function by targeting VEGFR with fibroblast growth factor signaling. Meanwhile, lenvatinib reduces the number of lowered M2-like macrophages in TAMs and increases the M1/M2 ratio. And lenvatinib increases the percentage of activated CD8+ T cells secreting IFN-γ and granzyme B (granzyme B is described as a critical soluble medium for cytotoxicity). In addition, lenvatinib upregulates plasmacytoid dendritic cell, the number of nuclear cells, especially CD8+ T cells, and their cytotoxic activity. Antitumor activity of lenvatinib plus anti-PD-1 combination therapy depends on lenvatinib’s activation of the IFN-γ signaling pathway [150]. In addition, lenvatinib alone and in combination therapy reduces the number of allogeneic tumor blood vessels, which may be another mechanism of lenvatinib combined with anti-PD-1 antitumor activity [150]. Apatinib is a small molecule targeted agent against VEGFR-2 that exhibits a dose- and time-dependent pattern for abnormal angiogenesis conditions. Low doses of apatinib plus anti-PD-L1 create a better immune support environment with more CD8+ T cells and fewer TAMs. Meanwhile, a more favorable pro-inflammatory microenvironment for immunotherapy appears 2 weeks after treatment [151].
Anti-EGFR agents
EGFR is a transmembrane tyrosine kinase receptor involved in tumor cell proliferation, invasion, and metastatic angiogenesis [152]. Tyrosine kinase inhibitors (EGFR-TKIs) inhibit EGFR and alter the tumor immune microenvironment [153]. Cetuximab is a chimeric IgG1 monoclonal antibody that inhibits the EGFR intracellular signaling pathway by binding to the extracellular domain of EGFR [154]. Cetuximab binds to receptors on NK cells, causing NK cell activation and inducing their lytic activity against tumor cells. Tumor antigens are released after the lysis of tumor cells, which are then presented to CD8+ T cells via DC. Therefore, the effect of cetuximab is to increase the invasion of cytotoxic CD8+ T cells into tumors, enhancing the antitumor effect. However, it also induces upregulation of PD-L1 expression on tumor cells through negative feedback effects [155, 156]. It can be inferred from these mechanisms that the combination of anti-PD-1/PD-L1 and cetuximab may work through complementary mechanisms of action. The reason is that cetuximab is able to activate the immune system for avelumab therapy by recruiting CD8+ T cells into TME. And PD-1/PD-L1 inhibitors block the PD-1/PD-L1 signaling pathway.
Anti-TGF-β agents
Dysregulation of TGF-β signal transduction pathways impairs multiple processes of the anticancer immune response, including antigen presentation, T cell infiltration, and tumor-killing activity. When anti-PD-1/PD-L1 alone does not work well in mouse colorectal tumor models, TGF-β blockers enhance the therapeutic efficacy of anti-PD-1/PD-L1. Blocking TGF-β1 and TGF-α2 significantly increased the Th1 immune response, upregulated IFN-γ production, and increased T-bet expression, a key transcription factor determining Th1 cell differentiation in tumor-infiltrating CD8+ T cells. An increasing number of Th1 cells promotes the polarization of TAMs towards M1-like macrophages and enhances the antitumor effect [157].
Anti-transcription factors
Bromodomain containing 4 (BRD4), a member of the bromodomain and extraterminal protein family, interacts with the acetylated lysine residues of histone tails on chromatins. Oncogenic transcription factors (such as c-MYC) are amplified by recruiting transcription mechanisms or indirectly by binding to enhancers, contributing to cancer cell proliferation [158]. AZD5153 is an inhibitor of BRD4, which depolarizes M2-like macrophages. MAF is a critical TF in regulating macrophage phenotypes associated with ovarian cancer. AZD5153 significantly reduces the binding of BRD4 to MAF-TF in mouse high-grade serous ovarian cancer. By inhibiting the expression of M2-like macrophages-related genes, the proportion of M2-like macrophages is reduced without reducing the total number of TAMs, for that TAMs are polarized to M1-like macrophages [159].
Nanotherapy
Cargo-free polymer nanoparticles (NPs) have a highly negative surface charge. When innate immune cells internalize NPs through scavenger receptors, including MARCO, they have an impact on the function of themselves. NP increases the production of TNF-α in tumor-bearing mice and reduces the expression of monocyte chemotaxis protein 1. The application of NP drugs reduces the aggregation of myeloid-derived suppressor cells at the tumor and metastatic sites. The combination of anti-PD-1/PD-L1 agents enhances this effect and promotes the efficacy of anti-PD-1/PD-L1 therapy [160]. Ginseng-derived nanoparticles (GDNPs) are isolated from Panax ginseng C.A. Mey. The expression of Ccl5 and Cxcl9 transcripts in M2-like macrophages increased significantly after GDNPs treatment. This promotes CCL5 and CXCL9 secretion, recruiting T lymphocytes to enhance tumor suppression. In addition, combination therapy with PD-1 monoclonal antibodies and GDNP reduced the M2/M1 ratio in tumors [161].
Anti-ILT4
Immunoglobulin-like transcript-4 (ILT4) is an inhibitory receptor of the immunoglobulin superfamily. ILT4 is mainly expressed in myeloid cells, including DCs, granulocytes, monocytes, macrophages, and platelets [162]. EGFR activation induces ILT4 in non-small cell lung cancer (NSCLC) cells. ILT4 migrates TAMs to TME by promoting the secretion of CCLs (chemokine (C-C motif) ligands), such as CCL2 and CCL5. In addition, ILT4 induce M2 polarization, upregulating its markers including CD163, CD206, IL-10, and Arg-1, and downregulating M1-like markers in TAMs, including CD80, CD86, IL-12, and TNF-α. And ILT4 directly reduces the proliferation vitality and killing ability of T cells. ILT4 blockades inhibit the above functions of ILT4. The combination therapy of ILT4 blockades and PD-L1 inhibitors showed synergistic effects. The combination therapy not only significantly inhibits the migration of TAMs to TME and the expression of its surface markers but also increases the proliferation of T cells [163].
Combining with chemotherapy
Chemotherapy mainly kills cancer cells and delays tumor growth by blocking the cell cycle, inhibiting DNA replication, interfering with cell metabolism, or inhibiting microtubule assembly. After cancer cell death, tumor antigens are presented by antigen-presenting cells, which leads to subsequent T cell recruitment, promotes the activation of the immune system, and thus promotes a highly effective antitumor immune response [8, 164, 165]. Induction of immunogenic cell death (ICD) is a critical way chemotherapy drugs work and can be induced by some anticancer drugs such as oxaliplatin. ICD requires cell surface CRT exposure, induction of EIF2α-dependent reticulum stress, HMGB1 and ATP release, and expression of type 1 IFNs (IFNα1 and IFNβ1) and chemokines (Cxcl9 and Cxcl0) [8, 165, 166]. The released molecules bind with its receptor to induce DC aggregation and enhance its antigen extraction ability, stimulating the adaptive antitumor immune response. In addition, chemotherapy drugs, such as gemcitabine and paclitaxel, increase the number of M1-like macrophages while reducing the number of M2-like macrophages and promote TAMs from M2-like macrophages to M1 repolarization [167]. 5-Fluorouracil (5-FU) selectively depletes bone marrow-derived suppressor cells in the body. 5-FU combined with oxaliplatin induces ICD in MSS colon cancer models and improves the efficacy of anti-PD-1, suggesting the possibility of using a combination of anti-PD-1 and chemotherapy to reverse immunotherapy resistance in MSS colon cancer [168–170]. Gemcitabine combined with anti-PD-1 antibody therapy increased CD8+ T cell infiltration compared with untreated and anti-PD-1 monotherapy. The same effect was observed in the treatment of pemetrexed. Chemotherapy exerts immunomodulatory effects by inducing immunogenic cell death, eliminating immunosuppressive cells, and enhancing effector cell function. Consequently, the TME modified by chemotherapy drugs favors anti-PD-1 antibody therapy.
Radiotherapeutic effect on therapeutic sensitivity of PD-1/PD-L1 inhibitor through modulating TAMs in solid cancers
The mechanism of action of radiotherapy is similar to that of some chemotherapy drugs. Radiation therapy (RT) induces double-stranded DNA damage and leads to cell death through apoptosis, necrosis, autophagy, mitosis catastrophe, or replicating aging. Then, after death, tumor cells release tumor-associated antigens, triggering and stimulating immune responses. Radiotherapy provides a supportive local immune microenvironment for antitumor immunity and enhances systemic antitumor immunity, resulting in the regression of unirradiated distant tumors [171–174]. When combined with immunomodulatory drugs, irradiation may enhance changes in infiltrating immune cells [175]. This combination therapy of RT and PD-1/PD-L1 inhibitor improved the long-term survival in preclinical studies and mouse models of melanoma, colorectal cancer, breast cancer, and NSCLC [175–177], while also preventing tumor recurrence. It has also shown good promise in some clinical trials: 1. RT increases the effectiveness of PD-L1 inhibition, and 2. In combination with PD-L1 inhibitors, RT increases the patients’ survival [178, 179].
Macrophages, which show a high degree of plasticity under immune stimulation, are a critical direct effector cell in combination therapy [180, 181]. Radiotherapy upregulates chemo-attractant stromal cell-derived factor 1 (SDF-1) and CSF1as well as CXCR4 to enhance the infiltration of TAMs in tumors [182, 183]. Studies have shown that radiotherapy increases the phagocytosis of TAMs, which is consistent with a significant decrease in PD-1 expression of TAMs after low irradiation doses (PD-1 inhibits the phagocytosis of TAMs and changes in M1 polarization). Meanwhile, compared with unirradiated TAMs, irradiation promotes the ability of TAMs antigen presentation. This is the ability of CD86 expression in the irradiation group to increase significantly and polarize towards M1-like macrophages [131, 181]. After radiotherapy, the proportion of HLA-DR high expression in TAMs was increased considerably. And the low expression of HLA and human MHC was associated with poor clinical results. Meanwhile, RT promoted the secretion of cytokines such as IL-23 p19 and IL-12 p70, and the changes in cytokine profile had an essential impact on the polarization of TAMs [181, 184].
In addition, irradiation has conflicting effects on macrophage phenotypes. In some studies, low-dose irradiation (2 Gy) reduces the number of M2-like macrophages and induces repolarization of M2-like macrophages to M1-like macrophages by increasing the expression ofiNOS, enhancing antitumor effects [181, 185]. In some other studies, irradiation was reported to lead to an increase in CD68+CD163+ M2-like macrophages around the tumor in NSCLC patients. The mechanism might be the release of ATP caused by RT-induced cancer cell death, which in turn was decomposed into adenosine. The accumulation of extracellular adenosine leads to the polarization of TAMs to M2-like macrophages [186–188]. In addition, RT induces ROS production. ROS-induced oxidation in the latency-associated peptide further activates TGF-β. TGF-β directly promotes the M2 polarization of TAMs. And TGF-β also upregulates the expression of immunosuppressive genes in M2-like macrophages, such as genes encoding IL-17 receptors (IL-17RB), to promote the development of Th17 cells. TGF-β also increases the expression of the outer nucleotides CD73 and CD39 of Th17 cells by down-regulating zinc finger protein growth factor independent-1 and inducing STAT3 expression, respectively. The overall manifestation is an increase in the number of Th17 cells and the expression of genes in Th17 cells responsible for converting ATP to adenosine [189]. HIF-1α has been shown to cause radiation resistance in endothelial cells, causing angiogenesis and tumor progression by promoting the expression of VEGF-A [190, 191]. RT stabilizes HIF-1α in cancer cells and thus increases cell content directly. RT also indirectly stabilizes HIF-1α by increasing TAMs [190].
The effectiveness of radiation therapy depends on several aspects. Tumor type and histotype: preoperative radiotherapy induces upregulation of PD-L1 in patients with cervical gland/adenosquamous cell carcinoma and soft tissue sarcom. While more patients with NSCLC or rectal cancer have decreased PD-L1 expression [181, 192–194]; Different combination regimens: a retrospective analysis of patients with metastatic melanoma reported a response rate of 64% in patients treated with both stereotactic radiosurgery and anti-PD-1 antibodies, higher than the 44% response rate in patients treated sequentially [195]; Irradiation dose: RT also modulates the immune system and TME in a dose-dependent manner. In some studies, low-dose RT promotes antitumor immunity. For example, low-dose RT of rectal cancer tissue differentiates TAMs towards the pro-inflammatory M1-phenotype, and high-dose RT at doses of 12–18 GY has also been shown to weaken the effect of antitumor immunity [181, 196]. In general, RT induces both immune activation and immunosuppression. When the impact of RT on TME is to enhance antitumor immunity, it will undoubtedly promote the effect of immunotherapy. If RT induces immunosuppressive effects, such as upregulating PD-L1, the combination of anti-PD-1/PD-L1 antibodies alleviates this immunosuppression. In addition, simultaneous administration helps increase the response rate and the effect of aspirin. These above suggests that the combination of radiotherapy and immunotherapy is promisingly more valuable [195, 197, 198].
Effect of listeria vaccine on cancer therapeutic efficiency of PD-1/PD-L1 inhibitors by regulating macrophages
As mentioned earlier, the low antigenicity of cancer cells and poor penetration and accumulation of immune cells in the TME are important reasons for the poor response to immune checkpoint therapy [199]. Cancer vaccines enhance immunogenicity, activate the patient’s immune system, and improve the effect of immune checkpoint treatment [200]. Cancer vaccines are another representative strategy for cancer immunotherapy, mainly divided into two main categories: preventive and therapeutic. Prophylactic vaccines induce immune memory by vaccinating healthy people to prevent the occurrence of specific cancers. The role of therapeutic vaccines is to strengthen or activate a patient’s immune system to treat cancer patients [201]. The development of cancer vaccines is based on the clinical phenomenon that patients with some infectious diseases are less likely to develop cancer than the general population [202]. For example, some specific antibodies produced by people with mumps reduce the incidence of ovarian cancer, and BCG vaccines used to prevent tuberculosis are now doing well in bladder cancer treatment [202, 203]. The mechanism of cancer vaccine treatment of tumors is to artificially stimulate and induce tumor antigen-specific T cells by using foreign antigens. As a result, TME is optimized, which induces cancer-specific immune responses [204].
However, immunosuppressive TME makes cancer vaccines less effective alone. The advantages and disadvantages of cancer vaccines and anti-PD-1/PD-L1 immunotherapy complement each other to a certain extent. Studies have shown that a Listeria-based HCC vaccine (Lmdd-MPFG), an oncology vaccine based on Listeria monocytogenes, elicits a strong anti-HBV-associated HCC immune response [205]. Its combination therapy with anti-PD-1/PD-L1 therapy has exerted a huge synergistic effect. There have been some changes in TME after vaccination. At the cellular level: Lmdd-MPFG vaccine is a potent macrophage polarizer. Lmdd-MPFG vaccine activates the NF-kB pathway via the TLR2 and MyD88 pathways and upregulates autophagic proteins, such as Atg16L1, Beclin1, LC3-II, p62, to enhance the autophagy process in M0 or M2-like macrophages. As a result, TAMs were repolarized from M2-like to M1-like macrophages. And CD8+ T cells with antitumor effects and DCs infiltration were significantly increased, while Treg cells (CD4+, CD25+, FoxP3+) were significantly reduced. The result is T cells resensitization to immune checkpoint blocking therapy [205]. At the cytokine level: an increase in M1-like macrophages were accompanied by an increase in gene expression of M1-like macrophages-related cytokines and chemokines, such as IFN-γ, iNOS, IL-23, CCl2, IL-1b, TNF-α. Meanwhile, the level of M2-like macrophages markers, such as IL-10, Arg-1, CD206, Fizz, TGF-β, Mgl-2, PDCD1LG2, and Ym-1 are reduced [205]. However, Lmdd-MPFG induces upregulation of PD-L1 expression levels in tumor tissue, while combined PD-1 antibodies enhance T cell responses by eliminating overexpression of PD-L1 in tumor tissues that may be induced by vaccines.
Roles of TAMs in immune therapy of PD-1/PD-L1 inhibitors for different solid cancers
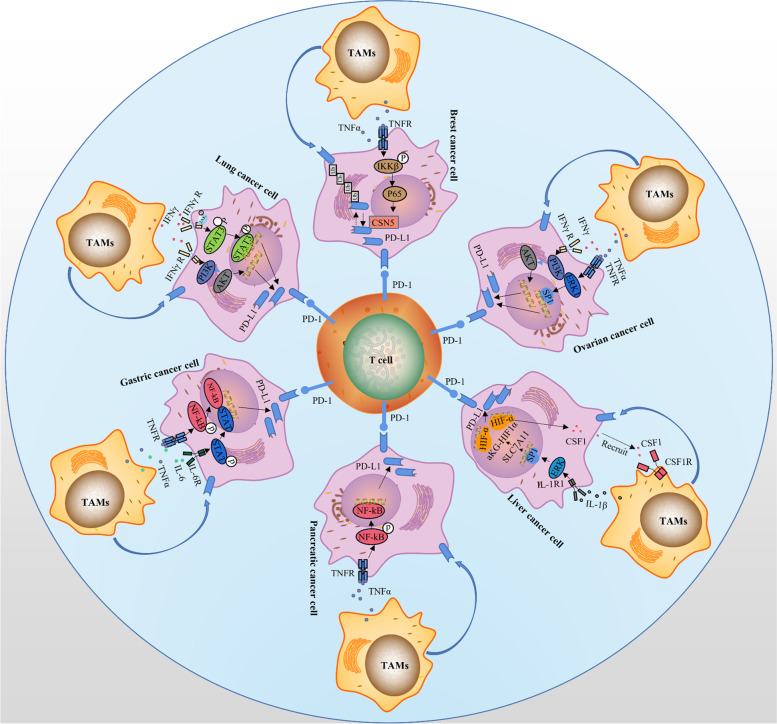
In different solid cancer types, TAMs often affect the expression of PD-1/PD-L1 through different pathways, which in turn affects the efficacy of PD-1/PD-L1 inhibitors (Fig. 4).
Fig. 4.
The effect of TAMs in different solid cancers. In pancreatic cancer, TAMs promote PD-L1 expression in cancer cells through TNF-α/NF-kB pathway. In liver cancer, TAMs promote PD-L1 expression in cancer cells through the IL-1β/ERK pathway. In gastric cancer, TAMs promote PD-L1 expression in cancer cells through IL-1/STAT and TNF-α/NF-kB pathways. In lung cancer, TAMs promote PD-L1 expression in cancer cells through IFN-γ/PI3K/AKT and IFN-γ/STAT3 pathways. In breast cancer, TAMs promote PD-L1 expression in cancer cells through TNF-α/IKK pathway. In ovarian cancer, TAMs promote PD-L1 expression in cancer cells through IFN-γ/PI3K/AKT and IFN-γ/ERK1 pathways
Pancreatic cancer
In pancreatic cancer, PD-L1 expression is correlated with CD163+ TAMs. TNF-α significantly increases the expression of PD-L1 mRNA, compared with other cytokines secreted by TAMs, such as IL-1a, IL-1b, IL-4, IL-6, IL-7, etc. The specific mechanism is that TNF-α increases the phosphorylation level of NF-kB. And real-time PCR confirms a clear positive correlation between PD-L1 protein expression and TNF-α mRNA level in PDAC tissues [206].
Liver cancer
In liver cancer, IL-1b, secreted by TAMs, upregulates the expression of SLC7A11 through the IL-1R1 pathway. SLC7A11 reduces the level of α-ketoglutarate by transferring intracellular glutamic acid to the extracellular, thereby reducing the degradation of HIF-1α and increasing its content in the cell. At the same time, SLC7A11 activates the AKT pathway and induces HIF-1α expression under low oxygen conditions [207]. HIF-1α, as a critical positive regulator of PD-L1 expression, binds with the HIF-1α binding site on the PD-L1 promoter, thereby upregulating PD-L1. In addition, SLC7A11 overexpression promotes the infiltration of TAMs in tumors through the CSF1 receptor (CSF-1R) axis [207, 208].
Gastric cancer
When co-cultured with gastric cancer cells, the expression of TNF-α and IL-6 in TAMs increased significantly. Cytokines of IL-1 and IL-1b do not have this trend. TNF-α and IL-6 induced PD-L1 expression through NF-kB and STAT3 signaling pathways [209].
Lung cancer
Studies have shown that in lung cancer, TAMs produce IFN-γ, IL 6, TNF-α, and IL 10 to induce A549 to express PD-L1. And IFN-γ induces tumor cells to express PD-L1 more effectively than other cytokines. It is the primary molecule induced by PD-L1 by TAMs in lung cancer. The mechanism that TAMs increase the secretion of IFN-γ is achieved by upregulating the JAK/STAT3 and PI3K/AKT signaling pathways [61].
Breast cancer
Compared with cytokines such as IL-6, IL-8, IL-1, and TNF-α secreted by macrophages, upregulates the expression of PD-L1 protein through non-transcriptional regulatory mechanisms (such as post-translational regulation) without increasing mRNA expression. The specific mechanism is that TNF-α binds to its ligand to activate IKKb-kinase, which induces a nuclear shift downstream p65. p65 interacts directly with COPS5, the promoter encoding CSN5, transcriptionally upregulating its expression. CSN5 is a subunit with deubiquitinase activity in the COP9 signaler. It interacts with PD-L1 and deubiquitinates it to stabilize it [210].
Ovarian cancer
In ovarian cancer, TAMs-derived IFN-γ, TNF-α, IL-10, and IL6 increase PD-L1 expression, and the density of membrane PD-L1 is positively correlated with high TAMs infiltration. The expression of PD-L1 was induced through IFN-γ via the PI3K pathway. And TNF-α induced the expression of PD-L1 through the ERK1/2 pathway [211].
Overall, existing evidence suggests that PD-L1 may be differentially regulated with respect to specific signaling pathways and transcription factors in different cell types. This provides guidance for the precise treatment of various cancers.
The clinical application value of TAMs in solid cancer therapy
In ICIs-mediated therapy, TAMs play a very important bifacial role, antitumor and pro-tumor. Because of this, targeting macrophage synergistic ICIs is a promising combination protocol. At present, macrophage-centred therapeutic strategies mainly divide into four aspects.
Reduces the recruitment of macrophages
CSF1and CCL2 play a crucial role in monocyte recruitment and TAMs generation [212]. For example, blocking CCL2/CCR2 restricts monocytes from entering the tumor [213, 214]. Inhibition of CSF1 reduces TAMs invasion as well as tumor proliferation and migration [215]. In addition to the CCR2-CCL2 signaling axis, CXCR4-CXCL12 (also known as stromal cell-derived factor-1, SDF-1) interaction is another signaling axis involved in the recruitment of monocytes/macrophages and implicated in the promotion of tumor invasiveness/regrowth [183]. Treatment with a CXCR4 inhibitor (AMD3100) inhibits its effect.
Depletion of existing macrophages in the TME
CSF-1R is a tyrosine kinase receptor expressed on mononuclear phagocytes [216]. After binding to CSF1, CSF-1R promotes the proliferation, function, and survival of macrophages. CSF-1R antibodies deplete TAMs by blocking the function of CSF-1R [216]. Certain drugs, such as Bisphosphonate, also induce apoptosis after being swallowed up by TAMs [217]. Mannose receptor (CD206) is overexpressed on M2-like macrophages, which is also one of the most commonly targeted receptors of macrophages [218]. Chimeric antigen receptor T-cell (CAR-T) immunotherapy specifically kills target cells. Construction of CAR-T specific against immunosuppressive subsets in TAMs reduces the number of TAMs in TME [219, 220].
Repolarization of existing macrophages in the TME
Multiple means polarize the TAMs to the M1 type. For example, immunomodulators, especially monoclonal antibodies, are widely used as monotherapy and as adjuvants conditioning TME for combinatorial treatments. An anti-MARCO (A pattern recognition scavenger receptor) induces anti-tumor activity in multiple tumor models by reprogramming the TAMs population into a pro-inflammatory phenotype and increasing tumor immunogenicity [221]. LILRB4 is a LILRB family receptor that is widely expressed on immune cells and enriched on TAMs [222]. After treatment with anti-LILRB4 antibodies, TAMs transitions to a less inhibitory phenotype [223]. In addition, as mentioned earlier, radiotherapy, targeted therapy and a variety of chemotherapy drugs have a similar effect [73, 150, 151, 167].
Macrophage cell therapy
Engineered receptors are used to arm monocytes to treat tumors. Based on the ability of macrophages to infiltrate tumors and their unique ability in TME, the method of genetically engineering macrophages with CARs to enhance their ability to kill tumors has great potential [224, 225]. While killing tumor cells, CAR macrophages (CAR-Ms) also inhibit the M2 polarization of TAMs and promotes M1 transformation. In addition, the expression of the CAR structure reverses the M2 polarized macrophages to the M1-like macrophages [225].
Monocytes are used as vehicles to deliver cytokines or nanoparticles to TME. Tie2-expressing monocytes have tumor-homing ability. It is used as a vehicle to deliver the anti-tumor cytokine IFNα to TME, which inhibits tumor angiogenesis and activates innate and adaptive immune cells [226].
Conclusions
As previously mentioned, TAMs are essential in treating solid tumors through multiple mechanisms. TAMs can regulate the expression of PD-L1 molecules in tumor cells through various pathways. Meanwhile, TAMs are significant targets of PD-1 / PD-L1 inhibitors and critical cells in mediating the role of traditional therapeutic options such as radiotherapy, chemotherapy, and targeted therapy. The above functions of TAMs are a critical basis for combining immunotherapy with conventional treatment regimens. However, the understanding of TAMs needs to be further improved. The urgent problems to be solved include 1. What are the key factors driving the phenotypic changes of TAMs in TME? 2. How to distinguish TAMs into subgroups with different functions and identify a subset of the required functions? Many studies have shown that combination therapy based on PD-1 / PD-L1 inhibitors with other traditional treatment regimens can synergistically benefit tumor patients. However, combination treatment regimens not only increase patient medical costs but also reduce the low toxicity of patients. Optimizing the combination treatment regimen, including drug, dose, timing, and order is a significant difficulty in developing combination treatment.
In conclusion, further studies on the classification and function of TAMs will help to improve the responsiveness of cancer patients to immune checkpoint therapy. Moreover, based on the immunosuppressive effects of TAMs, the development of drugs targeting TAMs to reduce the recruitment to TME and the clearance and repolarization of existing TAMs in TME are also hot research fields. Developing a good combination application program will also greatly promote the development of the tumor treatment field for the benefit of cancer patients.
Acknowledgements
Not applicable.
Abbreviations
- Anti-EGFR
Anti-epidermal growth factor receptor
- Anti-VEGFR
Anti-VEGF receptor
- Arg-1
Arginase 1
- ASPS
Alveolar soft part sarcoma
- BCC
Basal cell carcinoma
- BRD4
Bromodomain containing 4
- BTC
Biliary tract cancer
- CAR-T
Chimeric antigen receptor T-cell
- CAR-Ms
CAR macrophages
- CC
Cervical cancer
- CRC
Colorectal cancer
- CircRNAs
Circular RNAs
- COX-2
Cyclooxygenase 2
- CSCC
Cutaneous squamous cell carcinoma
- CSF1
Colony stimulating factor 1
- CSF-1R
CSF1 receptor
- CTCF
CCCTC-binding factor
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- DCs
Dendritic cells
- DNMTs
DNA methyltransferases
- EA
Esophageal adenocarcinoma
- ESCC
Esophageal squamous cell carcinoma
- ES-SCLC
Extensive-stage small cell lung cancer
- GC
Gastric cancer
- GEJC
Gastroesophageal junction cancer
- GDNPs
Ginseng-derived nanoparticles
- HATs
Histone acetyltransferase
- HCC
Hepatocellular cancer
- HDACs
Histone deacetylase
- HL
Hodgkin lymphoma
- HNSCC
Head and neck squamous cell cancer
- ICD
Immunogenic cell death
- ICIs
Immune checkpoint inhibitors
- IDO
Indolamine 2,3-dioxygenase
- ILT4
Immunoglobulin-like transcript-4
- iNOS
Inducible nitric oxide synthase
- Lmdd-MPFG
Listeria-based HCC vaccine
- LncRNAs
Long noncoding RNA
- LPS
Lipopolysaccharide
- MCC
Merkel cell cancer
- M-CSF
Macrophage colony-stimulating factor
- MiRNAs
MicroRNAs
- MM
Multiple myeloma
- MPM
Malignant pleural mesothelioma
- ncRNAs
Non-coding RNAs
- NKs
Natural killer cells
- NPC
Nasopharyngeal carcinoma
- NPs
Nanoparticles
- NSCLC
Non-small cell lung cancer
- OPN
Osteopontin
- PD-L2
Programmed death ligand 2
- PMBCL
Primary mediastinal B-cell lymphoma
- PTPRO
Protein tyrosine phosphatase, receptor type O
- RCC
Penal cell cancer
- RFA
Radiofrequency ablation
- ROS
Reactive oxygen species
- RT
Radiation therapy
- SC
Skin cancer
- SCLC
Small cell lung cancer
- scRNA-seq
Single-cell RNA sequencing
- TAMs
Tumor-associated macrophages
- TGF-β
Transforming growth factor-β
- TLR
Toll-like receptor
- TME
The tumor microenvironment
- TNBC
Triple-negative breast cancer
- TNF-α
Tumor necrosis factor-α
- UC
Urothelial cancer
- VEGF
Vascular endothelial growth factor
Authors’ contributions
ZQS provided direction and guidance throughout the preparation of this manuscript. HZ, LL and JBL finished the manuscript and designed the figures. ZQS, CZW, YL and WTY reviewed and made significant revisions to the manuscript. PYD, and SYH collected the related papers. All authors read and approved the final manuscript.
Funding
This study was supported by The National Natural Science Foundation of China (82173055, 81972663), The Science Project of Henan Natural Science Foundation (212300410074, 202300410446), The Youth Talent Innovation Team Support Program of Zhengzhou University (32320290), The Provincial and Ministry co-constructed key projects of Henan medical science and technology (SBGJ202102134), Key scientific and technological research projects of Henan Provincial Department of Science and Technology (212102310117), Henan Provincial Health Commission and Ministry of Health Co-construction Project, and Henan Provincial Health and Health Commission Joint Construction Project (LHGJ20200158), Henan Province young and middle-aged health science and technology innovation leading talent project (YXKC2022016), Henan Province Medical Affairs Technology Promotion Project (SYJS2022109).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved to publish this manuscript.
Competing interests
All authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Zhang, Lin Liu and Jinbo Liu contributed equally to this work.
Contributor Information
Zhenqiang Sun, Email: fccsunzq@zzu.edu.cn.
Yang Liu, Email: zlyyliuyang1440@zzu.edu.cn.
Chengzeng Wang, Email: czw202112@zzu.edu.cn.
References
- 1.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raskov H, Orhan A, Gaggar S, Gogenur I. Cancer-associated fibroblasts and tumor-associated macrophages in Cancer and Cancer immunotherapy. Front Oncol. 2021;11:668731. doi: 10.3389/fonc.2021.668731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng R, Lu J, Liu X, Peng XH, Wang J, Li XP. PD-L1 expression is highly associated with tumor-associated macrophage infiltration in nasopharyngeal carcinoma. Cancer Manag Res. 2020;12:11585–11596. doi: 10.2147/CMAR.S274913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 5.Santarpia M, Gonzalez-Cao M, Viteri S, Karachaliou N, Altavilla G, Rosell R. Programmed cell death protein-1/programmed cell death ligand-1 pathway inhibition and predictive biomarkers: understanding transforming growth factor-beta role. Transl Lung Cancer Res. 2015;4:728–742. doi: 10.3978/j.issn.2218-6751.2015.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twyman-Saint VC, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplon H, Reichert JM. Antibodies to watch in 2021. Mabs-Austin. 2021;13:1860476. doi: 10.1080/19420862.2020.1860476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Drug Evaluation of National Medical Products Administration.https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=89d1a5f2ef65811a1748b841508cbca9. Accessed 10 Jan 2023.
- 11.Center for Drug Evaluation of National Medical Products Administration. https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=16e941d5bc2a443e5353c10cdc1b0404. Accessed 10 Jan 2023.
- 12.Center for Drug Evaluation of National Medical Products Administration. https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=457d62d01a141c8fca2e536b49f16296. Accessed 10 Jan 2023.
- 13.Center for Drug Evaluation of National Medical Products Administration. https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=4a1aa099a4d8745593e4b2adc1081b1a. Accessed 10 Jan 2023.
- 14.Center for Drug Evaluation of National Medical Products Administration. https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=cca9ba26309c0ee4ff6027951a34bf9d. Accessed 10 Jan 2023.
- 15.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm., Accessed 10 Jan 2023.
- 17.Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, Chua C, Leong JY, Lim KH, Toh HC, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci U S A. 2017;114:E5900–E5909. doi: 10.1073/pnas.1706559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novikova MV, Khromova NV, Kopnin PB. Components of the hepatocellular carcinoma microenvironment and their role in tumor progression. Biochemistry (Mosc) 2017;82:861–873. doi: 10.1134/S0006297917080016. [DOI] [PubMed] [Google Scholar]
- 19.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates Cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Yang D, Li S, Gao Y, Jiang R, Deng L, Frankel FR, Sun B. Development of a Listeria monocytogenes-based vaccine against hepatocellular carcinoma. Oncogene. 2012;31:2140–2152. doi: 10.1038/onc.2011.395. [DOI] [PubMed] [Google Scholar]
- 22.Namgaladze D, Zukunft S, Schnutgen F, Kurrle N, Fleming I, Fuhrmann D, Brune B. Polarization of human macrophages by Interleukin-4 does not require ATP-citrate Lyase. Front Immunol. 2018;9:2858. doi: 10.3389/fimmu.2018.02858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 26.Liang S, Chen Z, Jiang G, Zhou Y, Liu Q, Su Q, Wei W, Du J, Wang H. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-kappaB/IL-6 signals. Cancer Lett. 2017;386:12–23. doi: 10.1016/j.canlet.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu S, Deng L, Liao X, Nie L, Qi F, Jin K, Tu X, Zheng X, Li J, Liu L, et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 2019;110:2110–2118. doi: 10.1111/cas.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kardoust PM, Shariat SF, Margulis V, Mori K, Lotan Y. Value of tumour-infiltrating immune cells in predicting response to intravesical BCG in patients with non-muscle-invasive bladder cancer: a systematic review and meta-analysis. BJU Int. 2021;127:617–625. doi: 10.1111/bju.15276. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, et al. M2 macrophage-derived exosomes promote cell migration and invasion in Colon Cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 35.Yin Z, Zhou Y, Ma T, Chen S, Shi N, Zou Y, Hou B, Zhang C. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J Cell Mol Med. 2020;24:5028–5038. doi: 10.1111/jcmm.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacconi C, Ungaro F, Correale C, Arena V, Massimino L, Detmar M, Spinelli A, Carvello M, Mazzone M, Oliveira AI, et al. Activation of the VEGFC/VEGFR3 pathway induces tumor immune escape in colorectal Cancer. Cancer Res. 2019;79:4196–4210. doi: 10.1158/0008-5472.CAN-18-3657. [DOI] [PubMed] [Google Scholar]
- 37.Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben DG, Shlomi T, Gil Z. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 39.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In'T VP, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 40.Molon B, Ugel S, Del PF, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernsmeier C, van der Merwe S, Perianin A. Innate immune cells in cirrhosis. J Hepatol. 2020;73:186–201. doi: 10.1016/j.jhep.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Bruns H, Buttner M, Fabri M, Mougiakakos D, Bittenbring JT, Hoffmann MH, Beier F, Pasemann S, Jitschin R, Hofmann AD, et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci Transl Med. 2015;7:247r–282r. doi: 10.1126/scitranslmed.aaa3230. [DOI] [PubMed] [Google Scholar]
- 44.Park J, Lee SE, Hur J, Hong EB, Choi JI, Yang JM, Kim JY, Kim YC, Cho HJ, Peters JM, et al. M-CSF from Cancer cells induces fatty acid synthase and PPARbeta/delta activation in tumor myeloid cells, Leading to Tumor Progression. Cell Rep. 2015;10:1614–1625. doi: 10.1016/j.celrep.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, Siegwart DJ, Wan Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 47.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 49.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 50.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 51.Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, Ricciardi-Castagnoli P. PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology. 2016;5:e1085146. doi: 10.1080/2162402X.2015.1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 54.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 57.Asaoka Y, Ijichi H, Koike K. PD-1 blockade in tumors with mismatchrepair deficiency. N Engl J Med. 2015:373;1979. [DOI] [PubMed]
- 58.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to Cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:eaal3604. [DOI] [PMC free article] [PubMed]
- 60.Dong L, Chen C, Zhang Y, Guo P, Wang Z, Li J, Liu Y, Liu J, Chang R, Li Y, et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning W, Zeng H, Zhang N, Du W, Chen C, Huang JA. PD-L1 induced by IFN-gamma from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol. 2017;22:1026–1033. doi: 10.1007/s10147-017-1161-7. [DOI] [PubMed] [Google Scholar]
- 62.Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764–1773. doi: 10.1136/gutjnl-2018-316324. [DOI] [PubMed] [Google Scholar]
- 63.Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. 2016;65:1895–1905. doi: 10.1136/gutjnl-2015-311292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natoli G. Maintaining cell identity through global control of genomic organization. Immunity. 2010;33:12–24. doi: 10.1016/j.immuni.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Jain N, Shahal T, Gabrieli T, Gilat N, Torchinsky D, Michaeli Y, Vogel V, Ebenstein Y. Global modulation in DNA epigenetics during pro-inflammatory macrophage activation. Epigenetics-Us. 2019;14:1183–1193. doi: 10.1080/15592294.2019.1638700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z, Zhang Y. Role of mammalian DNA methyltransferases in development. Annu Rev Biochem. 2020;89:135–158. doi: 10.1146/annurev-biochem-103019-102815. [DOI] [PubMed] [Google Scholar]
- 67.Tian YP, Zhu YM, Sun XH, Lai MD. Multiple functions of ten-eleven translocation 1 during tumorigenesis. Chin Med J. 2016;129:1744–1751. doi: 10.4103/0366-6999.185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang X, Wang X, Liu D, Yu L, Xue B, Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol. 2014;28:565–574. doi: 10.1210/me.2013-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daskalaki MG, Tsatsanis C, Kampranis SC. Histone methylation and acetylation in macrophages as a mechanism for regulation of inflammatory responses. J Cell Physiol. 2018;233:6495–6507. doi: 10.1002/jcp.26497. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Barozzi I, Termanini A, Prosperini E, Recchiuti A, Dalli J, Mietton F, Matteoli G, Hiebert S, Natoli G. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci U S A. 2012;109:E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Wang X, Zhu Y, Cao Y, Wang L, Li F, Zhang Y, Li Y, Zhang Z, Luo J, et al. The CTCF/LncRNA-PACERR complex recruits E1A binding protein p300 to induce pro-tumour macrophages in pancreatic ductal adenocarcinoma via directly regulating PTGS2 expression. Clin Transl Med. 2022;12:e654. doi: 10.1002/ctm2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao Q, Rong S, Repa JJ, St CR, Parks JS, Mishra N. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2014;34:1871–1879. doi: 10.1161/ATVBAHA.114.303393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knox T, Sahakian E, Banik D, Hadley M, Palmer E, Noonepalle S, Kim J, Powers J, Gracia-Hernandez M, Oliveira V, et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci Rep. 2019;9:6136. doi: 10.1038/s41598-019-42237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WT, Cavassani KA, Li X, Lukacs NW, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 77.Li L, Sun P, Zhang C, Li Z, Zhou W. MiR-98 suppresses the effects of tumor-associated macrophages on promoting migration and invasion of hepatocellular carcinoma cells by regulating IL-10. Biochimie. 2018;150:23–30. doi: 10.1016/j.biochi.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Wei X, Tang C, Lu X, Liu R, Zhou M, He D, Zheng D, Sun C, Wu Z. MiR-101 targets DUSP1 to regulate the TGF-beta secretion in sorafenib inhibits macrophage-induced growth of hepatocarcinoma. Oncotarget. 2015;6:18389–18405. doi: 10.18632/oncotarget.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poitz DM, Augstein A, Gradehand C, Ende G, Schmeisser A, Strasser RH. Regulation of the Hif-system by micro-RNA 17 and 20a - role during monocyte-to-macrophage differentiation. Mol Immunol. 2013;56:442–451. doi: 10.1016/j.molimm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Y, Yi C. MicroRNA-720 suppresses M2 macrophage polarization by targeting GATA3. Biosci Rep. 2016;36:e00363. doi: 10.1042/BSR20160105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, Wang H, Xu F, Shi L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239–1251. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
- 83.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C, Liu XJ, QunZhou XJ, Ma TT, Meng XM, Li J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int Immunopharmacol. 2016;32:46–54. doi: 10.1016/j.intimp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH, Pandolfi PP. Oncogenic role of fusion-circRNAs derived from Cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 86.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Song H, Yang Y, Sun Y, Wei G, Zheng H, Chen Y, Cai D, Li C, Ma Y, Lin Z, et al. Circular RNA Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol Ther. 2022;30:915–931. doi: 10.1016/j.ymthe.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Han X, Yang L, Fu J, Sun C, Huang S, Xiao W, Gao Y, Liang Q, Wang X, et al. Circular RNA circPPM1F modulates M1 macrophage activation and pancreatic islet inflammation in type 1 diabetes mellitus. Theranostics. 2020;10:10908–10924. doi: 10.7150/thno.48264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J, Cheng F, Rong G, Tang Z, Gui B. Circular RNA hsa_circ_0005567 overexpression promotes M2 type macrophage polarization through miR-492/SOCS2 axis to inhibit osteoarthritis progression. Bioengineered. 2021;12:8920–8930. doi: 10.1080/21655979.2021.1989999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo X, Chen R, Chen T. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951–2963. doi: 10.1002/jcb.26509. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Feng J, Fu H, Liu C, Yu Z, Sun Y, She X, Li P, Zhao C, Liu Y, et al. Coagulation factor X regulated by CASC2c recruited macrophages and induced M2 polarization in glioblastoma Multiforme. Front Immunol. 2018;9:1557. doi: 10.3389/fimmu.2018.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Lei Y, Wu M, Li N. Regulation of macrophage activation and polarization by HCC-derived Exosomal lncRNA TUC339. Int J Mol Sci. 2018;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Navin NE. Delineating cancer evolution with single-cell sequencing. Sci Transl Med. 2015;7:229f–296f. doi: 10.1126/scitranslmed.aac8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haque A, Engel J, Teichmann SA, Lonnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35–45. doi: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- 97.Yip SH, Sham PC, Wang J. Evaluation of tools for highly variable gene discovery from single-cell RNA-seq data. Brief Bioinform. 2019;20:1583–1589. doi: 10.1093/bib/bby011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan JM, Quintanal-Villalonga A, Gao VR, Xie Y, Allaj V, Chaudhary O, Masilionis I, Egger J, Chow A, Walle T, et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell. 2021;39:1479–1496. doi: 10.1016/j.ccell.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nalio RR, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Nunez NG, Tosello BJ, Richer W, Menger L, Denizeau J, et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell. 2022;185:1189–1207. doi: 10.1016/j.cell.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 100.Obradovic A, Chowdhury N, Haake SM, Ager C, Wang V, Vlahos L, Guo XV, Aggen DH, Rathmell WK, Jonasch E, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell. 2021;184:2988–3005. doi: 10.1016/j.cell.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Zuo C, Liu L, Hu Y, Yang B, Qiu S, Li Y, Cao D, Ju Z, Ge J, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol. 2021;75:1128–1141. doi: 10.1016/j.jhep.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 102.Chung W, Eum HH, Lee HO, Lee KM, Lee HB, Kim KT, Ryu HS, Kim S, Lee JE, Park YH, et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat Commun. 2017;8:15081. doi: 10.1038/ncomms15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pombo AA, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, Kancheva D, Martens L, De Vlaminck K, Van Hove H, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24:595–610. doi: 10.1038/s41593-020-00789-y. [DOI] [PubMed] [Google Scholar]
- 104.Ochocka N, Segit P, Walentynowicz KA, Wojnicki K, Cyranowski S, Swatler J, Mieczkowski J, Kaminska B. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun. 2021;12:1151. doi: 10.1038/s41467-021-21407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang K, Erkan EP, Jamalzadeh S, Dai J, Andersson N, Kaipio K, Lamminen T, Mansuri N, Huhtinen K, Carpen O, et al. Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer. Sci Adv. 2022;8:m1831. doi: 10.1126/sciadv.abm1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurten C, Kulkarni A, Cillo AR, Santos PM, Roble AK, Onkar S, Reeder C, Lang S, Chen X, Duvvuri U, et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat Commun. 2021;12:7338. doi: 10.1038/s41467-021-27619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang H, Yu D, Yang P, Guo R, Kong M, Gao Y, Yu X, Lu X, Fan X. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA sequencing. Clin Transl Med. 2022;12:e730. doi: 10.1002/ctm2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in Colon Cancer. Cell. 2020;181:442–459. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 109.Arlauckas SP, Garren SB, Garris CS, Kohler RH, Oh J, Pittet MJ, Weissleder R. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics. 2018;8:5842–5854. doi: 10.7150/thno.26888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan Y, Lu F, Fei Q, Yu X, Xiong P, Yu X, Dang Y, Hou Z, Lin W, Lin X, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124. doi: 10.1186/s13045-019-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, Yu EA, Schenk EL, Tan W, Zee A, et al. Therapy-induced evolution of human lung Cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teuwen LA, De Rooij L, Cuypers A, Rohlenova K, Dumas SJ, Garcia-Caballero M, Meta E, Amersfoort J, Taverna F, Becker LM, et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. 2021;35:109253. doi: 10.1016/j.celrep.2021.109253. [DOI] [PubMed] [Google Scholar]
- 113.Fei Q, Pan Y, Lin W, Zhou Y, Yu X, Hou Z, Yu X, Lin X, Lin R, Lu F, et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis. 2020;11:589. doi: 10.1038/s41419-020-02787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roussos P, Katsel P, Fam P, Tan W, Purohit DP, Haroutunian V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer's dementia. Alzheimers Dement. 2015;11:1163–1170. doi: 10.1016/j.jalz.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao Y, Li H, Chen J, Xu W, Yang G, Bao Z, Xia D, Lu G, Hu S, Zhou J. TREM-2 serves as a negative immune regulator through Syk pathway in an IL-10 dependent manner in lung cancer. Oncotarget. 2016;7:29620–29634. doi: 10.18632/oncotarget.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garrido-Martin EM, Mellows T, Clarke J, Ganesan AP, Wood O, Cazaly A, Seumois G, Chee SJ, Alzetani A, King EV, et al. M1(hot) tumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. J Immunother Cancer. 2020;8:e000778. [DOI] [PMC free article] [PubMed]
- 120.Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29:5045–5051. doi: 10.1007/s00330-019-06189-6. [DOI] [PubMed] [Google Scholar]
- 121.Hussain SM, Kansal RG, Alvarez MA, Hollingsworth TJ, Elahi A, Miranda-Carboni G, Hendrick LE, Pingili AK, Albritton LM, Dickson PV, et al. Role of TGF-beta in pancreatic ductal adenocarcinoma progression and PD-L1 expression. Cell Oncol (Dordr) 2021;44:673–687. doi: 10.1007/s13402-021-00594-0. [DOI] [PubMed] [Google Scholar]
- 122.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, Pan F, Topalian SL. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7:305. doi: 10.1186/s40425-019-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2. Arthritis Res Ther. 2005;7:114–117. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weigert A, Strack E, Snodgrass RG, Brune B. mPGES-1 and ALOX5/−15 in tumor-associated macrophages. Cancer Metastasis Rev. 2018;37:317–334. doi: 10.1007/s10555-018-9731-3. [DOI] [PubMed] [Google Scholar]
- 126.Komura N, Mabuchi S, Shimura K, Yokoi E, Kozasa K, Kuroda H, Takahashi R, Sasano T, Kawano M, Matsumoto Y, et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol Immunother. 2020;69:2477–2499. doi: 10.1007/s00262-020-02628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Liu H, Zhao Y, Yue D, Chen C, Li C, Zhang Z, Wang C. Tumor-associated macrophages (TAMs)-derived osteopontin (OPN) upregulates PD-L1 expression and predicts poor prognosis in non-small cell lung cancer (NSCLC) Thorac Cancer. 2021;12:2698–2709. doi: 10.1111/1759-7714.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21:3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, Chen Y, Jiang R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer. 2020;8:e000285. doi: 10.1136/jitc-2019-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cho HY, Choi EK, Lee SW, Jung KO, Seo SK, Choi IW, Park SG, Choi I, Lee SW. Programmed death-1 receptor negatively regulates LPS-mediated IL-12 production and differentiation of murine macrophage RAW264.7 cells. Immunol Lett. 2009;127:39–47. doi: 10.1016/j.imlet.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 131.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu N, Zhang J, Yin M, Liu H, Zhang X, Li J, Yan B, Guo Y, Zhou J, Tao J, et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol Ther. 2021;29:2321–2334. doi: 10.1016/j.ymthe.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roy S, Gupta P, Palit S, Basu M, Ukil A, Das PK. The role of PD-1 in regulation of macrophage apoptosis and its subversion by Leishmania donovani. Clin Transl Immunology. 2017;6:e137. doi: 10.1038/cti.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gubin MM, Esaulova E, Ward JP, Malkova ON, Runci D, Wong P, Noguchi T, Arthur CD, Meng W, Alspach E, et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint Cancer therapy. Cell. 2018;175:1014–1030. doi: 10.1016/j.cell.2018.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6:1260–1273. doi: 10.1158/2326-6066.CIR-17-0537. [DOI] [PubMed] [Google Scholar]
- 136.Xiong H, Mittman S, Rodriguez R, Moskalenko M, Pacheco-Sanchez P, Yang Y, Nickles D, Cubas R. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79:1493–1506. doi: 10.1158/0008-5472.CAN-18-3208. [DOI] [PubMed] [Google Scholar]
- 137.Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. doi: 10.1186/s12943-019-0974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13:25. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 141.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 142.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 143.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):i1–i9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 144.Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res. 2017;77:817–822. doi: 10.1158/0008-5472.CAN-16-2379. [DOI] [PubMed] [Google Scholar]
- 145.Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. 2012;3:21. doi: 10.3389/fimmu.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Tacchio M, Macas J, Weissenberger J, Sommer K, Bahr O, Steinbach JP, Senft C, Seifert V, Glas M, Herrlinger U, et al. Tumor vessel normalization, Immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, Angiopoietin-2, and VEGF. Cancer Immunol Res. 2019;7:1910–1927. doi: 10.1158/2326-6066.CIR-18-0865. [DOI] [PubMed] [Google Scholar]
- 147.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, et al. Low-dose Apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung Cancer. Cancer Immunol Res. 2019;7:630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 152.Seike M, Gemma A. Therapeutic biomarkers of EGFR-TKI. Gan To Kagaku Ryoho. 2012;39:1613–1617. [PubMed] [Google Scholar]
- 153.Liu Z, Han C, Dong C, Shen A, Hsu E, Ren Z, Lu C, Liu L, Zhang A, Timmerman C, et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci Immunol. 2019;4:eaav6473. doi: 10.1126/sciimmunol.aav6473. [DOI] [PubMed] [Google Scholar]
- 154.Baselga J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–S22. doi: 10.1016/S0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 155.Ferris RL, Lenz HJ, Trotta AM, Garcia-Foncillas J, Schulten J, Audhuy F, Merlano M, Milano G. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. 2018;63:48–60. doi: 10.1016/j.ctrv.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, Ferris RL. Anti-EGFR targeted monoclonal antibody isotype influences antitumor cellular immunity in head and neck Cancer patients. Clin Cancer Res. 2016;22:5229–5237. doi: 10.1158/1078-0432.CCR-15-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tauriello D, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Canellas A, Hernando-Momblona X, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 158.Qin ZY, Wang T, Su S, Shen LT, Zhu GX, Liu Q, Zhang L, Liu KW, Zhang Y, Zhou ZH, et al. BRD4 promotes gastric Cancer progression and metastasis through acetylation-dependent stabilization of snail. Cancer Res. 2019;79:4869–4881. doi: 10.1158/0008-5472.CAN-19-0442. [DOI] [PubMed] [Google Scholar]
- 159.Li X, Fu Y, Yang B, Guo E, Wu Y, Huang J, Zhang X, Xiao R, Li K, Wang B, et al. BRD4 inhibition by AZD5153 promotes antitumor immunity via depolarizing M2 macrophages. Front Immunol. 2020;11:89. doi: 10.3389/fimmu.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Hughes KR, Raghani RM, Ma J, Orbach S, Jeruss JS, Shea LD. Cargo-free immunomodulatory nanoparticles combined with anti-PD-1 antibody for treating metastatic breast cancer. Biomaterials. 2021;269:120666. doi: 10.1016/j.biomaterials.2021.120666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han X, Wei Q, Lv Y, Weng L, Huang H, Wei Q, Li M, Mao Y, Hua D, Cai X, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30:327–340. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao A, Liu X, Lin W, Wang J, Wang S, Si F, Huang L, Zhao Y, Sun Y, Peng G. Tumor-derived ILT4 induces T cell senescence and suppresses tumor immunity. J Immunother Cancer. 2021;9:e001536. doi: 10.1136/jitc-2020-001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen X, Gao A, Zhang F, Yang Z, Wang S, Fang Y, Li J, Wang J, Shi W, Wang L, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics. 2021;11:3392–3416. doi: 10.7150/thno.52435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tilsed CM, Fisher SA, Nowak AK, Lake RA, Lesterhuis WJ. Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front Oncol. 2022;12:960317. doi: 10.3389/fonc.2022.960317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 166.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 167.Ho T, Nasti A, Seki A, Komura T, Inui H, Kozaka T, Kitamura Y, Shiba K, Yamashita T, Yamashita T, et al. Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis. J Immunother Cancer. 2020;8:e001367. doi: 10.1136/jitc-2020-001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 169.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 170.Fumet JD, Isambert N, Hervieu A, Zanetta S, Guion JF, Hennequin A, Rederstorff E, Bertaut A, Ghiringhelli F. Phase Ib/II trial evaluating the safety, tolerability and immunological activity of durvalumab (MEDI4736) (anti-PD-L1) plus tremelimumab (anti-CTLA-4) combined with FOLFOX in patients with metastatic colorectal cancer. ESMO Open. 2018;3:e375. doi: 10.1136/esmoopen-2018-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 172.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 173.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26:1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 174.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 177.Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, Zhou C. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-Small cell lung Cancer. J Thorac Oncol. 2017;12:1085–1097. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 178.Lin J, Guo Q, Guo Z, Lu T, Chen G, Lin S, Chen M, Chen C, Lu J, Zong J, et al. Stereotactic body radiotherapy extends the clinical benefit of PD-1 inhibitors in refractory recurrent/metastatic nasopharyngeal carcinoma. Radiat Oncol. 2022;17:117. doi: 10.1186/s13014-022-02073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin J, Song AJ, Hoffman-Censits J, Leiby BE, Tuluc M, Shaw C, Harshyne L, Kean R, Bar-Ad V, Den RB, et al. A pilot study of radiation therapy in combination with Pembrolizumab in patients with metastatic renal cell Cancer. Am J Clin Oncol. 2020;43:82–86. doi: 10.1097/COC.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 180.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 181.Stary V, Wolf B, Unterleuthner D, List J, Talic M, Laengle J, Beer A, Strobl J, Stary G, Dolznig H, Bergmann M. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J Immunother Cancer. 2020;8:e000667. [DOI] [PMC free article] [PubMed]
- 182.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol. 2016;1:EAAG1266. doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Seliger B. Molecular mechanisms of HLA class I-mediated immune evasion of human tumors and their role in resistance to immunotherapies. Hla. 2016;88:213–220. doi: 10.1111/tan.12898. [DOI] [PubMed] [Google Scholar]
- 185.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 186.Lv M, Zhuang X, Shao S, Li X, Cheng Y, Wu D, Wang X, Qiao T. Myeloid-derived suppressor cells and CD68(+)CD163(+)M2-like macrophages as therapeutic response biomarkers are associated with plasma inflammatory cytokines: a preliminary study for non-Small cell lung Cancer patients in radiotherapy. J Immunol Res. 2022;2022:3621496. doi: 10.1155/2022/3621496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen BM, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166:839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 190.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/S1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 191.Kim YH, Yoo KC, Cui YH, Uddin N, Lim EJ, Kim MJ, Nam SY, Kim IG, Suh Y, Lee SJ. Radiation promotes malignant progression of glioma cells through HIF-1alpha stabilization. Cancer Lett. 2014;354:132–141. doi: 10.1016/j.canlet.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 192.Iijima M, Okonogi N, Nakajima NI, Morokoshi Y, Kanda H, Yamada T, Kobayashi Y, Banno K, Wakatsuki M, Yamada S, et al. Significance of PD-L1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J Gynecol Oncol. 2020;31:e19. doi: 10.3802/jgo.2020.31.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ, Buchwald ZS, Chowdhary M, Delman KA, Monson DK, et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology. 2018;7:e1442168. doi: 10.1080/2162402X.2018.1442168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, Nagata K, Nakagawa A, Kosaka Y, Otsuka K, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep. 2017;7:11373. doi: 10.1038/s41598-017-11949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, Kefford RF, Fogarty GB, Guminski A, Wang TW, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5:e1214788. doi: 10.1080/2162402X.2016.1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud A, Chan TA, Yamada Y, Beal K. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer. 2017;5:76. doi: 10.1186/s40425-017-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ribeiro GJ, Schmerling RA, Haddad CK, Racy DJ, Ferrigno R, Gil E, Zanuncio P, Buzaid AC. Analysis of the Abscopal effect with anti-PD1 therapy in patients with metastatic solid tumors. J Immunother. 2016;39:367–372. doi: 10.1097/CJI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 199.Maleki VS, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017;116:116–124. doi: 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 200.Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, Girish S, Wu B. Personalized Cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Igarashi Y, Sasada T. Cancer vaccines: toward the next breakthrough in Cancer immunotherapy. J Immunol Res. 2020;2020:5825401. doi: 10.1155/2020/5825401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cramer DW, Vitonis AF, Pinheiro SP, McKolanis JR, Fichorova RN, Brown KE, Hatchette TF, Finn OJ. Mumps and ovarian cancer: modern interpretation of an historic association. Cancer Causes Control. 2010;21:1193–1201. doi: 10.1007/s10552-010-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 2017;197:S142–S145. doi: 10.1016/j.juro.2016.10.101. [DOI] [PubMed] [Google Scholar]
- 204.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 205.Xu G, Feng D, Yao Y, Li P, Sun H, Yang H, Li C, Jiang R, Sun B, Chen Y. Listeria-based hepatocellular carcinoma vaccine facilitates anti-PD-1 therapy by regulating macrophage polarization. Oncogene. 2020;39:1429–1444. doi: 10.1038/s41388-019-1072-3. [DOI] [PubMed] [Google Scholar]
- 206.Tsukamoto M, Imai K, Ishimoto T, Komohara Y, Yamashita YI, Nakagawa S, Umezaki N, Yamao T, Kitano Y, Miyata T, et al. PD-L1 expression enhancement by infiltrating macrophage-derived tumor necrosis factor-alpha leads to poor pancreatic cancer prognosis. Cancer Sci. 2019;110:310–320. doi: 10.1111/cas.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, Wang Y, Liu D, Xie M, Ji X, et al. IL-1beta-induced elevation of solute carrier family 7 member 11 promotes hepatocellular carcinoma metastasis through up-regulating programmed death ligand 1 and Colony-stimulating factor 1. Hepatology. 2021;74:3174–3193. doi: 10.1002/hep.32062. [DOI] [PubMed] [Google Scholar]
- 208.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 209.Ju X, Zhang H, Zhou Z, Chen M, Wang Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-a signaling. Exp Cell Res. 2020;396:112315. doi: 10.1016/j.yexcr.2020.112315. [DOI] [PubMed] [Google Scholar]
- 210.Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y, Chang SS, Lin WC, Hsu JM, Hsu YH, et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD-L1 in ovarian Cancer cells. Cell Physiol Biochem. 2017;43:1893–1906. doi: 10.1159/000484109. [DOI] [PubMed] [Google Scholar]
- 212.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 214.Fang WB, Yao M, Brummer G, Acevedo D, Alhakamy N, Berkland C, Cheng N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. 2016;7:49349–49367. doi: 10.18632/oncotarget.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5:35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu S, Niu M, O'Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm. 2013;10:3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O'Connor RS, Minutolo NG, Casado-Medrano V, Lopez G, Matsuyama T, Powell DJ. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12:877. doi: 10.1038/s41467-021-20893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits Cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 222.van der Touw W, Chen HM, Pan PY, Chen SH. LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol Immunother. 2017;66:1079–1087. doi: 10.1007/s00262-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sharma N, Atolagbe OT, Ge Z, Allison JP. LILRB4 suppresses immunity in solid tumors and is a potential target for immunotherapy. J Exp Med. 2021;218:e20201811. doi: 10.1084/jem.20201811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ritchie D, Mileshkin L, Wall D, Bartholeyns J, Thompson M, Coverdale J, Lau E, Wong J, Eu P, Hicks RJ, Prince HM. In vivo tracking of macrophage activated killer cells to sites of metastatic ovarian carcinoma. Cancer Immunol Immunother. 2007;56:155–163. doi: 10.1007/s00262-006-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.