Abstract
Background
Although global vaccination against COVID-19 infection has its excellence, potential side effects are yet of concern. Several lines of evidence have proposed ADEM occurrence after SARS-CoV-2 infection. Moreover, a large number of case reports and case series have also suggested the casual association between ADEM and COVID-19 vaccination. To better understand the development of ADEM following COVID-19 vaccination and its potential association, we aimed to systematically review ADEM cases reported after COVID-19 vaccination.
Methods
We conducted a comprehensive systematic search using three databases including PubMed, Scopus, and Web of Science. Studies that reported ADEM after COVID-19 vaccination were eligible to include in our study. Observational studies, case reports, and case series which reported cases of ADEM with sufficient detail to confirm clinical diagnosis following COVID-19 vaccination were eligible to enter our study.
Results
Twenty studies were included in our systematic review after the abstract and full-text screening with a total of 54 cases. Among included patients, 45 (85.1 %) developed ADEM after the first dose of the COVID-19 vaccine, and seven (12.9 %) cases experienced ADEM after the second dose. The median time interval between vaccination and neurological symptoms was 14 days which ranged from 12 h to 63 days. Twelve (22.2 %) patients experienced symptoms of muscle weakness, ten (18.5 %) presented unconsciousness, nine (16.6 %) patients had urinary complaints, nine (16.6 %) had visual impairments, and five (9.2 %) experienced a seizure. After treatments, four (13.8 %) patients died. Forty-six patients had clinical improvement (85.1 %), also improvement in brain MRI was observed among 44 (81.4 %) patients.
Conclusion
In conclusion, it is not clear that ADEM could be a potential complication of COVID-19 vaccination based on the current evidence and further studies are needed. However, this rare condition should not trigger stopping the mass vaccination programs since the only way to eradicate the current pandemic of COVID-19 is to extend the number of immunized people.
Keywords: Acute disseminated encephalomyelitis, COVID-19 vaccination, ADEM, Neurological, SARS-CoV-2
1. Introduction
The coronavirus disease 19 (COVID-19) pandemic caused by the severe respiratory syndrome coronavirus 2 (SARS-CoV-2) was first presented in Wuhan, China, 2019 [1]. With the rapid development of vaccines, substantial protection against COVID-19 was provided [[2], [3]]. So far, 65.5 % of the world population has been efficaciously immunized with at least one dose of the COVID-19 vaccines to date [[1], [4]]. The most common worldwide administered vaccine types were Messenger RNA (mRNA) vaccines, including Pfizer/BioNTech (BNT162B2) and Moderna (mRNA-1273), alongside viral vector vaccine Oxford/AstraZeneca (ChAdOx1), respectively [5]. Although global vaccination against COVID-19 infection has its excellence, potential side effects are yet of concern. Along with general side effects reported after the COVID-19 vaccination such as fever, fatigue, and myalgia, some rare but severe neurological complications have also been recognized including cerebral venous sinus thrombosis (CVST), Guillain Barre Syndrome (GBS) and central nervous system (CNS) demyelination disorders [[2], [6], [7], [8]].
Acute disseminated encephalomyelitis (ADEM) is a rare autoimmune disease mostly occurring in children and young adults [[9], [10]]. It is well known for its acute-onset and rapidly progressive demyelinating process both in the brain and spinal cord [11]. Although the exact underlying mechanism of ADEM is still a matter of discussion, it is suggested that ADEM mainly occurs after bacterial and viral infection alongside vaccination [[12], [13], [14]]. Several studies have reported ADEM occurrence after SARS-CoV-2 infection [[15], [16], [17]]. Moreover, a large number of case reports and case series have also suggested the potential association between ADEM and COVID-19 vaccination [[18], [19], [20], [21], [22], [23]].
To better understand the development of ADEM following COVID-19 vaccination and its potential association, we aimed to systematically review ADEM cases reported after COVID-19 vaccination. Together, this systematic review study hopefully yields insights into post-COVID-19-vaccination presentations of ADEM and how it differs from the typical ADEM manifestations with the final purpose of early detection and efficient management of those at risk.
2. Methods and materials
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for the present systematic review[24].
2.1. Literature search
We conducted a comprehensive systematic search using three databases including PubMed, Scopus, and Web of Science. The following terms were used in our search strategy: ((Acute Disseminated Encephalomyelitis) or (ADEM)) and (COVID-19 or SARS-COV-2 or coronavirus or Coronavirus Disease or 2019-nCoV Disease) and (Vaccination or Vaccine or immunization). Additionally, we manually searched the reference list of review studies to identify relevant studies.
2.2. Eligibility criteria
Observational studies, case reports, and case series which reported cases of ADEM with sufficient detail to confirm clinical diagnosis following COVID-19 vaccination were eligible to enter our study. Review and non-English studies were excluded. Also, studies reported patients with prior history of any demyelinating disease were excluded.
2.3. Study selection
Two independent investigators (SH.R, F.N) screened the title and abstract of the studies and excluded non-relevant papers. Next, the remained studies underwent full-text evaluation for final selection. Any disagreements were resolved by consultation with the third investigator.
2.4. Data extraction
The following information was extracted for each case by the two reviewers (SH.R, F.N) using a prepared datasheet: Study demographic, age, sex, neurological symptoms, type of COVID-19 vaccine, dosage, the time interval between vaccination and neurological symptoms, MRI results, CSF findings, auto-antibodies results, SARS-CoV-2 PCR results, treatments, and Outcome.
2.5. Quality assessments
We used Joanna Briggs Institute Critical Appraisal tools to assess the quality of case series and case report studies which contains eight questions with answers based on “Yes” or “No” and a total score of 0 to 8[25].
3. Results
A total of 159 studies were retrieved from the databases after duplicate removal (Fig. 1 ). Next, 88 studies were excluded via title and abstract evaluation. Finally, after the full-text screening, 20 studies with a total of 54 cases were entered into our systematic review [[6], [18], [20], [21], [22], [23], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
Fig. 1.
PRISMA flow diagram depicting the flow of information through the different phases of a systematic review.
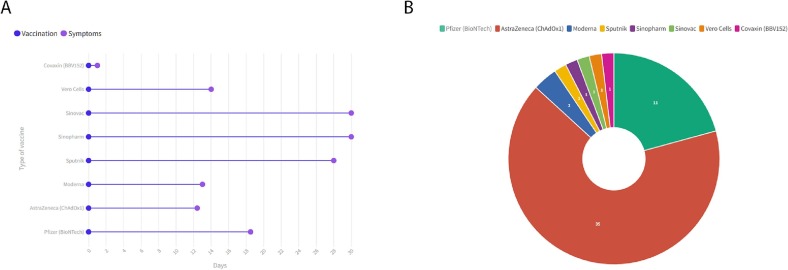
Of the 54 ADEM cases with a mean age of 43.1 ± 15.7 and range [19–88], 33 patients (61.1 %) were female and 21 patients (38.8 %) were male (Table 1 ). There was no pediatric case of ADEM among included studies. Patients were originally from India (n = 10), Italy (n = 4), Germany (n = 3), Australia (n = 2), Japan (n = 2), USA (n = 2), Saudi Arabia (n = 1), Argentina (n = 1), UK (n = 1), China (n = 1), Poland (n = 1), Iran (n = 1), and Turkey (n = 1). Thirty-five patients received AstraZeneca (ChAdOx1), 11 Pfizer (BioNTech), two Moderna, and one each received Sinopharm, Sputnik, Sinovac, Vero Cells, and Covaxin (BBV152). The type of vaccine was not reported for one patient [35]. Among included patients, 45 (85.1 %) developed ADEM after the first dose of the COVID-19 vaccine, and seven (12.9 %) cases experienced ADEM after the second dose. The median time interval between vaccination and neurological symptoms was 14 days which ranged from 12 h to 63 days (Fig. 2 ). Twelve (22.2 %) patients experienced symptoms of muscle weakness, ten (18.5 %) presented unconsciousness, nine (16.6 %) patients had urinary complaints, nine (16.6 %) had visual impairments, and five (9.2 %) experienced a seizure. Most of the studies performed serological tests. anti-MOG antibody was positive in CSF of 16 (29.6 %) cases and antinuclear antibodies (ANA) were positive only in one (1.8 %) patient. Fifty-one (94.4 %) of the included patients received glucocorticoids, 18 (33.3 %) underwent plasmapheresis, and 11 received IVIg (20.3 %). After treatments, four (13.8 %) patients died. Forty-six patients had clinical improvement (85.1 %), also improvement in brain MRI was observed among 44 (81.4 %) patients. The full clinical, serological, and imaging findings are detailed in Table 1.
Table 1.
Characteristics and clinical findings of inlcuded studies.
| First author | Country | Age | Sex | Neurological symptoms | Type of COVID-19 vaccine | Dosage of COVID-19 vaccine | Time interval between vaccination and neurological symptoms | MRI results | CSF findings | Auto-antibodies | SARS-CoV-2 PCR | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Quliti et al. 2022 | Saudi Arabia | 56 | Female | Gradual discomfort + generalized weakness + myalgias + difficultly in the articulation of speech + needed assistance to ambulate + anorexia + dysmetria | AstraZeneca (ChAdOx1) | 1st | 10d | MRI: the T2 and FLAIR sequences demonstrated large multifocal, bilateral, asymmetric, multiple hyperintensities in the subcortical and deep white matter involving the basal ganglia with no contrast enhancement | CSF: protein = 1.76, CSF glucose = 4.62; CSF WBC count = 1, RBC count = 7; (CSF differential cells) CSF segs = 20 %, CSF mono = 64 %, lymphocytes = 16 % | NR | Negative | Omeprazole + acetaminophen + hypertonic saline at 2 % + sodium correction over the next 24 h + MPS + physical and occupational therapy | Complete resolution of her symptoms, continued to improve and was able to mobilize freely without assistance, discharged from hospital |
| Ancau et al. 2021 | Germany | 61 | Male | Fever + headache + apathy + unconscious + foaming around the mouth + generalized seizure + comatose | AstraZeneca (ChAdOx1) | 1st | 2d | MRI: bilateral confluent cortical and subcortical FLAIR hyperintense lesions with hemorrhagic involvement of the basal ganglia | CSF: normal cell counts (1 leukocyte per μl) and moderate disturbance of the blood–brain-barrier + No CSF-specific oligoclonal bands or intrathecal IgG/-A/-M−synthesis were detected | against aquaporin-4 (AQP4) or myelin oligodendroyte glycoprotein (MOG) in cell-based assays (CBA) = negative + Screening for antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), antiphospholipid antibodies, neuronal and paraneoplastic antibodies = all negative. | Negative | Endotracheal intubation + MPS + PE | Slight improvement, reduction in size of the brain lesions, on clinical follow-up after 14 weeks of rehabilitation, the patient presented with a vegetative state |
| 25 | Female | Severe cephalgia + thoracic back pain + mild weakness + ascending numbness + complete paraplegic syndrome | AstraZeneca (ChAdOx1) | 1st | 9d | Spinal MRI: a longitudinal edema throughout the thoracic spinal cord exhibiting mild contrast enhancement as well as focal central hemorrhages + Cranial MRI: bi-hemispheric white matter lesions with focal contrast enhancement | CSF: erythrocytes 5,284 cells/μl, leukocytes241 cells/μl + highly elevated CSF/serum quotient for albumin of 164.7 × 10–3 + No CSF-specific oligoclonal bands were detected | Intrathecal IgM synthesis = positive, but IgG or IgA synthesis = negative / glial-, neuronal-targeting, and paraneoplastic autoantibodies (CBA for AQP4- and MOG-, immunofluorescence assays in the serum for ANA, ANCA, anti-double stranded DNA antibodies) = negative | Negative | MPS + PE | Cephalgia improved drastically and the sensory components slightly, clinical improvement of only sensory symptoms | ||
| 55 | Female | Progressive nausea + dizziness + meningism + severe spastic tetraparesis + increased intracerebral pressures + comatose + anisocoria | AstraZeneca (ChAdOx1) | 1st | 9d | Brain MRI: multiple FLAIR-hyperintense and hemorrhagic lesions in the right parietal and temporal lobes, bilaterally in fronto-temporal distribution as well as in the right occipital lobe and left fronto-basal region | CSF: mixed granulocytic and lymphocytic pleocytosis (10/μl) and a normal CSF/serum quotient for albumin of 7.4 × 10–3 + No CSF-specific oligoclonal bands were detected | Intrathecal IgM, IgA and IgG synthesis = positive / Both autoimmune (AQP4-, MOG-autoantibodies as measured by CBA), and paraneoplastic antibodies (immunofluorescence assays in the serum) = negative | Negative | emergency right-sided decompressive hemicraniectomy + MPS | Significant improvement of vigilance and motor function, died (due to progressive intracerebral hemorrhage of the brain stem) | ||
| Ballout et al. 2022 | USA | 81 | Male | Change in mental status + severe encephalopathy + viral-like illness + fever + fatigue + myalgia + acute inflammatory demyelinating process | Moderna | 1st | 13d | Brain MRI with gadolinium: on hospital Day 5 a diffusion restricting lesion involving the right dorsal medulla with corresponding T2 FlAIR hyperintensity, very faint left pontine, midbrain, and thalamic T2 FlAIR hyperintensity, and minimal T2 sulcal hyperintensity without apparent enhancement suggestive of a possible inflammatory or infectious process / Repeated Brain MRI with gadolinium: on hospital day 17 demonstrated multiple, non-enhancing, T2 hyperintense lesions involving bilateral frontoparietal lobes, lentiform nuclei, thalami, cerebral peduncles, pons, and right posterior medulla | 1st CSF: opening pressure = 26 cmH2O, glucose = 69 mg/dL (reference range 40–70 mg/dL), protein = 45 mg/dL (reference range 15–45 mg/dL), and WBC count = 3 cells/μL (reference range 0–5 cells/μL). / 2nd CSF: a mild lymphocytic pleocytosis with a WBC count of 11 cells/μL and protein of 52 mg/dL / A CSF autoimmune encephalitis panel + negative / 3rd CSF: pleocytosis of 69 cells/μL with 83 % lymphocytic predominance, protein of 45 mg/dL, and significantly elevated myelin basic protein (MBP) > 167.0 ng/mL (reference range 0–6.0 ng/mL). | anti-MOG antibody = negative | Negative | Vancomycin + IVIG + MPS + PE | Died (due to hemorrhagic shock of probable gastrointestinal origin) |
| Francis et al. 2022 | UK | 36 median | 14 Female | Transeverse myelitis + optic neuritis + Fever + Headache + dysesthesia + Posterma + Facial nerve palsy + paraplegia | 18 AstraZeneca (ChAdOx1) and 7 Pfizer (BioNTech) | 23 at 1st and two at 2nd | 20d | Brain MRI: Involving cerebrall peduncles, internal capsule, splenium, and spinal cord. Longitudinally extensive transverse myletis and periependymal FLAIR hyperintensities. | CSF: Protein (0.63 g/L, range 0.33–2.25), lymphocyte count was 36 × 106/L, and negative oligoclonal bands (OCBs) in MOGIgG + Patients. | Twelve patients were MOGIgG + and two patients were AQP4IgG+ | NR | IVMP + PE + IVIG | Only two patients had poor recovery |
| Ahmad et al. 2022 | USA | 61 | Female | General weakness and difficulty in communications | Pfizer (BioNTech) | 1st | 63d | Brain MRI: Signficant diffuse and symmetric acute leukoencephalopathy process involving the deep white matter extending downward through the brainstem into the cerebellar white matter tracts | White blood cell count of 10.1 K/uL and hemoglobin of 12.6 g/dL. Her comprehensive metabolic panel was significant for potassium of 3.2 mmol/L, bicarbonate of 11 mmol/L, chloride of 120 mmol/L. Additional tests, including procalcitonin, cortisol, glucose level, thyroid function tests, antinuclear antibody screen, and COVID RNA nasopharyngeal swab, were within normal limits. Her urinalysis was unremarkable, but her urine toxicology was positive for tetrahydrocannabinol. | Negative myelin oligodendrocyte glycoprotein (MOG) | Negative | MPS + IVIG | Significant improvement in the patient’s mentation. There was no further disease progression in brain MRI |
| Cao et al. 2021 | China | 24 | Female | Somnolence + memory decline + headache + low-grade fever + muscle stiffness + extremity weakness + reduced appetite + generalized tonic–clonic seizure | Vero Cells | 1st | 2w (14d) | Brain MRI: abnormal signals in the bilateral temporal cortex / Repeat brain MRI: an increased number of lesions, which were more striking in appearance on day 10; the lesions were improved by day 15 | 1st CSF: WBC count = 51 × 106/L / 2nd CSF: WBC count = 25 × 106/L | anti-aquaporin-4, anti-myelin basic protein, anti-MOG, anti-glial fibrillary acidic protein, autoimmune encephalitis, and paraneoplastic syndrome = all negative | Negative | Ceftriaxone + acyclovir + diazepam + levetiracetam + IVIG | MMSE scores improved, discharged, on a visit 1 month after discharge, felt no discomfort, and a repeat MRI showed complete resolution of brain lesions |
| Kania et al. 2021 | Poland | 19 | Female | Severe headache + fever + back and neck pain + nausea + vomiting + urinary retention + atopic dermatitis + depression + nuchal rigidity + bilateral Babinski signs | Moderna | 1st | 2w (14d) | Brain MRI: multiple, poorly demarcated, hyperintense lesions in T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) images located in both brain hemispheres, pons, the medulla oblongata, and cerebellum. Few of them were contrast‐enhanced lesions. Cervical and thoracic MRI revealed a widespread hyperintense area in T2‐weighted and FLAIR images extended from medulla oblongata to Th11 segment with overlapping few contrast‐enhancing lesions | CSF: WBC count = 294 × 106/L, lymphocytes = 91 %, monocytes = 8 %, neutrophils 1 %, protein levels = 648 mg/L, RBC count = 77/µL / Control lumbar puncture was done 12 days after the first one; CSF WBC count = 61 × 106/L and protein levels = 338 mg/L. | anti‐aquaporin‐4 and anti‐myelin oligodendrocyte glycoprotein = negative | NR | Ceftriaxone + acyclovir + MPS + PE | The clinical status improved after MPS, discharged from the hospital without any symptoms except a mild headache |
| Kenangil et al. 2021 | Turkey | 46 | Female | Tonic–clonic seizure | Sinovac | 2nd | 1 month (30d) | Cranial MRI: There were scattered hyperintense lesions in the left thalamus, bilateral corona radiata, left diencephalon, and right parietal cortex on T2 and FLAIR sequences on MRI. Some of these lesions showed mild restricted diffusion on DWI | CSF: acellular with normal protein content (45 mm/dL), an IgG index of 0.64 + no oligoclonal bands | ANA (1/100) + anti-SOX1 antibody = positive / anti-double-stranded DNA and extractable nuclear antigen (ENA) panel, anti-aquaporin-4 and anti-myelin oligodendrocyte (MOG) antibodies = negative | Negative | Steroids | Controlled MRI, without any new signs, symptoms, or seizures. |
| Lazaro et al. 2022 | Argentina | 26 | Female | Disorientation + inappropriate behavior + headache + gait imbalance + deferred memory + hypoprosexia + anosognosia + incoherent speech + visuospatial failures + Right upper limb weakness + gait ataxia | Sputnik | 1st | 4w (28d) | Brain MRI: nodular hyperintense lesions on T2-weighted image and fluid attenuated inversion recovery without restricted diffusion on diffusion. Marked vasogenic edema and T1-weighted image post contrast incomplete annular enhancement was observed | CSF: 3 cells, 50 g proteins/L, normal glucose + Oligoclonal bands (OCB) = positive / White blood cell count = 3–66 % mononuclear, Proteins = 50.6, Glucose = 78.3, Lactic acid = 1.74, Culture (bacterial, fungal and KOCH) = Negative, VDRL = Negative, Viral PCR (Herpes simplex I/I, Varicella Zoster, Cytomegalovirus, Epstein Barr, Enterovirus, Chagas, John Cunningham) = Negative, Mycobacterium Tuberculosis PCR = Negative, Oligoclonal Bands = Type 2 | Anti-myelin oligodendrocyte glycoprotein antibody (anti-MOG) IGG = negative | NR | MPS | The clinical course was favourable, neurological examination was normal, the MRI was repeated after three months, showing clear imaging improvement of all the lesions |
| Maramattom et al. 2022 | India | 64 | Male | Ascending paresthesias in the legs + epigastric band-like sensation + leg stiffness + hand paresthesias | AstraZeneca (ChAdOx1) | 2nd | 20d | Brain and spine MRI: bilateral corticospinal tract hyperintensities, Dorsal cord hyperintensity at D8–9, Whole-body PET/CT normal (multifocal cord hyperintensities and bilateral hemispheric corticospinal tract hyperintensities) | CSF: normal | NMDA/VKGC/NMO, MOG/paraneoplastic panel = negative | NR | IVIG + IVMP + rituximab | A repeat MRI at 1 month showed stabilization of the lesions and no new contrast enhancement (mRS 1 Level 2) |
| 46 | Male | Urinary complaints + progressive lower limb weakness + numbness + fever | AstraZeneca (ChAdOx1) | 1st | 4d | Brain and spine MRI: extensive supratentorial + infratentorial + long segment spinal cord hyperintensities + longitudinally extensive transverse myelitis (MRI brain: T2, FLAIR hyperintensities in bilateral middle cerebellar peduncle (left > right), pontine tegmentum, right paramedian medulla, and left thalamocapsular region) | CSF: 63 cells/mm3, Protein (52 mg/dl), sugar (93 mg/dl), CSF encephalitis panel: negative | Serum NMO, MOG, ANCA = negative | Negative | MPS + IVMP + PE | Improved significantly and was able to ambulate independently (Recovered, mRS 1 Level 2) | ||
| 42 | Female | Severe daily headache + photophobia + papilledema | AstraZeneca (ChAdOx1) | 1st | 5d | MRI: initial MRI: leptomeningeal and sulcal enhancement / 25 days later: large right temporal irregular enhancing lesion with significant perilesional edema | CSF: opening pressure 32 cm H2O, CSF parameters normal | Serum & CSF autoimmune encephalitis/NMO, MOG/viral encephalitis panel = all negative | NR | Decompression of lesion + Excisional biopsy + Oral prednisolone | Headache remitted spontaneously after the excision biopsy (mRS 1) | ||
| Miyamoto et al. 2022 | Japan | 54 | Female | Fever + headache + somnolence + urinary retention + decreased level of consciousness | Pfizer (BioNTech) | 2nd | 12d | Brain MRI: lesions in the bilateral basal ganglia, midbrain, and cerebral white matter | CSF: elevated protein levels (31.2 mg/mL) + increased cell count (23/µL, 91 % mononuclear cells) + elevated myelin basic protein (809.8 pg/mL) | anti-aquaporine-4 antibody + other encephalitis-related auto-antibodies (glutamate receptors, leucine-rich glioma-inactivated protein 1, contactin-associated protein 2, and glial fibrillary acidic protein) = all negative | NR | MPS + PE + IVIG | Discharged and recovered, able to perform activities of daily living independently. |
| Mumoli et al. 2021 | Italy | 45 | Male | Objective vertigo + fever + diffuse myalgia + feeling of burning on the back + backpain + (knees, thighs and perineum) numbness and hypoesthesia + urinary retention + loss of feet’s vibration sensation + gait difficulties and febrile status | AstraZeneca (ChAdOx1) | 1st | 12 h (0.5d) | Spinal cord MRI: a central non expansive short tau iversion recovery (STIR) signal lesions extended to spinal cord from D10 until conus without enhancement after administration of gadolinium | CSF: 43 cells (cut off < 25) associated with mild hyperproteinorachia (406 mg/l; cut off 305) + normal glycorrhachia and oligoclonal bands | IgG = positive / Autoimmune screening = normal / Acquaporin-4 antibodies = negative / anti-MOG = positive with a titer 1:2560 (positive ≥ 1:160) | Negative | Ceftriaxone + piperacillin/tazobactam + MPS | Brain and Spinal cord status was improved, the hyperintense streak in STIR has almost completely disappeared, and Anti MOG titer was stable. |
| Nagaratnam et al. 2022 | Australia | 36 | Female | Headache + photophobia + blurred vision + bilateral visual impairment + subjective colour desaturation + painful eye movements + fatigue + painful eye movements | AstraZeneca (ChAdOx1) | 1st | 14d | Brain MRI: multiple T2/ FLAIR hyperintense lesions involving the subcortical white matter, posterior limb of bilateral internal capsules, pons and left middle cerebellar peduncle. The largest lesion was in the right frontal centrum semiovale measuring 17 × 17 mm with multiple internal punctate foci of gadolinium contrast enhancement + There was no callosal involvement. Notably, there was no definite abnormal signal or enhancement of optic nerves / Spine MRI: evidence of demyelinating disease | CSF: a normal protein of 0.4 g/L (0.19 – 0.56 g/L), glucose of 4.8 mmol/L (2.8 – 4.5 mmol/L) with pleocytosis (white cell count 59 × 106/L) (<5 × 106/L) + CSF IgG was 0.06 g/L (<0.03 g/L) with serum IgG of 12.4 g/L (7.0 – 16 g/L) + oligoclonal IgG bands were present / Serum and CSF aquaporin 4 antibodies = negative | Serum myelin oligodendrocyte glycoprotein antibody (MOG) = negative | NR | MPS | Improvement in vision and discharged, repeat MRI Brain showed further improvement, visual evoked potentials showed improvement, no new symptoms to suggest a clinical relapse, consistent with a monophasic illness. |
| Netravathi et al. 2022 | India | 54 | Female | Progressive quadriparesis + altered sensorium + drowsiness | AstraZeneca (ChAdOx1) | 1st | 14d | Brain MRI: T2/FLAIR hyperintensities in the corpus callosum, bl periventricular and subcortical white matter, infratentorial region with patchy contrast enhancement | CSF: 8 cells- lymphocytic predominant, Protein:77 mg/dl, Glucos:98 mg/dl | ANA, ANCA, CRP -negative Serum NMO-MOG = negative | NR | MPS + PE + Prednisolone | Significant improvement |
| 35 | Female | Progressive paraparesis + altered sensorium + conscious + confused + paraparesis | AstraZeneca (ChAdOx1) | 1st | 9d | MRI: T2/FLAIR hyperintensities in mid brain, pons, left MCP, bl posterior internal capsule, thalamus, bl centrum semiovale and LETM from cervical cord to conus | CSF: 58 cells -lymphocytes P: 47.4 mg/dl, G: 106 mg/dl | ANA profile, ANCA, VDRL, RA factor = negative / serum MOG = positive / VEP, BERA, SSEP = normal | NR | MPS + Prednisolone | Significant improvement | ||
| 20 | Female | Paraesthesias + paraparesis + altered sensorium | Covaxin (BBV152) | 1st | 1d | MRI: few juxtacortical and short segment cervical T2/FLAIR hyperintensity at C5 level with subtle enhancement | CSF: 8 cells + lymphocytic predominant, P:24.9 mg/dl, G:61 mg/dl | ANA profile, ANCA,VDRL, RA factor, CRP = negative / Serum and CSF NMO-MOG = negative / CSF OCB = Positive / VEP, BERA, SSEP = normal | NR | MPS + PE + Prednisolone | Significant improvement | ||
| 33 | Female | Fever + vomiting + altered sensorium + persistent paraesthesias | AstraZeneca (ChAdOx1) | 1st | 14d | Brian MRI: T2/FLAIR hyperintensity in Bl fronto parietal region, no enhancement | CSF: 105 cells + lymphocytic predominant, P: 28.12 mg/dl, G: 70.4 mg/dl | Serum MOG = Strongly positive | NR | Acyclovir + MPS + Prednisolone | Significant improvement | ||
| 60 | Male | Tingling paraesthesias + motor weakness + behavioural and memory disturbances | AstraZeneca (ChAdOx1) | 2nd | 14d | Brain MRI: multiple focal lesions in right pons, midbrain, medial temporal lobes, splenium of corpus callosum, high parietal lobe with tumefaction and peripheral enhancement | CSF: 9 cells – 90 % lymphocytes, P:68.3 mg/dl, G:132 mg/dl, OCBs-negative | ANA,ANCA,B12,Homocysteine,VDRL = negative / ACE = normal / Serum NMO and MOG = negative / VEP = normal | NR | MPS + Prednisolone | Significant improvement | ||
| 45 | Male | Fever + urinary retention + difficulty in walking | AstraZeneca (ChAdOx1) | 1st | 10d | Brain and spine MRI: hyperintensities in brainstem, cervicodorsal cord and supratentorial regions with central cord swelling | CSF: 44 cells – 44 % lymphocytes, P:90.9 mg/dl, G:68 mg/dl + rabies CSF PCR = Negative | VEP-l-141,R-129,BERA = normal / N20 = normal / P37–40(mildly prolonged), ANA-U1RNP-1+,C-ANCA-, Serum MOG = strongly positive / S.NMO = Negative | NR | MPS + PE + cycles tab WYSOLONE + MG tab | Significant improvement | ||
| 52 | Female | Progressive slurring of speech + muscle weakness + swallowing difficulty | AstraZeneca (ChAdOx1) | 1st | 35d | Brain MRI: tumefactive demyelination in left frontal hemisphere with insular involvement along with left more than right midbrain involvement | CSF: 2 CELLS,P-40.5 mg/dl,G-56 mg/dl ESR-18 | ANA, ANCA = Negative / VDRL = Negative / S.NMO and MOG = Negative | NR | Rituximab + cycles Tab Wysolone + PE | Remained critically ill, requiring invasive ventilation, and died (after a prolonged intensive care unit stay and superimposed infection) | ||
| Permezel et al. 2021 | Australia | 63 | Male | Vertigo + abdominal pain + fatigue + ketoacidosis + silent myocardial infarction + declining cognition + emerging disorientation + impaired attention | AstraZeneca (ChAdOx1) | 1st | 12d | Brain and cervical spine MRI: numerous bilateral foci (>20) of high T2 and FLAIR signal in the cerebral white matter, with both periventricular and juxtacortical involvement | NR | NR | NR | Empiric antibiotics + antivirals + corticosteroids + PE | MRI brain was repeated on day 19 and demonstrated no changes, and died on day 20 of admission. |
| Rinaldi et al. 2021 | Italy | 45 | Male | Numbness + reduced visual acuity + dysarthria + dysphagia + clumsy right hand movements + urge incontinence | AstraZeneca (ChAdOx1) | 1st | 12d | Brian MRI: large, poorly marginated T2-weighted hyperintensities in the pons (which appeared swollen), right cerebellar peduncle, right thalamus, and multiple spinal cord segments (at the cervical, dorsal, and conus medullaris level). All lesions, except the thalamic one and a single dorsal spinal area, showed blurred gadolinium enhancement on T1-weighted images | CSF: mild lymphocytosis (44 leucocytes, 98 % mononuclear cells), normal proteins, no evidence of tumor cells on CSF cytology / CSF immunoelectrophoresis: the presence of three oligoclonal bands, with normal Link’s Index / Extensive panel for onco-neural antibodies on serum and CSF = negative | Anti-aquaporin-4 (AQP4), anti-myelin oligodendrocyte glycoprotein (MOG) antibodies, anti-nuclear, anti-extractable nuclear antigens, anti-neutrophil cytoplasmic, and anti-cardiolipin antibodies = all Negative | NR | MPS + prednisone | Clinically improved in a few days, and MRI significantly improved |
| Shimizu et al. 2021 | Japan | 88 | Female | Impaired consciousness + gaze-evoked nystagmus | Pfizer (BioNTech) | 2nd | 29d | Brian MRI: signal abnormalities in the bilateral middle cerebellar peduncles | CSF: bacterial and fungal cultures, a CSF oligoclonal band screen, and a test for autoantibodies against myelin basic protein = all negative | antinuclear-, autoimmune vasculitis-, onconeural-, and anti-ganglioside antibodies = all negative | NR | MPS | Impaired consciousness and gaze-evoked nystagmus were found to improve, further MRI brain scans revealed the signal abnormalities had decreased (Complete clinical recovery) |
| Simone et al. 2021 | Italy | 51 | Female | Acute urinary retention + bilateral hypoesthesia | NR | NR | 2w (14d) | MRI: enhancing T2 hyperintense lesions in the spinal cord with longitudinal extension, in the midbrain and in the optic nerves bilaterally | CSF: lymphocyte pleocytosis (50 cells/μL), negative oligoclonal bands | anti-MOG-IgG antibody = positive | Negative | MPS | Clinical improvement |
| Vogrig et al. 2021 | Italy | 56 | Female | Unsteadiness of gait + clumsiness of left arm + malaise + chills + diplopia + mild ataxia + left-ward deviation of gait + urinary retention | Pfizer (BioNTech) | 1st | 2w (14d) | Brain MRI: an area of hyperintensity on fluid attenuated inversion recovery (FLAIR) sequences involving the left cerebellar peduncle, with modest mass effect on the fourth ventricle, which was not present on the previous MRI examination. No contrast enhancement was observed and the lesion did not exhibit diffusion restriction. In addition, new supratentorial areas of hyperintensity on FLAIR sequences were observed, the largest in the left centrum semiovale (unremarkable) | CSF: pleocytosis (80 cells/mm3), protein and glucose levels = normal | MOG, AQP4, GM1, GM2, GM3, GM4, GD1a, GD1b, GD2, GD3, GT1a, GT1b, GQ1b = all negative | Negative | Prednisone | Spontaneously recovered and underwent regular follow-up |
| Yazdanpanah et al. 2022 | Iran | 37 | Male | Muscle weakness + dysphagia + drooling + nausea + vomiting + bilateral facial nerve paralysis | Sinopharm | 1st | 1 month (30d) | Brain MRI: typical imaging findings which presented as multifocal T2-FLAIR signal changes in the corticospinal tract, pons, and temporal lobe with diffusion restriction. | CSF: 2 WBCs, 32 RBCs, 56 mg/dL protein, and glucose of 97 mg/dL + IgG oligoclonal bands = negative | NR | Negative | PE + IVIG + antibiotic therapy + Heparin + Pantoprazole + Clindamycin + Paracetamol + MPS | Showed progressive recovery of motor function, and discharged (with an excellent general condition) |
PE: Plasma exchane, MPS: Methylprednisolone, IVIG: Intravenous immuneglobulin, IVMP: IV methylprednisolone, NR: Not reported.
Fig. 2.
The mean duration between vaccination and neurological symptoms on type of vaccine (A), and type of COVID-19 vaccine used among cases (B).
The result of the quality assessment revealed that 15 studies scored more than seven, and five studies scored below 6 (Table 2 ). The mean JBI score for all included studies was 7.15.
Table 2.
The Joanna Briggs Institute Critical Appraisal tools for Case Reports.
| Al-Quliti et al. 2022 | Ancau et al. 2021 | Ballout et al. 2022 | Francis et al. 2022 | Cao et al. 2021 | Ahmad et al. 2022 | Kania et al. 2021 | Kenangil et al. 2021 | Lazaro et al. 2022 | Maramattom et al. 2022 | Miyamoto et al. 2022 | Mumoli et al. 2021 | Nagaratnam et al. 2022 | Netravathi et al. 2022 | Permezel et al. 2021 | Rinaldi et al. 2021 | Shimizu et al. 2021 | Simone et al. 2021 | Vogrig et al. 2021 | Yazdanpanah et al. 2022 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Were patient’s demographic characteristics clearly described? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the patient’s history clearly described and presented as a timeline? | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Was the intervention(s) or treatment procedure(s) clearly described? | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were adverse events (harms) or unanticipated events identified and described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Total rank | 5 | 7 | 8 | 8 | 6 | 8 | 8 | 6 | 8 | 7 | 8 | 8 | 8 | 5 | 8 | 7 | 8 | 5 | 8 | 7 |
4. Discussion
Since the development of SARS-CoV-2 vaccines, several reports have noted the occurrence of ADEM after immunization. In the present study, we systematically collected a total of 54 reported cases of ADEM post-COVID-19 vaccination with the aim of reviewing clinical presentations, diagnostic features, therapeutic modalities, and final outcomes.
Generally, ADEM is a rare condition, which commonly occurred after a lag time of a few days to a mean of 26 days from a preceding infectious illness or immunization [[40], [41]]. The main presentations include the acute onset of polyfocal neurologic symptoms together with encephalopathy, often with subsequent rapid worsening which leads to hospitalization. Affected patients present with motor deficits involving a single limb or result in paraparesis or quadriparesis. Likewise, sensory deficits are common, and frequent brainstem involvements lead to oculomotor dysfunction and dysarthria. Further presentations may include headache, malaise, meningismus, ataxia, seizure, aphasia, optic neuritis, nystagmus, extrapyramidal movement disorders, urinary retention, and increased intracranial pressure, which could be simply determined by localization of the lesions [[42], [43]]. The lesions of ADEM are large, bilateral, and asymmetric which are poorly marinated, and could be observed as hyperintense lesions on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences [44].
There are no pre-specified biomarkers or confirmatory tests for establishing the diagnosis of ADEM. Thus, ADEM is considered a diagnosis of exclusion, and other inflammatory and demyelinating disorders should be ruled out before confirmation of ADAM diagnosis. It would be worth noting that ADEM is more common in children and diagnostic criteria have been purposed for children with the main two presentations of multifocal central nervous system symptoms and polyneuropathy [45]. For adults, there are no consensus diagnostic criteria yet and unlike for children, evidence of neuropathy is not a required feature for diagnosis, suggesting incomplete presentations for adult patients.
Since ADEM is more common in children [46] and COVID-19 vaccination for young adults is in its early phase, there may be a probability of an increased number of ADEM cases in the following months. Furthermore, ADEM represents a monophasic nature most frequently. Antibodies developed against MOG protein are a potential marker for the evolution of ADEM [47]. It has been reported that the risk of MOG-seropositive and further episodes of relapse is considerably higher in children [[48], [49]]. As a result, the vaccination of young individuals must be performed with strict supporting neurologic surveillance to detect ADEM cases more rapidly and cure them more efficiently.
Apart from the occurrence of ADEM after vaccination, a number of cases have been diagnosed as ADEM post-SARS-CoV-2 infection. In a literature search that has been conducted by Etemadifar and colleagues, a total of 31 ADEM cases were identified following SARS-CoV-2 infection. The average age was 52.3 years with no gender predominance of cases (16 females and 15 males). Moreover, a reduced level of consciousness and GCS was evident in 55.8 % of patients, 35.4 % represented muscle weakness, and 12.9 % developed seizures. Also, the outcome of death was reported in 25 % of patients [50]. By contrast, our cohort of cases was younger (average 46.5 years of age) and females comprised 65.5 % of the total population. We found that post-vaccination ADEM represented muscle weakness, unconsciousness, and seizure in 31.0 %, 27.6 %, and 10.3 % of patients. Furthermore, the mortality rate was 13.8 % in our cases. As it is evident, the rate of adverse events of ADEM following infection is higher than that that occurred post-vaccination. This could be potentially justified by the concomitant infection by SARS-CoV-2 which deteriorated the course of ADEM as compared to those who experienced ADEM after immunization with SARS-CoV-2 vaccines.
Interestingly, the plausibility of SARS-CoV-2 as a viral triggering for ADEM has been extended to other members of the coronaviridae family. In this regard, MERS and OC43 coronaviruses have also been linked to ADEM in recent years [[51], [52]]. Growing evidence has been biologically postulated that the shared epitope between SARS-CoV-2 antigens and neuronal proteins may promote subsequent autoimmune responses against the central nervous system through molecular mimicry [[53], [54]].
In addition to SARS-CoV-2 vaccines, there have been also some reports regarding the occurrence of ADEM following hepatitis, herpes papillomavirus (HPV), measles, mumps, and rubella (MMR), diphtheria, tetanus, and pertussis (DTAP), polio, and seasonal flu vaccines [[55], [56]]. Analyzing the data from the Vaccine Adverse Event Reporting System (VAERS) database showed that vaccines against seasonal flu and HPV were most frequently associated with ADAM [57]. A mean number of 40 annual ADEM events following vaccination was reported from 2005 to 2012 in the VAERS database [57].
The challenging differential diagnosis spectrum of ADEM mostly consists of CNS inflammatory demyelinating disorders such as the first attack of multiple sclerosis, autoimmune encephalitis, neuromyelitis optica, infectious meningoencephalitis, Bickerstaff encephalitis, and transverse myelitis. The lack of oligoclonal bands restricted to the CSF, the absence of periventricular lesions, variations in clinical symptoms, and the clinical evolution of ADEM are not in favor of Multiple Sclerosis (MS), although requiring a long follow-up for ruling out the dissemination in time which is the core characteristic of MS [58]. In addition, memory impairments, seizures, and psychiatric symptoms along with the presence of autoantibodies which are the typical manifestations of autoimmune encephalitis are not common features in ADEM [59]. The MOG antibody–associated disorder (MOGAD) consists of ADEM, transverse myelitis, and optic neuritis. However, there is clinical overlap between MOGAD, MS, and NMOSD, patients with high titer of anti-MOG antibodies should not be diagnosed with MS or NMOSD [60]. Furthermore, 16 (29.6 %) of our included subjects were positive for anti-MOG antibodies. A study by Wendel et al. found that patients with declining anti-MOG antibodies have a significantly lower risk of relapse [61]. The pathognomonic feature of 40–90 % of cases of neuromyelitis optica depending on the demographic is the presence of disease-specific aquaporin-4 (AQP4) antibody, which plays a key role in the pathogenesis of the disease [62]. Also, 10–40 % of cases with negative AQP4 antibody have positive MOG antibody which is mostly present in ADEM [61]. Among included studies, two cases by Francis et al. reported being positive for AQP4 antibody [39]. Based on the new criteria, MOGAD is typically linked to acute disseminated encephalomyelitis, optic neuritis, or transverse myelitis, and is less frequently connected with cerebral cortical encephalitis, brainstem presentations, or cerebellar presentations. MOGAD may manifest as either a monophasic or relapsing disease course, and diagnostic accuracy relies on the use of MOG-IgG cell-based assays [62]. It is essential to exclude diagnoses such as multiple sclerosis, although not all patients with multiple sclerosis require screening for MOG-IgG [62]. In differentiating the diagnosis of ADEM from infectious meningoencephalitis, evidence of meningismus together with the examination of CSF cytology and PCR might be helpful. Furthermore, Bickerstaff encephalitis and post-infection transverse myelitis are considered subtypes of ADEM, in which demyelination and inflammation are confined to the brainstem and spinal cord, respectively [56]. Therefore, in the era of the COVID-19 pandemic considering the sign and symptoms of systemic inflammation like a fever that occurred following SARS-CoV-2 infection or vaccination in conjunction with multifocal neurologic symptoms could confirm the diagnosis of ADEM.
Our study is the most up-to-date systematic review regarding clinical presentations, CSF findings, imagining information, and treatment outcomes of patients with ADEM after COVID-19 vaccination. Nevertheless, some limitations should be considered when interpreting our findings. Firstly, the lack of standard criteria for diagnosis of ADEM in adult patients may result in the variety of case definitions among our included patients. Secondly, incomplete data were reported for some variables which distorted making a comprehensive conclusion. Thirdly, the need for rapid reporting of the vaccine-associated adverse events led to the publication of studies with truncated follow-up duration which resulted in unknown information about the long-term consequences of the disease and the probable risk of relapse after a partial remitting.
In conclusion, it is not clear that ADEM could be a potential complication of COVID-19 vaccination based on the current evidence. However, this rare condition should not trigger stopping the mass vaccination programs and vaccine hesitancy since the only way to eradicate the current pandemic of COVID-19 is to extend the number of immunized people. Considering the causal and temporal association between SARS-CoV-2 vaccination and the occurrence of ADEM, neurologists must be aware of the serious neurological consequences that may arise after COVID-19 immunization in particular for the ChAdOx1 vaccine, and take immediate measures to avoid severe outcomes. Moreover, providing an integrated registry system for gathering detailed information on ADEM cases is highly recommended in order to report the cases in a standardized manner from all over the world.
5. Consent for publication
This manuscript has been approved for publication by all authors.
Funding
We do not have any financial support for this study.
Ethical approval
No need.
Author contributions
FN, MN, and HH: Designed the study and wrote the paper; FN and SHR: collected data, analyzed and interpreted the data, and wrote the draft version of the manuscript. The manuscript was revised and approved by all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sharma A., Ahmad Farouk I., Lal S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses. 2021;13(2):202. doi: 10.3390/v13020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernández A.F., Calina D., Poulas K., Docea A.O., Tsatsakis A.M. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 5.Calina D., Docea A., Petrakis D., Egorov A., Ishmukhametov A., Gabibov A., et al. Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review) Int J Mol Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maramattom B.V., Lotlikar R.S., Sukumaran S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol Sci. 2022;43(6):3503–3507. doi: 10.1007/s10072-022-06000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waheed S., Bayas A., Hindi F., Rizvi Z., Espinosa P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus. 2021;13(2):e13426-e doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607. [DOI] [PMC free article] [PubMed]
- 9.Pohl D., Alper G., Van Haren K., Kornberg A.J., Lucchinetti C.F., Tenembaum S., et al. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Supplement 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 10.Tenembaum S., Chitnis T., Ness J., Hahn J.S. Acute disseminated encephalomyelitis. Neurology. 2007;68(Issue 16, Supplement 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 11.Dubey D., Pittock S.J., Kelly C.R., McKeon A., Lopez-Chiriboga A.S., Lennon V.A., et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsiodras S., Kelesidis T., Kelesidis I., Voumbourakis K., Giamarellou H. Mycoplasma pneumoniae-associated myelitis: a comprehensive review. Eur J Neurol. 2006;13(2):112–124. doi: 10.1111/j.1468-1331.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 13.Marchioni E., Ravaglia S., Piccolo G., Furione M., Zardini E., Franciotta D., et al. Postinfectious inflammatory disorders: subgroups based on prospective follow-up. Neurology. 2005;65(7):1057–1065. doi: 10.1212/01.wnl.0000179302.93960.ad. [DOI] [PubMed] [Google Scholar]
- 14.Garg RK. Acute disseminated encephalomyelitis. Postgraduate Medical Journal. 2003;79(927):11. [DOI] [PMC free article] [PubMed]
- 15.Wang Y., Wang Y., Huo L., Li Q., Chen J., Wang H. SARS-CoV-2-associated acute disseminated encephalomyelitis: a systematic review of the literature. J Neurol. 2022;269(3):1071–1092. doi: 10.1007/s00415-021-10771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahapure K.S., Prabhune A.S., Chouvhan A.V. COVID-19-Associated Acute Disseminated Encephalomyelitis: A Systematic Review. Asian J Neurosurg. 2021;16(3):457–469. doi: 10.4103/ajns.AJNS_406_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manzano G.S., McEntire C.R.S., Martinez-Lage M., Mateen F.J., Hutto S.K. Acute Disseminated Encephalomyelitis and Acute Hemorrhagic Leukoencephalitis Following COVID-19: Systematic Review and Meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1080. doi: 10.1212/NXI.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Quliti K., Qureshi A., Quadri M., Abdulhameed B., Alanazi A., Alhujeily R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases. 2022;10(1):13. doi: 10.3390/diseases10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ÓSullivan C., Zach F., Moser T., Pilz G., Harrer A., Trinka E., et al. Misinterpretation of glioblastoma as ADEM: potentially harmful consequences of over-diagnosis of COVID-19 vaccine-associated adverse events. J Neurol. 2022;269(2):616–618. doi: 10.1007/s00415-021-10707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao L., Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2022;122(3):793–795. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazaro LG, Perea Cossio JE, Luis MB, Tamagnini F, Paguay Mejia DA, Solarz H, et al. Acute disseminated encephalomyelitis following vaccination against SARS-CoV-2: A case report. Brain Behav Immun Health. 2022;20:100439. [DOI] [PMC free article] [PubMed]
- 22.Miyamoto K., Koh J., Takahashi M., Niwa M., Ito H. A case of anti-MOG antibody-positive ADEM following COVID-19 mRNA vaccination. Neurol Sci. 2022;43(6):3513–3514. doi: 10.1007/s10072-022-06019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaratnam S.A., Ferdi A.C., Leaney J., Lee R.L.K., Hwang Y.T., Heard R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022;22(1) doi: 10.1186/s12883-022-02575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed]
- 25.M. P. Moola S MZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P. JBI Manual for Evidence Synthesis. JBI Manual for Evidence Synthesis. 2020.
- 26.Ancau M., Liesche-Starnecker F., Niederschweiberer J., Krieg S.M., Zimmer C., Lingg C., et al. Case Series: Acute Hemorrhagic Encephalomyelitis After SARS-CoV-2 Vaccination. Front Neurol. 2022;12 doi: 10.3389/fneur.2021.820049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballout A.A., Babaie A., Kolesnik M., Li J.Y., Hameed N., Waldman G., et al. A Single-Health System Case Series of New-Onset CNS Inflammatory Disorders Temporally Associated With mRNA-Based SARS-CoV-2 Vaccines. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.796882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kania K., Ambrosius W., Kupczyk E.T., Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann Clin Transl Neurol. 2021;8(10):2000–2003. doi: 10.1002/acn3.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mumoli L., Vescio V., Pirritano D., Russo E., Bosco D. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol Sci. 2022;43(2):763–766. doi: 10.1007/s10072-021-05761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netravathi M., Dhamija K., Gupta M., Tamborska A., Nalini A., Holla V.V., et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult Scler Relat Disord. 2022;60 doi: 10.1016/j.msard.2022.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozgen Kenangil G., Ari B.C., Guler C., Demir M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Permezel F., Borojevic B., Lau S., de Boer H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol. 2022;18(1):74–79. doi: 10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult Scler J. 2022;28(7):1151–1154. doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu M., Ogaki K., Nakamura R., Kado E., Nakajima S., Kurita N., et al. An 88-year-old woman with acute disseminated encephalomyelitis following messenger ribonucleic acid-based COVID-19 vaccination. eNeurologicalSci. 2021;25:100381. doi: 10.1016/j.ensci.2021.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simone A.M., Monti G., Amidei S., Costa M., Vaghi L., Devetak M., et al. Acute disseminated encephalomyelitis associated with anti-myelin oligodendrocyte glycoprotein (MOG-IGG) antibody in a patient with recent vaccination against SARS-CoV-2. J Neurol Sci. 2021;429:118167. [Google Scholar]
- 36.Vogrig A., Janes F., Gigli G.L., Curcio F., Negro I.D., D’Agostini S., et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazdanpanah F., Iranpour P., Haseli S., Poursadeghfard M., Yarmahmoodi F. Acute disseminated encephalomyelitis (ADEM) after SARS- CoV-2 vaccination: A case report. Radiol Case Rep. 2022;17(5):1789–1793. doi: 10.1016/j.radcr.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad H.R., Timmermans V.M., Dakakni T. Acute Disseminated Encephalomyelitis After SARS-CoV-2 Vaccination. Am J Case Rep. 2022;23:e936574. doi: 10.12659/AJCR.936574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francis A.G., Elhadd K., Camera V., Ferreira dos Santos M., Rocchi C., Adib-Samii P., et al. Acute Inflammatory Diseases of the Central Nervous System After SARS-CoV-2 Vaccination. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200063. doi: 10.1212/NXI.0000000000200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Seze J., Debouverie M., Zephir H., Lebrun C., Blanc F., Bourg V., et al. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol. 2007;64(10):1426. doi: 10.1001/archneur.64.10.1426. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz S., Mohr A., Knauth M., Wildemann B., Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56(10):1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 42.Atlas S.W., Grossman R.I., Goldberg H.I., Hackney D.B., Bilaniuk L.T., Zimmerman R.A. MR diagnosis of acute disseminated encephalomyelitis. J Comput Assist Tomogr. 1986;10(5):798–801. doi: 10.1097/00004728-198609000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Krupp L.B., Tardieu M., Amato M.P., Banwell B., Chitnis T., Dale R.C., et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler J. 2013;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 44.Tenembaum S.N. Pediatric demyelinating disease and anti-MOG antibody. Clin Exp Neuroimmunol. 2021;12(1):7–21. [Google Scholar]
- 45.Baumann M., Sahin K., Lechner C., Hennes E.M., Schanda K., Mader S., et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86(3):265–272. doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- 46.Hennes E.-M., Baumann M., Schanda K., Anlar B., Bajer-Kornek B., Blaschek A., et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900–908. doi: 10.1212/WNL.0000000000004312. [DOI] [PubMed] [Google Scholar]
- 47.Fisher K, Balasa A, Shukla N, Lotze T. Increased Likelihood of Relapse in Pediatric Anti-MOG Acute Disseminated Encephalomyelitis (ADEM) and Optic Neuritis (ON) vs. Seronegative ADEM and ON Patients (1061). AAN Enterprises; 2020.
- 48.Etemadifar M, Mansouri AR, Nouri H, Sedaghat N, Salari M, Maghsoudi M, et al. Post-COVID-19 acute disseminated encephalomyelitis: Case report and review of the literature. Neuroimmunology Reports. 2022;2:100066.
- 49.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ann Yeh E., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1):e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 51.Yapici-Eser H., Koroglu Y.E., Oztop-Cakmak O., Keskin O., Gursoy A., Gursoy-Ozdemir Y. Neuropsychiatric symptoms of COVID-19 explained by SARS-CoV-2 proteins’ mimicry of human protein interactions. Front Hum Neurosci. 2021;15:126. doi: 10.3389/fnhum.2021.656313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gammazza AM, Légaré S, Bosco GL, Fucarino A, Angileri F, Oliveri M, et al. Molecular mimicry in the post-COVID-19 signs and symptoms of neurovegetative disorders? The Lancet Microbe. 2021;2(3):e94. [DOI] [PubMed]
- 53.Huynh W., Cordato D.J., Kehdi E., Masters L.T., Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menge T., Hemmer B., Nessler S., Wiendl H., Neuhaus O., Hartung H.-P., et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 55.Pellegrino P, Carnovale C, Perrone V, Pozzi M, Antoniazzi S, Clementi E, et al. Acute Disseminated Encephalomyelitis Onset: Evaluation Based on Vaccine Adverse Events Reporting Systems. PLOS ONE. 2013;8(10):e77766. [DOI] [PMC free article] [PubMed]
- 56.Dale R., Branson J. Acute disseminated encephalomyelitis or multiple sclerosis: can the initial presentation help in establishing a correct diagnosis? Arch Dis Child. 2005;90(6):636–639. doi: 10.1136/adc.2004.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12(1):1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marignier R., Hacohen Y., Cobo-Calvo A., Pröbstel A.-K., Aktas O., Alexopoulos H., et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762–772. doi: 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 59.Wendel E.M., Thonke H.S., Bertolini A., Baumann M., Blaschek A., Merkenschlager A., et al. Temporal Dynamics of MOG Antibodies in Children With Acquired Demyelinating Syndrome. Neurol Neuroimmunol Neuroinflamm. 2022;9(6) doi: 10.1212/NXI.0000000000200035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wingerchuk D.M., Weinshenker B.G. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. CONTINUUM: Lifelong Learning. Neurology. 2013;19(4):944–967. doi: 10.1212/01.CON.0000433289.38339.a2. [DOI] [PubMed] [Google Scholar]
- 61.Jarius S., Paul F., Weinshenker B.G., Levy M., Kim H.J., Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85. doi: 10.1038/s41572-020-0214-9. [DOI] [PubMed] [Google Scholar]
- 62.Banwell B., Bennett J.L., Marignier R., Kim H.J., Brilot F., Flanagan E.P., et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023;22(3):268–282. doi: 10.1016/S1474-4422(22)00431-8. [DOI] [PubMed] [Google Scholar]