Abstract
Background
Neurological symptoms are common manifestation in acute COVID-19. This includes hyper- and hypokinetic movement disorders. Data on their outcome, however, is limited.
Methods
Cases with new-onset COVID-19-associated movement disorders were identified by searching the literature. Authors were contacted for outcome data which were reviewed and analyzed.
Results
Movement disorders began 12.6 days on average after the initial onset of COVID-19. 92% of patients required hospital admission (mean duration 23 days). In a fraction of patients (6 of 27; 22%; 4 males/2 females, mean age 66.8 years) the movement disorder (ataxia, myoclonus, tremor, parkinsonism) was still present after a follow-up period of 7.5 ± 3 weeks. Severe COVID-19 in general and development of encephalopathy were risk factors, albeit not strong predictors, for the persistence.
Conclusions
The prognosis of new-onset COVID-19-associated movement disorder appears to be generally good. The majority recovered without residual symptoms within several weeks or months. Permanent cases may be due to unmasking of a previous subclinical movement disorder or due to vascular/demyelinating damage. Given the relatively low response rate of one third only and the heterogeneity of mechanisms firm conclusions on the (long-term) outome cannot, however, be drawn.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-023-11661-x.
Keywords: COVID, Sars-CoV2, Movement disorder, Long covid, Outcome, Myoclonus ataxia, Parkinsonism
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2, 2019-nCoV) emerged in December 2019 and spread into a worldwide pandemic. More than 641 million people worldwide have been verifiably infected with Sars-CoV-2 (as of November 2022). About 10–30% of COVID-19 patients develop neurological symptoms in the acute phase, including a broad range of new-onset movement disorders [1]. Among these, ataxia and myoclonus are the most common manifestation; however, parkinsonism, dystonia, chorea and other movement disorders may also occur [2]. Little is known about the outcome of these patients, as most case reports were published relatively soon after the occurrence of symptoms. Long-term data are lacking. As a mission of the International Parkinson and Movement Disorders Society (MDS) Infection-Related Movement Disorder (IRMD) Study Group, we therefore collected detailed clinical information and outcome data of COVID-19-associated movement disorder cases.
Methods
We searched the literature using PubMed and Medrxiv, the preprint server for health sciences, to identify cases of COVID-19-associated new-onset movement disorders published until February 2022. We used the (combination of) search terms: movement disorder; parkinsonism/Parkinson’s disease; dystonia; chorea; tics; myoclonus; SARS-CoV2, and COVID-19. Further articles were identified by cross-referencing. The authors were contacted and asked to provide follow-up information using a standardized questionnaire. Additional cases were also accepted, including by submission of data via a publicly available page on the website of the International Parkinson Disease and Movement Disorders Society (https://www.movementdisorders.org/COVID-19-Pandemic-MDS/COVID-19-Repository-Submissions.htm).
Results
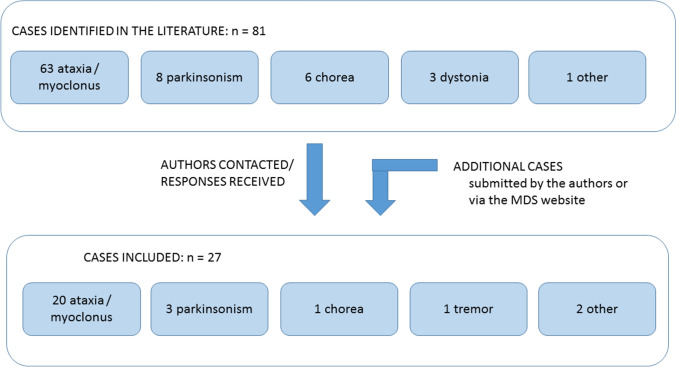
Reports of 81 patients with new-onset movement disorders were identified (63 ataxia/myoclonus, 8 parkinsonism, 6 chorea, 3 dystonia, other movement disorders), the authors of which were contacted by email. Information was received on 27 patients (8 female, 19 male) who were included in the final analysis (20 with ataxia/myoclonus [3–12], 3 with a parkinsonian syndrome [13–15], 1 with chorea [16], 1 with tremor, 1 with limb tremor and gait ataxia [17]), yielding an overall response rate of 33.3%. (Fig. 1) Submissions were received from the US, Spain, India (5 cases each), Germany (4 cases), Canada (2 cases), Pakistan (2 cases) and Italy, Belgium, France and Iran (1 case each). None of the cases had been vaccinated against COVID-19.
Fig. 1.
Identification of cases
The clinical information is summarized in Table 1. Briefly, the mean age at movement disorder onset for the whole group was 56.8 ± 12.6 years.
Table 1.
Clinical description of the study cohort (n = 27)
| Movement Disorder | Number of patients | Age | Sex (M/F) | Days of Hospital Stay | Days Between COVID and movement disorder | Follow Up# [weeks] |
|---|---|---|---|---|---|---|
| Myoclonus | 7 | 58.7 ± 13.1 | 3/4 | 20.4 ± 12.1 | 0,2,4,7,9,25,35 | 56.7 ± 32.3 |
| Myoclonus-Ataxia | 5 | 57.2 ± 12.4 | 4/1 | 12.2 ± 8.1 | 10,12,14,17; unknown in one case | 52.4 ± 31.2 |
| Ataxia | 5 | 53.2 ± 11.4 | 3/2 | 16.2 ± 10.2 | 0, 3, 12, 21; unknown in one case | 67.6 ± 20.1 |
| Opsoclonus-Myoclonus-Ataxia | 3 | 46.3 ± 13.0 | 3/0 | 5.3 ± 0.5 | 8, 10, 21 | 54.7 ± 3,8 |
| Hypokinetic rigid syndrome | 3 | 56 ± 7.5 | 2/1 | 48.3 ± 40.0 | 5, 33; ?44* | 67.0 ± 21.2 |
| Tremor | 1 | 62 | 1/0 | 37 | 10 | 8 |
| Serotonin Syndrome | 1 | 66 | 1/0 | 33 | 3 | 54 |
| Limb tremor and gait ataxia | 1 | 78 | 1/0 | 90 | 10 | 81 |
| Chorea | 1 | 58 | 1/0 | 13 | 2 | 3 |
*Onset not clear as the patient was intubated; movement disorder was first observed when the patient was extubated on day 44; # standardised from the outset
Severity of the COVID-19 infection ranged from mild to severe requiring hospital admission in all but two cases (92.6%; exceptions: one case with ataxia myoclonus and one with parkinsonism) with a mean cycle threshold score of 8.6 (data available for 18 patients) (with high scores indicating a low concentration of viral genetic material which is typically associated with a lower risk of infectivity).
The movement disorder developed an average of 12.6 ± 9.21 days after the initial onset of COVID-19 (information available for 24 patients). It was still persistent in six cases (four males, two females, mean age 66.8 years) after a mean duration of follow-up of 53 ± 23 days. Clinical details of these six cases are presented in Table 2. Briefly, their movement disorders manifested as ataxia, myoclonus, myoclonus-ataxia, tremor and parkinsonism. The movement disorder resolved in the remaining 21 patients within several weeks.
Table 2.
Summary of cases with persistent movement disorders
| Makhoul and Jankovic [14] | Makhoul and Jankovic (unpublished) | Muccioli et al. [7] | Desai and Chovatiya (unpublished) | Anand et al. [11], case 8 | Fearon et al. [15] | |
|---|---|---|---|---|---|---|
| Diagnosis | Unmasking of underlying still non-symptomatic iPD | Tremor | Immune‐mediated myoclonus | Post-stroke cerebellar ataxia | Myoclonic encephalopathy | Hypoxic–ischemic parkinsonism |
| Phenotype | Parkinsonism | Tremor | Myoclonus | Ataxia | Ataxia myoclonus, tremor, dystonia | Parkinsonism |
| Age | 64 | 62 | 61 | 67 | 71 | 46 |
| Sex | F | M | M | F | M | M |
| Admission required | No | Yes | Yes | Yes | Yes | Yes |
| Days in hospital | 0 | 37 | 38 | 17 | 18 | 98 |
| ICU care | No | Yes | Yes | Yes | No | Yes |
| BPAP | No | Yes | Yes | Yes | No | No |
| Previously vaccinated | No | No | No | No | No | No |
| Days between infection and onset of mov disord | 5 | 10 | 25 | 21 | 7 | 44* |
| Imaging findings | Decreased putaminal uptake on DaTscan | Brain MRI normal | Moderate cerebral small vessel disease | Cerebellar infarct | Diffuse frontal and temporal pachymeningeal enhancement, with resolution on follow-up imaging | Edema and haemorrhage, globus pallidus and dentates bilaterally, diffuse white matter changes |
| Treated with immune-modulatory drugs | No | Yes, steroids | No | Yes, steroids | No | No |
| Symptomatic treatment | Carbidopa/ levodopa, rasagiline | Propranolol, primidone, levetiracetam | Levetiracetam, clonazepam | None | Levetiracetam, valproic acid, zonisamide | Carbidopa/ levodopa |
| Course of mov. disord. | Steadily better | Steadily better | Steadily better | Steadily better | Steadily better | Static |
| Available follow-up (months) | 30 | 2 | 17 | 54 | 12 | 12 |
| Video previously published | Yes | No, published here (Video1) | Yes | No | No, published here (Video2) | Yes |
*Notably, this patient was intubated from day 5 after symptom onset until day 44
The mean duration of hospital admission was 22.9 ± 23.8 days for all cases (19.6 days for those who recovered vs 34.7 days for those with a persistent movement disorder). Oxygen supply was required in 51.8% of cases (56% of those admitted). Patients with an akinetic rigid syndrome stayed longer (48.3 ± 40 days) than patients with other phenotypes. Eight patients needed ICU treatment (29.6% of all cases; 32% of those admitted; 51.14% of those who needed oxygen; 19% of those who recovered vs 66.7% of those with a persistent movement disorder). Five patients required biphasic positive airway pressure (BPAP)(20% of those admitted); six patients were intubated (24% of those admitted).
Fourteen patients were treated with immunomodulatory drugs including steroids (nine patients), intravenous immunoglobulin (three patients) and a combination (one case). Most of the patients received symptomatic treatment for the particular type of movement disorder for a short period. At last follow-up, only two patients with parkinsonism continued to receive levodopa/carbidopa and only three patients with myoclonus were continuing to take levetiracetam and clonazepam. All other patients in our study did not require any symptomatic medicines for their movement disorders. One case [17] developed serotonin syndrome triggered by lopinavir/ritonavir (LPV/r) antiretroviral treatment.
Common comorbidities were present more frequently in those with a persistent movement disorder, i.e. hypertension (present in 83% of cases vs 43% of those who recovered); cardiac disease (14% vs 33%); cancer (33% vs 4%).
Brain imaging was performed in all cases and was reported to be normal in 63%. Abnormalities were detected in five cases whose movement disorder subsided, i.e. hemorrhagic leukoencephalitis (ataxia), bilateral cerebellar hyperintensities with cortical-meningeal enhancement (ataxia), cortical and brainstem ischemic lesions (myoclonus) and periventricular ischemic changes (chorea/myoclonus). On follow-up, the hemorrhagic changes had evolved into cerebellar gliosis and subcortical leukariosis, whereas the cerebellar edema and enhancement in the other ataxia patient completely resolved. No change was seen for the periventricular ischemic changes in both cases. Additional imaging included DaT imaging which showed bilateral asymmetric reduced nigro-striatal uptake in two cases with parkinsonism; this remained unchanged at ten months in the one who had a follow-up scan. Brain FDG PET in a myoclonus ataxia patient demonstrated putaminal and cerebellar hypermetabolism and diffuse cortical hypometabolism which normalised within eight weeks. Imaging findings from patients with persistent movement disorders are listed in the table. In summary, imaging did not reveal significant differences between the two groups that could explain the outcome. Data on CSF findings and neurophysiologic results were incomplete so they could not be used for correlation anaylsis.
Discussion
Movement disorders as a manifestation of COVID-19 appear to be rare; with myoclonus ataxia syndromes being the most common. However, parkinsonism, dystonia, chorea and other movement disorders have also been reported. To date, case reports largely lack follow-up data, yielding uncertainty about their long-term outcome. Here we provide follow-up information of 27 patients who had developed a new-onset organic movement disorder in the context of their COVID-19 infection. We found that the prognosis of the movement disorder is generally good, with the majority recovering without residual symptoms within several weeks or months. It remained unclear why ataxia and myoclonus recovered earlier than parkinsonism and chorea. Only in a fraction of patients (6 of 27; 22%) the movement disorder was persistent after a follow-up period of 7.5 ± 3 weeks. A severe clinical course of COVID-19 in general and the development of an encephalopathy were risk factors for the persistence of the movement disorder, albeit others who had a severe course recovered so this is not a strong predictor. Given the small number of patients, data on the presence or absence of comorbidities should be interpreted carefully.
Brain imaging studies were done in a heterogenous pattern (i.e. MRI vs CT, different sequences, at different times, with or without follow-up etc.)—which owes to the different clinical practices (and availability of imaging machines) across countries. It appears that results were unremarkable in two thirds of patients. Reported abnormalities included cortical, cerebellar and brainstem ischemic and meningeal enhancement. Follow-up information was scarce, available only for eight patients, in some of whom imaging abnormalities had resolved or remained static. DaT imaging was done in two case and revealed reduced uptake which remained unchanged after 10 months in the one where assessed.
Previous literature suggested that myoclonus may develop fairly soon after the initial infection, whereas parkinsonism manifests later [4, 18]. Our data do not recapitulate this observation. Thus, clinicians should be alert for the different types of movement disorders early and late after the initial infection.
Different hypotheses regarding the pathogenesis for acute COVID-19-associated movement disorders have been formulated, including anatomical, i.e. strategic, lesions (e.g. due to hypoxia), drug-induced, cytokine-mediated or as an autoimmune process. Accordingly, the prognosis may differ between cases. Thus, drug-induced cases may be more likely to recover once the triggering agent is removed [17], compared to someone with a secondary movement disorder associated with a hypoxic brain lesion.
About half of the patients were treated with some form of immunomodulatory treatment. In our series patients with hyperkinetic movement disorders received immunomodulation, while those with parkinsonism or isolated ataxia or those with suspected hypoxic ischemic encephalopathy did not receive immunomodulation.The decision whether to introduce this type of treatment was made by the treating physician based on clinical experience and local operating procedures. We are not aware of autoantibody positivity which may have influenced this decision; and based on the uncontrolled nature of our data conclusions in how far immunomodulatory treatment influenced time of recovery or prognosis cannot be drawn.
It is encouraging that almost three years into the pandemic only a handful of patients with persistent movement disorders have been reported. The concern is raised in view of the outbreak of encephalitis lethargica and postencephalitic parkinsonism in the 1920s which occurred after the 1918 influenza pandemic, commonly referred to as the Spanish flu. A second-hit mechanism has been proposed, however, the causal link remains a matter of debate, particularly with a lack of comprehensive surveillance data. For COVID-19, great vigilance is recommended in order to timely recognize and address potential neurological long-term manifestations of SARS-CoV2, including parkinsonism, especially in the long-term. Post-COVID movement disorders reflect a heterogeneity of mechanisms and thus one’s ability to draw firm conclusions on the (long-term) outome is limited.
The major limitation of our study is the relatively low response rate of 33%. Reasons for this could be the unavailability of follow-up as the patients may have died without informing the corresponding author, may have been admitted under other departments or a limited course of their disease. Notably, this leads to potential interpretation bias.
Finally, the cases reviewed here are all thought to be organic by nature. Notably, functional movement disorders associated with the SARS-CoV2 pandemic, lockdown and vaccine-related stressors have also been observed and reported recently. These include rapid-onset functional tic-like behaviours. The prognosis of the latter is good in affected adolescents, but less so in affected adults, based on a recent prospective follow-up study over 6 months [19].
Thus, our global multicentre collaborative study assessing long term outcome of post-COVID movement disorders suggests that the majority of the hyperkinetic movement disorders and immune mediated ataxias are self-limiting, while parkinsonism or ataxia due to hypoxia, ischemia or severe demyelination are more likely to take a chronic static persistent course requiring long-term management.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1: This 62-year old patient’s COVID-19 infection manifested with fever, headache, nausea, and diarrhea in July, 2021. His symptoms resolved within 10 days except for “shaking” in both hands. On admission he was confused, hallucinating and displayed generalized stereotyped movements of his whole body. EEG and brain MRI were normal. He gradually spontaneously improved but was left with residual tremor affecting both his upper and lower extremities while at rest and during action (shown in the video) but no evidence of bradykinesia (MP4 8004 KB)
Video 2: This 71-year old male patient presented due to confusion and gait disturbance. On admission he had generalized action-induced myoclonus affecting his limbs and face (see video) which improved over the course of 14 days (MP4 45776 KB)
Acknowledgements
This project was endorsed by the International Parkinson and Movement Disorders Society (MDS) Infection Related Movement Disorder study group.
Author contributions
SAS: research project: conception, organization, execution; statistical analysis: design, review and critique; manuscript: writing of the first draft. SD: research project: conception, organization, execution; manuscript: review and critique. OP: research project: conception, manuscript: review and critique. ECR: research project: conception; manuscript: review and critique. PA: research project: execution; manuscript: review and critique. GAB: research project: execution; manuscript: review and critique. AMC-A: research project: execution; manuscript: review and critique. HC: research project: execution; manuscript: review and critique. DC: research project: execution; manuscript: review and critique. FD: research project: execution; manuscript: review and critique. CF: research project: execution; manuscript: review and critique. FG: research project: execution; manuscript: review and critique. EG: research project: execution; manuscript: review and critique. AMG: research project: execution; manuscript: review and critique. MH: research project: execution; manuscript: review and critique. JJ: research project: execution; manuscript: review and critique. AEL: research project: execution; manuscript: review and critique. KM: research project: execution; manuscript: review and critique. LM: research project: execution; manuscript: review and critique. SAOS: research project: execution; manuscript: review and critique. VRO: research project: execution; manuscript: review and critique. JRP-S: research project: execution; manuscript: review and critique. RR: research project: execution; manuscript: review and critique. VR-C: research project: execution; manuscript: review and critique. CS: research project: execution; manuscript: review and critique. PS: research project: execution; manuscript: review and critique. LW: research project: execution; manuscript: review and critique. PP: research project: conception, organization, execution; statistical analysis: execution; manuscript: review and critique.
Funding
Open Access funding enabled and organized by Projekt DEAL. C Fearon was supported by Edmond J. Safra Fellowship in Movement Disorders from the Michael J. Fox Foundation. SAS was supported by the LMU Clinician Scientist Programme.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
None of the authors report any conflicts of interest.
Ethical standard statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. Informed consent was obtained from the individual contributors at their site to publish their cases.
References
- 1.Karuppan MKM, Devadoss D, Nair M, Chand HS, Lakshmana MK. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol Neurobiol. 2021 doi: 10.1007/s12035-020-02245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider SA, Hennig A, Martino D. Relationship between COVID-19 and movement disorders: a narrative review. Eur J Neurol. 2022;29:1243–1253. doi: 10.1111/ene.15217. [DOI] [PubMed] [Google Scholar]
- 3.Fadakar N, Ghaemmaghami S, Masoompour SM, et al. A First case of acute cerebellitis associated with coronavirus disease (COVID-19): a case report and literature review. Cerebellum. 2020;19:911–914. doi: 10.1007/s12311-020-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JL, Murphy KA, Sarna JR. Myoclonus and cerebellar ataxia associated with COVID-19: a case report and systematic review. J Neurol. 2021;268:3517–3548. doi: 10.1007/s00415-021-10458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimaldi S, Lagarde S, Harle JR, Boucraut J, Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from (18)F-FDG PET imaging and neuronal autoantibodies. J Nucl Med. 2020;61:1726–1729. doi: 10.2967/jnumed.120.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanguinetti SY, Ramdhani RA. Opsoclonus-myoclonus-ataxia syndrome related to the novel coronavirus (COVID-19) J Neuroophthalmol. 2021;41:e288–e289. doi: 10.1097/WNO.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muccioli L, Rondelli F, Ferri L, Rossini G, Cortelli P, Guarino M. Subcortical myoclonus in coronavirus disease 2019: comprehensive evaluation of a patient. Mov Disord Clin Pract. 2020;7:971–973. doi: 10.1002/mdc3.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following COVID-19. Mov Disord Clin Pract. 2020;7:974–976. doi: 10.1002/mdc3.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ros-Castello V, Quereda C, Lopez-Sendon J, Corral I. Post-hypoxic myoclonus after COVID-19 infection recovery. Mov Disord Clin Pract. 2020;7:983–984. doi: 10.1002/mdc3.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann KM, Harmel J, Wojtecki L. CORE-myoclonus syndrome: a proposed neurological initial manifestation of COVID-19. Mov Disord Clin Pract. 2021;8:637–638. doi: 10.1002/mdc3.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand P, Zakaria A, Benameur K, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med. 2020;48:1664–1669. doi: 10.1097/CCM.0000000000004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand P, Zhou L, Bhadelia N, Hamer DH, Greer DM, Cervantes-Arslanian AM. Neurologic findings among inpatients with COVID-19 at a safety-net US hospital. Neurol Clin Pract. 2021;11:e83–e91. doi: 10.1212/CPJ.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez-Guerrero A, Laespada-Garcia MI, Gomez-Grande A, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 14.Makhoul K, Jankovic J. Parkinson's disease after COVID-19. J Neurol Sci. 2021;422:117331. doi: 10.1016/j.jns.2021.117331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon C, Mikulis DJ, Lang AE. Parkinsonism as a sequela of SARS-CoV-2 infection: pure hypoxic injury or additional COVID-19-related response? Mov Disord. 2021;36:1483–1484. doi: 10.1002/mds.28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan M, Syed F, Ali L et al (2021) Chorea as a presentation of SARS-CoV-2 encephalitis: a clinical case report. J Mov Disord 14:245–247 [DOI] [PMC free article] [PubMed]
- 17.Mas Serrano M, Perez-Sanchez JR, Portela Sanchez S, et al. Serotonin syndrome in two COVID-19 patients treated with lopinavir/ritonavir. J Neurol Sci. 2020;415:116944. doi: 10.1016/j.jns.2020.116944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavallieri F, Fioravanti V, Bove F et al (2022) COVID-19 and Parkinsonism: a critical appraisal. Biomolecules 12(7):970 [DOI] [PMC free article] [PubMed]
- 19.Hull M, Jankovic J (2021) Increased incidence of functional (psychogenic) movement disorders in children and adults amidst the COVID-19 pandemic: a cross-sectional study. Neurol Clin Pract (epub ahead of print) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: This 62-year old patient’s COVID-19 infection manifested with fever, headache, nausea, and diarrhea in July, 2021. His symptoms resolved within 10 days except for “shaking” in both hands. On admission he was confused, hallucinating and displayed generalized stereotyped movements of his whole body. EEG and brain MRI were normal. He gradually spontaneously improved but was left with residual tremor affecting both his upper and lower extremities while at rest and during action (shown in the video) but no evidence of bradykinesia (MP4 8004 KB)
Video 2: This 71-year old male patient presented due to confusion and gait disturbance. On admission he had generalized action-induced myoclonus affecting his limbs and face (see video) which improved over the course of 14 days (MP4 45776 KB)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.