Abstract
Background:
Characterization of the dynamics of Zika virus (ZIKV) persistence following acute infection is needed to inform blood donor and diagnostic testing policies and understand the natural history of ZIKV infection. We characterized the natural history, persistence, and clinical outcomes of ZIKV infection through prospective study of initially asymptomatic ZIKV RNA+ blood donors.
Methods:
Fifty-three ZIKV-infected blood donors identified through ZIKV nucleic acid amplification technology (NAT) screening were enrolled into a one year follow-up study, with blood and body fluid samples and detailed symptom data collected at up to seven visits. All sample types were tested for ZIKV RNA by quantitative RT-PCR (qRT-PCR); follow-up plasma, whole blood (WB) and urine were also tested by replicate NAT testing. Plasma was tested for flavivirus-specific IgM and IgG by ELISA. ZIKV RNA persistence for each assay/sample type and serum antibody persistence from estimated date of plasma NAT-detectable infection were calculated from follow-up data using survival statistical methods.
Findings:
By qRT-PCR, plasma viremia was detectable for 9.9 days (95%CI: 8.1–12.0) whereas RBC- and WB-associated viral RNA persisted for 95.4 days (95%CI: 62.8129.1) and 73.5 days (95%CI: 39.8–107.5), respectively. Replicate NAT testing (≥1of 8 replicates positive) extended plasma detection to 34.8 days (95%CI: 19.9–56.2) and WB detection (≥1/2 positive results) to 104.8 days (95%CI: 76.7–129.9). Urine and saliva were qRT-PCR reactive up to 14.5 (95%CI: 10.5–20.3) and 26.4 days (95%CI: 19.7–38.7), respectively. ZIKV IgM persisted for 237.7 days (95%CI: 128.7–459.5) from estimated time since plasma NAT-detectable infection. ZIKV RNA tended to fall below detectable limits more rapidly in participants with preexisting DENV IgG. Of donors identified pre-seroconversion with symptom data at the first or second study visit, 64% (16/25) developed multiple ZIKV-related symptoms after asymptomatic index donations, compared to 36% (9/25) of donors detected post-seroconversion.
Interpretation:
Determinations of viral marker persistence is enhanced by follow-up of pre- and asymptomatic RNA+/Ab- blood donors. We found higher rates of post-donation symptomatic infection than in most previous reports. RBC-associated ZIKV RNA persists for several months following clearance from plasma and body fluids, and replicate highly sensitive NAT testing extends RNA detection in all compartments. WB testing can extend detection of acute infection for diagnostics and monitoring of pregnant women, sexual partners and travellers.
Introduction:
Zika virus (ZIKV), a mosquito-borne arbovirus also transmitted congenitally and through transfusion and sexual contact, spread rapidly throughout the Americas after its recognition in Brazil in 2014.1–5 Although asymptomatic or mildly symptomatic in most cases, ZIKV can cause Guillain-Barré syndrome and infection during pregnancy has been associated with intrauterine fetal death and congenital Zika syndrome.6
ZIKV diagnosis depends on detection of ZIKV RNA or ZIKV-specific antibody responses in blood or other body fluids.3,7,8 RNA assay sensitivities differ, impacting duration of RNA positivity in clinical samples.3,9 Further, clinical sensitivity is maximized by testing a compartment in which ZIKV RNA persists for the longest duration.7,10–13,14 Serologic assays for ZIKV antibodies are widely employed diagnostically but are problematic in that cross reactivity with antigenically similar flaviviruses (e.g. the four dengue viruses [DENV]) may confound interpretation.15,16 This is of particular concern in locations where these viruses co-circulate, and acute ZIKV or secondary DENV may anamnestically boost pre-existing cross-reactive antibody responses.17 Moreover, expanding DENV vaccination and potential introduction of ZIKV vaccines are likely to further complicate serologic diagnosis of these infections.16,18
These diagnostic challenges are of particular importance in monitoring pregnant women with fetuses at-risk for congenital Zika syndrome, detection of potential reinfections in DENV and ZIKV endemic areas, and assuring the removal of blood units containing ZIKV RNA from the blood supply. The peak rates of ZIKV RNA+ prevalence in blood donors reached 2.8% during the 2014 French Polynesia outbreak 19,and 1.8% in Puerto Rico in 2016. 2,20 These high rates of donor infections along with reported cases of transfusion-transmission1 prompted the US Food and Drug Administration (FDA) to issue guidance in 2016 requiring implementation of nucleic acid amplification technology (NAT) testing to screen blood donors.21 High throughput and highly sensitive ZIKV-specific NAT assays were developed by Roche Molecular Systems, Inc. (RMS) (the cobas® Zika test for use on the cobas® 6800/8800 Systems) and Grifols Diagnostic Solutions (Grifols) (the Procleix® Zika Virus Assay on the Panther® system). 2,5,22 Under US FDA Investigational New Drug (IND) applications, all blood donations in Puerto Rico were screened for ZIKV RNA beginning April 3, 2016. In the remainder of the US these assays were phased into use from May through December 2016.
This study (REDS-III US Zika Natural History Study) used donor NAT screening to identify and enroll asymptomatic ZIKV infected blood donors into a one-year follow-up study. The study design enabled identification of donors early in acute infection (i.e., before or shortly after development of ZIKV-specific immune responses), representing a highly informative population for characterization of laboratory parameters and incidence of clinical findings. We report the dynamics of viral and serological markers and clinical symptomatology following acute ZIKV infection in NAT-positive blood donors, with comprehensive data on viral persistence in blood compartments and body fluids in DENV-exposed and -naïve donors.
Methods:
Study design and participants
Donors were eligible for enrollment if confirmed ZIKV infected while donating at blood centers participating in our follow-up study (Banco de Sangre de Servicios Mutuos, OneBlood, Vitalant, New York Blood Center or American Red Cross) from April through December 2016, coinciding with a large ZIKV virus epidemic in PR and small autochthonous outbreak in South Florida.23,24 Of the five participating blood centers, BSSM, OneBlood and Vitalant had eligible ZIKV NAT-positive blood donors. Blood donor plasma samples were screened using either the RMS cobas® Zika or Grifols Procleix Zika Virus NAT assays. ZIKV infection in NAT-reactive donors was confirmed by repeat-reactivity on the screening NAT assay, reactivity on a confirmatory RT-PCR assay on index plasma, or ZIKV IgM seroconversion in index or follow-up serum samples; this supplemental testing was conducted as part of the manufacturers’ INDs.2,5 ZIKV quantitative viral load testing (qRT-PCR) was performed on plasma and residual RBC when available, and serological testing for ZIKV (IgM and IgG) and DENV (IgG) were performed on index and follow-up serum at Vitalant Research Institute
Eligible participants provided consent for follow-up study through a protocol approved by the UCSF Committee for Human Research. Follow-up visits were requested at weeks 1, 3, 6 and months 3, 6, 9, 12 following index NAT-reactive donations. Sample collection included 75ml of EDTA anticoagulated whole blood, 7ml whole blood in Tempus (Applied Biosystems) tubes (for RNA expression), 15ml urine, 3ml saliva, 1–5ml semen, and buccal and nasal swabs (Appendix p1). Samples were shipped overnight from the collecting blood center to the central testing laboratory in San Francisco using a dual-temperature compartment shipper with blood samples shipped at ambient temperature and body fluid samples on cold packs. Blood was processed into aliquots of whole blood, plasma, packed RBC (pRBC) and ficoll separated peripheral blood mononuclear cells (PBMC). All samples were cryopreserved immediately after processing and stored at either −80°C or in liquid nitrogen (PBMC). Cryopreservation is not expected to have a significant impact on detection of ZIKV RNA or serological markers and enabled batch testing of samples and development of sharable biorepository of these samples..
Estimation of date of infection in preseroconversion donations
We used parametric survival methods20 to estimate mean time to clearance of ZIKV RNA from plasma and other compartments, as well as time to appearance and clearance of IgM. Persistence is defined as the interval for which a marker of infection is detectable relative to initial NAT detectable infection in donors identified pre-seroconversion, or from index donations for all donors We estimated persistence of viral parameters relative to index donations and performed additional modeling for subjects who were ZIKV seronegative at the index visit. In these cases, we used index plasma VL and an estimate of ZIKV plasma RNA doubling time during ramp-up viremia in macaques to establish an estimated date of NAT-detectable infection. We then calculated time to appearance and loss of detectable RNA and antibodies relative to estimated date of plasma NAT-detectable infection.20
After evaluating several probability distributions for time to clearance of plasma RNA (data not shown) we determined that lognormal distribution fit the data better than alternatives. For each analysis, a subject was considered interval censored when the endpoint was reached between two visits, or right censored if the endpoint was not reached by the last contact. Confidence limits for mean times to RNA or IgM clearance and mean time to appearance of IgM were obtained by generating 10,000 bootstraps estimated from the parameters of the lognormal distribution. We calculated mean times to clearance from each pair of parameter estimates and obtained the overall means and 95% confidence limits from distribution of the 10,000 estimates. Data analysis took place using SAS software. Copyright © 2005–2017 SAS Institute Inc. (SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA). Comparisons of mean times between subgroups were accomplished by adding indicator variables for subgroup to the survival model. Missing data, such as no results if a study visit was missed, were treated as missing at random. We did not make use of imputation or other means to adjust for the missing values.
Symptom data collection
Blood donors at the time of index donation are asymptomatic and afebrile as required by AABB and FDA policies and donor center screening procedures. The ZIKV Symptom Questionnaire (appendix A), developed in collaboration with the US Centers for Disease Control and Prevention (CDC) (Atlanta, GA), was administered at each visit to record symptoms in the two weeks prior to the study visit and designed to capture symptoms ranging from mild ZIKV-compatible but nonspecific symptoms (e.g., fever, conjunctivitis, headache, and myalgia) to more severe neurologic disorders. Similar to previous studies of WNV-related symptoms in WNV+ blood donors, a cutoff of 3 or more symptoms was used to define incidence of symptomatic infection.25,26
ZIKV RNA testing on follow-up samples
ZIKV RNA VLs were measured in plasma, WB, pRBC, urine, saliva, and semen by qRT-PCR as previously described.3,22 To more sensitively determine the duration of ZIKV RNA persistence in plasma, whole blood and urine, multiple replicate NAT tests (8 for plasma and 2 for WB and urine) were performed on follow-up samples with the Grifols ZIKV assay.5
ZIKV and DENV serology
Anti-ZIKV IgM and IgG testing was performed using a modified ZIKV-capture ELISA developed by the CDC as previously described.2,5 ZIKV RNA+ index donation serum was also tested for pre-existing DENV IgG by the InBios Detect IgG ELISA; the DENV exposure status prior to ZIKV acquisition was corroborated by analysis of longitudinal DENV and ZIKV specific neutralization titers (data not shown). Cut-offs were set using 50 normal blood donor specimens and calculating the mean immune status ratio (ISR) and standard deviation (SD).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results:
Characteristics of study population
Between July 2016 and September 2017, of 4,522,115 donations screened, 453 donations were detected as initially reactive for ZIKV RNA by either the RMS or Grifols NAT assays and 405 were confirmed as ZIKV RNA positive. Of these, a subset of 217 were collected at REDS-III study participating blood centers and eligible with 53 enrolled into the follow-up study contributing a total of 267 follow-up study visits (Appendix p3). The mean duration of follow-up was 118 days (Standard Deviation 47.7 days) with a maximum of 399 days. Although some participants were lost to follow-up at various points after enrollment, the majority of study participants (40) completed at least 6 study visits The majority of participants were male (38/53, 72%) and 38 (72%) were DENV IgG positive (Appendix p2). The majority (42/53, 80%) were enrolled in PR and presumed to be autochthonous infections. The remaining study participants were from the continental US with 17% (9/53) of the overall cohort from Florida and 1 donor each from Texas and Nevada. All enrolled cases identified in the continental US had a history of travel to an area of active transmission; none were determined to be locally acquired infections. The numbers of outcome events for each parameter by study visit and the number of participants with missing data for each parameter by study visit is indicated in the Appendix pp2–3.
ZIKV RNA in plasma
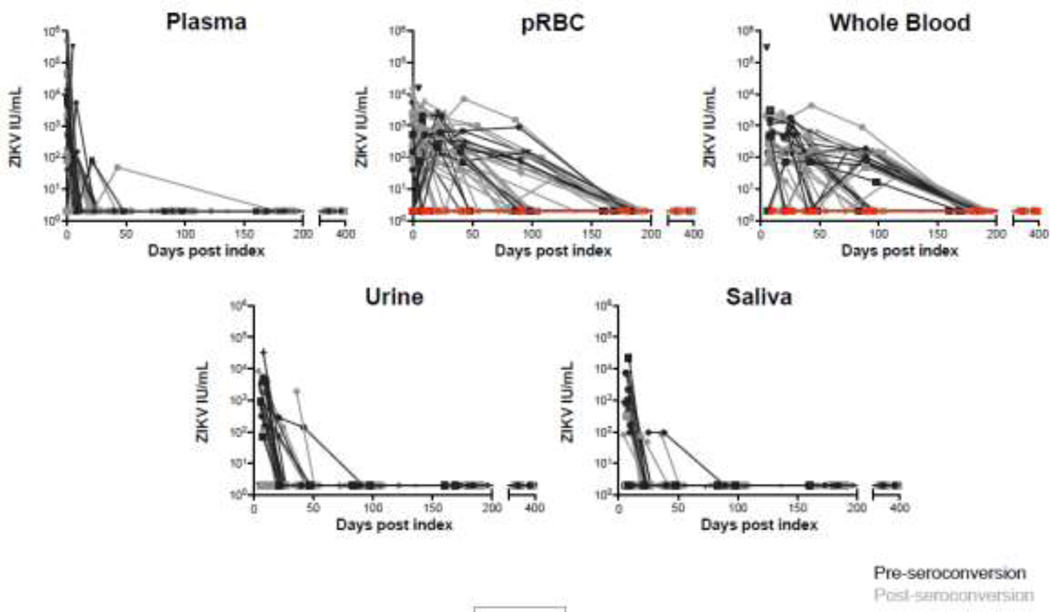
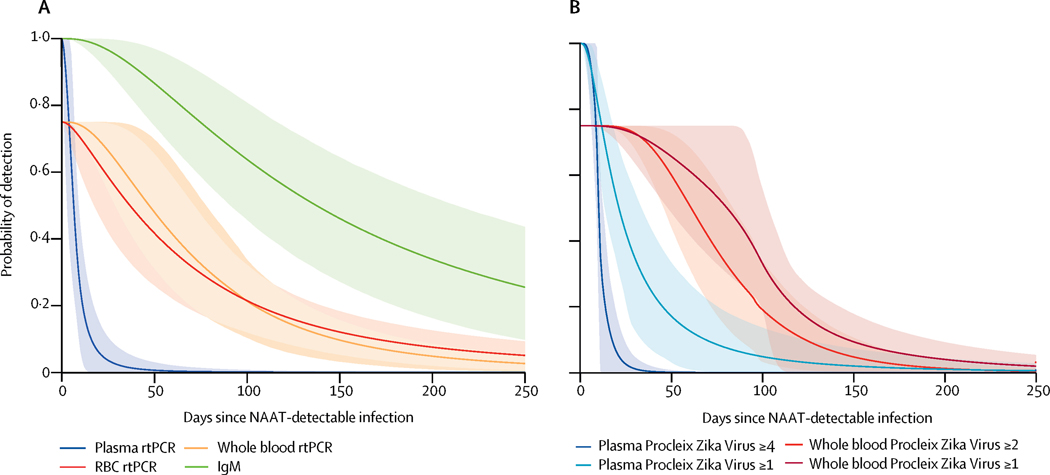
As part of the enrollment criteria, all 53 participants had detectable plasma RNA by sensitive qualitative NAT assays at index donation. Of these, 30 (57%) had quantifiable viral loads at index by qRT-PCR assay (figure 1) which is ~20-fold less sensitive than the qualitative donor screening NAT assays. 22Replicate testing of follow-up donor plasma by the Grifols NAT assay yielded 35/53 (66%) participants with ≥1/8 replicates positive, and 18 of these had ≥4/8 replicates positive in ≥1 follow-up visit. Restricting the analysis to the 25/53 (47%) donors identified pre-IgM seroconversion for whom we were able to estimate time from NAT-detectable infection (see methods), times from plasma NAT-detectable infection to loss of ZIKV RNA detectable in plasma by qRT-PCR was 9.9 days (95% CI 8.1–12.0), similar to the 11.1 days (95% CI 9.2–14.4) for RNA persistence defined as ≥4/8 positive replicates on the Grifols NAT assay. The interval to loss of RNA in plasma in ≥1/8 positive replicates was extended to 34.8 days (95% CI 19.9–56.2) (Figure 1 and Table 1). In participants with preexisting DENV IgG, the mean duration of plasma ZIKV RNA persistence was less than those who were DENV naïve (6.8 days (95%CI 3.9, 10.3) and 13.7 days (95%CI 5.5, 26.2) respectively) although the difference did not reach the level of statistical significance (Figure 2 and Table 2).
Figure 1.
Duration of ZIKV RNA persistence in blood compartments and body fluids detected by qRT-PCR by days post index donation. Black lines represent donors identified preseroconversion, grey lines post seroconversion, and red lines donors who never developed RBC associated RNA.
Table 1:
ZIKV viral and serological persistence in blood compartments and body fluids from time since plasma NAT-detectable infection for donors identified pre-IgM seroconversion and from NAT+ index donation for all donors combined by qRT-PCR, replicate Grifols TMA and ZIKV MAC ELISA. Number of participants with at least one follow-up visit positive in each compartment and assay are indicated. For all donors combined, donors seropositive at index are not informative to time to IgM and RBC detection estimation calculations and thus are not indicated.
| Donors pre-seroconversion at Index | All donors combined | ||||
|---|---|---|---|---|---|
|
|
|||||
| Participants positive at ≥1 follow-up visits | Interval since estimated date of infection* | Interval since index donation | |||
|
|
|||||
| Assay | Compartment | Interval | Mean (95% CI) days | Mean (95% CI) days. | |
| MAC ELISA IgM | plasma | seroconversion | 7·7 (6·1, 9·2) | - | |
| MAC ELISA IgM | plasma | seroreversion | 237·0 (128·7, 459·5) | 274·3 (189·4, 416·3) | |
| BSRI PCR | RBC | RBC detection | 2·0 (0·8, 3·3) | - | |
|
| |||||
| Grifols >1/8 pos | plasma | RNA clearance | 35 | 34·8 (19·9, 56·2) | 40·3 (27·1, 56·5) |
| Grifols >4/8 pos | plasma | RNA clearance | 18 | 11·1 (9·2, 14·4) | 9·5 (6·5, 13·8) |
| BSRI PCR | plasma | RNA clearance | 30 | 9·9 (8·1, 12·0) | 8·2 (5·1, 12·5) |
| BSRI PCR | RBC | RNA clearance | 45 | 95·4 (62·8, 129·1) | 87·1 (70·5, 104·3) |
| Grifols >1 pos | WB | RNA clearance | 46 | 104·8 (76·7, 129·9) | 107·3 (88·7, 128·9) |
| Grifols 2/2 pos | WB | RNA clearance | 41 | 74·2 (43·8, 104·9) | 84·0 (64·7, 107·1) |
| BSRI PCR | WB | RNA clearance | 40 | 73·5 (39·8, 107·5) | 84·3 (62·6, 108·3) |
| Grifols >1 pos | urine | RNA clearance | 34 | 23·0 (14·4, 36·2) | 13·5 (9·8, 18·1) |
| Grifols 2/2 pos | urine | RNA clearance | 24 | 19·1 (12·9, 27·3) | 17·3 (12·0, 24·4) |
| BSRI PCR | urine | RNA clearance | 23 | 14·5 (10·5, 20·3) | 12·4 (9·2, 16·3) |
| BSRI PCR | saliva | RNA clearance | 20 | 26·4 (19·7, 38·7) | 11·2 (8·1, 15·2) |
Plasma NAT detectable infection
RNA+ WB samples were not included in this determination as RNA+ plasma contributes to RNA positivity in WB
Figure 2:
Probability of ZIKV IgM and RNA detection in blood compartments in the 25 donors identified pre-seroconversion by method of detection. A. IgM persistence and RNA persistence by qRT-PCR in plasma, whole blood, and pRBC. B. RNA persistence by Grifols replicate testing in ≥4 and ≥1 of 8 replicates tested in plasma and ≥1 and 2 of 2 replicates tested in whole blood. 18 of 25 (72%) pre-IgM participants had RNA+ RBC in ≥1 time point and were thus included in modeling of time to loss of ZIKV RNA from RBCs. The fitted model indicates that the probability of detecting ZIKV RNA in RBCs is close to 100% near time zero for the 18/25 participants with RBC associated ZIKV RNA, however 7/25 participants never RNA+ in RBCs, thus maximum probability of the observed proportion detection in RBC is 72%. The upper and lower edges of the confidence bands in the survival curves represent the 2.5th and 97.5th percentiles of the predicted probabilities from fitting the survival model of time to loss of signal to 10,000 bootstrap datasets. Light shading indicates 95% CI. All 10,000 bootstrap probability estimates are close to 1.0 for times close to time zero.
Table 2.
Persistence of ZIKV plasma IgM and plasma, RBC and saliva RNA in participants with and without preexisting DENV IgG.
| Assay | Compartment | Interval | N | DENV lgG+ | N | DENV IgG− | p value |
|---|---|---|---|---|---|---|---|
| MAC ELISA IgM | plasma | seroreversion | 37 | 264·4 (168·7, 438·0) | 15 | 267·0 (170·5, 447·7) | 0.23 |
| BSRI PCR | plasma | RNA clearance | 38 | 6·8 (3·9, 10·3) | 15 | 13·7 (5·5, 26·2) | 0.14 |
| BSRI PCR | RBC | RNA clearance | 34 | 84·6 (63·2, 107·3) | 14 | 90·3 (67·6, 111·4) | 0.15 |
| BSRI PCR | saliva | RNA clearance | 14 | 18·2 (16·0, 23·3) | 6 | 32·5 (23·0, 47·5) | 0.02* |
ZIKV RNA in Whole Blood and RBC compartment
ZIKV RNA was detected in WB in 40/53 (75%) participants in at least one follow-up visit by qRT-PCR and in 46/53 participants (87%) with ≥1/2 Grifols NAT+ replicates (Table 1). For the 25 donors identified pre-seroconversion with detectable ZIKV RNA in WB, the duration of detectable ZIKV RNA in WB from dates of estimated plasma NAT-detectable infection were 73.5 days (95% CI 39.8–107.5) by qRT-PCR and 74.2 days (95% CI 43.8–104.9) when 2/2 replicates tested positive by the Grifols NAT assay; with a criteria that ≥1/2 RNA+ replicate test positive, persistence was extended to 104.8 days (95% CI 76.7–129.9) (Figure 2). In 45/53 (85%) donors, ZIKV RNA was detected in pRBC by qRT-PCR at one or more study visits. In the subset of 25 donors identified pre-seroconversion, ZIKV RNA became detectable in pRBC a mean of 2 (95% CI 0.8–3.3) days after plasma NAT-detectable infection and remained detectable for a mean of 95.4 days (95% CI 62.8–129). Of note, 7 of these 25 participants (28%) never developed pRBC associated RNA (Figure 1). RNA+ WB samples were not included in this determination of time to RBC detection or persistence as RNA+ plasma contributes to RNA positivity in WB. Participants with preexisting DENV IgG cleared pRBC ZIKV RNA faster than DENV naïve participants (84.6 days (95% CI 63.2, 107.3) vs 90.3 days (95%CI 67.6, 111.4), although this difference was not statistically significant.
ZIKV RNA in body fluids
ZIKV RNA was detected in urine by qRT-PCR and ≥1 replicate Grifols NAT assays in 23/53 (43%) and 34/53 (64%) participants, respectively. In the 25 pre-seroconversion donors with positive urine results, the mean duration of persistence from estimated plasma NAT-detectable infection was 14.5 days (95% CI 10.5–20.3) by qRT-PCR and 23.0 days (14.4–36.2) by replicate NAT testing (Table 1). ZIKV RNA was detected in saliva in 20/53 (38%) participants. For the 25 participants identified pre-seroconversion, RNA was detected for a mean of 26.4 days (95% CI 19.7, 38.7) from estimated dates of plasma NAT-detectable infection. Participants with preexisting DENV IgG tended to clear ZIKV RNA from saliva sooner than those lacking DENV IgG: 18.2 days (95% CI 16.0, 23.3) vs 32.5 days (95% CI 23.0, 47.5), respectively (Table 2). In 12 male participants who provided semen samples, 3 had detectable RNA in semen at one or more visits; all collection time points that tested positive for ZIKV RNA in semen also had detectable RNA in whole blood.3
Serological Parameters
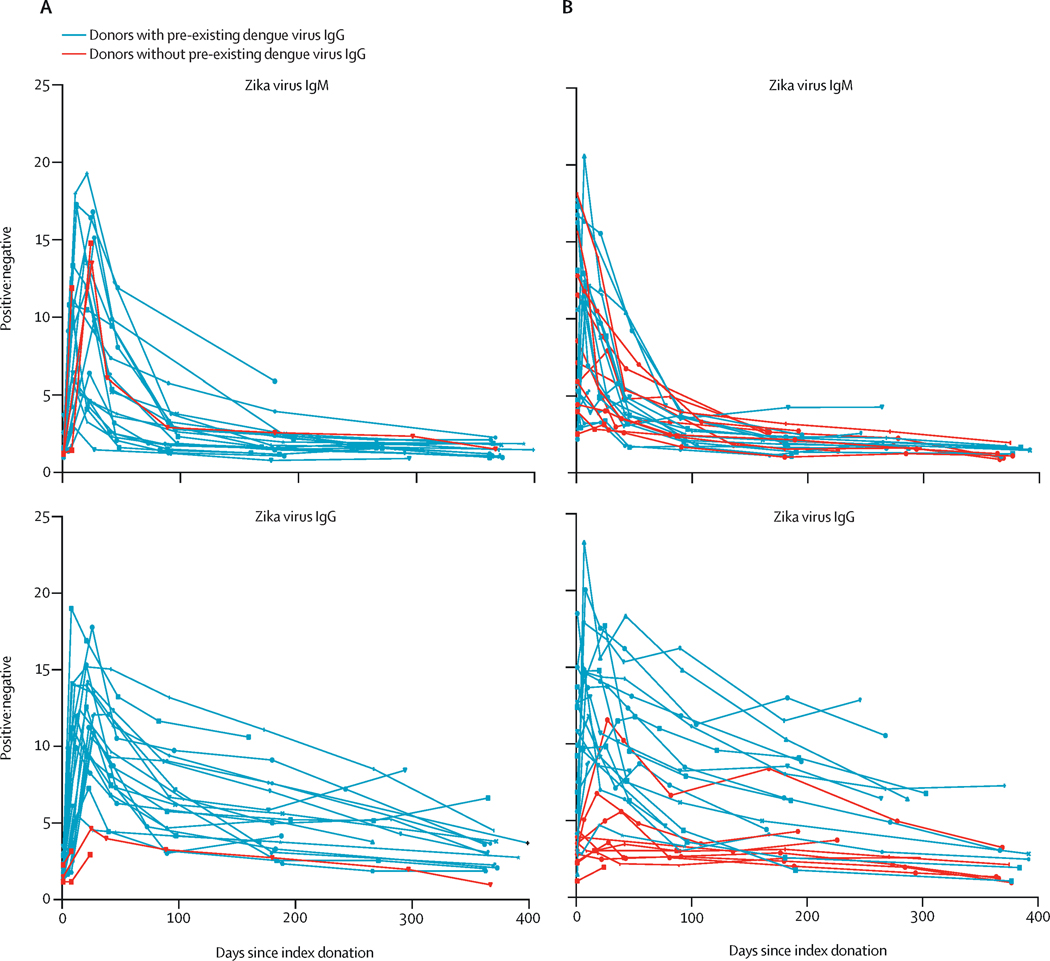
All donors seroconverted to ZIKV IgM with the exception of one participant enrolled in very early infection with only one early study visit that occurred before the window of seroconversion. In donors who were seronegative at index, the IgM response was brisk with the majority of donors seroconverting by the first follow-up visit (Figure 3). The mean time from plasma NAT-detectable infection to IgM seroconversion was 7.7 days (95% CI 6.1, 9.2) (Table 1). IgG responses also evolved rapidly (Figure 3); however, detection of ZIKV-specific IgG in DENV-experienced individuals was hampered by the use of an assay that lacked ZIKV specificity. In the absence of pre-existing DENV IgG, DENV naïve participants generally had weaker ZIKV IgG responses presumably due to a lack of DENV memory and lack of anamnestic boosting of cross-reactive antibodies (Figure 3). During study follow-up IgM and IgG reactivity levels declined over time (Figure 3). Of the 43 participants with at least a 6-month follow-up visit, 34 seroreverted on IgM testing. Of these, the mean time to IgM seroreversion was 237.0 days (95%CI 128.7–459.5) for donors who were ZIKV IgM seronegative at time of donation (Table 1). Seven donors seroreverted on IgG testing; 2 of these were DENV-naïve.
Figure 3:
ZIKV IgM and IgG responses over time. The ratio of positive signal to negative background (P/N) of ZIKV-specific IgM for each donor by time after NAT+ index donation for 25 donors identified pre-IgM seroconversion (A) and 28 donors post-IgM seroconversion at index (B). Donors with pre-existing DENV IgG are indicated in black, and DENV IgG- in red. Negative values are ≤2, Positive values are >3.0.
Clinical Outcomes
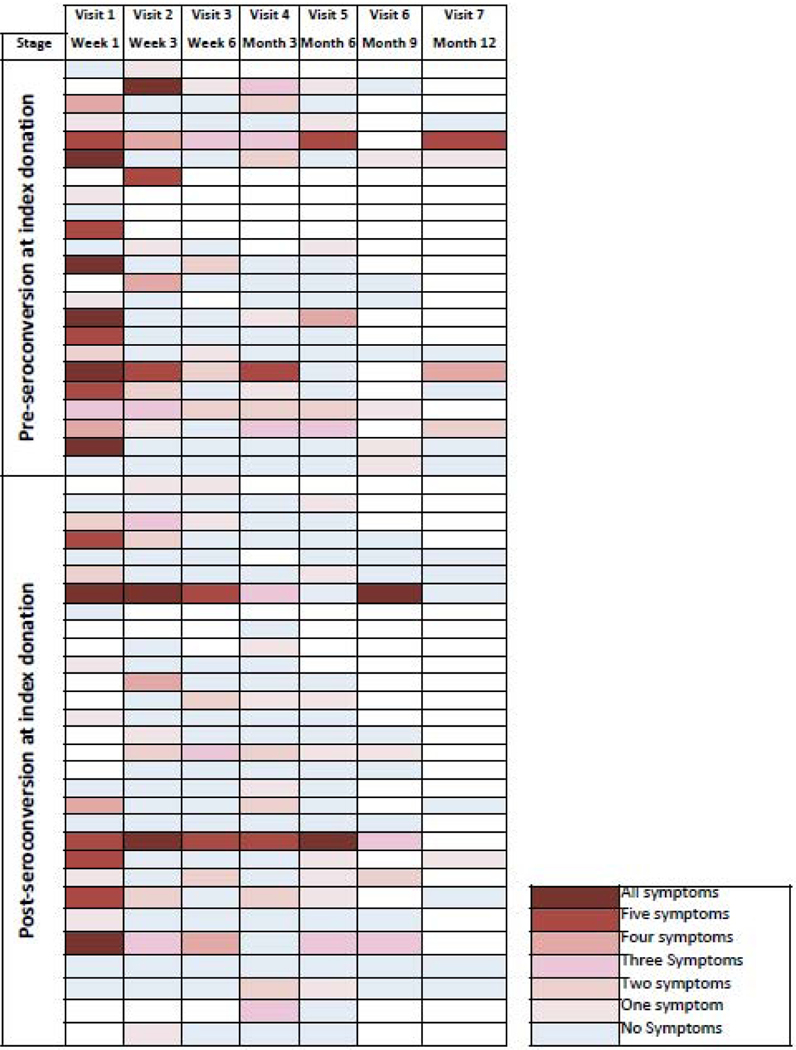
Most (16/25; 64%) enrolled ZIKV+ donors with pre-IgM phase index donations developed multiple (≥50%) of the 6 ZIKV-associated symptoms (fever, rash, joint or bone pain, body or muscle pain, painful or red eyes, headache) by the second follow-up visit; only 5/25 (20%) had no symptoms (Figure 4). These symptoms resolved on subsequent follow-up visits (generally by the next visit) indicating they were both incident and transient, and hence consistent with ZIKV infection. In contrast, only 9/25 (36%) of post-IgM seroconversion donors with first or second follow up visits reported multiple symptoms by the second visit.
Figure 4.
Heat map indicating the number of symptoms reported at each study visit. Each row represents a study participant, grouped by ZIKV IgM status at index donation; IgM- on top, IgM+ on bottom. Columns represent study visits with the color intensity reflecting the frequency of symptoms reported. Symptom categories: fever; rash; joint or bone pain; body or muscle pain; painful or red eyes; headache.
Discussion:
Our findings from a one-year longitudinal follow-up study of 53 initially asymptomatic ZIKV-infected donors identified through blood NAT screening provide significant insights into the natural history of ZIKV infection, including persistence of ZIKV RNA in different blood compartments and body fluids, serological persistence and clinical outcomes.
Our cohort, which includes persons identified and enrolled in different stages of infection, with and without pre-existing DENV immunity, differs from most previous reports of ZIKV persistence which were restricted to infected individuals presenting with clinical disease.10,27 Previous studies have reported RNA persistence and rates of symptomatic infection in ZIKV NAT+ blood donors, but estimates were based on time from donation without normalization to estimated time since infection.27
Although assumptions are made regarding the application of data from macaques, time from infection to detectable viremia, as estimated from the macaque data and viral loads at index donation for donors detected in the seronegative ramp-up stage of infection, is generally a very small fraction of time from infection to clearance of RNA or IgM. As such, any differences between humans and Macaques would minimally impact estimates of persistence unless the difference ramp-up viral load dynamics (doubling time) between the two species was very large.
Our finding of an approximately 12 day mean time of detectable RNA in plasma to clearance by the Grifols ZIKV NAT assay is almost identical to the 11.7 days (95% CI 10.0,14.5) calculated using similar methodology for the RMS NAT assay based on a larger cohort of 140 PR blood donors who enrolled into the RMS-sponsored short-term ZIKV IND study (of which most of this cohort is a subset ). 20
Donors with preexisting DENV immunity cleared RNA faster from plasma, RBC and saliva than DENV naïve donors and reached statistical significance in saliva, although no difference was observed for time to Zika IgM seroreversion for DENV-experienced participants (Table 2). This observation of faster clearance of ZIKV in the context of pre-existing DENV immunity is similar to that seen in some DENV patients after secondary infection and in a DENV-exposed non-human primate cohort of ZIKV infection.28 Additionally, DENV pre-exposure has been shown to be associated with reduced ZIKV transmission efficiency and reduced risk of ZIKV infection and symptoms. 29 The protective role of DENV IgG may thus facilitate ZIKV clearance. Most of the 15 DENV-naive donors were identified after seroconversion (12/15), which could have been up to ~30 days after infection based on our estimates of highly sensitive plasma NAT detectability as the period where donors would have screened plasma NAT+; in contrast most of the 38 DENV-experienced donors were identified pre-seroconversion (21/38), or within ~5 days from estimated time since NAT-detectable infection. Thus our estimates are enriched for DENV-exposed donors compared to the DENV-naïve donors, providing additional support for these differences in RNA persistence. Although not significant in our cohort, differences in persistence of ZIKV RNA may have important implications for the diagnostic detection periods in flavivirus endemic and non-endemic areas.
Early analysis of confirmatory testing results from PR IND testing showed higher ZIKV RNA levels in pRBC compared to plasma post-IgM seroconversion (Appendix p5), leading us to focus on relative persistence in these compartments in our longitudinal follow up study. We found that a high proportion of participants had RBC-associated ZIKV RNA in early infection, following IgM seroconversion, and after approximately three months of follow-up, similar to that reported in WNV infection.30 Persistence in pRBC was longer than in WB; as plasma viremia wanes during the course of infection the aviremic plasma dilutes RBC-associated RNA signal in WB but not in pRBC. Three month duration of RBC-associated persistence suggests it may be the result of infection of erythroid progenitors during acute infection, with RNA persisting on progeny RBC consistent with the life span of mature RBCs. Alternatively, passive binding of ZIKV or immune complexed virus to RBC cannot be ruled out. The several-day delay in detection of RBC-associated ZIKV RNA after highly sensitive detection in plasma supports this hypothesis in that plasma viremia must reach sufficient levels early in infection to infect progenitor cells in the bone marrow. Of interest, ~25% of participants did not develop RBC-associated ZIKV RNA through mechanisms that are under investigation.
These findings of RNA persistence associated with RBC, similar to previous observations in symptomatic travelers7 and in follow-up studies of WNV infected donors,31–33 have important implications for ZIKV diagnostic testing in that WB testing extends the diagnostic window for clinical cases, travelers and for monitoring pregnant women up to three months. However, a proportion of the population does not develop RBC-associated RNA, and hence testing RNA negative on whole blood should not be considered definitive evidence of absence of recent ZIKV infection and should be confirmed with serologic testing.
Our RBC findings indicate that many “tail-end” ZIKV RNA-positive donations are likely missed by donor NAT screening which is currently limited to testing plasma. This raises the question of whether RBC-associated ZIKV RNA is infectious particularly if RBC components are transfused into immune-suppressed patients. However it may not be infectious, given both the relatively low levels of virus associated with RBC (~1 ZIKV RNA molecule per 104−5 RBCs) and the presence of high levels of ZIKV-specific neutralizing Abs that develop concurrent with clearance of plasma viremia.34 Moreover, no cases have been reported of infectious RBC transfusions after routine plasma screening, despite very large epidemics in Puerto Rico and French Caribbean Islands where NAT screening of blood donor plasma was performed. As no transfusion-transmitted cases attributable to plasma RNA-negative components have been reported, RBC-associated ZIKV RNA in the absence of ZIKV RNA in plasma is likely not infectious, and hence NAT screening of plasma is likely sufficient to protect the blood supply.
Participants were followed for one year to allow characterization of developing humoral and cellular immunity. Understanding the antibody profile a year after initial exposure enables discrimination of new infection from recurring re-infections, may facilitate development of ZIKV incidence assays to discriminate recent vs remote infections, and may allow comparison and discrimination of natural infection vs vaccine immune responses. 17
Of donors enrolled into the REDS-III study who were detected in the pre-ZIKV-IgM/IgG stages of infection, 64% reported symptoms meeting the case definition for clinical Zika by the second follow-up visit. Donors in later infection would have likely developed symptoms prior to index donation resulting in either not presenting to donation or deferral at the time of donation, and hence were excluded from consideration of incidence of symptomatic infection and analyses of pre-symptomatic viral, immune and other biomarkers that may correlate with disease manifestations. These longitudinal symptom data from initially asymptomatic ZIKV RNA+ blood donors demonstrate a higher rate of symptomatic ZIKV infections than reported from the 2007 Yap Island ZIKV outbreak that correlated incidence of ZIKV from a serosurvey with rates of reported disease.35 However, given that symptoms are not always present and may be mild, ZIKV infection should not be ruled out in the absence of reported symptoms.
The findings from our study are relevant to the application of ZIKV molecular and serological assays for monitoring pregnant women for acquisition of ZIKV infection and consequent potential risk of congenital infection and disease. Based on our results and others documenting enhanced and prolonged detection of ZIKV RNA by testing whole blood rather than plasma 7,8, a number of large cohort studies of infection of pregnant women, including the large NIH funded ZIP Study and the European ZikaAlliance Study, have included whole blood in their testing algorithms. On the other hand, our data demonstrating extended detection of ZIKV IgM beyond 6 months and in some cases over a year after acquisition has led to the decision not to rely on IgM or other serological testing in these pregnant women cohort studies, and a recommendation by CDC to not rely on ZIKV IgM testing to indicate recent acquisition of infection in pregnant women in routine clinical practice including testing of pregnant women. 36–38 Results from these larger studies should provide the basis for updated recommendation for diagnosis of ZIKV infections in clinical cases and for monitoring pregnant women and traveler for recent acquisition of infection.
In conclusion, we capitalized on the unique capacity to identify and enroll asymptomatic blood donors to provide insight into the natural history of ZIKV infection, relative persistence of ZIKV in blood compartments and body fluids and the characterization of immune markers for up to one year following infection. Better understanding of persistence relative to the sensitivity of detection assays and the finding of longer duration of ZIKV RNA in RBC-containing blood fractions have important implications for blood screening and diagnostic testing. The current study enabled development of a comprehensive repository of well-characterized samples to support 1) validation of screening and diagnostic ZIKV assays, 2) studies of host immune responses to ZIKV to inform vaccine development and novel therapeutic approaches, 3) analyses of mechanisms of clearance or persistence and 4) analyses of predictive markers of symptom development. Studies made possible by access to these samples and data are in progress.
Supplementary Material
Research in context.
Evidence before this study
Since the emergence and rapid spread of Zika throughout the Americas after it’s recognition in Brazil in 2014, assessment of the natural history of ZIKV infection has been undertaken but studies have been largely limited to clinical cases with short-term follow-up. While there have been previous reports of ZIKV viral and serological persistence in patients presenting with clinical disease, there have been limited longitudinal studies of initially asymptomatic infected blood donors to determine the relative persistence of ZIKV in different blood and body fluid compartments and their impact on diagnostic detection periods. A literature review was performed in PubMed prior to study initiation with search terms: ”ZIKV infection, persistence”, “ZIKV transfusion transmission”, “virus persistence”, “asymptomatic infection”, ”ZIKV natural history”. Based on this review, cases of transfusion transmission of ZIKV have been described, as well as studies documenting extended ZIKV RNA persistence in whole blood relative to serum, with most studies report persistence relative to symptom onset.
Added value of this study
Our cohort, which includes persons identified and enrolled in all stages of infection, with and without pre-existing DENV immunity, differs from most previous reports of ZIKV persistence which were restricted to infected individuals presenting with clinical disease. Previous studies have reported RNA persistence and rates of symptomatic infection in ZIKV NAT+ blood donors, but estimates were based on time from donation without normalization to estimated time since infection. We capitalized on the unique capacity to identify and enroll asymptomatic blood donors to provide insight into the natural history of ZIKV infection, the relative persistence of ZIKV in blood compartments and body fluids and the characterization of immune markers for up to one year following infection. The unique capacity to identify and enroll ZIKV NAT+ blood donors in early acute infection pre-seroconversion enables more accurate estimations of persistence normalized to time of NAT detectable infection in DENV exposed and naïve participants.
Implications of all the available evidence
A better understanding of persistence of ZIKV in blood compartments and body fluids relative to the sensitivity of detection assays has important implications for blood screening and diagnostic testing. The finding of longer duration of ZIKV RNA in RBC-containing blood fractions extends the period of diagnosis for clinical cases, travelers and for monitoring pregnant women. Longitudinal follow-up of infected persons enables characterization of developing humoral and cellular immunity, which is of particular importance in areas where antigenically similar Flaviviruses co-circulate.
Acknowledgments:
This project has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100001I and from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under Contract #HHSO100201600010C. The authors would like to acknowledge Honey Dave, Melanie Dimapasoc, Nelly Gefter, Joseph Lau, Kai Lu, Inderdeep Singh, Kristen Valanoski, Bonnie Yip, Daniel Hindes and Edwin Velez for their invaluable technical assistance.
MS, MCL, SK and MPB conceived of and designed the study. MS wrote the first drafts of the Article with contributions from SB, GS, SAG, LLP, and JML. DB performed statistical analysis and modeling, SB, GS and THL designed and performed laboratory tests and contributed to data analysis and manuscript review. RB oversaw IRB review and contributed to data management and analysis. ZK managed and performed QC of study databases. JOA oversaw participant recruitment and sample collection activities. PCW, SAG, LLP and LML were investigators for IND studies and oversaw donor screening testing. MPB and MCL acquired funding. All authors reviewed the manuscript for intellectual content and assisted in the interpretation of results.
Footnotes
Conflicts of interest
Dr Linnen is an employee of Grifols Diagnostic Solutions Inc. and shareholder of Grifols. Drs Galel and Lee are employees and shareholder of Roche Diagnostics, which sponsored the clinical trial that identified the potential subjects for this study.
Conflicts of interest
Dr Linnen is an employee of Grifols Diagnostic Solutions Inc. and shareholder of Grifols. Drs Galel and Lee are employees of Roche Molecular Systems. The remaining coauthors declare no conflicts of interest.
MCL is now an employee of Cerus Corporation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barjas-Castro ML, Angerami RN, Cunha M, S., et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016; 56(7): 1684–8. [DOI] [PubMed] [Google Scholar]
- 2.Galel SA, Williamson PC, Busch MP, et al. First Zika-positive donations in the continental United States. Transfusion 2017; 57(3pt2): 762–9. [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Richard V, Teissier A, et al. Detection of ZIKV RNA in semen of asymptomatic blood donors. Clinical Microbiology and Infection 2017; 23(12): 1001.e1-.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med 2016; 374(16): 1552–63. [DOI] [PubMed] [Google Scholar]
- 5.Williamson PC, Linnen JM, Kessler DA, et al. First cases of Zika virus–infected US blood donors outside states with areas of active transmission. Transfusion 2017; 57(3pt2): 770–8. [DOI] [PubMed] [Google Scholar]
- 6.Cao-Lormeau V-M, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet 2016; 387(10027): 1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill 2016; 21(26). [DOI] [PubMed] [Google Scholar]
- 8.Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika Virus in Body Fluids - Preliminary Report. N Engl J Med 2017; 379(13): 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Moraes FM, Espósito DLA, Klein TM, da Fonseca BAL. Searching for the best real-time RT-PCRs to detect Zika virus infections: the importance of comparing several protocols. Brazilian Journal of Medical and Biological Research 2018; 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santiago GA, Vazquez J, Courtney S, et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun 2018; 9(1): 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean Michel M, Catherine M, Christophe P, et al. Zika Virus Infection and Prolonged Viremia in Whole-Blood Specimens. Emerging Infectious Disease journal 2017; 23(5): 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray KO, Gorchakov R, Carlson AR, et al. Prolonged Detection of Zika Virus in Vaginal Secretions and Whole Blood. Emerging Infectious Diseases 2017; 23(1): 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2015; 68: 53–5. [DOI] [PubMed] [Google Scholar]
- 14.Stone M, Lanteri MC, Bakkour S, et al. Relative analytical sensitivity of donor nucleic acid amplification technology screening and diagnostic real-time polymerase chain reaction assays for detection of Zika virus RNA. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Priyamvada L, Hudson W, Ahmed R, Wrammert J. Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology. Emerg Microbes Infect 2017; 6(5): e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons G, Stone M, Busch MP. Arbovirus Diagnostics: From Bad to Worse due to Expanding Dengue Virus Vaccination and Zika Virus Epidemics. Clin Infect Dis 2018; 66(8): 1181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai W-Y, Youn HH, Brites C, et al. Distinguishing Secondary Dengue Virus Infection From Zika Virus Infection With Previous Dengue by a Combination of 3 Simple Serological Tests. Clinical Infectious Diseases 2017; 65(11): 1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larocca RA, Abbink P, Peron JP, et al. Vaccine protection against Zika virus from Brazil. Nature 2016; 536: 474 EP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance 2014; 19(14): 20761. [DOI] [PubMed] [Google Scholar]
- 20.Williamson PC, Biggerstaff BJ, Simmons G, et al. An observational cohort study of evolving viral and serological stages of RNA positive blood donors and estimation of incidence of infection during the 2016 Puerto Rican Zika epidemic. Lancet Infectious Diseases 2019; (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Administration USFaD. Guidance for industry: revised recommendations for reducing the risk of Zika virus transmission by blood and blood components. Silver Spring (MD). CBER Office of Communication, Outreach, and Development; August 2016. [Google Scholar]
- 22.Stone M, Lanteri MC, Bakkour S, et al. Relative analytical sensitivity of donor nucleic acid amplification technology screening and diagnostic real-time polymerase chain reaction assays for detection of Zika virus RNA. Transfusion 2017; 57(3pt2): 734–47. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy M. US officials issue travel alert for Miami area as Zika cases rise to 15. BMJ 2016; 354. [DOI] [PubMed] [Google Scholar]
- 24.Frieden TR, Schuchat A, Petersen LR. Zika virus 6 months later. JAMA 2016; 316(14): 1443–4. [DOI] [PubMed] [Google Scholar]
- 25.Custer B, Kamel H, Kiely NE, Murphy EL, Busch MP. Associations between West Nile virus infection and symptoms reported by blood donors identified through nucleic acid test screening. Transfusion 2009; 49(2): 278–88. [DOI] [PubMed] [Google Scholar]
- 26.Lanteri MC, O’Brien KM, Purtha WE, et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. The Journal of clinical investigation 2009; 119(11): 3266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallian P, Cabie A, Richard P, et al. Zika virus in asymptomatic blood donors, Martinique. Blood 2017; 129(2): 263–6. [DOI] [PubMed] [Google Scholar]
- 28.Pantoja P, Perez-Guzman EX, Rodriguez IV, et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017; 8: 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Barraquer I, Costa F, Nascimento EJM, et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019; 363(6427): 607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rios M, Daniel S, Chancey C, Hewlett IK, Stramer SL. West Nile virus adheres to human red blood cells in whole blood. Clin Infect Dis 2007; 45(2): 181–6. [DOI] [PubMed] [Google Scholar]
- 31.Lai L, Lee TH, Tobler L, et al. Relative distribution of West Nile virus RNA in blood compartments: implications for blood donor nucleic acid amplification technology screening. Transfusion 2012; 52(2): 447–54. [DOI] [PubMed] [Google Scholar]
- 32.Lanteri MC, Lee TH, Wen L, et al. West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: implication for transfusion and transplantation safety. Transfusion 2014; 54(12): 3232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute west nile virus infection. The Journal of infectious diseases 2008; 198(7): 984–93. [DOI] [PubMed] [Google Scholar]
- 34.Robbiani DF, Bozzacco L, Keeffe JR, et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017; 169(4): 597–609.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy MR, Chen T-H, Hancock WT, et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine 2009; 360(24): 2536–43. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention NCfEaZIDN, Division of Vector-Borne Diseases (DVBD). Testing Guidance for Zika Virus. 2019. https://www.cdc.gov/zika/laboratories/types-oftests.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fzika%2Fhcproviders%2Ftypes-of-tests.html. [Google Scholar]
- 37.Meaney-Delman D, Oduyebo T, Polen KND, et al. Prolonged Detection of Zika Virus RNA in Pregnant Women. 2016; 128(4): 724–30. [DOI] [PubMed] [Google Scholar]
- 38.Driggers RW, Ho CY, Korhonen EM, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med 2016; 374(22): 2142–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.