Abstract
Background
Including adequate concentrations of antioxidants in dog diets has been recommended to reduce their vulnerability to the action of free radicals and reactive oxygen species (ROS). Oxidative stress in dogs has been associated with a wide range of diseases and disorders, as well as with ageing. There are few reports about the influence of diet on dog's antioxidant profile and oxidative stress.
Objective
The objective of this study was to evaluate the effect of four types of dry dog food on the oxidative/antioxidant profile of dogs.
Methods
Six Beagle dog males were used. The study included four experimental diets (dry foods A–D). Each dry food was supplied for 5 weeks to all dogs, for a total of 24 weeks, including an adaptation week between one food and another. For each dry dog food, the total phenolic content (TPC), total antioxidant capacity (TAC) and cytotoxicity were evaluated. Each week, a blood sample was collected to measure ROS and TAC of plasma. A crossover repeated measures design was used. Mixed models were adjusted, and means were compared using the Tukey test.
Results
Food A had the highest values for TPC and TAC. Food C had the lowest levels of ROS, whereas food B had the highest TAC in the blood plasma. The dog had a significant influence on the redox state of its blood plasma, even when the same dog was fed the different dry foods.
Conclusion
Dry dog food influences the oxidative/antioxidant profile of dog's blood plasma; however, this seems to be unrelated to the antioxidant profile of the food.
Keywords: antioxidant, cryopreservation, dogs, reactive oxygen species, sperm
Oxidative stress in canines has been associated with a wide range of diseases and disorders, as well as with ageing. Including adequate concentrations of antioxidants in dog diets has been recommended to reduce their vulnerability to the reactive oxygen species (ROS). The results of this study showed that dry dog food influences the oxidative/antioxidant profile of canine blood plasma.
1. INTRODUCTION
During the storage of food for animal consumption, different processes that alter their natural properties may occur; one of them is the peroxidation of lipids. This process causes the rancidity of food and produces a change in flavour, aroma and colour, as well as a decrease in the shelf life of the product (Błaszczyk et al., 2013). To counteract this, natural or synthetic antioxidants are often added in small amounts. These substances increase the durability of the food product and improve or modify its properties, including its appearance, taste or structure, without diminishing its nutritional value (Silva & Lidon, 2016). In addition, including adequate concentrations of antioxidants in dog diets has been recommended in order to counterbalance the action of free radicals and reactive oxygen species (ROS) (Pacheco et al., 2018). The imbalance between ROS production and their elimination by the body antioxidant systems is known as oxidative stress (Macotpet et al., 2013). ROS can severely alter the structure of molecules, such as proteins, lipids and deoxyribonucleic acid (DNA) (Valko et al., 2006). These alterations can cause cell degeneration and ageing (Hermans et al., 2007). Oxidative damage plays an important role in the pathogeny of many inflammatory diseases, such as diabetes mellitus, kidney disease and cancer in mammals, and some alterations of the cardiovascular and nervous system (Halliwell, 2007; Peddireddy et al., 2012; Sánchez‐Pérez et al., 2005; Sharifi, 2009; Valko et al., 2006). Oxidative stress in dogs has been associated with osteoarthritis (Barrouin‐Melo et al., 2016) and carcinogenesis (Macotpet et al., 2013; Winter et al., 2009), one of the leading causes of death for this species (Bonnett et al., 1997).
Several studies have investigated the influence of age, exercise and animal welfare on dog oxidative stress (Dunlap et al., 2006; Pasquini et al., 2008; Passantino et al., 2014). However, there are few reports about the influence of the type of diet on dog redox state (Pacheco et al., 2018). A total antioxidant capacity (TAC) assay is the most commonly used analytical method to determine the antioxidant balance in a biological sample (Rubio et al., 2016). Extensive research has measured blood plasma TAC in healthy dogs (Nemec et al., 2000), dogs with heart disease (Hetyey et al., 2007), dermal disease (Martínez‐Subiela et al., 2014), infectious disease (Ciftci et al., 2014; Kocaturk et al., 2015) and after undergoing surgical procedures (Lee & Kim, 2014) or vaccination (Rudoler et al., 2015). However, there are limited information regarding the effect of diet in the TAC of dog blood plasma. On the other hand, it has been reported that the nutritional composition of dry dog food is not related to their antioxidant contribution, as measured by the TAC and the total phenol content (Restrepo et al., 2019).
The aim of this study was to evaluate the effect of dry dog food with different nutritional profiles on the oxidative/antioxidant profile of blood plasma in dogs. We hypothesize that the oxidative/antioxidant profile of dog's plasma will be affected by the type of dry dog food supplied, due to the differences in the antioxidant profile of each of the diet.
2. MATERIALS AND METHODS
2.1. Animals and experimental design
Six healthy 1‐year‐old Beagle males were used. A crossover repeated measures design was used, because all dogs were simultaneously fed with the same dry dog food for 5 weeks, and this was repeated until the four dry dog foods and 20 weeks were completed. Each dry food had a different nutritional profile (Table 1). The order in which the diets were supplied was randomly assigned. Every week and for 20 weeks, each dependent variable was evaluated, for a total of 20 repetitions per dog and 120 repetitions throughout the experiment. A week was included at the beginning of each period (Bruni et al., 2020), in which the previous food was gradually changed until reaching 100% of the food to be tested, as follows: 25% on the first day, 50% on the second day, 75% on the third day and 100% from the fourth to the seventh day. Therefore, the entire experiment lasted 24 weeks. This “adaptation week” was the washout period in our experimental design. All foods were formulated, prepared and provided by Solla S.A. (Itagüi, Colombia).
TABLE 1.
Nutritional profiles of dry dog foods
| Nutrient/food | A | B | C | D |
|---|---|---|---|---|
| Moisture (%) | 7.56 | 8.01 | 7.62 | 7.50 |
| Protein (%) | 20.08 | 17.33 | 22.92 | 24.00 |
| Fat (%) a | 8.55 | 11.64 | 13.69 | 16.00 |
| Fiber (%) | 3.86 | 2.44 | 2.05 | 2.60 |
| Ash (%) | 7.09 | 6.67 | 6.38 | 6.20 |
| NFE | 52.86 | 53.91 | 47.34 | 43.7 |
| Metabolizable energy (kcal/kg) | 3200 | 3620 | 3810 | 3870 |
| Calcium (%) | 1.50 | 1.70 | 1.18 | 1.00 |
| Phosphorus (%) | 1.08 | 1.09 | 0.87 | 0.80 |
| Ca:P ratio | 1.39 | 1.56 | 1.36 | 1.25 |
| Sodium (%) | 0.33 | 0.20 | 0.20 | 0.20 |
| Potassium (%) | 0.9 | 0.7 | 0.6 | 0.6 |
| Vitamin A (UI/kg) | 6494 | 7792 | 9090.9 | 11,688.3 |
| Vitamin D (UI/kg) | 780 | 936 | 1092.0 | 1170.0 |
| Vitamin E (UI/kg) | 56 | 67 | 100.0 | 111.1 |
| Thiamine (mg/kg) | 2.7 | 3 | 3.78 | 4.05 |
| Riboflavin (mg/kg) | 5.6 | 7 | 7.84 | 8.4 |
| Pantothenic acid (mg/kg) | 14 | 17 | 19.6 | 21 |
| Niacin (mg/kg) | 18 | 22 | 25.2 | 27 |
| Pyridoxine (mg/kg) | 2 | 2 | 2.52 | 2.7 |
| Folic acid (mg/kg) | 0.3 | 0.36 | 0.42 | 0.45 |
| Vitamin B12 (mg/kg) | 0.035 | 0.042 | 0.049 | 0.0525 |
| Choline (mg/kg) | 1700 | 2040 | 2380 | 2550 |
| Butylhydroxytoluene‐BHT (%) | 0.015 | 0.015 | 0.015 | 0.015 |
Fat sources: beef fat (food A); chicken fat (food B); chicken fat and fish oil (food C); chicken fat, fish oil and seaweed extract (food D). NFE: nitrogen‐free extract. Ca: calcium. P: phosphorus. Additional ingredients: A, B, C and D: corn, poultry swine hydrolysate, sodium chloride, zinc sulphate, calcium carbonate, calcium phosphate, Yucca schidigera, copper sulphate, mixed tocopherols, calcium pantothenate, manganese sulphate, biotin, vitamin K, calcium iodate, sodium selenite. A, B and C: wheat bran, meat meal, propionic acid, citric acid. B, C and D: organic zinc, vitamin C, organic selenium. A and B: soybean hulls, soybean meal, rice polishing, bentonite, choline chloride, methionine. A and D: ferrous sulphate. B and C: corn gluten meal, potassium sorbate, iron sulphate, organic copper, organic manganese. C and D: fish meal, rice hulls, beet pulp, lignocellulose, zeolite, potassium chloride, choline chloride, yeast extract (Saccharomyces cerevisiae), probiotics. A: molasses, rice bran. B: titanium dioxide. D: poultry hydrolysate, poultry meal, oats, cassava starch, egg, pea protein, wheat protein, algae extract, l‐lysine, organic iron, l‐carnitine, lutein, betacarotene, taurine, tribasic copper.
Dogs were assigned to individual feeding modules with two areas: a resting and feeding area (2 m2) equipped with feeders, and an outside area (4.8 m2) equipped with automatic drinkers. Dogs interacted socially with other dogs in playgrounds, where they stayed for 6 h each day. During the experiment, each animal was weighed every week at the same hour of the day. Rations were updated based on energy input and requirements of the National Research Council (Nutrient Requirements of Dogs and Cats, 2006). Additionally, both the total offered food and the rejected food were weighed. Dogs were fed twice a day (7:30 and 15:30 h) and had free access to water throughout the study. Prior to the experiment, all dogs were vaccinated, dewormed and clinically examined to ensure optimal health.
2.2. Assessment of the antioxidant profile of dry dog foods
2.2.1. Total phenolic content (TPC)
We measured total phenolic content (TPC) through the Folin–Ciocalteu method (Singleton & Rossi, 1965). Each food was evaluated in triplicate, macerating 0.8 g of dry dog food and adding 10 ml of type I water. The mixtures were subjected to a vortex for 30 s and then centrifuged for 10 min at 3000g in a Z 206 A centrifuge (Hermle, Germany). Then 50 μl of sample were added to 125 μl of Folin–Ciocalteu reagent, and 400 μl of sodium carbonate 7.1% (p/v), adjusting with distilled water to 1000 μl. A standard curve was generated using gallic acid (Merck, Germany) as the sample. Readings were made at 760 nm using a UV–Vis spectrophotometer (Jenway, 6405, Essex, England).
2.2.2. Discoloration test with ABTS•+ cation radical
Each dry dog food TAC was measured using the ABTS (2,20‐azino‐bis 3‐ethylbenzothiazoline‐6‐sulfonic acid) assay (Arts et al., 2004), macerating 0.8 g of dry dog food and adding 10 ml of type I water. The mixtures were subjected to a vortex for 30 s and then centrifuged for 10 min at 3000g in a Z 206 A centrifuge (Hermle, Germany). A total of 10 μl of the supernatant and 990 μl of the ABTS•+ radical solution (Sigma‐Aldrich, USA) were used. After 30 min of reaction at room temperature in the dark, a 6405 UV/Vis spectrophotometer (Jenway, USA) measured the change in absorbance in triplicate with respect to a reference solution composed of 10 μl of buffer solution and 990 μl of the ABTS•+ radical solution. The radical was generated by the oxidation of 3.5 mM of ABTS with 1.25 mM of potassium persulphate. After 24 h of reaction, PBS absorbance was adjusted to pH 7.4 up to 0.70 units, at a λ of 732 nm and was compared against a Trolox (Sigma‐Aldrich, USA) standard curve.
2.2.3. Oxygen radical absorbance capacity (ORAC) assay
The TAC for each food was also measured using the oxygen radical absorbance capacity (ORAC) assay (Zapata et al., 2014), macerating 0.8 g of dry dog food and adding 10 ml of type I water. The mixtures were subjected to a vortex for 30 s and then centrifuged for 10 min at 3000g in a Z 206 A centrifuge (Hermle, Germany). A fluorescein solution of 1 × 10−5 M in PBS (75 mM, pH 7.4) and an AAPH solution of 0.6 M (Sigma‐Aldrich, USA) in PBS (75 mM, pH 7.4) were used. Each reaction tube was filled with 21 μl of fluorescein, 2.899 μl of PBS, 30 μl of sample (supernatant) y and 50 μl of AAPH. The assay was conducted under controlled conditions at 37°C and pH 7.4, and Trolox (Merck, Darmstadt, Germany) was used as the control antioxidant. The readings were performed by triplicate on an LS 55 spectrofluorometer (Perkin Elmer, Waltham, USA) at excitation/emission lengths of 493/515 nm. The ORAC value was calculated using the differences in areas under the fluorescein decay curve between a target and the sample, and it was then compared against a Trolox standard curve.
2.3. Diet cytotoxicity analysis
For MTT (3‐(4,5‐dimethylthiazol‐2‐yl) 2,5 diphenyltetrazolium bromide) assay (Mosmann, 1983), CHO‐K1 cells were obtained from an ATCC (American Type Culture Collection). These cells were kept in RPMI 1640 medium (Sigma‐Aldrich, USA) supplemented with 5% bovine foetal serum (Gibco, Thermo Fisher Scientific, USA) and 1% penicillin–streptomycin solution (100 IU penicillin and 100 μg/ml of streptomycin), in a humid atmosphere at 37.5°C with 5% CO2. About 6000 cells were seeded in a 96‐well cell culture plate and exposed to 10 different concentrations (from 0.1 to 10 mg/ml) of each of the experimental diets for 24 h, ensuring the availability of the food during an entire cell cycle. A total of 10 μl of MTT (Sigma‐Aldrich, USA) at a concentration of 5 mg/ml was then added to the wells. After a 3.5‐h incubation period, 100 μl of isopropanol acid (0.8% v/v of smoking HCl, 10% v/v of triton, 89.2% v/v of isopropyl alcohol) were added to dissolve the formazan crystals. The reading was performed at 570 nm on a Multiskan Spectrum reader (Thermo Scientific).
2.4. Measurement of the redox state in blood plasma
Blood samples were collected once a week from each dog, during the 5 weeks of each treatment for a total of 20 weeks. Blood samples were obtained through a venous puncture of the cephalic vein. The blood plasma was extracted by centrifugation at 3000 × g for 10 min (Pacheco et al., 2018) and stored at −80°C. ROS production was measured using the 2.7‐dichlorodihydrofluorescein diacetate (H2DCFDA) assay (Aitken et al., 2013). Each sample was made up of 30 μl of blood plasma, 240 μl of buffer solution (pH 7.4) and 30 μl of a 40 mM H2DCFDA solution (Intervet International BV, Boxmeer, the Netherlands). Readings were performed by quadruplication on an LS 55 spectrofluorometer (Perkin Elmer, Massachusetts, USA). The blood plasma TAC was measured using the ABTS assay described above.
2.5. Statistical analysis
To evaluate sources of variation, a linear model was adjusted for each dependent variable. In each statistical model, the random effect of the dog ID, the fixed effects of the dry food, the dog's weight and the interactions between the sampling date and the dog ID and the dry food and the dog ID were included. The normality of the data was assessed using the Shapiro–Wilk test. Means were compared by the Tukey test. For the evaluation of the cellular cytotoxicity of dry foods, a polynomial regression analysis was performed. The level of significance for all assessments was p < 0.05. All data were analysed using SAS version 9.2 software (SAS Inst. Inc., Cary, NC, USA).
3. RESULTS
3.1. Antioxidant profile, consumption and antioxidant contribution of dry dog foods
The average weight of the dogs during their participation in the study was 9.83 ± 0.15 kg. The results of consumption and rejection for each food and for each dog are presented in Table 2. Dogs showed no signs of physical or behavioural discomfort during adaptation week for any of the diets. The description of the antioxidant profile of the foods used in the study is shown in Table 3. Considering the average daily consumption of each food and its antioxidant profile, the daily antioxidant contribution of each food was calculated (Table 4). Food A presented the highest TPC and the highest TAC evaluated through the ORAC and ABTS assays (Table 3), which was reflected in a higher daily antioxidant contribution (Table 4).
TABLE 2.
Consumption and rejections for each food
| Food A | Food B | Food C | Food D | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dog | C (g) | R (g) | C (g) | R (g) | C (g) | R (g) | C (g) | R (g) | C (g) | R (g) |
| 1 | 255.1 ± 12.5 | 34.8 ± 12.5 | 259.4 ± 10.8 | 30.5 ± 10.8 | 240.1 ± 12.29 | 133.0 ± 23.6 | 248.6 ± 13.8 | 141.3 ± 13.8 | 250.8 | 84.9 |
| 2 | 230.1 ± 9.6 | 21.9 ± 9.6 | 235.7 ± 6.7 | 16.3 ± 6.7 | 213.3 ± 15.5 | 118.2 ± 30.0 | 182.0 ± 10.3 | 169.9 ± 10.3 | 215.2 | 81.5 |
| 3 | 262.2 ± 13.9 | 33.8 ± 13.9 | 274.7 ± 9.6 | 21.3 ± 9.6 | 207.9 ± 8.6 | 171.4 ± 17.9 | 207.8 ± 14.8 | 188.2 ± 14.8 | 238.1 | 103.6 |
| 4 | 288.6 ± 13.1 | 21.4 ± 13.1 | 250.7 ± 11.7 | 59.3 ± 11.7 | 219.2 ± 8.4 | 175.9 ± 27.8 | 241.2 ± 14.7 | 168.8 ± 14.7 | 249.9 | 106.3 |
| 5 | 252.0 ± 0.0 | 0.0 ± 0.0 | 252.0 ± 0.0 | 0.0 ± 0.0 | 326.2 ± 19.4 | 5.3 ± 5.3 | 252.1 ± 11.9 | 99.91 ± 11.9 | 270.5 | 26.30 |
| 6 | 153.0 ± 1.0 | 1.0 ± 1.0 | 154.0 ± 0.0 | 0.0 ± 0.0 | 195.1 ± 13.6 | 28.3 ± 15.1 | 173.4 ± 8.1 | 80.6 ± 8.1 | 168.8 | 27.4 |
| Average (g) | 240.1a | 18.8C | 237.7ab | 21.2C | 233.6ab | 105.4B | 217.5b | 141.4A | 232.2 | 71.7 |
Note: C: food consumption. R: food rejection. The results of consumption and rejection are expressed as the mean ± standard error of the mean (SEM). The averages of consumption and rejection were calculated based on the total of each food that was made available to each dog. Different lower case letters indicate significant statistical difference (p < 0.05) between food consumption. Different upper case letters indicate significant statistical difference (p < 0.05) between food rejections. Bold and italics text indicates general averages of consumption and rejection, both for each dog regardless of diet (rows) and for each diet regardless of dog (columns). The value in bold but without italics at the end of the table, means the general average of consumption and rejection.
TABLE 3.
Antioxidant profile of the dry dog foods
| Food | Total phenolic content a (mg gallic acid/100 g sample) | Total antioxidant capacity by ABTS b (μmol Trolox/100 g sample) | Total antioxidant capacity by ORAC c (μmol Trolox/100 g sample) |
|---|---|---|---|
| A | 225.97 ± 1.06 | 3718.09 ± 81.54 | 2755.16 ± 99.97 |
| B | 133.25 ± 4.39 | 2648.21 ± 61.4 | 1551.93 ± 79.85 |
| C | 130.82 ± 0.88 | 3122.91 ± 30.77 | 1792.56 ± 58.05 |
| D | 155.18 ± 2.15 | 3156.58 ± 61.49 | 2644.59 ± 126.56 |
Abbreviation: ORAC, oxygen radical absorbance capacity.
Total phenolic content was measured by Folin–Ciocalteu method.
ABTS: total antioxidant capacity measured by discoloration test with ABTS•+ cation radical.
ORAC: total antioxidant capacity measured by the ORAC assay. The results are expressed as the mean ± standard error of the mean (SEM).
TABLE 4.
Daily antioxidant contribution per food
| Food | Consumption a (g) | Phenol contribution b (mg gallic acid/day) | ABTS contribution c (μmol Trolox/day) | ORAC contribution d (μmol Trolox/day) |
|---|---|---|---|---|
| A | 240.18 | 542.74 ± 2.55 | 8930.14 ± 195.84 | 6617.37 ± 240.11 |
| B | 237.76 | 316.82 ± 10.44 | 6296.43 ± 145.99 | 3689.90 ± 189.85 |
| C | 233.64 | 305.65 ± 2.06 | 7296.31 ± 71.89 | 4188.10 ± 135.63 |
| D | 217.52 | 337.55 ± 4.68 | 6866.31 ± 133.76 | 5752.61 ± 275.30 |
Abbreviation: ORAC, oxygen radical absorbance capacity.
Average consumption of each food per dog.
Average of phenols consumed per dog.
Average of ABTS units consumed per dog.
Average of ORAC units consumed per dog. The results are expressed as the mean ± standard error of the mean (SEM).
3.2. Diet cytotoxicity analysis
Results for the MTT assay show that none of the foods produced a cellular viability of less than 80% in the concentrations tested, indicating that none of them had a cytotoxic effect. An increase in cellular viability was observed for food D in concentrations greater than 1.0 mg/ml. The polynomial regression analysis of the MTT assay was chosen due to better fitness and showed a reduction in cellular viability with an increase in the concentration of foods A–C, resulting in the negative regression coefficients found (Figure 1). On the other hand, a positive regression coefficient was obtained for food D. For this regression analysis, coefficients of determination (R 2) greater than 0.3 for all diets were found (R 2: 0.30–0.81).
FIGURE 1.
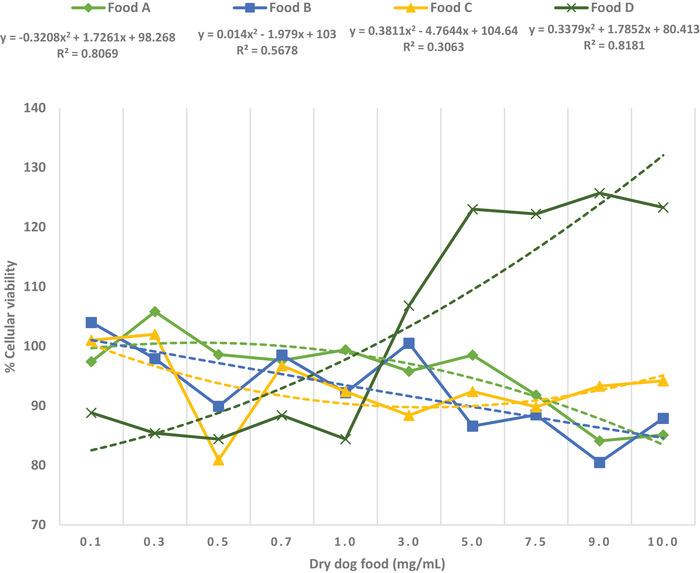
Polynomial regression analysis for the MTT assay. Polynomial regression equations are presented according to the following formula: y = β 2 x 2 + β 1 x + β 0, where β 0 is the y‐intercept, β 1 is the slope for x, and β 2 is the slope for x 2. R 2: coefficient of determination. The dotted lines correspond to the trendlines for the regression for each food.
3.3. Redox state of blood plasma
A total of 480 blood samples were evaluated, obtaining ROS and ABTS values of 0.065 ± 0.002 relative fluorescence units and 11,651.94 ± 231.03 μmol Trolox/L sample, respectively. The statistical models used for both variables were significant (p < 0.05), with R 2 values of 0.90 and 0.98, respectively. Food, dog ID, sampling date and the interactions between sampling date and dog ID, and the dry food and the dog ID, affected plasma ROS and ABTS values (p < 0.05). This suggests that much of the variability found in the redox state of the dog blood plasma may be attributed to these effects. The results for ROS and ABTS in the blood plasma by food are described in Table 5. Food A produced the highest levels of ROS and the lowest levels of TAC, whereas food C had the lowest ROS production, and food B had the highest TAC. Regarding the analysis per dog of the redox state of blood plasma, dog number 5 had the lowest ROS, whereas dog number 1 had the highest TAC and the second lowest ROS production (Table 6). It might be thought that dog number 1 was the animal that was subjected to the least oxidative stress. Moreover, it was observed that the redox state of the blood samples was dog‐dependent, even for the same type of food (Figures 2 and 3). However, a more stable behaviour was observed in the TAC of blood plasma when dogs were fed with food C, whereas ROS production was not different between dogs when they were fed with foods B and C.
TABLE 5.
Blood plasma oxidative/antioxidant state by food
| Food | Reactive oxygen species (RFU) | Total antioxidant capacity by ABTS A (μmol Trolox/100 g sample) |
|---|---|---|
| A | 0.075 ± 0.005 a | 7107.37 ± 145.88 c |
| B | 0.070 ± 0.002 a | 17,119.58 ± 561.03 a |
| C | 0.040 ± 0.001 b | 12,581.72 ± 76.45 b |
| D | 0.068 ± 0.005 a | 12,525.81 ± 196.51 b |
Abbreviation: RFU: relative fluorescence units.
Total antioxidant capacity measured by discoloration test with ABTS.+ cation radical. The results are expressed as the mean ± standard error of the mean.
Different letters in the columns indicate significant statistical difference (p < 0.05).
TABLE 6.
Effect of the dog on the blood plasma redox/antioxidant state
| Dog | Reactive oxygen species (RFU) | Total antioxidant capacity by ABTS A (μmol Trolox/100 g sample) |
|---|---|---|
| 1 | 0.060 ± 0.004 b | 12,096.64 ± 606.06 a |
| 2 | 0.068 ± 0.003 b | 11,034.65 ± 549.46 b |
| 3 | 0.065 ± 0.002 b | 11,896.97 ± 551.45 a |
| 4 | 0.079 ± 0.008a | 11,973.65 ± 545.22 a |
| 5 | 0.049 ± 0.002 c | 11,092.02 ± 576.48 b |
| 6 | 0.068 ± 0.008 b | 11,817.7 ± 571.24 ab |
Abbreviation: RFU: relative fluorescence units.
ATotal antioxidant capacity measured by discoloration test with ABTS.+ cation radical. The results are expressed as the mean ± standard error of the mean.
Different letters in the columns indicate significant statistical difference (p < 0.05).
FIGURE 2.
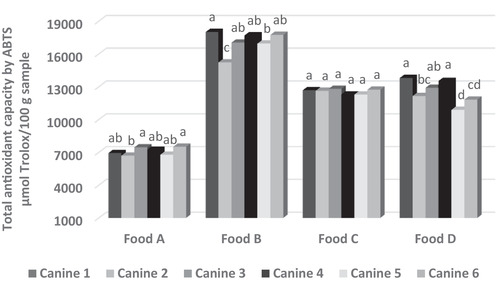
Effect of food on blood plasma total antioxidant capacity (TAC) by dog. Different letters in the columns within the same food indicate significant statistical difference (p < 0.05). ABTS: total antioxidant capacity measured by discoloration test with ABTS•+ cation radical
FIGURE 3.
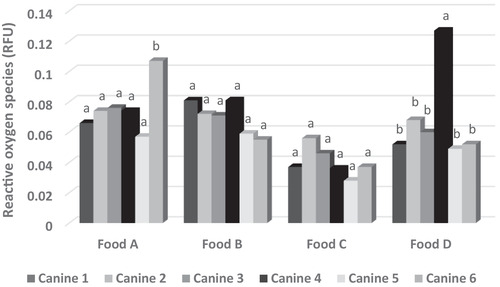
Effect of food on blood plasma reactive oxygen species (ROS) by dog. Different letters in the columns within the same food indicate significant statistical difference (p < 0.05). RFU, relative fluorescence units
4. DISCUSSION
Oxidative stress refers to the imbalance between oxidants and antioxidants in favour of ROS. Elevated oxidative stress can result in oxidative damage at the cellular level, leading to deleterious and pathological consequences (Passantino et al., 2014). For this reason, the incorporation of adequate concentrations of antioxidants in diets has been recommended in order to reduce the vulnerability of cell membranes to the action of free radicals, reactive species of nitrogen and oxygen (Pacheco et al., 2018). However, individual antioxidant levels in foods do not necessarily reflect the food TAC, which depends on synergistic and redox interactions between the different molecules present in the food. Several methods have been developed to measure the food TAC (Pellegrini et al., 2003). It was found in this study that food A followed by food D had the highest values for total phenols, ABTS and ORAC, whereas food B obtained the lowest values. This may be due to variations in composition and raw materials used in different dry dog foods, with differences reported between fibre content, raw protein and fat (Restrepo et al., 2019). For example, lipids in dry dog foods can represent from 5% to 40% of the diet, where fat may be animal‐based, plant‐based or sometimes a mixture of both (Bauer, 2007). In general, trends for the development of high‐quality dry dog foods are associated with a high fat content as an energy source, mainly in the form of polyunsaturated fatty acids (PUFAs), which increase the final digestibility of the diets (Marx et al., 2017). However, PUFAs are known to be more susceptible to oxidation (Pacheco et al., 2018). In a previous research, it was observed higher average values for TPC and ABTS in the dry dog foods with higher fibre content, suggesting a positive correlation between raw fibre content and TPC (Restrepo et al., 2019). The ABTS is a single‐electron transfer‐based assay, the limitations of which are its pH dependency and its reliance on the percentage of product decrease rather than the kinematics (Bartosz, 2010). It has been reported that when using a hydrophilic assay, as in the present study, most of the antioxidant capacity can be attributed to protein (10%–28%), uric acid (7%–60%) and ascorbic acid (2%–27%), whereas the effect of lipophilic components, such as vitamin E, is minimal (Aldini et al., 2010). Hence, a lower ABTS value for food B may be explained by the fact that it is the food with the lowest protein content. However, ABTS•+ is soluble in both aqueous and organic solvents and is not affected by ionic strength, so can be used in multiple media to determine both hydrophilic and lipophilic antioxidant capacities of body fluids (Awika et al., 2003). ORAC assay was not performed on the blood plasma of dogs, because being a hydrogen atom transfer method, the presence of reducing agents, including metals, is a complication and can lead to erroneously high apparent reactivity (Prior et al., 2005). In addition, the ORAC assay is very sensitive to the temperature, more laborious, costly and time‐consuming than the ABTS assay (Schaich et al., 2015).
TAC has become a prominent indicator of quality and functionality in human food, and it is frequently used to promote the consumption of products with a high antioxidant capacity (Pompella et al., 2014). It has even been reported that consumption above 10,000 μmol Trolox/day (ORAC units) is linked to a lower risk or incidence of hypertension, cerebral infarction, overall mortality, stroke and endometrial cancer in humans (Prior, 2015). The results of the antioxidant contribution in this study, presented as the foods ORAC units, are below this value. However, in pet nutrition, TAC is not often reported and possibly not often considered in the formulation of diets (Restrepo et al., 2019). In 2001, a commercial dry dog food product with a high antioxidant component, measured in ORAC units (μmol Trolox/g), was first introduced into the pet food industry to assist in the treatment of cognitive decline and age‐related behavioural changes in older dogs (Zicker, 2005). Moreover, it has been reported that a diet rich in antioxidants, based on the number of ORAC units in fruits and vegetables in a serving, decreases cognitive decline and oxidative damage caused by ageing in Beagle dogs (Dowling & Head, 2012). However, it is known that dry foods manufacturing process (extrusion, baking or other) can affect the oxidative stability of the kibble (Hołda & Głogowski, 2016). Deep fat frying can be problematic due to the combination of high temperatures and water that accelerate reactions, such as triacylglycerol hydrolysis, fatty acid oxidation, double‐bond isomerization and polymerization of fatty acids (Zhang et al., 2012). Meats are also highly susceptible to lipid oxidation because cooking releases protein‐bound metals, inactivates antioxidant enzymes and physically disrupts lipid membranes (Bou et al., 2010). At each stage of the feed manufacturing process, there could be a loss of nutrients; however, this loss is taken into account. For this reason, the design of the formula includes a higher level of vitamins, PUFAs and antioxidants, so that the final product contains the desired amounts.
On the other hand, some studies have quantified the safety of ingredients in dog food by measuring in vitro cytotoxicity in different types of cells relevant to toxicity tests (Koči et al., 2015; Ortega et al., 2016; Zhang et al., 2015). Moreover, the NRC has released directions on how to evaluate the safety of substances used in horse, dog and cat foods (National Research Council (NRC), 2008). Cytotoxicity of dry pet foods has also been associated with fungal contamination and mycotoxins (Singh et al., 2018). The oxidation of lipids also generates potentially cytotoxic fatty acid degradation products that can form genotoxic advanced lipid oxidation end products (Kanner, 2007); highly unsaturated fatty acids such as the omega‐3s are the most susceptible to oxidation and potentially generate the most toxic degradation products, such as aldehydes, which are able to form conjugates with proteins, cell membranes and DNA (Vieira et al., 2017). In this study, none of foods had a cytotoxic effect according to the viability analyses that were performed, not even those supplemented with fish oil. This could be because the stability of lipids and the resulting lipid oxidation products are dependent upon the degree and nature of the degree of unsaturation of fatty acids, antioxidant content, prooxidants, food processing operations and storage conditions (Vieira et al., 2017). Additionally, an increase in cell viability was observed for food D, which could be explained by cell proliferation due to the nutritional contribution of this food. The methods used to determine viability, including the MTT assay used in this research, have been reported to be also common for the detection of cell proliferation (Adan et al., 2016). It would be very interesting for future studies, to evaluate the relationship between the content of PUFAs in dry dog foods, lipid peroxidation and their cytotoxicity.
It has been reported that supplementation of the diet of dogs with sources of vitamins, minerals and polyphenols produces an increase in the TAC of their blood plasma (Corsato‐Alvarenga & Aldrich, 2018; Hesta et al., 2009; Sechi et al., 2017). Vitamin E supplementation, for example, has been shown to increase plasma TAC and to improve clinical signs and effects on oxidative stress, in dogs with atopic dermatitis (Kapun et al., 2014). Essential fatty acids also play a role in scavenging free radicals produced within cells, which participate to the protection of cell constituents (Sagols & Priymenko, 2011). Daily supplementation in omega‐3 essential fatty acids increases the cellular concentration of superoxide dismutase, making it more available to neutralize the free radicals produced by cellular metabolism (Luostarinen et al., 1997). Omega‐3 fatty acids can be found mainly in fish oils (Sagols & Priymenko, 2011), such as those used in foods C and D. In our study, food D had the highest concentrations of vitamins and minerals, and food A had the highest concentration of polyphenols and TAC. However, the higher TAC and the lowest ROS production in the blood plasma were found when dogs were fed with foods B and C, respectively. This shows that there was no consistent relationship between the antioxidant contribution of food and the redox state of dog blood plasma. This may be because antioxidants do not completely prevent oxidation. After some time, the antioxidant activity may become saturated. Therefore, the simultaneous use of multiple antioxidants becomes key to achieving a synergistic action (Silva & Lidon, 2016).
The TAC is a suitable biochemical parameter for measuring the general antioxidant state of plasma and bodily fluids that result from consumption and/or production of antioxidants, and the antioxidant usage due to normal or increased levels of ROS production. Hence, the TAC assesses the capacity of both known and unknown antioxidants and their synergetic interaction, thus giving an estimate on the equilibrium between oxidants and antioxidants in vivo (Ghiselli et al., 2000). Even though the TAC measurement with the ABTS radical allows for a simultaneous identification of hydrophilic and lipophilic antioxidants (Prior et al., 2005), the result presented above may be explained by the fact that this study did not measure all antioxidant components. For instance, it does not take into account the role of important enzymes such as superoxide dismutase glutathione peroxidase and catalases (Rubio et al., 2016a). The variability in the results with different TAC measuring methods in dogs can be explained by the inherent limitations of each method (Rubio et al., 2016a). These limitations may even account for the differences found while using the same method, such as the ABTS assay (Rubio et al., 2016). Other possible limitations of this study were the small sample size and potential carry‐over effects of previous treatment. However, in the week, from the fourth to the seventh day of this week, 100% of the new diet was supplied. The sample for the evaluation of the redox status of the blood plasma was always taken at the end of the adaptation week with the new diet, so that the dogs already had an 11‐day consumption of the food evaluated. Only males of the same breed and age were chosen for the study, to avoid possible variations in the redox state of the plasma, related to these characteristics. However, it would be very interesting for future studies to include effects, such as breed, age and sex, in the evaluation of the redox status of blood plasma, related to the type of diet.
Another aspect that may have influenced the results is the percentage of consumption and rejection of each of the tested foods. The fact that foods C and D had lower consumption and higher rejection percentages than food B may account for the reduced TAC in the blood plasma of the dogs fed with dry foods C and D, although they had a higher concentration of vitamins and minerals in their formulation. Differences in food consumption may be due to its different components and proportions of the same. For example, many different fat sources, particularly the highly polyunsaturated fats and/or the lower saturated fats, can condition flavour preferences (Ackroff et al., 2005). Other researchers have suggested that, if dogs are offered a range of nutritionally variable foods, they make food choices that maintain a specific ratio of macronutrients (Hewson‐Hughes et al., 2013). These studies imply that food consumption reflects a physiological need rather than choices based on palatability or food availability (Hewson‐Hughes et al., 2016). This could suggest that foods C and D could meet the nutritional needs of dogs, with less food consumed, which is also related to the energy density of the diet; foods C and D have a higher metabolizable energy and, therefore, a lower consumption, whereas for foods A and B, the opposite was observed, because dogs compensate for dietary energy by increasing intake (Alexander et al., 2017).
On the other hand, endogenous antioxidant system is complex and includes enzymatic antioxidants (superoxide dismutase, glutathione peroxidase), free radical traps (vitamins A, C and E) and metal chelating agents (Freeman et al., 2005). Some of these antioxidants may become prooxidants and be potentially harmful, depending on the antioxidant concentration, reactivity, redox potential and the nature of their neighbouring molecules (Carocho & Ferreira, 2013; Villanueva & Kross, 2012a). This may explain why the food with the best antioxidant profile (food A) did not result in the most optimal redox state of the dog blood plasma, and why the food that exhibited the lowest TAC (food B) showed the highest blood ROS levels. Ideally, the preferred method to evaluate oxidative stress is to directly measure ROS. Nevertheless, ROSs are difficult to measure with standard biochemical techniques due to ROS high reactivity and short half‐life (Macotpet et al., 2013). The reports on the reference values for oxidative stress in dogs are severely limited and contradictory because the oxidative state is variable and may be modified by several factors (Todorova et al., 2005). In this study, for example, an influence of the dog on the antioxidant capacity and ROS production in its blood plasma was detected, even when the same dog was fed the different dry foods. This may support the idea that there is a genetic component that causes different responses to antioxidants (Villanueva & Kross, 2012b). Furthermore, the interaction between sampling date and dog ID affected plasma ROS and ABTS values, possibly because sampling date was subject to the type of dry dog food and its consumption.
5. CONCLUSIONS
Dry dog food affects the oxidative/antioxidant profile of blood plasma in dogs. However, it appears that this is not directly related to the oxidative/antioxidant profile of the food, but it seems to be affected by the nutritional profile and the consumption of the food, as well as by a great influence of the dog. This leads us to think that the process of determining the food that most favours the oxidative/antioxidant equilibrium in dogs still has important knowledge gaps to be addressed.
AUTHOR CONTRIBUTIONS
Conceptualization; investigation: Giovanni Restrepo, Benjamín Alberto Rojano, Juan Camilo Duque, Carolina Mesa, Oliver Restrepo, Luis Miguel Gomez, Alexandra Usuga. Data curation: Giovanni Restrepo, Alexandra Usuga. Formal analysis: Giovanni Restrepo, Luis Miguel Gomez. Funding acquisition: Juan Camilo Duque, Luis Miguel Gomez. Methodology: Giovanni Restrepo, Juan Camilo Duque, Carolina Mesa, Oliver Restrepo, Luis Miguel Gomez, Alexandra Usuga. Project administration: Juan Camilo Duque, Luis Miguel Gomez. Resources: Benjamín Alberto Rojano, Juan Camilo Duque, Carolina Mesa, Luis Miguel Gomez. Software: Giovanni Restrepo, Luis Miguel Gomez. Writing – original draft: Giovanni Restrepo, Juan Camilo Duque, Luis Miguel Gomez, Alexandra Usuga. Writing – review and editing: Giovanni Restrepo, Juan Camilo Duque, Carolina Mesa, Luis Miguel Gomez, Alexandra Usuga.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. This research had the endorsement of the Institutional Committee for the Care and Use of Animals (CICUA) of the CES University (Project Code: 233, Act No. 50). The study proceeded in accordance with the Colombian Law 84 of December 1989, Chapter VI (on the use of live animals in experiments and research), Article 26 and Law 1774 of 2016, Article 1.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1064.
ACKNOWLEDGEMENT
The authors would like to thank Solla S.A, Medellín, Antioquia, Colombia, for its financial support.
Usuga, A. , Rojano, B. A. , Duque, J. C. , Mesa, C. , Restrepo, O. , Gomez, L. M. , & Restrepo, G. (2023). Dry food affects the oxidative/antioxidant profile of dogs. Veterinary Medicine and Science, 9, 687–697. 10.1002/vms3.1064
Contributor Information
Alexandra Usuga, Email: ausuga@ces.edu.co.
Benjamín Alberto Rojano, Email: brojano@unal.edu.co.
Juan Camilo Duque, Email: jcduque@solla.com.
Carolina Mesa, Email: cmesa@solla.com.
Oliver Restrepo, Email: orestrepor@solla.com.
Luis Miguel Gomez, Email: lgomezosorio@gmail.com.
Giovanni Restrepo, Email: grestre0@unal.edu.co.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ackroff, K. , Lucas, F. , & Sclafani, A. (2005). Flavor preference conditioning as a function of fat source. Physiology and Behavior, 85(4), 448–460. 10.1016/j.physbeh.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Adan, A. , Kiraz, Y. , & Baran, Y. (2016). Cell proliferation and cytotoxicity assays. Current Pharmaceutical Biotechnology, 17(14), 1213–1221. 10.2174/1389201017666160808160513 [DOI] [PubMed] [Google Scholar]
- Aitken, R. J. , Smith, T. B. , Lord, T. , Kuczera, L. , Koppers, A. J. , Naumovski, N. , Connaughton, H. , Baker, M. A. , & De Iuliis, G. N. (2013). On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology, 1(2), 192–205. 10.1111/j.2047-2927.2012.00056.x [DOI] [PubMed] [Google Scholar]
- Aldini, G. , Yeum, K. J. , Niki, E. , & Russell, R. M. (2010). Biomarkers for antioxidant defense and oxidative damage: Principles and practical applications. In Aldini G., Yeum K.‐J., Niki E., & Russell R. M. (Eds.), Biomarkers for antioxidant defense and oxidative damage: Principles and practical applications. Wiley‐Blackwell. 10.1002/9780813814438 [DOI] [Google Scholar]
- Alexander, J. E. , Colyer, A. , & Morris, P. J. (2017). The effect of reducing energy density, via the addition of water to dry diet, on body weight and activity in dogs. Journal of Nutritional Science, 6, e42. 10.1017/jns.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts, M. J. T. J. , Sebastiaan Dallinga, J. , Voss, H. P. , Haenen, G. R. M. M. , & Bast, A. (2004). A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chemistry, 88(4), 567–570. 10.1016/j.foodchem.2004.02.008 [DOI] [Google Scholar]
- Awika, J. , Rooney, W. L. , Wu, X. L. , Prior, R. , & Cisneros‐Zevallos, L. (2003). Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. Journal of Agricultural and Food Chemistry, 51(23), 6657–6662. 10.1021/jf034790i [DOI] [PubMed] [Google Scholar]
- Barrouin‐Melo, S. M. , Anturaniemi, J. , Sankari, S. , Griinari, M. , Atroshi, F. , Ounjaijean, S. , & Hielm‐Björkman, A. K. (2016). Evaluating oxidative stress, serological‐ and haematological status of dogs suffering from osteoarthritis, after supplementing their diet with fish or corn oil. Lipids in Health and Disease, 15(1), 139. 10.1186/s12944-016-0304-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosz, G. (2010). Non‐enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radical Research, 44(7), 711–720. 10.3109/10715761003758114 [DOI] [PubMed] [Google Scholar]
- Bauer, J. E. (2007). Responses of dogs to dietary omega‐3 fatty acids. Journal of the American Veterinary Medical Association, 231(11), 1657–1661. 10.2460/javma.231.11.1657 [DOI] [PubMed] [Google Scholar]
- Błaszczyk, A. , Augustyniak, A. , & Skolimowski, J. (2013). Ethoxyquin: An antioxidant used in animal feed. International Journal of Food Science, 2013, 1–12. 10.1155/2013/585931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett, B. N. , Egenvall, A. , Olson, P. , & Hedhammar, Å. (1997). Mortality in insured Swedish dogs: Rates and causes of death in various breeds. Veterinary Record, 141(2), 40–44. 10.1136/vr.141.2.40 [DOI] [PubMed] [Google Scholar]
- Bou, R. , Hanquet, N. , Codony, R. , Guardiola, F. , & Decker, E. A. (2010). Effect of heating oxyhemoglobin and methemoglobin on microsomes oxidation. Meat Science, 85(1), 47–53. 10.1016/J.MEATSCI.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Bruni, N. , Martello, E. , Fusi, E. , Meineri, G. , & Giardini, A. (2020). Study of faecal parameters and body condition in dogs with a diet supplemented with Lactobacillus acidophilus D2/CSL (CECT 4529). Italian Journal of Animal Science, 19(1), 704–711. 10.1080/1828051X.2020.1783378 [DOI] [Google Scholar]
- Carocho, M. , & Ferreira, I. C. F. R. (2013). A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology, 51(1), 15–25. 10.1016/j.fct.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Ciftci, G. , Ural, K. , Aysul, N. , Cenesiz, S. , Guzel, M. , Pekmezci, D. , & Sogut, M. Ü. (2014). Investigation of the 8‐hydroxy‐2’‐deoxyguanosine, total antioxidant and nitric oxide levels of serum in dogs infected with Babesia vogeli . Veterinary Parasitology, 204(3–4), 388–391. 10.1016/j.vetpar.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Corsato‐Alvarenga, I. , & Aldrich, C. G. (2018). The effect of sorghum fractions on apparent total tract digestibility and antioxidant capacity by dogs. PLoS One, 13(10), e0206090. 10.1371/journal.pone.0206090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling, A. L. S. , & Head, E. (2012). Antioxidants in the canine model of human aging. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1822(5), 685–689. 10.1016/j.bbadis.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap, K. L. , Reynolds, A. J. , & Duffy, L. K. (2006). Total antioxidant power in sled dogs supplemented with blueberries and the comparison of blood parameters associated with exercise. Comparative Biochemistry and Physiology – A Molecular and Integrative Physiology, 143(4), 429–434. 10.1016/j.cbpa.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Freeman, L. M. , Rush, J. E. , Milbury, P. E. , & Blumberg, J. B. (2005). Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. Journal of Veterinary Internal Medicine, 19(4), 537–541. 10.1111/j.1939-1676.2005.tb02724.x [DOI] [PubMed] [Google Scholar]
- Ghiselli, A. , Serafini, M. , Natella, F. , & Scaccini, C. (2000). Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radical Biology and Medicine, 29(11), 1106–1114. 10.1016/S0891-5849(00)00394-4 [DOI] [PubMed] [Google Scholar]
- Halliwell, B. (2007). Oxidative stress and cancer: Have we moved forward? Biochemical Journal, 401(1), 1–11. 10.1042/BJ20061131 [DOI] [PubMed] [Google Scholar]
- Hermans, N. , Cos, P. , Maes, L. , De Bruyne, T. , Vanden Berghe, D. , Vlietinck, A. J. , & Pieters, L. (2007). Challenges and pitfalls in antioxidant research. Current Medicinal Chemistry, 14(4), 417–430. 10.2174/092986707779941005 [DOI] [PubMed] [Google Scholar]
- Hesta, M. , Ottermans, C. , Krammer‐Lukas, S. , Zentek, J. , Hellweg, P. , Buyse, J. , & Janssens, G. P. J. (2009). The effect of vitamin C supplementation in healthy dogs on antioxidative capacity and immune parameters. Journal of Animal Physiology and Animal Nutrition, 93(1), 26–34. 10.1111/j.1439-0396.2007.00774.x [DOI] [PubMed] [Google Scholar]
- Hetyey, C. S. , Manczur, F. , Dudás‐Györki, Z. , Reiczigel, J. , Ribiczey, P. , Vajdovich, P. , & Vörös, K. (2007). Plasma antioxidant capacity in dogs with naturally occurring heart diseases. Journal of Veterinary Medicine Series A: Physiology Pathology Clinical Medicine, 54(1), 36–39. 10.1111/j.1439-0442.2007.00911.x [DOI] [PubMed] [Google Scholar]
- Hewson‐Hughes, A. K. , Colyer, A. , Simpson, S. J. , & Raubenheimer, D. (2016). Balancing macronutrient intake in a mammalian carnivore: Disentangling the influences of Flavour and Nutrition. Royal Society Open Science, 3(6), 160081. 10.1098/rsos.160081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson‐Hughes, A. K. , Hewson‐Hughes, V. L. , Colyer, A. , Miller, A. T. , McGrane, S. J. , Hall, S. R. , Butterwick, R. F. , Simpson, S. J. , & Raubenheimer, D. (2013). Geometric analysis of macronutrient selection in breeds of the domestic dog, Canis lupus familiaris . Behavioral Ecology, 24(1), 293–304. 10.1093/beheco/ars168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hołda, K. , & Głogowski, R. (2016). Selected quality properties of lipid fraction and oxidative stability of dry dog foods under typical storage conditions. Journal of Thermal Analysis and Calorimetry, 126(1), 91–96. 10.1007/s10973-016-5543-2 [DOI] [Google Scholar]
- Kanner, J. (2007). Dietary advanced lipid oxidation endproducts are risk factors to human health. Molecular Nutrition and Food Research, 51(9), 1094–1101. 10.1002/mnfr.200600303 [DOI] [PubMed] [Google Scholar]
- Kapun, A. P. , Salobir, J. , Levart, A. , Kalcher, G. T. , Svete, A. N. , & Kotnik, T. (2014). Vitamin E supplementation in canine atopic dermatitis: Improvement of clinical signs and effects on oxidative stress markers. Veterinary Record, 175(22), 560. 10.1136/vr.102547 [DOI] [PubMed] [Google Scholar]
- Kocaturk, M. , Tvarijonaviciute, A. , Martinez‐Subiela, S. , Tecles, F. , Eralp, O. , Yilmaz, Z. , & Ceron, J. J. (2015). Inflammatory and oxidative biomarkers of disease severity in dogs with parvoviral enteritis. Journal of Small Animal Practice, 56(2), 119–124. 10.1111/jsap.12250 [DOI] [PubMed] [Google Scholar]
- Koči, J. , Jeffery, B. , Riviere, J. E. , & Monteiro‐Riviere, N. A. (2015). In vitro safety assessment of food ingredients in canine renal proximal tubule cells. Toxicology in Vitro, 29(2), 289–298. 10.1016/j.tiv.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , & Kim, M. C. (2014). Comparison of oxidative stress status in dogs undergoing laparoscopic and open ovariectomy. Journal of Veterinary Medical Science, 76(2), 273–276. 10.1292/jvms.13-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luostarinen, R. , Wallin, R. , & Saldeen, T. (1997). Dietary (n‐3) fatty acids increase superoxide dismutase activity and decrease thromboxane production in the rat heart. Nutrition Research, 17(1), 163–175. 10.1016/S0271-5317(96)00242-4 [DOI] [Google Scholar]
- Macotpet, A. , Suksawat, F. , Sukon, P. , Pimpakdee, K. , Pattarapanwichien, E. , Tangrassameeprasert, R. , & Boonsiri, P. (2013). Oxidative stress in cancer‐bearing dogs assessed by measuring serum malondialdehyde. BMC Veterinary Research, 9(1), 101. 10.1186/1746-6148-9-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Subiela, S. , Bernal, L. J. , Tvarijonaviciute, A. , Garcia‐Martinez, J. D. , Tecles, F. , & Cerón, J. J. (2014). Canine demodicosis: The relationship between response to treatment of generalised disease and markers for inflammation and oxidative status. Veterinary Dermatology, 25(2), 72–e24. 10.1111/vde.12108 [DOI] [PubMed] [Google Scholar]
- Marx, F. R. , Trevizan, L. , Saad, F. M. O. B. , Lisenko, K. G. , Reis, J. S. , & Kessler, A. M. (2017). Endogenous fat loss and true total tract digestibility of poultry fat in adult dogs. Journal of Animal Science, 95(7), 2928–2935. 10.2527/jas2017.1393 [DOI] [PubMed] [Google Scholar]
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63. 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) . (2008). Safety of dietary supplements for horses, dogs, and cats. In Safety of dietary supplements for horses, dogs, and cats. National Academies Press. 10.17226/12461 [DOI] [Google Scholar]
- Nemec, A. , Drobnič‐Košorok, M. , Skitek, M. , Pavlica, Z. , Galac, S. , & Butinar, J. (2000). Total àntioxidant capacity (TAC) values and their correlation with individual antioxidants in serum of healthy beagles. Acta Veterinaria Brno, 69(4), 297–303. 10.2754/avb200069040297 [DOI] [Google Scholar]
- Nutrient Requirements of Dogs and Cats . (2006). In Nutrient requirements of dogs and cats. National Academies Press. 10.17226/10668 [DOI] [Google Scholar]
- Ortega, M. T. , Jeffery, B. , Riviere, J. E. , & Monteiro‐Riviere, N. A. (2016). Toxicological effects of pet food ingredients on canine bone marrow‐derived mesenchymal stem cells and enterocyte‐like cells. Journal of Applied Toxicology, 36(2), 189–198. 10.1002/jat.3158 [DOI] [PubMed] [Google Scholar]
- Pacheco, G. F. E. , Bortolin, R. C. , Chaves, P. R. , Moreira, J. C. F. , Kessler, A. M. , & Trevizan, L. (2018). Effects of the consumption of polyunsaturated fatty acids on the oxidative status of adult dogs. Journal of Animal Science, 96(11), 4590–4598. 10.1093/jas/sky313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini, A. , Luchetti, E. , Marchetti, V. , Cardini, G. , & Iorio, E. L. (2008). Analytical performances of d‐ROMs test and BAP test in canine plasma. Definition of the normal range in healthy Labrador dogs. Veterinary Research Communications, 32(2), 137–143. 10.1007/s11259-007-9014-x [DOI] [PubMed] [Google Scholar]
- Passantino, A. , Quartarone, V. , Pediliggeri, M. C. , Rizzo, M. , & Piccione, G. (2014). Possible application of oxidative stress parameters for the evaluation of animal welfare in sheltered dogs subjected to different environmental and health conditions. Journal of Veterinary Behavior: Clinical Applications and Research, 9(6), 290–294. 10.1016/j.jveb.2014.06.009 [DOI] [Google Scholar]
- Peddireddy, V. , Siva Prasad, B. , Gundimeda, S. D. , Penagaluru, P. R. , & Mundluru, H. P. (2012). Assessment of 8‐oxo‐7, 8‐dihydro‐2′ ‐deoxyguanosine and malondialdehyde levels as oxidative stress markers and antioxidant status in non‐small cell lung cancer. Biomarkers, 17(3), 261–268. 10.3109/1354750X.2012.664169 [DOI] [PubMed] [Google Scholar]
- Pellegrini, N. , Serafini, M. , Colombi, B. , Del Rio, D. , Salvatore, S. , Bianchi, M. , & Brighenti, F. (2003). Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. Journal of Nutrition, 133(9), 2812–2819. 10.1093/jn/133.9.2812 [DOI] [PubMed] [Google Scholar]
- Pompella, A. , Sies, H. , Wacker, R. , Brouns, F. , Grune, T. , Biesalski, H. K. , & Frank, J. (2014). The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition, 30(7–8), 791–793. 10.1016/j.nut.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Prior, R. L. (2015). Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. Journal of Functional Foods, 18, 797–810. 10.1016/j.jff.2014.12.018 [DOI] [Google Scholar]
- Prior, R. L. , Wu, X. , & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53(10), 4290–4302. 10.1021/jf0502698 [DOI] [PubMed] [Google Scholar]
- Restrepo, B. G. , Usuga, S. A. , Gomez, L. M. , Mesa Pineda, C. , Restrepo Rojas, O. , Duque, J. C. , & Rojano, B. A. (2019). Relación entre la composición, la capacidad antioxidante y el contenido fenólico de alimentos concentrados para perros. Revista de Investigaciones Veterinarias Del Perú, 30(3), 1083–1091. 10.15381/rivep.v30i3.16721 [DOI] [Google Scholar]
- Rubio, C. P. , Hernández‐Ruiz, J. , Martinez‐Subiela, S. , Tvarijonaviciute, A. , Arnao, M. B. , & Ceron, J. J. (2016). Validation of three automated assays for total antioxidant capacity determination in canine serum samples. Journal of Veterinary Diagnostic Investigation, 28(6), 693–698. 10.1177/1040638716664939 [DOI] [PubMed] [Google Scholar]
- Rubio, C. P. , Hernández‐Ruiz, J. , Martinez‐Subiela, S. , Tvarijonaviciute, A. , & Ceron, J. J. (2016a). Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Veterinary Research, 12(1), 166. 10.1186/s12917-016-0792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoler, N. , Harrus, S. , Martinez‐Subiela, S. , Tvarijonaviciute, A. , van Straten, M. , Cerón, J. J. , & Baneth, G. (2015). Comparison of the acute phase protein and antioxidant responses in dogs vaccinated against canine monocytic ehrlichiosis and naive‐challenged dogs. Parasites & Vectors, 8(1), 175. 10.1186/s13071-015-0798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagols, E. , & Priymenko, N. (2011). Oxidative stress in dog with heart failure: The role of dietary fatty acids and antioxidants. Veterinary Medicine International, 2011, 180206. 10.4061/2011/180206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Pérez, Y. , Carrasco‐Legleu, C. , García‐Cuellar, C. , Pérez‐Carreón, J. , Hernández‐García, S. , Salcido‐Neyoy, M. , Alemán‐Lazarini, L. , & Villa‐Treviño, S. (2005). Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Letters, 217(1), 25–32. 10.1016/j.canlet.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Schaich, K. M. , Tian, X. , & Xie, J. (2015). Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. Journal of Functional Foods, 14, 111–125. 10.1016/J.JFF.2015.01.043 [DOI] [Google Scholar]
- Sechi, S. , Fiore, F. , Chiavolelli, F. , Dimauro, C. , Nudda, A. , & Cocco, R. (2017). Oxidative stress and food supplementation with antioxidants in therapy dogs. Canadian Journal of Veterinary Research = Revue Canadienne de Recherche Veterinaire, 81(3), 206–216. [PMC free article] [PubMed] [Google Scholar]
- Sharifi, N. (2009). Commentary: Antioxidants for cancer: New tricks for an old dog? The Oncologist, 14(3), 213–215. 10.1634/theoncologist.2008-0219 [DOI] [PubMed] [Google Scholar]
- Silva, M. , & Lidon, F. (2016). Food preservatives – An overview on applications and side effects. Emirates Journal of Food and Agriculture, 28(6), 366. 10.9755/ejfa.2016-04-351 [DOI] [Google Scholar]
- Singh, S. D. , Sheik Abdul, N. , Phulukdaree, A. , Tiloke, C. , Nagiah, S. , Baijnath, S. , & Chuturgoon, A. A. (2018). Toxicity assessment of mycotoxins extracted from contaminated commercial dog pelleted feed on canine blood mononuclear cells. Food and Chemical Toxicology, 114, 112–118. 10.1016/j.fct.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Singleton, V. L. , & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic‐phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158. [Google Scholar]
- Todorova, I. , Simeonova, G. , Kyuchukova, D. , Dinev, D. , & Gadjeva, V. (2005). Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comparative Clinical Pathology, 13(4), 190–194. 10.1007/s00580-005-0547-5 [DOI] [Google Scholar]
- Valko, M. , Rhodes, C. J. J. , Moncol, J. , Izakovic, M. , & Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress‐induced cancer. Chemico‐Biological Interactions, 160(1), 1–40. 10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Vieira, S. A. , Zhang, G. , & Decker, E. A. (2017). Biological implications of lipid oxidation products. JAOCS, Journal of the American Oil Chemists’ Society, 94(3), 339–351. 10.1007/s11746-017-2958-2 [DOI] [Google Scholar]
- Villanueva, C. , & Kross, R. D. (2012a). Antioxidant‐induced stress. International Journal of Molecular Sciences, 13(2), 2091–2109. 10.3390/ijms13022091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva, C. , & Kross, R. D. (2012b). Antioxidant‐induced stress. International Journal of Molecular Sciences, 13(2), 2091–2109. 10.3390/ijms13022091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, J. L. , Barber, L. G. , Freeman, L. , Griessmayr, P. C. , Milbury, P. E. , & Blumberg, J. B. (2009). Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. Journal of Veterinary Internal Medicine, 23(2), 311–316. 10.1111/j.1939-1676.2009.0273.x [DOI] [PubMed] [Google Scholar]
- Zapata, S. , Piedrahita, A. , & Rojano, B. (2014). Capacidad atrapadora de radicales oxígeno (ORAC) y fenoles totales de frutas y hortalizas de Colombia. Perspectivas En Nutrición Humana, 16(1), 25–36. [Google Scholar]
- Zhang, L. W. , Koci, J. , Jeffery, B. , Riviere, J. E. , & Monteiro‐Riviere, N. A. (2015). Safety assessment of potential food ingredients in canine hepatocytes. Food and Chemical Toxicology, 78, 105–115. 10.1016/j.fct.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Saleh, A. S. M. , Chen, J. , & Shen, Q. (2012). Chemical alterations taken place during deep‐fat frying based on certain reaction products: A review. Chemistry and Physics of Lipids, 165(6), 662–681. 10.1016/j.chemphyslip.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Zicker, S. C. (2005). Cognitive and behavioral assessment in dogs and pet food market applications. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 29(3), 455–459. 10.1016/j.pnpbp.2004.12.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


