Abstract
Background
Avian coccidiosis is thought to be one of the most expensive infectious diseases in the poultry industry.
Objectives
Safe and alternative anti‐coccidial drugs are herbal extracts because they do not result in tissue residue and drug resistance. The objective of the present study was to evaluate the anti‐coccidial effect of the herbal mixture, a complex of two plants (Echinacea purpurea, Glycyrrhiza glabra) in broiler chickens in comparison with toltrazuril.
Methods
One hundred twenty broiler chickens were used in this experiment and divided into 4 equally numbered groups. All the groups, except Group D, were experimentally infected with mixed Eimeria spp. (E. Tenella, E. maxima, E. necatrix and E. brunetti) on day 14. Group A treated with an herbal mixture [Glycyrrhiza glabra Extract 5% (standardised to 5.4% glycyrrhizic acid) and Echinacea purpurea Extract 2% (standardised to 4% total phenolic content based on chlorogenic acid); Coxinin‐EC®; Shamim Teb Sepid Giti]. Group B treated with toltrazuril. Group C was experimentally infected with mixed Eimeria spp. but they did not have any treatment, this group was our positive control. Performance indices, faecal oocyst excretion, and intestinal lesion score were determined during the experiment.
Results
Positive control group had the poorest results and more mortality than other groups. Group D was not infected and was healthy all the experiment period. Treatment with herbal complex significantly reduced the negative performance and pathogenic effects associated with Eimeria spp. at a level that was comparable with toltrazuril.
Conclusions
In summary, the anti‐coccidial activity of the studied herbal complex suggests its use as an alternative anti‐coccidial agent to chemotherapeutic drugs for controlling coccidiosis in poultry.
Highlights
-
‐
Coccidiosis is an important infectious disease that causes serious financial loss to the poultry industry.
-
‐
Chemical anti‐coccidial drugs and vaccines are the main control strategies to combat the disease. However, these tools have some constraints.
-
‐
Herbal remedies are suitable alternatives to chemical compounds for control of losses associated with coccidiosis in poultry.
-
‐
An herbal mixture (Echinacea purpurea, Glycyrrhiza glabra) has promising effects for controlling of coccidiosis in broiler chickens.
Keywords: chicken, coccidiosis control, herbal medicine
Herbal remedies are suitable alternatives to chemical compounds for control of losses associated with coccidiosis in poultry. An herbal mixture (Echinacea purpurea, Glycyrrhiza glabra) has promising effects for controlling of coccidiosis in broiler chickens.
1. INTRODUCTION
Avian coccidiosis is a remarkable enteric disease in poultries with the ability to inflict a large economic impact on farm profitability (Kostadinović et al., 2019). It is known for its extraordinary effects and huge damage to poultry industries among the parasitic diseases around the world (Blake et al., 2020). The economic loss to the poultry enterprise due to coccidiosis is considerable, which is specifically because of prophylactic or healing in‐feed medications and additionally due to the impact of the ailment on birds’ health (Kostadinović & Lević, 2018; Puvača et al., 2019). The global cost of the pathogen to the poultry industry is estimated to be over US$2.4 billion per annum (Shirley & Chapman, 2005). There are seven species of avian coccidian. Eimeria species multiply inside the bird's intestinal tract, inflicting considerable tissue damage. The harm caused to tissue can interfere with nutrient absorption, feeding and digestion (Chapman, 2009). This can lead to blood loss, poor skin pigmentation, dehydration and escalated vulnerability to other diseases including necrotic enteritis (Shirley & Millard, 1986). Control of coccidiosis is substantially established by using chemotherapeutic agents. During the fight against coccidiosis, to avert and control the disease, the poultry industry relied heavily on anti‐coccidial drugs. The intensive use of anti‐coccidial drugs has led to the development of resistance (Abbas et al., 2011; Noack et al., 2019). So far, chemotherapeutic drugs and anti‐coccidial feed additives have managed coccidiosis but have been complicated by the emergence of drug resistance and their toxic effects on animal health (Peek & Landman, 2011). Furthermore, drug residues in poultry meat and other products are a potential constraint to the consumer (Sundar et al., 2017). Also, some achievement is done with vaccines for controlling avian coccidiosis, especially in broiler breeders (Tewari & Maharana, 2011). But, proscribing issue for using vaccines in opposition to coccidia is that the inclusion of numerous species of Eimeria in one vaccine can purpose similarly despair in weight benefit and feed conversion (Alfaro et al., 2007). Therefore, alternative tactics are being required for more effective and safer control of coccidiosis in chickens in both developed and underdeveloped countries (Crespy & Williamson, 2004). Among alternative methods, herbal compounds are the most potential candidates for the control of avian coccidiosis (Nidaullah, 2010). Based on the directions provided by world health organisations (WHO), FDA and EMEA for veterinary medicine in any possible case, synthetic drugs should be replaced with plant‐based materials to lower the amount of synthetic drugs and their metabolites (residues) in the animal products. Due to their influential properties and complex bioactivity, medicinal plant supplements and their essential oils can be considered the best alternatives to synthetic drugs (Arafat & Abbas, 2018). During the last decade the consumption of medicinal plant supplements and their other products has expanded according to their antioxidation (Elmahallawy et al., 2021), hypocholesterolemic (Yong et al., 2020) and antibacterial activity (Li et al., 2018). Moreover, a correlation between some of the components and diverse stimulatory effects on the digestive system and digestive enzyme production has been detected (Vinus et al., 2018). Medicinal plant components also have been proved to exhibit anti‐parasitic (Gohel et al., 2019), antiviral (Alagbe et al., 2018), antioxygenic, anti‐mycotic (Abed et al., 2021) and insecticidal effects.
In this study, a comparative model was designed to assess the effectiveness of herbal medicine based on two plant extracts (Glycyrrhiza glabra, and Echinacea purpurea) for the treatment of coccidiosis in broilers. The goal of the current study was to evaluate the effect of a commercial multi‐plant extract compound, in experimental coccidiosis in broiler chickens.
2. MATERIALS AND METHODS
2.1. Birds, housing
In total, 120 one‐day‐old Ross 308 broiler chicks were purchased from a local hatchery. The chicks were kept on a slotted floor in a specific place for raising birds. The birds were fostered under standard environmental conditions, following the breeding standards of the Ross breed. The temperature was adjusted on 33°C on the first days of chick arrival, which was held until the end of the first week. Then the temperature was gradually reduced to 21°C on day 22 and held at this level by the end of the period. During the experiment water, and food were provided as much as desired and there were no anti‐coccidial or coccidiostats drugs in the food. Gohar Daneh Shargh Corporation provided a standard commercial diet for feeding the birds. At the end of the second week, the birds were transferred from the litter to the cages, and birds were grouped randomly. The birds were divided into 4 groups of 30 and 3 replicates of 10 within each group. Group A takes an herbal mixture [Glycyrrhiza glabra Extract 5%) standardised to 5.4% glycyrrhizic acid) and Echinacea purpurea Extract 2% (standardised to 4% total phenolic content based on chlorogenic acid); Coxinin‐EC®; Shamim Teb Sepid Giti] at a dose of 1 cc in 1 L of water for 4 days. The second group (Group B) receives toltrazuril 2.5% (Behroodatrak Co.) with the dose of 1 cc/L for 4 days. In the third group (Group C), birds become infected with Eimeria oocysts but do not receive treatment; this is positive control group. The fourth group (Group D) is the one that faces no challenge and needs no treatment. All procedures in this study including birds and their care were approved by institutional Ethics Committee.
2.2. Weighing birds, measuring feed consumption and calculating feed conversion ratio
The birds were weighed collectively until the fourteenth day. But at the end of the second week, the birds were grouped, and the weighing procedure was performed for groups, and then the average weight of each group was calculated. The weighing of the birds continued until day 42 of breeding (end of the experiment).
By feeding oocysts and starting the challenge in birds, the amount of daily feed consumed in each replicate of the groups was recorded separately.
To calculate the feed conversion ratio by calculating the amount of feed consumed and also the increase in bird weight during the week, the feed conversion ratio was calculated.
2.3. Challenge birds using Eimeria oocysts
A mixture of sporulated Eimeria oocysts was purchased from a local supplier. From the prepared mixture, a direct slide sample was taken and under the microscope, the diversity of Eimeria sporulated oocyst species was observed. Species of this mixture included 50% of Eimeria tenella, 25% of Eimeria maxima and the remaining 25% included other species such as Eimeria acervulina, Eimeria mitis and Eimeria necatrix. To challenge the birds, about 250,000 mixture oocysts of Eimeria spp. were fed to each bird through an oral gavage on day 14 of rearing. The estimated challenge dose was based on the infective inoculums that cause obvious lesions and negligible mortality in the birds.
2.4. Registration of clinical symptoms and investigation of possible casualties
During the experimental period, birds in all groups underwent daily care for clinical signs, especially those related to coccidiosis, and the symptoms were recorded. Symptoms include decreased daily feed intake, lethargy and somnolence of the birds, and symptoms of dysentery. In addition, in cases where casualties were observed in the groups, the carcass was necropsied as soon as possible and the cause of death and injury were recorded.
2.5. Treatment using drugs
Treatment was started by observing symptoms in birds that included lethargy, reduced feed intake, dysentery and death. In the present experiment, on the fourth day after feeding oocysts, specific symptoms of the disease were observed in Groups A, B and C, then treatment was started. The medicines – to the extent mentioned in the grouping section of the birds – were provided to the birds of each group on a daily basis. The length of the treatment period is based on the manufacturer's recommendation.
2.6. Calculation of output per gram (OPG) in different groups
In order to reach this goal, according to the standard method and by using the McMaster counting slide, the number of oocysts per gram of faeces was counted (Haug et al., 2006). On this matter, 3 g of faeces from birds of each group were mixed with 42 ccs of water and shaken vigorously to obtain a uniform mixture. Then 15 cc of this mixture is centrifuged at a speed of 2000 rpm for 10 min and in the next step, the supernatant is discarded while water and saturated salt are added to the formed sediment to bring the volume to 15 cc. In the next step, we pour some part of it on the cells of the McMaster slide, and by placing the slide on a flat surface for 5 min, the oocysts have a chance to float. The oocysts are counted with a 10× magnification of the compound microscope and the average obtained from the two cells of the McMaster slide is expressed as OPG in that sample.
During the trial period, birds from each group were sampled and faecal oocysts were counted on four different days: the first time on the fifth day after the challenge; the second time on the seventh day after the challenge; the third time on the ninth day after the challenge; the fourth time is the twelfth day after the challenge. The increasing and decreasing trend in the number of excreted oocysts in the faeces of birds were recorded.
2.7. Lesion scoring
The descriptive method of Johnson and Reid (1970) was used on this matter. In this method, based on the lesions observed in different parts of the intestine, a score of 0 (healthy) to +4 (the most severe lesions) is considered. It should be noted that for scoring, four areas of the intestine, namely the beginning of the intestine including the duodenum, the middle part of the intestine including the jejunum and ileum, the end of the intestine including the colon and finally, the cecum were examined. At the end of treatment, two birds from each replicate were slaughtered humanely and lesions of different parts of the intestine were recorded, and then the average lesions in the carcasses were obtained.
2.8. Statistical analysis
The data on body weight, FCR and OPG parameters were analysed by ANOVA coupled with post hoc tests (SPSS® Software version 16). The discrepancy was considered statistically significant at the p value < 0.05 for all the analysed data.
3. RESULT
3.1. Weekly weight gains of birds
The average weight of birds in different groups during the breeding period (42 days) is given in Table 1.
TABLE 1.
Mean ± SEM weight of birds in four groups of study during the 42 days
| Group | First day | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 |
|---|---|---|---|---|---|---|---|
| Herbal mixture (Group A) | 48 ± 0.9 | 171 ± 6 | 408 ± 16 | 645 ± 50a | 867 ± 42a | 1430 ± 46a | 1910 ± 124a |
| Toltrazuril (Group B) | 48 ± 0.9 | 171 ± 6 | 408 ± 16 | 650 ± 50a | 865 ± 34a | 1310 ± 77a | 1833 ± 82a |
| Challenged and not treated (Group C) | 48 ± 0.9 | 171 ± 6 | 408 ± 16 | 550 ± 33a | 751 ± 42a | 1259 ± 44a | 1655 ± 118a |
|
No challenge No treatment (Group D) |
48 ± 0.9 | 171 ± 6 | 408 ± 16 | 670 ± 50a | 955 ± 82a | 1431 ± 103a | 1881 ± 151a |
Note: Means denoted by different superscript letters show significant differences between groups in each column (p < 0.05).
Accordingly, the most noticeable weight gains were seen in the groups that did not have challenges by Eimeria oocysts, Group D, and consistently had the most remarkable average weight during the period. The least weight gain had a place with the Group C which was not treated during the challenge. The weight contrast between the other treated groups is not noticeable. The important thing to mention about coccidiosis is that this disease will lead to a decrease in weight gain in meat herds, which causes significant economic damage. In the present experiment, Group D gained weight more than the other groups, so the control and prevention of coccidiosis can reduce the damage caused by this disease.
3.2. Feed conversion ratio
The results of the feed conversion ratio (weekly) in different groups from the beginning of the period to the end of the experiment period are given in Table 2. As can be seen in Table 2, the best results were seen during the rearing period – except on day 42 – in Group D. The best feed conversion ratio on day 42, was observed in the group that received an herbal mixture. It is vital about the adverse effect of coccidiosis on feed conversion ratio, which is very clear in the table above on day 21 and prompted a huge contrast in the feed conversion ratio of Group D with other study groups. Yet, with the use of medication in Groups A and B, the condition of birds has also improved and does not show a distinction from the unchallenged group.
TABLE 2.
Weekly FCR values of birds of four groups during the study
| Group | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 |
|---|---|---|---|---|---|---|
| Herbal mixture (Group A) | 2.71 | 2.62 | 3.1 ± 0.1a | 2.81 ± 0.05a | 1.67 ± 0.03a | 1.75 ± 0.13a |
| Toltrazuril (Group B) | 2.71 | 2.62 | 3.5 ± 0.05b | 3.48 ± 0.38b | 2.31 ± 0.11b | 1.98 ± 0.09a |
| Challenged and not treated (Group C) | 2.71 | 2.62 | 5.4 ± 0.13c | 5.43 ± 0.22c | 2.75 ± 0.13c | 2.42 ± 0.19b |
|
No challenge No treatment (Group D) |
2.71 | 2.62 | 2.3 ± 0.15d | 2.39 ± 0.2d | 1.4 ± 0.1d | 1.8 ± 0.15a |
Note: Means denoted by different superscript letters show significant differences between groups in each column (p < 0.05).
3.3. Registration of clinical signs and possible casualties
The birds were perfectly healthy on the day of feeding the oocysts and had no particular clinical problems. Stool consistency was also normal. Oocysts were fed on day 14 in Groups A, B and C. Two days after the challenge, diarrhoea was seen in the birds. Yet, the birds were clinically and appetizingly normal. On the fourth day after the challenge, chocolate‐coloured dysentery and diarrhoea were seen in various groups and lethargy and anorexia were seen in birds. The decrease in food consumption was also clear in the groups. Four days after the challenge and after observing signs, the treatment began in Groups A and B. Three days after taking the medication, the stool status got back to normal, and the overall state of the birds was normal.
In the group receiving herbal mixture (Group A), only one death was observed before treatment. In Group B, three deaths were observed, one related to before starting treatment and the other two related to one day after starting treatment. In Group C, two deaths occurred on the second and third days of treatment. No casualties were observed in Group D. Necropsies were performed on all carcasses and specific coccidiosis lesions were observed in all carcasses. The most obvious finding was related to the cecum, where a severe lesion of +4 was observed.
3.4. Results of scoring lesions
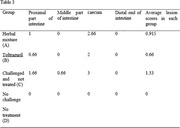
The best time to score lesions in coccidiosis is days 5–7 after infection. Based on lesions observed in different parts of the intestine. The average scores in each part are given in Table 3.
TABLE 3.
Lesion scoring of the intestine in different groups
| Group | Proximal part of intestine | Middle part of intestine | caecum | Distal end of intestine | Average lesion scores in each group |
|---|---|---|---|---|---|
| Herbal mixture (Group A) | 1 | 0 | 2.66 | 0 | 0.915 |
| Toltrazuril (Group B) | 0.66 | 0 | 2 | 0 | 0.66 |
| Challenged and not treated (Group C) | 1.66 | 0.66 | 3 | 0 | 1.33 |
|
No challenge No treatment (Group D) |
0 | 0 | 0 | 0 | 0 |
The results showed that in the Group D, no lesion was observed in the intestines. The score of Group D was zero or equivalent to a healthy gut. Most injuries were observed in Group C, (a score of 1.33), which is quite expected. It is notable that the intestinal lesions score of Group A, compared to Group B, decreased and showed acceptable performance of an herbal mixture.
3.5. Counting the number of oocysts per gram of faeces (OPG)
The results of counting excreted oocysts from birds in each group in four sampling stages (days 5, 7, 9 and 12 after the challenge) in different groups are presented in Table 4. The results of counting the number of oocysts per gram of faeces show that the herbal mixture has established a good performance and has reduced the number of oocysts per gram of faeces. In the case of Toltrazuril, the results of drug use have been satisfactory and the number of oocysts excreted from birds has decreased with drug use. The point to consider in the results is that with the use of drugs (herbal mixture and toltrazuril), a sharp decrease in faecal oocysts is observed on day 9 after the challenge. But in the untreated group, a sharp decrease in excreted oocysts was observed on day 12 after the challenge.
TABLE 4.
Mean ± SEM OPG values of different groups at 5, 7, 9 and 12 days post‐challenge
| Group | Day 5 | Day 7 | Day 9 | Day 12 |
|---|---|---|---|---|
| Herbal mixture (Group A) | 72,000 ± 1000a | 180,000 ± 3000a | 84,000 ± 2000a | 70,000 ± 2000a |
| Toltrazuril (Group B) | 45,000 ± 2000b | 210,000 ± 3000b | 89,000 ± 2000b | 50,000 ± 1000b |
| Challenged and not treated (Group C) | 32,000 ± 500c | 150,000 ± 3000c | 132,500 ± 3000c | 32,700 ± 700c |
|
No challenge No treatment (Group D) |
0d | 0d | 0d | 0d |
Note: Means denoted by different superscript letters show significant differences between groups in each column (p < 0.05).
4. DISCUSSION
Broiler herds infected with coccidiosis experience an important reduction in weight gain that will lead to huge economic losses. Coccidiosis is an infectious disease that damages intestinal epithelial cells and results in dreadful haematochezia (Chand et al., 2016; Kadykalo et al., 2018). Prolonged use of anti‐coccidial chemicals promotes drug resistance and results in tissue residues in chickens; therefore, a healthy anti‐coccidial treatment based on herbs is essential (Nahed et al., 2022; Naidoo et al., 2008). Based on the observation of this study, birds in the non‐challenged Group D had a difference in weight gain in comparison to birds of other groups, hence the importance of control and prevention in management and reduction of the damages of the coccidiosis can be concluded (Scheurer et al., 2013). Our experiment was held in highly hygienic conditions. Environmental factors such as density and stress status of the herds are essential in detecting performance responses to plant extracts used as treatment. Continuing failures of vaccines, broad emergence of anti‐coccidial drug resistance, and residual toxic effects of drugs are the reasons behind the efforts on finding alternative methods to control coccidiosis. On this matter, various botanicals have been proved to be beneficial for their use as anti‐coccidial agents or immunomodulatory.
Common clinical signs were observed during the present study such as anorexia, paleness, ruffled feathers, depression and huddling together that complied with the observation provided in the studies by Dubey (2019) and Tanweer et al. (2014). Based on the results of this experiment, Group D ingests more feed in comparison to other groups. The result of the current experiment is backed by Hashmi et al. (1994) and Tipu et al. (2002) who mentioned that coccidiosis infection leads to a reduction in feed intake (Hayat et al., 1991). Notable weight loss and reduced FCR are the results of all Eimeria isolates infection (Logan et al., 1993). The FCR reduction happens because of the process in which the organism damages the absorptive mucosal surface and contests for micronutrients. The outcome is metabolic disturbance and therefore unfavourably impacts nutrient utilisation (Ali et al., 2019). All seven Eimeria species evolving in the chick's digestive tract in a specific site can induce a wide range of symptoms from mild subclinical enteric infection to subacute high mortality. The clinical conclusion of coccidial infection can be affected by Eimeria species, strains, stress, environmental factors, host genetics, simultaneous infections, infective dose and flock size (Nahed et al., 2022; Taylor et al., 2022). It is believed that weight gain is the more sensitive variable to coccidiosis and anti‐coccidial treatments (Gerhold et al., 2016). When infective sporozoites arrive at the cecum mucosa by penetrating villus epithelial cells, this results in widespread damage to the cecum epithelia, a bloody stool and a huge number of oocyst excretion (Dutta et al., 1990; Kawazoe & Di Fabio, 1994). In this study, infected broilers were very depressed, revealing ruffled feathers and a lesser feed intake, which may have been due to altered gut homeostasis that led to poor feed intake and metabolism and thus reduced weight gains (Abbas et al., 2013). Interestingly, FCR and body weight at day 42 in Group A were better than in other groups that would be linked to growth‐promoting properties of the herbal complex. The lower OPG recorded in the infected group given the herbal extracts (Group A) was comparable with that obtained in the group being administered the toltrazuril (Group B) and was probably the effect of the phenolic compounds in the herbal extracts. Phenols can interact with cytoplasmic membranes and change their cation permeability, leading to impairment of crucial processes in the coccidia cells and, finally, their death (Arczewska‐Wlosek & Swiatkiewicz, 2012; Sikkema et al., 1995). Our findings showed a significant reduction in oocysts output in the herbal‐treated group (Group A) in comparison with the toltrazuril‐treated group (Group B) and the result of the current experiment is backed by Qaid et al. (2021). Birds in Groups A and B had higher OPG levels compared with Group C. This would be linked to the over‐multiplication of the uninhibited Eimeria species (Pop et al., 2019). Birds in groups A and B had better growth performance than group C, despite the higher OPG levels. This may be linked to the effect of diminished injury to intestinal epithelial cells or stimulation of enterocyte renewal, which can provide the substrate for Eimeria species multiplication (Pop et al., 2019). Based on the investigation of Arczewska‐Wlosek and Swiatkiewicz (2012), the usage of an herbal extract mixture comprising Echinacea purpurea, Thymus vulgaris, Allium sativum, Origanum vulgare and Salvia officinalisi to some extents reduces the negative effects of Eimeria experimental infections (E. acervulina, E. tenella, E. maxima and E. necatrix) in broiler chickens. In a recent study by Abbas et al. (2010) and Conway et al. (2002), broiler chickens, which experimentally infected with Eimeria, showed more cecal lesion score and lower weight gain and higher oocyst counts as compared to the non‐medicated group, which exactly support our findings (Abbas et al., 2010; Conway et al., 2002). All species of Echinacea are herbaceous, perennial plants of the family Asteraceae. Echinacea is well‐thought‐out one of the top 10 species of herbs known for their disease‐resistance activities (Miller & Yu, 2004). Echinacea consists of nine spp., three of which (Echinacea purpurea, Echinacea pallida, and Echinacea angustifolia) have been used medicinally all over the world. Echinacea is used for the treatment of upper respiratory tract infections in humans, helping to decrease the duration of the cold symptoms (Caruso & Gwaltney, 2005). It also has many other pharmacological potentials, including wound‐healing and anti‐inflammatory actions (Speroni et al., 2002). Based on the findings of another study, the extracted juice of Echinacea purpurea enhanced humoral and cellular immunity in mice by increasing the number of peripheral lymphocytes and monocytes. Moreover, it can also boost the immune system by increasing total immunoglobulins (IgM, IgG) levels (Mishima et al., 2004). Positive effects of this herbal mixture including Echinacea purpurea in the treatment of coccidiosis in broilers are also recorded in this study. The Echinacea purpurea’s anti‐coccidial effect has been referred to as its immunomodulating properties, broadly reported (Arczewska‐Włosek et al., 2018; Fabia et al., 2021). E. purpurea extract triggers the innate immune response by activating macrophages (Goel et al., 2002), macrophage‐derived cytokine creation and activation of natural killer cells and polymorphonuclear leukocytes, therefore, acting as an immunostimulant (Currier & Miller, 2001; Srinivasu et al., 2020). Glycyrrhiza glabra extract has bioactive components, such as glycyrrhizin and flavonoids, which have medicinal and pharmacological activities (Pop et al., 2019). The Glycyrrhiza polysaccharide is known to have a solid immune action and extensive involvement in some processes of the immune system. Significantly, the usage of licorice in broiler diets developed the immune organs such as the bursa or, spleen, and as a result of that immune efficacy, livability and health status improve. These herb extracts have been found to show antioxidant and immunogenic potentials, which might increase the feed efficiency, blood biochemical indices of the poultry birds, growth performance and carcass traits and act as a potential solution for solving respiratory, immune and digestive problems in poultries (Alagawany et al., 2019; Hussain et al., 2017).
It is concluded from the present study that the herbal drug containing Glycyrrhiza glabra and Echinacea purpurea show improved performance and anti‐coccidiosis effects in the broilers challenged by Eimeria spp. More research is recommended to ascertain the effectiveness of this drug for controlling coccidiosis in layers, breeders, and turkeys. Furthermore, extraction of active ingredients in these herbs for development of newer drugs may lead to better anti‐coccidial efficacy.
AUTHOR CONTRIBUTION
Conceptualisation: Ali Ghafouri. Methodology: Ali Ghafouri, Abolfazl Ghaniei and Soheil Sadr. Formal analysis and investigation: Abolfazl Ghaniei and Soheil Sadr. Writing – original draft preparation: Soheil Sadr, Amir Ebrahim Tavanaee Tamannaei, Ali Charbgoo and Shakila Ghiassi. Writing – review and editing: Abolfazl Ghaniei and Soheil Sadr. Resources: Morteza Abuali. Supervision: Abolfazl Ghaniei.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING INFORMATION
This study was financially supported by research deputy of Ferdowsi University of Mashhad (no. 102181).
ETHICAL APPROVAL
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.971
ACKNOWLEDGEMENT
We would like to thank the research deputy of the Ferdowsi University of Mashhad for supporting the research process.
Ghafouri, S. A. , Ghaniei, A. , Tamannaei, A. E. T. , Sadr, S. , Charbgoo, A. , Ghiassi, S. , & Abuali, M. (2023). Evaluation of therapeutic effects of an herbal mixture (Echinacea purpurea and Glycyrrhiza glabra) for treatment of clinical coccidiosis in broilers. Veterinary Medicine and Science, 9, 829–836. 10.1002/vms3.971
Amir Ebrahim Tavanaee Tamannaei, Soheil Sadr and Ali Charbgoo contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abbas, R. , Iqbal, Z. , Blake, D. , Khan, M. , & Saleemi, M. (2011). Anticoccidial drug resistance in fowl coccidia: The state of play revisited. World's Poultry Science Journal, 67(2), 337–350. [Google Scholar]
- Abbas, R. , Iqbal, Z. , Mansoor, M. , Sindhu, Z. , Zia, M. , & Khan, J. (2013). Role of natural antioxidants for the control of coccidiosis in poultry. The Pakistan Veterinary Journal, 33, 401–407. [Google Scholar]
- Abbas, R. Z. , Iqbal, Z. , Khan, M. N. , Zafar, M. A. , & Zia, M. A. (2010). Anticoccidial activity of Curcuma longa L. in broilers. Brazilian Archives of Biology and Technology, 53, 63–67. [Google Scholar]
- Abed, A. , Radwan, I. , El‐Aziz, M. , & Ali, A. (2021). Antifungal activity of natural essential oils against molds and yeasts associated with respiratory problems in broiler chickens. Advances in Animal and Veterinary Sciences, 9(3), 348–355. [Google Scholar]
- Alagawany, M. , Elnesr, S. S. , Farag, M. R. , El‐Hack, A. , Mohamed, E. , Khafaga, A. F. , Taha, A. E. , Tiwari, R. , Yatoo, M. , & Bhatt, P. (2019). Use of licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: Current knowledge and prospects. Animals, 9(8), 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagbe, J. , Alagbe, J. , & Tijani, T. (2018). Effects of dried Centella asiatica leaf meal as an herbal feed additive on the growth performance, haematology and serum biochemistry of broiler chicken. Pacific International Journal, 1(4), 172–180. [Google Scholar]
- Alfaro, D. , Silva, A. , Borges, S. , Maiorka, F. , Vargas, S. , & Santin, E. (2007). Use of Yucca schidigera extract in broiler diets and its effects on performance results obtained with different coccidiosis control methods. Journal of Applied Poultry Research, 16(2), 248–254. [Google Scholar]
- Ali, M. , Chand, N. , Khan, R. U. , Naz, S. , & Gul, S. (2019). Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. Journal of Applied Animal Research, 47(1), 79–84. [Google Scholar]
- Arafat, N. , & Abbas, I. (2018). Coccidia of Japanese quail: From identification, prevalence, infection, and immunization. The Journal of Parasitology, 104(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Arczewska‐Wlosek, A. , & Swiatkiewicz, S. (2012). The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. Journal of Animal and Feed Sciences, 21, 133–142. [Google Scholar]
- Arczewska‐Włosek, A. , Świątkiewicz, S. , Ognik, K. , & Józefiak, D. (2018). Effect of dietary crude protein level and supplemental herbal extract blend on selected blood variables in broiler chickens vaccinated against coccidiosis. Animals, 8(11), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. P. , Knox, J. , Dehaeck, B. , Huntington, B. , Rathinam, T. , Ravipati, V. , Ayoade, S. , Gilbert, W. , Adebambo, A. O. , & Jatau, I. D. (2020). Re‐calculating the cost of coccidiosis in chickens. Veterinary Research, 51(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso, T. J. , & Gwaltney Jr, J. M. (2005). Treatment of the common cold with echinacea: A structured review. Clinical Infectious Diseases, 40(6), 807–810. [DOI] [PubMed] [Google Scholar]
- Chand, N. , Faheem, H. , Khan, R. U. , Qureshi, M. S. , Alhidary, I. A. , & Abudabos, A. M. (2016). Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environmental Science and Pollution Research, 3(14), 14414–14421. [DOI] [PubMed] [Google Scholar]
- Chapman, H. (2009). A landmark contribution to poultry science – Prophylactic control of coccidiosis in poultry. Poultry Science, 88(4), 813–815. [DOI] [PubMed] [Google Scholar]
- Conway, D. , Mathis, G. , & Lang, M. (2002). The use of diclazuril in extended withdrawal anticoccidial programs: 1. Efficacy against Eimeria spp. in broiler chickens in floor pens. Poultry Science, 81(3), 349–352. [DOI] [PubMed] [Google Scholar]
- Crespy, V. , & Williamson, G. (2004). A review of the health effects of green tea catechins in in vivo animal models. The Journal of Nutrition, 134(12), 3431S–3440S. [DOI] [PubMed] [Google Scholar]
- Currier, N. L. , & Miller, S. C. (2001). Echinacea purpurea and melatonin augment natural‐killer cells in leukemic mice and prolong life span. The Journal of Alternative & Complementary Medicine, 7(3), 241–251. [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. (2019). Coccidiosis in livestock, poultry, companion animals, and humans. Experimental Parasitology, 141, 134–137. [Google Scholar]
- Dutta, G. , Mohan, A. , & Tripathi, R. (1990). Study of the gametocytocidal/sporontocidal action of qinghaosu (artemisinin) by electron microscopy. The Journal of Parasitology, 76, 849–852. [PubMed] [Google Scholar]
- Elmahallawy, E. K. , Fehaid, A. , El‐Shewehy, D. M. , Ramez, A. M. , Alkhaldi, A. A. , Mady, R. , Nasr, N. E. , Arafat, N. , Hassanen, E. A. , & Alsharif, K. F. (2021). S‐Methylcysteine ameliorates the intestinal damage induced by Eimeria tenella infection via targeting oxidative stress and inflammatory modulators. Frontiers in Veterinary Science, 8, 754991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabia, K. , Wolski, D. , Bienko, M. , Radzki, R. P. , & Szymanczyk, S. (2021). The problem of coccidiosis in broiler chickens and laying hens: Selected control methods and alternative solutions. Medycyna Weterynaryjna, 77(09), 425–429. [Google Scholar]
- Gerhold, R. , Fuller, A. , & McDougald, L. (2016). Coccidiosis in the chukar partridge (Alectoris chukar): A survey of coccidiosis outbreaks and a test of anticoccidial drugs against Eimeria kofoidi . Avian Diseases, 60(4), 752–757. [DOI] [PubMed] [Google Scholar]
- Goel, V. , Chang, C. , Slama, J. V. , Barton, R. , Bauer, R. , Gahler, R. , & Basu, T. K. (2002). Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. International Immunopharmacology, 2(2‐3), 381–387. [DOI] [PubMed] [Google Scholar]
- Gohel, B. , Garg, D. , Patil, S. , Savsani, H. , Trivedi, S. , & Kadam, S. (2019). Efficacy of Ocimum sanctum (Tulsi) and Aloe vera leaves powder as phytogenic growth promoter in diet of broiler chickens. Journal of Entomology and Zoology Studies, 7(2), 379–383. [Google Scholar]
- Hashmi, H. , Issot, N. , & Maqbool, A. (1994). Experimental induction of coccidiosis in broiler chicks with Eimeria tenella and comparative efficacy of different prophylactic measures against the disease. Journal of Animal Health and Production, 14, 55–63. [Google Scholar]
- Haug, A. , Williams, R. , & Larsen, S. (2006). Counting coccidial oocysts in chicken faeces: A comparative study of a standard McMaster technique and a new rapid method. Veterinary Parasitology, 136(3‐4), 233–242. [DOI] [PubMed] [Google Scholar]
- Hayat, C. , Nabi, I. , Hayat, B. , Iqbal, Z. , & Khan, M. (1991). Comparative chemoprophylactic effect of different anticoccidials on performance of broilers. Pakistan Veterinary Journal (Pakistan), 100(6), 101162. [Google Scholar]
- Hussain, K. , Iqbal, Z. , Abbas, R. Z. , Khan, M. K. , & Kashif Saleemi, M. (2017). Immunomodulatory activity of Glycyrrhiza glabra extract against mixed Eimeria infection in chickens. International Journal of Agriculture & Biology, 19(4), 10.17957/IJAB/15.0397 [DOI] [Google Scholar]
- Johnson, J. , & Reid, W. M. (1970). Anticoccidial drugs: Lesion scoring techniques in battery and floor‐pen experiments with chickens. Experimental Parasitology, 28(1), 30–36. [DOI] [PubMed] [Google Scholar]
- Kadykalo, S. , Roberts, T. , Thompson, M. , Wilson, J. , Lang, M. , & Espeisse, O. (2018). The value of anticoccidials for sustainable global poultry production. International Journal of Antimicrobial Agents, 51(3), 304–310. [DOI] [PubMed] [Google Scholar]
- Kawazoe, U. , & Di Fabio, J. (1994). Resistance to diclazuril in field isolates of Eimeria species obtained from commercial broiler flocks in Brazil. Avian Pathology, 23(2), 305–311. [DOI] [PubMed] [Google Scholar]
- Kostadinović, L. , & Lević, J. (2018). Effects of phytoadditives in poultry and pigs diseases. Journal of Agronomy, 5. [Google Scholar]
- Kostadinović, L. , Popović, S. , Pelić, D. L. , Čabarkapa, I. , Đuragić, O. , & Lević, J. (2019). Medicinal plants as natural alternative to coccidial synthetic drugs in broiler chicken production. Journal of Agronomy, 5. [Google Scholar]
- Li, B. , Zhang, J. Q. , Han, X. G. , Wang, Z. L. , Xu, Y. Y. , & Miao, J. F. (2018). Macleaya cordata helps improve the growth‐promoting effect of chlortetracycline on broiler chickens. Journal of Zhejiang University‐Science B, 19(10), 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, N. , McKenzie, M. , Conway, D. , Chappel, L. , & Hammet, N. (1993). Anticoccidial efficacy of semduramicin.: 2. Evaluation against field isolates including comparisons with salinomycin, maduramicin, and monensin in battery tests. Poultry Science, 72(11), 2058–2063. [DOI] [PubMed] [Google Scholar]
- Miller, S. C. , & Yu, H.‐ C . (2004). Echinacea: The genus Echinacea. CRC Press. [Google Scholar]
- Mishima, S. , Saito, K. , Maruyama, H. , Inoue, M. , Yamashita, T. , Ishida, T. , & Gu, Y. (2004). Antioxidant and immuno‐enhancing effects of Echinacea purpurea . Biological and Pharmaceutical Bulletin, 27(7), 1004–1009. [DOI] [PubMed] [Google Scholar]
- Nahed, A. , Abd El‐Hack, M. E. , Albaqami, N. M. , Khafaga, A. F. , Taha, A. E. , Swelum, A. A. , El‐Saadony, M. T. , Salem, H. M. , El‐Tahan, A. M. , & AbuQamar, S. F. (2022). Phytochemical control of poultry coccidiosis: A review. Poultry Science, 101(1), 101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo, V. , McGaw, L. J. , Bisschop, S. , Duncan, N. , & Eloff, J. N. (2008). The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Veterinary Parasitology, 153(3‐4), 214–219. [DOI] [PubMed] [Google Scholar]
- Nidaullah, H. , Durrani, F. R. , Ahmad, S. , Jan, I. U. , & Gu, S. (2010). Aqueous extract from different medicinal plants as anticoccidial, growth promotive and immunostimulant in broilers. ARPN Journal of Agricultural and Biological Science, 5(1), 53–59. [Google Scholar]
- Noack, S. , Chapman, H. D. , & Selzer, P. M. (2019). Anticoccidial drugs of the livestock industry. Parasitology Research, 118(7), 2009–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek, H. , & Landman, W. (2011). Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Veterinary Quarterly, 31(3), 143–161. [DOI] [PubMed] [Google Scholar]
- Pop, L. M. , Varga, E. , Coroian, M. , Nedișan, M. E. , Mircean, V. , Dumitrache, M. O. , Farczádi, L. , Fülöp, I. , Croitoru, M. D. , & Fazakas, M. (2019). Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites & Vectors, 12(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvača, N. , Ljubojević Pelić, D. , Čabarkapa, I. , Popović, S. , Tomičić, Z. , Nikolova, N. , & Lević, J. (2019). Quality of broiler chickens carcass fed dietary addition of garlic, black pepper and hot red pepper. Journal of Agronomy, Technology and Engineering Management, 2(1), 218–227. [Google Scholar]
- Qaid, M. M. , Al‐Mufarrej, S. I. , Azzam, M. M. , & Al‐Garadi, M. A. (2021). Anticoccidial effectivity of a traditional medicinal plant, Cinnamomum verum, in broiler chickens infected with Eimeria tenella . Poultry Science, 100(3), 100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurer, W. , Spring, P. , & Maertens, L. (2013). Effect of 3 dietary phytogenic products on production performance and coccidiosis in challenged broiler chickens. Journal of Applied Poultry Research, 22(3), 591–599. [Google Scholar]
- Shirley, M. , & Chapman, H. D. (2005). Eight decades of research on Eimeria in poultry. In Proceedings of the IX International Coccidiosis Conference , Brazil. [Google Scholar]
- Shirley, M. , & Millard, B. (1986). Studies on the immunogenicity of seven attenuated lines of Eimeria given as a mixture to chickens. Avian Pathology, 15(4), 629–638. [DOI] [PubMed] [Google Scholar]
- Sikkema, J. , de Bont, J. A. , & Poolman, B. (1995). Mechanisms of membrane toxicity of hydrocarbons. Microbiological Reviews, 59(2), 201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroni, E. , Govoni, P. , Guizzardi, S. , Renzulli, C. , & Guerra, M. (2002). Anti‐inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. Journal of Ethnopharmacology, 79(2), 265–272. [DOI] [PubMed] [Google Scholar]
- Srinivasu, B. , Preetam, V. C. , Gurram, S. , & Reddy, A. R. (2020). Comparative evaluation of herbal coccidiostat with chemotherapeutic coccidiostats on performance of broilers to control coccidiosis. Tropical Animal Health and Production, 52(4), 1985–1989. [DOI] [PubMed] [Google Scholar]
- Sundar, S. , Harikrishnan, T. , Latha, B. R. , Chandra, G. S. , & Kumar, T. (2017). Anticoccidial drug resistance in chicken coccidiosis and promising solutions: A review. Journal of Entomology and Zoology Studies, 5(4), 1526–1529. [Google Scholar]
- Tanweer, A. J. , Saddique, U. , Bailey, C. , & Khan, R. (2014). Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitology Research, 113(8), 2951–2960. [DOI] [PubMed] [Google Scholar]
- Taylor, J. , Walk, C. , Misiura, M. , Sorbara, J.‐O. B. , Giannenas, I. , & Kyriazakis, I. (2022). Quantifying the effect of coccidiosis on broiler performance and infection outcomes in the presence and absence of control methods. Poultry Science, 101, 101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari, A. , & Maharana, B. (2011). Control of poultry coccidiosis: Changing trends. Journal of Parasitic Diseases, 35(1), 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipu, M. A. , Pasha, T. , & Ali, Z. (2002). Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers. International Journal of Poultry Science, 1(4), 91–93. [Google Scholar]
- Vinus, R. D. , Sheoran, N. , Maan, N. , & Tewatia, B. (2018). Potential benefits of herbal supplements in poultry feed: A review. The Pharma Innovation Journal, 7(6), 651–656. [Google Scholar]
- Yong, T. , Chen, M. , Li, Y. , Song, X. , Huang, Y. , Chen, Y. , Jia, R. , Zou, Y. , Li, L. , & Yin, L. (2020). Anticoccidial effect of Fructus Meliae toosendan extract against Eimeria tenella . Pharmaceutical Biology, 58(1), 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


