Abstract
BACKGROUND:
Fine-needle aspiration (FNA) results classified as the non-diagnostic category of the Milan system for Reporting Salivary Gland Cytopathology (MSRSGC) may be infrequently encountered in children. Clinical management may be challenging due to lack of data regarding outcomes and underlying causes.
METHODS:
We retrospectively analyzed 106 consecutive pediatric salivary gland FNAs (2000–2020; 45% performed under image guidance). The outcomes of patients with non-diagnostic results were analyzed. Clinical, FNA procedural, and histopathologic parameters were compared between diagnostic and non-diagnostic cases. A root cause analysis was performed using the fishbone diagram and the 5 Whys method.
RESULTS:
A total of 103 initial FNAs were identified. The non-diagnostic rates for initial and repeat biopsy were 16% (16/103) and 67% (2/3), respectively. Initial non-diagnostic FNAs were most frequently managed by clinical/radiologic follow-up only (56%, 9/16), followed by direct surgery (19%, 3/16) and repeat FNA (19%, 3/16). By histologic and clinical/radiologic follow-up, the risk of malignancy for non-diagnostic cases was zero. Palpation guidance (p<0.05), inadequate sampling determined by rapid on-site evaluation (p<0.01), and lesions with cystic, vascular, or diffuse nature (p<0.05) were significantly associated with non-diagnostic results. By root cause analysis, proceduralist sampling error and lack of ultrasound guidance were the most common primary and secondary causes, respectively.
CONCLUSIONS:
Pediatric salivary gland lesions of the non-diagnostic MSRSGC category have minimal risk of malignancy and may be successfully managed by clinical/radiologic follow-up. The root causes for non-diagnostic results were often multifactorial and primarily related to proceduralist sampling, characteristics of the lesions, and lack of ultrasound guidance.
Keywords: Pediatric, fine-needle aspiration, salivary gland, Milan, non-diagnostic, root cause analysis
Précis:
In children, salivary gland lesions with non-diagnostic fine-needle aspiration results have minimal risks of malignancy and may be managed primarily by clinical and radiologic follow-up. The root causes for non-diagnostic results are most often related to proceduralist sampling, characteristics of the lesions, and lack of ultrasound guidance.
INTRODUCTION
Salivary gland tumors in pediatric patients are rare and more likely to be malignant compared to adults (30% - 75% versus 20% - 40%),1–7 particularly when occurring in younger children.6,8 A wide spectrum of pathologic entities may be encountered, ranging from developmental and inflammatory conditions to benign and malignant neoplasms.9–13 For preoperative evaluation of a salivary gland mass in children, fine-needle aspiration (FNA) biopsy is routinely used to assess malignant potential and guide clinical management.14 Recent studies indicate that the overall sensitivity and specificity of FNA cytopathology for salivary gland neoplasms in pediatric patients are comparable to those observed in adults6,8,15.
Since the introduction of the Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) in 2018, its clinical utility for standardization of reporting and preoperative risk stratification has been widely validated in adults. Recent studies in pediatric patients, although limited by scale, also support MSRSGC as a valuable tool for the preoperative assessment of salivary gland lesions in children.6,15,16 However, a significant fraction of salivary gland aspirates may provide non-diagnostic results. In a meta-analysis by Jalaly et al,17 the overall rate of non-diagnostic samples was 10%. The risk of malignancy (ROM) in non-diagnostic aspirates estimated by MSRSGC is approximately 25%,18 although the actual ROMs reported in recent studies tended to be lower, with a mean ROM of 17% (range, 0–50%).17 For the clinical management of these patients, current MSRSGC guidelines recommend a repeat FNA following an initial non-diagnostic aspirate, and subsequent non-diagnostic FNAs can be managed by additional imaging studies for follow-up, core needle or open biopsy for histopathologic diagnosis, or surgical excision.18 Although limited, recent data have provided practical experience (mostly in adults) to inform clinical management of non-diagnostic FNAs.19,20 However, the outcomes of non-diagnostic FNA in pediatric patients have not been systematically analyzed in the literature. In addition, understanding of the root causes of non-diagnostic aspirates is essential for improving the diagnostic yield of salivary gland FNAs.
In this study, we sought to describe the clinical outcomes of pediatric patients with initial non-diagnostic salivary gland FNAs. We aimed to establish the determinants of non-diagnostic results and perform a root cause analysis for individual cases to formulate recommendations for improving diagnostic yield.
MATERIALS AND METHODS
Patient population and data collection
This study received institutional review board approval from Vanderbilt University. During a period of 20 years between April 2000 and April 2020, a total of 106 pediatric salivary gland FNAs in patients aged 21 or younger were retrieved from the pathology files at Vanderbilt University Medical Center. The FNAs of lymph nodes were included only when radiologic evidence confirmed an intra-salivary gland location. Patient’s demographics, presentation, imaging studies, lesion location and size, FNA procedural information, status of rapid on-site evaluation (ROSE), cytopathologic findings, histologic and clinical follow-up were retrospectively reviewed. FNA diagnoses were retrospectively recategorized according to the MSRSGC.21 Any of the MSRSGC categories other than category I (non-diagnostic) was considered diagnostic. One cytopathologist (H.W.) independently reviewed the slides of all non-diagnostic cases and confirmed the assignment of non-diagnostic category using the criteria defined by MSRSGC.21 We followed those patients with an initial non-diagnostic FNA result to determine the outcomes, including whether they received a repeat FNA, surgery, clinical follow-up only, or lost to follow-up. If a second FNA returned non-diagnostic, the same process was repeated to determine the final outcomes (Fig 1).
Figure 1.
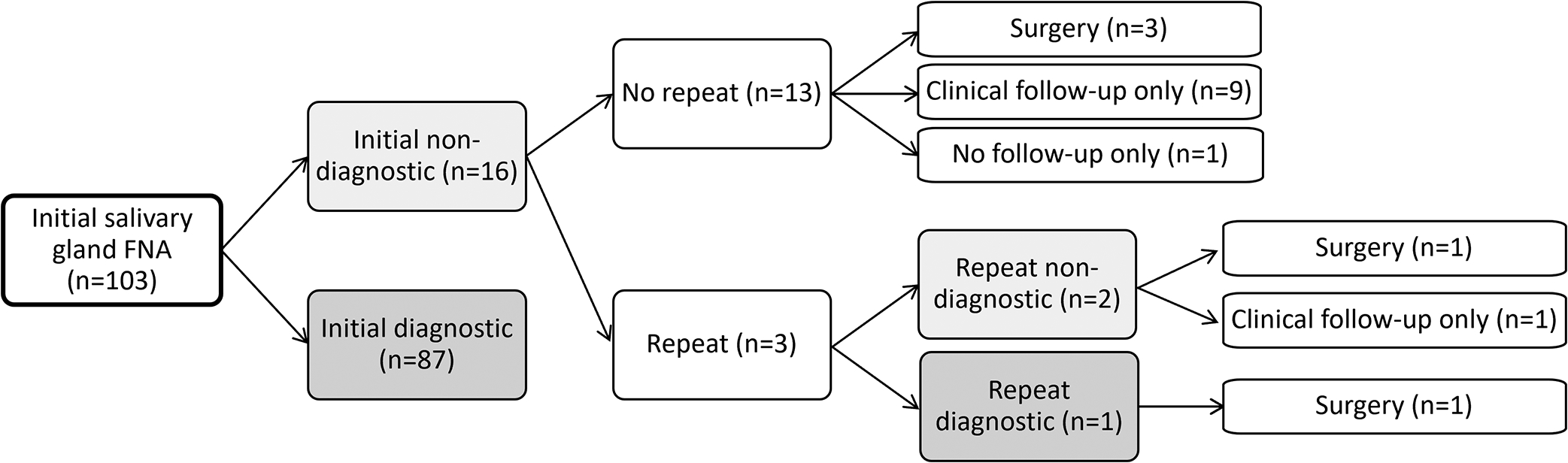
Flow diagram of follow-up results after an initial fine-needle aspiration of pediatric salivary gland lesions. Only patients with non-diagnostic aspirates were included.
FNA procedure
At our institution, the FNA biopsies are primarily performed by cytopathologists or interventional radiologists under palpation or ultrasound guidance. In most cases, ROSE of the material obtained by FNA biopsy with or without ultrasound guidance is performed by either a cytopathologist or an experienced cytotechnologist. During ROSE, one air-dried and one alcohol-fixed direct smears are prepared from each pass. The air-dried slide is stained with Diff-Quik for ROSE, and the other alcohol-fixed slide is stained later with hematoxylin and eosin stain. The needle is rinsed in saline or RPMI medium for processing as a cytospin preparation, a formalin-fixed cell block, or sending for flow cytometry analysis and/or microbiology cultures, depending on the cytomorphologic findings and amount of material available.
Root cause analysis
The “5 Whys” method was used to identify the true root cause of each case. A fishbone (Ishikawa) diagram was used to perform root cause analysis of the non-diagnostic cases. We considered four main categories of root causes in the fishbone diagram: 1) man, 2) material, 3) machine, and 4) method (Fig 2).
Figure 2.
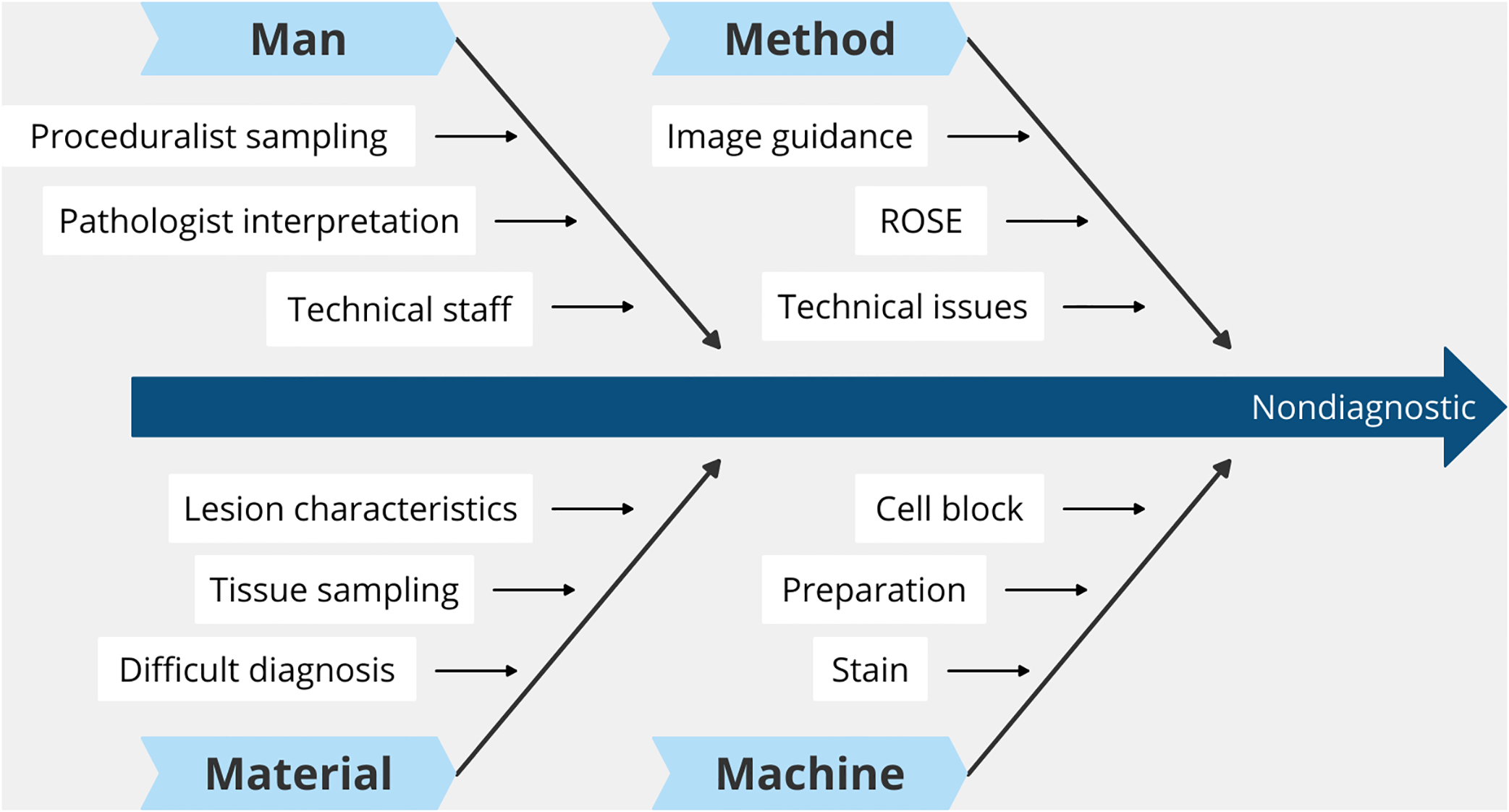
Fishbone diagram of root causes for non-diagnostic fine-needle aspiration of pediatric salivary gland lesions.
Statistical Analysis
A Mann–Whitney U test was used for analysis of patient age, lesion size, and number FNA passes between diagnostic and non-diagnostic groups, whereas a Fisher’s exact test was used for analysis of all other parameters.
Results
Study population
A total of 18 (17%) non-diagnostic cases were identified from the 106 pediatric salivary gland FNAs (95 patients) performed between April 2000 to April 2020. The mean age in the diagnostic and non-diagnostic groups were 13.1 years (range 0–21) and 13.4 years (range 0–21), respectively. Both groups demonstrated a slight female gender dominance, with parotid gland being the most common location of lesions. The average size of the lesions was 2.5 cm (range 0.5–7.3) and 2.9 cm (range 1.2–7.1) in the diagnostic and non-diagnostic groups, respectively. The percentage of FNAs performed under image guidance (including ultrasound and CT) was 45% (42 of 93 cases with detailed procedural information available for analysis) and increased from 26% (10/38) in the period of 2000–2010 to 58% (32/55) in the period of 2011–2020.
Outcomes of non-diagnostic FNA
Among the 103 initial FNAs, 16 (16%) were deemed non-diagnostic. Among these patients, 3 had a repeat FNA. The other 13 non-diagnostic cases were followed by either surgery for definitive diagnosis (n=3), clinical follow-up only (n=9), or lost to follow-up (n=1). Two of the three repeat FNAs were again non-diagnostic and followed by either surgery (n=1) or clinical follow-up only (n=1). The third repeat FNA returned as diagnostic (benign neoplasm, MSRSGC category 4a) and was followed by surgery. Among the 10 patients who were ultimately followed without surgery, 6 received follow-up imaging studies, 3 had CT and/or MRI before FNA, and 1 was followed with close observation only. The reasons for surgery for patients with non-diagnostic FNAs (n=3) included subsequent MRI findings, rapid growth in lesion size, and physician recommendation. The clinical presentation, radiographic findings, and outcomes of patients with non-diagnostic FNAs were summarized in Table 1. The histologic diagnoses following surgical resection (n=5) were pleomorphic adenoma (n=3), brachial cleft remnant (n=1), and benign/reactive lymph node (n=1). When the outcome was based on surgical and/or clinical follow-up, the most common final clinical diagnosis was vascular anomaly (n=5, including hemangioma and vascular malformation) and benign/reactive lymph node (n=5), followed by pleomorphic adenoma (n=3), resolved lesions (n=2), and branchial cleft remnant (n=1) (Table 2). No malignancy was found in patients with non-diagnostic FNAs in our study. In comparison, 15 of 54 lesions with histologic follow-up were malignant. Examples of non-diagnostic aspirates and outcomes are illustrated in Fig 3 to 6.
Table 1.
Analysis of outcomes for non-diagnostic pediatric salivary gland FNA.
| Patient # | Age | Sex | Clinical presentation | Imaging studies | Outcome | |||
|---|---|---|---|---|---|---|---|---|
| Repeat FNA | Surgery | Clinical FU only | Final diagnosis | |||||
| 1 | 14 | M | Left posterior auricular nodule | CT: nodule (1 cm) in superficial lobe of left parotid, likely lymph node | No | No | Yes (MRI) | Benign lymph node |
| 2 | 6 | M | Right submandibular mass | N/A | No | No | No | Unknown |
| 3 | 15 | F | Left neck mass | MRI: loculated mass (2.6 cm) in left parotid with rim enhancement | No | No | Yes | Benign lymph node |
| 4 | 19 | F | Left facial and cervical lymphadenopathy | N/A | No | No | Yes | Benign lymph node |
| 5 | 19 | M | Left facial swelling | CT: Mild diffuse enlargement of left parotid | Yes (non-diagnostic) | No | Yes | Lesion resolved |
| 5 | 19 | M | For repeat FNA | Same as above | No | No | Yes (MRI) | Lesion resolved |
| 6 | 12 | F | Right facial swelling | CT: mass (1.5 cm) in right parotid, likely pleomorphic adenoma | No | No | Yes (antibiotics) | Lesion resolved |
| 7 | 17 | F | Left jaw nodule | N/A | No | No | Yes (MRI) | Hemangioma |
| 8 | 0 | M | Left facial mass | MRI: enhancing mass (4.2 cm) in left parotid and masticator spaces, likely vascular lesion | No | No | Yes (steroids) | Hemangioma |
| 9 | 3 | F | Left facial mass | CT: fluid-filled lesion (3.6 cm) in left parotid gland, likely benign cyst | No | Yes | No | Branchial cleft remnant |
| 10 | 0 | F | Left facial mass | MRI: Enhancing mass (7.1 cm) in left parotid region | No | No | Yes (MRI) | Hemangioma |
| 11 | 18 | F | Right facial mass | CT: multiloculated hypodensity (3.6 cm) posterior to parotid gland, likely lymphatic malformation | No | No | Yes (MRI) | Vascular malformation |
| 12 | 18 | M | Right facial mass | MRI: enhancing mass (1.3 cm) in right parotid | Yes (diagnostic) | Yes (after repeat FNA) | No | Pleomorphic adenoma |
| 13 | 7 | M | Left facial mass | MRI: enhancing mass (3 cm) in the left masseter muscle and parotid gland | No | No | Yes (MRI) | Vascular malformation |
| 14 | 18 | F | Left facial mass | CT: solid soft tissue mass (2.2 cm) in superficial lobe of left parotid | Yes (non-diagnostic) | No | Yes | Pleomorphic adenoma |
| 14 | 19 | F | For repeat FNA | MRI: well-circumscribed, enhancing mass (2.4 cm) in superficial lobe of left parotid | No | Yes | No | Pleomorphic adenoma |
| 15 | 19 | F | Right pre-auricular nodule | MRI: non-enhancing cystic lesions (up to 1.2 cm) throughout the right and left parotid | No | No | Yes (PET-CT) | Benign lymph nodes |
| 16 | 20 | M | Left neck mass | CT: necrotic mass (4.3 cm), likely lymph node | No | Yes | No | Benign lymph nodes |
FNA, fine‐needle aspiration; FU, follow-up; CT, computed tomography; MRI, magnetic resonance imaging; PET-CT, positron emission tomography–computed tomography.
Table 2.
Final diagnoses (based on surgical and/or clinical follow-up) in cases with non-diagnostic FNA.
| Final diagnosis | Frequency | ||
|---|---|---|---|
| Surgical | Non-surgical | Combined | |
| Vascular anomaly | ‐ | 5 | 5 |
| Benign lymph node | 1 | 4 | 5 |
| Pleomorphic adenoma | 3 | ‐ | 3 |
| Lesion resolved after FNA | - | 2 | 2 |
| Branchial cleft remnant | 1 | ‐ | 1 |
| Unknown | ‐ | 1 | 1 |
FNA, fine‐needle aspiration.
Figure 3.
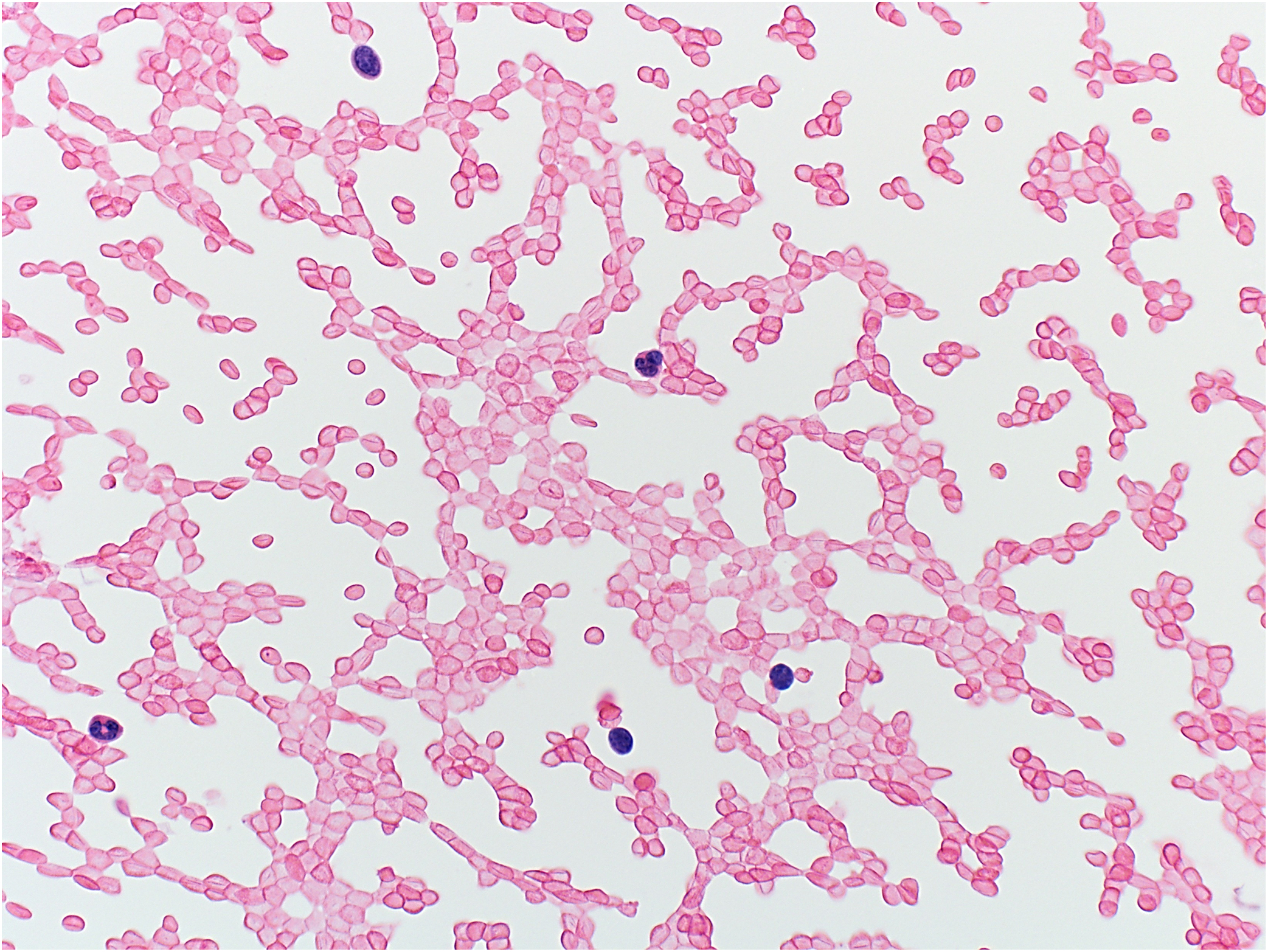
Example of non-diagnostic aspirate: blood only (hematoxylin and eosin stain, original magnification ×400). The patient was lost to follow-up.
Figure 6.
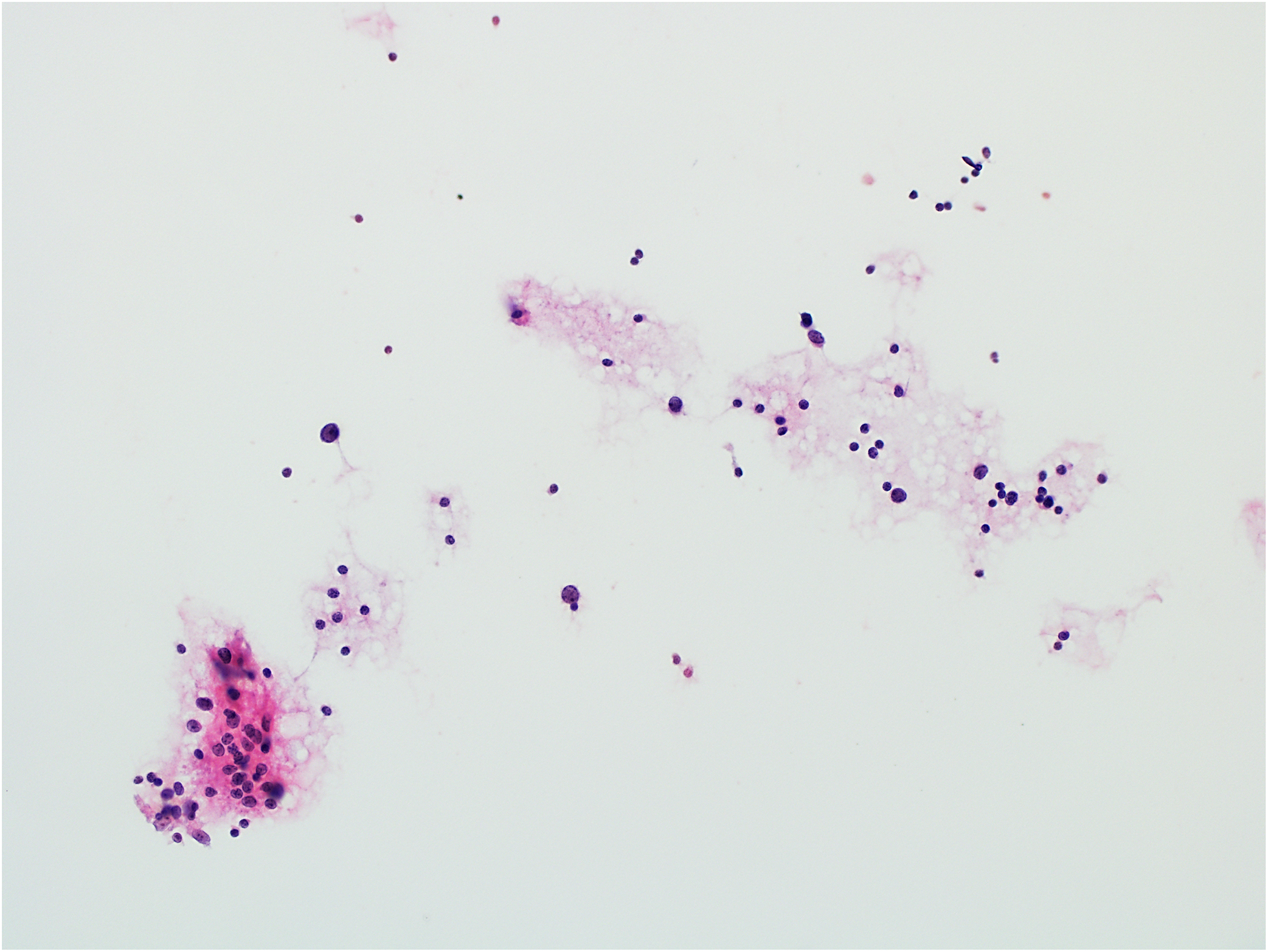
Example of non-diagnostic aspirate: scattered reactive-appearing lymphocytes (hematoxylin and eosin stain, original magnification ×200). The histologic follow-up diagnosis was pleomorphic adenoma.
Determinants of non-diagnostic FNA
We further examined the determinants of non-diagnostic FNA by comparing patient demographics, lesion characteristics, and FNA procedural parameters between diagnostic (n=88) and non-diagnostic (n=18) groups (Table 3). We found that palpation guided FNA (p<0.05), inadequate sampling determined by ROSE (p<0.01), and lesions with cystic, vascular, or diffuse nature (p<0.05) were significantly associated with non-diagnostic results. We found no statistically significant association with age, gender, lesion location, proceduralist, procedure location, sedation status, number of FNA passes, malignancy, or neoplasm.
Table 3.
Analysis of determinants for non-diagnostic pediatric salivary gland FNA.
| Characteristic | Diagnostic FNA (n=88) | Non-diagnostic FNA (n=18) | P value* |
|---|---|---|---|
| Age (mean [range]) (y) | 13.1 (0–21) | 13.4 (0–21) | 0.276 |
| Male:female | 43:45 | 8:10 | 0.606 |
| Anatomic site | |||
| Parotid | 64/88, 73% | 16/18, 89% | 0.233 |
| Submandibular | 22/88, 25% | 2/18, 11% | 0.233 |
| Other | 2/88, 2% | 0/18, 0 | 1 |
| Size (mean [range]) (cm) | 2.5 (0.5–7.3) | 2.9 (1.2–7.1) | 0.610 |
| Type of FNA procedure | |||
| Palpation-guided | 39/78, 50% | 13/16, 81% | 0.028 |
| Ultrasound-guided | 37/78, 47% | 3/16, 19% | 0.051 |
| CT-guided | 2/78, 3% | 0/16, 0 | 1 |
| FNA proceduralist | |||
| Cytopathologist | 37/78, 47% | 12/16, 75% | 0.056 |
| Interventional radiologist | 34/78, 44% | 4/16, 25% | 0.263 |
| Otolaryngologist | 7/78, 9% | 0/16, 0 | 0.599 |
| FNA procedure location | |||
| Outpatient clinic | 39/78, 50% | 11/15, 73% | 0.156 |
| Radiology suite | 35/78, 45% | 4/15, 73% | 0.257 |
| Operating room | 4/78, 5% | 0/15, 0 | 1 |
| Sedation | 26/77, 34% | 3/15, 20% | 0.374 |
| ROSE performed | 73/77, 95% | 16/18, 89% | 1 |
| Inadequate | 24/73, 33% | 15/16, 94% | <0.001 |
| FNA passes (mean [range]) | 2.8 (1–7) | 2.9 (1–4) | 0.667 |
| Malignancy | |||
| Based on histologic follow-up | 15/49, 31% | 0/5, 0 | 0.306 |
| Based on all follow-up* | 17/84, 20% | 0/17, 0 | 0.069 |
| Neoplasm | |||
| Based on histologic follow-up | 33/49, 67% | 3/5, 60% | 1 |
| Based on all follow-up* | 35/84, 42% | 3/17, 18% | 0.098 |
| Cystic, vascular or diffuse lesions | |||
| Based on histologic follow-up | 4/49, 8% | 1/5, 20% | 0.397 |
| Based on all follow-up* | 18/84, 21% | 9/17, 53% | 0.014 |
FNA, fine‐needle aspiration; ROSE, rapid onsite evaluation;
Mann–Whitney U test was used for analysis of patient age and lesion size, whereas Fisher’s exact test was used for analysis of all other parameters.
Root cause analysis
The primary and secondary root causes identified in each FNA are provided in Table 4. Most cases (83%, 15/18) were associated with more than one root cause. The most common category of primary root cause was man-related (n=11, 61%). Of these 11 cases, all were due to proceduralist sampling errors. No interpretation error was identified upon independent retrospective slide review of individual cases. The remaining primary root causes were material-related (n=7, 41%). Among these, 4 were due to either vascular or cystic nature of the lesions (2 hemangiomas, 1 vascular malformation, and 1 branchial cleft remnant), 2 due to either lack of a discrete mass palatable during the FNA procedures (unknown lesion which resolved after FNA), and 1 due to the small size of the lesion (benign lymph node). When both primary and secondary root causes are combined for analysis, the most frequent category of cause was method-related (n=13; all were secondary), followed by man- (n=11; all were primary) and material-related (n=9; 7 were primary). The 13 method-related secondary causes included 12 FNAs performed without image guidance and 1 without ROSE. Two machine-related causes were identified, and both were due to lack of cell blocks. The primary and secondary root causes identified are summarized in Table 5.
Table 4.
Root cause analysis of each non-diagnostic pediatric salivary gland FNA.
| Patient # | Final diagnosis | ROSE | Reason for nondiagnosis | Primary cause | Secondary cause |
|---|---|---|---|---|---|
| 1 | Benign lymph node | Inadequate | Blood only | Material: small lesion (1 cm) | Method: no image guidance |
| 2 | Unknown | Inadequate | Blood only | Man: sampling | N/A |
| 3 | Benign lymph node | Inadequate | Rare anucleated squamous cells, histiocytes and debris | Man: sampling | N/A |
| 4 | Benign lymph node | Inadequate | Benign salivary gland tissue | Man: sampling | Method: no image guidance |
| 5 | Lesion resolved | Inadequate | Fibroconnective tissue and blood | Material: no discrete mass | Method: no image guidance |
| 5 (repeat FNA) | Lesion resolved | N/A | Few anucleated squamous cells | Material: no discrete mass | Method: no image guidance, no ROSE |
| 6 | Lesion resolved | Inadequate | Blood only | Man: sampling | Method: no image guidance |
| 7 | Hemangioma | Inadequate | Scant salivary gland tissue | Man: sampling | Material: vascular lesion; Method: no image guidance |
| 8 | Hemangioma | Inadequate | Fibroconnective tissue and blood | Material: vascular lesion | Machine: no cell block |
| 9 | Branchial cleft remnant | N/A | Blood and few macrophages | Material: cystic lesion | N/A |
| 10 | Hemangioma | Inadequate | Blood and rare stromal elements | Material: vascular lesion | Machine: no cell block |
| 11 | Vascular malformation | Inadequate | Rare anucleated squamous cells and debris | Man: sampling | Material: vascular lesion |
| 12 | Pleomorphic adenoma | Inadequate | Benign salivary gland acinar and ductal cells | Man: sampling | Method: no image guidance |
| 13 | Vascular malformation | Adequate | Benign salivary gland tissue and rare lymphocytes | Material: vascular lesion | Method: no image guidance |
| 14 | Pleomorphic adenoma | Inadequate | Blood, few acinar cells, scant ductal cells, and rare proteinaceous material | Man: sampling | Method: no image guidance |
| 14 (repeat FNA) | Pleomorphic adenoma | Inadequate | Few acinar cells and lymphocytes | Man: sampling | Method: no image guidance |
| 15 | Benign lymph node | Inadequate | Blood and debris | Man: sampling | Method: no image guidance |
| 16 | Benign lymph node | Inadequate | Paucicellular specimen with acinar cells and blood | Man: sampling | Method: no image guidance |
FNA, fine‐needle aspiration; ROSE, rapid on-site evaluation.
Table 5.
Root causes identified in cases with non-diagnostic FNA.
| Root cause | Frequency | ||
|---|---|---|---|
| Primary | Secondary | Total | |
| Method | 0 | 13 | 13 |
| No image guidance | 0 | 12 | 12 |
| No ROSE | 0 | 1 | 1 |
| Man | 11 | 0 | 11 |
| Sampling | 11 | 0 | 11 |
| Material | 7 | 2 | 9 |
| Vascular lesion | 3 | 2 | 5 |
| No discrete mass | 2 | 0 | 2 |
| Cystic lesion | 1 | 0 | 1 |
| Small lesion (1cm) | 1 | 0 | 1 |
| Machine | 0 | 2 | 2 |
| No cell block | 0 | 2 | 2 |
FNA, fine‐needle aspiration; ROSE, rapid on-site evaluation.
Discussion
In our retrospective cohort study that included 106 pediatric salivary gland FNAs, the rate of non-diagnostic results on initial biopsy was 16% (16/103). This rate increased to 67% (2/3) on repeat biopsy of the same lesion. However, the small number of repeat samples did not allow meaningful comparison of diagnostic yield between initial and repeat FNAs. As alternative management strategies, CT and MRI were diagnostic modalities frequently utilized in our institution following a non-diagnostic FNA. In fact, most of the salivary gland lesions with an initial non-diagnostic FNA (69%, 9/13) were subsequently managed by clinical/radiologic follow-up only, and 7 of these 9 patients received either MRI (n=6) or PET-CT (n=1). For the remaining 2 patients, one also received MRI prior to FNA biopsy. This follow-up strategy led to successful diagnosis and management of 5 vascular anomalies and 4 benign lymph nodes.
Notably, the overall ROM for non-diagnostic aspirates (including initial and repeat FNAs) was zero in our cohort, regardless of the methods of calculation (either by histologic follow-up alone or by combination with clinical/radiological follow-up). Similar findings were also reported in another pediatric series by Satturwar et al15 that included 32 salivary gland FNAs. In a recent international, multi-institutional pediatric series by Maleki et al, the ROM for non-diagnostic category was 5.9% (1 of 17 cases).16 In contrast to this low malignancy risk, MSRSGC estimates the general ROM in non-diagnostic aspirates to be approximately 25% and recommends repeat FNA, imaging studies such as contrast enhanced CT/MRI, alternative biopsy approaches, or surgery.18 Recent studies with large number of cases reporting on the application of MSRSGC have shown variable ROMs for the non-diagnostic category, ranging from 0 to 20%.17,19,22–28 These case series, together with two minimally overlapping meta-analyses,17,22 are summarized in Table 6. Only studies that incorporated at least 300 total FNAs with at least 100 histologic follow-up were included in this summary. When data extracted from all non-pediatric studies were combined, the mean ROM for non-diagnostic category was 15%, a rate lower than that originally estimated by MSRSGC and yet substantially higher than that observed in our pediatric cohort. Again, the relatively small numbers of cases in our cohort and other pediatric series15,16 have limited the statistic power. Nonetheless, the observed minimal or low malignancy risk in non-diagnostic cases in children may have important implications for clinical management and therefore merits further investigation with greater number of patients from different institutions. If substantiated by additional pediatric series, this observation may provide evidence for consideration of a more conservative approach following an initial non-diagnostic FNA biopsy.
Table 6.
Frequency and ROM for non-diagnostic category in MSRSGC reported in the literature.
| Series | Age (yr), mean (range) | No. of FNAs | No. of resection | Non-diagnostic FNA | |
|---|---|---|---|---|---|
| Frequency, % | ROM, % | ||||
| Adult/general population a | |||||
| Farahani 2019 (review)b | 51 (0–100) | 26981 | 16456 | 4 | 17 |
| Jalaly 2020 (review)c | 54 (1–100) | 16394 | 8468 | 10 | 17 |
| Mazzola 2020 | 59 | 503 | 503 | 8 | 20 |
| Altinboga 2021 | 55 (7–95) | 578 | 198 | 15 | 13 |
| Castrodad-Rodríguez 2021 | 58 (10–99) | 380 | 176 | 17 | 0 |
| Higuchi 2021 | 56 (0– 96) | 1608 | 1608 | 18 | 13 |
| Hirata 2021 | 52 (11–90) | 480 | 216 | ‐ | 5 |
| Hosseini 2021 | 63 (14–94) | 343 | 162 | 6 | 13 |
| Reerds 2021 | 55 (0–98) | 12,898 | 12,898 | 19 | 13 |
| Total | ‐ | 60,165 | 40,685 | 10 | 15 |
| Pediatric population | |||||
| Wang 2021 and current study | 13 (0–21) | 106 | 54 | 17 | 0 |
| Satturwar 2021 | 12 (0–18) | 32 | 20 | 16 | 0 |
| Maleki 2022 | 13 (0–21) | 477 | 237 | 10 | 6 |
ROM, risk of malignancy; MSRSGC, Milan System for Reporting Salivary Gland Cytopathology; FNA, fine‐needle aspiration;
Case series with at least 300 FNAs and 100 resection specimens not included in the reviews by Farahani et al or Jalaly et al are summarized in this table;
44 publications between 1966 and 2017 were included;
37 publications between 2017 and 2020 were included.
Among the three factors significantly associated with non-diagnostic aspirates in our study, palpation guided FNAs have been consistently shown by others to yield lower diagnostic rates compared to ultrasound guided FNAs in the setting of adult palpable head and neck lesions.29–31 At least two potential underlying mechanisms for higher diagnostic yield conferred by ultrasound guided FNAs were considered by some authors,30 including 1) accurate needle positioning within the lesion during aspiration, and 2) visualization and targeting of heterogeneous areas of a lesion for selective sampling of solid areas and avoidance of cystic or necrotic tissue. We consider the same reasoning is also applicable in pediatric patients. In keeping with this notion, lesions of cystic, vascular, or diffuse nature were found to be a significant determinant of non-diagnostic aspirates in our study.
Lastly, systematic exploration of the exact reasons for non-diagnostic FNAs via root cause analysis enabled us to recognize and categorize the root causes in each case. The most commonly identified category for primary cause was man (n=11); all were related to sampling rather than interpretation and were assisted by ROSE with impression of inadequate material. In 2 cases, no secondary causes were identified. Both lesions were large, and the aspiration was guided by ultrasound. In the other 9 cases, at least one secondary cause was identified in each, most commonly due to lack of ultrasound guidance. Clinically, these 9 lesions were large and most were palpable. While proceduralist sampling skill was determined to be the main issue for these cases, utilization of ultrasound for better needle positioning and selection of solid areas within the lesion for aspiration may potentially improve the diagnostic yield. The value of ultrasound was also emphasized in MSRSGC’s recommendation regarding a repeat FNA. Despite the application of ROSE in most non-diagnostic cases and the use of multiple passes, all these FNAs eventually failed to obtain adequate diagnostic material. This underscores the challenge of performing FNAs in some of the pediatric salivary gland lesions. Careful review of clinical findings and any available radiologic information regarding the nature of the lesion by the proceduralist prior to FNA biopsy is necessary for consistently achieving high diagnostic rates.
In conclusion, our retrospective pediatric cohort of salivary gland FNAs demonstrated that the initial rate of non-diagnostic results was approximately 16%, and the rate for repeat aspirates could potentially be higher. In contrast to adults, the ROM for lesions with a non-diagnostic MSRSGC category in children was zero, and most patients with an initial non-diagnostic FNA were successfully managed by clinical/radiologic follow-up only rather than repeat FNA or surgery. The underlying mechanism for non-diagnostic aspirates was often multifactorial, but primarily related to proceduralist sampling, characteristics of the lesions, and lack of ultrasound guidance. The diagnostic rate could potentially be improved by close correlation of clinical and radiologic information and utilization of ultrasound guidance.
Figure 4.
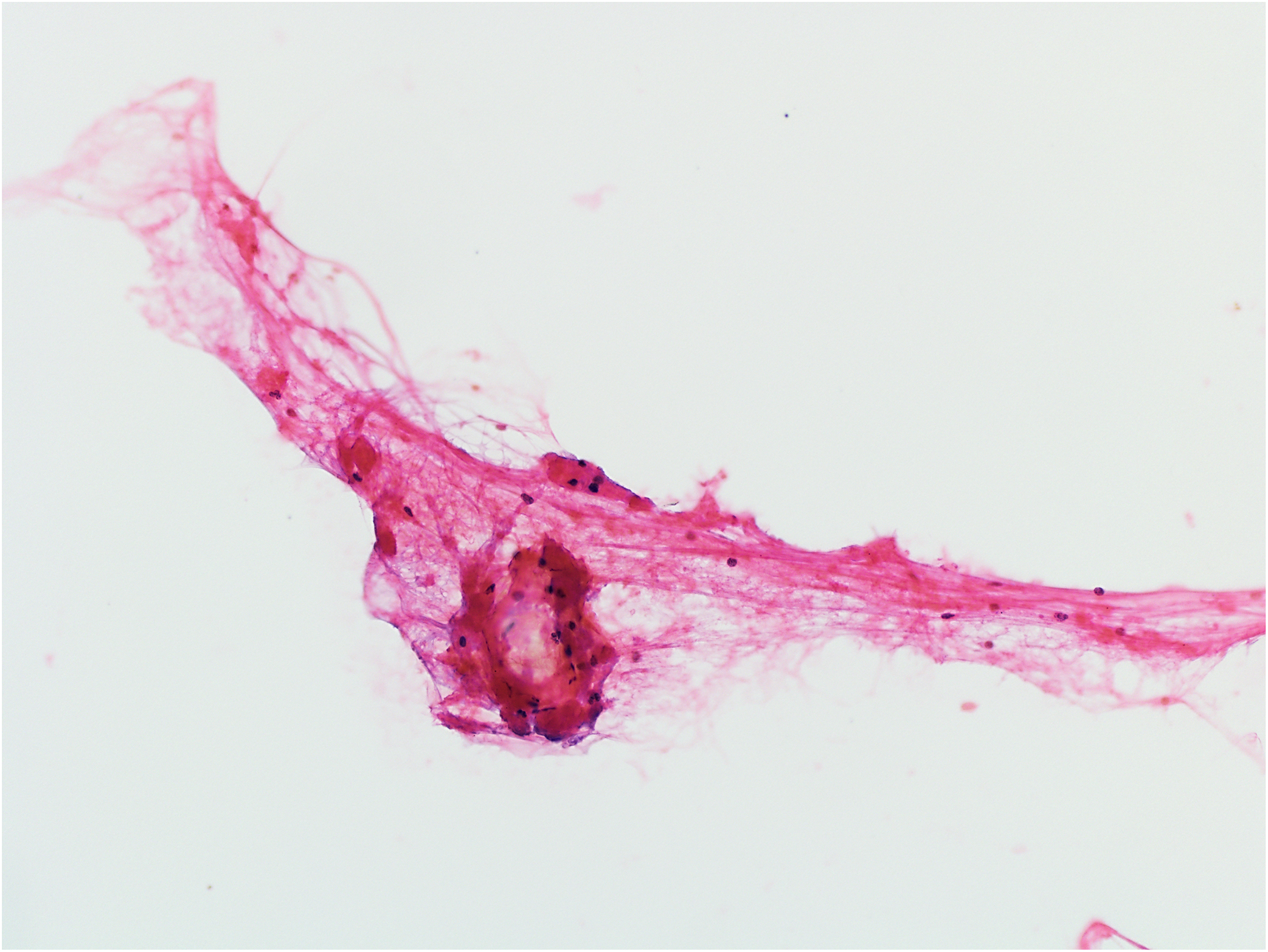
Example of non-diagnostic aspirate: scant fibroconnective tissue (hematoxylin and eosin stain, original magnification ×200). The clinical follow-up diagnosis supported by magnetic resonance imaging studies was benign intraparotid lymph node.
Figure 5.
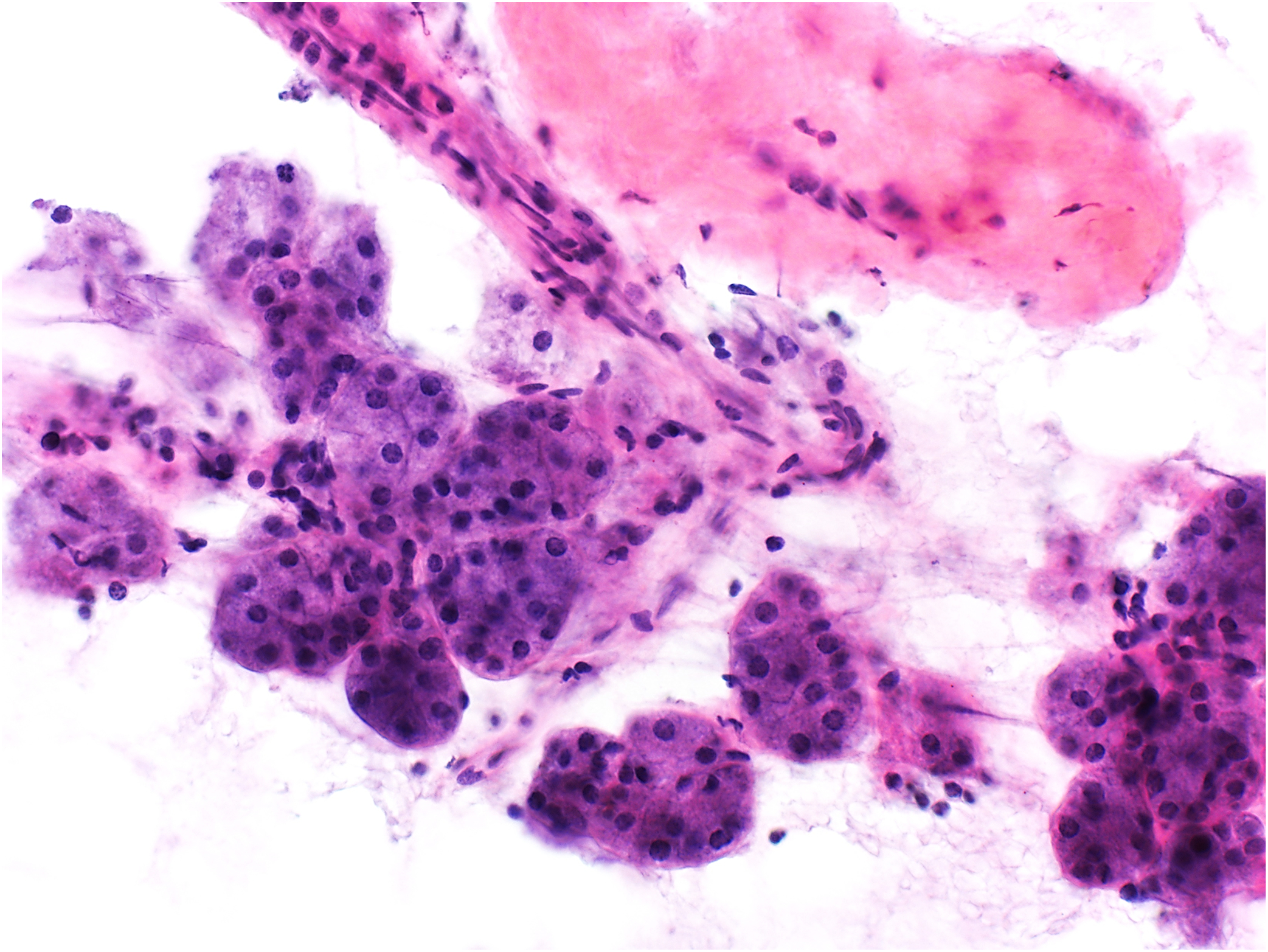
Example of non-diagnostic aspirate: benign salivary gland acinar cells (hematoxylin and eosin stain, original magnification ×400). The histologic follow-up diagnosis was pleomorphic adenoma.
Acknowledgments
This study is not supported by any funding or grants.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Ord RA, Carlson ER. Pediatric Salivary Gland Malignancies. Oral Maxillofac Surg Clin North Am. 2016;28(1):83–89. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida EJ, Garcia J, Eisele DW, Chen AM. Salivary gland malignancies in children. Int J Pediatr Otorhinolaryngol. 2014;78(2):174–178. [DOI] [PubMed] [Google Scholar]
- 3.Carlson ER, Schlieve T. Salivary Gland Malignancies. Oral Maxillofac Surg Clin North Am. 2019;31(1):125–144. [DOI] [PubMed] [Google Scholar]
- 4.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146(1):51–58. [DOI] [PubMed] [Google Scholar]
- 5.Wang XD, Meng LJ, Hou TT, Huang SH. Tumours of the salivary glands in northeastern China: a retrospective study of 2508 patients. Br J Oral Maxillofac Surg. 2015;53(2):132–137. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Weiss VL, Borinstein SC, et al. Application of the Milan System for Reporting Pediatric Salivary Gland Cytopathology: Analysis of histologic follow-up, risk of malignancy, and diagnostic accuracy. Cancer Cytopathol. 2021. Jul;129(7):555–565. doi: 10.1002/cncy.22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pusztaszeri M, Baloch Z, Vielh P, Faquin WC. Application of the Milan system for reporting risk stratification in salivary gland cytopathology. Cancer Cytopathol. 2018;126(1):69–70. [DOI] [PubMed] [Google Scholar]
- 8.Ronchi A, Montella M, Zito Marino F, et al. Diagnostic accuracy of FNA cytology for diagnosis of salivary gland tumors in pediatric patients. Cancer Cytopathol. 2019;127(8):529–538. [DOI] [PubMed] [Google Scholar]
- 9.Lennon P, Silvera VM, Perez-Atayde A, Cunningham MJ, Rahbar R. Disorders and tumors of the salivary glands in children. Otolaryngol Clin North Am. 2015;48(1):153–173. [DOI] [PubMed] [Google Scholar]
- 10.Luna MA, Batsakis JG, el-Naggar AK. Salivary gland tumors in children. Ann Otol Rhinol Laryngol. 1991;100(10):869–871. [DOI] [PubMed] [Google Scholar]
- 11.Ellies M, Laskawi R. Diseases of the salivary glands in infants and adolescents. Head Face Med. 2010;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentz BG, Hughes CA, Ludemann JP, Maddalozzo J. Masses of the salivary gland region in children. Arch Otolaryngol Head Neck Surg. 2000;126(12):1435–1439. [DOI] [PubMed] [Google Scholar]
- 13.Orvidas LJ, Kasperbauer JL, Lewis JE, Olsen KD, Lesnick TG. Pediatric parotid masses. Arch Otolaryngol Head Neck Surg. 2000;126(2):177–184. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Yoon TM, Lee JK, Lim SC. Clinical utility of fine needle aspiration cytology in pediatric parotid tumors. Int J Pediatr Otorhinolaryngol. 2013;77(8):1272–1275. [DOI] [PubMed] [Google Scholar]
- 15.Satturwar SP, Fuller MY, Monaco SE. Is Milan for kids?: The Milan System for Reporting Salivary Gland Cytology in pediatric patients at an academic children’s hospital with cytologic-histologic correlation. Cancer Cytopathol. 2021Nov;129(11):884–892. doi: 10.1002/cncy.22455. [DOI] [PubMed] [Google Scholar]
- 16.Maleki Z, Saoud C, Viswanathan K, et al. Application of the Milan System for Reporting Salivary Gland Cytopathology in pediatric patients: An international, multi-institutional study. Cancer Cytopathol. 2022. Jan 26. doi: 10.1002/cncy.22556 [DOI] [PubMed] [Google Scholar]
- 17.Jalaly JB, Farahani SJ, Baloch ZW. The Milan system for reporting salivary gland cytopathology: A comprehensive review of the literature. Diagn Cytopathol. 2020;48(10):880–889. [DOI] [PubMed] [Google Scholar]
- 18.Baloch Z, Field AS, Katabi N, Wenig BM. The Milan system for reporting salivary gland cytopathology. In: The Milan system for reporting salivary gland cytopathology. Springer; 2018:1–9. [Google Scholar]
- 19.Reerds STH, Van Engen-Van Grunsven ACH, van den Hoogen FJA, Takes RP, Marres HAM, Honings J. Accuracy of parotid gland FNA cytology and reliability of the Milan System for Reporting Salivary Gland Cytopathology in clinical practice. Cancer Cytopathol. 2021;129(9):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DN, Antic T, Reeves W, et al. Histopathologic and clinical outcomes of Milan System categories “non-diagnostic” and “non-neoplastic” of salivary gland fine needle aspirations. J Am Soc Cytopathol. 2021;10(4):349–356. [DOI] [PubMed] [Google Scholar]
- 21.Bongiovanni M, Faquin WC, Rossi ED. The Milan system for reporting salivary gland cytopathology. 2018. [Google Scholar]
- 22.Farahani SJ, Baloch Z. Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta-analysis of published literature. Diagn Cytopathol. 2019;47(2):67–87. [DOI] [PubMed] [Google Scholar]
- 23.Mazzola F, Tomasoni M, Mocellin D, et al. A multicenter validation of the revised version of the Milan system for reporting salivary gland cytology (MSRSGC). Oral Oncol. 2020;109:104867. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy Altinboga A, Yildirim F, Ahsen H, Kiran MM, Kesici GG, Yuce G. The effectiveness of the Milan system for risk stratification of salivary gland lesions: The 10-year cytohistopathological correlation results of salivary gland FNA cytology at a tertiary center. Diagn Cytopathol. 2021;49(8):928–937. [DOI] [PubMed] [Google Scholar]
- 25.Castrodad-Rodriguez CA, Lajara S, Khader SN, et al. Application of the Milan System for Reporting Salivary Gland Cytopathology: Experience of an academic institution in a tertiary academic medical center. Cancer Cytopathol. 2021;129(3):204–213. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi K, Urano M, Akiba J, et al. A multi-institutional study of salivary gland cytopathology: Application of the Milan System for Reporting Salivary Gland Cytopathology in Japan. Cancer Cytopathol. 2022. Jan;130(1):30–40. doi: 10.1002/cncy.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirata Y, Higuchi K, Tamashiro K, et al. Application of the Milan System for Reporting Salivary Gland Cytopathology: A 10-Year Experience in a Single Japanese Institution. Acta Cytol. 2021;65(2):123–131. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini SM, Resta IT, Baloch ZW. Diagnostic performance of Milan system for reporting salivary gland cytopathology: A prospective study. Diagn Cytopathol. 2021;49(7):822–831. [DOI] [PubMed] [Google Scholar]
- 29.Robitschek J, Straub M, Wirtz E, Klem C, Sniezek J. Diagnostic efficacy of surgeon-performed ultrasound-guided fine needle aspiration: a randomized controlled trial. Otolaryngol Head Neck Surg. 2010;142(3):306–309. [DOI] [PubMed] [Google Scholar]
- 30.Conrad R, Yang SE, Chang S, et al. Comparison of Cytopathologist-Performed Ultrasound-Guided Fine-Needle Aspiration With Cytopathologist-Performed Palpation-Guided Fine-Needle Aspiration: A Single Institutional Experience. Arch Pathol Lab Med. 2018;142(10):1260–1267. [DOI] [PubMed] [Google Scholar]
- 31.Wu M A comparative study of 200 head and neck FNAs performed by a cytopathologist with versus without ultrasound guidance: evidence for improved diagnostic value with ultrasound guidance. Diagn Cytopathol. 2011;39(10):743–751. [DOI] [PubMed] [Google Scholar]


