Objective:
Gallbladder cancer (GBC) mortality remains high and chemoresistance is increasing. This review consolidates what is known about the mechanisms of chemoresistance to inform and accelerate the development of novel GBC-specific chemotherapies.
Methods:
Studies related to GBC-related chemoresistance were systematically screened in PubMed using the advanced search function. Search terms included GBC, chemotherapy, and signaling pathway.
Results:
Analysis of existing studies showed that GBC has poor sensitivity to cisplatin, gemcitabine (GEM), and 5-fluorouracil. DNA damage repair-related proteins, including CHK1, V-SCR, and H2AX, are involved in tumor adaptation to drugs. GBC-specific chemoresistance is often accompanied by changes in the apoptosis and autophagy-related molecules, BCL-2, CRT, and GBCDRlnc1. CD44+ and CD133+ GBC cells are less resistant to GEM, indicating that tumor stem cells are also involved in chemoresistance. In addition, glucose metabolism, fat synthesis, and glutathione metabolism can influence the development of drug resistance. Finally, chemosensitizers such as lovastatin, tamoxifen, chloroquine, and verapamil are able improve the therapeutic effect of cisplatin or GEM in GBC.
Conclusions:
This review summarizes recent experimental and clinical studies of the molecular mechanisms of chemoresistance, including autophagy, DNA damage, tumor stem cells, mitochondrial function, and metabolism, in GBC. Information on potential chemosensitizers is also discussed. The proposed strategies to reverse chemoresistance should inform the clinical use of chemosensitizers and gene-based targeted therapy for this disease.
Key Words: gallbladder cancer, chemoresistance, chemosensitizers, reversal strategies, combination chemotherapy
Gallbladder cancer (GBC) incidence is low; however, the prognosis is poor and mortality is extremely high.1 Adenocarcinoma, which largely occurs in epithelial cells that line the gallbladder, is the most common form of this disease. Patients with a medical history of bile duct obstruction are at higher risk of developing GBC (odds ratio=4.4).2,3 In particular regions, including Chile, Northern India, and New Mexico, GBC incidence is very high.4,5 In China, GBC is relatively rare and most cases are distributed in the northwest and northeast.6 GBC incidence is increasing each year, however, and women have higher incidence and mortality rates than men.6–8 To date, there has been a lack of effective prevention methods and treatments for this disease.9
GBC chemoresistance is increasing. While surgery is the most effective treatment for GBC, early diagnosis is rare. Most patients lack the option of surgery upon diagnosis, and local recurrence following both laparoscopic and open surgery is >9%.10 For many individuals who receive successful surgical treatment, GBC was accidentally discovered during other operations.11,12
Chemotherapy is the primary option for GBC patients who are not eligible for surgery. Regimens based on gemcitabine (GEM) and cisplatin have been widely used in the past 10 years, but the drug response rate remains undesirable, and drug resistance is a challenge.13 While combination treatment using chemotherapy and targeted drugs has improved the response rate, efficacy remains poor.14 Chemotherapy regimens based on characteristic genes may improve GBC treatment outcomes.15,16 The current review summarizes the findings of 91 papers and 10 clinical trials that are specifically focused on GBC-related chemosensitivity (Fig. 1). The highlighted results should inform strategies to reverse chemotherapy resistance in GBC patients.
FIGURE 1.
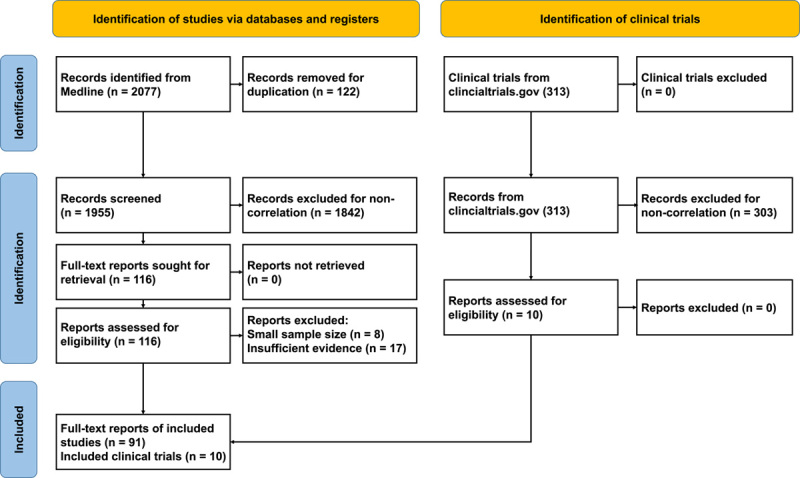
Flow chart of the screened literature.
METHODS
Analysis of medical studies covering January 1, 2005 through May 1, 2022, from journals indexed in Medline using the advanced search function yielded a set of relevant records. Text words related to GBC (“gallbladder cancer,” “gallbladder neoplasm,”), chemotherapy (“chemotherapy,” “drug therapy”) were used in the search strategy. The full-text articles were screened and systematically categorized by criteria which as detailed in the selection flow diagram (Fig. 1). After stepwise screening and assessing, 91 original research studies were selected. Then we extracted the key genes, signaling pathways, cell lines used for experimental verification, chemotherapeutic drugs, survival information of patients, sample size from these articles (Table 1).
TABLE 1.
Genes That Interfere With GBC-Specific Sensitivity to Chemotherapeutics
| Gene symbol | Cell lines | Drugs | Research types | Event | Sensitivity to drugs (in vitro) | Tumor volume vs. control (in vivo) | Sample size; median survival time (mo) |
|---|---|---|---|---|---|---|---|
| CRT | GBC-SD+NOZ | GEM17 | vtr+cli | KD OE | Up Down | — — | 7; 28 25; 16 |
| LncRNA-RP11-147L13.8 | GBC-SD+NOZ | GEM18 | vtr+cli | KD OE | Down Up | — — | 48; 30 48; 50 |
| Lnc-RNA-MYLK-AS1 | GBC-SD+NOZ+EH-GB1+SGC-996 | GEM19 | vtr+cli | KD OE | Up Down | — — | 60; 60 60; 20 |
| Lnc-RNA-SSTR5-AS1 | GBC-SD+NOZ | GEM20 | vvo+vtr+cli | KD OE | Up Down | Smaller — | 54; 50 56; >80 |
| MiR-205-5p | GBC-SD | GEM21 | vvo+vtr+cli | KD OE | Down Up | — Smaller | 68; 25 68; 40 |
| ELP5 | GBC-SD+NOZ | GEM22 | vvo+vtr+cli | KO OE | Down Up | Bigger — | 10; 60 50; 22 |
| MBD1 | GBC-SD+SGC-996 | GEM23 | vtr+cli | KD — | Up — | — — | 26; 25 58; 15 |
| MiR-218-5p | GBC-SD+NOZ | GEM24 | vvo+vtr+cli | KD OE | Down Up | — Smaller | 42; 10 40; 20 |
| MiR-125b-5p | NOZ+GBC-SD+SGC-996 | CDDP25 | vvo+vtr+cli | KD OE | Down Up | — Smaller | 41; 10 41; 20 |
| aPKCι | GBC-SD+NOZ | GEM26 | vvo+vtr+cli | KD — | Up — | Smaller — | 23; 50 49; 5 |
| MiR-433 | GBC-SD | GEM27 | vtr | — OE | — Down | — — | — — |
| GLI2 | TYGBK-1+NOZ+TGBC2TKB | GEM28 | vvo+vtr+cli | KD — | Down — | Smaller — | 9; 50 5; >60 |
| UCP2 | G-415 | GEM29 | vvo+vtr+cli | KD — | Up — | — — | — — |
| NOX1 | GBC-SD+SGC-996 | CDDP30 | vvo+vtr | KD OE | Up Down | Smaller — | — — |
| FXR | SGC-996+QGC939+GBC-SD | CDDP31 | vvo+vtr+cli | — Activate | — Up | — Smaller | — — |
| MiR-1207-5p | GBC-SD | CDDP32 | vtr | Inhibit Activate | Up Down | — — | — — |
| V-SRC | HAG-1 | CDDP33 | vtr | — OE | — Down | — — | — — |
| MiR-31 | GBC-SD+NOZ | CDDP34 | vvo+vtr | — OE | — Up | — Smaller | — — |
| C-ERBB-2 | HAG-2 | CDDP35 | vtr | — OE | — Up | — — | — — |
| OLFM4 | GBC-SD | CDDP36 | vvo+vtr+cli | KD — | Up — | Smaller — | 10; 20 30; 10 |
| GBCDRlnc1 | GBC-SD+NOZ | DOX+5-FU37 | vvo+vtr+cli | KD OE | Up Down | Smaller — | 22; 15 23; 8 |
| MiR-1231 | GBC-SD+NOZ | DTX38 | vtr+cli | — OE | — Up | — — | — — |
| MiR-335 | GBC-SD+SGC-996 | 5-FU39 | vtr | — OE | — Up | — — | — — |
| XRCC1 | GBC-SD | 5-FU40 | vtr+cli | KD — | Up — | — — | 86; 13 129; 9 |
| PLAC8 | SGC-996GR+SGC-996OR | GEM+OXA41 | vtr | KD — | Up — | — — | — — |
| DUSP1 | SGC996+GBC-SD | CDDP+5-FU42 | vtr | — OE | — Down | — — | — — |
5-FU indicates 5-fluorouracil; CDDP, cisplatin; cli, clinical research; Dox, doxorubicin; DTX, docetaxel; GBC, gallbladder cancer; GEM, gemcitabine; KD, knockdown; KO, knockout; OE, overexpress; OXA, oxaliplatin; vtr, in vitro; vvo, in vivo.
At the same time, 10 clinical trials from Clincialtrials.gov involving chemotherapy sensitization are included in this review. Descriptive statistics report the results.
THE RESPONSE RATE OF FIRST-LINE GBC CHEMOTHERAPY DRUGS
GBC has high rates of malignancy and multidrug resistance. The current chemotherapy drugs include cisplatin (CDDP), GEM, 5-fluorouracil (5-FU), epirubicin, oxaliplatin (OXA), S-1, capecitabine, and Irinotecan.43,44 Clinical trial results indicate that the response rate of commonly used chemotherapy drug combinations remain low: 26.1%, 15.5%, 30.7%, 14.3%, and 29.8% for GEM+CDDP, GEM, GEM+OXA, 5-FU+FA, and GEM+S-1, respectively.13,16,45
The response rate of GEM+CDDP as a first-line chemotherapy regimen (26.1%) was 10.6% higher than that of GEM alone (15.5%).13 However, it is worth noting that in a clinical trial of 410 patients with advanced biliary tract tumors, patients treated with GEM+CDDP had a greater survival advantage than those treated with GEM alone. In addition, the combination treatment was also not associated with a substantial increase in side effects.46 The anticancer effect of GEM can be inhibited by drug metabolism, but the key factors involved in this process are not yet fully understood.47
GBC has a low sensitivity to first-line chemotherapy drugs, and some researchers have identified its occurrence at the cellular and molecular levels (Table 1). In addition to first-line chemotherapy regimens, other drugs used to treat GBC include 5-FU, OXA, doxorubicin, docetaxel, and molecular-targeted drugs.48 Several factors contribute to the responsiveness of GBC to these medications (Fig. 2).
FIGURE 2.
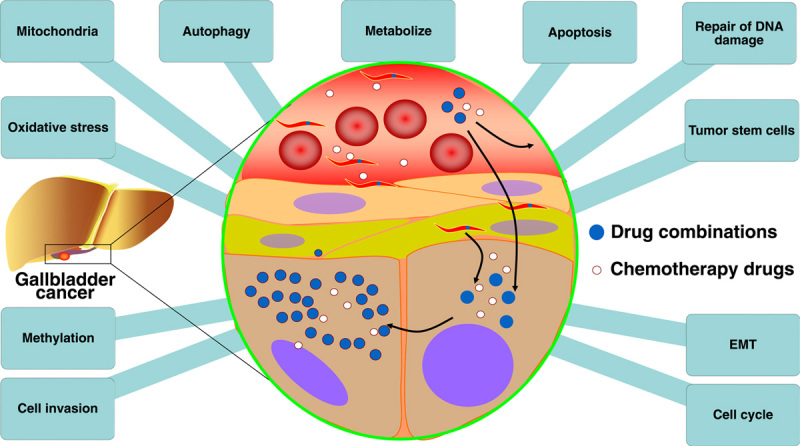
Factors affecting gallbladder cancer drug resistance. EMT indicates epithelial-to-mesenchymal transition.
BOTH PROGRAMMED AND NONPROGRAMMED CELL DEATH CONTRIBUTE TO CHEMORESISTANCE
Programmed cell death usually includes both apoptosis and autophagy, while nonprogrammed cell death is broader and includes death caused by DNA damage. Platinum-based chemotherapy drugs can cause cellular DNA damage. Cells then recognize the damage and initiate DNA repair responses to maintain genome stability.49 However, this mechanism of self-repair can drive cancer cells to develop resistance to chemotherapy drugs. Targeting DNA damage response pathways has been used as a therapeutic strategy for colorectal cancer, and DNA damage response inhibitors are being further evaluated in preclinical and clinical studies.50 Lovastatin can inhibit cholesterol biosynthesis in GBC and also increase the sensitivity of cancer cells to cisplatin by inhibiting the expression of CHK1, CHK2, and H2AX, during the DNA damage response.51 Gallbladder adenocarcinoma cells with high V-SCR expression are highly resistant to cisplatin, primarily as a result of enhanced DNA repair responses.33 These findings suggest that DNA damage repair responses may play an important role in the chemoresistance mechanism of GBC.
Apoptosis occurs in various tumor chemotherapy processes, and cancer cell sensitivity to apoptosis-related anticancer drugs is dependent on the type of drug and the function of antiapoptotic proteins such as B-cell lymphoma-2 (Bcl-2). This protein not only regulates the apoptosis of normal cells, but also contributes to pancreatic cancer resistance to GEM.52 Apoptosis occurs through extrinsic and intrinsic pathways, and chemotherapy-induced apoptosis is associated with intrinsic, mitochondria-mediated pathways.53 Moreover, apoptosis is often associated with DNA damage repair. If repair fails, the mitochondrial apoptosis cascade becomes activated and cell death occurs.54
CRT knockdown not only inhibits GBC cell proliferation and promotes apoptosis, but also increases the sensitivity of gallbladder carcinomas to GEM.17 Upregulated SSTR5-AS1 promotes GEM resistance in GBC by inhibiting apoptosis.20 MiR-1231 overexpression attenuates the proliferative potential of GBC cells and both induces apoptosis and enhances GBC sensitivity to docetaxel.38 OLFM4 downregulation reduces the expression of ARL6IP1, an antiapoptotic factor, and sensitizes GBC cells to cisplatin in vitro and in vivo.36 while XRCC1 knockdown significantly increases the sensitivity of CD133+ GBC cells to 5-FU by promoting apoptosis.40
Autophagy, also known as type II programmed cell death, was first proposed by Ashford and Porter who used electron microscopy to identify autophagic structures in human hepatocytes. There are 3 main autophagy-related signaling pathways involved in mediating tumor drug resistance: HSF1-mediated autophagy, reactive oxygen species (ROS)-mediated autophagy, and Met inhibition.55,56 Current studies indicate that GBC-specific drug resistance is related to autophagy activity. GBC resistance-related lncRNA1 (GBCDRlnc1) is a key regulator of chemoresistance.37 Knockdown of this gene inhibits autophagy at the initial stage, enhancing GBC cell sensitivity to doxorubicin both in vitro and in vivo.37
ENDOGENOUS FACTORS LEAD TO THE MALIGNANT PROGRESSION AND DRUG RESISTANCE OF CANCER CELLS
One of the endogenous factors involved in chemoresistance is cancer stem cells. CD133+ GBC cells are thought to have the properties of cancer stem cells, capable of promoting tumor formation, proliferation, invasion, and drug resistance.57 While cancer stem cells exist in a variety of tumors, and the markers for identification, including CD44, CD133, and LGR5, have been widely used, the mechanism of chemoresistance in GBC stem cells remains unknown.58,59 Traditional tumor therapy can inhibit tumor cell proliferation, while resting cancer stem cells may develop mutations and promote drug resistance.60
CD44+ and CD133+ GBC cells are less resistant to GEM when miR-205-5p expression is increased.21 While purified CD133+ GBC cells are highly resistant to conventional chemotherapy, arsenic trioxide can effectively induce apoptosis and reduce CD133 gene expression.61 Thus, inhibiting tumor stem cell marker gene expression and attenuating cancer stem cell properties is expected to increase the sensitivity of GBC to chemotherapeutic drugs while reducing the effective dose. Finally, sesamin can potently affect the stem cell-like characteristics of GBC side population cells, attenuating their resistance to cisplatin, and inhibiting tumor growth both in vitro and in vivo.62
Cell cycle-regulated molecules are also involved in the GBC response to chemotherapeutics. For example, aberrant activation of cyclin-dependent kinases is a hallmark of cancer cell cycle dysregulation.63 Studies have confirmed that the abnormal function of Rb, FAT1, FZR1, and other proteins in cell cycle process can promote tumor chemotherapy resistance.64–66 Indeed, alterations in upstream signaling pathways, such as PI3K/Akt (protein kinase B), can cause tumor resistance to chemotherapeutics by affecting the cell cycle. CRT can mediate PI3K/Akt signaling to increase the proportion of GBC cells in the G0/G1 stage and elevate GBC sensitivity to GEM.17 In addition, ectopic expression of MiR-433 gene can significantly reduce cyclin M expression and lead to GEM resistance in GBC patients.27 In addition, MiR-335 overexpression causes the GBC cell cycle to arrest in the G0/G1 phase, increasing cell sensitivity to 5-fluoropyrimidine treatment.39 Thus, it is important to consider the roles of both cancer protein and genes in mediating drug resistance.
Epithelial-to-mesenchymal transition (EMT) is the process by which epithelial cells separate from their neighbors and acquire the migratory properties of mesenchymal cells.67 Of note, EMT and drug resistance in GBC are closely related to this process. A research team from Fudan University Shanghai Cancer Center found that MBD1 promotes the malignant behaviors, including invasion, proliferation and migration, and EMT, of GBC cells and induces chemoresistance to GEM.23 Other studies have confirmed that GLI2, a transcription factor in the Hedgehog (Hh) signaling pathway, promotes cell invasion by activating GBC cell EMT, increasing cancer cell sensitivity to GEM.28 Noncoding genes can also affect GBC-mediated drug resistance properties by altering cell invasiveness. For example, researchers from Zhongshan Hospital of Fudan University found that lncRNA-RP11-147L13.8 overexpression inhibited GBC cell migration and invasion and increased GBC cell sensitivity to GEM.68
MITOCHONDRIAL DYSFUNCTION ALTERS DRUG RESISTANCE IN GBC CELLS
Mitochondrial dysfunction and enhanced oxidative stress can improve the efficacy of chemotherapeutic drugs in GBC patients. ROS are primarily produced by the respiratory chain of the mitochondrial inner membrane and the rate of ROS production is regulated by the mitochondrial inner membrane transmembrane potential and related functional proteins. While, mitochondrial metabolism supports tumor anabolism by providing key metabolites for macromolecule synthesis.69 Inhibiting this process increases intracellular oxygen levels and enhances the lethality of chemotherapeutic drugs.70 Interestingly, the knockdown of UCP2 in GBC inhibits the noncanonical nuclear factor-kappaB(NF-κB)/β-catenin axis, and increases mitochondrial ROS, ultimately enhancing the GEM sensitivity of GBC cells.29 MCL1 is also involved in leptin-promoted mitochondrial fusion and can, in turn, inhibit GEM-induced cell death.71 Similarly, typical protein kinase Cι (aPKCι) inhibits ROS production in a kinase-independent manner by competing with Nrf2 for Keap1 binding, conferring GEM resistance to GBC cells.26 Finally, increased NOX1 expression increases intracellular ROS levels, which activate the HIF-1α/MDR1 pathway and promote GBC chemoresistance to cisplatin.30 In summary, mitochondrial dysfunction and oxidative stress are involved in GBC sensitivity to chemotherapeutic drugs.
ABNORMAL METABOLISM REGULATES THE DRUG SENSITIVITY OF GBC CELLS
Studies indicate that cell metabolism, including glucose metabolism, fat synthesis, cholesterol synthesis, hormone production, and glutathione metabolism can regulate GBC-specific drug resistance. For example, tamoxifen inhibits GBC cell proliferation by interfering with glucose metabolism, increasing cisplatin sensitivity.72 Animal experiments have shown that ovariectomies can eliminate estrogen and prevent GBC-specific resistance to cisplatin in nude mice.72 In UCP2 knockout mice, glycolysis is inhibited and GEM treatment sensitivity is increased in GBC cells.29 Lovastatin, a hypolipidemic drug, combined with cisplatin, can also enhance the efficacy of drug treatment in GBC mouse models.51 Finally, emodin and cisplatin combination therapy significantly increases GBC cell chemosensitivity by inducing glutathione.73 In summary, UCP2 and lovastatin can impact GBC chemosensitivity by affecting metabolic processes.
EPIGENETICS-INVOLVED CHEMORESISTANCE IN GBC
Studies have also identified a role for epigenetics in GBC-specific chemoresistance. Hypermethylation of the ELP5 promoter is shown to inhibit ELP5 expression, while DNA demethylating agents sensitize GBC cells to GEM by inducing ELP5.43,74 Some noncoding RNAs are also regulators of GBC-specific drug resistance. For example, long noncoding RNA-SSTR5-AS1 promotes GEM resistance in GBC by regulating NONO.20 In addition, lnc-RNA-MYLK-AS1 promotes GEM resistance by upregulating EZH expression.19 Other studies report that MiR-218-5p promotes GBC sensitivity to GEM by attenuating PRKCE expression.24,75 However, MiR-125b-5p enhances GBC sensitivity to cisplatin by downregulating Bcl-2.25,41 GBC sensitivity to cisplatin was shown to increase when MiR-1207-5p expression decreased in the peripheral blood.24,32 These findings suggest that hypermethylation of ELP5 and noncoding RNAs plays a role in regulating GBC-specific chemoresistance.
SIGNALING PATHWAYS INVOLVED IN GBC SENSITIVITY TO CISPLATIN, GEM, AND 5-FU
Most studies have used cisplatin and GEM to assess the drug resistance mechanism of GBC and have primarily focused on a single gene. Using a single target gene is problematic because the biological functions of cells are often accompanied by changes in signaling pathways. These changes play an important role in the development of drug resistance in cancer cells.76 Identification of the key pathway associated with GBC chemoresistance will inform the development of chemosensitizers. The current study provides a review of chemotherapy-related pathways in GBC and synthesizes the effects of altering these signaling pathways on GBC cell sensitivity (Table 2).
TABLE 2.
Signaling Pathways That Interfere With GBC-specific Sensitivity to Chemotherapeutics
| Pathway | Cell lines | Drugs | Research types | Event | Sensitivity to drugs (in vitro) | Year |
|---|---|---|---|---|---|---|
| p38 | SGC996+GBC-SD | CDDP+5-FU42 | vtr | — Inhibit | — Down | 2018 |
| HIF1α/MDR1 | GBC-SD+SGC-996 | CDDP30 | vtr | — Activate | — Down | 2015 |
| PI3K/Akt | GBC-SD+NOZ | GEM17 | vtr | — Inhibit | — Up | 2021 |
| NF-κB/β-catenin | G-415 | GEM29 | vtr | — Inhibit | — Up | 2020 |
| Hedgehog | TYGBK-1+NOZ+TGBC2TKB | GEM28 | vtr | — Inhibit | — Down | 2021 |
| MEK/ERK+PI3K—AKT | GBC-SD+SGC-996 | 5-FU77 | vtr | — Inhibit | — Up | 2020 |
| Mitochondrial-Dependent Apoptosis Pathway | GBC-SD | Epirubicin78 | vtr | — Inhibit | — Down | 2011 |
| NF-κB | GBC-SD+EH-GB1+EH-GB2 | GEM79 | vtr | — Inhibit | — Up | 2015 |
| Hippo-YAP1 | GBC-PDOs | GEM80 | vtr | — Inhibit | — Up | 2020 |
| EGFR/HER2 | TGBC1-TKB | GEM81 | vtr | — Inhibit | — Up | 2010 |
5-FU indicates 5-fluorouracil; CDDP, cisplatin; cli, clinical research; Dox, doxorubicin; DTX, docetaxel; GBC, gallbladder cancer; GEM, gemcitabine; KD, knockdown; KO, knockout; OE, overexpress; OXA, oxaliplatin; vtr, in vitro; vvo, in vivo.
Multiple pathways are involved in GBC sensitivity to cisplatin and 5-FU. For example, signaling through p38 mitogen-activated protein kinase, a member of the mitogen-activated protein kinase family, can be activated by multiple environmental stresses and inflammatory cytokines.42 DUSP1 also enhances GBC chemoresistance to cisplatin and 5-FU by regulating p38 and the DNA damage/repair system.34,42 In addition, NOX1 overexpression in a GBC cell line (SGC-996) activates the HIF-1α/MDR1 pathway and mediates GBC-specific resistance to cisplatin.30 The NF-κB pathway often acts as a downstream effector pathway for PI3K-AKT signaling. Activated PI3K-AKT (protein kinase B) can activate NF-κB, upregulate expression of the MDR1 gene and its protein product, P-gp, and promote drug resistance in tumor cells.79,82 NF-κB also induces tumor drug resistance by upregulating expression of apoptosis-related genes such as Bcl-xl, which acts together with P-gp.83 Bufalin inhibits mitogen-activated protein kinase, extracellular regulated protein kinases (MEK/ERK) and PI3K/AKT signaling by inhibiting the expression of pc-Met and improves GBC cell sensitivity to 5-FU.42,77 In summary, several related signaling pathways regulate GBC-specific sensitivity to cisplatin and 5-FU.
GEM is the first-line agent for GBC chemotherapy and the PI3K/Akt, NF-κB, Hh and epidermal growth factor receptor (EGFR)/HER2 pathways mediate the response of GBC to this drug. For example, GBC is less resistant to GEM after inactivation of the PI3K/Akt pathway,17 and maslinic acid enhances the antitumor activity of GEM both in vitro and in vivo by inhibiting NF-κB signaling in human GBC cells.79 UCP2 knockdown inhibits the NF-κB/β-catenin axis in GBC, and treatment with a UCP2 inhibitor increases GEM sensitivity in animal xenograft models.29 However, inhibition of GLI2 in the Hh signaling pathway reduces GBC sensitivity to GEM.28 Targeted inhibition of the EGFR/HER2 pathway can also enhance the antiproliferative effect of GEM in the biliary tract and GBC.81 Interestingly, GBC cells show increased sensitivity to GEM after inhibition of Hippo-Yes-associated protein 1 (YAP1) signaling.80 These findings inform methods for addressing GBC-specific chemoresistance.
The signaling pathways involved in GBC-specific sensitivity to chemotherapeutics are depicted in Figure 3. The expression of some of these key molecules is confirmed in GBC. However, only MEK/ERK, PI3K/AKT, NF-κB, Hh, and EGFR/HER2 signaling can increase GBC sensitivity after the pathway is blocked, and the corresponding target drugs are also limited.84 The discovery of novel drug resistance targets will inform the development of chemosensitizers that offer new treatment options for GBC patients.
FIGURE 3.
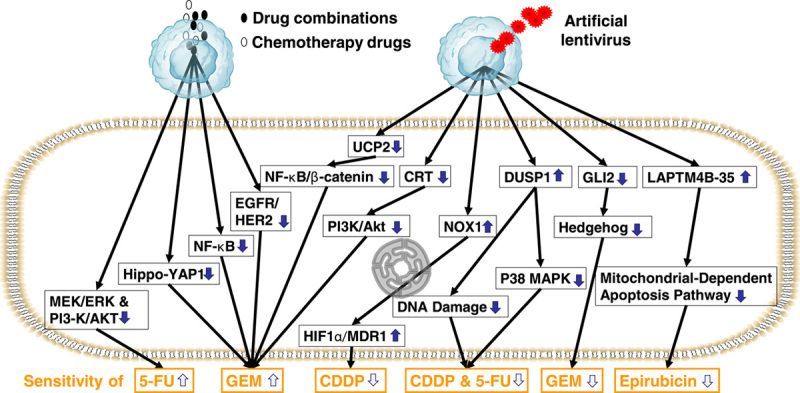
Signaling pathways associated with the gallbladder cancer response to chemotherapy drugs.
STRATEGIES TO REVERSE GBC-SPECIFIC DRUG RESISTANCE
Sensitizers can enhance the efficacy of radiotherapy or chemotherapy; however, some can be associated with adverse effects. Developing effective chemosensitizers is important to reduce the effective dose of chemotherapy drugs and counter GBC-specific chemoresistance (Table 3).
TABLE 3.
Potential Sensitizers for GBC Chemotherapeutics
| Sensitizer | Sensitized drugs | Targets | Cell lines | Sensitivity to drugs (in vitro) | Sensitivity to drugs (in vivo) | Year |
|---|---|---|---|---|---|---|
| Maslinic acid | GEM79 | NF-κB Pathway | EH-GB1+EH-GB2+GBC-SD | Up | Up | 2015 |
| Chloroquine | GEM85 | Autophagy | NOZ+SGC-996+GBC-SD | Up | Up | 2020 |
| Lenvatinib | GEM86 | PI3K/AKT | NOZ+GBC-SD | Up | — | 2021 |
| α-Mangostin | GEM87 | AMPK | NOZ+GBC-SD | Up | Up | 2020 |
| Hispidulin | GEM+5-FU88 | HIF-1α/P-gp | GBC-SD | Up | — | 2015 |
| Bufalin | 5-FU77 | MEK/ERK+PI3K/AKT | SGC-996+GBC-SD | Up | — | 2020 |
| Lovastatin | CDDP51 | Mevalonate Pathway | NOZ+GBC-SD | Up | Up | 2019 |
| Leptin | GEM71 | CEBPD | RCB-1130 | Down | — | 2021 |
| Tamoxifen | CDDP72 | Nrf2 | NOZ+SGC-996+GBC-SD | Up | Up | 2020 |
| Icariin | GEM89 | NF-κB | SGC-996+GBC-SD | Up | Up | 2013 |
| Metformin | CDDP90 | PI3K/AKT/ERK Pathway | SGC-996+GBC-SD | Up | Up | 2018 |
| OSI-027 | 5-FU91 | mTOR | RBE+GBC-SD | Up | — | 2016 |
| Cordycepin | GEM+5-FU92 | AMPK/mTORC1 | GBC-SD | Up | — | 2014 |
| Phenoxodiol | GEM93 | Akt/mTOR | SGC-996+GBC-SD | Up | Up | 2014 |
| Verapamil | CDDP+CBP+OXA94 | GSH+MRP1 | SGC-996+GBC-SD | Up | — | 2013 |
| Emodin | CDDP83 | Survivin | SGC-996 | Up | Up | 2011 |
| Emodin | CDDP95 | ABCG2 | SGC-996+GBC-SD | Up | Up | 2013 |
| Sesamin | CDDP62 | NF-κB-IL-6-Stat3-Twist | SGC-996+GBC-SD | Up | Up | 2014 |
| Emodin | CDDP73 | MRP1 | SGC-996 | Up | — | 2010 |
| Somatostatin | DOX96 | ICBP90 | GBC-SD | Up | — | 2008 |
5-FU indicates 5-fluorouracil; CDDP, cisplatin; cli, clinical research; Dox, doxorubicin; DTX, docetaxel; GBC, gallbladder cancer; GEM, gemcitabine; KD, knockdown; KO, knockout; OE, overexpress; OXA, oxaliplatin; vtr, in vitro; vvo, in vivo.
Lovastatin, tamoxifen, metformin, emodin, sesamin, lenvatinib, and verapamil can improve the therapeutic effect of cisplatin in GBC patients. This drug sensitizes GBC cells to cisplatin-induced apoptosis and inhibits CHK1, CHK2, and H2AX activation during the DNA damage response.51 Tamoxifen inhibits proliferation by interfering with glucose metabolism. When used as the primary drug for breast cancer hormone therapy, tamoxifen also sensitizes cisplatin.72 Metformin synergistically enhances the antitumor activity of cisplatin in GBC through PI3K/AKT/ERK signaling.90 Emodin can also deplete glutathione by generating ROS, inhibiting the expression of survivin, ABCG2, and MRP1, and finally enhancing the anticancer effect of cisplatin on GBC cells.73,83,95 Sesamin attenuates cisplatin resistance and inhibits the growth of human GBC stem-like side population cells.62 Combination therapy with lenvatinib and GEM induces apoptosis, inhibits cell proliferation and colony formation, and reduces GEM resistance in GBC patients.97 Verapamil also induces the production of intracellular ROS, reduces glutathione levels, and enhances the chemosensitivity of GBC cells to platinum drugs.94
Potential GEM synergists include maslinic acid, chloroquine, lenvatinib, α-Mangostin, icariin, phenoxodiol, and cordycepin. Studies indicate that maslinic acid and icariin can enhance the antitumor activity of GEM in vitro and in vivo by inhibiting NF-κB signaling.79,89 The autophagy inhibitor, chloroquine, combined with GEM can sensitize cells to chemotherapy.85 Lenvatiniba and phenoxydiol were also shown to inhibit AKT signaling, decreasing the GBC-specific resistance to GEM.86,93 and α-Mangostin was found to sensitize cancer cells to GEM by inhibiting neoadipogenic adipogenesis.87 Low concentrations of cordycepin can enhance GBC-SD cell sensitivity to GEM and 5-FU.92 This may be attributed to the activation of adenosine monophosphate-activated protein kinase and the degradation of MDR.92 Conversely, leptin can make GBC cells resistant to GEM by activating MCL1.71
While doxorubicin, 5-FU, and S-1 do not have an obvious effect on GBC, potential sensitizers paired with chemotherapeutics can be effective. For example, hispidulin sensitizes GEM and 5-FU to inhibit GBC cell proliferation by downregulating HIF-1α/P-gp signaling.88 and bufalin reduces the stem cell-like properties of GBC by inhibiting MEK/ERK and PI3K/AKT signaling and improves the efficacy of 5-FU.77 Chloroquine and the mTOR inhibitor, OSI-027, can also improve the effect of 5-FU in GBC patients.91,98 Notably, simvastatin enhances GBC sensitivity to the oral anticancer drug, S-1, by modulating apoptosis.99 and somatostatin enhances GBC cell sensitivity to doxorubicin.96,100
There are few current chemotherapeutic options for advanced GBC. Several clinical trial research projects involving potential chemosensitizers are ongoing (Table 4).101,102 While some of these studies have completed phases I and II, only one has entered phase III based on existing positive results.101 Many potential GBC chemosensitizers have also been identified in basic research labs. In addition, a large-scale clinical study of 314 people who are receiving treatment with drugs that target TGF-β or PD-L1 in combination with conventional chemotherapeutic agents (NCT04066491), is underway. Targeted drugs combined with cytotoxic chemotherapy drugs can partially enhance the effect of chemotherapy. While chemotherapy-associated side effects and a lack of effective targets remain to be addressed, the development of sensitizers provides hope to specific GBC patient populations.102 In addition, many drugs with known pharmacological properties and targets have auxiliary effects on GBC-related chemotherapeutics. However, the safety and effectiveness of these drugs require additional verification. It is hoped that these medications will enter clinical trials as complementary therapies to high-cost targeted drugs.
TABLE 4.
Clinical Trials Associated With Chemotherapy Sensitization in GBC
| Potential sensitizer | Targets | N | Phase | Status | Results | Trial ID |
|---|---|---|---|---|---|---|
| Everolimus | mTOR | 152 | Phase 2 | Unknown status | — | NCT02836847 |
| Leucovorin calcium | — | 42 | Phase 2 | Completed | — | NCT00009893 |
| 3-AP | RNR | 33 | Phase 2 | Completed | Negative | NCT00075504 |
| M7824 | TGF-β+PD-L1 | 314 | Phase 2 Phase 3 | Active, not recruiting | — | NCT04066491 |
| MEK 162 | MEK | 0 | Phase 1 | Withdrawn | — | NCT02105350 |
| Selumetinib | MEK 1/2 | 57 | Phase 2 | Active, not recruiting | — | NCT02151084 |
| NUC-1031 | — | 21 | Phase 1 | Completed | Positive | NCT02351765 |
| Copanlisib | PI3K | 24 | Phase 2 | Completed | Negative | NCT02631590 |
| MK-2206 | Akt | 0 | Phase 2 | Withdrawn | — | NCT01859182 |
| ADH-1 | N-Cadherin | 17 | Phase 1 | Completed | — | NCT01825603 |
5-FU indicates 5-fluorouracil; CDDP, cisplatin; cli, clinical research; Dox, doxorubicin; DTX, docetaxel; GBC, gallbladder cancer; GEM, gemcitabine; KD, knockdown; KO, knockout; OE, overexpress; OXA, oxaliplatin; vtr, in vitro; vvo, in vivo.
Data were obtained from https://clinicaltrials.gov (accessed August 20, 2022).
The only drug with positive clinical trial results is NUC-1031, created through a phosphoramidate transformation of GEM. This medication was combined with cisplatin to improve the efficiency of chemotherapeutic drugs (NCT02351765) for patients with advanced GBC. The researchers confirmed that this treatment regimen has a good safety profile.99 While only 21 participants were involved in this study, the overall response rate reached 33%, and the median progression-free survival and overall survival were 7.2 and 9.6 months, respectively.99 These results suggest that this regimen has a potential anti-GBC function. Unfortunately, NuCana announced in March 2022 that the 828-patient phase III clinical trial study (NuTide: 121) was terminated (NCT04163900). This study compared 2 options (NUC-1031 combined with cisplatin and GEM combined with cisplatin) for the treatment of cholangiocarcinoma, GBC, and ampullary cancer. However, the treatment was terminated at the first mid-term assessment because the regimen appeared unlikely to achieve the primary goal of improving overall survival by at least 2.2 months.
These studies identify the potential clinical value of chemosensitizers for GBC treatment. While current clinical trials have mixed results, several new chemosensitizers are worthy of preclinical evaluation.
CONCLUSIONS
GBC has several mechanisms of resistance to chemotherapy drugs, including autophagy, DNA damage, cell cycle, tumor stem cells, mitochondrial function, and metabolism. There are several potential reversal strategies for GBC patients who respond poorly to chemotherapy that require validation by clinical trials (Fig. 4). On the basis of the results of genetic testing, patients can be grouped by coding genes, noncoding genes, and signaling pathways, each of which has several potential chemotherapy sensitization regimens. Each chemotherapy regimen can then be designed to match the patient’s genetic profile.
FIGURE 4.
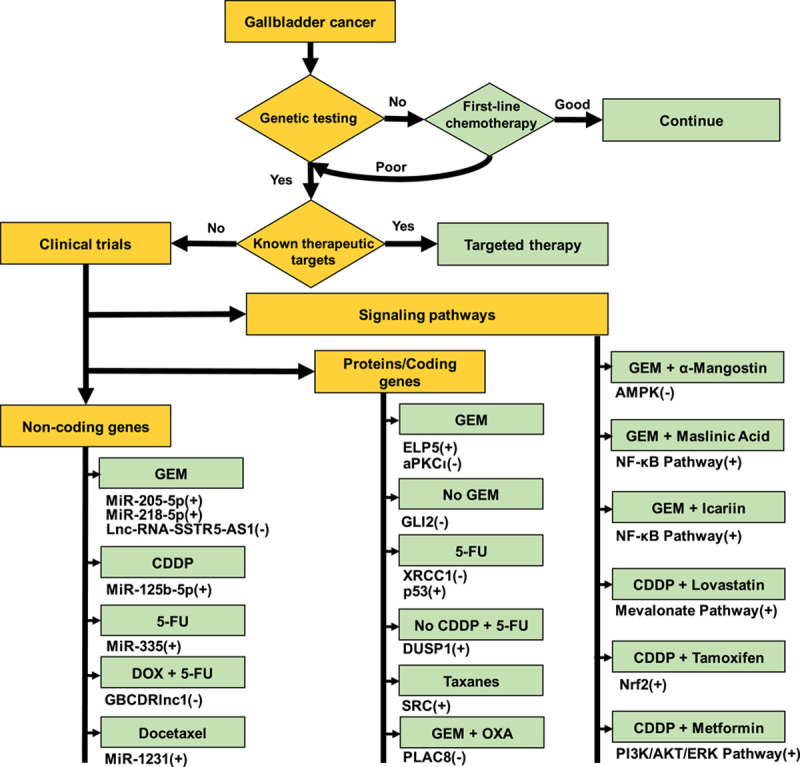
Potential strategies to reverse gallbladder cancer-specific chemoresistance. 5-FU indicates 5-fluorouracil; CDDP, cisplatin; GEM, gemcitabine; OXA, oxaliplatin.
PROBLEMS AND FUTURE PERSPECTIVES
The efficacy of chemotherapy among patients with advanced GBC remains poor and is usually accompanied by a high rate of chemoresistance. This is primarily the result of tumor-specific genetic changes. Basic research has helped to identify several potential chemosensitizing targets and sensitizers. However, work is still required to translate these findings into clinical use. Genetically testing and matching individual cancers with known chemotherapy regimens or sensitizers will inform individualized treatment based on the characteristics of specific tumor genes. In recent years, several new chemoresistance targets have been identified and many novel chemotherapeutic drugs have been developed. Testing potential chemosensitizers in clinical trials should help to accelerate the progression from discovery to drug development and clinical use. In summary, clinical data on the efficacy of GBC-specific chemotherapeutic sensitizers remains limited and few clinical studies are ongoing. An enhanced understanding of the molecular mechanism of GBC can inform the safety evaluation and efficacy verification of any new drugs included in clinical trials.
The widespread application of whole genome sequencing and single-cell sequencing technology has promoted further study of the molecular mechanisms and key factors involved in the response to chemotherapy drugs. These techniques have helped in the formulation of GBC-specific chemotherapy regimens. Importantly, the use of effective chemosensitizers in combination with first-line chemotherapy may reverse GBC-specific drug resistance.
ACKNOWLEDGMENTS
The authors are very grateful to Zhuying Lin for her valuable suggestions on the modification of the figures in this article.
Footnotes
J.L. and S.Y. contributed equally to this work.
J.L.: conceptualization, writing – original draft. S.Y.: investigation. Z.L.: writing – review and editing. W.H.: project administration. X.L.: data curation. R.L.: supervision, funding acquisition. J.T.: funding acquisition, project administration. W.W.: conceptualization, writing – review and editing. All authors approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 82060536, 81660477, and 81960499); Project of Yunling Scholar (Ruhong Li) and Postgraduate Project of the Scientific Research Fund of the Education Department of Yunnan Province (Grant No. 2021Y345).
The authors declare no conflicts of interest.
Contributor Information
Jinbao Lai, Email: laijinbao@kmmu.edu.cn.
Songlin Yang, Email: 327057710@qq.com.
Zhuying Lin, Email: llz_y@hotmail.com.
Wenwen Huang, Email: 475402515@qq.com.
Xiao Li, Email: lx1992584@163.com.
Ruhong Li, Email: lrh272@126.com.
Jing Tan, Email: tanjing@kmmu.edu.cn.
Wenju Wang, Email: wangwenju@kmmu.edu.cn.
REFERENCES
- 1.Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet. 2021;397:428–444. [DOI] [PubMed] [Google Scholar]
- 2.Mhatre S, Richmond RC, Chatterjee N, et al. The role of gallstones in gallbladder cancer in India: a Mendelian randomization study. Cancer Epidemiol Biomarkers Prev. 2021;30:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–1138. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SS, Van De Wyngard V, Pfeiffer RM, et al. Inflammatory profiles in Chilean Mapuche and non-Mapuche women with gallstones at risk of developing gallbladder cancer. Sci Rep. 2021;11:3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 8.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 9.Hu ZI, Lim KH. Evolving paradigms in the systemic treatment of advanced gallbladder cancer: updates in year 2022. Cancers (Basel). 2022;14:1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Kwon JH, Lee JW. Oncologic and long-term outcomes of laparoscopic and open extended cholecystectomy for gallbladder cancer. J Clin Med. 2022;11:2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetze TO, Bechstein WO, Bankstahl US, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC)—a phase III study of the German registry of incidental gallbladder carcinoma platform (GR)-the AIO/CALGP/ACO-GAIN-trial. BMC Cancer. 2020;20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paliogiannis P, Scognamillo F, Attene F, et al. Preneoplastic and neoplastic gallbladder lesions occasionally discovered after elective videocholecystectomy for benign disease. A single centre experience and literature review. Ann Ital Chir. 2013;84:281–285. [PubMed] [Google Scholar]
- 13.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Kalyan Mohanti B, Pal Chaudhary S, et al. Modified gemcitabine and oxaliplatin or gemcitabine+cisplatin in unresectable gallbladder cancer: results of a phase III randomised controlled trial. Eur J Cancer. 2019;123:162–170. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Cao Y, Yang X, et al. Mutational spectrum and precision oncology for biliary tract carcinoma. Theranostics. 2021;11:4585–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950–1958. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, Qi L, Du Z, et al. Calreticulin: a potential diagnostic and therapeutic biomarker in gallbladder cancer. Aging (Albany NY). 2021;13:5607–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Zhan M, Li Q, et al. FXR agonists enhance the sensitivity of biliary tract cancer cells to cisplatin via SHP dependent inhibition of Bcl-xL expression. Oncotarget. 2016;7:34617–34629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Tian M, Zhang D, et al. Long Non-coding RNA myosin light chain kinase antisense 1 plays an oncogenic role in gallbladder carcinoma by promoting chemoresistance and proliferation. Cancer Manag Res. 2021;13:6219–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue Z, Yang B, Xu Q, et al. Long non-coding RNA SSTR5-AS1 facilitates gemcitabine resistance via stabilizing NONO in gallbladder carcinoma. Biochem Biophys Res Commun. 2020;522:952–959. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GF, Wu JC, Wang HY, et al. Overexpression of microRNA-205-5p exerts suppressive effects on stem cell drug resistance in gallbladder cancer by down-regulating PRKCE. Biosci Rep. 2020;40:20194509–20194523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Chen W, Zhang H, et al. MiR-31 regulates the cisplatin resistance by targeting Src in gallbladder cancer. Oncotarget. 2016;7:83060–83070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wensheng L, Bo Z, Qiangsheng H, et al. MBD1 promotes the malignant behavior of gallbladder cancer cells and induces chemotherapeutic resistance to gemcitabine. Cancer Cell Int. 2019;19:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen ED, Liu B, Yu XS, et al. The effects of miR-1207-5p expression in peripheral blood on cisplatin-based chemosensitivity of primary gallbladder carcinoma. Onco Targets Ther. 2016;9:3633–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schinzari G, Rossi E, Mambella G, et al. First-line treatment of advanced biliary ducts carcinoma: a randomized phase II study evaluating 5-FU/LV plus oxaliplatin (Folfox 4) versus 5-FU/LV (de Gramont Regimen). Anticancer Res. 2017;37:5193–5197. [DOI] [PubMed] [Google Scholar]
- 26.Tian L, Lu Y, Yang T, et al. aPKCι promotes gallbladder cancer tumorigenesis and gemcitabine resistance by competing with Nrf2 for binding to Keap1. Redox Biol. 2019;22:101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Zhang W, Lu B, et al. miR-433 accelerates acquired chemoresistance of gallbladder cancer cells by targeting cyclin M. Oncol Lett. 2018;15:3305–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichimiya S, Onishi H, Nagao S, et al. GLI2 but not GLI1/GLI3 plays a central role in the induction of malignant phenotype of gallbladder cancer. Oncol Rep. 2021;45:997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Shi L, Lin W, et al. UCP2 promotes proliferation and chemoresistance through regulating the NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer. Biochem Pharmacol. 2020;172:113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan M, Wang H, Chen T, et al. NOX1 mediates chemoresistance via HIF1α/MDR1 pathway in gallbladder cancer. Biochem Biophys Res Commun. 2015;468:79–85. [DOI] [PubMed] [Google Scholar]
- 31.Boudny V, Murakami Y, Nakano S, et al. Expression of activated c-erbB-2 oncogene induces sensitivity to cisplatin in human gallbladder adenocarcinoma cells. Anticancer Res. 1999;19(6b):5203–5206. [PubMed] [Google Scholar]
- 32.Zheng B, Wang J, Fan K, et al. lncRNA RP11-147L13.8 suppresses metastasis and chemo-resistance by modulating the phosphorylation of c-Jun protein in GBC. Mol Ther Oncolytics. 2021;23:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masumoto N, Nakano S, Fujishima H, et al. v-src induces cisplatin resistance by increasing the repair of cisplatin-DNA interstrand cross-links in human gallbladder adenocarcinoma cells. Int J Cancer. 1999;80:731–737. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Ye Z, Gu F, et al. DUSP1 enhances the chemoresistance of gallbladder cancer via the modulation of the p38 pathway and DNA damage/repair system. Oncol Lett. 2018;16:1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan HX, Wang S, Zhao H, et al. Sonic hedgehog signaling may promote invasion and metastasis of oral squamous cell carcinoma by activating MMP-9 and E-cadherin expression. Med Oncol. 2014;31:41. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z, Yang S, Zhou Y, et al. OLFM4 depletion sensitizes gallbladder cancer cells to cisplatin through the ARL6IP1/caspase-3 axis. Transl Oncol. 2022;16:101331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Q, Wang S, Jin L, et al. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong YQ, Ni JL, Fang Q, et al. MiR-1231 enhances docetaxel sensitivity to gallbladder carcinoma cells by downregulating FOXC2. Eur Rev Med Pharmacol Sci. 2020;24:12116–12123. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Chen LC, Qian JY, et al. MiR-335 promotes cell proliferation by inhibiting MEF2D and sensitizes cells to 5-Fu treatment in gallbladder carcinoma. Eur Rev Med Pharmacol Sci. 2019;23:9829–9839. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Miao X, Zhang Y, et al. XRCC1 is a promising predictive biomarker and facilitates chemo-resistance in gallbladder cancer. Front Mol Biosci. 2020;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Zhan M, Chen T, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep. 2016;7:43109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian L, Su H, Wang G, et al. Anti-tumor activity of bufalin by inhibiting c-MET mediated MEK/ERK and PI3K/AKT signaling pathways in gallbladder cancer. J Cancer. 2020;11:3114–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu S, Jiang C, Lin R, et al. Epigenetic activation of the elongator complex sensitizes gallbladder cancer to gemcitabine therapy. J Exp Clin Cancer Res. 2021;40:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki T, Takeda T, Okamoto T, et al. Chemotherapy for biliary tract cancer in 2021. J Clin Med. 2021;10:3108–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581–4586. [DOI] [PubMed] [Google Scholar]
- 46.Weigt J, Malfertheiner P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2010;4:395–397. [DOI] [PubMed] [Google Scholar]
- 47.Binenbaum Y, Na'ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2015;23:55–68. [DOI] [PubMed] [Google Scholar]
- 48.Tella SH, Kommalapati A, Borad MJ, et al. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020;21:e29–e41. [DOI] [PubMed] [Google Scholar]
- 49.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catalano F, Borea R, Puglisi S, et al. Targeting the DNA damage response pathway as a novel therapeutic strategy in colorectal cancer. Cancers (Basel). 2022;14:1338–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu Y, Duan J, et al. Cholesterol depletion sensitizes gallbladder cancer to cisplatin by impairing DNA damage response. Cell Cycle. 2019;18:3337–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Liu H, Xue R, et al. BH3 mimetic ABT-199 enhances the sensitivity of gemcitabine in pancreatic cancer in vitro and in vivo. Dig Dis Sci. 2018;63:3367–3375. [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Mao Q, Zhang B, et al. Thiazolidinedione-based structure modification of celastrol provides thiazolidinedione-conjugated derivatives as potent agents against non-small-cell lung cancer cells through a mitochondria-mediated apoptotic pathway. J Nat Prod. 2022;85:1147–1156. [DOI] [PubMed] [Google Scholar]
- 54.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–248. [DOI] [PubMed] [Google Scholar]
- 55.Chen C, Gao H, Su X. Autophagy-related signaling pathways are involved in cancer (Review). Exp Ther Med. 2021;22:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu J, Tang Z, Gong W, et al. Isolation and identification of tumor-initiating cell properties in human gallbladder cancer cell lines using the marker cluster of differentiation 133. Oncol Lett. 2017;14:7111–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Cai H, Sun L, et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68:4009–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Gong P, Chen T, et al. Colorectal cancer stem cell states uncovered by simultaneous single-cell analysis of transcriptome and telomeres. Adv Sci (Weinh). 2021;8:2004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ai Z, Pan H, Suo T, et al. Arsenic oxide targets stem cell marker CD133/prominin-1 in gallbladder carcinoma. Cancer Lett. 2011;310:181–187. [DOI] [PubMed] [Google Scholar]
- 62.Kong X, Ma MZ, Zhang Y, et al. Differentiation therapy: sesamin as an effective agent in targeting cancer stem-like side population cells of human gallbladder carcinoma. BMC Complement Altern Med. 2014;14:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong QY, Kok ZH, Bui NL, et al. A unique CDK4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacol Res. 2020;156:104686. [DOI] [PubMed] [Google Scholar]
- 64.Rubin SM, Sage J. Manipulating the tumour-suppressor protein Rb in lung cancer reveals possible drug targets. Nature. 2019;569:343–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu TN, Huang CM, Huang CS, et al. Targeting FAT1 inhibits carcinogenesis, induces oxidative stress and enhances cisplatin sensitivity through deregulation of LRP5/WNT2/GSS signaling axis in oral squamous cell carcinoma. Cancers (Basel). 2019:11, 1883–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishizawa J, Sugihara E, Kuninaka S, et al. FZR1 loss increases sensitivity to DNA damage and consequently promotes murine and human B-cell acute leukemia. Blood. 2017;129:1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giannelli G, Koudelkova P, Dituri F, et al. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798–808. [DOI] [PubMed] [Google Scholar]
- 68.Zheng B, Wang J, Fan K, et al. Erratum: lncRNA RP11-147L13.8 suppresses metastasis and chemo-resistance by modulating the phosphorylation of c-Jun protein in GBC. Mol Ther Oncolytics. 2021;23:531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasan K, Werner M, Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun X, Fan T, Sun G, et al. 2-Deoxy-D-glucose increases the sensitivity of glioblastoma cells to BCNU through the regulation of glycolysis, ROS and ERS pathways: In vitro and in vivo validation. Biochem Pharmacol. 2022;199:115029. [DOI] [PubMed] [Google Scholar]
- 71.Wang WJ, Lai HY, Zhang F, et al. MCL1 participates in leptin-promoted mitochondrial fusion and contributes to drug resistance in gallbladder cancer. JCI Insight. 2021;6:135438–135454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang S, Wang H, Chen W, et al. Tamoxifen inhibits cell proliferation by impaired glucose metabolism in gallbladder cancer. J Cell Mol Med. 2020;24:1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, Sun YP, Huang XZ, et al. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem Pharmacol. 2010;79:1134–1140. [DOI] [PubMed] [Google Scholar]
- 74.Xu S, Zhan M, Jiang C, et al. Genome-wide CRISPR screen identifies ELP5 as a determinant of gemcitabine sensitivity in gallbladder cancer. Nat Commun. 2019;10:5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Zhan M, Xu SW, et al. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017;8:e2770–e2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou L, He XD, Yu JC, et al. Overexpression of LAPTM4B-35 attenuates epirubucin-induced apoptosis of gallbladder carcinoma GBC-SD cells. Surgery. 2011;150:25–31. [DOI] [PubMed] [Google Scholar]
- 77.Yu Y, Wang J, Xia N, et al. Maslinic acid potentiates the antitumor activities of gemcitabine in vitro and in vivo by inhibiting NF-κB-mediated survival signaling pathways in human gallbladder cancer cells. Oncol Rep. 2015;33:1683–1690. [DOI] [PubMed] [Google Scholar]
- 78.Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin X, Zhang X, Wang Q, et al. Perifosine downregulates MDR1 gene expression and reverses multidrug-resistant phenotype by inhibiting PI3K/Akt/NF-κB signaling pathway in a human breast cancer cell line. Neoplasma. 2012;59:248–256. [DOI] [PubMed] [Google Scholar]
- 80.García P, Rosa L, Vargas S, et al. Hippo-YAP1 Is a Prognosis Marker and Potentially Targetable Pathway in Advanced Gallbladder Cancer. Cancers (Basel). 2020;12:778–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bizama C, García P, Espinoza JA, et al. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41:222–234. [DOI] [PubMed] [Google Scholar]
- 82.Wang FT, Wang H, Wang QW, et al. Inhibition of autophagy by chloroquine enhances the antitumor activity of gemcitabine for gallbladder cancer. Cancer Chemother Pharmacol. 2020;86:221–232. [DOI] [PubMed] [Google Scholar]
- 83.Ye J, Qi L, Liang J, et al. Lenvatinib induces anticancer activity in gallbladder cancer by targeting AKT. J Cancer. 2021;12:3548–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Y, Fan Y, Hu Y, et al. α-Mangostin suppresses the de novo lipogenesis and enhances the chemotherapeutic response to gemcitabine in gallbladder carcinoma cells via targeting the AMPK/SREBP1 cascades. J Cell Mol Med. 2020;24:760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao H, Xie J, Peng J, et al. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder cancer cells by targeting HIF-1α. Exp Cell Res. 2015;332:236–246. [DOI] [PubMed] [Google Scholar]
- 86.Zhang DC, Liu JL, Ding YB, et al. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin. 2013;34:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bi T, Zhu A, Yang X, et al. Metformin synergistically enhances antitumor activity of cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway. Cytotechnology. 2018;70:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Q, Mou LJ, Tao L, et al. Inhibition of mTOR suppresses human gallbladder carcinoma cell proliferation and enhances the cytotoxicity of 5-fluorouracil by downregulating MDR1 expression. Eur Rev Med Pharmacol Sci. 2016;20:1699–1706. [PubMed] [Google Scholar]
- 89.Wu WD, Hu ZM, Shang MJ, et al. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int J Mol Sci. 2014;15:12778–12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Huang X, Huang Z, et al. Phenoxodiol enhances the antitumor activity of gemcitabine in gallbladder cancer through suppressing Akt/mTOR pathway. Cell Biochem Biophys. 2014;70:1337–1342. [DOI] [PubMed] [Google Scholar]
- 91.Wang H Li X Chen T, et al. . Mechanisms of verapamil-enhanced chemosensitivity of gallbladder cancer cells to platinum drugs: glutathione reduction and MRP1 downregulation. [DOI] [PubMed]
- 92.Wang W, Sun Y, Li X, et al. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncol Rep. 2011;26:1143–1148. [DOI] [PubMed] [Google Scholar]
- 93.Qin YY, Li JY, Li SG, et al. Mechanism research in somatostatin reverting the chemosensitivity of GBC-SD cell line. Zhonghua Wai Ke Za Zhi. 2008;46:381–383. [PubMed] [Google Scholar]
- 94.Li XX, Dong Y, Wang W, et al. Emodin as an effective agent in targeting cancer stem-like side population cells of gallbladder carcinoma. Stem Cells Dev. 2013;22:554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai JP, Chen W, Hou X, et al. Simvastatin enhances the chemotherapeutic efficacy of S-1 against bile duct cancer: E2F-1/TS downregulation might be the mechanism. Anticancer Drugs. 2013;24:1020–1029. [DOI] [PubMed] [Google Scholar]
- 96.Tang J, Liang X, Ma R, et al. Effects of autophagy on 5-fluorouracil cytotoxicity for gallbladder carcinoma GBC-SD cell. Zhonghua Yi Xue Za Zhi. 2014;94:612–616. [PubMed] [Google Scholar]
- 97.Quan ZW, Yang Y, Li JY, et al. The mechanisms of somatostatin induced enhanced chemosensitivity of gallbladder cancer cell line to doxorubicin: cell cycle modulation plus target enzyme up-regulation. Biomed Pharmacother. 2010;64:451–457. [DOI] [PubMed] [Google Scholar]
- 98.Ocean AJ, Christos P, Sparano JA, et al. Phase II trial of the ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehydethiosemicarbazone plus gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2011;68:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McNamara MG, Bridgewater J, Palmer DH, et al. A phase Ib study of NUC-1031 in combination with cisplatin for the first-line treatment of patients with advanced biliary tract cancer (ABC-08). Oncologist. 2021;26:e669–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan ES, Cao B, Kim J, et al. Phase 2 study of copanlisib in combination with gemcitabine and cisplatin in advanced biliary tract cancers. Cancer. 2021;127:1293–1300. [DOI] [PubMed] [Google Scholar]
- 101.Belkouz A, Wilmink JW, Haj Mohammad N, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Crit Rev Oncol Hematol. 2020;151:102975. [DOI] [PubMed] [Google Scholar]
- 102.Canale M, Monti M, Rapposelli IG, et al. Molecular targets and emerging therapies for advanced gallbladder cancer. Cancers (Basel). 2021;13:5671–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]


