Abstract
Background
Lymphoma with Mott cell change, or Mott cell lymphoma (MCL), is an uncommon variant of canine lymphoma. Because of its rare occurrence, there has been no comprehensive study describing the disease so far. Miniature dachshunds, a popular breed in Japan, sometimes experience MCL.
Objectives
To investigate the clinical characteristics and outcomes of MCL in miniature dachshunds.
Methods
Medical records were retrospectively reviewed to identify miniature dachshunds diagnosed with MCL and other types of lymphoma. Data on clinical and laboratory findings, treatments and outcomes were collected. Survival times were compared between miniature dachshunds with MCL and other types of lymphoma.
Results
Of the 87 miniature dachshunds diagnosed with lymphoma, 9 (10%) had cytological characteristics of MCL. All 9 miniature dachshunds with MCL were categorised as having alimentary lymphoma (small and/or large intestine, 6 dogs; mesenteric lymph node, 3 dogs). The median age was 3.1 years (range, 2.0–9.4 years). All nine dogs were treated with chemotherapeutic protocols used for large cell lymphoma or alkylating agents such as melphalan or chlorambucil. The overall response rate to initial chemotherapy was 78%, and the median progression‐free survival was 105 days. Overall survival in these nine dogs ranged from 6 to >1513 days (median, 240 days), which was significantly longer than in 29 miniature dachshunds with alimentary large cell lymphoma other than MCL (median, 57 days; p = 0.0491).
Conclusions
MCL in miniature dachshunds can be recognised as a peculiar type of B‐cell lymphoma occurring in relatively young dogs as an alimentary form and has a longer survival compared with typical alimentary large cell lymphoma.
Keywords: alimentary, breed, canine, dog, gastrointestinal, intestinal
The present retrospective study described the clinical characteristics and outcomes of lymphoma with Mott cell change, or Mott cell lymphoma (MCL) in miniature dachshunds. The response rate to initial chemotherapy was 78%, and the median overall survival was 240 days, showing a better prognosis than that of large cell alimentary lymphoma in this study population. MCL in miniature dachshunds can be recognized as a peculiar type of lymphoma which appears to be prevalent in the breed.

1. INTRODUCTION
Mott cells are plasma cells characterised by cytoplasmic vesicles containing immunoglobulin (Ig), termed Russell bodies. Accumulation of Ig in Mott cells is caused by impaired Ig secretion. In recent years, several case reports of ‘lymphoma with Mott cell differentiation’, characterised as B‐cell lymphoma accompanied by abundant Mott cells, have been published in companion animals. So far, seven lymphoma cases with Mott cell differentiation have been reported in dogs (De Zan et al., 2009; Kodama et al., 2008; Seelig et al., 2011; Snyman et al., 2013; Stacy et al., 2009), and one case each in a cat (Kanehara et al., 2016), ferret (Gupta et al., 2010), and hedgehog (Cazzini et al., 2019). No comprehensive study has characterised this rare variant in dogs. In particular, the outcomes of dogs with Mott cell lymphoma (MCL) are not well understood because many were euthanised soon after diagnosis. Miniature dachshunds, a popular breed in Japan, sometimes experience MCL. Furthermore, miniature dachshunds that develop MCL appeared to show better treatment outcomes than dogs with alimentary large cell lymphoma. The purpose of this retrospective study was to clarify the clinical characteristics and prognosis of MCL in miniature dachshunds.
2. MATERIALS AND METHODS
2.1. Case selection
Medical records at the Veterinary Medical Center of the University of Tokyo were reviewed to identify miniature dachshunds diagnosed with lymphoma between 1 April 2008 and 31 August 2017. The inclusion criteria was cytological or histological diagnosis of lymphoma of any anatomical location. Specimens for cytology were obtained by fine needle aspiration (FNA) or impression smears of tissue samples collected during biopsy or surgery. In the cases that underwent endoscopy, cytological specimens were prepared using the squash smear technique, in which the biopsy sample was squashed by applying pressure using two glass slides as previously described (Maeda et al., 2017). Each cytological specimen was air‐dried and stained with Wright‐Giemsa. MCL was diagnosed when cytology revealed lymphoma admixed with Mott cells with abundant Russel bodies in the cytoplasm. When Mott cells were frequently observed and accounted for more than 3% of the lymphoma cells, the case was diagnosed as MCL. Cases in which cytological specimens were unavailable were excluded.
2.2. Histopathological examination and immunohistochemistry
Endoscopically or surgically collected biopsy samples were submitted for histopathological examination. Tissues were fixed in 10% buffered formalin and embedded in paraffin. Four micrometre‐thick sections were deparaffinised and stained with haematoxylin and eosin. Histopathological specimens were subjected to immunohistochemistry as needed, according to the discretion of the pathologist. The following primary antibodies were used for immunohistochemistry: rabbit anti‐CD3 antibody (ready to use; Dako, Tokyo, Japan), rabbit anti‐CD20 antibody (1:100; Biocare Medical, Pacheco, CA, USA), mouse anti‐CD79 antibody (1:50; clone HM57; GeneTex, Irvine, CA, USA), rabbit anti‐PAX5 antibody (1:50; Thermo Fisher Scientific, Waltham, MA, USA), rabbit anti‐lambda light chain antibody (1:5000; Dako, Tokyo, Japan) and rabbit anti‐kappa light chain antibody (1:1000; Dako, Tokyo, Japan). Horseradish peroxidase (Envision+ System; Dako, Tokyo, Japan) was used as secondary antibody and visualised with DAB chromogen.
2.3. Data collection
Data obtained from the medical records of each miniature dachshund with lymphoma included age, sex, anatomical location of lymphoma, chemotherapeutic agents used, response to therapy, overall survival time (OS) and progression‐free survival time (PFS). Data on the clinical signs, physical examination findings, complete blood cell count (CBC), blood biochemistry and diagnostic imaging including thoracic and abdominal radiography, abdominal ultrasonography and whole‐body computed tomography (CT) were obtained. Additional data such as serum concentration of ionised calcium, parathyroid hormone (PTH), and PTH‐related protein (PTHrP), serum protein electrophoresis and polymerase chain reaction results for lymphocyte antigen receptor gene rearrangement (PARR) were retrieved when available. Specimens for histopathological examination and immunohistochemistry were reviewed. Detailed data on chemotherapeutic agents, response to treatment, and clinical outcomes were obtained. The following criteria were used to evaluate the response to treatment: complete remission (CR), 100% reduction in the size of all measurable disease; partial remission (PR), 50% or more but not CR; stable disease (SD), <50% reduction or <25% increase in the size of overall measurable disease; and progressive disease (PD), 25% or more increase in the size of overall measurable disease or the appearance of new lesions. CR, PR or SD were documented for a minimum of 1 week while receiving chemotherapy.
2.4. Statistical analysis
Mann–Whitney U tests were used to compare numerical values. Kaplan–Meier curves and 2‐sided log‐rank tests were used to compare survival data. For analysis, the data were excluded if the dogs were still alive at the time of analysis or lost to follow‐up. OS was defined as the date of diagnosis to the date of death from any cause. PFS was defined as the date from which treatment started to the date of PD or death from any cause. Statistical significance was set at p < 0.05. All statistical analyses were performed using commercial statistical software (JMP Pro 13.0.0, SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Study population
Between April 2008 and August 2017, a total of 3080 miniature dachshunds were referred, and 113 (3.7%) of these were diagnosed with lymphoma. Of the 113 Miniature Dachshunds with lymphoma, 26 dogs were excluded because the cytological specimens could not be evaluated, and the remaining 87 dogs were included in this study.
The anatomical classifications of lymphoma in the 87 miniature dachshunds were alimentary (n = 61, 70%), multicentric (n = 13, 15%), hepatic (n = 4, 5%), cutaneous (n = 2, 2%), nasal (n = 2, 2%) and renal (n = 2, 2%) forms. The other three dogs were identified as having lymphoma in the spleen, skeletal muscle and orbit. Of the 87 miniature dachshunds, 9 (10%) were diagnosed with MCL (1 dog was histologically confirmed), all of which were categorised as alimentary lymphoma (Table 1). MCL accounted for 15% (9/61) of alimentary lymphomas in miniature dachshunds in this study. Miniature dachshunds with alimentary lymphoma other than MCL included 31 dogs with large cell lymphoma (16 dogs were confirmed by histopathology with or without immunohistochemistry) and 21 dogs with small cell lymphoma (all dogs were confirmed by histopathology with or without immunohistochemistry).
TABLE 1.
Patient characteristics, treatments, and outcomes of 9 miniature dachshunds diagnosed with alimentary Mott cell lymphoma
| Case No. | Sex | Age (years) | Site(s) of lesions | Initial chemotherapy | Response | PFS (days) | OS (days) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | FS | 2.0 | Small intestine, MLN | L‐CHOP | CR | 205 | >1513 | Alive |
| 2 | M | 2.5 | Small intestine, MLN | L‐CHOP | PR | 37 | 75 | Died of tumour progression |
| 3 | M | 5.8 | Small intestine, MLN | Melphalan, P | PD | ND | 6 | Died of tumour progression |
| 4 | F | 2.3 | Small intestine, MLN | L‐CHOP | PR | 136 | 180 | Died of tumour progression |
| 5 | FS | 3.1 | Large intestine | L‐COP | CR | 98 | >824 | Alive |
| 6 | F | 2.3 | Small and large intestine, MLN | Melphalan, P | PR | 36 | 160 | Died of tumour progression |
| 7 | M | 9.4 | MLN | CHOP | CR | 189 | 449 | Died of tumour progression |
| 8 | MC | 4.4 | MLN | L, Chlorambucil, P | SD | 60 | 259 | Died of tumour progression |
| 9 | M | 3.1 | MLN | L‐CHOP | PR | 112 | 240 | Died of tumour progression |
Abbreviations: C, cyclophosphamide; CR, complete response; F, female; FS, female spayed; H, hydroxydaunorbicin (doxorubicin); L, L‐asparaginase; M, male; MC, male castrated; MLN, mesenteric lymph node; ND, not determined; O, vincristine; OS, overall survival time; P, prednisolone; PD, progressive disease; PFS, progression free survival; PR, partial response; SD, stable disease.
Five males (one castrated) and four females (two spayed) had MCL, and their median age was 3.1 years (range, 2.0–9.4 years). Twenty males (12 castrated) and 11 females (9 spayed) had large cell alimentary lymphoma, and their median age was 10.2 years (range, 1.0–15.4 years). Miniature dachshunds with small cell alimentary lymphoma included 11 males (8 castrated) and 10 females (9 spayed), and their median age was 8.4 years (range, 1.2–14.5 years). The miniature dachshunds diagnosed with MCL were significantly younger than those with large cell or small cell lymphoma (p < 0.001 and p = 0.0028, respectively), whereas no significant difference in age was found between dogs with large cell lymphoma and those with small cell lymphoma (p = 0.1047).
3.2. Clinical features
Of the nine miniature dachshunds diagnosed with MCL, four had solitary or multiple masses in the small intestine, one in the large intestine, one in the small and large intestines, with or without mesenteric lymph node involvement. In the remaining three cases, remarkable enlargement of the mesenteric lymph nodes was observed without obvious intestinal mass formation. The frequent clinical signs in the nine miniature dachshunds with MCL were weight loss (n = 7), diarrhoea or soft stool (n = 6), gastrointestinal haemorrhage (n = 5), lethargy (n = 5), vomiting (n = 4) and anorexia (n = 4). One patient showed no clinical signs. On physical examination, abdominal masses were palpable in all cases, and swelling of the peripheral lymph nodes was observed in three cases.
3.3. Laboratory findings
CBC and blood biochemistry examinations were performed in all nine miniature dachshunds with MCL. Moderate regenerative anaemia (24% and 29%; reference range, 37–62%) and mild to moderate non‐regenerative anaemia (29% and 35%) was observed in two each of the nine dogs. Leucocytosis (median, 20,295/μl; range, 17,100–56,890/μl; reference range, 5100–16,800/μl) due to neutrophilia was observed in six of the nine dogs. Lymphocytosis was not noted in any of the cases. Thrombocytosis (574,000 and 576,000/μl; reference range, 148,000–84,000/μl) was observed in two dogs. Hyperproteinaemia (7.4 and 9.0 g/dl; reference range, 5.0–7.2 g/dl) was observed in two dogs. Serum protein electrophoresis was performed in one of the two cases with hyperproteinaemia, but monoclonal gammopathy was not observed. Hypoalbuminaemia (2.2 and 2.5 g/dl; reference range, 2.6–4.0 g/dl) was observed in two dogs. Hypercalcaemia (median, 13.8 mg/dl; range, 13.2–16.9 mg/dl; reference range, 9.3–12.1 mg/dl) was observed in three dogs. Ionised calcium was measured in one of the three cases with hypercalcaemia (16.9 mg/dl), and an elevated ionised calcium was confirmed (1.89 mmol/L; reference range, 1.24–1.56 mmol/L). PTH and PTHrP levels were measured in the same case: PTH was within the reference range (11.1 pg/ml; reference range, 8.0–35.0 pg/ml) and PTHrP was below the detection limit (<1.0 pmol/L). Elevation in C‐reactive protein (median, 6.7 mg/dl; range, 0.9–20 mg/dl; reference range, 0–0.7 mg/dl) was observed in all dogs.
3.4. Results of diagnostic imaging
Thoracic radiography was performed in all nine dogs, and abnormal findings were noted in five cases: enlargement of sternal lymph nodes (n = 5), mediastinal lymph nodes (n = 2) and tracheobronchial lymph nodes (n = 1). Abdominal radiography was performed in seven of the nine dogs, and the presence of intra‐abdominal masses was detected in all cases. Gastrointestinal masses and/or enlarged mesenteric lymph nodes were detected by ultrasonography in all nine cases. Circumferential, hypoechoic or mixed echogenic intestinal wall thickening with effacement of wall layering was a typical finding of the intestinal masses. The enlarged mesenteric lymph nodes were hypoechoic or mixed echogenic and round in shape. The anatomical location of the enlarged lymph nodes could not be precisely assigned because they were massive and fused with the surrounding lymph nodes. CT scans performed in three cases revealed localised gastrointestinal masses with mild contrast enhancement and markedly enlarged mesenteric lymph nodes, such as the jejunal lymph nodes (Figure 1).
FIGURE 1.

Representative findings on abdominal computed tomography of the miniature dachshunds diagnosed with Mott cell lymphoma (Case 1). A localised jejunal mass (M) and severely enlarged jejunal lymph node (LN) can be observed. Adhesion is suggested between the mass and the lymph nodes (arrowheads).
3.5. Cytological findings
In the nine miniature dachshunds diagnosed with MCL, cytology of FNA samples from the intestinal masses or enlarged lymph nodes showed heterogeneous discrete round cell populations (Figure 2). These cells were classified into two types based on their morphologies. The first cell type had a morphology consistent with Mott cells containing a large amount of densely packed, clear to pale blue cytoplasmic globules consistent with Russell bodies. The number and size of Russell bodies varied from case to case. The size of each Mott cell varied depending on the amount of Russell bodies in the cytoplasm. The nuclei of Mott cells were displaced by abundant Russell bodies with irregularly condensed nuclear chromatin. The proportion of Mott cells in the lymphoid cell population ranged from 4% to 87% (median, 34%). The second cell type was lymphoid cells, mainly small to medium lymphocytes, which had round nuclei with partially clumped chromatin and scant to moderate clear cytoplasm. A few large immature lymphoid cells with round nuclei and fine chromatin patterns, several eccentric nucleoli, and scant basophilic cytoplasm were also found. Notably, some of the small lymphocytes showed a morphological appearance resembling plasma cells, with an eccentric dense nucleus and deeply blue cytoplasm containing a small amount of Russell bodies, showing a morphological tendency to Mott cells. Mitotic figures were rare in most cases but were occasionally found.
FIGURE 2.

Representative findings on cytology of the miniature dachshunds diagnosed with Mott cell lymphoma (Case 1). A large number of Mott cells with abundant cytoplasmic Russell bodies admixed with a population of small to large lymphoid cells can be observed in the specimen obtained by fine needle aspiration of the jejunal mass. The size and number of intracellular Russell bodies varies among Mott cells. Some Mott cells are collapsed due to the presence of a large amount of Russell bodies. Wright‐Giemsa stain, × 400.
3.6. Histopathological and immunohistochemical findings
Histopathological and immunohistochemical findings were obtained in one patient with MCL (Case 3). In this case, the duodenal mass and enlarged mesenteric lymph nodes were surgically resected, and histopathological examination was performed. Histopathological examination of the duodenal mass revealed transmural proliferation of lymphoid cells composed of small to large lymphocytes accompanied by abundant Mott cells (Figure 3a and b). Histopathological examination of the mesenteric lymph node also confirmed the diffuse proliferation of lymphocytes and Mott cells, similar to those found in the duodenal mass. The histopathological diagnosis was lymphoplasmacytic lymphoma (LPL) with Mott cell change.
FIGURE 3.

Histologic sections of the jejunal mass (case 3). (a) Neoplastic proliferation of round cells infiltrating from the lamina propria (LP) to the lamina muscularis mucosae (LMM), the submucosa (SM), the tunica muscularis (TM) can be observed. Haematoxylin and eosin stain, × 100. (b) Most of the tumour cells are small lymphocytes, but some are medium to large. The presence of numerous Mott cells with abundant cytoplasmic Russell bodies is also recognised (some of the Mott cells are indicated by arrows). Haematoxylin and eosin stain, × 400.
Immunohistochemical staining of formalin‐fixed, paraffin‐embedded tissue sections was routinely performed on samples from the duodenal mass and mesenteric lymph nodes of the dog. The small, medium and large lymphocytes strongly expressed CD20, CD79a and PAX5 and did not express CD3. The Mott cells weakly expressed CD79a and did not express CD3, CD20 or PAX5. Strong cytoplasmic positivity for the Ig lambda light chain was consistently present in the Mott cells and was scattered among the lymphocytes. The Mott cells were negative for Ig kappa light chain expression.
3.7. PARR analysis
PARR analyses using FNA samples of the lesions were performed in seven of the nine miniature dachshunds with MCL. Clonal gene rearrangement of the Ig heavy chain (IgH) gene was identified in four of the seven cases. Clonal T‐cell receptor gamma chain (TCRγ) gene rearrangement was not detected in any case.
3.8. Treatment and outcomes
All nine miniature dachshunds diagnosed with MCL received initial chemotherapy protocols as follows: CHOP protocol (consisting of vincristine, cyclophosphamide, doxorubicin and prednisolone; Garrett et al., 2002) (n = 5), COP protocol (n = 1), melphalan and prednisolone (n = 2) and chlorambucil and prednisolone (n = 1). Seven patients received L‐asparaginase concurrently with the initial treatment. Among the five patients who underwent the CHOP protocol, two achieved CR and three achieved PR. One patient with COP showed CR. One of the two dogs treated with melphalan and prednisolone achieved PR, while the other dog developed bacterial peritonitis due to perforation of the intestinal mass and was categorised as PD. The remaining dog that received chlorambucil and prednisolone persisted with SD after treatment. The overall response rate after various remission induction chemotherapies was 78%. The median PFS after the first induction treatment was 105 days (range, 36–205 days).
Recurrence was detected after discontinuation of chemotherapeutic protocols in all three cases that achieved CR. After relapse identification, re‐induction chemotherapy was administered in these cases. In case 1, recurrence was observed 205 days, 496 days and 770 days after diagnosis. The CHOP protocol was used for the first re‐induction therapy, and the COP protocol (cyclophosphamide was replaced by chlorambucil) was used for the second and third re‐induction therapies. All three re‐induction therapies resulted in CR. In case 5, recurrence was confirmed 98, 418 and 542 days after diagnosis, and CR was achieved using the COP or CHOP protocol. In case 7, re‐induction with the COP protocol resulted in SD.
When the cases with lymphoma became refractory to the previous treatment, a variety of second‐line or rescue protocols were employed in five cases: nimustine (n = 3), lomustine (n = 2), CHOP (n = 2), DMAC protocol (consisting of dexamethasone, melphalan, actinomycin‐D and cytarabine; Alvarez et al., 2006) (n = 1), L‐asparaginase (n = 1), dacarbazine (n = 1), mitoxantrone (n = 1) and chlorambucil (n = 1). None of the rescue chemotherapies led to CR or PR in any case.
By the time of the final analysis, seven miniature dachshunds with MCL had died due to tumour progression, while the remaining two dogs were alive without recurrence on the day of their last visit. No dog was euthanised. The median OS for the nine dogs with MCL was 240 days (range, 6–1513 days).
3.9. Comparison of the prognosis
Of the 31 miniature dachshunds diagnosed with large cell alimentary lymphoma, two cases were lost to follow‐up after the first visit and were excluded from the evaluation of prognosis. The initial treatments for the remaining 29 dogs were as follows: L‐asparaginase and prednisolone (n = 14); (L‐)CHOP (n = 4); L‐COP (n = 3); chlorambucil and prednisolone (n = 3); surgery, L‐asparaginase and prednisolone (n = 1); prednisolone only (n = 1); and no treatment (n = 3). By the time of the final analysis, 24 of the 29 dogs had died due to tumour progression, while five dogs were alive on the day of their last visit. The median OS for patients with large cell alimentary lymphoma was 57 days (range, 0 to >3801 days).
Of the 21 Miniature Dachshunds with small cell alimentary lymphoma, one case was excluded due to being lost to follow‐up after the first visit. The initial treatment for the remaining 20 dogs was as follows: prednisolone only (n = 17), chlorambucil and prednisolone (n = 2), melphalan and prednisolone (n = 1). By the end of the study, nine of the 20 dogs had died because of tumour progression, three dogs had died from other causes, and the remaining eight dogs were alive on the day of their last visit. The median OS for small cell alimentary lymphoma cases was 1440 days (range, 15 to >2667 days).
The OS for the miniature dachshunds diagnosed with MCL was significantly longer than that for miniature dachshunds with large cell alimentary lymphoma (p = 0.0491). On the other hand, there was no significant difference in OS between miniature dachshunds with MCL and miniature dachshunds with small cell alimentary lymphoma (p = 0.0779) (Figure 4).
FIGURE 4.
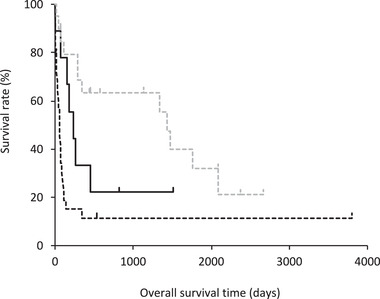
Kaplan–Meier survival curve of overall survival time in the miniature dachshunds with alimentary Mott cell lymphoma (MCL; n = 9, black solid line), large cell alimentary lymphoma (n = 29, black dashed line) and small cell alimentary lymphoma (n = 20, grey dashed line). The median overall survival time in the miniature dachshunds with MCL, large cell alimentary lymphoma, and small cell alimentary lymphoma was 240 days, 57 days and 1440 days, respectively. Dogs diagnosed with small cell alimentary lymphoma had a significantly longer overall survival time than those with large cell alimentary lymphoma (p < 0.0001). Dogs with MCL showed a significantly longer overall survival time than those with large cell alimentary lymphoma (p = 0.0491). There was no significant difference in overall survival time between miniature dachshunds with MCL and miniature dachshunds with small cell alimentary lymphoma (p = 0.0779).
4. DISCUSSION
The first report of canine MCL was from Japan, described a case of a 1‐year‐old miniature dachshund with a lesion in the small intestine (Kodama et al., 2008). On the other hand, six cases of canine MCL in various breeds have been reported from the other countries, with the median age of 6 years (range, 2–9 years) and diverse anatomical forms of the disease including multicentric, gastric, small intestinal and hepatic form lymphomas (De Zan et al., 2009; Seelig et al., 2011; Snyman et al., 2013; Stacy et al., 2009). A recent study from Japan reported that miniature dachshunds accounted for a large proportion of canine gastrointestinal B‐cell lymphomas, and that the most common subtype was LPL, sometimes with the lesion containing Mott cells (Kojima et al., 2021). In the present study, nine cases of MCL were found in relatively young (median age, 3.1 years old) miniature dachshunds, and all of them had an alimentary form. This suggests that MCL is a rare disease but can be found as an alimentary form of lymphoma in relatively young adult miniature dachshunds.
In our study, the median OS of miniature dachshunds diagnosed with large cell and small cell alimentary lymphoma was 57 and 1440 days, respectively. Small cell gastrointestinal lymphoma is generally considered an indolent disease (Couto et al., 2018; Lane et al., 2018), whereas large cell gastrointestinal lymphoma has a grave prognosis with short median OS of 13, 62, 77 and 147 days (Frank et al., 2007; Nakagawa et al., 2021; Rassnick et al., 2009; Sogame et al., 2018). In comparison, miniature dachshunds with alimentary MCL in the present study had a better prognosis with a median OS of 240 days. Several possible reasons for the difference in prognosis between alimentary MCL and alimentary large cell lymphoma can be considered as follows.
The first possibility is related to the degree of maturity of the cells in the MCL. Cytological examination of the MCL revealed that the tumour tissue predominantly consisted of small‐ to medium‐sized cells and mitotic figures were rare. In addition, the morphology of Mott cells indicates the capability of producing Igs, which is consistent with functional maturation towards plasma cells. These morphological features indicate that MCL is a lymphoma of relatively mature B cells, which may explain the less aggressive biological behaviour. A recent report on the histopathology and immunophenotyping study of canine gastrointestinal lymphoma indicated that MCL was classified as LPL according to the WHO classification, which is a distinct subtype of diffuse large B‐cell lymphoma, the most common subtype of canine lymphoma (Kojima et al., 2021; Valli et al., 2011).
Another possible reason for the favourable prognosis of MCL could be responsiveness to treatment. The response rate to systemic chemotherapy for canine large cell gastrointestinal lymphoma was reported to be 56% (Nakagawa et al., 2021; Rassnick et al., 2009). In contrast, a response rate as high as 78% was obtained for the alimentary MCL in this study. Typical canine large cell gastrointestinal lymphomas are mostly derived from T cells; however, MCL is derived from B cells (Coyle & Steinberg, 2004; Kojima et al., 2021). It is reasonable that differences in cell origin influence the responsiveness to treatment and prognosis, as shown in multicentric canine lymphoma (Marconato et al., 2011; Teske et al., 1994).
Two cases included in our study were confirmed to have survived for more than 2 years (still alive at 824 and 1513 days after diagnosis). These two dogs experienced relapse three times, and CR could be achieved by re‐induction therapy with COP or modified COP protocol in which cyclophosphamide was replaced by chlorambucil. In addition, a previous study reported that a miniature dachshund with intestinal MCL was successfully treated with palliative surgery and COP chemotherapy for more than 10 months (Kodama et al., 2008). The clinical course of these cases indicated that the COP protocol (especially vincristine) sustained its efficacy for a prolonged period in some cases of MCL in miniature dachshunds without the development of chemotherapy resistance. The sustained efficacy of the COP protocol in the treatment of miniature dachshunds with MCL is considered a peculiar phenomenon in chemotherapy for canine lymphoma.
In the present study, hyperproteinaemia was observed in two cases, and serum protein electrophoresis performed in one case, revealed a polyclonal rather than monoclonal gammopathy. In a previous report, the presence of M‐protein was detected by immunofixation electrophoresis in two dogs with MCL, although monoclonal gammopathy was not found on serum protein electrophoresis (Seelig et al., 2011). Since the presence of M‐proteinaemia provides evidence of clonal immunoglobulin production, the immunofixation electrophoresis was considered to be a possible diagnostic aid for MCL.
Hypercalcaemia has been occasionally reported in dogs with MCL (Snyman et al., 2013; Stacy et al., 2009). Ionised calcium was measured in only one of the three dogs with hypercalcaemia in this study and was confirmed to be elevated. Although there was no obvious osteolysis or elevation of PTHrP, it was concluded that hypercalcaemia was a paraneoplastic syndrome of unknown mechanism because screening tests ruled out all other diseases causing hypercalcaemia, and calcium levels were normalised after remission of MCL.
The term ‘B‐cell lymphoma with Mott cell differentiation’ was used in the first case report by Kodama et al. in 2008 and has been used in the succeeding reports describing lymphoid neoplasms in which the lymphoid cells are admixed with Mott cells (De Zan et al., 2009; Seelig et al., 2011; Snyman et al., 2013; Stacy et al., 2009). However, ‘differentiation’ is known as a physiological process in which stem or progenitor cells undergo acquisition of specialised phenotypes and functions in each organ system. Mott cells are generated by the accumulation of globular cytoplasmic inclusions composed of immunoglobulin because of the abnormal cellular indigestion of the endoplasmic reticulum (Kopito & Sitia, 2000). Since Mott cells are not included in the normal B‐cell differentiation lineage, we propose using ‘Mott cell change’ instead of ‘Mott cell differentiation’. Therefore, in the present study, ‘lymphoma with Mott cell change’ or ‘Mott cell lymphoma’ was used to designate lymphoid neoplasms in which the lymphoid cells are admixed with Mott cells.
Limitations of the present study include its retrospective nature and small sample size. Most cases of MCL were diagnosed using cytology alone, but ideally, histopathological examination should have been performed. In addition, special stains including periodic acid‐Schiff and techniques such as immunohistochemistry and immunocytochemistry should have been applied for more reliable identification of the neoplastic cells and their cytoplasmic vesicles. Similarly, only 16 of the 31 dogs with large cell lymphoma were histologically confirmed. Staging was not comprehensive in some of the cases, and post‐mortem examination was not performed in any case, making group comparisons unreliable. Treatment protocols, procedures and intervals for assessing treatment responses were also not standardised. Of note, 6 of the 9 miniature dachshunds diagnosed with MCL were treated with (L‐)CHOP/COP protocol, whereas more than half of the dogs with large cell alimentary lymphoma were treated with L‐asparaginase and prednisolone. It is possible that the protocol selection could have some impact on the difference in survival times of dogs in each group. Previous studies reported the median OS of dogs with large cell gastrointestinal lymphoma treated with CHOP/COP protocol to be 60–77 days (Rassnick et al., 2009; Sogame et al., 2018). On the other hand, the median PFS and OS of dogs treated with continuous L‐asparaginase administration were 50 days and 147 days, respectively, which were not inferior to those with COP/CHOP protocol (Nakagawa et al., 2021). Therefore, it is assumed that the significant difference in OS between the miniature dachshunds with MCL and large cell alimentary lymphoma was not solely due to protocol selection. Prospective studies with a larger number of cases are strongly warranted in the future to clarify appropriate treatment protocols and prognosis in MCL in miniature dachshunds.
In conclusion, MCL was found mostly in young adult miniature dachshunds with an alimentary form lymphoma in which intestinal masses and enlarged mesenteric lymph nodes were observed. The response rate to initial chemotherapy was 78%, and the median OS was 240 days, showing a better prognosis than that of large cell alimentary lymphoma in this study population. MCL in miniature dachshunds can be recognised as a peculiar type of B‐cell lymphoma which appears to be prevalent in the breed.
AUTHOR CONTRIBUTIONS
Aki Ohmi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. Miho Tanaka: conceptualisation; data curation; formal analysis; investigation; methodology. Jun Rinno: data curation; formal analysis; investigation. Masaya Tsuboi: data curation; formal analysis; investigation; methodology. James K. Chambers: Data curation, Formal analysis, Investigation, Methodology, Supervision. Kazuyuki Uchida: data curation; formal analysis; investigation; methodology; project administration; supervision; validation. Yuko Goto‐Koshino: data curation; formal analysis; investigation; methodology. Hirotaka Tomiyasu: supervision; validation. Koichi Ohno: supervision; validation. Hajime Tsujimoto: supervision; validation; writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest. None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of this paper.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a retrospective study on client‐owned dogs, treatment was undertaken in the context of advanced veterinary medicine and written‐informed consent was obtained from the owners.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.975.
ACKNOWLEDGEMENTS
We thank all the dogs and their owners, and all the staff of the Veterinary Medical Center of the University of Tokyo and Department of Veterinary Internal Medicine.
Ohmi, A. , Tanaka, M. , Rinno, J. , Tsuboi, M. , Chambers, J. K. , Uchida, K. , Goto‐Koshino, Y. , Tomiyasu, H. , Ohno, K. , & Tsujimoto, H. (2023). Clinical characteristics and outcomes of Mott cell lymphoma in nine miniature dachshunds. Veterinary Medicine and Science, 9, 609–617. 10.1002/vms3.975
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alvarez, F. J. , Kisseberth, W. C. , Gallant, S. L. , & Couto, C. G. (2006). Dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) protocol for dogs with relapsed lymphoma. Journal of Veterinary Internal Medicine/American College of Veterinary Internal Medicine, 20(5), 1178–1183. [DOI] [PubMed] [Google Scholar]
- Cazzini, P. , Richardson, J. , Smith, N. , Lodzinska, J. , Robinson, A. L. , & Philbey, A. W. (2019). Lymphoma with Mott cell differentiation and validation of immunohistochemical lymphoid markers in an African pygmy hedgehog (Atelerix albiventris). Veterinary Clinical Pathology, 48(4), 725–729. [DOI] [PubMed] [Google Scholar]
- Couto, K. M. , Moore, P. F. , Zwingenberger, A. L. , Willcox, J. L. , & Skorupski, K. A. (2018). Clinical characteristics and outcome in dogs with small cell T‐cell intestinal lymphoma. Veterinary and Comparative Oncology, 16(3), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, K. A. , & Steinberg, H. (2004). Characterization of lymphocytes in canine gastrointestinal lymphoma. Veterinary Pathology, 41(2), 141–146. [DOI] [PubMed] [Google Scholar]
- De Zan, G. , Zappulli, V. , Cavicchioli, L. , Martino, L. D. , Ros, E. , Conforto, G. , & Castagnaro, M. (2009). Gastric B‐cell lymphoma with Mott cell differentiation in a dog. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians, Inc, 21(5), 715–719. [DOI] [PubMed] [Google Scholar]
- Frank, J. D. , Reimer, S. B. , Kass, P. H. , & Kiupel, M. (2007). Clinical outcomes of 30 cases (1997‐2004) of canine gastrointestinal lymphoma. Journal of the American Animal Hospital Association, 43(6), 313–321. [DOI] [PubMed] [Google Scholar]
- Garrett, L. D. , Thamm, D. H. , Chun, R. , Dudley, R. , & Vail, D. M. (2002). Evaluation of a 6‐month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. Journal of Veterinary Internal Medicine/American College of Veterinary Internal Medicine, 16(6), 704–709. [DOI] [PubMed] [Google Scholar]
- Gupta, A. , Gumber, S. , Schnellbacher, R. , Bauer, R. W. , & Gaunt, S. D. (2010). Malignant B‐cell lymphoma with Mott cell differentiation in a ferret (Mustela putorius furo). Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians, Inc, 22(3), 469–473. [DOI] [PubMed] [Google Scholar]
- Kanehara, T. , Matsui, N. , Murakami, M. , Maruo, K. , Mori, T. , Hirata, A. , Yanai, T. , & Sakai, H. (2016). B‐cell lymphoma with Mott cell differentiation in a cat. Veterinary Clinical Pathology, 45(2), 356–360. [DOI] [PubMed] [Google Scholar]
- Kodama, A. , Sakai, H. , Kobayashi, K. , Mori, T. , Maruo, K. , Kudo, T. , Yanai, T. , & Masegi, T. (2008). B‐cell intestinal lymphoma with Mott cell differentiation in a 1‐year‐old miniature dachshund. Veterinary Clinical Pathology, 37(4), 409–415. [DOI] [PubMed] [Google Scholar]
- Kojima, K. , Chambers, J. K. , Ii, T. , Nibe, K. , Mizuno, T. , & Uchida, K. (2021). Histopathological features and immunophenotyping of canine transmural gastrointestinal lymphoma using full‐thickness biopsy samples. Veterinary Pathology, 58(6), 1033. 10.1177/03009858211030523 [DOI] [PubMed] [Google Scholar]
- Kopito, R. R. , & Sitia, R. (2000). Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Reports, 1(3), 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, J. , Price, J. , Moore, A. , Dandrieux, J. R. S. , Clifford, C. , Curran, K. , Choy, K. , & Cannon, C. (2018). Low‐grade gastrointestinal lymphoma in dogs: 20 cases (2010 to 2016). The Journal of Small Animal Practice, 59(3), 147–153. [DOI] [PubMed] [Google Scholar]
- Maeda, S. , Tsuboi, M. , Sakai, K. , Ohno, K. , Fukushima, K. , Kanemoto, H. , Hiyoshi‐Kanemoto, S. , Goto‐Koshino, Y. , Chambers, J. K. , Yonezawa, T. , Uchida, K. , & Matsuki, N. (2017). Endoscopic cytology for the diagnosis of chronic enteritis and intestinal lymphoma in dogs. Veterinary Pathology, 54(4), 595–604. [DOI] [PubMed] [Google Scholar]
- Marconato, L. , Stefanello, D. , Valenti, P. , Bonfanti, U. , Comazzi, S. , Roccabianca, P. , Caniatti, M. , Romanelli, G. , Massari, F. , & Zini, E. (2011). Predictors of long‐term survival in dogs with high‐grade multicentric lymphoma. Journal of the American Veterinary Medical Association, 238(4), 480–485. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T. , Kojima, M. , Ohno, K. , Chambers, J. K. , Uchida, K. , Ohmi, A. , Goto‐Koshino, Y. , Tomiyasu, H. , & Tsujimoto, H. (2021). Efficacy and adverse events of continuous l‐asparaginase administration for canine large cell lymphoma of presumed gastrointestinal origin. Veterinary and Comparative Oncology, 20, 102–108. 10.1111/vco.12749 [DOI] [PubMed] [Google Scholar]
- Rassnick, K. M. , Moore, A. S. , Collister, K. E. , Northrup, N. C. , Kristal, O. , Chretin, J. D. , & Bailey, D. B. (2009). Efficacy of combination chemotherapy for treatment of gastrointestinal lymphoma in dogs. Journal of Veterinary Internal Medicine/American College of Veterinary Internal Medicine, 23(2), 317–322. [DOI] [PubMed] [Google Scholar]
- Seelig, D. M. , Perry, J. A. , Zaks, K. , Avery, A. C. , & Avery, P. R. (2011). Monoclonal immunoglobulin protein production in two dogs with secretory B‐cell lymphoma with mott cell differentiation. Journal of the American Veterinary Medical Association, 239(11), 1477–1482. [DOI] [PubMed] [Google Scholar]
- Snyman, H. N. , Fromstein, J. M. , & Vince, A. R. (2013). A rare variant of multicentric large B‐cell lymphoma with plasmacytoid and mott cell differentiation in a dog. Journal of Comparative Pathology, 148(4), 329–334. [DOI] [PubMed] [Google Scholar]
- Sogame, N. , Risbon, R. , & Burgess, K. E. (2018). Intestinal lymphoma in dogs: 84 cases (1997‐2012). Journal of the American Veterinary Medical Association, 252(4), 440–447. [DOI] [PubMed] [Google Scholar]
- Stacy, N. I. , Nabity, M. B. , Hackendahl, N. , Buote, M. , Ward, J. , Ginn, P. E. , Vernau, W. , Clapp, W. L. , & Harvey, J. W. (2009). B‐cell lymphoma with Mott cell differentiation in two young adult dogs. Veterinary Clinical Pathology, 38(1), 113–120. [DOI] [PubMed] [Google Scholar]
- Teske, E. , van Heerde, P. , Rutteman, G. R. , Kurzman, I. D. , Moore, P. F. , & MacEwen, E. G. (1994). Prognostic factors for treatment of malignant lymphoma in dogs. Journal of the American Veterinary Medical Association, 205(12), 1722–1728. [PubMed] [Google Scholar]
- Valli, V. E. , Myint, M. S. , Barthel, A. , Bienzle, D. , Caswell, J. , Colbatzky, F. , Durham, A. , Ehrhart, E. J. , Johnson, Y. , Jones, C. , Kiupel, M. , Labelle, P. , Lester, S. , Miller, M. , Moore, P. , Moroff, S. , Roccabianca, P. , Ramos‐Vara, J. , Ross, A. , … Vernau, W. (2011). Classification of canine malignant lymphomas according to the world health organization criteria. Veterinary Pathology, 48(1), 198–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


