Abstract
Background
The long‐term clinical and biofhemical effects of basal‐bolus insulin treatment with lispro and NPH in dogs with diabetes mellitus are undocumented.
Objectives
To perform a prospective pilot field study of the long‐term effects of lispro and NPH on clinical signs and serum fructosamine concentrations (SFC) in dogs with diabetes mellitus.
Methods
Twelve dogs received combined lispro and NPH insulins treatment twice a day and were examined every 2 weeks for 2 months (visits 1–4), and every 4 weeks for up to 4 additional months (visits 5–8). Clinical signs and SFC were recorded at each visit. Polyuria and polydipsia (PU/PD) were scored as absent (0) or present (1).
Results
Median (range) PU/PD scores of combined visits 5–8 (0, 0–1) were significantly lower than median scores of combined visits 1–4 (1, 0–1, p = 0.03) and at enrolment (1, 0–1, p = 0.045). Median (range) SFC of combined visits 5–8 (512 mmol/L, 401–974 mmol/L) was significantly lower than SFC of combined visits 1–4 (578 mmol/L, 302–996 mmol/L, p = 0.002) and at enrolment (662 mmol/L, 450–990 mmol/L, p = 0.03). Lispro insulin dose was significantly and negatively, albeit weakly, correlated with SFC concentration during visits 1 through 8 (r = –0.3, p = 0.013). Median duration of follow up was 6 months (range 0.5–6) and most dogs (8, 66.7%) were followed for 6 months. Four dogs withdrew from the study within 0.5–5 months because of documented or suspected hypoglycaemia, short NPH duration or sudden unexplained death. Hypoglycaemia was noted in 6 dogs.
Conclusions
Long‐term lispro and NPH combination therapy may improve clinical and biochemical control of some diabetic dogs with comorbidities. Risk of hypoglycaemia should be addressed with close monitoring.
Keywords: basal‐bolus insulin treatment, canine, hypoglycaemia, post‐prandial hyperglycaemia
A prospective pilot field study of the undocumented long‐term effects of lispro and NPH on clinical signs and serum fructosamine concentrations (SFC) in dogs with diabetes mellitus and comorbidities was conducted. A significant improvement in clinical signs (i.e., polyuria and polydipsia) and SFC was documented; however, 4/12 dogs withdrew from the study within 0.5‐5 months because hypoglycemia, short NPH duration, or sudden unexplained death. Therefore, Lispro and NPH combination therapy may improve clinical and biochemical control of diabetic dogs with comorbidities but considering the limited sample size, and various effects of diets and comorbidities, future larger studies are warranted to establish specific recommendations, and the risk of hypoglycemia should be addressed with close monitoring.
1. INTRODUCTION
Well‐regulated diabetes mellitus (DM) in dogs, is defined as a state in which there is an absence of clinical signs such as polyuria (PU), polydipsia (PD), polyphagia and weight loss along with an absence of hypoglycaemia, while blood glucose (BG) concentrations are maintained below the renal threshold of about 200 mg/dl for the majority of the day (Behrend et al., 2018). Insulin is the mainstay of DM treatment in dogs, and numerous insulin products can be used for this purpose (Behrend et al., 2018; Bertalan et al., 2020; Fracassi et al., 2015; Fracassi et al., 2018; Hess & Drobatz, 2013; Palm et al., 2009). Combination insulin treatment with lispro and NPH is designed to mimic physiologic insulin secretion (Bertalan et al., 2020). Lispro is a rapid‐onset, short duration insulin (Bertalan et al., 2020), that mimics the first (bolus) phase of physiologic insulin secretion, which in dogs begins within 2–4 min after glucose stimulation and peaks within 8–10 min (Misler et al., 2009). NPH activity mimics the second (basal) physiologic phase of insulin secretion, which develops within 20 min after glucose stimulation and contributes to the longer duration of the insulin effect (Bertalan et al., 2020; Misler et al., 2009). Recently, a study examining the effect of combination insulin treatment with lispro insulin and NPH, administered twice daily at mealtime in dogs with well‐regulated spontaneous DM, reported that the addition of lispro insulin to NPH significantly decreased post‐prandial hyperglycaemia (PPH) and serum fructosamine concentrations (SFC) after 2 weeks of treatment (Bertalan et al., 2020). However, this basal‐bolus insulin treatment (BBIT) was assessed in a homogenous group of well‐controlled diabetic dogs, with no known co‐morbidities (Bertalan et al., 2020). Moreover, BBIT was implemented for a short time‐period of 2 weeks only, precluding a long‐term evaluation of the effect of this treatment protocol on SFC and clinical signs in dogs with DM (Bertalan et al., 2020).
Long‐term field studies in heterogeneous groups of older dogs with DM and comorbidities are challenging because the clinical needs of older dogs are likely to change over time in a manner that could be unrelated to the study treatment or even to the primary disease studied (Hess et al., 2000). However, comorbidities are common in dogs with DM (Hess et al., 2000), and long‐term field studies describing the effects of insulin treatments should therefore include dogs with DM and comorbidities. Monitoring insulin treatment in dogs with DM can also be trying, and serial blood or interstitial glucose measurements can be laborious and yield inaccurate results (Del Baldo et al., 2020; Fleeman & Rand, 2003). Serum fructosamine concentration is another imperfect tool for monitoring of glycaemic regulation, but it is well suited for monitoring of trends in glycaemic control, and unlike serial blood or interstitial glucose measurements it is easy to measure and relatively inexpensive (Baldo et al., 2020; Behrend et al., 2018). While all methods for monitoring glycaemic control have some shortcomings, there is consensus in the veterinary literature about the need to interpret all glucose concentration quantifications in light of clinical signs and that documentation of absence or presence of clinical signs is essential for defining the success of insulin treatment (Behrend et al., 2018). The aim of this pilot, prospective, field study was therefore to describe the effects of the lispro and NPH BBIT treatment protocol, over a period of several months, on clinical signs and SFC in a heterogeneous group of diabetic dogs with variably controlled DM, including dogs with and without concurrent illnesses.
2. MATERIALS AND METHODS
A prospective, interventional, longitudinal, pilot field study of 12 dogs with naturally occurring DM was performed between 30 November 2014 and 25 May 2019. The study was approved by the Institutional Ethics Committee. Dogs were enrolled after their owners signed the approved consent form. Diabetic dogs from the patient population of a Veterinary Teaching Hospital and dogs specifically referred for participation in the study, were enrolled. Dogs were included if they were diagnosed with DM based on the presence of ≥2 of the following clinical signs: PU and PD, polyphagia and weight loss. Dogs also had to have documented persistent fasting (10–12 h) hyperglycaemia (BG>200 mg/dl) (Cobas 6000, Roche, Mannheim, Germany; ACCU‐CHEK® Performa, Roche, Mannheim, Germany), glucosuria (Combur10 Test* UX, Urinalysis Strips, Roche Diagnostics GmbH, Mannheim, Germany), SFC (Cobas 6000, Roche, Mannheim, Germany) greater than 375 mmol/L (Reference interval 0–375 μmol/L), and at least 2 weeks of twice daily exogenous insulin treatment. Serum fructosamine concentration was measured using colorimetric nitro blue tetrazolium reduction method (Cobas 6000, Roche, Mannheim, Germany). Complete blood count (ADVIA 120, Siemens Medical Solutions Diagnostics GmbH, formerly Bayer HealthCare GmbH, Erfurt, Germany), serum biochemical analysis (Cobas 6000, Roche, Mannheim, Germany), urinalysis and aerobic urine culture performed on urine obtained by cystocentesis were completed in all dogs within the 2‐week period prior to inclusion in the study, alongside any other diagnostic tests deemed necessary to diagnose suspected comorbidities by the attending clinician. Presence of all concurrent illnesses and prescribed treatments were recorded. Dogs were excluded from the study if the owners could not commit to the BBIT protocol and follow‐up visits, if the dog had clinical signs suggestive of hypoglycaemia within the 2 weeks prior to enrolment in the study, or if hypoglycaemia (blood or interstitial glucose <70 mg/dl) was documented during the 2 weeks prior to enrolment in the study. These data were extracted from the medical files and from collection of historical data at visit 0, prior to enrolment and commencement of the BBIT.
The study period lasted up to 6 months and dogs were examined every 2 weeks for the first 2 months, and then every 4 weeks for up to 4 additional months, for a maximum total of 8 follow‐up visits after enrolment. The history, clinical signs, and results of a complete physical examination were recorded and SFC was measured at the time of enrolment (visit 0) and on each of the follow‐up visits. Body condition score (BCS) was assigned by a single investigator during the physical examination at enrolment, based on evaluation of the dog's silhouette, size and location of major fat deposits and palpation, using a 9‐point scoring system (Laflamme, 1997). The historical recorded information related to the time prior to enrolment included the duration of DM, insulin treatment (type, dose and duration), clinical signs, comorbidities and prescribed medications other than insulin (type, dose and duration). On the day of enrolment (visit 0), NPH was given SC, twice daily, at the same dose prescribed during the 2 weeks prior to enrolment, or if NPH was not prescribed previously, at a dose of 0.35 U/kg (in one dog that received glargine insulin prior to enrolment). In addition, at the time of enrolment lispro insulin was administered at a starting dose of 0.05–0.1 U/Kg (rounded down to half unit dose increments), SC twice a day. Both insulin injections were administered to each dog SC every 12 h, within seconds of each other, in random order, at the time of a meal, for the duration of the study. Dogs were fed every 12 h and were not fed between meals. Compliance of owners with the treatment protocol was recorded at each visit.
Owners were required to document changes in their dog's daily drinking and urination habits, appetite, activity level and any sign that could be indicative of hypoglycaemia (i.e., weakness, disorientation, collapse or seizures). Owners were instructed to contact the researchers at any time if they had any concerns or when any signs suggestive of hypoglycaemia were noted.
At each of the follow‐up visits (visits 1–8) PU and PD were scored as notably absent (similar to drinking and urination patterns prior to development of DM; 0) or notably present (1), to reflect whether PU and PD were largely eliminated or not. Appetite was scored as normal (0) or abnormal (hyporexia or polyphagia, 1). Weight was also documented at each follow‐up visit and based on the initial assigned BCS, and was scored as desired unchanged or increased weight (0), indicative of good or improving diabetic control, respectively, or undesired weight change (1) indicative of uncontrolled DM. Serial blood or interstitial glucose measurements (FreeStyle Libre, Abbott, Alameda, CA) were not required for inclusion in the study and were performed at home or in the hospital at the discretion of the attending clinician and depending on owner consent, as would be the case in a primary care setting. Spot BG measurements were obtained during each visit, at the time of SFC measurement and within 4–6 h from the time of meal and insulin administration.
Hypoglycaemia was defined as an interstitial or BG <70 mg/dl documented at home by the owner of the dog, or during follow‐up visits at the hospital. Changes in insulin doses were made at the discretion of the attending clinician. Dogs could be removed from the study at any time, if the owners or the attending clinician deemed this to be in the best interest of the dog.
Results are reported as counts and percentages or median and range, and non‐parametric statistical analysis was performed for all tests because of the small sample size. All continuous variables were not normally distributed as determined visually and by the Skewness and Kurtosis tests for normality. The Wilcoxon signed‐rank test was used for a pairwise comparison of median PU, PD, appetite and weight change scores at the time of enrolment to the medians of all combined scores recorded during visits 1–4 and visits 5–8. The Wilcoxon signed‐rank test was also used for a pairwise comparison of median SFC at the time of enrolment to the median SFC during visits 1–4 and visits 5–8. Spearman's correlation was utilised to assess the association between each (i.e., NPH or lispro) insulin dose during visits 1 through 8 and SFC during the same time period. A p value of <0.05 was considered significant for all tests. All statistical evaluations were performed using a statistical software package (Stata 14.0 for Mac, Stata Corporation, College Station, TX). A sample size calculation was not performed for this exploratory study; however, 12 dogs were enrolled, and this is considered an acceptable number of dogs for a small‐scale preliminary study such as this one (Moore et al., 2011). Previous literature reports median SFC before and 2 weeks after lispro treatment in NPH treated dogs with no concurrent illness (Bertalan et al., 2020). However, means and standard deviations of SFC, needed for a sample size calculation, have not been reported for this treatment protocol in dogs and therefore data needed for a sample size calculation are not yet available.
3. RESULTS
3.1. Dogs’ demographics and historically relevant data
Twelve dogs were enrolled in the study, including 9 (75%) neutered females and 3 (25%) neutered males. Dog breeds included mixed breeds (5 dogs, 42%), Labrador Retriever (2, 17%), and one dog (8%) of each of the following breeds: Golden Retriever, Keeshond, Samoyed, Maltese, and Border Collie. At the time of enrolment, median age was 10.5 years (range, 6–15 years) and median weight was 12.3 kg (range, 5.9–26 kg). Median BCS at enrolment was 5 (range, 3–6). Median duration of DM prior to enrolment was 4 months (range, 1–60 months). Prior to enrolment in the study, most dogs (11/12, 92%) were treated with SC NPH insulin administered twice daily, and one dog (8%) was treated with glargine insulin administered twice daily. One dog also received 0.1 U/kg of regular insulin given SC every 12 h, in combination with NPH insulin, as two separate injections, for 3 weeks prior to enrolment. Dogs were fed Hill's® Prescription Diet w/d (Hill's® Prescription Diet w/d / Hill's Prescription Diet d/d Dog Food with Duck and Rice, Hill's Pet Nutrition, Inc. KS, USA) (8 dogs, 66.7%), ROYAL CANIN® Diabetic (ROYAL CANIN® Diabetic. ROYAL CANIN SAS, France) (2 dogs, 16.7%), PRO PLAN® Canine OM Obesity Management (PRO PLAN® Canine OM Obesity Management, NESTLE PURINA, Italy) (1 dog, 8.3%), and Hill's Prescription Diet d/d Dog Food with Duck and Rice with 5 g of mixed soluble and insoluble fibres per meal (1 dog, 8.3%), to best treat all concurrent illnesses and address individual preferences. All dogs ate the recommended daily meal for their weight according to clinical needs assessment (i.e., desired weight gain), divided into 2 meals per day, given immediately prior to insulin administration. Diets were not changed throughout the study period.
Ten of 12 (83.3%) dogs had previously diagnosed comorbidities at the time of enrolment into the study. Concurrent illnesses included pituitary dependent hyperadrenocorticism (3 of 12 dogs, 25%), and in one dog (8%) each, the following conditions: chronic polypoid cystitis with urolithiasis, chronic inflammatory enteropathy, chronic lymphocytic hepatitis, chronic pancreatitis and polyarthritis, hypothyroidism, severe periodontal disease and chronic urinary tract infection. Treatment for chronic comorbidities was carried out without changes throughout the study period. Historical episodes of diabetic ketoacidosis (3 dogs, 25%), or hyperglycaemic hyperosmolar syndrome (1 dog, 8%) were documented within 3 months of enrolment.
In addition to insulin, dogs received the following medications: amoxicillin‐clavulanate (Synulox, Zoetis, Haupt Pharma Latina, Italy), 15 mg/kg, PO, twice daily (4 dogs, treatment duration ranged between 3 and 6 weeks), metronidazole benzoate (Flagyl, Sanofi, Unither Liquid Manufacturing, France), 15 mg/kg PO, twice daily (2 dogs), and trilostane (Vetoryl, Dales Pharmaceuticals, Snaygill Industrial Estate, UK), 2–4 mg/kg, PO, twice daily (2 dogs, given for 12 and 13 months prior to inclusion). Additionally, one dog each received one of the following medications: prednisone (Prednisone, REKAH Pharmaceutical Prod. LTD), 0.25 mg/kg, PO, once daily; levothyroxine (Eltroxin, Aspen Bad Oldesloe GmbH, Germany, given for >2 years prior to inclusion), 0.01 mg/kg, PO, twice daily, meloxicam (Metacam, Boehringer Ingelheim Animal Health USA Inc., GA, USA), 0.1 mg/kg, PO, once daily, firocoxib (Previcox, Patheon INC., Canada), 5 mg/kg, PO, once daily; and mirtazapine (Mirtazapine, TEVA Pharmaceuticals Industries LTD), 0.65 mg/kg, PO, once daily. Trilostane treatment in one dog with pituitary dependent hyperadrenocorticism was declined due to financial constraints.
3.2. Basal‐bolus insulin treatment implementation, responses and complications
Median duration of follow up was 6 months (range 0.5–6), and most dogs (8, 66.7%) were enrolled in the study for a 6‐month period. The 4 (33.3%) remaining dogs were enrolled in the study for 5 months (2 dogs), 3 months (1 dog) or 0.5 months (1 dog). One dog was removed from the study after 5 months of enrolment because of nonclinical but documented hypoglycaemia which persisted after decreasing the doses of both insulins. At the time of removal from the study, BG in this dog was 51 mg/dl and the NPH and lispro insulin doses were 0.53 U/kg and 0.09 U/kg SC twice daily, respectively. A second dog was removed from the study 3 months after enrolment because of clinical signs consistent with hypoglycaemia. The NPH and lispro insulin doses at the time were 0.6 U/kg and 0.07 U/kg SC twice daily, respectively and BG was not measured at the time. These dogs were removed in order to discontinue lispro treatment and further decrease the NPH dose. A third dog was removed from the study after 5 months of enrolment due to short action duration of NPH, documented with a continuous glucose monitor. This third dog also had clinical signs suggestive of poorly regulated DM including severe polyphagia and weight loss with PU and PD. The fourth dog, which was enrolled for less than 6 months was found dead at home 15 days after enrolment into the study, 2 days after the most recent follow‐up visit, 6 h after insulin injections were administered and after the owner was absent for 2 h. The dog had a history of urinary tract infections and episodic unexplained weakness 6 weeks prior to enrolment into the study with a documented BG of 127 mg/dl at the time of weakness. The NPH and lispro insulin doses at the time of enrolment and throughout the study were 0.6 U/kg and 0.06 U/kg SC twice daily, respectively. The SFC of this fourth dog at the time of enrolment was 619 mmol/L and the last SFC measured during visit 1 and 2 days prior to death was 549 mmol/L. At the time of visit 1, there was also one BG measurement of 208 mg/dl (4 h after the time of both insulin injections). An autopsy was not performed.
Median PU/PD, appetite and weight change scores, as well as SFC at visit 0 and for each of visits 1–8 are reported in Table 1. Median PU and PD scores of combined visits 5–8 were significantly lower (improved) than median scores of combined visits 1–4 and median scores at visit 0 (Table 2). Additionally, median SFC of combined visits 5–8 was significantly lower than median SFC of combined visits 1–4 and median SFC at visit 0, and median SFC of combined visits 1–4 was also significantly lower than median SFC at visit 0 (Table 2). Changes in SFC over time are presented in Figure 1. Median weight score of visits 1–4 and 5–8 was lower (improved) compared to visit 0 (Table 2), but the difference was not significant. No significant differences were detected between the median appetite scores at visit 0, and combined visits 1–4, or combined visits 5–8 (Table 2).
TABLE 1.
Median polyuria, polydipsia, appetite, weight scores and serum fructosamine concentrations at the time of enrolment (visit 0) and at visits 1–8 of dogs treated with lispro and neutral protamine Hagedorn insulins
| Visit # a | Number of dogs |
PU/PD scoreb [median (range)] |
Appetite scorec [median (range)] |
Weight scored [median (range)] |
SFC (μmol/L) [median (range)] |
|---|---|---|---|---|---|
| 0 | 12 | 1 (0–1) | 0 (0–1) | 0.5 (0–1) | 662 (450–990) |
| 1 | 12 | 1 (0–1) | 0 (0–1) | 1 (0–1) | 627.5 (549–988) |
| 2 | 11 | 1 (0–1) | 0.5 (0–1) | 1 (0–1) | 590 (440–937) |
| 3 | 11 | 1 (0–1) | 0 (0–1) | 0 (0–1) | 568 (448–996) |
| 4 | 11 | 1 (0–1) | 0 (0–1) | 0 (0–1) | 544 (302–927) |
| 5 | 11 | 0 (0–1) | 0 (0–1) | 0 (0–1) | 554 (405–887) |
| 6 | 10 | 0 (0–1) | 0 (0–1) | 0 (0–1) | 526.5 (433–927) |
| 7 | 9 | 1 (0–1) | 0 (0–1) | 1 (0–1) | 509 (432–974) |
| 8 | 8 | 0 (0–1) | 0 (0–0) | 0 (0–1) | 500 (401–791) |
PU/PD, polyuria and polydipsia; SFC, serum fructosamine concentration.
Visit 0 was the last assessment prior to commencement of the study treatment protocol. Visit 1 was the first recorded visit following 2 weeks of combined lispro and NPH insulin treatment.
bPolyuria and polydipsia were categorised as absent (0) or present (1).
cAbnormal appetite, whether increased (polyphagia) or decreased (hyporexia) received the score of 1, and normal appetite received a score of 0.
dUndesired weight change received the score of 1, while stable weight or desired weight gain received a score of 0.
TABLE 2.
Median scores of polyuria, polydipsia, weight, appetite and serum fructosamine concentration at visit 0, visits 1–4 and visits 5–8 of dogs treated with lispro and neutral protamine Hagedorn insulins
| PU/PD scoreb | Appetite scorec | Weight scored | SFC (μmol/L) | |||||
|---|---|---|---|---|---|---|---|---|
|
Visit number a |
n | Median (range) | n | Median (range) | n | Median (range) | n | Median (range) |
| 0 | 12 | 1 (0–1) | 12 | 0 (0–1) | 12 | 0.5 (0–1) | 12 | 662 (450–990) |
| 1–4 | 44 | 1 (0–1) | 44 | 0 (0–1) | 44 | 0 (0–1) | 45 | 578 (302–996)h |
| 5–8 | 31 | 0 (0–1)e | 33 | 0 (0–1)f | 36 | 0 (0–1)g | 38 | 512 (401–974)i |
n, number of observations; PU/PD, polyuria and polydipsia; SFC, serum fructosamine concentration.
Visit 0 was the last assessment prior to commencement of the study treatment protocol. Visit 1 was the first recorded visit following 2 weeks of combined lispro and NPH insulin treatment.
bPolyuria and polydipsia were categorised as absent (0) or present (1).
cAbnormal appetite, whether increased (polyphagia) or decreased (hyporexia) received the score of 1, and normal appetite received a score of 0.
dUndesired weight change received the score of 1, while stable weight or desired weight gain received a score of 0.
eMedian PU/PD scores of combined visits 5–8 were significantly lower (improved) than median scores of combined visits 1–4 (p = 0.03) and median scores at visit 0 (p = 0.045).
fMedian appetite scores of visits 1–4 or visits 5–8 were not significantly different from the median appetite score of visit 0 (p = 0.16 and p = 0.31, respectively), nor were any significant differences found between median appetite scores of visits 5–8 compared to visits 1–4 (p = 0.41).
gMedian weight scores of visits 1–4 and visits 5–8 were insignificantly lower compared to visit 0 (p = 0.31 and p = 0.26, respectively), and no significant differences were found between median weight scores of visits 1–4 compared to visits 5–8 (p = 0.63).
hMedian serum fructosamine concentration of combined visits 1–4 was significantly lower than median serum fructosamine concentration at visit 0 (p = 0.03).
iMedian serum fructosamine concentration of combined visits 5–8 was significantly lower than median serum fructosamine concentration of combined visits 1–4 (p = 0.002) and median serum fructosamine concentration at visit 0 (p = 0.03).
FIGURE 1.
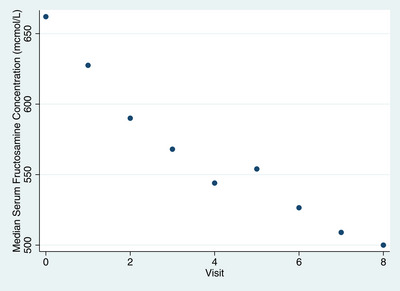
Scatter plot of changes in median serum fructosamine concentration (presented on the Y axis) recorded at 8 follow‐up visits (presented on the X axis) of diabetic dogs treated with NPH and lispro insulins
Spot BG measurements were obtained during each visit, at the time of SFC measurement, to screen for possible hypoglycaemia. Hyperglycaemia was noted in 78/83 (94%) of these measurements, normoglycaemia was documented in 3/83 (3.6%) of these measurements, and hypoglycaemia was detected in 2/83 (2.4%) of these measurements. Serial BG measurements were performed in 25/83 (30%) visits in 6 dogs, either as in hospital 8 to 12‐h BG curves in 16/83 (19%) visits in 3 dogs, or using a continuous glucose monitor in 9/83 (11%) visits in 3 other dogs, at the attending clinician and owner discretion. Nonclinical hypoglycaemia was documented in 5 dogs. Hypoglycaemia (ranging from 45–60 mg/dl) was documented as part of serial BG measurements in 3 dogs, noted on a spot BG measurement (BG = 51 and BG = 60 mg/dl) in 1 dog and recorded using a continuous glucose monitor in another dog (55 mg/dl).
The median NPH dose prescribed during visits 1–8 was 0.48 U/kg SC given twice daily (range, 0.26–1.25 U/kg) and the median lispro dose was 0.08 U/kg SC given twice daily (range 0.05–0.19 U/kg; doses presented in Table 3). Lispro insulin dose in visits 1 through 8 was significantly and negatively, albeit weakly, correlated with SFC concentration during visits 1 through 8 (r = –0.3, p = 0.013), while NPH insulin dose was not correlated with SFC concentrations during the same time frame. All 12 owners exhibited excellent compliance to the protocol in regard to insulin administration. However, 2 owners reported clinical signs suggestive of hypoglycaemia only during a hospital follow‐up visit and not at the time of their occurrence, as instructed.
TABLE 3.
NPH and lispro insulin doses throughout visits 1–8 of dogs treated with lispro and neutral protamine Hagedorn insulins
| Visit |
NPH insulin dose Median (range)* |
Lispro insulin dose Median (range)* |
|---|---|---|
| 0** | 0.445 (0.3–1.04) | ‐ |
| 1 | 0.445 (0.3–1.04) | 0.07 (0.06–0.19) |
| 2 | 0.48 (0.27–0.85) | 0.08 (0.06–0.19) |
| 3 | 0.555 (0.26–0.82) | 0.08 (0.06–0.14) |
| 4 | 0.49 (0.29–0.96) | 0.08 (0.07–0.13) |
| 5 | 0.53 (0.29–1.17) | 0.08 (0.07–0.13) |
| 6 | 0.49 (0.29–1.25) | 0.08 (0.06–0.125) |
| 7 | 0.47 (0.3–0.95) | 0.08 (0.06–0.09) |
| 8 | 0.495 (0.29–0.95) | 0.08 (0.06–0.09) |
Units/kg, doses refer to the prescribed treatment during the 2–4 weeks prior to the evaluation visit.
**Visit 0 was the visit in which basal‐bolus insulin treatment protocol was initiated. Visit 1 was the first recorded visit following 2 weeks of combined NPH and lispro insulin treatment. All dogs received insulin treatment every 12 h, prior and following inclusion. One dog was treated with glargine insulin (0.75 unit/kg every 12 h) prior to inclusion.
4. DISCUSSION
This is the first reported longitudinal study of the effects of several months of NPH and lispro BBIT treatment on clinical signs and SFC in dogs with DM and frequent comorbidities. Serum fructosamine concentration was significantly higher before the introduction of lispro insulin compared to SFC measured after the addition of lispro insulin to the treatment protocol at visits 1–4 and 5–8. Furthermore, SFC at visits 1–4 was significantly higher than SFC at visits 5–8. Similarly, PU/PD improved with introduction of lispro insulin to the treatment protocol as evident by significantly higher clinical scores at the onset of the study and during visits 1–4, compared to visits 5–8. These findings suggest that the addition of lispro insulin to NPH may improve glycaemic regulation and clinical signs associated with DM in certain diabetic dogs with and without comorbidities, and that this improvement is more evident after two months of treatment. However, considering the limited sample size of this pilot study, and various effects of diets and comorbidities, future larger studies, prospectively comparing different insulin treatments, are warranted to establish specific recommendations. Additionally, one dog died, and insulin type was changed early in several dogs due to hypoglycaemic episodes or short activity duration of NPH, indicating this specific BBIT combination is not universally suited to all diabetic dogs, and close monitoring is required to avoid potentially fatal complications.
Visits 1–4 and 5–8 were combined for the purpose of data analysis for several reasons. The initial lispro dose was chosen as a dose perceived to be low and safe, allowing for gradual dose adjustments, as needed over time. Therefore, data from the first few visits do not necessarily reflect optimal treatment goals. Grouping the data also minimalised the number of statistical tests performed and helped avoid a type I statistical error.
Most dogs included in the current study were diagnosed with comorbidities and were considered to have uncontrolled DM, treated for several months (median 4; range 1–60 months) at enrolment. It is therefore concluded that the BBIT with NPH and lispro insulins contributed to the overall improvement in clinical signs and SFC. However, this study was not designed to compare different treatment protocols, and other treatment protocols could also be effective. Future studies, comparing this BBIT protocol to other treatment regimens are indicated to expand available treatment options and allow future tailoring of the best treatment protocol to each individual patient.
A previous study investigating an identical BBIT protocol demonstrated a decrease in SFC in a group of dogs with clinically controlled DM and PPH, concluding that BBIT lowers PPH and contributes to better glycaemic control over a 2‐week period (Bertalan et al., 2020). Interestingly, in the present study, an increased lispro dose, but not NPH dose, was significantly (although weekly) correlated with a decrease in SFC concentrations. It is possible that the lispro correlated decrease in SFC was associated with a decrease in PPH, however, PPH was not quantified in this study. This study was designed before continuous glucose monitors were documented to effectively detect PPH in dogs (Howard et al., 2021; Shiraiwa et al., 2005). Future studies of the BBIT will have the advantage of incorporating continuous glucose monitors in the study design and monitoring PPH in the home environment of the dog.
Four dogs were enrolled in the study for fewer than 6 months, including 2 dogs that remained in the study for 5 months. Two of these dogs had suspected or documented hypoglycaemia, and one dog in which hypoglycaemia cannot be excluded was found dead at home. Previous studies investigating NPH activity in diabetic dogs, reported hypoglycaemia in 7% of BG measurements (Fracassi et al., 2018; Lorenzen, 1992), similar to the hypoglycaemia rate herein (8.4%). Pharmacodynamic studies of NPH demonstrate that BG concentrations nadir about 2–4 h after insulin administration (Fracassi et al., 2018; Palm et al., 2009). It is possible that in some dogs lispro insulin activity overlaps with that of NPH, increasing the risk for hypoglycaemia. Given the potentially fatal complications of hypoglycaemia, it is recommended that dogs treated with this combination of insulin products be monitored closely with a continuous glucose monitor. It is also recommended that when adding lispro insulin to NPH treatment of dogs with DM, the initial dose of lispro not exceed 0.05 U/kg. Finally, if hypoglycaemia is suspected the dose of NPH can also be decreased. If hypoglycaemic episodes persist despite dose reductions, especially in dogs without evident PPH, replacing this NPH and lispro insulins combination with other BBIT combinations or a single‐insulin treatment is indicated.
The insulin combination of NPH and lispro was chosen for this study because this is the only BBIT protocol published in dogs with spontaneous DM (Bertalan et al., 2020). However, due to the potential short activity of NPH in some dogs, other intermediate insulin types may prove beneficial in the BBIT. Lente insulin, for example, has longer duration of action and a later nadir compared to NPH insulin (Fleeman et al., 2009; Fracassi et al., 2018). However, lente insulin has limited availability in some parts of the world, and NPH is as safe and was slightly more effective in achieving good glycaemic control compared to lente insulin in one study. (Fracassi et al., 2018). Times of BG nadirs with lente treatment are also similarly variable (2–12 h) and hypoglycaemia was reported in 38% of lente‐treated dogs, thereby suggesting that the same challenges encountered with NPH, can occur with lente insulin BBIT (Fracassi et al., 2018). Thus, future studies comparing BBIT protocols using different intermediate and longer‐acting insulins are warranted.
Concurrent diseases were documented in most dogs included in the study. Comorbidities may contribute to insulin resistance, generally suspected at insulin doses greater than 1.5 U/kg. (Hess, 2010; RW, 2015) In the present cohort, the BBIT led to improved clinical signs, and decreased SFC, with NPH insulin doses ranging between 0.3 and 0.95 U/kg at the end of the study. These lower doses suggest that while some of the comorbidities were well controlled with specific interventions (i.e., trilostane treatment for hyperadrenocorticism), or did not cause major insulin resistance, lispro and NPH combination treatment can be a beneficial treatment option in diabetic dogs with comorbidities
This study has several limitations. First, the number of dogs enrolled in this pilot study is small, and insignificant findings might be due to small sample size. Second, PPH was not evaluated because continuous glucose monitors had not yet been validated for this use in dogs, at the time of the study. Therefore, it is unknown if the improvement in clinical signs and decrease in SFC are due to a decrease in PPH or other reasons. Third, continuous interstitial glucose monitoring or serial BG curves would have added data regarding hypoglycaemia, and overall DM control; however, both the flash‐glucose monitoring system devices and serial BG curves have many shortcomings (Del Baldo et al., 2020; Howard et al., 2021), affecting their use as monitoring tools in the primary clinical setting. Although clinical signs, used in this study as an end point, are subjective, clinical control (as perceived by owners) remains the major treatment goal in diabetic dogs (Behrend et al., 2018; Briggs et al., 2000). In first opinion practices, clinical signs were the sole monitoring tool in >70% of visits (Cartwright et al., 2019); therefore, clinical assessment and serial SFC measurements reported in this study can be useful for implementation of this protocol in a wide first opinion practice setting. Fourthly, laboratory analytes that could potentially affect SFA (e.g., albumin and triglycerides) were unavailable, precluding investigating their impact on SFC. Additionally, various comorbidities could have caused DM‐associated clinical signs (i.e., PU/PD caused by hyperadrenocorticism) or contributed to insulin‐resistance, impacting the ability to accurately assess the isolated effect of the BBIT on clinical signs. Another study limitation is that dogs with hypoglycaemia were removed from the study, and this could have skewed the results. It is possible that dogs could have been retained in the study with a further reduction in the lispro insulin dose, but because hypoglycaemia can be fatal, the choice was made to remove these dogs from the study, discontinue lispro insulin, and reduce the NPH dose, rather than risk potentially fatal hypoglycaemia. Lastly, diet and exercise were not standardised. Still, all dogs but one received DM‐appropriate diets and this study design does allow for generalisability of the conclusions to the effectiveness of the BBIT in improving clinical signs and decreasing SFC in dogs fed a variety of diets, addressing the many and common concurrent disorders diagnosed in diabetic dogs. None of the diets were changed during the study, and clinical signs and SFC trends of each dog were compared to those measured in other visits, all while consistently feeding the same diet to each dog. There was no specific monitoring for any snacks that might have been given at home, although owners received strict instructions to feed only the prescribed diet and introduce no dietary changes during the study period. With the availability and validation of continuous glucose monitoring systems, future larger cohort studies of this protocol could investigate the rate of hypoglycaemia and the specific effects of this protocol on PPH (Corradini et al., 2016; Del Baldo et al., 2020).
5. CONCLUSIONS
A combination of NPH and lispro insulins reduced SFC and improved clinical signs in a subset of diabetic dogs with and without concurrent illness and with various diet preferences. The BBIT protocol was well accepted by owners and adding insulin injections had no negative effect on owner compliance. However, short action duration of NPH insulin, and potentially overlapping effects of NPH and lispro insulins are potential disadvantages of the BBIT, warranting investigations into additional and different combinations of BBIT. Caution is indicated with implementation of this treatment protocol, and the risk of hypoglycaemia should be addressed by lowering the staring dose of lispro insulin to 0.05 U/kg and with close monitoring for hypoglycaemia.
AUTHOR CONTRIBUTIONS
Sharon Kuzi: data curation; investigation; methodology; project administration; resources; writing—original draft; writing ‐ review & editing. Michal Mazaki‐TOVI: Data curation, investigation, methodology, writing ‐ review & editing. Shai Hershkovitz: data curation; investigation. Einat Yas: data curation; investigation. Rebecka S.Hess: Conseptualization, methodology, resources, writing ‐ original draft, writing ‐ review & editing.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING
The study was made possible by a gift from Ms. Catharine Adler.
ETHICS STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Institutional Ethics Committee of the Hebrew University Veterinary Teaching Hospital (approval # KSVM‐VTH/24_2014).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1077.
Kuzi, S. , Mazaki‐Tovi, M. , Hershkovitz, S. , Yas, E. , & Hess, R. S. (2023). Long‐term field study of lispro and neutral protamine Hagedorn insulins treatment in dogs with diabetes mellitus. Veterinary Medicine and Science, 9, 704–711. 10.1002/vms3.1077
DATA AVAILABILITY STATEMENT
The data presented in this study are available in supplementary material Excel table. cd_value_code=text
REFERENCES
- Baldo, F. D. , Magna, L. , Dondi, F. , Maramieri, P. , Catrina, O. M. , Corradini, S. , Linari, G. , Golinelli, S. , Tardo, A. M. , Bonfanti, U. , & Fracassi, F. (2020). Comparison of serum fructosamine and glycated hemoglobin values for assessment of glycemic control in dogs with diabetes mellitus. American Journal of Veterinary Research, 81, 233–242. [DOI] [PubMed] [Google Scholar]
- Behrend, E. , Holford, A. , Lathan, P. , Rucinsky, R. , & Schulman, R. (2018). AAHA diabetes management guidelines for dogs and cats. Journal of the American Animal Hospital Association, 54, 1–21. [DOI] [PubMed] [Google Scholar]
- Bertalan, A. V. , Drobatz, K. J. , & Hess, R. S. (2020). Effects of treatment with lispro and neutral protamine Hagedorn insulins on serum fructosamine and postprandial blood glucose concentrations in dogs with clinically well‐controlled diabetes mellitus and postprandial hyperglycemia. American Journal of Veterinary Research, 81, 153–158. [DOI] [PubMed] [Google Scholar]
- Briggs, C. E. , Nelson, R. W. , Feldman, E. C. , Elliott, D. A. , & Neal, L. A. (2000). Reliability of history and physical examination findings for assessing control of glycemia in dogs with diabetes mellitus: 53 cases (1995‐1998). Journal of the American Veterinary Medical Association, 217, 48–53. [DOI] [PubMed] [Google Scholar]
- Cartwright, J. A. , Cobb, M. , & Dunning, M. D. (2019). Pilot study evaluating the monitoring of canine diabetes mellitus in primary care practice. Veterinary Record Open, 6, e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini, S. , Pilosio, B. , Dondi, F. , Linari, G. , Testa, S. , Brugnoli, F. , Gianella, P. , Pietra, M. , & Fracassi, F. (2016). Accuracy of a flash glucose monitoring system in diabetic dogs. Journal of Veterinary Internal Medicine, 30, 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Baldo, F. , Canton, C. , Testa, S. , Swales, H. , Drudi, I. , Golinelli, S. , & Fracassi, F. (2020). Comparison between a flash glucose monitoring system and a portable blood glucose meter for monitoring dogs with diabetes mellitus. Journal of Veterinary Internal Medicine, 34, 2296–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleeman, L. M. , & Rand, J. S. (2003). Evaluation of day‐to‐day variability of serial blood glucose concentration curves in diabetic dogs. Journal of the American Veterinary Medical Association, 222, 317–321. [DOI] [PubMed] [Google Scholar]
- Fleeman, L. M. , Rand, J. S. , & Morton, J. M. (2009). Pharmacokinetics and pharmacodynamics of porcine insulin zinc suspension in eight diabetic dogs. The Veterinary Record, 164, 232–237. [DOI] [PubMed] [Google Scholar]
- Fracassi, F. , Corradini, S. , Hafner, M. , Boretti, F. S. , Sieber‐Ruckstuhl, N. S. , & Reusch, C. E. (2015). Detemir insulin for the treatment of diabetes mellitus in dogs. Journal of the American Veterinary Medical Association, 247, 73–78. [DOI] [PubMed] [Google Scholar]
- Fracassi, F. , Linari, G. , Del Baldo, F. , Di Cunzolo, A. , D'Angelo, S. , Malerba, E. , Carotenuto, G. , Bonfanti, U. , & Corradini, S. (2018). Comparison of lente insulin and NPH insulin therapy for the treatment of newly diagnosed diabetic dogs: A randomised study. The Veterinary Record, 183, 262. [DOI] [PubMed] [Google Scholar]
- Hess, R. S. (2010). Insulin resistance in dogs. The Veterinary Clinics of North America. Small Animal Practice, 40, 309–316. [DOI] [PubMed] [Google Scholar]
- Hess, R. S. , & Drobatz, K. J. (2013). Glargine insulin for treatment of naturally occurring diabetes mellitus in dogs. Journal of the American Veterinary Medical Association, 243, 1154–1161. [DOI] [PubMed] [Google Scholar]
- Hess, R. S. , Saunders, H. M. , Van Winkle, T. J. , & Ward, C. R. (2000). Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993‐1998). Journal of the American Veterinary Medical Association, 217, 1166–1173. [DOI] [PubMed] [Google Scholar]
- Howard, L. A. , Lidbury, J. A. , Jeffery, N. , Washburn, S. E. , & Patterson, C. A. (2021). Evaluation of a flash glucose monitoring system in nondiabetic dogs with rapidly changing blood glucose concentrations. Journal of Veterinary Internal Medicine, 35(6), 2628–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme, D. (1997). Development and validation of a body condition score system for dogs. Canine Practice, 22, 10–15. [Google Scholar]
- Lorenzen, F. H. (1992). The use of isophane insulin for the control of diabetes mellitus in dogs. Acta Veterinaria Scandinavica, 33, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler, S. , Zhou, Z. , Dickey, A. S. , Silva, A. M. , Pressel, D. M. , & Barnett, D. W. (2009). Electrical activity and exocytotic correlates of biphasic insulin secretion from beta‐cells of canine islets of Langerhans: Contribution of tuning two modes of Ca2+ entry‐dependent exocytosis to two modes of glucose‐induced electrical activity. Channels (Austin), 3, 181–193. [DOI] [PubMed] [Google Scholar]
- Moore, C. G. , Carter, R. E. , Nietert, P. J. , & Stewart, P. W. (2011). Recommendations for planning pilot studies in clinical and translational research. Clinical and Translational Science, 4, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm, C. A. , Boston, R. C. , Refsal, K. R. , & Hess, R. S. (2009). An investigation of the action of neutral protamine Hagedorn human analogue insulin in dogs with naturally occurring diabetes mellitus. Journal of Veterinary Internal Medicine, 23, 50–55. [DOI] [PubMed] [Google Scholar]
- RW, N. (2015). Canine diabetes mellitus. In Feldman E. C. N. R., Reush C., & Scott‐Moncrieff J. C. (Eds.), Canine and feline endocrinology (pp. 213–253, 4th edn.). St. Louis: Elsevier Saunders. [Google Scholar]
- Shiraiwa, T. , Kaneto, H. , Miyatsuka, T. , Kato, K. , Yamamoto, K. , Kawashima, A. , Kanda, T. , Suzuki, M. , Imano, E. , Matsuhisa, M. , Hori, M. , & Yamasaki, Y. (2005). Postprandial hyperglycemia is a better predictor of the progression of diabetic retinopathy than Hba1c in Japanese type 2 diabetic patients. Diabetes Care, 28, 2806–2807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in supplementary material Excel table. cd_value_code=text


