Abstract
BACKGROUND:
Diagnosis and management of salivary gland tumors in pediatric patients can be challenging. The utility of fine-needle aspiration (FNA) cytopathology and performance of the Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) in this age group have not been systematically assessed. The paucity of data has contributed to the controversial role of FNA cytopathology in the presurgical management of these patients.
METHODS:
We retrospectively analyzed 104 pediatric salivary gland FNAs (2000–2020). Distribution percentages, risk of neoplasm (RON), and risk of malignancy (ROM) were assessed for each category of the MSRSGC. Overall diagnostic accuracy was calculated.
RESULTS:
The overall sensitivity, specificity, negative predictive value, and positive predictive value of pediatric salivary gland FNA specimens at the study institution were 73%, 97%, 89% and 92%, respectively. The RON for nondiagnostic, nonneoplastic, AUS, benign neoplasms, SUMP, SM, and malignant were 60%, 11%, 100%, 100%, 100%, 100%, and 100%, respectively, while the ROM were 0%, 11%, 100%, 6%, 67%, 100%, and 100%, respectively. The percentage of nonneoplastic FNAs was much higher compared to the adult population (52% vs 8%) due to high prevalence of nonneoplastic lesions in pediatric patients. All neoplasms in patients aged 0–10 were malignant, while benign neoplasms only occurred in patients aged 11 or older, suggesting an inverse correlation between malignancy rate and age.
CONCLUSIONS:
FNA cytopathology demonstrates excellent diagnostic performance in differentiating malignant versus benign pediatric salivary gland lesions. The MSRSGC is a valuable tool for standardization of reporting and preoperative risk stratification of these lesions.
Keywords: pediatric, fine-needle aspiration (FNA), Milan System for Reporting Salivary Gland Cytopathology (MSRSGC), risk of malignancy (ROM), risk of neoplasm (RON), salivary gland
Précis
FNA cytopathology demonstrates excellent diagnostic performance in pediatric salivary gland lesions. The MSRSGC is a valuable tool for standardization of reporting and preoperative risk stratification of these lesions.
INTRODUCTION
Salivary gland tumors in children and adolescents are rare. Nonetheless, they represent a large, heterogenous spectrum of entities with diverse clinicopathologic characteristics, rendering accurate diagnosis and appropriate management of these patients challenging.1–8 A wide range of malignancy rates have been reported for pediatric salivary gland tumors in the literature, varying from approximately 30% to 75%.6,7,9 Such considerable difference among studies is likely due to, in part, small number of cases included in some series, variable definitions adopted for the age range of pediatric patients, and inconsistent case selection criteria for tumor types. Nevertheless, the general malignancy rate in children and adolescents is consistently higher compared with the adult patients (approximately 20% to 40%).1,10–12 While surgery is the treatment of choice for these tumors, pathologic diagnosis dictates the extent of resection and the need for neck dissection.5–7
Fine-needle aspiration (FNA) cytopathology has been proven to be an excellent diagnostic test in the presurgical evaluation of salivary gland lesions in adults to assess malignant potentials,13,14 and have received wide acceptance by the surgical community internationally.1,15,16 The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) is 6-tier diagnostic category system recently published to standardize terminology for the diagnosis of salivary gland FNA and provide the risk of malignancy (ROM) for each diagnostic category along with recommendations for management.17–20 These categories include 1) nondiagnostic, 2) nonneoplastic, 3) atypia of undetermined significance (AUS), 4a) benign neoplasm, 4b) salivary gland neoplasms of uncertain malignant potential (SUMP), 5) suspicious for malignancy (SM), and 6) malignant. Despite the success of FNA cytopathology in the presurgical management of adult salivary gland lesions, its utility in the pediatric population is currently controversial due to a paucity of study data regarding its diagnostic accuracy in this age group and concerns for complications related to sedation or general anesthesia in younger children.2,5 Ronchi et al recently reported the first series of pediatric salivary gland tumors with presurgical cytological diagnoses (n=34) and follow-up histopathology (n=28), and demonstrated the sensitivity and specificity of FNA cytopathology in pediatric patients are similar to those observed in adults.9 However, to the best of our knowledge, the utility of MSRSGC has not been systematically assessed in pediatric patients in the literature, and data regarding estimation of ROM for each MSRSGC category is currently lacking.
In this study, we aimed to present our institution’s experience in pediatric salivary gland lesions and correlate the MSRSGC diagnoses with histopathology in subsequent surgical resections.
MATERIALS AND METHODS
Data Collection
This institutional review board-approved study was carried out to retrieve cases of pediatric salivary gland FNAs performed between April 2000 and February 2020 from the Vanderbilt University Medical Center archives. Based on the age limit of pediatrics adopted by the American Academy of Pediatrics,21 patients who were 21 years and younger at the time of FNA procedure were included in our cohort. For each case, the information collected when available included sex, age, location and size of the lesion, radiologic findings, FNA diagnoses, surgical pathology follow-up diagnoses, and long-term outcome. The lesions located adjacent to but without clear association with salivary gland demonstrated by radiologic studies were excluded. The FNAs of lymph nodes were included only when radiologic evidence confirmed an intra-salivary gland location.
Classification
All FNAs were re-classified retrospectively into of one the MSRSGC diagnostic categories. Histopathologic diagnoses were correlated with FNA diagnoses. Frequency of FNA diagnoses, risk of neoplasm and malignancy rate were calculated for each MSRSGC category.
ROM and RON calculations
Two methods were used to calculate the ROM in this study.18,22 The first method is based on histologic follow-up (ROM-H), likely representing an overestimation of the actual ROM. The second method divides the total number of malignant cases by the number of cytologic specimens (ROM-C), likely representing an underestimation of the actual ROM. The actual ROM, in general, is expected to be between the ROM-H and the ROM-C. The risk of neoplasm (RON) was calculated on the basis of the histologic follow‐up, similar to ROM-H.
Diagnostic accuracy
The sensitivity and specificity of FNA cytopathology in the diagnosis of pediatric salivary gland lesions were calculated. True positive was defined as any histologically malignant lesion with a malignant, SM or SUMP diagnosis. False negative was defined as any histologically malignant lesion with a benign cytopathology diagnosis. True negative was defined as any histologically benign lesion with a benign cytopathology diagnosis. False positive was defined as any histologically benign lesion with a malignant, SM or SUMP diagnosis.
Results
Patient Demographics and Lesion Characteristics
The study cohort included 104 salivary gland FNAs from 94 pediatric patients, including 43 male and 51 female patients aged from 0 to 21 years (mean, 13.1 years). Among these, 54 salivary gland lesions (57%) were eventually followed by definitive surgery at the study institution, while 35 (37%) had only clinical follow-up without resection. Of the 104 FNA specimens, 74 (71%) were from lesions located in the parotid gland, 28 (27%) in the submandibular gland, and 2 (2%) in the minor salivary glands. Based on imaging or gross examination of the resected specimens, the average size of these lesions was 2.6 cm (range, 0.5–7.3 cm).
MSRSGC Classification, Histologic Follow-up, RON, and ROM
The 104 cases were reclassified according to the MSRSGC as follows: nondiagnostic, 17 (16%); nonneoplastic, 54 (52%); AUS, 4(4%); benign neoplasm, 18 (17%); SUMP, 3 (3%); SM, 2 (2%); and malignant, 6 (6%). Among the 54 resected lesions, the most frequent MSRSGC category was benign neoplasm and nonneoplastic (tied; n=18 each, 33%), followed by nondiagnostic (n=5, 9%), AUS (n=4, 7%), malignant (n=4, 7%), SUMP (n=3, 6%) and SM (n=2, 4%). All patients with AUS, benign neoplasm, SUMP and SM, as well as 4 of 6 patients with malignant category, underwent resection following the FNA cytopathologic diagnoses. Histologic examination of the surgical specimens confirmed a neoplastic diagnosis for all cases in these five MSRSGC categories (RON = 100% in each). Based on histologic follow-up, the ROM was 100% for AUS (4/4 cases), SM (2/2 cases) and malignant (4/4 cases), 6% for benign neoplasm (1/18 cases), and 67% for SUMP (2/3 cases). In contrast, a minority of the FNAs classified as nonneoplastic (5/17 cases, 33%) and nondiagnostic (18/54 cases, 29%) were followed by resection, with a neoplastic diagnosis established in 3/5 (all 3 were benign) and 2/18 cases (all 2 were malignant), respectively. The cytopathologic diagnoses, RON and ROM in this cohort are summarized in Table 1.
Table 1.
Cytopathologic Diagnoses of 104 Pediatric Salivary Gland Fine-Needle Aspiration Biopsies, Surgical Follow-up, Risk of Neoplasm, and Risk of Malignancy.
| Milan System Diagnostic Category | No. of FNAs (% of Total) | No. of FNAs Followed by Resection | Histologic Correlation | RON (%) | ROM-H (%) | ROM-C (%) | ||
|---|---|---|---|---|---|---|---|---|
| Nonneoplastic | Benign Neoplasm | Malignant Neoplasm | ||||||
| Nondiagnostic | 17 (16) | 5 | 2 | 3 | 0 | 60 | 0 | 0 |
| Nonneoplastic | 54 (52) | 18 | 16 | 0 | 2 | 11 | 11 | 4 |
| AUS | 4 (4) | 4 | 0 | 0 | 4 | 100 | 100 | 100 |
| Benign neoplasm | 18 (17) | 18 | 0 | 17 | 1 | 100 | 6 | 6 |
| SUMP | 3 (3) | 3 | 0 | 1 | 2 | 100 | 67 | 67 |
| SM | 2 (2) | 2 | 0 | 0 | 2 | 100 | 100 | 100 |
| Malignant | 6 (6) | 4 | 0 | 0 | 4 | 100 | 100 | 67 |
| Total | 104 | 54 | 18 | 21 | 15 | N/A | N/A | N/A |
Abbreviations: FNA, fine‐needle aspiration; RON, risk of neoplam, ROM, risk of malignancy; ROM‐H, risk of malignancy based on histologic follow‐up; ROM‐C, risk of malignancy based on cytologic specimens; AUS, atypia of undetermined significance; SUMP, salivary gland neoplasm of uncertain malignant potential; SM, suspicious for malignancy.
Diagnostic performance of FNA cytopathology
Based on histologic follow-up, the overall sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FNA cytopathology in our study in differentiating malignant versus benign salivary gland lesions were 73%, 97%, 89% and 92%, respectively. When AUS and SUMP categories were excluded, the sensitivity, specificity, PPV and NPV were 67%, 100%, 100% and 92%, respectively.
While 3 of the 15 (20%) histologically malignant lesions were classified by FNA cytopathology as nonneoplastic (n=2) or benign neoplasm (n=1), the remainder (12/15, 80%) were appropriately diagnosed as malignant (4/15, 27%), SM (2/15, 13%), SUMP (2/15, 13%) or AUS (4/15, 27%). A total of two malignant FNAs were not followed by resection. Both cases involved the parotid glands, and cytopathologic diagnoses of rhabdomyosarcoma were made. A definitive initial diagnosis with negative FOXO-1 fusion status was successfully established by immunohistochemistry and fluorescence in situ hybridization studies in one case using the cell-block preparations. Chemotherapy was appropriately initiated based on the FNA diagnosis alone. In another case, a cytopathologic diagnosis of metastatic rhabdomyosarcoma involving an intraparotid lymph node was similarly established which was further supported by clinical follow-up.
Among the histologically confirmed malignant FNAs, mucoepidermoid carcinoma (MEC) was the most common (5/15, 33%,), followed by metastatic melanoma (4/15, 27%, Fig 1), lymphoma/leukemia (3/15, 20%, Fig 2), secretary carcinoma (2/15, 13%, Fig 3) and acinic cell carcinoma (1/15, 7%; Table 2). The vast majority of benign neoplasms were pleomorphic adenoma (20/21, 95%). All histologically benign lesions were appropriately diagnosed by FNA cytopathology and classified as nondiagnostic, nonneoplastic, benign neoplasm or SUMP (Table 3).
Figure 1.
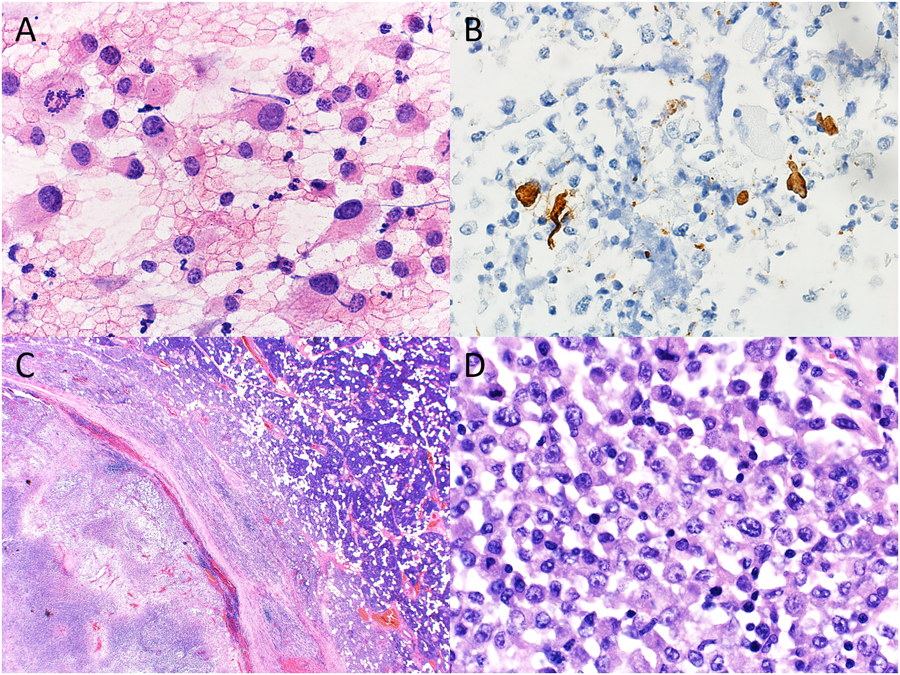
Cytopathologic-histologic correlation of a case of metastatic melanoma with unknown primary site involving an intraparotid lymph node from an 18-year-old male. (A) Fine-needle aspiration (FNA) of an enlarged intraparotid lymph node demonstrated a population of large, pleomorphic cells with granular, hyperchromatic nuclei and conspicuous nucleoli (H&E, original magnification X600). (B) Immunohistochemistry performed on the cell block preparation demonstrated positivity for HMB45 in a small subset of tumor cells (immunohistochemistry, original magnification X400). (C) Surgical resection of the parotid gland showed an intraparotic lymph node with effaced architecture and extensive necrosis (H&E, original magnification X20). (D) Clusters of pleomorphic neoplastic cells with cytologic features similar to those on FNA smear were best seen in the subcapsular region (H&E, original magnification X600).
Figure 2.
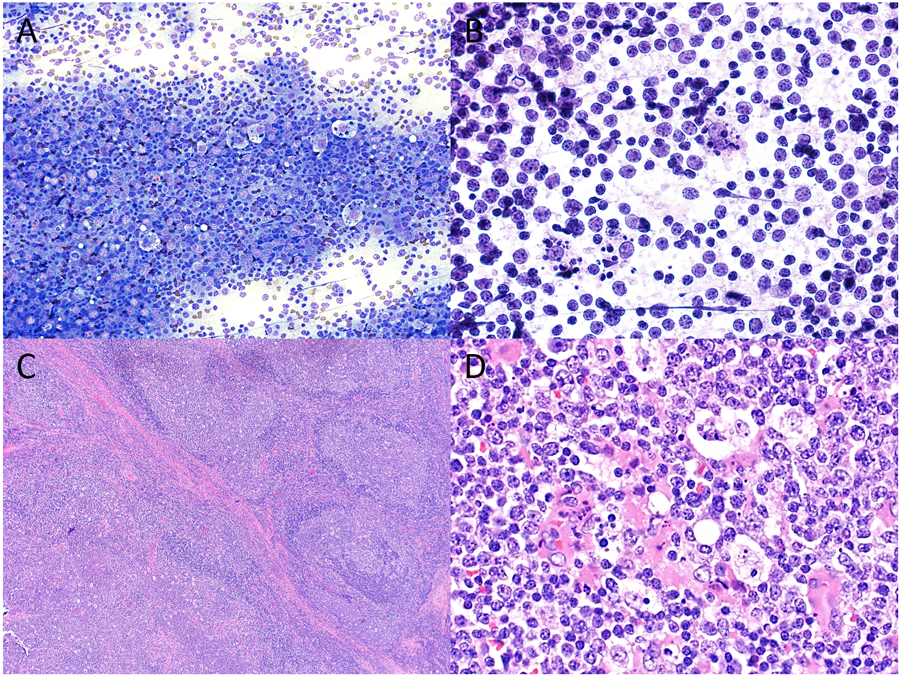
Cytopathologic-histologic correlation of a case of pediatric follicular lymphoma arising in an intraparotid lymph node from a 14-year-old male. Fine-needle aspiration (FNA) demonstrated a mixed population of mature lymphocytes intermixed with numerous tangible body macrophages and scattered eosinophils on both (A) Diff-Quik (original magnification X200) and (B) H&E preparations (original magnification X600), cytologic features that overlap with those seen in reactive lymph nodes. However, flow cytometry detected a population of CD10-positive monotypic B cells, prompting a diagnosis of “atypical lymphoid infiltrate”. (C) Surgical resection showed partial effacement of nodal architecture with expansile follicles (lower left) and a rim of residual normal lymph node architecture at the periphery (upper right) (H&E, original magnification X40). (D) A monotonous population of small to intermediate sized lymphocytes with intermixed tangible macrophages were best seen within expansile follicles (H&E, original magnification X600).
Figure 3.
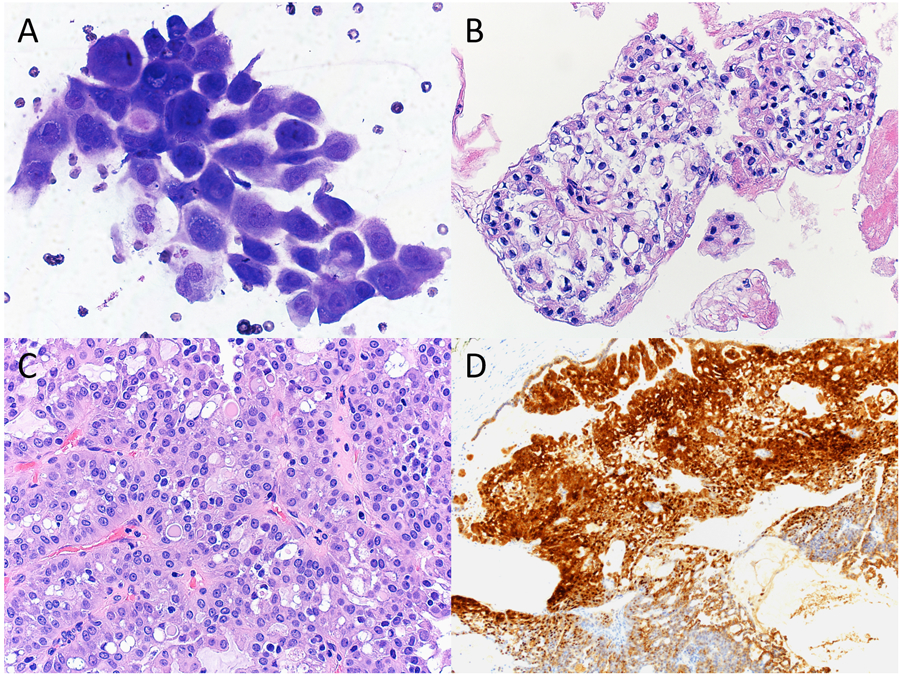
Cytopathologic-histologic correlation of a case of secretory carcinoma arising in the parotid gland from a 11-year-old female. (A) Fine-needle aspiration (FNA) demonstrated frequent clusters of polygonal epithelial cells forming acinar-like structures. Tumor cells exhibited ovoid-to-round nuclei, finely granular cytoplasm with abundant small vacuoles, and occasional intracytoplasmic mucin (Diff-Quik, original magnification X600). (B) Clusters of tumor cells forming papillary structures with clear to eosinophilic cytoplasm and more frequent intracytoplasmic mucin were present in the cell block preparation (H&E, original magnification X400). (C) Surgical resection showed proliferation of bland tumor cells with papillary and acinar architectures. Intracytoplasmic eosinophilic colloid-like material was evident (H&E, original magnification X400). (D) By immunohistochemistry, the tumor cells were positive for S-100 (immunohistochemistry, original magnification X100).
Table 2.
Cytopathologic-Histologic Correlation of Resected Malignant Salivary Gland Lesions.
| Milan System Diagnostic Category | No. of Malignant Salivary Gland Lesions | ||||||
|---|---|---|---|---|---|---|---|
| MEC | Metastatic Melanoma | Secretory Carcinoma | ACC | AML | NLPHL | PFL | |
| Nondiagnostic | - | - | - | - | - | - | - |
| Nonneoplastic | - | - | - | - | 1 | 1 | - |
| AUS | 2 | - | - | 1 | - | - | 1 |
| Benign neoplasm | 1 | - | - | - | - | - | - |
| SUMP | 1 | - | 1 | - | - | - | - |
| SM | 1 | - | 1 | - | - | - | - |
| Malignant | - | 4* | - | - | - | - | - |
| Total | 5 | 4* | 2 | 1 | 1 | 1 | 1 |
Abbreviations: AUS, atypia of undetermined significance; SUMP, salivary gland neoplasm of uncertain malignant potentialneoplasm; SM, suspicious for malignancy. MEC, mucoepidermoid carcinoma; ACC, acinic cell carcinoma; AML, acute myeloid leukemia; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma; PFL, pediatric follicular lymphoma.
Two patients with metastatic melanomas received repeated FNA biopsies with malignant cytology diagnoses.
Table 3.
Cytopathologic-Histologic Correlation of Resected Benign Salivary Gland Lesions.
| Milan System Diagnostic Category | No. of Benign Salivary Gland Lesions | ||||
|---|---|---|---|---|---|
| Pleomorphic Adenoma | Pilomatricoma | Benign Lymph Nodes | Chronic Sialadenitis | Benign Cysts | |
| Nondiagnostic | 3 | - | 1 | - | 1 |
| Nonneoplastic | - | - | 9 | 3 | 4 |
| AUS | - | - | - | - | - |
| Benign neoplasm | 16 | 1 | - | - | - |
| SUMP | 1 | - | - | - | - |
| SM | - | - | - | - | - |
| Malignant | - | - | - | - | - |
| Total | 20 | 1 | 10 | 3 | 5 |
Abbreviations: AUS, atypia of undetermined significance; SUMP, salivary gland neoplasm of uncertain malignant potentialneoplasm; SM, suspicious for malignancy.
The three false negative cases included one low-grade MEC, one nodular lymphocyte predominant Hodgkin lymphoma, and one intraparotid lymph node involved by acute myeloid leukemic infiltrate. The clinicopathologic characteristics of these three cases were summarized in Table 4.
Table 4.
Clinical and Cytopathologic Characteristics of the False-Negative Cases (n=3).
| Case | Age/Sex | Location | Size (cm) | Imaging | Milan System Diagnostic Category | Cytopathologic Findings | Ancillary testing | Follow-up Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 11/F | Parotid | 5.5 | N/A | Benign neoplasm | Benign epithelial cells, lack of mucin, favor pleomorphic adenoma | N/A | MEC, low grade |
| 2 | 12/F | Submandibular | 3.6 | Left submandibular gland swelling with central hypodensity | Nonneoplastic | Few glandular cells, acute and chronic inflammatory cells and acinar groups | N/A | Involved by AML |
| 3 | 18/M | Parotid | 7.3 | Large enhancing right parotid gland mass with adjacent lymphadenopathy | Nonneoplastic | Abundant lymphocytes with variation in size and scattered tingible body macrophages, favor reactive intraparotid lymph node | Flow cytometry analysis shows a polytypic population of lymphocytes | NLPHL |
Abbreviations: MEC, mucoepidermoid carcinoma; AML, acute myeloid leukemia; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma.
Incidence of MSRSGC Diagnoses and Malignancy by Age
Among the MSRSGC categories, benign neoplasm, SUMP and SM FNAs were diagnosed only in patients aged 11 to 21 years, while nondiagnostic, nonneoplastic, AUS and malignant were encountered in all pediatric age groups (Fig 4A). In particular, the nondiagnostic and nonneoplastic FNAs demonstrated comparatively even distribution over the entire age range. While the malignant FNAs were occasionally diagnosed before the age of 11, a majority of the malignant and all SM FNAs (6/8 cases) were encountered in the older pediatric patients who were aged 11 to 21 years. Based on such difference, we divided the pediatric cohort into two age groups, younger children (aged 0–10 years) versus older pediatric patients (aged 11–21 years), and found that the percentage distribution of diagnoses in these two groups exhibited considerable differences (Fig 4B). Notably, the younger group was composed of only 4 MSRSGC categories, with the nonneoplastic diagnoses comprising more than 2/3 of the cases compared to 45% in the older group. In contrast, the benign neoplasm category comprised 24% in the older group compared to 0% in the younger. In concordance with the distribution of MSRSGC categories by age, all histologically benign neoplasms (20/20 cases) as well as most of the histologically malignant neoplasms (13/15 cases) occurred in the older group. When a salivary gland neoplasm did occur in younger children, it was invariably malignant in our cohort.
Figure 4.
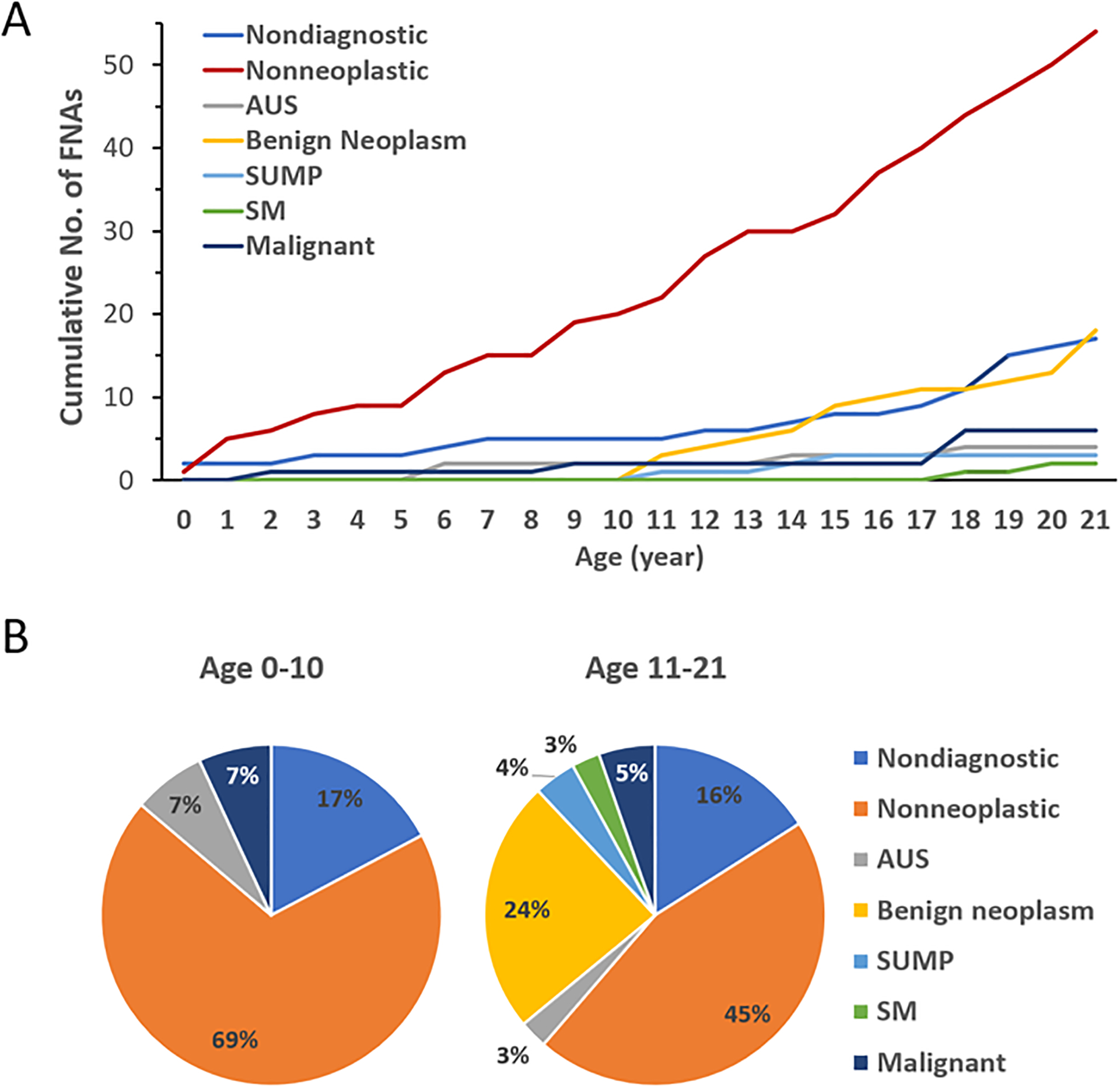
Incidence of MSRSGC diagnoses by age. (A) Accumulative curves of different MSRSGC categories by age from 0 to 21 years. (B) Distribution of MSRSGC categories in two age groups (age 0–10 versus age 11–21 years).
Discussion
Diagnosis and management of salivary gland tumors in pediatric patients can be challenging. While these tumors are rare, epidemiology and clinical outcome studies indicate a much higher frequency of malignancy in pediatric salivary gland tumors than in adults.1,2,6,7 As a valuable diagnostic tool for presurgical evaluation of adult salivary gland tumors,1,13–16 FNA cytopathology has met resistance in the management of pediatric patients partly due to a paucity of studies in the literature.9,23
To evaluate the utility of FNA cytopathology and performance of the MSRSGC in pediatric pateints, we retrospectively categorized the salivary gland FNA specimens collected over the past 20 years based on the criteria proposed by the MSRSGC, analyzed the histologic follow-up when available, and assessed the malignancy rate for each diagnostic category. The sensitivity and specificity of FNA in differentiating benign and malignant salivary gland lesions in our pediatric cohort were 73% and 97%, respectively, which differed from 92% and 86% reported by Ronchi et al,9 but was comparable to the adult studies demonstrating an overall sensitivity of 79%−84% and specificity of 95%−97%.19,24,25 With a prevalence of malignancy of 28% (15/54 cases), the PPV and NPV in our study were 89% and 92%, respectively, which was also comparable to 90% and 94% in adults.19,24 Thus, in keeping with the pediatric series by Ronchi et al,9 our findings provide strong evidence for high diagnostic adequacy of salivary gland FNA in pediatric patients.
In this pediatric cohort, 16% of the FNA cases (17/104) were categorized as nondiagnostic, which was higher than the maximum target rate of 10% suggested by the MSRSGC for adults18,20 and the rate of 4% reported in a meta-analysis review.25 Most cases classified as nondiagnostic in this study had scant cellularity and/or insufficient amount of lesional cells present on aspirates. Rapid on-site evaluation of FNA specimens were performed in 12 of the 16 in-house cases with nondiagnostic diagnoses. Histologic follow-up was available in 5 cases, including 3 pleomorphic adenomas, 1 reactive lymph node, and 1 branchial cleft cyst. Clinical and/or radiologic (non-histologic) follow-up was available in additional 11 cases, including 4 vascular anomalies, 2 reactive lymph nodes, 1 benign cyst, 1 inflammatory lesion, 1 mixed connective tissue disease, 1 temporomandibular joint-pain-dysfunction syndrome, and 1 spontaneously resolved lesion. The fluid-rich nature of these lesions, particularly vascular anomalies and benign cysts, may in part account for the higher likelihood of sampling hypocellular aspirates or the lack of abnormal cells. With no malignant neoplasms encountered, the ROM-H was zero in the nondiagnostic category, much lower than the MSRSGC target rate of 25%.
The most frequent MSRSGC category in this study was nonneoplastic, accounting for 52% of FNAs (54/104), which was strikingly higher than the overall rate of 8% reported.25 For comparison, benign neoplasm constitutes the most frequently encountered MSRSGC category in adults (67%).25 On the basis of histologic follow-up, the ROM-H was 11% (2/18), comparable to the MSRSGC target rate of 10%. The remainder were nonneoplastic lesions, including reactive lymph nodes (50%, 9/18), benign cysts (22%, 4/18) and chronic sialadenitis (11%, 2/18). When cases with clinical follow-up were included (n=31), reactive lymph nodes remained the most common (47%, 23/49), followed by benign cysts (20%, 10/49), chronic sialadenitis (10%, 5/49), infection (8%, 4/49), vascular anomaly (6%, 3/49), and others. The prevalence of these nonneoplastic salivary gland lesions in the pediatric population may therefore account for the higher frequency of nonneoplastic FNAs.
Notably, the ROM-H for AUS was 100% (4/4 cases), much higher than the MSRSGC target rate of 20%. These 4 cases included 2 MEC, 1 acinic cell carcinoma and 1 pediatric follicular lymphoma. Although the number of cases was small, it did raise the consideration that indeterminate salivary gland lesions might associate with increased likelihood of malignancy compared with adults. Similarly, the ROH-H for SUMP and SM was 67% (2/3 cases) and 100% (2/2 cases), respectively, compared to the MSRSGC target rates of 35% and 60%, respectively. Additional studies from different institutions with larger sample size will be necessary to assess these observations. If substantiated, these findings would indicate that indeterminate pediatric FNAs represent a significant management burden for the clinicians, and that special considerations or recommendations may be warranted for these groups.
Analysis of the incidence of MSRSGC diagnoses and malignancy by age in our cohort confirmed the observation that the probability of a salivary neoplasm being malignant inversely correlates with the patient’s age. In fact, all neoplasms in the younger groups were malignant, while benign neoplasms only occurred in pediatric patients aged 11 or older. Correspondingly, the incidence of MSRSGC diagnoses varied considerably between these two age groups, with benign neoplasm, SUMP and SM diagnoses being encountered in the older group only. Such age-specific incidence of pediatric salivary gland neoplasms may have significant clinical implication, and one must exercise greater caution while evaluating a salivary gland tumor in a young child.
The current study has multiple limitations. It is a retrospective study which may have selection bias. Our pediatric cohort was derived from a single tertiary medical center serving mostly a regional population, therefore may not be representative internationally or equally applicable to practices in other regions. Although the numbers of FNAs and subsequent resections in our study are the largest to date, a much greater number of cases are necessary for further calculation of ROM in difference age groups.
In conclusion, the current series demonstrate the MSRSGC as an effective, tiered classification system for reporting salivary gland cytopathology in pediatric pateints for appropriate clinical management, and that the sensitivity and specificity of FNA cytopathology are comparable to those in adults. Thus, our study provides additional evidence supporting the utility of FNA cytopathology in the presurgical evaluation of pediatric salivary gland lesions. Further studies are needed for the establishment of specific diagnostic and management guidelines in the pediatric population.
Acknowledgments
This study is not supported by any funding or grants.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
This paper was not invited for submission.
References
- 1.Carlson ER, Schlieve T. Salivary Gland Malignancies. Oral Maxillofac Surg Clin North Am. 2019;31(1):125–144. [DOI] [PubMed] [Google Scholar]
- 2.Lennon P, Silvera VM, Perez-Atayde A, Cunningham MJ, Rahbar R. Disorders and tumors of the salivary glands in children. Otolaryngol Clin North Am. 2015;48(1):153–173. [DOI] [PubMed] [Google Scholar]
- 3.Luna MA, Batsakis JG, el-Naggar AK. Salivary gland tumors in children. Ann Otol Rhinol Laryngol. 1991;100(10):869–871. [DOI] [PubMed] [Google Scholar]
- 4.Zamani M, Gronhoj C, Schmidt Jensen J, von Buchwald C, Charabi BW, Hjuler T. Survival and characteristics of pediatric salivary gland cancer: A systematic review and meta-analysis. Pediatr Blood Cancer. 2019;66(3):e27543. [DOI] [PubMed] [Google Scholar]
- 5.Aro K, Leivo I, Makitie A. Management of salivary gland malignancies in the pediatric population. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):116–120. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida EJ, Garcia J, Eisele DW, Chen AM. Salivary gland malignancies in children. Int J Pediatr Otorhinolaryngol. 2014;78(2):174–178. [DOI] [PubMed] [Google Scholar]
- 7.Ord RA, Carlson ER. Pediatric Salivary Gland Malignancies. Oral Maxillofac Surg Clin North Am. 2016;28(1):83–89. [DOI] [PubMed] [Google Scholar]
- 8.Ellies M, Laskawi R. Diseases of the salivary glands in infants and adolescents. Head Face Med. 2010;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronchi A, Montella M, Zito Marino F, et al. Diagnostic accuracy of FNA cytology for diagnosis of salivary gland tumors in pediatric patients. Cancer Cytopathol. 2019;127(8):529–538. [DOI] [PubMed] [Google Scholar]
- 10.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146(1):51–58. [DOI] [PubMed] [Google Scholar]
- 11.Tumors of the Salivary Glands, Atlas of Tumor Pathology: Third Series, Fascicle 17 G. L. Ellis and P. L. Auclair. Armed Forces Institute of Pathology, Washington D.C. ISBN: 1 881041 26 3 (Printed). 1996. Price: $69.00. ISBN: 1 881041 41 7 (CD-ROM). 1998. Price: $65.00. J Pathol The Journal of Pathology. 2000;192(4):564–565. [Google Scholar]
- 12.Wang XD, Meng LJ, Hou TT, Huang SH. Tumours of the salivary glands in northeastern China: a retrospective study of 2508 patients. Br J Oral Maxillofac Surg. 2015;53(2):132–137. [DOI] [PubMed] [Google Scholar]
- 13.Liu CC, Jethwa AR, Khariwala SS, Johnson J, Shin JJ. Sensitivity, Specificity, and Posttest Probability of Parotid Fine-Needle Aspiration: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2016;154(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colella G, Cannavale R, Flamminio F, Foschini MP. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68(9):2146–2153. [DOI] [PubMed] [Google Scholar]
- 15.Sood S, McGurk M, Vaz F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S142–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pynnonen MA, Gillespie MB, Roman B, et al. Clinical Practice Guideline: Evaluation of the Neck Mass in Adults. Otolaryngol Head Neck Surg. 2017;157(2_suppl):S1–S30. [DOI] [PubMed] [Google Scholar]
- 17.Pusztaszeri M, Baloch Z, Vielh P, Faquin WC. Application of the Milan system for reporting risk stratification in salivary gland cytopathology. Cancer Cytopathol. 2018;126(1):69–70. [DOI] [PubMed] [Google Scholar]
- 18.Rossi ED, Faquin WC. The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): An international effort toward improved patient care-when the roots might be inspired by Leonardo da Vinci. Cancer Cytopathol. 2018;126(9):756–766. [DOI] [PubMed] [Google Scholar]
- 19.Pusztaszeri M, Rossi ED, Baloch ZW, Faquin WC. Salivary Gland Fine Needle Aspiration and Introduction of the Milan Reporting System. Adv Anat Pathol. 2019;26(2):84–92. [DOI] [PubMed] [Google Scholar]
- 20.Bongiovanni M, Faquin WC, Rossi ED. The Milan system for reporting salivary gland cytopathology. 2018.
- 21.Hardin AP, Hackell JM, Committee On P, Ambulatory M. Age Limit of Pediatrics. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan K, Sung S, Scognamiglio T, Yang GCH, Siddiqui MT, Rao RA. The role of the Milan System for Reporting Salivary Gland Cytopathology: A 5-year institutional experience. Cancer Cytopathol. 2018;126(8):541–551. [DOI] [PubMed] [Google Scholar]
- 23.Fuller M, Monaco S. Is Milan for Kids? The Milan System for Reporting Salivary Gland Cytopathology in Pediatric Patients at an Academic Children’s Hospital. Paper presented at: American Society of Cytopathology 66th Annual Scientific Meeting 2018; Washington, D.C. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011;136(1):45–59. [DOI] [PubMed] [Google Scholar]
- 25.Farahani SJ, Baloch Z. Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta-analysis of published literature. Diagn Cytopathol. 2019;47(2):67–87. [DOI] [PubMed] [Google Scholar]


