Abstract
Background
To evaluate the prognostic value of the pre-treatment aspartate transaminase (AST)/alanine transaminase (ALT) ratio in hepatocellular carcinoma (HCC) patients receiving radiofrequency ablation (RFA)/microwave ablation (MWA) combined with simultaneous TACE.
Methods
The data for 117 patients were retrospectively analyzed in this study. The endpoint of prognosis was overall survival (OS). The Youden index was used to choose the optimal cut-off value of the pre-treatment AST/ALT ratio for OS prediction. Univariate and multivariate analyses were used to identify independent risk factors, then integrated to establish the nomogram.
Results
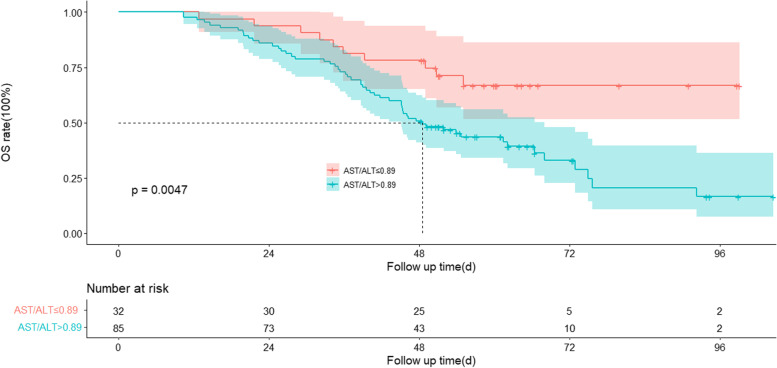
The AST/ALT ratio cut-off value for OS prediction was 0.89, and patients with a higher AST/ALT ratio had poorer OS. The median OS for the high-value AST/ALT group was not reached, while the median OS for the low-value AST/ALT group was 48.5 months (P = 0.0047). The univariate and multivariate analysis showed that AST/ALT ratio, AFP, and tumor numbers were independent prognostic indicators for OS. The integrated nomogram showed higher predictive accuracy for OS (C-index 0.674, 95%CI: 0.600–0.748).
Conclusions
The preoperative AST/ALT ratio could be a prognostic indicator for HCC patients receiving thermal ablation combined with simultaneous TACE.
Keywords: TACE, Thermal ablation, Hepatocellular carcinoma, Enzymes
Background
Hepatocellular carcinoma (HCC) is still one of the most prevalent malignant tumors worldwide. The prognosis of HCC is poor, and the mortality rate of HCC continues to increase at an annual rate of approximately 2%-3% [1]. The main HCC treatments include surgical resection, liver transplantation, ablation, transarterial chemoembolization (TACE), and systemic treatment [2]. Although surgeries, including surgical resection and liver transplantation, are curative treatments, less than 20% of patients are suitable candidates [3]. Locoregional therapies include TACE, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), microwave ablation (MWA), high-intensity focused ultrasound (HIFU), and others. TACE is one of the recommended therapies for intermediate-stage HCC according to the Barcelona clinic liver cancer (BCLC) staging classification [2]. However, the tumor necrosis rate is low, and the intrahepatic recurrence rate is high applying TACE alone [4]. Percutaneous thermal ablation, including RFA and MWA, is considered the optimum therapy choice for focal unresectable early-stage HCC. It can cause coagulation necrosis of tumors and allow a long-term survival rate comparable to surgical resection [5]. Ablation is hard to achieve complete tumor necrosis when it comes to large lesions or tumors adjacent to a large vessel. The combination of TACE with MWA or RFA has advantages compared to monotherapy and can complement the disadvantages of each other [3, 6, 7]. However, the number of prognostic and predictive models in HCC patients undergoing thermal ablation combined with simultaneous TACE is limited.
The serum transaminases, such as aspartate transaminase (AST) and alanine transaminase (ALT), are routine laboratory markers for liver function. In 2015, Bezan showed that a high preoperative AST/ALT ratio (termed the De Ritis ratio) was a prognostic factor for nonmetastatic renal cell carcinoma [8]. Since then, some studies have examined the prognostic value of the AST/ALT ratio in various cancers, such as prostate cancer, oropharyngeal cancer, colorectal carcinoma, and HCC [9–11]. Zhang et al. [12] showed that high AST/ALT ratio was significantly correlated with poorer OS in primary HCC patients. In addition, Liu et al. [13] depicted that high aspartate aminotransferase-to-alanine aminotransferase ratio (AAR) implied poor OS in HCC patients undergoing TACE. However, to the best of our knowledge, there are no studies on AST/ALT in predicting the prognosis of HCC patients who received thermal ablation combined with simultaneous TACE.
This study aimed to examine the value of pre-treatment AST/ALT in the prognosis of HCC patients who received thermal ablation combined with simultaneous TACE and establish a prognosis model by developing a nomogram incorporating AST/ALT ratio into BCLC staging classification.
Methods
Patients
The data for 117 patients who received percutaneous thermal ablation combined with simultaneous TACE procedure in Zhongshan Hospital, affiliated with Fudan University, from November 2010 to January 2017 were retrospectively collected. The inclusion criteria were as follows: (1) diagnosis of hepatocellular carcinoma based on pathological diagnosis or imaging diagnosis; (2) no major vascular invasion, portal vein thrombosis, or extrahepatic metastasis; (3) Patients with primary or recurrent HCC who were not candidates or refused surgical resection; (4) Child–Pugh Class A or B; (5) platelet count > 50 × 10^9/L, prothrombin time ≤ 18 s. The exclusion criteria for the study were as follows: (1) incomplete medical records; (2) lost to follow-up; (3) major vascular invasion,portal vein thrombosis os extrahepatic metastasis; (4) Child–Pugh Class C.
Pre-treatment evaluation
Enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen was performed two weeks before the operation to determine the location, number, size, and relationship between the tumor and adjacent tissues. Ultrasound (US) was performed to judge the visibility of tumors. Blood routine, coagulation function, liver and kidney function, tumor markers, and other related laboratory tests were done within one week before the operation.
Thermal ablation combined with simultaneous TACE procedure
The patient was placed in the supine position during the operation with conscious sedation and/or local anesthesia. Based on the pre-treatment radiological image and the angiography result, a 14G water‑cooled MWA antenna (Nanjing Vison‑China Medical Devices R and D Center, China) or 17G RFA electrode needle (MedSphere Shanghai, China) was inserted into the tumor through percutaneous puncture with US guidance. The microwave power was set between 80-100W. The radiofrequency adopted the automatic impedance mode with the power set at 120W. The maximum temperature was set at 110 °C. Single-point ablation or multi-point overlapping ablation was used according to the size of the lesions and the ablation zone needed to cover the lesion completely. Suppose the lesion was more than 0.5 cm away from difficult locations (large blood vessels, bile ducts, and hollow organs). In that case, the ablation area should exceed the edge of the tumor by 0.5–1.0 cm to achieve a safe margin. If the tumor was close to the difficult locations, it is not mandatory to have the ablation area exceed the 0.5–1.0 cm tumor region. After the ablation was completed, the power was lowered to 50W, and needle tract ablation was carried out to prevent needle tract bleeding and tumor implantation metastasis.
The femoral artery or radial artery was punctured with a 5F-sheath using the modified Seldinger method. A 4-5F RH catheter was used to select the celiac trunk and/or superior mesenteric angiography for angiography to observe the results of ablation, residual lesions, bleeding, and arteriovenous fistula. Microcatheter super-selective catheterizations were performed in the target artery. The emulsion, which was made with platinum-based chemotherapy (100-150 mg) (Jiangsu Hengrui Medicine, Lianyungang, China) + epirubicin (30-50 mg) (Farmorubicin; Pfizer, Wuxi, China) + lipiodol (5–10 ml) (Guerbet, Roissy, France) + Embosphere 500 μm or less (Merit Medical Systems, Inc., South Jordan, UT, USA), was injected into the target artery with digital subtraction angiography (DSA) fluoroscopy. Super-selective gelatin sponge particles were given for embolization if arterial-portal venous fistula or arterial-hepatic venous fistula occurred. A microcatheter was used to select the target artery and embolize with coils for arterial hemorrhage.
Follow-up
Patients received abdominal enhanced CT or MRI and laboratory tests (blood routine, liver and kidney function, tumor markers, etc.) at 1-, 3-, 6-, 9-, and 12-month after the operation. Patients were followed up every six months thereafter if no active intrahepatic lesions or extrahepatic metastasis were found in the imaging examination within one year. If there were active intrahepatic lesions or extrahepatic metastasis, TACE, ablation, systemic therapy, or other treatments tailored to the patient’s situation were chosen. The end of follow-up is the follow-up deadline of December 31, 2020, or the patient’s date of death. The endpoint of the analysis was overall survival (OS), defined as the time from receiving thermal ablation combined with simultaneous TACE for the first time to the end of follow-up.
Statistical analysis
SPSS statistical software (version 24.0) was used for data analysis in this study. Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as percentages. The Youden Index was used to calculate the cut-off value of the pre-treatment AST/ALT ratio. The Kaplan–Meier method was used to draw a survival curve to calculate the cumulative survival rate. Log-rank test was used for univariate analysis, and the Cox regression model was used for multivariate analysis. Factors in univariate analysis with P < 0.05 were included in multivariate analysis. P < 0.05 was considered statistically significant in both the univariate and multivariate analysis. The package of rms in R (Version 4.1.0) was used to draw a nomogram of prognosis according to the results of multivariable analysis, and further evaluated the performance of the nomogram by the C-index.
Results
Analysis at baseline
Clinicopathological characteristics of patients were presented in Table 1. Generally, among the 117 included patients, 96 (82.1%) were male, and 21 (17.9%) were female. The median age was 60 ± 10 years. Most patients received MWA combined with simultaneous TACE (n = 79, 67.5%) and the other patients underwent RFA combined with simultaneous TACE (n = 38, 32.5%).
Table 1.
Baseline characteristics of the research patients (n = 117)
| General information | Data |
|---|---|
| Age(y) | 60 ± 10 |
| Sex [n (%)] | |
| Male | 96 (82.1%) |
| Female | 21 (17.9%) |
| Operation mode [n (%)] | |
| RFA + TACE | 38 (32.5%) |
| MWA + TACE | 79 (67.5%) |
| Hepatectomy before treatment [n (%)] | |
| Yes | 25 (21.4%) |
| No | 92 (78.6%) |
| Hepatitis [n (%)] | |
| HBV | 102 (87.2%) |
| HCV | 4 (3.4%) |
| Cirrhosis [n (%)] | |
| yes | 80 (68.4%) |
| no | 37 (31.6%) |
| ALB(g/l) | 40.0 ± 5.0 |
| AFP (ng/ml) | 497.5 ± 1776.8 |
| AST/ALT ratio | 1.3 ± 1.3 |
| Tumor numbers | |
| 1 | 68 (58.1%) |
| 2 | 18 (15.4%) |
| ≥ 3 | 31 (26.5%) |
| Tumor maximum diameter(mm) | 27.7 ± 12.9 |
| BCLC stages | |
| A | 86 (73.5%) |
| B | 31 (26.5%) |
| Child–Pugh grades | |
| A | 113 (96.6%) |
| B | 4(3.4%) |
RFA radiofrequency ablation, TACE transarterial chemoembolization, MWA microwave ablation, HBV hepatitis B virus, HCV hepatitis C virus, ALB albumin, AFP alpha fetoprotein, AST aspartate transaminase, ALT alanine transaminase, BCLC Barcelona Clinic Liver Cancer
Relationships between AST/ALT ratio and other characteristics
The median AST/ALT ratio was 1.3. Stratified by the Youden index, the optimal cut-off value for OS prediction was 0.89. All patients included in the study were divided into two groups according to AST/ALT ratio: a high-value group (AST/ALT > 0.89, n = 85) and a low-value group (AST/ALT ≤ 0.89, n = 32). The low-value AST/ALT group was associated with a lower ALT count (p < 0.001) and lower AFP (p = 0.013). In addition, a statistically significant association was found in sex between two groups (p = 0.01) (Table 2).
Table 2.
Correlation between AST/ALT ratio and clinicopathological variables of patients with hepatocellular carcinoma
| Variables | AST/ALT ≤ 0.89 (n = 32) |
AST/ALT > 0.89 (n = 85) |
p |
|---|---|---|---|
| Sex, female/male | 1/31 | 20/65 | 0.01* |
| Age, < 60/ ≥ 60 | 18/14 | 31/54 | 0.061 |
| Operation mode, MWA + TACE/RFA + TACE | 19/13 | 60/25 | 0.248 |
| Hepatectomy before treatment, No/Yes | 22/1 | 70/15 | 0.11 |
| HBV, No/Yes | 3/29 | 12/73 | 0.709 |
| HCV, No/Yes | 30/2 | 83/2 | 0.643 |
| Cirrhosis, No/Yes | 14/18 | 23/62 | 0.084 |
| ALB, < 35/ ≥ 35 | 2/3 | 11/74 | 0.486 |
| ALT, ≤ 40/ > 40 | 15/17 | 76/9 | < 0.001* |
| AST, ≤ 35/ > 35 | 21/11 | 56/29 | 0.979 |
| AFP, < 200/ ≥ 200 | 29/3 | 58/27 | 0.013* |
| Number, < 3/ ≥ 3 | 26/6 | 60/25 | 0.244 |
| Tumor maximum diameter, < 30/ ≥ 30 | 23/9 | 51/34 | 0.235 |
| Tumor maximum diameter, < 50/ ≥ 50 | 32/0 | 74/11 | 0.075 |
| BCLC, A/B | 27/5 | 59/26 | 0.102 |
| Child–Pugh grade, A/B | 32/0 | 81/4 | 0.498 |
RFA radiofrequency ablation, TACE transarterial chemoembolization, MWA microwave ablation, HBV hepatitis B virus, HCV hepatitis C virus, ALB albumin, AFP alpha fetoprotein, AST aspartate transaminase, ALT alanine transaminase, BCLC Barcelona Clinic Liver Cancer
*Data are statistically significant results
Analysis of the primary endpoint: OS
The median follow-up time was 49.8 ± 20.6 months. By the end of follow-up, 65 patients had died. The median OS was 55.1 months (95% CI: 41.0–69.1 months). The 1-, 3-, 5-, and 7-year cumulative survival rates were 98.0%, 75.0%, 50.0%, and 32.0%, respectively. The median OS for the high-value AST/ALT group was not reached, while the median OS for the low-value AST/ALT group was 48.5 months (P = 0.0047) (Fig. 1). Furthermore, univariate analysis showed that AFP (P = 0.015), tumor numbers (P < 0.001), maximum tumor diameter (P = 0.01), and BCLC stage (p < 0.001) were statistically correlated with OS. In multivariate analysis, AFP (HR: 1.961; 95%CI: 1.140–3.374; P = 0.015), AST/ALT ratio (HR: 2.075; 95%CI: 1.039–4.145; P = 0.039) and tumor numbers (HR: 3.074; 95%CI: 1.832–5.160; P < 0.001) were identified as independent prognostic indicators for OS (Table 3).
Fig. 1.
Kaplan–Meier survival curve for patients with hepatocellular carcinoma according to the AST/ALT ratio
Table 3.
Univariate and multivariate analyses of clinicopathological characteristics of patients with hepatocellular carcinoma for OS
| Parameter | Patient (n = 117) | OS | ||
|---|---|---|---|---|
| Univariate P-value |
Multivariate P-value |
HR (95%CI) | ||
| Age (y) | ||||
| < 60 | 49 | |||
| ≥ 60 | 68 | 0.669 | ||
| Sex | ||||
| Male | 96 | |||
| Female | 21 | 0.13 | ||
| Operation mode | ||||
| RFA + TACE | 38 | |||
| MWA + TACE | 79 | 0.77 | ||
| Hepatectomy before treatment | ||||
| YES | 25 | |||
| NO | 92 | 0.237 | ||
| Cirrhosis | ||||
| YES | 80 | |||
| NO | 37 | 0.12 | ||
| ALB (g/l) | ||||
| < 35 | 13 | |||
| ≥ 35 | 104 | 0.057 | ||
| AFP (ng/ml) | ||||
| < 200 | 87 | |||
| ≥ 200 | 30 | 0.015* | 0.015* | 1.961(1.140–3.374) |
| AST/ALT ratio | ||||
| ≤ 0.89 | 32 | |||
| > 0.89 | 85 | 0.005* | 0.039* | 2.075(1.039–4.145) |
| Tumor numbers | ||||
| < 3 | 86 | |||
| ≥ 3 | 31 | < 0.001* | < 0.001* | 3.074(1.832–5.160) |
| Tumor maximum diameter(mm) | ||||
| < 50 | 106 | |||
| ≥ 50 | 11 | 0.01* | 0.22 | |
| BCLC stage | ||||
| A | 86 | |||
| B | 31 | < 0.001* | 0.891 | |
| Child–Pugh grades | ||||
| A | 113 | |||
| B | 4 | 0.156 | ||
OS overall survival, RFA radiofrequency ablation, TACE transarterial chemoembolization, MWA microwave ablation, ALB albumin, AFP alpha fetoprotein, AST aspartate transaminase, ALT alanine transaminase, BCLC Barcelona Clinic Liver Cancer
*Data are statistically significant results
Prognostic nomograms integrating AFP, tumor number, AST/ALT ratio and BCLC
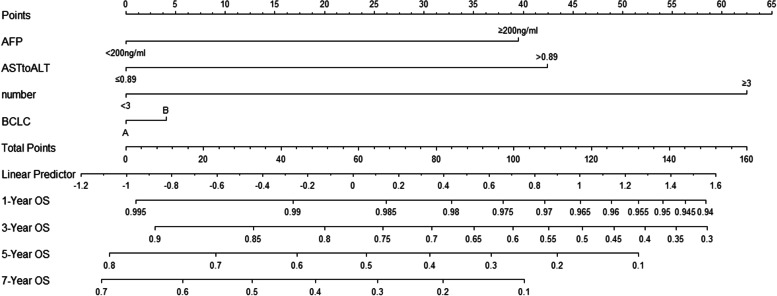
A model was established by integrating AFP, tumor number, AST/ALT ratio, and BCLC (Fig. 2). The C-index for OS prediction of the formulated nomogram was 0.674 (95%CI: 0.600–0.748), which was higher than BCLC (0.607, 95%CI:0.548–0.666) and other indicators and combinations (Table 4).
Fig. 2.
Nomogram predicts 1-,3-,5-and 7-year OS in hepatocellular carcinoma patients
Table 4.
The identification ability and independent prognostic factors of the nomogram of patients with hepatocellular carcinoma: concordance indices in overall survival prediction
| Variables | OS | |
|---|---|---|
| C-index | 95%CI | |
| AST/ALT ratio | 0.573 | 0.516–0.630 |
| BCLC | 0.607 | 0.548–0.666 |
| AFP | 0.564 | 0.505–0.623 |
| number | 0.62 | 0.563–0.677 |
| BCLC + AFP + number | 0.657 | 0.584–0.730 |
| BCLC + AFP + number + AST/ALT ratio | 0.674 | 0.600–0.748 |
OS overall survival, AST aspartate transaminase, ALT alanine transaminase, AFP alpha fetoprotein, BCLC Barcelona Clinic Liver Cancer
Discussion
This study found that the AST/ALT ratio was an easily accessible and novel prognostic factor for HCC patients receiving thermal ablation combined with simultaneous TACE. A high AST/ALT ratio was significantly associated with poor outcomes expressed as OS. Furthermore, the nomogram integrating AST/ALT ratio, AFP, tumor number, and BCLC had improved predictive ability compared with the BCLC staging classification alone.
Since Fernando De Ritis first described AST/ALT ratio as an enzymatic test for viral hepatitis in 1957 [14], researchers have taken it as a potential prognostic biomarker in various diseases [8–13, 15, 16]. Also, Granito et al. [17] evaluated the prognostic significance of changes in liver parameters as predictors of treatment response to cTACE. They found that a postprocedure increase of transaminases (AST increase ≥ 46%, ALT increase ≥ 52%) compared with baseline values was shown to be a reliable predictor of response to cTACE. The results proved the transaminase value as prognostic indicators of HCC treatments. AST and ALT are important enzymes in the liver and are widely used to evaluate liver function. AST is mainly found in the mitochondria of liver cells, while ALT is presented primarily outside the mitochondria of liver cells. The serum AST and ALT can be impacted by many non-tumor-related factors, such as coronary heart disease, chronic hepatitis, and drugs. Therefore, the combination of AST and ALT as AST/ALT ratio is more stable and appropriate than they were used as single predictors [18]. Cancer cells have a higher rate of aerobic glycolysis to generate more energy to support their high proliferative status than normal cells [19]. AST plays a vital role in aerobic glycolysis due to its ability to relocate nicotinamide adenine dinucleotide hydrogen into mitochondria through malate-aspartate shuffling [20]. Thus, more AST is activated than ALT in high proliferative cancer tissues. Moreover, glutaminolysis and pyruvate production are catalyzed by ALT, which are also strengthened in tumor cells [21]. Hence, the AST/ALT ratio may reflect the metabolic status in cancers, possibly related to tumor growth and progression.
Therefore, we hypothesized that the AST/ALT ratio maybe a potential prognostic indicator for HCC patients receiving thermal ablation combined with simultaneous TACE in our study. According to the optimal cut-off value of AST/ALT ratio, we stratified patients into two groups, and found the subgroup with lower value of AST/ALT ratio (≤ 0.89) was related to the lower ALT count and lower AFP. In addition, High AST/ALT ratio was associated with poor OS, consistent with the literature. Wu et al. [18] analyzed 18 studies and concluded that an elevated serum AST/ALT ratio before treatment was significantly associated with poor clinical outcomes of OS in patients with solid tumors. Another study also depicted that a high AST/ALT ratio implied poor OS in HCC patients accepting TACE [13].
AFP is the most frequently used biomarker for HCC [22]. It has been proven that constantly increased serum AFP level was correlated with aggressive histological morphology of HCC, such as vascular invasion, satellitosis and poorly differentiated [23–25]. A previous study had demonstrated that elevated AFP meant dismal prognosis [26]. However, excluding the liver transplantion, an AFP threshold has not been used as a guide for HCC treatment. Till now, the decision -making process mainly relies on imaging features and clinical characteristics without consideration of markers of tumor aggressiveness. This approach may hinder the optimal management of HCC patients, since there are increasing evidences suggesting that cancer biology is a helpful predictor of treatment outcomes [27]. Potentially, AFP represents an easily accessable biomarker to modify the decision-making process currently adopted for the management of HCC. Herein, we integrated AFP, number, BCLC, and AST/ALT ratio and improved the predictive accuracy of BCLC system in terms of C-index.
There were some shortcomings in our study. First, this was a single-center retrospective study with small sample size. Due to the characteristics of the retrospective study, some biases were hard to be avoided. Second, this study did not exclude patients who have previously received locoregional treatment, which might impact the result. Third, some patients received additional treatments after first-time thermal ablation combined with simultaneous TACE, which might have a confounding effect on the tumor response and outcome.
Despite the limitations mentioned above, our study still had clinical value. First, it was the first time to show the predictive value of AST/ALT ratio in HCC patients receiving thermal ablation combined with simultaneous TACE. Second, AST/ALT ratio was a conventional blood parameter, and its convenience, easy accessibility and inexpressiveness revealed its excellent performance in risk stratification for HCC patients. In addition, AST/ALT ratio could become an effective tool for future clinical studies and patient management.
Conclusions
This study investigated the prognostic role of the AST/ALT ratio in the outcome of HCC patients undergoing thermal ablation combined with simultaneous TACE. The AST/ALT ratio was an independent prognostic factor for HCC patients receiving thermal ablation combined with simultaneous TACE, and a high preoperative AST/ALT ratio indicated poor OS. Besides, the constructed nomogram including AFP, number, BCLC, and AST/ALT ratio was confirmed as a well-performed prognostic model for OS. This finding may help improve the management of HCC patients who accept thermal ablation combined with simultaneous TACE through early identification of patients with potential poor outcomes.
Acknowledgements
Thanks to Ning Pu for his statistical advice on this study.
Abbreviations
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- HCC
Hepatocellular carcinoma
- RFA
Radiofrequency ablation
- MWA
Microwave ablation
- OS
Overall survival
- PFS
Progression-free survival
- AFP
Alpha fetoprotein
- TACE
Transarterial chemoembolization
- PEI
Percutaneous ethanol injection
- HIFU
High-intensity focused ultrasound
- BCLC
Barcelona Clinic Liver Cancer
- mRECIST
The modified response evaluation criteria in solid tumors
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- ALB
Albumin
Authors’ contributions
L.X.L. and Z.P.Y. conceived and designed the project. F.H.W., S.S.G., M.F.W., and D.Y.Z. collected the data. H.Y.S., L.X.L., and Y.D. analyzed and interpreted the data. F.H.W. drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by China Postdoctoral Science Foundation [2022M710756], the Shanghai Sailing Program [21YF1443500], Shanghai Municipal Health Commission (No.201940409), National Natural Science Foundation of China [82202969] and Youth Fund of Zhongshan Hospital Fudan University [LCBSHZX010].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from individual or guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feihang Wang, Shanshan Gao, and Mengfei Wu contributed equally to this work.
Contributor Information
Zhiping Yan, Email: zhipingyan@fudan.edu.cn.
Lingxiao Liu, Email: liulingxiao2022@163.com.
References
- 1.Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7(308):319. doi: 10.1016/j.gendis.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Xie H, Zhou L, Chen X, Zheng S. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. doi: 10.1155/2019/8619096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Ni CF. Current status of the combination therapy of transarterial chemoembolization and local ablation for hepatocellular carcinoma. Abdom Radiol (NY). 2019;44:2268–2275. doi: 10.1007/s00261-019-01943-2. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 6.Gui CH, Baey S, D’cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46:763–771. doi: 10.1016/j.ejso.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wu M, Gao S, Song H, et al. Percutaneous thermal ablation combined with simultaneous transarterial chemoembolization for hepatocellular carcinoma </=5 cm. J Cancer Res Ther. 2019;15:766–772. doi: 10.4103/jcrt.JCRT_250_19. [DOI] [PubMed] [Google Scholar]
- 8.Bezan A, Mrsic E, Krieger D, et al. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J Urol. 2015;194:30–35. doi: 10.1016/j.juro.2015.01.083. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, He Z, Ma S, Liu R. AST/ALT ratio as a significant predictor of the incidence risk of prostate cancer. Cancer Med. 2020;9:5672–5677. doi: 10.1002/cam4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knittelfelder O, Delago D, Jakse G, et al. The AST/ALT (De Ritis) ratio predicts survival in patients with oral and oropharyngeal cancer. Diagnostics (Basel). 2020;10(11):973. doi: 10.3390/diagnostics10110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheipner L, Smolle MA, Barth D, et al. The AST/ALT ratio is an independent prognostic marker for disease-free survival in stage II and III colorectal carcinoma. Anticancer Res. 2021;41:429–436. doi: 10.21873/anticanres.14792. [DOI] [PubMed] [Google Scholar]
- 12.Zhang LX, Lv Y, Xu AM, Wang HZ. The prognostic significance of serum gamma-glutamyltransferase levels and AST/ALT in primary hepatic carcinoma. BMC Cancer. 2019;19:841. doi: 10.1186/s12885-019-6011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Jia BS, Zou BW, et al. Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Medicine (Baltimore) 2017;96:e8512. doi: 10.1097/MD.0000000000008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritis de F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta. 1957;2:70–74. doi: 10.1016/0009-8981(57)90027-X. [DOI] [PubMed] [Google Scholar]
- 15.Shijo H, Okazaki M. Prediction of portal vein invasion by hepatocellular carcinoma: a correlations between portal vein tumor thrombus and biochemical tests. Jpn J Clin Oncol. 1991;21:94–99. doi: 10.1093/oxfordjournals.jjco.a039452. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Dai J, Zhang Y, et al. Baseline HBV-DNA load plus AST/ALT ratio predicts prognosis of HBV-related hepatocellular carcinoma after hepatectomy: a multicentre study. J Viral Hepat. 2021;28:1587–1596. doi: 10.1111/jvh.13606. [DOI] [PubMed] [Google Scholar]
- 17.Granito A, Facciorusso A, Sacco R, et al. TRANS-TACE: prognostic role of the transient hypertransaminasemia after conventional chemoembolization for hepatocellular carcinoma. J Pers Med. 2021;11(10):1041. doi: 10.3390/jpm11101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Chen L, Wang Y, Tan W, Huang Z. Prognostic value of aspartate transaminase to alanine transaminase (De Ritis) ratio in solid tumors: a pooled analysis of 9,400 patients. Onco Targets Ther. 2019;12:5201–5213. doi: 10.2147/OTT.S204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 2015;21:711–725. doi: 10.3748/wjg.v21.i3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friday E, Oliver R, 3rd, Welbourne T, Turturro F. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: relationship to mitochondrial membrane potential. J Cell Physiol. 2011;226(511):519. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Chen R, Wei Q, Xu X. The Landscape Of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We? Int J Biol Sci. 2022;18:536–551. doi: 10.7150/ijbs.64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 24.Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- 25.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 26.Cabibbo G, Maida M, Genco C, et al. Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. World J Hepatol. 2012;4:256–261. doi: 10.4254/wjh.v4.i9.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trevisani F, Garuti F, Neri A, Alpha-fetoprotein for diagnosis, prognosis, and transplant selection. Semin Liver Dis. 2019;39:163–177. doi: 10.1055/s-0039-1677768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.