Abstract
Coronavirus disease 2019 (COVID-19) has presented a serious worldwide threat to public health since its emergence in late 2019. From a safety point of view, drug repurposing has received particular attention. Several clinical studies have demonstrated that the use of fluvoxamine, a selective serotonin reuptake inhibitor with potent sigma-1 receptor agonism, in the early-stage of infection might be associated with the prevention of clinical deterioration in individuals with SARS-CoV-2 infection, although several reports have shown that a low dose of fluvoxamine may be ineffective. There is increasing evidence that SARS-CoV-2 can cross the blood–brain barrier, resulting in a number of psychiatric and neurologic symptoms in COVID-19 survivors. Importantly, about half of COVID-19 survivors experience a variety of long-term sequelae, including psychiatric and neurologic symptoms, known as long COVID. In this priority review, the author presents an overview of the potential use of fluvoxamine in the treatment of COVID-19 and long COVID.
Keywords: Antidepressants, COVID-19, Fluvoxamine, Post-acute COVID-19 syndrome, Serotonin, Sigma-1 receptor
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has seriously affected public health worldwide since the first report of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan city, China, in December 2019. Vaccines for SARS-CoV-2 are crucial in preventing the spread of the virus and avoiding clinical deterioration in infected individuals. However, there are some safety concerns regarding COVID-19 vaccines (i.e., risk of myocarditis and pericarditis in young men, risk of immune thrombotic thrombocytopenia, and risk of ischemic stroke in the older population) [1–6]. Furthermore, it seems that immunological imprinting caused by a history of COVID-19 vaccination might modulate the immune response to subsequent infection or vaccination against new SARS-CoV-2 variants [7, 8].
In response to drug safety concerns, drug repurposing has received particular attention [9–15]. Several clinical findings suggest that the use of fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), could be associated with a reduced risk of clinical deterioration in patients with COVID-19. Since the first report by Lenze et al. [16] in November 2020, there have been many studies investigating the effects of fluvoxamine in individuals infected with SARS-CoV-2. However, the efficacy of fluvoxamine for COVID-19 remains controversial.
Infection with SARS-CoV-2 can cause a number of long-term health issues, known as long COVID or post-COVID conditions [17–19], and COVID-19 survivors can experience a variety of sustained sequelae. According to the World Health Organization, long COVID-19 is defined as the symptoms that usually occur after 3 months from the onset of SARS-CoV-2 infection, with symptoms lasting for at least 2 months [20]. Long COVID frequently impacts multiple organs, including respiratory and non-respiratory organs such as the brain [18, 21]. Currently, there are no specific prophylactic or therapeutic drugs to treat long COVID [22].
This review presents an overview of the potential of fluvoxamine in the treatment and clinical management of COVID-19 and long COVID.
Mechanisms of action of fluvoxamine
Serotonin transporter
The main target of all SSRIs, including fluvoxamine, is serotonin transporters. Serotonin transporters are localized throughout the body, including in the brain, lungs, and platelets in the blood [11, 23–28]. The order of affinity of SSRIs for human serotonin transporters is paroxetine > sertraline > fluoxetine and escitalopram > citalopram > fluvoxamine (Table 1) [29]. Thus, fluvoxamine is less potent than other SSRIs. Preclinical and clinical data indicate that SSRIs may mediate potent anti-inflammatory activity in mice and patients with depression [30–33]. Although all SSRIs can block serotonin transporters in the body, they do not all produce similar beneficial effects in patients with SARS-CoV-2 infection [34]. Therefore, the inhibition of serotonin transporters may not play a major role in the prophylactic effects of fluvoxamine in patients with COVID-19, although a partial role is possible [11].
Table 1.
Affinity and pharmacology of SSRIs for serotonin transporter, sigma-1 receptor and action at ASM
| SSRIs references | Ki (nM) for serotonin transporter (human) | Ki (nM) for sigma-1 receptor (rat) | Action at sigma-1 receptor (mouse and rat) | Functional inhibitor at ASM |
|---|---|---|---|---|
| Fluvoxamine | 2.3 | 36a or 17.0b | Agonist | Yes |
| Sertraline | 0.26 | 57a or 31.6b | Antagonist | Yes |
| Fluoxetine | 1.1 | 240a or 191.2b | Agonist | Yes |
| Escitalopram | 1.1 | 288.3b | Agonist | Yes |
| Citalopram | 1.6 | 292a or 403.8b | Agonist | Yes |
| Paroxetine | 0.10 | 1893a or 2041b | Yes | |
| References (author/year) | Owen et al. (2001) [29] |
aNarita et al. (1996) [37] bIshima et al. (2014) [41] |
Hashimoto et al. (2022) [11] Nishimura et al. (2008) [40] Ishima et al. (2014) [41] |
Kornhuber et al. (2011) [59] Gulbins et al. (2013) [60] Gulbins et al. (2018) [61] |
Sigma-1 receptor
Fluvoxamine as a potent sigma-1 receptor agonist
The sigma-1 receptor, cloned in 1997, contains an endoplasmic reticulum (ER)-retention signal [35]. Hayashi and Su [36] demonstrated that the sigma-1 receptor can function as a novel ER molecular chaperone in cells, and that agonists of the sigma-1 receptor have neuroprotective effects against ER stress associated with systemic inflammation. In 1996, we demonstrated that some SSRIs, including fluvoxamine, sertraline, fluoxetine, and citalopram, have high to moderate affinity to sigma-1 receptors in the rat brain (Table 1) [37]. By contrast, paroxetine had weak affinity to sigma-1 receptor (Table 1). Subsequent studies using mice and a cell culture system showed that three SSRIs (fluvoxamine, fluoxetine, and escitalopram) are agonists of the sigma-1 receptor, whereas sertraline may be an antagonist (Table 1) [38–41]. Among the commercially available SSRIs, fluvoxamine is the most potent agonist for sigma-1 receptor [42–45].
Rosen et al. [46] reported that sigma-1 receptor plays an important role in systemic inflammation using a lipopolysaccharide (LPS)-induced sepsis mouse model. LPS-induced mortality of sigma-1 receptor knockout mice was higher than that of wild-type mice. Blood levels of the proinflammatory cytokines of knockout mice after LPS administration were also higher than those of control mice, suggesting a protective role of sigma-1 receptor in systemic inflammation [46]. Importantly, fluvoxamine could attenuate LPS-induced lethal septic shock in mice [46]. Taken together, these data suggest that sigma-1 receptor may play a crucial role in systemic inflammation, and that activation of sigma-1 receptor by fluvoxamine might protect against ER stress associated with systemic inflammation due to SARS-CoV-2 infection, resulting in reduced mortality [9–11].
Sigma-1 receptor in the replication of SARS-CoV-2
Sigma-1 receptor is known to play an important role in the early-stage of viral RNA replication [47], suggesting a key role for sigma-1 receptor in the initial steps of virus-induced host cell reprogramming [11, 48, 49]. Two studies using human protein–protein interaction maps revealed that many compounds that interact with sigma-1 receptor were inhibitors of SARS-CoV-2 replication [50], and that knockdown of SIGMAR1 caused robust reductions in SARS-CoV-2 replication [51], suggesting a key role of sigma-1 receptor in SARS-CoV-2 replication. These findings suggest that sigma-1 receptor could be a therapeutic target in patients with early-stage COVID-19 [9–11, 52, 53].
Acid sphingomyelinase (ASM)
Acid sphingomyelinase (ASM) and ceramide are known to play a crucial role in viral infections [11, 54–56]. Since ASM is involved in ceramide generation, the inhibition of ASM could reduce virus entry into epithelial cells. Furthermore, it is suggested that SARS-CoV-2 can activate the ASM/ceramide system, resulting in the formation of ceramide-enriched membrane domains that may facilitate viral entry [11, 57, 58]. All SSRIs, including fluvoxamine, fluoxetine, sertraline, and paroxetine, are shown to inhibit ASM (Table 1), indicating the role of ASM in the pharmacological effects of these SSRIs [59–61].
An observational study in France showed that the use of ASM inhibitors was associated with a reduced risk of intubation or death in severely hospitalized patients with COVID-19 (n = 277) [62]. Among the SSRIs, escitalopram was significantly associated with reduced clinical deterioration in COVID-19 patients, although other SSRIs (i.e., fluoxetine, sertraline, and paroxetine) did not reach statistical significance [62]. Unfortunately, this study did not include fluvoxamine-treated patients. Given the role of ASM and sigma-1 receptor in the entry and replication of SARS-CoV-2 in cells, it is possible that escitalopram may be a potential prophylactic drug for patients with COVID-19 [11].
Effects of fluvoxamine on COVID-19 patients
In November 2020, Lenze et al. [16] showed that COVID-19 patients (n = 80) treated with fluvoxamine did not undergo clinical deterioration compared with the placebo group (n = 72) (Table 2). In this study (STOP COVID), the patients received a dose of fluvoxamine (50 mg) or a placebo in the evening immediately after baseline assessment and confirmation of eligibility. Then, patients were treated with fluvoxamine (100 mg twice daily for 2 days) or the placebo, as tolerated. Subsequently, fluvoxamine (100 mg 3 times daily) was administered up to day 15. The schedule and dose range of fluvoxamine administration was determined based on the occupancy of sigma-1 receptor in the human brain after oral administration of fluvoxamine [63]. Although this study only involved a small sample size, the results encouraged subsequent non-randomized observational studies and randomized placebo-controlled studies. The same research group performed the STOP COVID 2 study. The patients received a dose of fluvoxamine (50 mg) or a placebo in the evening immediately after baseline assessment. Then, the patients were treated with fluvoxamine (100 mg twice daily) or placebo until day 15 [64]. But this study was terminated early because of futility (Table 2).
Table 2.
A summary of the randomized clinical trials on the effects of fluvoxamine in patients with COVID-19
| Author/year/references | Study name | Country | Number of participants | Dose and duration | Outcome | Efficacy |
|---|---|---|---|---|---|---|
| Lenze et al. (2020) [16] | STOP COVID | USA | Outpatients with COVID-19 | Fluvoxamine (50 mg) (n = 80) or placebo (n = 72) was administered in the evening immediately after the baseline assessment. Then, fluvoxamine (100 mg twice daily for two days) or placebo was administered for tolerance, and subsequently increased to a dose of fluvoxamine (100 mg thrice daily) until day 15 | Clinical deterioration (1: shortness of breath or hospitalization for shortness of breath or pneumonia and 2: oxygen saturation less than 92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater) of fluvoxamine group (0/80) was significantly lower than that of placebo group (6/72) | Benefit |
| Lenze et al. (2021) [64] | STOP COVID 2 | USA and Canada | Outpatients with COVID-19 | Fluvoxamine (50 mg) (n = 272) or placebo (n = 275) was administered on day 1. Then, fluvoxamine (100 mg twice daily) or placebo was administered as tolerated until day 15 | This study was terminated early for futility | No benefit |
| Reis et al. (2022) [66] | TOGETHER | Brazil | High-risk symptomatic unvaccinated patients with COVID-19 | Fluvoxamine (100 mg twice daily) (n = 741) and placebo (n = 756) for 10 days | Fluvoxamine group showed significantly lower proportion of emergency setting for more than 6 h or transferred to tertiary hospital due to COVID-19 compared with placebo group | Benefit |
| Seo et al. (2022) [70] | Korea | Patients with mild or moderate COVID-19 who were admitted to the community treatment centers | Fluvoxamine (50 mg) (n = 26) or placebo (n = 26) was administered on day 1. Then, fluvoxamine (100 mg twice daily for about 10 days) or placebo was administered for tolerance until discharge from the community treatment centers | A single-blind trial showed that there was no significant differences in clinical deterioration between the fluvoxamine group and placebo group | No benefit | |
| Bramante et al. (2022) [71] | COVID-OUT | USA | Patients with COVID-19 | Fluvoxamine (50 mg twice daily) (n = 334) and placebo (n = 327) for 14 days | Fluvoxamine did not prevent the primary events such as hypoxemia, an emergency department visit, hospitalization, or death associated with COVID-19 | No benefit |
| McCarthy et al. (2023) [72] | ACTIV-6 | USA | Outpatients with mild to moderate COVID-19 | Fluvoxamine (50 mg twice daily) (n = 674) and placebo (n = 614) for 10 days | The median time to sustained recovery was 12 days in the fluvoxamine group and 13 days in the placebo group. There was no significant difference between the two groups | No benefit |
Using a prospective observational study, Seftel and Boulware [65] demonstrated the incidence of hospitalization in the fluvoxamine-treated group (0/65) and the observation alone group (6/48) of COVID-19 patients. The incidence of hospitalization was statistically significant difference between the two groups [65]. Reis et al. [66] reported a randomized, placebo-controlled trial of fluvoxamine (100 mg twice daily for 10 days) in unvaccinated adult patients with a risk factor for severe disease progression [66]. The number of patients observed in an emergency room for 6 h or hospitalized in the fluvoxamine group (n = 741) was significantly lower compared with the placebo group (n = 756) (Table 2) [66]. This TOGETHER study suggested that the use of fluvoxamine (100 mg twice daily for 10 days) among early-diagnosed, high-risk, COVID-19 patients could reduce clinical deterioration.
An open-label, prospective cohort trial using intensive care unit patients with COVID-19 in Croatia showed that the mortality rate among the fluvoxamine group (100 mg three times daily for 15 days) (30/51: 58.8%) was significantly lower than that of the matched control group (39/51: 76.5%), although there were no differences in the number of days on ventilator support, the duration of the intensive care unit stays, or the total hospital stay between the two groups [67]. Furthermore, Oskotsky et al. [68] performed a retrospective prospective study of COVID-19 patients treated with SSRIs using electronic health records from healthcare centers (n = 87) in the USA. The relative risk of mortality was significantly lower among COVID-19 patients treated with fluoxetine or fluvoxamine compared with untreated COVID-19 patients. By contrast, there was no significant association between SSRIs (i.e., citalopram, paroxetine, sertraline) other than fluvoxamine or fluoxetine and the risk of death [68]. Moreover, a prospective observational real-world study in Honduras showed that the rates of mortality and hospitalization, and the oxygen requirements of fluvoxamine-treated patients (n = 594), were lower than those of patients not treated with fluvoxamine (n = 63) [69].
Seo et al. [70] reported a single-blind randomized control trial of fluvoxamine (50 mg on day 1 and 100 mg twice daily for 10 days) in adult Korean patients with mild to moderate COVID-19 (Table 2). There was no difference in clinical deterioration between the fluvoxamine group (n = 26) and the placebo group (n = 26). Furthermore, Bramante et al. [71] reported a double-blind, randomized, placebo-controlled trial of fluvoxamine in patients with COVID-19. Fluvoxamine (n = 334, 50 mg twice daily for 14 days) did not prevent the occurrence of primary events such as hypoxemia, an emergency department visit, hospitalization, or death associated with COVID-19 compared with the placebo group (n = 327) (Table 2). In this trial, the authors investigated the effects of other candidates, such as metformin and ivermectin, which did not prevent the occurrence of primary events [71].
In 2023, McCarthy et al. [72] reported the results of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) platform randomized clinical trial (Table 2). Outpatients with mild to moderate COVID-19 (n = 1288) were randomly assigned to the fluvoxamine group (50 mg twice daily for 10 days; n = 674) and the placebo group (n = 614). The medial time to sustained recovery was 12 days (interquartile range [IQR], 11–14 days) in the fluvoxamine group and 13 days (IQR, 12–13 days) in the placebo group, with no significant difference between these two groups. The results of this study showed that fluvoxamine did not improve the time to sustained recovery in outpatients with mild to moderate COVID-19 [72].
Recently, two randomized placebo control trials using a large sample size found no evidence to confirm the beneficial effects of fluvoxamine in patients with COVID-19 [71–74]. These two trials used a low dose of fluvoxamine (50 mg twice daily) because of acute common side effects (i.e., nausea). In the treatment of psychiatric disorders (major depressive disorder [MDD], obsessive–compulsive disorder [OCD], social anxiety disorder [SAD], panic disorder), an initial low dose of fluvoxamine (50 mg daily) is used, and then higher doses (100 to 300 mg daily) are used for maintenance. Fluvoxamine has been approved for use in patients with OCD and SAD, but not MDD in the USA. A recent real-world case control study in the USA demonstrated that the odds ratio for acquiring COVID-19 in OCD patients (n = 4558) administered fluvoxamine was lower than that in OCD patients not receiving fluvoxamine (n = 77,511), indicating the protective effects of fluvoxamine in patients with OCD [74]. Considering the maximum dose of fluvoxamine permissible for patients with OCD (300 mg/day), it appears that administration of this drug may prevent clinical deterioration after SARS-CoV-2 infection in OCD patients.
Given the role of sigma-1 receptor in the beneficial effects of fluvoxamine, it seems that a dose of 50 mg twice daily of fluvoxamine is too low for activation of the sigma-1 receptor in the human body. Therefore, the author would like to recommend that high doses of fluvoxamine (100 mg two or three times daily for 10–15 days) may be suitable for patients with early-stage COVID-19. However, further multicenter double-blind, randomized clinical trials with a large sample size of patients administered fluvoxamine (100 mg two or three daily for 10–15 days) are needed.
Effects of fluvoxamine on long COVID
Detection of SARS-CoV-2 in the brain
Increasing evidence indicates that SARS-CoV-2 can enter the brain. In 2020, two articles reported the detection of SARS-CoV-2 in the brains of patients who died from COVID-19 [75, 76]. Rhea et al. [77] reported that intravenous or intranasal administration of radio-iodinated S1 protein of SARS-CoV-2 crossed the blood–brain barrier (BBB) in mice by adsorptive transcytosis. It was also reported that SARS-CoV-2 can cross the BBB via a transcellular pathway accompanied by basement membrane disruption without alteration of the tight junctions [78]. Using an in vitro BBB cell culture system, Petrovski et al. [79] demonstrated that the spike protein S1 could cross the human brain endothelial cell barrier. Interestingly, a study using the UK Biobank showed that long-term deleterious effects linked to the olfactory cortex after SARS-CoV-2 infection might be associated with the earliest and most common symptoms, such as loss of smell and taste, among COVID-19 survivors [80, 81]. Furthermore, Song et al. [82] detected SARS-CoV-2 in the cortical neurons of patients who died from COVID-19, and pathological changes in the brain were associated with infection with minimal immune cell infiltrates. In addition, a recent study using autopsies from patients who died from COVID-19 demonstrated that SARS-CoV-2 was widely distributed among multiple respiratory and non-respiratory tissues including the brain [83]. Interestingly, they found few histopathological changes in the brain, despite a substantial viral burden. It is noteworthy that SARS-CoV-2 can produce systemic infection and persist in the body for months [83]. Taken together, these findings indicate that SARS-CoV-2 may enter the brain after infection, resulting in a number of psychiatric and neurologic symptoms [84, 85]. Analysis of the cellular distribution of SARS-CoV-2 and its persistence in the human brain may provide an understanding of the long-lasting psychiatric and neurologic symptoms in COVID-19 survivors. Finally, it is suggested that spike protein, which is derived from SARS-CoV-2 and generated from COVID-19 vaccines, might be able to cross the BBB, resulting in neuroinflammation and blood clots in the brain [86].
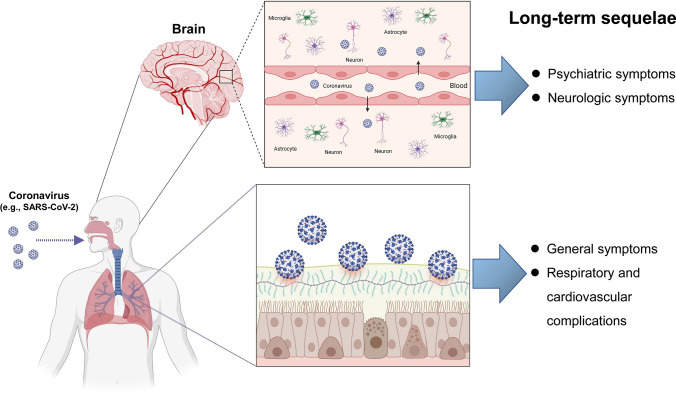
Infection with coronaviruses, such as SARS-CoV-2, can cause nerve damage through direct infection pathways (i.e., blood circulation and neuronal pathways), ACE2 (angiotensin-converting enzyme 2), hypoxia, immune injury, and other mechanisms (Fig. 1) [84]. Furthermore, systemic inflammation induced by SARS-CoV-2 infection could contribute to a decrease in neurotrophic factors and monoamines, and activation of microglia in the brain, resulting in long-term psychiatric and neurologic symptoms in COVID-19 survivors [84, 87, 88].
Fig. 1.
Detection of SARS-CoV-2 in the brain and long-term sequelae in COVID-19 survivors. Coronaviruses, such as SARS-CoV-2, can cause nerve damage through the direct infection pathway (blood circulation and neuronal pathways), and other mechanisms [84]. Several studies using autopsies from patients who died from COVID-19 showed that SARS-CoV-2 was detectable in multiple respiratory and non-respiratory tissues, including the brain [75, 76, 82, 83]. Furthermore, SARS-CoV-2 infection can result in long-term psychiatric symptoms (i.e., depression, anxiety, sleep problems) and neurologic symptoms (i.e., difficulty thinking or concentrating, headaches, dizziness, cognitive impairment) in COVID-19 survivors. The other most common symptoms of long COVID were general symptoms (i.e., tiredness or fatigue), and respiratory and cardiovascular complications (i.e., difficulty breathing or shortness of breath, cough, chest pain, fast-beating or pounding heart). Part of this figure was designed using resources from Biorender.com
Effects of SSRIs on long COVID
A systematic review using COVID-19 survivors (n = 250,351) demonstrated that the proportion of COVID-19 survivors experiencing at least one post-acute sequelae of COVID-19 was 54% at 6 months or more (long-term) [89]. The most common symptoms were general symptoms (i.e., tiredness or fatigue), respiratory and heart symptoms (i.e., difficulty breathing or shortness of breath, cough, chest pain, fast-beating or pounding heart), neurologic symptoms (i.e., difficulty thinking or concentrating, headaches, dizziness, cognitive impairment), and psychiatric symptoms (i.e., depression, anxiety, sleep problems) (Fig. 1) [90]. A recent meta-analysis using participants (n = 1,285,407) from 32 countries demonstrated that about half of COVID-19 survivors have a high burden of long-term sequelae in the 12 months after hospital discharge [21]. It is therefore important to treat these long-term sequelae in COVID-19 survivors. However, the precise mechanisms underlying long COVID remain unclear. Unfortunately, there are no potential therapeutic drugs for long-lasting sequelae in COVID-19 survivors, although vaccination has been strongly associated with a low risk of long COVID [91].
A recent retrospective population-based study demonstrated that the use of SSRI at baseline (at or prior to COVID-19 infection) was associated with a significant reduction in the risk of post-acute sequelae of COVID-19 [92]. They found a statistically significant 26% reduction (0.74 [95% CI 0.63–0.88], P = 5 × 10˗4) in the relative risk of long COVID among patients (n = 2021) receiving SSRI (i.e., fluvoxamine, fluoxetine, escitalopram, citalopram) with sigma-1 receptor agonism compared with patients (n = 14,584) without SSRI. They also found a statistically significant 25% reduction (0.75 [95% CI 0.62–0.90], P = 0.003) in the relative risk of long COVID among patients (n = 1328) receiving SSRI (i.e., sertraline, paroxetine) without sigma-1 receptor agonism compared with patients (n = 14,584) without SSRI. There were no statistically significant differences in relative risk between the group with SSRI with sigma-1 receptor agonism and the group with SSRI without sigma-1 receptor agonism. In this study, citalopram was included as an SSRI with sigma-1 receptor agonism. However, citalopram did not show agonist activity at the sigma-1 receptor in nerve growth factor-induced neurite outgrowth in PC12 cells [41], suggesting that citalopram should be included as an SSRI without sigma-1 receptor agonism. Therefore, further re-analysis of the data is needed to ascertain the role of sigma-1 receptor agonism on the SSRI-induced significant reduction in relative risk for long COVID. Nonetheless, the current data suggest that baseline use of SSRIs with or without sigma-1 receptor agonism was associated with a significant reduction in the risk of long COVID. In addition, Fenton et al. [93] suggested that SSRIs with anti-inflammatory activity may be effective in the treatment of depression in patients with long COVID.
Khani and Entezari-Maleki [94] reported that fluvoxamine could be a new potential therapeutic drug for long COVID. Considering the crucial role of fluvoxamine for serotonin transport, sigma-1 receptor, and ASM, it is possible that fluvoxamine might possess prophylactic or therapeutic effects in the case of long COVID [95]. Therefore, it may be of interest to investigate whether fluvoxamine could improve persistent symptoms in COVID-19 survivors.
Limitation
This study has limitation. From the results of several meta-analyses, there is an ongoing debate over the beneficial effects of fluvoxamine for COVID-19 [96–103]. A recent meta-analysis using 6 randomized clinical trials and 5 observational studies demonstrated that the medium dose (100 mg twice daily) of fluvoxamine, but not low dose (50 mg twice daily), was associated with a 21% reduction in the risk of hospitalization, and a 28% reduction in the risk of mortality [104]. A recent prospective interventional open-label cohort study in Uganda demonstrated that the use of fluvoxamine (100 mg twice daily for 10 days) among inpatients with COVID-19 was associated with reduced mortality and increased complete symptom resolution [105]. From the limited placebo-controlled randomized clinical data, we cannot conclude the beneficial effects of fluvoxamine for COVID-19. However, several non-randomized, observational studies suggest that baseline use of SSRIs, with or without sigma-1 receptor agonism, was associated with a reduction in the risk of clinical deterioration after infection and a reduction in the risk of long COVID. Collectively, it seems that fluvoxamine could be a potential candidate to reduce clinical deterioration in COVID-19 patients and long-lasting sequelae in COVID-19 survivors [10, 11, 106, 107].
Conclusion
As discussed above, it is possible that SARS-CoV-2 can enter the brain and induce neuroinflammation, resulting in acute symptoms (i.e., loss of smell and taste) and long COVID symptoms, including a number of psychiatric and neurologic symptoms. Although the precise mechanisms underlying long COVID remain unclear, invasion of SARS-CoV-2 in the brain may contribute to long-term psychiatric and neurologic symptoms in COVID-19 survivors.
The current clinical data suggest that the use of SSRIs at baseline might be associated with a low risk of subsequent hospitalization by infection and long COVID. Considering the role of sigma-1 receptor in mechanisms of action of SARS-CoV-2 [10, 11], the potent sigma-1 receptor agonist fluvoxamine could be a potential therapeutic or prophylactic drug for COVID-19 and long COVID. Oral use of fluvoxamine has the advantages of a favorable safety profile, it is inexpensive and widely available, and it can be used in children, adolescents, adults, and older individuals [11]. Finally, the author would like to propose that fluvoxamine (i.e., 100 mg two or three times daily for 10–14 days) might be an excellent candidate drug to help deal with a future COVID pandemic.
Acknowledgements
The author would like to thank our collaborators who are listed as the co-authors of our papers in the reference list. The author thanks Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
KH did the reference search and wrote the manuscript. The author read and approved the final manuscript.
Declarations
Competing interests
Dr. Hashimoto is the inventor of filed patent applications on “The use of R-Ketamine in the treatment of psychiatric diseases”, “(S)-norketamine and salt thereof as pharmaceutical”, “R-Ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder”, “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases”, and “R-Ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder” by the Chiba University. Dr. Hashimoto has also received speakers’ honoraria, consultant fee, or research support from Abbott, Boehringer-Ingelheim, Daiichi-Sankyo, Meiji Seika Pharma, Seikagaku Corporation, Dainippon-Sumitomo, Taisho, Otsuka, Murakami Farm and Perception Neuroscience.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRN-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlstad Ø, Hovi P, Husby A, Härkänen T, Selmer RM, Pihlström N, et al. SARS-CoV-2 vaccination and mycarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7(6):600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, et al. Cerebral venous thrombosis after vaccination against. Lancet. 2021;398(10306):1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavord S, Hunt BJ, Horner D, Bewley S, Karpusheff J, Guideline Committee Vaccine induced immune thrombocytopenia and thrombosis: summary of NICE guidance. BMJ. 2021;375:n2195. doi: 10.1136/bmj.n2195. [DOI] [PubMed] [Google Scholar]
- 5.Kim YE, Huh K, Park YJ, Peck KR, Jung J. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. 2022;328(9):887–889. doi: 10.1001/jama.2022.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanou MI, Palaiodimou L, Aguiar de Sousa D, Theodorou A, Bakola E, Katsaros DE, et al. Acute arterial ischemic stroke following COVID-19 vaccination: a systematic review and meta-analysis. Neurology. 2022 doi: 10.1212/WNL.0000000000200996. [DOI] [PubMed] [Google Scholar]
- 7.Brazil R. How your first brush with COVID warps your immunity. Nature. 2023;613(7944):428–430. doi: 10.1038/d41586-023-00086-1. [DOI] [PubMed] [Google Scholar]
- 8.Aydillo T, Rombauts A, Stadlbauer D, Aslam S, Abelenda-Alonso G, Escalera A, et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat Commun. 2021;12(1):3781. doi: 10.1038/s41467-021-23977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci. 2021;271(1):249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Suzuki T, Hashimoto K. Old drug fluvoxamine, new hope for COVID-19. Eur Arch Psychiatry Clin Neurosci. 2022;272(1):161–163. doi: 10.1007/s00406-021-01326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27(4):1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng YL, Salim CK, Chu JJH. Drug repurposing for COVID-19: approaches, challenges and promising candidates. Pharmacol Ther. 2021;228:107930. doi: 10.1016/j.pharmthera.2021.107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesan P. Repurposing drugs for treatment of COVID-19. Lancet Respir Med. 2021;9(7):e63. doi: 10.1016/S2213-2600(21)00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fico G, Isayeva U, De Prisco M, Oliva V, Solè B, Montejo L, et al. Psychotropic drug repurposing for COVID-19: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2023;66:30–44. doi: 10.1016/j.euroneuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenze E, Mattar C, Zorumski CF, Zorumski CF, Stevens A, Schweiger J, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19. A randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondratiuk AL, Pillay TD, Kon OM, Lalvani A. A conceptual framework to accelerate the clinical impact of evolving research into long COVID. Lancet Infect Dis. 2021;21(6):756–757. doi: 10.1016/S1473-3099(21)00136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, Ballouz T, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–1615. doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorman EA, O'Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequalae, and management. Lancet. 2022;400(10358):1157–1170. doi: 10.1016/S0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- 20.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng N, Zhao YM, Yan W, Li C, Lu QD, Liu L, et al. A systematic review and meta-analysis of long-term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28(1):423–433. doi: 10.1038/s41380-022-01614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaffer L. Lots of long COVID treatment leads, but few are proven. Proc Natl Acad Sci USA. 2022;119(36):e2213524119. doi: 10.1073/pnas.2213524119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adnot S, Houssaini A, Abid S, Marcos E, Amsellem V. Serotonin transporter and serotonin receptors. Handb Exp Pharmacol. 2013;218:365–380. doi: 10.1007/978-3-642-38664-0_15. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Goromaru T. High-affinity [3H]6-nitroquipazine binding to the 5-hydroxytryptamine transport system in rat lung. Biochem Pharmacol. 1991;41(11):1679–1682. doi: 10.1016/0006-2952(91)90169-6. [DOI] [PubMed] [Google Scholar]
- 25.Takano A, Suhara T, Sudo Y, Inoue M, Hashimoto K, Zhang MR, et al. Comparative evaluation of two serotonin transporter ligands in the human brain: [11C](+)McN5652 and [11C]cyanoimipramine. Eur J Nucl Med. 2002;29(10):1289–1297. doi: 10.1007/s00259-002-0884-4. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Goromaru T. High-affinity binding of [3H]6-nitroquipazine to 5-hydroxytryptamine transporter in human platelets. Eur J Pharmacol. 1990;187(3):295–302. doi: 10.1016/0014-2999(90)90356-b. [DOI] [PubMed] [Google Scholar]
- 27.Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91(1):119–128. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- 28.Mercado CP, Killic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10(4):231–241. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5):345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 30.Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alterations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2013;103(4):853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Dong C, Zhang JC, Yao W, Ren Q, Yang C, Ma M, et al. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-alpha, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2016;144:7–12. doi: 10.1016/j.pbb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci. 2015;16(4):7796–7801. doi: 10.3390/ijms16047796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Jannot AS, Neuraz A, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26(9):5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 35.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA. 1996;93(15):8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interaction of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307(1):117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32(3):514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- 39.Ishima T, Fujita Y, Kohno M, Kunitachi S, Hotio M, Takatsu Y, et al. Improvement of phencyclidine-induced cognitive deficits in mice by subsequent subchronic administration of fluvoxamine, but not sertraline. Open Clin Chem J. 2009;2:7–11. doi: 10.2174/1874241600902010007. [DOI] [Google Scholar]
- 40.Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS ONE. 2008;3(7):e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishima T, Fujita Y, Hashimoto K. Interactions of new antidepressants with sigma-1 receptor chaperons and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–173. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 42.Hindmarch I, Hashimoto K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum Psychopharmacol. 2010;25(3):193–200. doi: 10.1002/hup.1106. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci. 2015;127(1):6–9. doi: 10.1016/j.jphs.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Albayrak Y, Hashimoto K. Sigma-1 receptor agonists and their clinical implication in neuropsychiatric disorders. Adv Exp Med Biol. 2017;964:153–161. doi: 10.1007/978-3-319-50174-1_11. [DOI] [PubMed] [Google Scholar]
- 46.Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11(478):eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friesland M, Mingorance L, Chung J, Chisari FV, Gastaminza P. Sigma-1 receptor regulates early steps of viral RNA replication at the inset of hepatitis C virus infection. J Virol. 2013;87(11):6377–6390. doi: 10.1128/JVI.03557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasallo C, Gastaminza P. Cellular stress response in hepatitis C virus infection: mastering a two-edged sword. Virus Res. 2015;209:100–117. doi: 10.1016/j.virusres.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Vela JM. Repurposing sigma-1 receptor ligands for COVID-19 therapy? Front Pharmacol. 2020;11:582310. doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H, et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370(6521):eabe9403. doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sykhatme W. Fluvoxamine: a review of its mechanisms of actions and its role in COVID-19. Front Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brimson JM, Prasanth MI, Malar DS, Brimson S, Thitilertdecha P, Tencomnao T. Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: the possible role of the sigma-1 receptor and autophagy. Expert Opin Ther Targets. 2021;25(6):435–449. doi: 10.1080/14728222.2021.1952987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Wang A, Wu Y, Gulbins E, Grassmé H, Zhao Z. Acid sphingomyelinase-ceramide system in bacterial infections. Cell Physiol Biochem. 2019;52(2):280–301. doi: 10.33594/000000021. [DOI] [PubMed] [Google Scholar]
- 55.Beckmann N, Becker KA. Ceramide and related molecules in viral infections. Int J Mol Sci. 2021;22(11):5676. doi: 10.3390/ijms22115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Törnquist K, Asghar MY, Srinivasan V, Korhonen L, Lindholm D. Sphingolipids as modulators of SARS-CoV-2 infection. Front Cell Dev Biol. 2021;9:689854. doi: 10.3389/fcell.2021.689854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, et al. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26(1):9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- 58.Kornhuber J, Hoertel N, Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2022;27(1):307–314. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE. 2011;6(8):e23852. doi: 10.1371/journal.pone.0023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. 2013;19(7):934–938. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- 61.Gulbins A, Schumacher F, Becker KA, Wilker B, Soddemann M, Boldrin F, et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol Psychiatry. 2018;23(12):2324–2346. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Lenze EJ, et al. Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharmacol Ther. 2021;110(6):1498–1511. doi: 10.1002/cpt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishikawa M, Ishiwata K, Ishii K, Kimura Y, Sakata M, Naganawa M, et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C]SA4503. Biol Psychiatry. 2007;62(8):878–883. doi: 10.1016/j.biopsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Lenze E. Fluvoxamine for early treatment of Covid-19 (Stop Covid 2). ClinicalTrials.gov Identifier: NCT04668950.
- 65.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2):ofab050. doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis G, Moreira Silva EADS, Medeiros Silva DC, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calusic M, Marcec R, Luksa L, Jurkovic I, Kovac N, Mihaljevic S, et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Bri J Clin Pharmacol. 2022;88(5):2065–2073. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oskotsky T, Maric I, Tang A, Oskotsky B, Wong RJ, Aghaeepour N, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11):e2133090. doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pineda E, Singh J, Pineda MV, Umanzor JG, Baires F, Benitez LG, et al. Impact of fluvoxamine on outpatient treatment of COVID-19 in Honduras in a prospective observational real-world study. Front Pharmacol. 2022;13:1054644. doi: 10.3389/fphar.2022.1054644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seo H, Kim H, Bae S, Park S, Chung H, Sung HS, et al. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022;54(1):102–113. doi: 10.3947/ic.2021.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022;387(7):599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarthy MW, Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Felker GM, et al. Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2023;329(4):296–305. doi: 10.1001/jama.2022.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhimraj A, Gallagher JC. Lack of benefit of fluvoxamine for COVID-19. JAMA. 2023;329(4):291–292. doi: 10.1001/jama.2022.23954. [DOI] [PubMed] [Google Scholar]
- 74.Diaz AD, Baweja R. Protective effect of fluvoxamine for COVID-19 in obsessive–compulsive disorder: a real-world case-control study. Prim Care Comp CNS Disord. 2022;24(5):22br03337. doi: 10.4088/PCC.22br03337. [DOI] [PubMed] [Google Scholar]
- 75.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neurological features of Covid-19. N Engl J Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24(3):368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, et al. SAES-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target. 2021;6(1):337. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petrovszki D, Walter FR, Vigh JP, Kocsis A, Valkai S, Deli MA, Dér A. Penetration of the SARS-CoV-2 spike protein across the blood-brain barrier, as revealed by a combination of a human cell culture model system and optical biosensing. Biomedicines. 2022;10(1):188. doi: 10.3390/biomedicines10010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sollmann N, Beer AJ, Kirchhoff F. SARS-CoV-2 infection and the brain: direct evidence for brain changes in milder cases. Signal Transduct Target Ther. 2022;7(1):230. doi: 10.1038/s41392-022-01072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3):e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erickson MA, Rhea EM, Knopp RC, Banks WA. Interactions of SARS-CoV-2 with the blood–brain barrier. Int J Mol Sci. 2021;22(5):2681. doi: 10.3390/ijms22052681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oldfield PR, Hibberd J, Bridle BW. How does severe acute respiratory syndrome-coronavirus-2 affect the brain and its implications for the vaccines currently in use. Vaccines (Basel) 2021;10(1):1. doi: 10.3390/vaccines10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiat. 2021;78(6):682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Theoharides TC. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. 2022;59(3):1850–1861. doi: 10.1007/s12035-021-02696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendation. Nat Rev Microbiol. 2023 doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its management. Int J Biol Sci. 2022;18(12):4768–4780. doi: 10.7150/ijbs.75056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sidky H, Sahner DK, Girvin AT, Hotaling N, Michael SG, Kurilla MG, et al. Assessing the effect of selective serotonin reuptake inhibitors in the prevention of post-acute sequelae of COVID-19. medRxiv. 2022 doi: 10.1101/2022.11.09.22282142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fenton C, Lee A. Antidepressants with anti-inflammatory properties may be useful in long COVID depression. Drugs Ther Perspect. 2023;39(2):65–70. doi: 10.1007/s40267-022-00975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khani E, Entezari-Maleki T. Fluvoxamine and long COVID-19: a new role for sigma-1 receptor (S1R) agonists. Mol Psychiatry. 2022;27(9):3562. doi: 10.1038/s41380-022-01545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hashimoto Y, Suzuki T, Hashimoto K. Comments to “Fluvoxamine and long COVID-19: a new role for sigma-1 receptor (S1R) agonists” by Khani and Entezari-Maleki. Mol Psychiatry. 2022;27(9):3563–3564. doi: 10.1038/s41380-022-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo CM, Harari O, Chernecki C, Thorlund K, Forrest JI. Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials. Am J Trop Med Hyg. 2022;106(5):1315–1320. doi: 10.4269/ajtmh.21-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhuta S, Khokher W, Kesireddy N, Iftikhar S, Beran A, Mhanna M, et al. Fluvoxamine in nonhospitalized patients with acute COVID-19 infection and the lack of efficacy in reducing rates of hospitalization, mechanical ventilation, and mortality in placebo-controlled trials: a systematic review and meta-analysis. Am J Ther. 2022;29(3):e298–e304. doi: 10.1097/MJT.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 98.Lee TC, Vigod S, Bortolussi-Courval É, Hanula R, Boulware DR, Lenze EJ, et al. Fluvoxamine for outpatients management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(4):e226269. doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheema HA, Jafar U, Elrashedy AA, Shahid A, Awan RU, Ehsan M, et al. Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients. J Infect. 2022;85(6):702–769. doi: 10.1016/j.jinf.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu LC, Chao CM, Chang SP, Lan SH, Lai CC. Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: a systematic review and meta-analysis. J Infect Public Health. 2022;15(11):1259–1264. doi: 10.1016/j.jiph.2022.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marcec R, Dodig VM, Likic R. A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference. J Infect. 2023;86(2):154–225. doi: 10.1016/j.jinf.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trkulja V. There is no reasonable evidence to support efficacy of fluvoxamine in prevention of disease deterioration in COVID-19 outpatients: a comment on two recent meta-analyses advocating its use. J Infect. 2023;86(2):154–225. doi: 10.1016/j.jinf.2022.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trkulja V. Why we should not recommend or offer fluvoxamine to COVID-19 patients? Eur J Clin Pharmacol. 2023;79(2):321–322. doi: 10.1007/s00228-022-03447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng J, Rayner D, Ba Ramaraju H, Abbas U, Carcia C, Heybati K, et al. Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis. Clin Microbiol Infect. 2023 doi: 10.1016/j.cmi.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirenga BJ, Mugenyi L, Sánchez-Rico M, Kyobe H, Muttamba W, Mugume R, et al. Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: a prospective interventional open-label cohort study. Mol Psychiatry. 2023;1–8. 10.1038/s41380-023-02004-3. [DOI] [PMC free article] [PubMed]
- 106.Mahdi M, Hermán L, Réthelyi JM, Bálint BL. Potential role of the antidepressants fluoxetine and fluvoxamine in the treatment of COVID-19. Int J Mol Sci. 2022;23(7):3812. doi: 10.3390/ijms23073812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mills FP, Reis G, Wilson LA, Thorlund K, Forrest JI, Guo CM, et al. Early treatment with fluvoxamine among patients with COVID-19: a cost-consequence model. Am J Trop Med Hyg. 2022;108(1):101–106. doi: 10.4269/ajtmh.22-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]