Abstract
An estimated 70% of patients with colorectal cancer will develop liver metastases during the course of their disease. While the first-line treatment for hepatic metastases is resection, most patients with colorectal liver-only or liver-dominant metastases (CRLM) present with unresectable disease and are not surgical candidates. In the past decade, locoregional liver-directed therapies have demonstrated safety and efficacy in the treatment of patients with unresectable CRLM and chemotherapy-refractory disease. These treatments can be used to attempt conversion to surgical resectability, can control local disease progression, and have the potential to prolong survival. However, they have not yet become the standard of care in many practices. Each treatment has unique risks, and the clinical data are heterogeneous and thus difficult to interpret. In this article, we will review the most recent, high-impact literature on 3 common locoregional therapies used in the treatment of patients with unresectable CRLM: hepatic artery infusion pump chemotherapy, stereotactic body radiation therapy, and selective internal radiation therapy with yttrium-90 embolization. Ultimately, for this patient population, clinical decision-making requires a multidisciplinary discussion which should take into account individual patient characteristics and clinical expertise available at the treatment facility.
Keywords: Colorectal cancer, liver metastases, unresectable, regional therapy, yttrium 90, stereotactic body radiation therapy, hepatic artery infusion pump chemotherapy
Introduction
Colorectal cancer is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths globally, with an incidence that is projected to increase by 60% by the year 2030.1 For those with colorectal cancer, the liver is the most common site of metastatic disease. Approximately 25% of patients initially present with synchronous liver metastases, while an estimated 70% of patients will develop liver metastases during the course of their disease.1,2
The first-line treatment for patients with colorectal liver-only or liver dominant metastases (CRLM) is resection, yet 70% to 80% of patients present with unresectable disease.2,3 The 5-year survival rate for patients with unresectable CRLM remains poor, at approximately 5%.4,5 For these patients, the National Comprehensive Cancer Network (NCCN) guidelines recommend systemic chemotherapy with consideration of additional biologic therapies.6 However, systemic therapies are frequently difficult for patients to tolerate, with approximately 30% of patients discontinuing treatment before completing the full number of cycles.7,8 Moreover, despite receiving adequate chemotherapy, many patients develop progressive disease.9
Over the past decade, locoregional liver-directed therapies have demonstrated safety and efficacy in the treatment of patients with unresectable CRLM with chemotherapy-refractory disease. However, these therapies have not yet become the standard of care in many practices.2 Additionally, establishing a consistent and effective management approach is challenging, due to differing practice patterns among institutions as well as a paucity of comparative studies within the literature.9,10 To address this knowledge gap, this article reviews 3 common locoregional therapies used in the treatment of patients with unresectable CRLM: hepatic artery infusion pump chemotherapy (HAIP), stereotactic body radiation therapy (SBRT), and selective internal radiation therapy with yttrium-90 embolization (Y90). Herein, we will examine recent high-impact literature that reports how these locoregional treatments influence overall survival (OS), progression-free survival (PFS), and conversion to resection, along with their commonly associated adverse events (AEs).
Methods
We performed a comprehensive systematic literature review to identify recent publications discussing the role of HAIP, SBRT, or Y90 in the treatment of unresectable CRLM. We reviewed only literature that focused on a molecularly unselected patient population, which is an important distinction since select tumor mutations in unresectable CRLM may demonstrate durable responses to specific systematic or liver-directed therapies.11 Specific outcomes of interest included OS; PFS, or local control if PFS data were not reported; conversion to resection; and AEs. AEs were graded using either the Common Toxicity Criteria Adverse Events version 3.0, with specific focus on AEs of grade 3 (serious) or higher, or the Clavien-Dindo classification of surgical complications.12,13 We included studies published after 2010 and included phase 1, phase 2, and phase 3 trials; systematic reviews; meta-analyses; case-control studies; and prospective or retrospective cohort studies (Figure). Consensus guidelines, single-case reports, and meeting abstracts were excluded from this review. A total of 26 papers met inclusion criteria and were analyzed.
Figure.
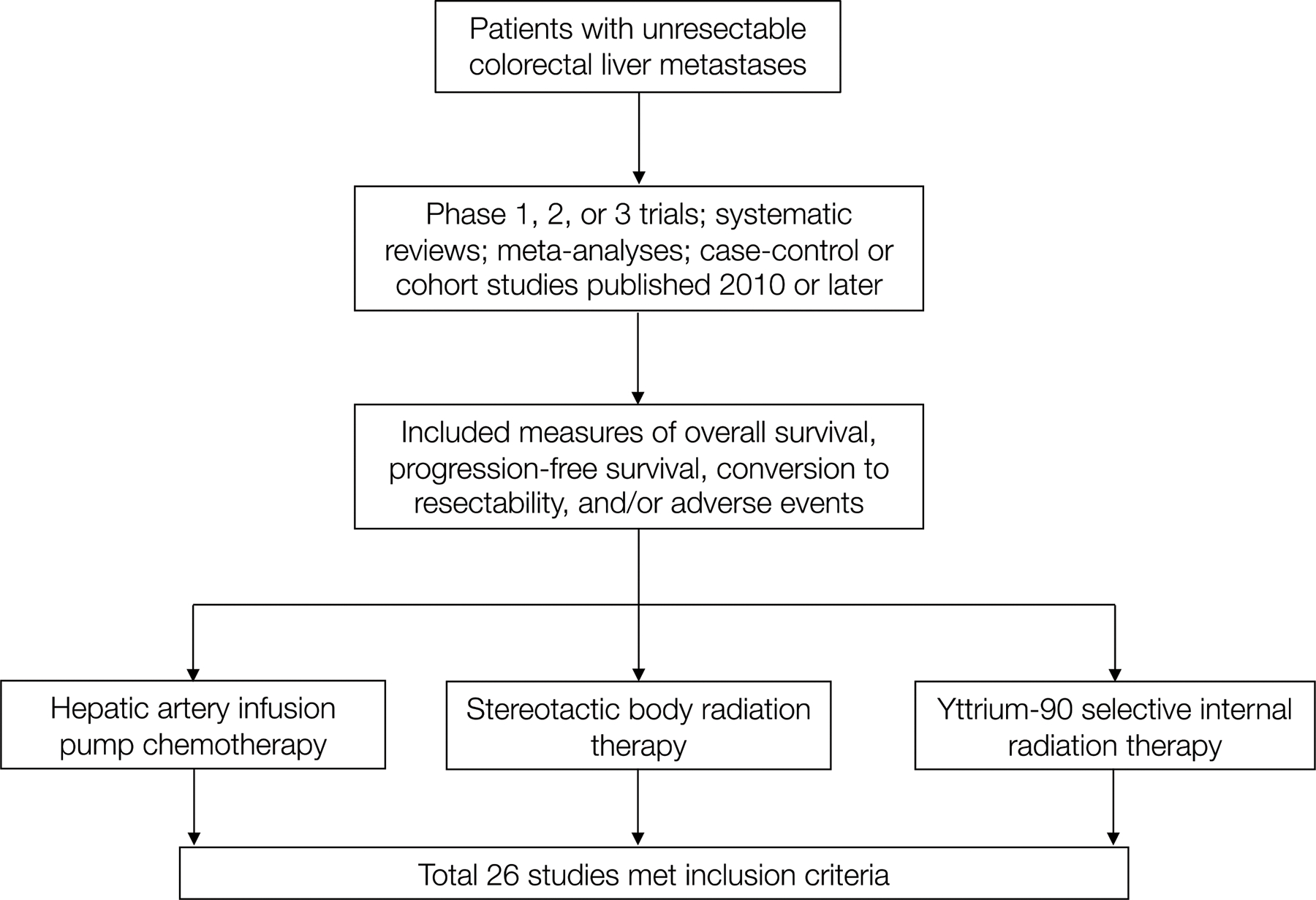
Schema of Literature Review Process
Hepatic Artery Infusion Pump Chemotherapy
Since its inception in the 1950s, HAIP has been refined as a safe and effective strategy to control disease progression or expand resectability in patients with unresectable CRLM.14,15 This locoregional therapy capitalizes on the unique blood supply of the liver, as the hepatic artery predominantly supplies liver metastases, while the portal vein perfuses normal hepatocytes. Infusion of chemotherapy directly into the hepatic artery allows selective drug delivery of maximal cytotoxic concentrations to metastatic lesions with relative sparing of the normal liver parenchyma and minimization of systemic AEs. HAIP is typically administered via the gastroduodenal artery by a surgically implanted pump or a percutaneously placed catheter connected to an external pump.15,16 Furthermore, HAIP allows for high first-pass hepatic extraction and concomitant administration of systemic therapy. The NCCN recommends that HAIP be considered for selected patients with unresectable CRLM; however, it should be implemented only at institutions with surgical and medical oncology expertise in HAIP administration (category 2B recommendation).6
We examined 8 peer-reviewed studies focusing on the role of HAIP in the treatment of unresectable CRLM (Table 1). This literature review includes 3 prospective phase 2 trials, 2 retrospective multicenter reviews, 2 retrospective single-institution reviews, and 1 meta-analysis. The included studies examined patients who may have received prior chemotherapy but were not previously treated with resection/ablation or HAIP. The publication years ranged from 2015 to 2021 and the number of patients included in each study ranged from 59 to 3000.
Table 1.
Hepatic Artery Infusion Pump Chemotherapy for Treatment of Unresectable CRLM
| Study | Design | Treatment | N | Prior treatments | Median OS, months (95% CI) | Median PFS, months (95% CI) | Conversion to resection/ablation | Adverse events |
|---|---|---|---|---|---|---|---|---|
| D’Angelica et al (2015)17 | Prospective, phase 2 | HAIP + chemotherapy | 49 | Chemotherapy | 38 (28 to not reached) | 13 (7–16) | 47% | 41% grade ≥3 |
| Zacharias et al (2015)24 | Meta-analysis | HAIP | 3000 | None or chemotherapy | First-line: 21.4 (19.4–23.4) Second-line or later: 13.2 (12.2–14.2) |
NR | 15% | 55% grade ≥3 |
| Lévi et al (2016)18 | Prospective, phase 2 | HAIP + chemotherapy | 64 | Chemotherapy | 25.5 (18.8–32.1) | 9.3 (7.8–10.9) | 29.7% | 77% grade ≥3 |
| Dhir et al (2017)20 | Retrospective, single-center | HAIP + chemotherapy vs chemotherapy alone | 86 | Chemotherapy | 32.8 vs 15.3 (0.21– 0.72) | NR | No group difference | Not reported |
| Lim et al (2017)21 | Retrospective, multicenter | HAIP + chemotherapy: first/second- vs third/fourth-line | 61 | Chemotherapy | 13.5 vs 8.3 (0.4–1.1) | 9 vs 6 (0.2–0.7) | 16.4% | 16% grade ≥3 |
| Pak et al (2018)19 | Prospective, phase 2 | HAIP + chemotherapy | 64 | Chemotherapy | 38 (28.8–53.7) | 13 (9–16) | 52% | ≥20% grade ≥3 |
| Boilève et al (2020)22 | Retrospective, single-center | HAIP + chemotherapy | 89 | Chemotherapy | 20 (15–24) | 9 (8–11) | 27% | 79% grade ≥3 |
| Muaddi et al (2021)23 | Retrospective, multicenter | HAIP + chemotherapy | 154 | Chemotherapy | 19.5 (IQR, 10.5–31) | 3-year PFS, 4.1%* | 7.8% | 8.4% biliary sclerosis 4.6% Clavien-Dindo ≥3b during hospitalization |
CRLM, colorectal liver-only or liver-dominant metastases; HAIP, hepatic artery infusion pump chemotherapy; OS, overall survival; PFS, progression-free survival; IQR, interquartile range; NR, not reported.
95% CI and/or P value not reported
The three phase 2 trials examined survival and resection outcomes among patients with unresectable CRLM who received HAIP in addition to systemic chemotherapy.17–19 Each of these studies, which included 49, 64, and 64 patients respectively, demonstrated relatively similar median OS (25.5–38 months) and PFS (9.3–13 months). More importantly, up to 52% of patients demonstrated conversion to resectability, therefore offering these patients a chance for cure. In the phase 2 study conducted by D’Angelica et al,17 all patients received HAIP in addition to systemic chemotherapy. However, most patients (65%) were receiving HAIP and chemotherapy as their second- or third-line therapy for unresectable CRLM. Overall, 47% of patients achieved conversion to resection over a median timeframe of 6 months, and conversion was the only factor associated with prolonged OS and PFS in multivariate analyses.
Four retrospective analyses were reviewed, demonstrating promising trends in median OS and conversion to resection when utilizing HAIP for unresectable CRLM. Dhir et al20 performed a single-institution retrospective case-control study examining 86 patients who received either HAIP plus chemotherapy or chemotherapy alone. OS was statistically longer for patients who received HAIP plus chemotherapy (32.8 months) compared to those who did not receive HAIP (15.3 months; 95% CI, 0.21–0.72). There was no difference in conversion to resection rates between treatment groups. Lim et al21 performed a multicenter retrospective comparison of 61 patients who either received HAIP plus chemotherapy as first- or second-line treatment for unresectable CRLM vs third- or fourth-line treatment. The authors reported an improvement in median PFS in patients receiving HAIP plus chemotherapy as an earlier treatment - 9 vs 6 months (95% CI, 0.18–0.66), but the improvement in median OS was not statistically significant. Among all patients, the conversion to resection rate was 16.4%. Two additional retrospective cohort studies including 89 to 154 patients in each study receiving HAIP plus chemotherapy were reviewed.22,23 Median OS and the rate of conversion to resection was 19.5 months and 7.8%, respectively, in one study, and 20 months and 27%, respectively, in the other.
A recent meta-analysis pooled data from 90 studies that examined 3,000 patients who underwent hepatic artery–directed therapies; it found a median OS for HAIP as first-line treatment of 21.4 months (95% CI, 19.4–23.3) vs 13.2 months (95% CI, 12.2–14.2) as a second-line or later therapy.24 Overall, the conversion to resection rate was highest among patients receiving HAIP (15%) compared with other hepatic artery therapies such as transcatheter arterial chemoembolization (4%) or radioembolization (2%).
The main drawbacks of HAIP are the requirements for technical expertise, an experienced team of oncologists, and the potential for biliary toxicity, which may necessitate dose adjustment, coadministration with dexamethasone, or stent placement.14 In the studies examined, the rate of grade ≥ 3 AEs ranged from 8.4% to 79%, with the most common complications including diarrhea (29%), transaminitis (16%), pump-related complications (14.3%), abdominal pain (12%), biliary sclerosis (8.4%), vomiting (6%), and neutropenia (2%). 17,23 Another possible disadvantage when considering HAIP is that its use may restrict future use of additional locoregional therapies, such as Y90 or transarterial chemoembolization.
In conclusion, in studies of patients who had received prior chemotherapy for unresectable CRLM, the addition of HAIP may improve survival and rates of conversion to resection. However, providers must weigh the potential benefits of HAIP against its risks of toxicity and the need for referral to institutions with HAIP infrastructure and expertise.
Stereotactic Body Radiation Therapy
SBRT aims to precisely deliver large, hypofractionated doses of radiation to target lesions while minimizing its delivery to adjacent normal tissues.25,26 Through image guidance, this noninvasive locoregional modality induces cell death and coagulation necrosis of the targeted tissue, causing a gradual reduction in tumor size and/or complete replacement by fibrosis.27,28 Multiple hepatic lesions can be treated simultaneously; however, practitioners must ensure that adequate liver volume is spared from unintended radiation spread.29 The NCCN states that SBRT is a reasonable treatment option for patients with CRLM who are not candidates for resection, ablation, or participation in a clinical trial.6
We analyzed 9 peer-reviewed studies that investigated the clinical outcomes of patients treated with SBRT for unresectable CRLM (Table 2). This literature review included 5 retrospective cohort studies, 1 systematic review, and 3 prospective studies. The publication years ranged from 2010 to 2021, and the number of patients examined ranged from 11 to 656 per study. The patient populations across studies varied markedly with respect to previous lines of chemotherapy, prior hepatic interventions, and the presence or absence of extrahepatic metastases. Additionally, we observed substantial variation in the main outcome reported, which included a mixture of median OS, percent survival over time, median PFS, and percent local control. This heterogeneity contributed to the complexity in interpretation of these studies.
Table 2.
Stereotactic Body Radiation Therapy for Treatment of Unresectable CRLM
| Study | Design | Treatment | N | Prior treatments | Median OS (±SD#), months (95% CI) | Median PFS, months (95% CI) | Adverse events |
|---|---|---|---|---|---|---|---|
| van der Pool et al (2010)37 | Prospective, single-center cohort | SBRT | 20 | Any | 34* | 11* | 2 cases ≥ grade 3 |
| Kress et al (2012)30 | Retrospective, single-center cohort | SBRT | 11 | Any | 16.1* | 1-year LC: 72% | 1 case ≥ grade 3 |
| Scorsetti et al (2015)36 | Prospective, phase 2 | SBRT | 42 | Any | 29.0 ±3.7 (21.8–36.2) | 12 ±4.2 (3.8–20.2) | None ≥ grade 3 |
| McPartlin et al (2017)38 | Prospective, phase 1 and 2 | SBRT | 60 | Any | 16.0 (11.9–20.5) | 10.8* | 1 case ≥ grade 3 |
| Doi et al (2017)31 | Retrospective, single-center cohort | SBRT | 24 | Any | 45* | NR | Not reported |
| Petrelli et al (2018)35 | Systematic review | SBRT | 656 | Any | 31.5* | 11.5* | 8.7% ≥ grade 3 |
| Vernaleone et al (2019)32 | Retrospective, single-center cohort | SBRT | 38 | Any | 20.1 (±2.0) | 6.6 (±0.9) | None ≥ grade 3 |
| Flamerique et al (2020)33 | Retrospective, single-center cohort | SBRT | 22 | Any | 24* | NR | 1 case ≥ grade 3 |
| Py et al (2021)34 | Retrospective, single-center cohort | SBRT | 67 | Any | 53 (38–66) | 1-year LC: 81.9% (70.2%-89.2%) 5-year LC: 13.1% (6.0%-23.0%) |
3% ≥ grade 3 |
CRLM, colorectal liver-only or liver-dominant metastases; SBRT, stereotactic body radiation therapy; OS, overall survival; PFS, progression-free survival; LC, local control; NR, not reported.
95% CI and/or P value not reported.
Where available.
We examined 5 retrospective cohort analyses reporting outcomes among patients with unresectable CRLM treated with SBRT; the study populations ranged from 11 to 67 patients.30–34 Median OS ranged from 16.1 to 53 months. Only 1 study reported median PFS, which was 6.6 months (SD ±0.93),32 whereas other studies reported a 1-year local control rate between 73% and 91.9%.30,34 A final systematic review that pooled data from 18 studies was assessed.35 Of 656 patients receiving SBRT for unresectable CRLM, Petrelli et al35 reported a median OS of 31.5 months and a median PFS of 11.5 months. Three prospective single-arm analyses including between 20 and 60 patients in each study who received SBRT were also reviewed.36–38 In these studies, median OS ranged from 16 to 34 months and median PFS from 10.8 to 12 months.
Overall, SBRT is well tolerated as it is a noninvasive modality with short treatment sessions typically lasting less than an hour each.29 In the studies examined, the rate of grade ≥ 3 AEs ranged from 3% to 10%, with the most common complications including nausea (5%), gastrointestinal ulcers (5%), thrombocytopenia (2%), and transient transaminitis (2%).31,33,38 Another possible disadvantage when considering this locoregional therapy is that tumor response can be limited by histologic subtype and prior use of chemotherapy, both of which have been linked to increased rates of local failure.29
In conclusion, SBRT may be an attractive option for patients with chemotherapy-refractory, unresectable CRLM in whom more invasive locoregional therapies that require a percutaneous approach are contraindicated. It is also an appealing option for patients who require the treatment of multiple hepatic tumors if an adequate liver volume can be spared from unintentional radiation spread. Unfortunately, due to a paucity of high-impact studies, interpretation of the clinical data is limited. For this reason, further research is warranted regarding the use of SBRT among patients with unresectable CRLM who are unable to receive more invasive liver-directed therapies.
Selective Internal Radiation Therapy With Yttrium 90 Embolization
Treatment with Y90 involves selectively injecting radioactive yttrium-90 microparticles via a catheter into the hepatic artery branch that feeds a tumor.39,40 These microparticles then become permanently lodged in the tumor vasculature, consequently delivering high-dose beta radiation to the surrounding tissue to induce tumor necrosis.38–41 The NCCN states that Y90 radioembolization can be considered in select patients with unresectable CRLM who have chemotherapy-resistant or refractory disease and predominant hepatic metastases.6
We examined 9 peer-reviewed studies that focused on the role of Y90 radioembolization in the treatment of unresectable CRLM (Table 3). This included 1 systematic review, 1 prospective and 3 retrospective cohort studies, 1 prospective case series, and 3 reports that collectively discussed a total of 3 prospective randomized control studies. The papers were published between 2014 and 2019 and included between 52 and 1103 patients in each study.
Table 3.
Yttrium-90 Selective Internal Radiation Therapy for Treatment of Unresectable CRLM
| Study | Design | Treatment | N | Prior treatments | Median OS, months (95% CI) | Median PFS, months | Adverse events |
|---|---|---|---|---|---|---|---|
| Saxena et al (2014)49 | Systematic review | Y90 | 979 (20 studies) | Chemo ± hepatic intervention | 12 (range, 8.3–36.0) | 9 (range, 6–16) | Not reported |
| Saxena et al (2015)45 | Retrospective, single-center cohort | Y90 | 302 | Chemo ± hepatic intervention | 10.5* | NR | None ≥ grade 3 |
| Hickey et al (2015)46 | Retrospective, multicenter cohort | Y90 | 531 | Any | 10.6 (8.8–12.4) | NR | 13% ≥ grade 3 |
| Abbott et al (2015)47 | Retrospective, single-center cohort | Y90 | 68 | Chemo ± hepatic intervention | 11.6* | NR | 7.3% ≥ grade 3 |
| Golfieri et al (2015)48 | Prospective, single-center case series | Y90 | 52 | Chemo ± hepatic intervention | 11.0 (8.0–14.0) | NR | 6% ≥ grade 3 |
| Van Hazel et al (2016)43 | Prospective, multicenter RCT | Chemo vs chemo + Y90 | 530 | None | NR | 10.2 (chemo) vs 10.7 (chemo + Y90); P=0.4 | 74.4% (chemo) vs 85.4% (chemo + Y90) grade ≥ 3; P=0.5 |
| Wasan et al (2017)42 | 3 multicenter RCTs (combined analysis) | Chemo vs chemo + Y90 | 1103 | None | 23.3 (chemo) vs 22.6 (chemo + Y90); P=0.6 | 10.3 (chemo) vs 11.0 (chemo + Y90); P=0.1 | Y90 group greater odds of grade ≥ 3 |
| Gibbs et al (2018)44 | 2 multicenter RCTs (combined analysis) | Chemo vs chemo + Y90, stratified by primary tumor side | 739 | None | RSP: 22.0 (chemo + Y90) vs 17.1 (chemo); P=0.01 LSP: 24.6 (chemo + Y90) vs 26.6 (chemo); P=0.3 |
RSP: 10.8 (chemo + Y90) vs 8.7 (chemo); P=0.06 LSP: 11.4 (chemo + Y90) vs 10.8 (chemo); P=0.4 |
RSP: No difference in grade ≥ 3 LSP: Y90 group greater odds of grade ≥ 3 |
| White et al (2019)2 | Prospective, multicenter cohort | None | 399 | Chemo ± hepatic intervention | 7.6 (6.9–8.3) | 3.0 (2.8–3.1) | 143 (36%) experienced an AE; 8% were ≥ grade 3 |
CRLM, colorectal liver-only or liver-dominant metastases; RCT, randomized controlled trial; Y90, yttrium-90 selective internal radiation therapy; chemo, chemotherapy; OS, overall survival; PFS, progression-free survival; RSP, right-sided primary tumor; LSP, left-sided primary tumor; NR, not reported; AE, adverse event.
95% CI and/or P value not reported.
SIRFLOX, FOXFIRE, and FOXFIRE-Global were all multicenter phase 3 randomized control trials showing that Y90 radioembolization in addition to FOLFOX-based chemotherapy does not improve OS or PFS when compared with chemotherapy alone as first-line treatment for unresectable CRLM.42,43 A subsequent subgroup analysis of the SIRFLOX and FOXFIRE-Global trials by Gibbs et al44 showed a 4.9-month increase in median OS (P=0.008) in only those patients with right-sided primary tumors.44
Failure of the aforementioned phase 3 trials to show definitive superiority of Y90 radioembolization as a first-line treatment has shifted focus away from this locoregional therapy as an option for patients with unresectable CRLM who have failed 1 or more lines of chemotherapy. While several single-arm studies have been published specifically on this topic, the patient populations across studies are markedly variable with respect to previous lines of chemotherapy, prior hepatic resection or ablation, and the presence of extrahepatic metastases. Patients in these studies were generally high-functioning, with 94% to 100% of patients having an ECOG status of 0 or 1 among single-arm studies reporting the statistic.45–48 In studies regarding the use of salvage Y90 radioembolization for unresectable CRLM, median OS ranged from 7.6 to 11.6 months.44–49 Only 1 study reported median PFS for salvage Y90 radioembolization, which was 3 months (95% CI, 2.8–3.1).2 These outcomes are clearly inferior to those obtained with HAIP and SBRT, and thus we infer that Y90 radioembolization should be reserved for salvage therapy only in patients with unresectable CRLM.
Overall, Y90 radioembolization is safe and well tolerated. However, the delivery of Y90 microparticles to tissues other than the tumor can lead to complications.40 Fortunately, the beta radiation is quite precise, penetrating on average only 2.5 mm from its source and thereby limiting its effects to the intended delivery site.40 Serious complications can include gastrointestinal ulcers, radiation pneumonitis, and radioembolization-induced liver disease, which includes portal hypertension or damage to the biliary tree.40 More common postprocedural complaints include nausea, abdominal pain, and generalized fatigue.47 An analysis of three phase 3 trials reported that less than 6% of patients developed grade ≥ 3 AEs associated with Y90 radioembolization. Another analysis examining the use of Y90 radioembolization in patients who failed previous lines of chemotherapy reported that between 0% and 13% of participants developed grade ≥ 3 AEs.2,45–48
In conclusion, Y90 radioembolization is not recommended as a first-line treatment option for patients with unresectable CRLM. However, it is a potentially safe therapy in the salvage setting. While Y90 radioembolization is generally well tolerated, interpretation of the clinical data reported is limited due to the heterogeneous patient populations and lack of comparison groups. Nonetheless, in a patient population with few remaining treatment options, this therapy has the potential to improve OS (within the right-sided metastases population) with a relatively low incidence of serious AEs. Future areas of research may focus on studying Y90 radioembolization as a first-line therapy for unresectable CRLM in patients with right-sided primary tumors and conducting phase 3 trials comparing it to other locoregional treatments or supportive care for patients with unresectable CRLM refractory to chemotherapy. Additionally, other uses of Y90 radioembolization reported in the literature deserve further large-scale scientific inquiry, including its use to downsize CRLM for resection and to induce contralateral liver hypertrophy.50–51
Conclusions
Locoregional liver-directed therapies are an attractive option for patients with unresectable CRLM. In general, these therapies are well tolerated and AE profiles are minimal. Unfortunately, the lack of large-scale, prospective phase 3 trials complicates the interpretation of available data. HAIP is considered a safe and effective strategy to control disease progression or expand resectability. However, providers must weigh these potential benefits with risks of toxicity and the need for referral to institutions with HAIP infrastructure and expertise. SBRT may be an attractive option for patients with unresectable CRLM for whom locoregional therapies that require a percutaneous approach are contraindicated. Lastly, although Y90 radioembolization was not shown to be effective as first-line treatment for patients with unresectable CRLM, it has shown some potential in patients with chemotherapy-refractory disease and in those with right-sided primary tumors. Ultimately, for patients with unresectable CRLM, clinical decisions require multidisciplinary discussions that carefully consider the patient’s disease process, comorbidities, and functional status in addition to the available clinical expertise at the treating facility.
sources of funding:
This work was supported by a training grant from the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR001860). The funding agency was not involved in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Footnotes
Conflicts of interest: The authors do not report any conflicts of interest.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 2.White J, Carolan-Rees G, Dale M, et al. Analysis of a National Programme for Selective Internal Radiation Therapy for Colorectal Cancer Liver Metastases. Clin Oncol. 2019;31(1):58–66. doi: 10.1016/j.clon.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, De Gramont A, Figueras J, et al. The Oncosurgery Approach to Managing Liver Metastases from Colorectal Cancer: A Multidisciplinary International Consensus. Oncologist. 2012;17(10):1225–1239. doi: 10.1634/theoncologist.2012-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Eynde M, Hendlisz A. Treatment of colorectal liver metastases: a review. Rev Recent Clin Trials. 2009;4(1):56–62. doi: 10.2174/157488709787047558 [DOI] [PubMed] [Google Scholar]
- 6.Colon Cancer (Version 2.2021). National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Published 2021. Accessed April 15, 2021. [Google Scholar]
- 7.Fairchild AH, White SB. Decision Making in Interventional Oncology: Intra-arterial Therapies for Metastatic Colorectal Cancer-Y90 and Chemoembolization. Semin Intervent Radiol. 2017;34(2):87–91. doi: 10.1055/s-0037-1601854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji JH, Park SH, Lee J, et al. Prospective phase II study of neoadjuvant FOLFOX6 plus cetuximab in patients with colorectal cancer and unresectable liver-only metastasis. Cancer Chemother Pharmacol. 2013;72(1):223–230. doi: 10.1007/s00280-013-2190-1 [DOI] [PubMed] [Google Scholar]
- 9.Levy J, Zuckerman J, Garfinkle R, et al. Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2018;20(10):905–915. doi: 10.1016/j.hpb.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Young AL, Adair R, Culverwell A, et al. Variation in referral practice for patients with colorectal cancer liver metastases. Br J Surg. 2013;100(12):1627–1632. doi: 10.1002/bjs.9285 [DOI] [PubMed] [Google Scholar]
- 11.Dendy MS, Ludwig JM, Kokabi N, et al. Genomic mutations and histopathologic biomarkers in Y90 radioembolization for chemorefractory colorectal liver metastases. Oncotarget. 2018;9(65):32523–32533. doi: 10.18632/oncotarget.25992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Published 2006. Accessed August 8, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- 13.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 14.Anteby R, Kemeny NE, Kingham TP, et al. Getting Chemotherapy Directly to the Liver: The Historical Evolution of Hepatic Artery Chemotherapy. J Am Coll Surg. 2021;232(3):332–338. doi: 10.1016/j.jamcollsurg.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg. 2019;154(8):768–776. doi: 10.1001/jamasurg.2019.1694 [DOI] [PubMed] [Google Scholar]
- 16.Leung M, Gholami S. The state of hepatic artery infusion chemotherapy in the management of metastatic colorectal cancer to the liver. Chinese Clin Oncol. 2019;8(5):54. doi: 10.21037/cco.2019.09.01 [DOI] [PubMed] [Google Scholar]
- 17.DʼAngelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chem otherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261(2):353–360. doi: 10.1097/SLA.0000000000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lévi FA, Boige V, Hebbar M, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(2):267–274. doi: 10.1093/annonc/mdv548 [DOI] [PubMed] [Google Scholar]
- 19.Pak LM, Kemeny NE, Capanu M, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol. 2018;117(4):634–643. doi: 10.1002/jso.24898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhir M, Jones HL, Shuai Y, et al. Hepatic Arterial Infusion in Combination with Modern Systemic Chemotherapy is Associated with Improved Survival Compared with Modern Systemic Chemotherapy Alone in Patients with Isolated Unresectable Colorectal Liver Metastases: A Case-Control Study. Ann Surg Oncol. 2017;24(1):150–158. doi: 10.1245/s10434-016-5418-6 [DOI] [PubMed] [Google Scholar]
- 21.Lim A, Le Sourd S, Senellart H, et al. Hepatic Arterial Infusion Chemotherapy for Unresectable Liver Metastases of Colorectal Cancer: A Multicenter Retrospective Study. Clin Colorectal Cancer. 2017;16(4):308–315. doi: 10.1016/j.clcc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Boilève A, De Cuyper A, Larive A, et al. Hepatic arterial infusion of oxaliplatin plus systemic chemotherapy and targeted therapy for unresectable colorectal liver metastases. Eur J Cancer. 2020;138:89–98. doi: 10.1016/j.ejca.2020.07.022 [DOI] [PubMed] [Google Scholar]
- 23.Muaddi H, D’Angelica M, Wiseman JT, et al. Safety and feasibility of initiating a hepatic artery infusion pump chemotherapy program for unresectable colorectal liver metastases: A multicenter, retrospective cohort study. J Surg Oncol. 2021;123(1):252–260. doi: 10.1002/jso.26270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharias AJ, Jayakrishnan TT, Rajeev R, et al. Comparative Effectiveness of Hepatic Artery Based Therapies for Unresectable Colorectal Liver Metastases: A Meta-Analysis. PLoS One. 2015;10(10):e0139940. doi: 10.1371/journal.pone.0139940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkpatrick JP, Kelsey CR, Palta M, et al. Stereotactic body radiotherapy: A critical review for nonradiation oncologists. Cancer. 2014;120(7):942–954. doi: 10.1002/cncr.28515 [DOI] [PubMed] [Google Scholar]
- 26.Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol. 2014;20(12):3100–3111. doi: 10.3748/wjg.v20.i12.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maturen KE, Feng MU, Wasnik AP, et al. Imaging effects of radiation therapy in the abdomen and pelvis: evaluating “innocent bystander” tissues. Radiographics. 33(2):599–619. doi: 10.1148/rg.332125119 [DOI] [PubMed] [Google Scholar]
- 28.Haddad MM, Merrell KW, Hallemeier CL, et al. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol. 2016;41(10):2061–2077. doi: 10.1007/s00261-016-0768-x [DOI] [PubMed] [Google Scholar]
- 29.Loi M, Desideri I, Dominici L, et al. Thermal Ablation versus SBRT in liver tumours: pros and cons. Med Oncol. 2020;37(6):1–8. doi: 10.1007/s12032-020-01377-7 [DOI] [PubMed] [Google Scholar]
- 30.Kress MAS, Collins BT, Collins SP, Dritschilo A, Gagnon G, Unger K. Stereotactic body radiation therapy for liver metastases from colorectal cancer: Analysis of safety, feasibility, and early outcomes. Front Oncol. 2012;2(FEB):1–7. doi: 10.3389/fonc.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doi H, Uemoto K, Suzuki O, et al. Effect of primary tumor location and tumor size on the response to radiotherapy for liver metastases from colorectal cancer. Oncol Lett. 2017;14(1):453–460. doi: 10.3892/ol.2017.6167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernaleone M, Bonomo P, Di Cataldo V, et al. Robotic stereotactic radiotherapy for liver oligometastases from colorectal cancer: a single-center experience. Radiol Medica. 2019;124(9):870–876. doi: 10.1007/s11547-019-01042-8 [DOI] [PubMed] [Google Scholar]
- 33.Flamarique S, Campo M, Asín G, Pellejero S, Viúdez A, Arias F. Stereotactic body radiation therapy for liver metastasis from colorectal cancer: size matters. Clin Transl Oncol. 2020;22(12):2350–2356. doi: 10.1007/s12094-020-02375-x [DOI] [PubMed] [Google Scholar]
- 34.Py JF, Salleron J, Courrech F, et al. Long-term outcome of Stereotactic Body Radiation Therapy for patient with unresectable liver metastases from colorectal cancer. Cancer Radiother. 2021;25(4):350–357. doi: 10.1016/j.canrad.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 35.Petrelli F, Comito T, Barni S, Pancera G, Scorsetti M, Ghidini A. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol. 2018;129(3):427–434. doi: 10.1016/j.radonc.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 36.Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141(3):543–553. doi: 10.1007/s00432-014-1833-x [DOI] [PubMed] [Google Scholar]
- 37.Van Der Pool AEM, Méndez Romero A, Wunderink W, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010;97(3):377–382. doi: 10.1002/bjs.6895 [DOI] [PubMed] [Google Scholar]
- 38.McPartlin A, Swaminath A, Wang R, et al. Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int J Radiat Oncol Biol Phys. 2017;99(2):388–395. doi: 10.1016/j.ijrobp.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 39.Gulec SA, Fong Y. Yttrium 90 microsphere selective internal radiation treatment of hepatic colorectal metastases. Arch Surg. 2007;142(7):675–682. doi: 10.1001/archsurg.142.7.675 [DOI] [PubMed] [Google Scholar]
- 40.Sangro B, Martínez-Urbistondo D, Bester L, et al. Prevention and treatment of complications of selective internal radiation therapy: Expert guidance and systematic review. Hepatology. 2017;66(3):969–982. doi: 10.1002/hep.29207 [DOI] [PubMed] [Google Scholar]
- 41.Justinger C, Gruden J, Kouladouros K, et al. Histopathological changes resulting from selective internal radiotherapy (SIRT). J Surg Oncol. 2018;117(5):1084–1091. doi: 10.1002/jso.24967 [DOI] [PubMed] [Google Scholar]
- 42.Wasan HS, Gibbs P, Sharma N, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159–1171. doi: 10.1016/S1470-2045(17)30457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 (Plus or Minus Bevacizumab) versus mFOLFOX6 (Plus or Minus Bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(15):1723–1731. doi: 10.1200/JCO.2015.66.1181 [DOI] [PubMed] [Google Scholar]
- 44.Gibbs P, Heinemann V, Sharma N, et al. Effect of Primary Tumor Side on Survival Outcomes in Untreated Patients With Metastatic Colorectal Cancer When Selective Internal Radiation Therapy Is Added to Chemotherapy: Combined Analysis of Two Randomized Controlled Studies. Clin Colorectal Cancer. 2018;17(4):e617–e629. doi: 10.1016/j.clcc.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 45.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is Yttrium-90 Radioembolization a Viable Treatment Option for Unresectable, Chemorefractory Colorectal Cancer Liver Metastases? A Large Single-Center Experience of 302 Patients. Ann Surg Oncol. 2015;22(3):794–802. doi: 10.1245/s10434-014-4164-x [DOI] [PubMed] [Google Scholar]
- 46.Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: Safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016;57(5):665–671. doi: 10.2967/jnumed.115.166082 [DOI] [PubMed] [Google Scholar]
- 47.Abbott AM, Kim R, Hoffe SE, et al. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin Colorectal Cancer. 2015;14(3):146–153. doi: 10.1016/j.clcc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 48.Golfieri R, Mosconi C, Giampalma E, et al. Selective transarterial radioembolisation of unresectable liver-dominant colorectal cancer refractory to chemotherapy. Radiol Medica. 2015;120(8):767–776. doi: 10.1007/s11547-015-0504-6 [DOI] [PubMed] [Google Scholar]
- 49.Saxena A, Bester L, Shan L, et al. A systematic review on the safety and efficacy of yttrium-90 radioembolization for unresectable, chemorefractory colorectal cancer liver metastases. J Cancer Res Clin Oncol. 2014;140(4):537–547. doi: 10.1007/s00432-013-1564-4 [DOI] [PubMed] [Google Scholar]
- 50.Garlipp B, Gibbs P, Van Hazel GA, et al. Secondary technical resectability of colorectal cancer liver metastases after chemotherapy with or without selective internal radiotherapy in the randomized SIRFLOX trial. Br J Surg. 2019;106(13):1837–1846. doi: 10.1002/bjs.11283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teo J-Y, Allen JC, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford). 2016;18(1):7–12. doi: 10.1016/j.hpb.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


