Abstract
Background
There are still many offered donor livers that are declined during the allocation process. Machine perfusion offers the option to evaluate (especially marginal) donor organs and to better decide whether a graft has the potential of being transplanted or not. There is a lack of clear detailed data on why organs are declined and how many donor livers would have the potential of being evaluated in the machine.
Material/Methods
We retrospectively reviewed 1356 donor livers between 2016 and 2018, which were offered by Eurotransplant and were declined during the allocation process; 284 grafts were from donor after cardiac death (DCD) and 1072 donations were from after brain death (DBD). The analysis was performed independently and blinded by senior transplant surgeons.
Results
There were 904 (66.6%) donor livers with potential to be evaluated as suitable grafts in machine perfusion, whereas 417 (30.8%) organs were definitely not-transplantable, mainly due to liver cirrhosis, (untreated) donor malignancy, cardiac diseases of the donor leading to a hepatic congestion, and/or systemic infections in the donor. Donors in blood group “AB” were disproportionally often rejected. Due to missing data, 35 (2.6%) organs could not be sufficiently evaluated.
Conclusions
Our data suggest that many declined donor livers have potential of being evaluated by machine perfusion. Comprehensive use of machine perfusion is necessary and useful to improve the current organ shortage.
Keywords: Liver Transplantation, Tissue and Organ Procurement
Background
The potential value of machine perfusion, either hypothermic [1] or normothermic [2], has been shown recently. Beside logistical advantages, there were lower risks of non-anastomotic biliary strictures, especially in donation after cardiac death (DCD) donors [1], as well as a lower level of graft injury reflected by the liver enzymes [2]. Markmann et al showed a significant decrease of ischemic biliary complications applying a portable liver machine perfusion, especially in DCD donors [3].
However, machine perfusion becomes a more and more important technique in modern transplantation programs. One reason for being restrictive in establishing a machine perfusion program might be the immense cost per application, which are, depending on the cost coverage system of the individual countries, often not clarified and are a matter of debate. On the contrary, Raigani et al found that the costs for machine perfusion in an US cohort of extended-criteria and subsequently discarded livers were comparable to the monthly cost of care for waiting list patients with high MELD scores [4].
The organ demand in the Eurotransplant area is still high: in 2021, 2411 patients were registered on the waiting list vs 1514 performed liver transplantations from deceased donors (data provided in the Annual Report 2021 by Eurotransplant).
In recent years, the percentage of discarded livers within the Eurotransplant region varied from 18.4% to 26.7% (see Table 1, data from the “Annual reports” provided by Eurotransplant). However, it is unclear why a particular liver was discarded. The questions in this regard are what were the reasons for discarding the livers and do the discarded livers have a theoretical potential for evaluation in machine perfusion?
Table 1.
Numbers and percentage of reported, offered, accepted and transplanted donor livers per year between 2012 and 2019 within the Eurotransplant region (study period in bold letters).
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|
| Reported | 1953 | 1862 | 1919 | 1965 | 1935 | 1939 | 2168 | 2093 |
| Offered | 1945 | 1855 | 1916 | 1962 | 1934 | 1935 | NA | NA |
| Accepted | 1886 | 1784 | 1854 | 1898 | 1864 | 1883 | NA | NA |
| Transplanted | 1595 | 1466 | 1536 | 1569 | 1525 | 1519 | 1643 | 1536 |
| Percentage transplanted organs (of reported livers) | 81.6 | 78.7 | 80 | 79.8 | 78.8 | 78.3 | 75.7 | 73.3 |
| Percentage transplanted organs (of accepted livers) | 84.6 | 82.2 | 82.8 | 82.6 | 81.8 | 80.7 | NA | NA |
NA – not applicable.
The aim of the study was to provide a practical evaluation of the discarded liver within the Eurotransplant region for absolute reasons for liver declining or for the eventual justification evaluating this liver during machine perfusion.
Material and Methods
The donor data were provided from the Eurotransplant database.
All initially offered and during the allocation, procurement, and pre-transplantation process discarded donor livers between January 2016 and December 2018 were recorded and the following data, if applicable, captured:
Age, weight, height, body mass index, sex, country and/or region, blood group, graft type (whole liver or split), HIV antibodies, HIV antigen, HBs antigen, HBs antibody, HCV antibody, HBc antibody, Lues serology, CMV IgG, CMV IgM, Toxoplasmosis serology, cause of death, ICD-10 code and description, additional diagnosis, length of ICU stay, episodes of cardiac arrest (yes/no), duration of cardiac arrest, malignancy (yes/no), specification of malignancy, alcohol abuse (yes/no), results of abdominal ultrasound, pathology results (if applicable), laboratory values: sodium (last value before procurement), ASAT (last value before procurement), ALAT (last value before procurement), gamma-GT, bilirubin (last value before procurement), Quick (last value before procurement), INR (last value before procurement), peak sodium, peak ASAT, and peak ALAT.
The data of the livers after brain death donation were analyzed independently by 2 senior surgeons (FR/HMT) who are self-reliantly involved in the decision process of liver acceptance or declination at the transplantation center. The analysis was performed blinded to the decision of the other investigator. In case of split decision, a third senior surgeon (US) made the final decision about the potential use of a particular organ for machine perfusion.
DCD grafts were analyzed separately, since acceptance of DCD livers is not allowed in all (European) countries. These donor organs were initially analyzed blinded by the investigators and then discussed between all 3 transplant surgeons to find a consensus decision.
The study was approved by the local ethics committee (registration number 2021-2217).
Definite reasons for declining a donor liver were:
– Malignancy < 5 years between treatment of the tumor and organ donation or an advanced malignant disease as defined in the “8th Edition of the Guide to the quality and safety of organs for transplantation” [5];
– Cirrhosis from the macroscopic aspect described by the procurement surgeon;
– Histologic proven septum-forming fibrosis (F3 fibrosis) or cirrhosis in the frozen section;
– Liver congestion based on cardiac diseases;
– Shock liver after trauma or resuscitation.
Severe steatosis, mild or moderate fibrosis (≤F2 fibrosis), or highly elevated liver enzymes alone were not reasons for declining an organ, but the combination of several parameters might have been led to the decision to decline the liver, even for machine perfusion, at the discretion of the individual surgeon.
Data Analysis
The data were analyzed descriptively. A statistical analysis was not provided since the character of the data was unsuited to statistical comparison.
Results
We analyzed the data of 1356 donor livers. Out of these, there were 284 DCD grafts and 1072 donations after brain death (DBD). The main characteristics are summarized in Table 2. Figure 1 shows the workflow and the numeric results of the individual analyses.
Table 2.
Data of the whole study cohort.
| Whole study cohort | Donation after brain death | Non-heart beating donation | |
|---|---|---|---|
| Age (years) | 59 (0–92) | 60 (0–92) | 56 (0–77) |
| Weight (kg) | 80 (2.6–180) | 80 (2.6–180) | 80 (4–130) |
| Height (cm) | 173 (48–200) | 172 (48–200) | 175 (57–200) |
| Body mass index (kg/m2) | 27 (10–68) | 27 (10–68) | 26 (12–45) |
| Gender | |||
| Male | 810 | 635 | 175 |
| Female | 546 | 437 | 109 |
| Country | |||
| Germany | 484 | 484 | – |
| Belgium | 219 | 126 | 93 |
| Netherlands | 219 | 31 | 188 |
| Austria | 149 | 146 | 3 |
| Hungary | 119 | 119 | – |
| Slovenia | 34 | 34 | – |
| Croatia | 66 | 66 | – |
| Outside ET area | 66 | 66 | – |
| Blood group | |||
| 0 | 479 | 364 | 115 |
| A | 586 | 460 | 126 |
| B | 162 | 135 | 27 |
| AB | 129 | 113 | 16 |
| Type of organ | |||
| Whole liver | 1351 | 1067 | 284 |
| Split liver | 5 | 5 | – |
| Virology | |||
| HIV antibody positive | 2 | 1 | 1 |
| HIV antigene positive | 2 | – | 2 |
| HBs antigene positive | 10 | 10 | – |
| HBs antibody positive | 181 | 136 | 45 |
| HBc antibody positive | 105 | 95 | 10 |
| HCV antibody positive | 12 | 33 | 3 |
| Lues positive | 6 | 6 | – |
| Cause of death | |||
| Trauma | 236 | 188 | 48 |
| Hypoxia | 284 | 158 | 126 |
| Cerebro-vascular accident | 788 | 688 | 100 |
| Duration of ICU stay (days) | 48 | 38 | 10 |
| Peak sodium (mmol/l) | 148 (129–195) | 150 (129–195) | 144 (133–171) |
| Peak AST (U/l) | 79 (9–8.000) | 73.5 (9–8.000) | 97 (10–6574) |
| Peak ALT (U/l) | 53 (6–5.479) | 48 (6–5.479) | 74 (11–5095) |
Figure 1.
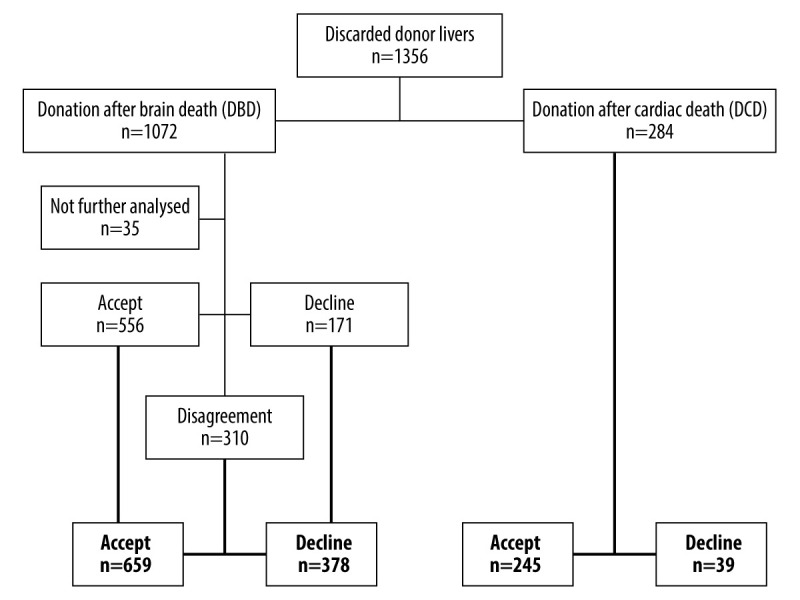
Flowchart of the analyzed organs.
Analysis of Donor Livers – Donation After Brain Death
From the 1072 DBD livers, we could not sufficiently judge 35 since there was a lot of missing information in the electronic database from donations outside the Eurotransplant area (n=34) and 1 potentially good donor liver could not be procured since the donor had massive intrabdominal adhesions due to previous operations. In the first analysis, 556 livers were assessed as suitable for machine perfusion, whereas 171 livers were declined. That resulted in 310 organs, but the initial investigators disagreed. After evaluation of the third investigator, 659 DBD livers were finally found to be suitable for machine perfusion and 378 were definitely declined.
Reasons for Declining Organs – Donations After Brain Death
There were 378 donor livers not suitable for any liver transplantation. Reasons for declination were:
-
– Diseases of the liver itself (n=231):
Liver cirrhosis (n=70), septum-forming fibrosis (n=91), polycystic liver disease (n=2), JAK-2-mutation (n=1).
-
– Histologic proven:
Necrosis (n=16), severe steatosis with zones of parenchymal collapse (n=19), steatohepatitis (n=12), destructive cholangitis/severe inflammation (n=20).
-
– Vascular reasons (n=5):
Thrombosis of the hepatic artery (n=2), portal venous and venous thrombosis (n=1), aortic dissection with involvement of the hepatic artery (n=1), portal vein thrombosis (n=1).
-
– Resulting conditions of the donor disease (n=43):
Liver congestion with or without dilatative cardiomyopathy (n=11), shock liver with or without extracorporeal membrane oxygenation (n=32).
-
– Diseases of the donor (n=30):
Severe liver trauma (n=4), sepsis (n=3), bacterial meningitis (n=2), herpes encephalitis (n=2), recently performed liver resection (n=2), endocarditis (n=2), amyloidosis (n=1), necrotizing pancreatitis (n=1), acute liver failure (n=1), extensively prolonged resuscitation (n=1), acute respiratory distress syndrome (n=1), HELLP syndrome (n=1), liver insufficiency due to Marcumar therapy (n=1), recently operated bowel perforation (n=1), smoke inhalation injury (n=1), fungal pneumonia after bone marrow transplantation (n=1), ventriculitis (n=1), pulmonary hypertension (n=1), infection with multi-resistant bacteria (n=1), hepatitis C with highly elevated viral load (n=1), and chronic obstructive pulmonary disease + state after open aortic repair (n=1).
-
– Donor malignancy (n=66):
Renal cell carcinoma (n=13), colorectal carcinoma/neuroendocrine tumor of the colon (n=7), bronchial carcinoma (n=5), breast cancer (n=3), cholangiocarcinoma (n=3), prostate cancer (n=3), non-Hodgkin lymphoma (n=3), leiomyosarcoma (n=2), phaeochromocytoma (n=2), malignant melanoma (n=2), polycythemia (n=2), liposarcoma (n=2), endometrial cancer (n=1), B-cell lymphoma (n=1), cervical cancer (n=1), ependymoma (n=1), mesothelioma (n=1), pancreatic cancer (n=1), oral cancer (n=1), plasmocytoma (n=1), gastric cancer (n=1), cancer of the adrenal gland (n=1), metastasized gastrointestinal stromal tumor (n=1) and carcinoma of unknown origin with manifestation in lymph nodes (n=5), in liver metastases (n=1), and in various locations (n=2).
-
– Procurement or allocation related reasons (n=3):
Bad perfusion (n=2), left-lateral split (n=1).
Evaluation of Potentially Suitable Livers – Donations After Brain Death
From the donor organs that were assessed to be suitable for machine (n=659), there were 17 livers without any criteria for marginality. These grafts were considered adequate for transplantation according to our analysis but the reasons for denial could not be identified.
From the remaining donor organs, 139 had a severe steatosis or F2-fibrosis, 11 had an HCV-positive state with corresponding slight fibrosis, 13 were organs from donors with an age of “0” (donor weight between 2.6 and 6 kg) and the remaining 496 livers had a combination of increased age and/or long-lasting resuscitation episodes and/or significantly elevated liver enzymes and/or significantly increased sodium and/or steatosis in ultrasound and/or no available frozen section in time.
Analysis of Donor Livers – Donation After Cardiac Death
Reasons for Declining Organs – Donations After Cardiac Death
There were 39 donor livers not suitable for any liver transplantation. Reasons for declination were:
-
– Resulting conditions of the donor disease (n=17):
Heart failure with hepatic congestion (n=4); instable donor with need of ECMO therapy (n=4); shock liver (n=4); recurrent pulmonary embolism ± cava-filter (n=2); prolonged and recurrent resuscitation (n=1); right heart failure (n=1); cardiogenic shock+air embolism (n=1).
-
– Diseases of the donor (n=19):
Florid systemic infection (n=5); recent aortic dissection with reconstruction of the aortic arch (n=3); active MRSA infection (n=2); recently perforated duodenal ulcer (n=2); smoke inhalation injury (n=1); active severe pancreatitis (n=1); hemorrhagic shock due to an unclear coagulation disorder (n=1); endocarditis/pulmonary embolism/mitral valve reconstruction in the same donor (n=1); necrosis of the intestine (n=1); HIV infection (n=1); hepatic sarcoidosis (n=1).
-
– Donor malignancy (n=3):
Suspicious carcinoma in lung and adrenal glands in the same donor (n=1); multiple intrahepatic lesions (n=1); suspicious pulmonary lesion, whereby the whole procurement was abandoned (n=1).
Evaluation of Potentially Suitable Livers – Donations After Cardiac Death
From the potential donor organs after cardiac death, 245 livers could have been transplanted on the basis of the available data but the reasons for declining the organs were not available for this study. Furthermore, 67 donor livers would have had the potential for an evaluation in the machine. Reasons for this were:
Steatosis ± elevated transaminases (n=18); highly elevated transaminases after resuscitation (n=17); alcohol abuse and steatosis ± resuscitation (n=4); high BMI and elevated transaminases ± resuscitation (n=3); alcohol abuse and hepatitis B or C (n=3); highly elevated transaminases (n=2); unclear histology of suspected malignancy (n=2); prolonged resuscitation (n=2); child <5 kg (n=1); high BMI and older donor (n=1); normal liver enzymes but ischemic cardiomyopathy (n=1); COPD and cardiac revascularization (n=1); other reasons (n=12).
Discussion
This study is, to our knowledge, the most meticulous analysis of discarded organs regarding a potential analysis for machine perfusion. This “real life analysis” showed that there is a potential for machine perfusion of approximately 300 livers per year (904 donor organs in a study period of three years), organs that have not been used for liver transplantation yet.
There are several reasons for declining organs without having the advantage of evaluating them in a machine. Especially marginal organs can benefit from this and data from the UK suggest that the percentage of liver transplantations can be increased by 15% after implementation of machine perfusion [6]. A recent paper by Clavien et al described an evaluation period of 3 days with a subsequent uneventful transplantation [7]. Of course, this is an extreme situation but well describes the potential of a normothermic machine application.
Some criteria for marginal organs should be discussed in the following sections:
Steatosis
The demographic development and the increasing proportion of obese people lead naturally to more steatotic donor livers, which have to be assessed for their suitability for liver transplantation. In general, microsteatosis is mostly seen as uncritical and potentially reversible, whereby severe macrosteatosis is considered to be a risk factor for graft dysfunction up to life-threatening graft failure.
A recently published review by Lai et al showed that machine perfusion might attenuate the graft damage from macrosteatotic livers measured by the peak of transaminases. “Hard” outcome parameters like recipient’s death or need for retransplantation were rare events and therefore not sufficiently ratable in this study due to the relatively small number of included patients [8].
The future in this context will be a pharmacological modulation of the livers, as shown by Raigani et al in an animal model. They showed a better graft function after defatting initially steatotic livers [9]. As shown by the same group, metabolic and lipidomic profiling during normothermic machine perfusion might have the potential to guide the resuscitation and rehabilitation of steatotic donor livers [10]. Boteon et al manipulated the lipid metabolism of human livers and decreased the lipid content of the potential graft [11].
In our analysis, graft steatosis was obviously a major concern, which subsequently led to organ rejection. In our opinion, severe (macro-)steatosis is indeed a major reason for declining a donor organ. Therefore, we see a main application for the usage of machine perfusion in steatotic organs which are otherwise not transplanted with a clear conscience. Machine perfusion makes it on the one hand possible to assess the organ even if a severe steatosis was diagnosed and, on the other hand, will give the centers the opportunity to affect the steatosis in terms of a “defatting procedure”.
Unclear Findings in Frozen Sections
In some donors, there are unclear findings during organ procurement, in worst case suspicious for a malignant disease. In an Italian analysis of 11 271 organ donors, in 595 of these a malignant disease was already treated in the history or actually [12]. In an autopsy study, unsuspected neoplasia rate was 7% [13]. Organs with an unclear finding during an organ procurement are usually rejected if the histologic analysis does not come to an unambiguous benign result. In some cases, time-consuming histologic analysis is necessary. This time is, considering the cold ischemia time, often unavailable and could be bridged using machine perfusion.
Elevated Liver Enzymes/State After Prolonged Resuscitation
A prolonged resuscitation associated with elevated liver enzymes are suspect for a significant hepatocellular damage due to the period of warm ischemia time, which often leads to rejection of the potential donor organ since the risk for primary non-function or ischemic cholangiopathy is considered to be too high. This is not only the case in DCD (see below) but also in DBD (with hypoxic brain damage as cause of death leading to organ donation). In these cases, machine perfusion can also be an option to assess the near-term graft function and therefore minimizing the risk of a fatal graft failure. Furthermore, the risk of non-anastomotic biliary strictures is minimized when machine perfusion is applied [1].
Donation After Cardiac Death
This is predominantly a domain for machine perfusion. However, the different allocation rules in Eurotransplant countries allowing or prohibiting DCD can make the allocation difficult. Furthermore, logistical reasons are easily conceivable in this particular mode of organ donation. Croome et al reported that ischemic cholangiopathy after non-heart beating donation as problematic, suggesting machine perfusion as a suitable tool for reduction of complications and improving prediction of risk after non-heart beating donation [14]. This is not only the case for donor livers after non-heart beating donation, but also for kidneys [15].
Blood Groups
We found in our analysis a remarkable distribution of blood groups showing a higher proportion of discarded organs in blood group “AB” (9.5% of all discarded donor livers). Usually, blood group “AB” is found in around 5% of the Mid-European population [16]. Most centers are restrictive when accepting organs for “AB” recipients since the waiting list dynamic shows that the small number of AB recipients goes along with the highest probability getting transplanted in a MELD-based allocation system when having blood group “AB” [17]. Therefore, marginal organs for “AB” recipients are discarded more often as the centers take the chance of picking a better organ for a particular “AB” patient.
Machine perfusion can offer the following opportunities for “AB” donor livers:
It might give the centers the chance to gain time for finding a suitable “AB” recipient even outside the Eurotransplant region and therefore reduces the probability losing transplantable organs.
It can be possible to prepare suitable but blood-group incompatible recipients.
Limitations
Of course, a retrospective study designs always has limitations. In this particular study, we were faced with the following problems:
Not all macroscopic evaluations of the procurement surgeon were available. This might have led to a better judgement of the organ from our point of view.
We could not calculate a donor-risk index or an Eurotransplant donor-risk index since the cold ischemia time is needed to calculate these.
We had no insight into the center-specific reasons for declining the organ. We are aware that specific donor-recipient constellations may lead to non-acceptance of a particular organ. In some cases of good organs, recipient-related reasons might have led to a rejection of a particular organ without getting the graft re-allocated in time. Of course, such logistic problems could be solved using machine perfusion.
Conclusions
In summary, we showed that 904 declined donor livers would have had the potential to be evaluated with machine perfusion (donation after brain death: 659; non-heart beating donation: 245). Our data suggest that comprehensive use of machine perfusion is necessary and useful to improve the current organ shortage.
Acknowledgements
The authors thank Eurotransplant, the ELIAC, and especially Marieke de Rosner van Rosmalen for their support with data acquisition.
Abbreviations
- DCD
donation after cardiac death
- DBD
donation after brain death
- HELLP
hemolysis, elevated liver enzymes, low platelet count syndrome
- HIV
human immunodeficiency virus
- HBs
hepatitis B surface
- HCV
hepatitis C virus
- HBc
hepatitis B core
- CMV
cytomegaly virus
- IgG
immunoglobulin G
- ICD-10
International Statistical Classification of Diseases and Related Health Problems
- 10th revisionICU
Intensive Care Unit
- ASAT
aspartate aminotransferase
- ALAT
alanine aminotransferase
- gamma-GT
gamma-glutamyltransferase
- INR
international normalized ratio
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: HMT is funded by the German Research Foundation (DFG) under the research group program FOR 5151 “QuaLiPerF (Quantifying Liver Perfusion-Function Relationship in Complex Resection – A Systems Medicine Approach)” under grant number 436883643 and under grant number 465194077 (Priority Program SPP 2311, subproject SimLivA)
References
- 1.van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation – a randomized trial. N Engl J Med. 2021;384(15):1391–401. doi: 10.1056/NEJMoa2031532. [DOI] [PubMed] [Google Scholar]
- 2.Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 3.Markmann JF, Abouljoud MS, Ghobrial RM, et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157(3):189–98. doi: 10.1001/jamasurg.2021.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raigani S, De Vries RJ, Carroll C, et al. Viability testing of discarded livers with normothermic machine perfusion: Alleviating the organ shortage outweighs the cost. Clin Transplant. 2020;34(11):e14069. doi: 10.1111/ctr.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Directorate for the Quality of Medicines & HealthCare. Guide to the quality and safety of organs for transplantation. 8th Edition. 2022. [Google Scholar]
- 6.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–45. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 7.Clavien PA, Dutkowski P, Mueller M, et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat Biotechnol. 2022;40(11):1610–16. doi: 10.1038/s41587-022-01354-7. [DOI] [PubMed] [Google Scholar]
- 8.Lai Q, Ruberto F, Pawlik TM, et al. Use of machine perfusion in livers showing steatosis prior to transplantation: A systematic review. Updates Surg. 2020;72(3):595–604. doi: 10.1007/s13304-020-00797-4. [DOI] [PubMed] [Google Scholar]
- 9.Raigani S, Carroll C, Griffith S, et al. Improvement of steatotic rat liver function with a defatting cocktail during ex situ normothermic machine perfusion is not directly related to liver fat content. PLoS One. 2020;15(5):e0232886. doi: 10.1371/journal.pone.0232886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raigani S, Karimian N, Huang V, et al. Metabolic and lipidomic profiling of steatotic human livers during ex situ normothermic machine perfusion guides resuscitation strategies. PLoS One. 2020;15(1):e0228011. doi: 10.1371/journal.pone.0228011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boteon YL, Attard J, Boteon A, et al. Manipulation of lipid metabolism during normothermic machine perfusion: Effect of defatting therapies on donor liver functional recovery. Liver Transpl. 2019;25(7):1007–22. doi: 10.1002/lt.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eccher A, Lombardini L, Girolami I, et al. How safe are organs from deceased donors with neoplasia? The results of the Italian Transplantation Network. J Nephrol. 2019;32(2):323–30. doi: 10.1007/s40620-018-00573-z. [DOI] [PubMed] [Google Scholar]
- 13.Sens MA, Zhou X, Weiland T, Cooley AM. Unexpected neoplasia in autopsies: Potential implications for tissue and organ safety. Arch Pathol Lab Med. 2009;133(12):1923–31. doi: 10.5858/133.12.1923. [DOI] [PubMed] [Google Scholar]
- 14.Croome KP, Taner CB. The changing landscapes in DCD liver transplantation. Curr Transplant Rep. 2020;7(3):194–204. doi: 10.1007/s40472-020-00283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jochmans I, Brat A, Davies L, et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet. 2020;396(10263):1653–62. doi: 10.1016/S0140-6736(20)32411-9. [DOI] [PubMed] [Google Scholar]
- 16.Chandler T, Hiller J, Peine S, Stargardt T. Blood donation and donors: Insights from a large German teaching hospital (2008–2017) Vox Sang. 2020;115(1):27–35. doi: 10.1111/vox.12853. [DOI] [PubMed] [Google Scholar]
- 17.Barone M, Avolio AW, Di Leo A, et al. ABO blood group-related waiting list disparities in liver transplant candidates: Effect of the MELD adoption. Transplantation. 2008;85(6):844–49. doi: 10.1097/TP.0b013e318166cc38. [DOI] [PubMed] [Google Scholar]


