Abstract
Purpose
The purpose of the current study, The National Institute of Child Health and Human Development (NICHD) Study of Health in Early and Adult Life (SHINE), was to build on the landmark Study of Early Child Care and Youth Development (SECCYD), a longitudinal birth cohort initiated in 1991, by conducting a health-focused follow-up of the now adult participants. This effort has produced an invaluable resource for the pursuit of life course research examining links between early life risk and resilience factors and adulthood health and disease risk.
Participants
Of the 927 NICHD SECCYD participants available for recruitment in the current study, 705 (76.1%) participated in the study. Participants were between 26 and 31 years and living in diverse geographic locations throughout the USA.
Findings to date
In descriptive analyses, the sample exhibited risk on health status indicators, especially related to obesity, hypertension and diabetes. Of particular concern, the prevalence of hypertension (29.4%) and diabetes (25.8%) exceeded national estimates in similar-age individuals. Health behaviour indicators generally tracked with the parameters of poor health status, showing a pattern of poor diet, low activity and disrupted sleep. The juxtaposition of the sample’s relatively young age (mean=28.6 years) and high educational status (55.6% college educated or greater) with its poor health status is noteworthy, suggesting a dissociation between health and factors that are typically health protective. This is consistent with observed population health trends, which show a worsening of cardiometabolic health status in younger generations of Americans.
Future plans
The current study, SHINE, lays the groundwork for future analyses in which the uniquely robust measures collected as a part of the original NICHD SECCYD will be leveraged to pinpoint specific early life risk and resilience factors as well as the correlates and potential mechanisms accounting for variability in health and disease risk indicators in young adulthood.
Keywords: EPIDEMIOLOGY, MENTAL HEALTH, PREVENTIVE MEDICINE, PUBLIC HEALTH, SOCIAL MEDICINE, CARDIOLOGY
Strengths and limitations of this study.
The current study, The Study of Health in Early and Adult Life, leveraged the original National Institute of Child Health and Human Development Study of Early Child Care and Youth Development to extend and maximise the value of this longitudinal birth cohort by collecting adulthood measures of health, thereby, creating an invaluable resource for the pursuit of life course research relating early life exposures to adulthood health and disease risk.
Gold standard methods were used for the measurement of each health status and health behaviour indicator.
Extensive recruitment methods were used to engage participants living in different locations throughout the USA and adaptations to the study procedures were developed (eg, ‘self-administered’ study protocol) to allow flexibility with participation, especially needed through the COVID-19 pandemic.
The nature of the data collection required that data collection teams work in the field to implement the study protocols, thus, resulting in many challenges, including the management of numerous staff persons, physical distance from the participants and varied data collection environments.
Introduction
The National Institute of Child Health and Human Development (NICHD) Study of Early Child Care and Youth Development (SECCYD) is a landmark study of child development conducted in the USA between 1991 and 2009.1 It was initiated by NICHD to characterise impacts of early childcare environments on domains of child social, emotional and cognitive development as well as aspects of physical development and health. Families were enrolled at the child’s birth from diverse geographic locations and followed annually over the course of the study. The breadth and depth of measurement available in the NICHD SECCYD has made it a unique resource for developmental scientists, supporting a wealth of discovery in broad areas of child health and well-being. To date, well over one thousand scientific research articles have been published leveraging these data1–6 with additional efforts employed to follow the members of this longitudinal birth cohort who are now in young adulthood.
The value of the NICHD SECCYD continues to grow over time, most especially in its potential to inform timely research questions relating early life environments to adulthood health and disease risk.7 8 Burgeoning areas of research suggest the origins of adulthood health and disease are rooted in early life environments.9–14 In these studies, markers indexing childhood exposures such as maladaptive family interactions (eg, abuse) and lower socioeconomic status (eg, low parental education) have been identified as early life risk factors for long-term disease and mortality outcomes, as well as intermediate health conditions (eg, obesity).15–20 The epidemiological studies reporting these associations, however, typically lack the depth of measurement present in a study such as the NICHD SECCYD, precluding opportunities to pinpoint the processes and mechanisms underlying these effects, but see studies.21–24 As examples, areas of measurement uniquely available in the NICHD SECCYD include repeated, multimethod assessments of attachment security, parenting sensitivity, childcare quality and nuances of early educational environments as well as child-level assessments of intelligence, temperament and social relationships. Moreover, a focus on upstream factors relevant to later life health is a growing imperative as traditional disease-focused approaches targeting the remediation of poor health in adulthood are simply not working. The USA, compared with other high-income countries, ranks the lowest in life expectancy, the highest in infant mortality and has the highest percentage of adults who are overweight or obese,25–27 itself a significant predictor of morbidity and mortality.28–30 All the while, spending on healthcare exceeds US$3 trillion per year.31 32 These worsening trends underscore the profound need to move away from conventional strategies for intervention to instead consider how early life risk and resilience factors may be leveraged in the context of primary prevention efforts.
The objective of the current study was to actualise the potential of the NICHD SECCYD by conducting a follow-up assessment of the now adult participants (ages 26–31 years). This follow-up, rebranded The Study of Health in Early and Adult Life (SHINE) focused on the collection of detailed health information, using gold standard methods for the assessment of blood pressure and anthropometrics, the ascertainment of blood and hair samples, the implementation of 24-hour diet recall interviews and 7 day actigraphy for activity/sleep monitoring and the completion of comprehensive self-report questionnaires in multiple areas of health and well-being. The availability of these measures will make possible the pursuit of prospective research questions linking the wealth of existing data characterising the early life environments of the participants as children and adolescents with the newly collected data characterising the health status of the participants now as adults. Here, in the current report, we present results describing these adulthood health measures and outline our analytical plans to test a series of life course models integrating the NICHD SECCYD and SHINE data. Additionally, we also discuss our unique experiences and lessons learnt during the SHINE data collection in which we faced many challenges conducting in-person health assessments among participants living in distant locations throughout the USA and in a period overlapping with the COVID-19 pandemic.
Cohort description
Sample overview
Participants in the current study were originally recruited at birth as a part of the NICHD SECCYD, a prospective study of children and their families followed between birth and adolescence to examine trajectories of child health and development.1 Families were from 10 geographically diverse study sites in the USA: Seattle, Washington; Madison, Wisconsin; Irvine, California; Pittsburgh, Pennsylvania; Wellesley, Massachusetts; Little Rock, Arkansas; Philadelphia, Pennsylvania; Morganton, North Carolina; Lawrence, Kansas; and Charlottesville, Virginia. In the first 11 months of 1991, all mother–infant dyads of babies born within preselected 24-hour intervals at participating hospitals were screened. The exclusion criteria were mother<18 years old, non-English speaking or had a substance use disorder; serious medical problems (mother or infant); lived>1 hour from the study site; child being placed for adoption; concurrent participation in another study; and refusal to participate in initial screening. Additional sampling requirements were imposed (eg, 10% recruitment of single parent households) to ensure that the sociodemographic composition of the final sample (N=1364 families; n=659 girls (48.3%) and n=705 boys (51.7%)) was proportionate to the population of the geographies from which they were recruited, according to the 1990 US Census.
Following completion of the final data collection time point in the original NICHD SECCYD at age 15 years, 946 adolescent participants and their parents agreed to be recontacted for future research studies. Additional research contacts occurred at participant ages 17–18 years,33 age 22 years34 and ages 26–27 years,35 36 after which time 930 young adults remained in the sample. This reduction in sample size was due to 14 participants who rescinded their consent for future contact and 2 participants who died. Among these 930 participants, 3 additional participants died subsequently, leaving 927 participants available for recruitment in the current study. All participant deaths were confirmed by death records, obituaries or verbal confirmation by parents.
The current study, a follow-up to the NICHD SECCYD rebranded SHINE, located these now young adults (n=927, ages 26–31) to complete an in-person study visit. The SHINE data collection occurred between 2018 and 2022. Extensive social, behavioural and health data were collected with the goal of testing effects of early life exposures, and the mechanisms of these effects, on trajectories of health and disease risk over time. The current study is the first among the existing NICHD SECCYD data collection efforts to engage the participants as adults (age≥18 years) with an in-depth, in-person protocol focused on the assessment of cardiometabolic health specifically. The current study is also unique in its design and methodological approach as it was led by a single research team at the University of Washington (UW) who oversaw the in-person data collection at numerous locations throughout the USA. To execute the study from a distance, and during the COVID-19 pandemic, many useful adaptations were developed, some originating from experiences of failure that are shared here as lessons to other investigators interested in conducting similar work (see details in Challenges and lessons learnt section below).
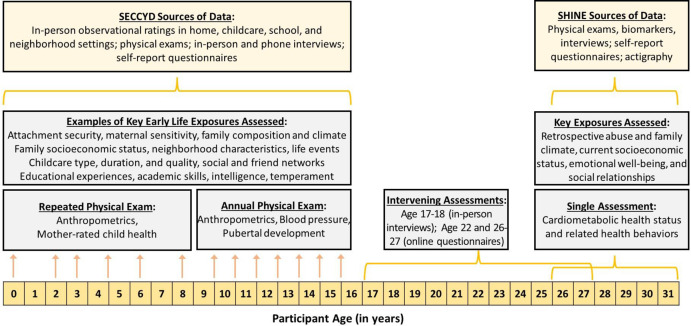
Figure 1 summarises available data from the current study, SHINE, as well as the original NICHD SECCYD in the context of the timeline of these data collections.
Figure 1.
Description of the data collections in the SECCYD (repeated assessments between birth and age 15.5) and SHINE (single assessment in young adulthood), as well as single follow-up assessments at ages 17–18, 22 and 26–27 years. SECCYD, Study of Early Child Care and Youth Development; SHINE, Study of Health in Early and Adult Life.
Informed consent and assent for the original NICHD SECCYD were obtained from parents and children, respectively. Informed consent for the NICHD SECCYD follow-up study, SHINE, was obtained from the now adult target participants. All methods were carried out in accordance with relevant guidelines and regulations.37 Participants were financially compensated in the original NICHD SECCYD and at each follow-up, including the current study, based on time and burden and in alignment with the Institutional Review Boards and UW Human Subjects Division.
Participation rates
The 927 participants (468 (50.5%) women, 459 men (49.5%)) available for recruitment in the current study were contacted using information from prior assessments. Contacts were initiated via email, phone, text or social media, including Facebook and LinkedIn. Efforts to update participant contact information included reaching out to alternative contacts such as parents or grandparents, searching social media sites, mailing postcards to physical addresses and using paid, secured services offered through White Pages, LexisNexis and TransUnion. Over time, various ad hoc strategies were used to further incentivise participation, including increasing the study payment (eg, from US$250 to US$300 to US$400 over time for completion of the full protocol), offering payment for screening, engaging participants through newsletters and e-cards, and developing alternate protocols that allowed flexibility in completing only portions of the study or in completing some portions of the study remotely and independently.
Extensive recruitment efforts resulted in the following participation rates. In the full sample, 705 (of 927; 76.1%) individuals participated in the study. Of the 222 non-participants, 31 (13.9%) declined, primarily due to being too busy, 6 (2.7%) rescinded their consent for future contact, 79 (35.6%) were initially engaged but did not follow-up, 90 (40.5%) were unresponsive to all contact efforts (using contact information that was presumed to be valid but was not verified), 13 (5.9%) had no contact information and 3 (1.4%) were confirmed to be incarcerated during the period of recruitment. With respect to sex assigned at birth, 378 (of 468; 80.8%) women and 327 (of 459; 71.2%) men participated in the study, reflecting a significant difference in rates of participation with women more likely to participate (χ2(1, N=927)=11.5, p<0.001). Five participants no longer identified as the sex assigned at birth. Instead, two participants identified as transgender male, one as transgender female and two as non-binary. Finally, participation rates by original recruitment site were as follows: Seattle, Washington (88.1%); Madison, Wisconsin (75.5%); Irvine, California (76.2%); Pittsburgh, Pennsylvania (76.6%); Wellesley, Massachusetts (67.0%); Little Rock, Arkansas (85.2%); Philadelphia, Pennsylvania (71.9%); Morganton, North Carolina (65.3%); Lawrence, Kansas (79.6%); and Charlottesville, Virginia (78.3%). However, reports of rates by original recruitment site are misleading insofar as a sizeable proportion of participants (221 (31.3%)) had relocated and completed the protocol at a different main or ancillary site or chose to complete one of the remote protocols even if they were within travel distance. Of note, all participants were offered paid travel accommodations to reduce barriers to in-person participation.
Data collection overview
All participants were engaged using an introductory letter describing the study, followed by phone and email contacts. Exclusions were temporary, including pregnancy or breast feeding and current/recent cold or influenza, and participants were followed and rescreened as necessary to identify changes in eligibility. Women who were not using medications affecting their menstrual cycle, and who could predict the start of their period within 5 days, were scheduled to participate in the early follicular phase between menstrual cycle days 2 and 7. All participants were invited to participate in the full study protocol with paid accommodations for travel offered when necessary. However, based on participant preferences and circumstances, alternate study protocols were also developed to reduce the time/burden of study participation. Of the 705 participants, 551 (78.2%) participated in the full study protocol which entailed an in-person home visit (3–4 hours) and 2 postvisit activities occurring over a 1-week to 2-week period. A total of 16 participants (2.3%) participated in the partial study protocol, which entailed a standard subset of study activities. A total of 99 participants (14.0%) participated in the self-administered study protocol, which entailed a standard subset of study activities that could be performed by the participant remotely and independently. Finally, 39 (5.5%) completed the study questionnaires only. See details in the ‘Data collection protocols’ section below.
The structure of the data collection both retained and built on the 10 original recruitment sites. At each of these 10 main sites, a data collector and mobile phlebotomist were hired and trained to administer the study protocol. The study visits occurred primarily in the homes of the participants. However, based on the locations of participants, at times, a central location for data collection was established (eg, in a rented professional office space) and the participants would travel to the data collection team. After each study visit, all associated research materials were returned to the UW research team in Seattle, Washington, who managed and oversaw the data collection efforts at all locations throughout the study period. At the study visit, a standard paper form was used to record the collected data in real time (eg, blood pressure readings) and to document compliance with each step of the data collection protocol. The information on this form was entered into the online data capture tool, REDCap, while the visit was still on-going, making it immediately available to the UW research team to review and intervene (if needed) before the visit ended. Limited paperwork, including the form referenced above, and the hair samples were shipped by regular mail and the processed blood samples were immediately placed on dry ice and shipped overnight by FedEx. The UW research team also conducted all of the postvisit research activities as well as the three study protocols that did not have an in-person component.
All data collectors and mobile phlebotomists received intensive training led by MEB and the UW research team. The data collector training included human subjects research training (online), formal orientation to the study procedures (online) and a 2-day in-person training session at UW. The first day focused on training for each protocol segment and the second day required the successful execution of the full study protocol on a practice participant to receive certification. The data collectors generally had college degrees with at least 3 years of experience working in a research setting. All data collectors worked concurrently in relevant areas of social or health sciences (eg, nursing, social work, psychology, public health). The mobile phlebotomist training included human subjects research training (online), formal training on the blood collection and blood processing procedures (online) and a 1-day in-person training session at UW. The in-person training required the successful execution of the blood collection and blood processing procedures on a practice participant to receive certification. The mobile phlebotomists generally had at least 2 years of experience working in mobile phlebotomy as well as experience performing blood processing. All mobile phlebotomists worked concurrently in relevant medical settings and all were required to maintain their professional credentials in their respective states. For both the data collector and mobile phlebotomist, as needed, additional training was offered in-person and online and practice supplies were provided for independent practice before beginning data collection. After data collection began, all data collection materials and samples were inspected by the UW research team, research visits were observed periodically via Zoom and constructive feedback was provided throughout the period of data collection. Over time, two data collectors and two mobile phlebotomists left their positions and were replaced, repeating the same training process described above.
The 10 main data collection sites were set up over time in this order: Seattle, Washington (started in January 2018); Madison, Wisconsin (started in May 2018); Irvine, California (started in May 2018); Pittsburgh, Pennsylvania (started in October 2018); Wellesley, Massachusetts (started in November 2018); Little Rock, Arkansas (started in March 2019); Philadelphia, Pennsylvania (started in March 2019); Morganton, North Carolina (started in June 2019); Lawrence, Kansas (started in July 2019); and Charlottesville, Virginia (started in February 2020). Once a site was set up, it generally remained open. However, intermittent disruptions were experienced based on turnover among the data collectors/mobile phlebotomists and the COVID-19 pandemic. All sites were open for at least 2 years, ranging between 2 and 4 years.
In addition, based on the locations of participants outside of these 10 main sites, several ancillary sites were set up. A data collector and mobile phlebotomist from one of the main sites travelled to the indicated ancillary site to conduct the study protocol among a preidentified ‘cluster’ of participants over a period of days. The ancillary data collection sites included the following: San Francisco Bay Area (2019); San Jose, California (2019); Denver, Colorado (2020); Atlanta, Georgia (2020); Washington, District of Columbia (2020); New York, New York (two times in 2021); Portland, Oregon (2019, 2021); Kansas City, Missouri (2021); and Nashville, Tennessee (2021).
Data collection protocols
Full study protocol
The full study protocol entailed an in-person home visit (3–4 hours) and two postvisit activities occurring over a 1-week to 2-week period. The in-person home visit was scheduled in the morning between 7:00 and 10:00 local time and included the measurement of blood pressure and anthropometrics (height, weight, and waist and hip circumferences), the collection of blood and hair samples, participation in one 24-hour diet recall interview and the completion of self-report questionnaires in areas of health and well-being. The postvisit activities included participation in two additional 24-hour diet recall interviews (by phone) and completion of activity/sleep monitoring using an activity monitor worn 24 hours/day over a 7-day period.
Partial study protocol
The ‘partial’ study protocol included participation in three 24-hour diet recall interviews (by phone), completion of the self-report questionnaires (online) and completion of activity/sleep monitoring using an activity monitor worn 24 hours/day over a 7-day period. Therefore, in the ‘partial’ study protocol, data are missing for the assessment of blood pressure and anthropometrics as well as the collection of blood and hair samples. Among the 16 (2.3%) participants who completed this protocol, 6 (37.5%) did so because they were living outside of the USA and the remainder expressed miscellaneous reasons for their preference for this protocol.
Self-administered study protocol
The ‘self-administered’ study protocol included the study protocol segments that could be performed by the participant remotely and independently, although with support and oversight by the UW research team. These segments included the measurement of blood pressure and anthropometrics (height, weight, and waist and hip circumferences), participation in three 24-hour diet recall interviews (by phone), completion of the self-report questionnaires (online) and completion of activity/sleep monitoring using an activity monitor worn 24 hours/day over a 7-day period. Participants were provided all the supplies/equipment (eg, blood pressure monitor, flat scale, tape measure and activity monitor) and detailed instructions necessary to complete this protocol at home. Real-time support was provided by the UW research team over phone/email at the time of the collection. Participants were also provided videos produced by the UW research team that demonstrated the correct method of taking the blood pressure and anthropometric measures. The self-administered study protocol was developed, in part, in response to the COVID-19 pandemic, allowing data collection to continue without having in-person contact with the participants. Therefore, in the ‘self-administered’ protocol, data are missing for the collection of blood and hair samples. Among the 99 (14.0%) participants who completed this protocol, all occurring during the pandemic, the majority (90 (90.9%)) did so because they lived in locations that were distant from one of the main or ancillary data collection sites and they did not want to travel to the site, even though paid travel accommodations were offered. The remainder expressed miscellaneous reasons for their preference for this protocol.
Questionnaires only study protocol
The ‘questionnaires-only’ study protocol included completion of the self-report questionnaires online with real-time support and follow-up offered by the UW research team. Therefore, in the ‘questionnaires only’ protocol, data are missing for all of the other study assessments (ie, blood pressure, anthropometrics, blood and hair samples, 24-hour diet recall interviews and the activity monitor). Among the 39 (5.5%) participants who completed this protocol, the majority (29 (74.4%)) responded to the questionnaire link without communicating with the study team directly and the remainder expressed miscellaneous reasons for their preference for this protocol.
Across the study protocols, with respect to the main data collection components, complete data are available for 647 (91.8%) participants for the blood pressure assessment, 664 (94.2%) participants for the anthropometric assessment, 527 (74.8%) participants for the blood collection, 468 (66.4%) participants for the hair collection, 700 (99.3%) for the self-report questionnaires, 664 (94.2%) participants for at least one 24-hour diet recall interview and 581 (82.4%) participants for the valid wear of the activity monitor.
Available data
Blood pressure assessment
The circumference of the participant’s bare upper arm was measured first to enable selection of the correct cuff size. Next, the participant was directed to sit at a table in a relaxed position with legs uncrossed, feet flat on the floor and no talking for a 5 min rest period. Following the rest period, the preselected cuff was correctly positioned on the left arm with the arm resting on the table at heart level. A research grade, automated blood pressure monitor was used, preprogrammed to take three consecutive measurements with 1 min in between readings. The cuff was then repositioned on the right arm and the measurements were repeated.
Anthropometric assessment
The participant was directed to remove shoes, all excess clothing and accessories and any items from pockets. First, a research grade flat scale was positioned on a hard surfaced floor to measure weight. Next, a research grade stadiometer was assembled and positioned against an open wall to measure height. The height measurement was taken with the participant’s heels, hips, shoulders and head aligned along the back of the stadiometer. Finally, a tension-controlled tape measure was positioned at the midpoint between the iliac crest and lowest rib to measure waist circumference (on the exhalation) and repositioned at the widest point of the hips to measure hip circumference.
Blood sample collection
The participant’s blood was drawn from the arm in a seated or supine position by a trained phlebotomist. The blood draw occurred in the morning between 7:00 and 10:00 following an overnight fast starting at 21:00. Other restrictions included cessation of exercise, alcohol intake and non-essential cold/allergy and headache medications 12 hours prior, the cessation of caffeine 8 hours prior and the cessation of nicotine 1 hour prior. Following the draw, the blood was processed and aliquoted on site. The samples were then placed on dry ice and packaged for shipment by FedEx overnight to the UW research team who received and stored them at −80°C for later analysis. In batch, assays were performed in areas of cardiometabolic health (eg, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin, haemoglobin A1c) and inflammation (eg, C reactive protein).
Hair sample collection
The target area on the participant’s head (posterior vertex) was identified and 2–3 ‘bundles’ of hair were tied off in this region, together equalling in quantity the diameter of a standard writing pen. These bundles were then cut close to the scalp and affixed to a pre-prepared foil envelope for shipment by regular mail to the UW research team who inspected and stored the hair samples at room temperature for later analysis. If necessary, hair outside of the target area was taken, excluding facial hair or hair along the hairline. Participants also completed a self-report questionnaire regarding hair washing and use of hair care products and styling tools. In batch, assays were performed to assess hair cortisol, indexing the activity of the hypothalamic–pituitary–adrenal axis as a marker of psychological stress experienced over the preceding months.
Diet recall interviews
The participant’s dietary intake over the prior 24 hours was assessed using the computer-based Automated Self-Administered 24-Hour Dietary Assessment (ASA24).38 One ASA24 interview was conducted in-person and two others were conducted over the phone, all occurring over a 1-week period with one interview referencing a weekend day. Data collected through these interviews was scored using the Healthy Eating Index-2015 (HEI-2015) scoring system developed by the US Department of Agriculture.39 This scoring system produces an overall diet quality score as well as 13 values related to key nutrients or food components based on the US Department of Health & Human Services 2015–2020 Dietary Guidelines.
Activity monitor wear
The participant was directed to wear an activity monitor on the right hip during the day for the assessment of activity and on the wrist of the nondominant hand during the night for the assessment of sleep. The duration of wear was 24 hours per day for seven consecutive days, including 2 weekend days. The participant completed a log during this period, recording wake and sleep times each day. Additional instructions were provided regarding the removal of the device when exposed to water. The activity monitor was provided by mailing it to the participant along with a postage-paid box for the participant to use to return the monitor following wear. On return of the activity monitor, the data were then exported from the device and scored using proprietary software to derive activity variables such as moderate/vigorous activity, number of steps, and sedentary time as well as sleep variables such as sleep latency, duration and awakenings.
Self-report questionnaires
The participant completed a comprehensive set of self-report questionnaires using the online data capture tool, REDCap. The participant was assisted by the UW research team (either in-person or remotely depending on the study protocol) who provided oversight, general support and referrals to diverse support services. In summary, the questionnaires pertained to sociodemographic and neighbourhood characteristics; medical, reproductive and psychiatric history; health behaviours in areas of smoking, exercise, nutrition and sleep; cognitive function in areas of executive functioning and decision-making; stress, adverse events and psychological well-being, including depression and anxiety symptoms; and family composition and family, social and romantic relationships.
In these areas, specific questionnaires were selected based on psychometric evaluation showing high reliability and validity. As well, a subset of questionnaires was retained due to their use in the original NICHD SECCYD. Although too numerous to list, examples of these questionnaires include: health behaviours (eg, Dietary Screening Questionnaire,40 Pittsburgh Sleep Quality Index (PSQI)41); stress and adversity (eg, Perceived Stress Scale,42 Stress and Adversity Inventory for Adults43), psychological well-being (eg, Center for Epidemiologic Studies Depression,44 Adult Self Report45) and social relationships (eg, Dyadic Adjustment Scale,46 Experiences in Close Relationships—Relationship Structures Questionnaire47).
Challenges and lessons learned
The study’s original efforts to implement phlebotomy services in the numerous locations of the study participants included use of advertised mobile phlebotomy companies. These efforts failed, however, as such companies were not able to provide well-trained phlebotomists, were not able to provide coverage for the geographical locations of the participants and were not positioned to properly train their employees to perform the study protocol. As a result, the study transitioned to an independent contractor model in which mobile phlebotomists were sought through indeed.com, interviewed and trained remotely and then brought to UW to be certified in the proper implementation of the blood collection and blood processing protocols. As independent contractors, the mobile phlebotomists were provided liability insurance, a centrifuge if needed and all indicated supplies. Otherwise, expenses such as gas mileage were covered in their contracts as a part of their per participant payment. Although the independent contractor model required more time for recruitment, training and on-going administrative tasks related to contracts and invoicing, it was superior to other options and produced a higher quality blood sample collection.
In a related issue, the study’s original efforts to freeze and ship the blood samples included attempts to find common in-field storage locations that could store and send the samples in batch as they accumulated. These efforts failed, however, as few lab entities offered such services, it was impossible to cover the geographical locations of the participants, and any available services were cost prohibitive. As a result, the study transitioned to the use of dry ice with individual blood sample shipments sent by the mobile phlebotomist after each study visit. The challenges associated with this approach included limited availability of dry ice in some geographical areas, human error in measuring the correct quantity of dry ice, and variability in the proximity of FedEx facilities that accept packages containing dry ice. Despite these challenges, this approach overall was superior to other options. In all, seven shipments arrived thawed, three due to human error (not enough dry ice) and four due to FedEx delays. However, an add-on protocol in which participants were asked to do a second blood draw if needed was used to redraw samples for five participants, leaving only two participants with ruined samples. Additional compensatory efforts were developed to use ‘extra’ dry ice and to avoid shipments around holiday times and bad weather.
A final main challenge, not unique to the current study, pertained to the COVID-19 pandemic. In mid-2020, following a university mandated 3-month discontinuation of all in-person research, the current study faced the decision of whether to resume in-person research. At this crossroads, the ‘self-administered’ study protocol was developed to offer participants an option to complete the study assessments that were able to be completed remotely and independently. As a part of this protocol, extensive work was put into the construction of custom shipping boxes to send supplies and equipment (eg, blood pressure monitor, flat scale) and the development of a website that housed videos and special instructions regarding the correct collection of each measure. Although this protocol, by definition, meant the blood and hair sample collections would be missing, it allowed the study to move forward with another assessment approach in its toolkit. Moreover, the ‘self-administered’ protocol remains broadly useful for all research conducted from a distance. Subsequently, additional add-on protocols were devised to collect the in-person data missed during this period.
Patient and public involvement
No patient or public involvement.
Findings to date
The sociodemographic characteristics of the sample are described in table 1. On average, the participants were 28.6 years of age (range: 26.2–31.3 years). With respect to ethnicity, 6.4% were Hispanic and 93.6% non-Hispanic, while the examination of race showed 14.9% belonged to historically marginalised groups, including 10.2% black, 1.1% Asian/Pacific Islander and 0.2% American Indian/Alaska Native (Eskimo, Aleutian) as well as 3.4% who were mixed race. Most participants (71.6%) reported being in a current romantic relationship and 26.1% had at least one child. Overall, the sample was well educated with 55.6% of participants, including 58.5% of women and 52.2% of men, having a college degree or greater. This compares to 40.0% in the population, according to 2019 US Census reports of educational attainment among individuals between 25 and 34 years.48 Notably, 13.6% of participants were current students, full or part-time. Of these, 69.5% were pursuing degrees at the college level or greater. If the anticipated degrees are obtained, the number of participants with a college degree or greater will grow to 59.6% of the full sample and 64.0% of women and 54.3% of men. In addition, 39.4% of participants reported an individual income of US$50 000 or greater and 30.1% of participants reported a household income of US$100 000 or greater. Only 2.9% of participants indicated that paying for basics such as food was ‘very’ or ‘extremely’ difficult and only 9.6% of participants were living below the poverty line. However, 38.3% of participants reported they would not be able to maintain their current standard of living for more than 2 months if they lost their income, reflecting some financial instability. In sum, inspection of the sociodemographic characteristics of the sample revealed an overall pattern of relative socioeconomic advantage among the participants on most parameters of education and income.
Table 1.
Description of sociodemographic characteristics in the full sample and in women and men separately
| Total* (n=705) N (%) or mean (SD), range |
Women* (n=378) N (%) or mean (SD), range |
Men* (n=327) N (%) or mean (SD), range |
|
| Age (in years) | 28.6 (1.2), 26.2–31.3 | 28.7 (1.2), 26.2–31.2 | 28.6 (1.2), 26.4–31.3 |
| Race/ethnicity | |||
| Hispanic | 45 (6.4%) | 19 (5.0%) | 26 (8.0%) |
| White, non-Hispanic | 555 (78.7%) | 303 (80.1%) | 252 (77.0%) |
| Black, non-Hispanic | 72 (10.2%) | 40 (10.6%) | 32 (9.8%) |
| Asian/PI, non-Hispanic | 8 (1.1%) | 4 (1.1%) | 4 (1.2%) |
| AI/AN, non-Hispanic | 1 (0.2%) | 0 (0.0%) | 1 (0.3%) |
| Mixed race, non-Hispanic | 24 (3.4%) | 12 (3.2%) | 12 (3.7%) |
| Family composition | |||
| People living in home | 2.7 (1.4), 1–10 | 2.8 (1.4), 1–10 | 2.5 (1.3), 1–7 |
| Married or living as married | 241 (34.4%) | 150 (39.7%) | 91 (28.3%) |
| Current romantic relationship | 501 (71.6%) | 295 (78.0%) | 206 (64.0%) |
| One or more children | 183 (26.1%) | 123 (32.5%) | 60 (18.6%) |
| Education | |||
| Less than HS diploma | 7 (1.0%) | 1 (0.3%) | 6 (1.9%) |
| HS diploma/GED | 88 (12.6%) | 41 (10.8%) | 47 (14.6%) |
| Some college, AA, certificate, trade | 216 (30.8%) | 115 (30.4%) | 101 (31.3%) |
| College degree or greater | 389 (55.6%) | 221 (58.5%) | 168 (52.2%) |
| Student status | |||
| Part-time | 34 (4.9%) | 24 (6.4%) | 10 (3.1%) |
| Full-time | 61 (8.7%) | 43 (11.4%) | 18 (5.6%) |
| Employment | |||
| Part-time, for pay | 85 (12.1%) | 59 (15.6%) | 26 (8.1%) |
| Full-time, for pay | 516 (73.7%) | 256 (67.7%) | 260 (80.7%) |
| Individual income | |||
| <US$10 000 | 78 (11.1%) | 48 (12.7%) | 30 (9.3%) |
| US$10 000–US$29 999 | 167 (23.9%) | 104 (27.5%) | 63 (19.6%) |
| US$30 000–US$49 999 | 179 (25.6%) | 89 (23.5%) | 90 (27.9%) |
| US$50 000–US$99 999 | 213 (30.4%) | 111 (29.4%) | 102 (31.7%) |
| US$100 000+ | 63 (9.0%) | 26 (6.9%) | 37 (11.5%) |
| Household income | |||
| <US$20 000 | 75 (10.8%) | 43 (11.4%) | 32 (10.0%) |
| US$20 000–US$49 999 | 163 (23.5%) | 90 (23.9%) | 63 (22.9%) |
| US$50 000–US$99 999 | 248 (35.7%) | 127 (33.8%) | 121 (37.9%) |
| US$100 000–US$149 999 | 126 (18.1%) | 74 (19.7%) | 52 (16.3%) |
| US$150 000+ | 83 (11.9%) | 42 (11.2%) | 41 (12.9%) |
| Financial disadvantage | |||
| Very/extreme difficulty paying for basics | 20 (2.9%) | 14 (3.7%) | 6 (1.9%) |
| <2-month safety net if lost income | 268 (38.3%) | 149 (39.6%) | 119 (37.1%) |
| Adjusted household income† | US$46 176 (US$36 509), US$1667–US$287 500 | US$43 312 (US$33 467), US$1667–US$162 500 | US$49 552 (US$39 586), US$2500–US$287 500 |
| Income-to-needs ratio† | 4.7 (3.5), 0.3–22.0 | 4.6 (3.5), 0.3–17.8 | 4.8 (3.5), 0.3–22.0 |
| Income below the poverty line | 67 (9.6%) | 38 (10.1%) | 29 (9.1%) |
| Income 1.0–1.9 times the poverty line | 95 (13.7%) | 62 (16.5%) | 33 (10.3%) |
| Income 2.0–2.9 times the poverty line | 86 (12.4%) | 42 (11.2%) | 44 (13.8%) |
| Income≥3 times the poverty line | 447 (64.3%) | 234 (62.2%) | 213 (66.8%) |
*Missing data: five participants did not complete the questionnaire items pertaining to family composition, education, employment and individual income. Seven participants did not complete the questionnaire items pertaining to student status. Ten participants did not complete the questionnaire items pertaining to household income.
†Definitions: adjusted household income is the total household income divided by the number of individuals identified as being dependent on the income. Income-to-needs ratio is the total household income divided by the US Census poverty threshold for the number of individuals identified as being dependent on the income without respect to their relation to one another.
PI, Pacific Islander; AI, American Indian; AN, Alaska Native; HS, high school; GED, general equivalency diploma; AA, Associates degree.
Information pertaining to the cardiometabolic health status of the sample is described in table 2. Each health status indicator is first presented as a continuous variable and then as a categorical variable, coded according to established clinical guidelines. On average, the participants were overweight (mean body mass index (BMI)=27.8) with 52.7% of women and 63.4% of men in overweight/obese categories. Within the obese category, 5.9% of women and 6.4% of men were considered class III or severely obese. Compared with national estimates in similar-age groups (20–39 years), the percent of obese participants in the current study (29.8%) was comparable to the percent obese in the National Health Interview Survey (ie, 28.5%)49 but was lower than in the National Health and Nutrition Examination Survey (NHANES) (ie, 39.8%).50 In addition, in line with the distribution of BMI, 53.0% were in the high/very high range for waist circumference, reflecting significant central adiposity in the sample. With respect to blood pressure, 23.6% of women and 35.9% of men were hypertensive according to the American College of Cardiology and American Heart Association guidelines (systolic blood pressure≥130 mm Hg or diastolic blood pressure≥80 mm Hg), exceeding national estimates reported in NHANES (ie, 13.0% in women and 31.2% in men) among individuals 18–39 years.51 Moreover, a substantial number of women, 6.4% and 29.0%, were in the prediabetic (A1c 5.7%–6.4%) and diabetic (A1c>6.4%) ranges, respectively, for haemoglobin A1c as were 7.0% and 22.1% of the men, respectively. As with hypertension, these numbers exceed national estimates reported in NHANES (ie, 13% diabetic) among individuals 18 years of age or older.52 In sum, inspection of the health status indicators in the sample revealed a distinct pattern of poor cardiometabolic health with a sizeable proportion of the sample displaying values in clinically meaningful risk ranges, especially in areas of obesity, hypertension and diabetes.
Table 2.
Description of health status indicators in the full sample and in women and men separately
| Total* (n=705) N (%) or mean (SD), range |
Women* (n=378) N (%) or mean (SD), range |
Men* (n=327) N (%) or mean (SD), range |
|
| Body mass index (BMI) | |||
| BMI (kg/m2) | 27.8 (7.1), 16.7–65.9 | 27.5 (7.2), 16.7–59.3 | 28.2 (6.9), 16.8–65.9 |
| Underweight, <18.5 | 13 (1.9%) | 9 (2.5%) | 4 (1.3%) |
| Normal, 18.5–24.9 | 268 (40.4%) | 158 (44.8%) | 110 (35.3%) |
| Overweight, 25.0–29.9 | 185 (27.9%) | 79 (22.4%) | 106 (34.1%) |
| Obese, >30.0 | 198 (29.8%) | 107 (30.3%) | 91 (29.3%) |
| Class I obesity, 30.0–34.9 | 101 (15.2%) | 56 (15.9%) | 45 (14.5%) |
| Class II obesity, 35.0–39.9 | 56 (8.4%) | 30 (8.5%) | 26 (8.4%) |
| Class III obesity, 40.0+ | 41 (6.2%) | 21 (5.9%) | 20 (6.4%) |
| Waist circumference (WC) | |||
| WC (cm) | 92.2 (17.8), 62.2–180.0 | 87.9 (16.4), 62.2–149.4 | 97.1 (18.2), 64.6–180.0 |
| High: 80–88 cm, women; 94–102 cm, men | 115 (17.7%) | 60 (17.3%) | 55 (18.2%) |
| Very high: >88 cm, women; >102 cm, men | 229 (35.3%) | 146 (42.2%) | 83 (27.4%) |
| Blood pressure (BP) | |||
| Systolic BP (SBP) (mm Hg) | 115.2 (12.9), 83.7–167.3 | 109.5 (11.0), 83.7–153.3 | 121.6 (11.8), 84.7–167.3 |
| Diastolic BP (DBP) (mm Hg) | 73.1 (10.2), 45.7–104.0 | 72.4 (10.1), 48.7–99.3 | 73.9 (10.2), 45.7–104.0 |
| SBP≥130 mm Hg or DBP≥80 mm Hg | 190 (29.4%) | 81 (23.6%) | 109 (35.9%) |
| Total cholesterol | |||
| Total cholesterol (mg/dL) | 169.1 (33.7), 83–296 | 167.4 (31.7), 85–289 | 171.1 (35.8), 83–296 |
| Total cholesterol, ≥200 mg/dL | 94 (17.8%) | 44 (15.5%) | 50 (20.5%) |
| High-density lipoprotein (HDL) | |||
| HDL (mg/dL) | 53.9 (13.3), 24–106 | 57.2 (12.6), 29–96 | 50.1 (13.1), 24–106 |
| HDL<50 mg/dL, women; <40 mg/dL, men | 131 (24.9%) | 78 (27.6%) | 53 (21.7%) |
| Low-density lipoprotein (LDL) | |||
| LDL (mg/dL) | 97.5 (29.5), 23–223 | 93.6 (27.4), 23–192 | 102.1 (31.2), 26–224 |
| LDL≥130 mg/dL | 70 (13.3%) | 26 (9.2%) | 44 (18.0%) |
| Fasting triglycerides | |||
| Triglycerides (mg/dL) | 88.7 (55.8), 27–538 | 83.4 (47.4), 27–393 | 94.8 (63.6), 29–538 |
| Triglycerides, ≥150 mg/dL | 48 (9.1%) | 21 (7.4%) | 27 (11.1%) |
| Fasting glucose | |||
| Glucose (mg/dL) | 91.5 (18.9), 63–293 | 89.5 (18.1), 63–293 | 93.7 (19.5), 66–273 |
| Glucose≥100 mg/dL | 41 (7.8%) | 12 (4.2%) | 29 (11.9%) |
| Fasting insulin | |||
| Insulin (μIU/mL) | 10.0 (8.3), 0.1–70.2 | 10.2 (8.0), 0.6–67.6 | 9.8 (8.6), 0.1–70.2 |
| Insulin≥20 μIU/mL | 43 (8.2%) | 24 (8.5%) | 19 (7.8%) |
| Haemoglobin (Hb) A1c | |||
| HbA1c (%) | 5.3 (2.3), 0.6–23.3 | 5.3 (2.2), 0.6–17.1 | 5.2 (2.4), 1.3–23.3 |
| HbA1c normal, <5.7% | 355 (67.4%) | 182 (64.3%) | 173 (70.9%) |
| HbA1c pre-diabetes, 5.7%–6.4% | 36 (6.8%) | 19 (6.7%) | 17 (7.0%) |
| HbA1c diabetes, >6.4% | 136 (25.8%) | 82 (29.0%) | 54 (22.1%) |
| C reactive protein (CRP) | |||
| CRP (mg/L) | 4.5 (4.4), 0.01–21.2 | 5.0 (4.6), 0.01–21.2 | 4.0 (4.0), 0.01–17.8 |
| CRP≥10 mg/L | 65 (12.3%) | 39 (13.8%) | 26 (10.7%) |
*Missing data: a total of 41 participants do not have anthropometric data, 95.1% because they participated in a protocol that did not collect these data and 4.9% for a miscellaneous reason. A total of 58 participants do not have blood pressure data, 94.8% because they participated in a protocol that did not collect these data and 5.2% for a miscellaneous reason. A total of 178 participants do not have blood samples, 86.0% because they participated in a protocol that did not collect these data and 14.0% for a miscellaneous reason (eg, refused the blood draw, sample thawed in transit). Sources of data: for BMI, 2.3% of values were derived from self-reported height and weight in the ‘partial’ study protocol and 14.9% of values were derived from measurements taken in the ‘self-administered’ study protocol. For WC and BP, 15.3% of values were derived from measurements taken in the ‘self-administered’ protocol. Clinical guidelines: clinical guidelines were used to code the health status indicators according to the Centers for Disease Control and Prevention (CDC) for BMI, fasting glucose, fasting insulin and HbA1c; British Heart Foundation for WC; American College of Cardiology and American Heart Association (AHA) for BP; AHA for total cholesterol, HDL, LDL and fasting triglycerides; and CDC/AHA for CRP.
Information pertaining to relevant health behaviours that may account for the health status of the sample is described in table 3. With respect to cigarette smoking, 27.5% of participants identified as current or past smokers. The number of current smokers (14.9%) was comparable to national estimates (ie, 14.1%) among similar-age individuals (25–44 years) as was the pattern of smoking between women and men (13.0% vs 17.1.%, respectively) with men more likely to smoke.53 Based on 24-hour diet recalls, the HEI-2015,39 a marker of diet quality reflecting the degree of alignment with dietary guidelines, was low (mean HEI-2015=50.2) as compared with an ideal score of 100, indicating complete alignment with dietary guidelines. This value was also lower than national estimates (ie, 53 between 19 and 30 years and 58 between 31 and 59 years), but in line with the poor diets of Americans in general.39 In parallel, intake of fruits and vegetables was low with only 6.9% and 17.9%, respectively, meeting the daily recommendation for intake in these food groups. This is also in line with the low intake of fruits (ie, 12.3%) and vegetables (ie, 10.0%) in the US population.54 Finally, using actigraphy, patterns of activity and sleep were examined. On average, the time engaged in moderate, vigorous or very vigorous activity was 1.3 hours/day while sedentary time was 4.5 hours/day with only 15.9% of participants walking 10 000+ steps per day. On average, the participants slept 7.3 hours/night, 38.6% slept less than the recommended 7–9 hours of sleep/night and 41.0% had sleep efficiency scores<85%, indicating disrupted sleep. Moreover, the global PSQI41 showed 45.6% had a score of 6 or greater, reflecting significant sleep problems. In sum, inspection of the health behaviour indicators in the sample revealed a general pattern of behaviours related to poor dietary habits, low levels of activity and disrupted sleep which tracks and may explain the poor health status of the sample on parameters of cardiometabolic risk.
Table 3.
Description of health behaviour indicators in the full sample and in women and men separately
| Total* (n=705) N (%) or mean (SD), range |
Women* (n=378) N (%) or mean (SD), range |
Men* (n=327) N (%) or mean (SD), range |
|
| Smoking | |||
| Current | 104 (14.9%) | 49 (13.0%) | 55 (17.1%) |
| Current cigarettes/day | 7.5 (6.3), <1–20 | 7.1 (5.5), <1–20 | 7.9 (7.0), <1–20 |
| Past | 88 (12.6%) | 25 (6.6%) | 63 (19.5%) |
| Past cigarettes/day | 7.9 (8.3), <1–45 | 5.8 (4.9), <1–15 | 8.7 (9.2), <1–45 |
| Age last quit (in years) | 24.6 (3.2), 12–29 | 25.0 (3.2), 19–29 | 24.5 (3.2), 12–29 |
| Current/past | 192 (27.5%) | 74 (19.6%) | 118 (36.6%) |
| Never | 507 (72.5%) | 303 (80.4%) | 204 (63.4%) |
| 24-hour diet recall | |||
| Health Eating Index-2015 | 50.2 (11.0), 22.4–86.7 | 51.3 (10.9), 22.7–86.7 | 49.0 (11.0), 22.4–84.3 |
| Vegetable, cup equivalents | 1.8 (1.1), 0.0–6.8 | 1.8 (1.0), 0.0–6.8 | 1.9 (1.1), 0.2–5.7 |
| Fruit, cup equivalents | 0.7 (0.8), 0.0–10.5 | 0.7 (0.7), 0.0–4.3 | 0.7 (1.0), 0.0–10.5 |
| Vegetable, meets daily guideline† | 119 (17.9%) | 74 (21.0%) | 45 (14.5%) |
| Fruit, meets daily guideline† | 46 (6.9%) | 24 (6.8%) | 22 (7.1%) |
| Actigraphy, activity level† | |||
| Very vigorous activity (min/day) | 2.1 (4.7), 0.0–35.9 | 2.0 (4.2), 0.0–33.0 | 2.2 (5.2), 0.0–35.9 |
| Vigorous activity (min/day) | 8.9 (16.7), 0.0–177.5 | 8.7 (15.9), 0.0–117.0 | 9.1 (17.7), 0.0–177.5 |
| Moderate activity (min/day) | 65.0 (44.0), 1.8–333.2 | 61.5 (42.0), 8.7–308.3 | 69.3 (46.0), 1.8–333.2 |
| Number of steps per day | 7368.9 (2,782.8), 1233.9–16 330.6 |
7189.7 (2,546.6), 1692.4–16 330.6 |
7579.8 (3,028.7), 1233.9–15 656.7 |
| Number of steps 10 000+ | 92 (15.9%) | 38 (12.1%) | 54 (20.3%) |
| Sedentary time (min/day) | 271.2 (108.6), 23.7–609.3 | 252.8 (98.5), 31.3–609.3 | 292.8 (115.9), 23.7–604.5 |
| Actigraphy, sleep† | |||
| Sleep efficiency† | 85.0 (6.1), 52.8–97.3 | 85.2 (5.6), 61.1–97.3 | 84.7 (6.7), 52.8–97.1 |
| Sleep efficiency<85% | 238 (41.0%) | 123 (38.8%) | 115 (43.6%) |
| Total sleep time (hours) | 7.3 (1.0), 4.0–11.7 | 7.5 (1.0), 4.5–11.7 | 7.0 (1.0), 4.0–10.2 |
| Sleep<7 hours | 224 (38.6%) | 94 (29.7%) | 130 (49.2%) |
| Number of awakenings | 19.8 (7.4), 2.2–48.3 | 19.7 (7.2), 2.2–47.0 | 19.8 (7.7), 3.0–48.3 |
| Average awakening length (min) | 3.7 (1.4), 1.3–13.4 | 3.7 (1.2), 1.3–8.5 | 3.7 (1.6), 1.6–13.4 |
| Sleep fragmentation† | 30.1 (8.9), 7.0–69.8 | 29.1 (7.7), 9.8–59.1 | 31.3 (10.1), 7.0–69.8 |
| Self-report, sleep | |||
| PSQI Global Sleep Quality Index | 5.8 (3.3), 0–18 | 5.9 (3.5), 0–18 | 5.7 (3.1), 0–16 |
| PSQI Global Sleep Quality Index≥6 | 319 (45.6%) | 180 (47.6%) | 139 (43.2%) |
*Missing data: six participants did not complete the questionnaire items pertaining to smoking or sleep. A total of 41 participants do not have diet data, 95.1% because they participated in a protocol that did not collect these data and 4.9% for a miscellaneous reason. A total of 124 participants do not have actigraphy data, 31.5% because they participated in a protocol that did not collect these data and 68.5% for a miscellaneous reason (eg, did not wear monitor for sufficient length of time).
†Definitions: for vegetables, the daily guideline of 2.5 cups was used for women based on a 2000 calorie diet and the daily guideline of 3.0 cups was used for men based on a 2400 calorie diet. For fruit, the daily guideline of 2.0 cups of fruit was used for both women and men as the recommendation for fruit does not differ between 2000 and 2400 calorie diets. For actigraphy for both activity and sleep indicators, a minimum wear time of 2 days and nights was required. Sleep efficiency is the number of minutes asleep divided by the number of minutes in bed. Sleep fragmentation is an index of restlessness during sleep derived from movement.
PSQI, Pittsburgh Sleep Quality Index.
In summary, in descriptive analyses, findings to date revealed that the sample was well educated and growing in their educational attainment as 13.6% were current students. Despite this, the sample showed considerable risk on health status indicators, especially related to obesity, hypertension and diabetes. Of particular concern, the prevalence of hypertension and pre-diabetes and diabetes exceeded national estimates in similar-age individuals. The examination of health behaviour indicators generally tracked with the parameters of poor health status, showing a pattern of poor diet, low activity and disrupted sleep. The juxtaposition of the sample’s relatively young age (26–31 years) and high educational status (55.6% college educated or greater) with its poor health status may suggest a dissociation between health and factors that are typically health protective. This is consistent with observed population health trends, which show a worsening of cardiometabolic health status in younger generations of Americans, especially among Millennials,55 the generation to which the current sample (born in 1991) belongs.
Strengths and limitations
A primary strength of the current study, SHINE, was its leveraging of the original NICHD SECCYD to extend and maximise the value of this longitudinal birth cohort. The addition of adulthood measures of health allows innumerable opportunities for the pursuit of life course research relating early life environments to adulthood health and disease risk. Additional strengths include the gold standard methods that were used for the measurement of each health status and health behaviour indicator. Extensive recruitment methods were also used to engage participants living in different locations throughout the USA and adaptations to the study procedures were developed (eg, ‘self-administered’ study protocol) to allow flexibility with participation. Taken together, these approaches balanced the standards of high-quality research with the imperative to reach participants in distant locations and to reduce barriers to participation, including during the challenging times of the COVID-19 pandemic.
There were also several limitations in the current study. Of the 1364 families that participated in the original NICHD SECCYD, only 927 (68.0%) adult children were available for inclusion in the current study based on their prior consent to be recontacted, as well as 5 having died. Of this number, 705 (76.1%) participated in the current SHINE study. Analyses showed retention in SHINE was predicted by higher maternal education at birth (b=0.152, p<0.001), but not income-to-needs ratio at birth (b=−0.007, p=0.779), with a 16% increase in the odds of retention among participants with more highly educated mothers. This pattern of greater educational attainment was also observed among the now adult children and will need to be considered when interpreting future study findings. Another pattern observed in the current study was that more women than men participated despite efforts to target men specifically. In addition, the nature of the study required that data collection teams work in the field to implement the study protocols. In this context, the numerous staff persons, distance from the participants, and varied data collection environments made oversight by the UW team an on-going challenge. A related issue concerns the remote protocols that by definition were administered with less oversight by the UW team. However, as described above, numerous training and quality assurance measures were implemented to ensure fidelity across all the study protocols. Finally, the data collection for the study occurred between 2018 and 2022, overlapping with the height of the COVID-19 pandemic. In addition to the challenges of conducting in-person research during the pandemic, the pandemic itself may have had differential impacts on participants who participated during this period and should also be considered when interpreting future study findings.
Future plans
The current study lays the groundwork for future analyses relating early life environments to adulthood health and disease risk. Within this broad framework, two specific areas of inquiry will be pursued initially. First, building on a large literature describing the graded relationship between socioeconomic status and health,56–59 an in-depth examination is planned to delineate the specific features of educational attainment that are health protective. This objective stems from a National Institutes of Health (NIH) initiative to support research that ‘further elucidates the pathways involved in the relationship between education and health outcomes and to identify the specific aspects and qualities of education that are responsible for this relationship’.60 The current study is well positioned to contribute to this area by testing links between key aspects of education in early life, such as childhood academic skills and classroom experiences, and childhood health concurrently as well as adulthood health prospectively. In addition, this work will consider the potential moderating role of education in offsetting early childhood adversity experiences as well as other contributing factors such as high-quality childcare, parental education and child intelligence and temperament.
Second, building on a large literature examining early life adversity exposures and poor health,9–14 an in-depth examination is planned that focuses on the potential mediating role of growth and pubertal development trajectories in accounting for early life adversity effects on adulthood cardiometabolic health.61–63 This objective also stems from an NIH initiative to support research that identifies specific vulnerability factors and mechanisms by which early life adversity exposures transmit risk for poor health.64 The current study is well positioned to contribute to this area by testing empirically the mechanistic role of pubertal development in a single longitudinal data set, thereby integrating previously separate literatures (1) relating early life adversity to earlier and faster rates of pubertal maturation65–69 and (2) relating earlier pubertal maturation to poor cardiometabolic outcomes.70–75 This work will also consider concurrent trajectories of prepubertal weight gain, relevant health behaviours and resilience factors. For both main areas of inquiry, the many strengths of the original NICHD SECCYD and recent SHINE data collection will allow testing of these life course models with adequate accounting of covariates and alternative explanatory factors and will overcome common challenges present in these literatures, including long latency periods between the exposures and outcomes of interest as well as poor integration of relevant developmental and epidemiologic approaches.
Context
The original NICHD SECCYD and recent SHINE data collection may be placed in the larger landscape of cohort studies around the globe. Great Britain initiated the first National Birth Cohort studies (1946, 1958 and 1970) followed more recently by the Avon Longitudinal Study of Parents and Children (1991) and the United Kingdom Millennium Cohort Study (2000).76 In the USA, the National Longitudinal Surveys (1979, 1986 and 1997) and the Early Childhood Longitudinal Study (1998) were launched later as were efforts such as the Minnesota Twin Family Study (1989) and the Adolescent Brain and Cognitive Development Study (2015). Other notable cohort studies include the Dunedin Multidisciplinary Health and Development Study (Dunedin Study, 1979) in New Zealand and the Mater-University of Queensland Study of Pregnancy in Australia (1981).
Each of these studies, unique in time, place and scope, reflects the value of the longitudinal cohort design in which causal inferences may be drawn between exposures and their impacts in areas of child health and development. On the other hand, common challenges emerge, including problems with selective attrition and sample representativeness, the maintenance of long-term funding and the accommodation of new lines of research into the existing study.76 In context, the NICHD SECCYD/SHINE follow-up is generally smaller in size compared with other cohorts and even at its inception was not population-based. Rather, recruitment parameters ensured participants represented the geographies of their respective locations including across urban and rural settings. Additionally, problems with attrition have been experienced. In contrast, relative strengths of the NICHD SECCYD/SHINE follow-up include its depth of measurement, which is unique compared with other cohorts, including, for example, multimethod assessments of attachment, Tanner staging of pubertal development and the current gold standard measures of health status and health behaviours.
Collaboration
Data and materials from the NICHD SECCYD are available online77 (icpsr.umich.edu/web/ICPSR/series/233). Researchers interested in working with the team of investigators who led the SHINE follow-up data collection are invited to contact MEB and GIR. Potential collaborative efforts will be considered under specific conditions, including, but not limited to, the proposed scope of work and assurances related to data security and integrity.
Conclusions
The landmark NICHD SECCYD, as described above, is a unique resource that has supported research in diverse areas of child health and well-being since its inception in 1991. With the addition of the follow-up data collection—SHINE, through which the health status of the now adult participants has been characterised, new opportunities to test life course models linking early life environments to adulthood health and disease risk have emerged. These opportunities are timely given the wealth of evidence suggesting the origins of adulthood health begin in childhood as well as the growing imperative to move toward prevention focused efforts to reverse worsening US population health trends. The initial examination of these newly available data reveals a distinct pattern of poor health, especially relating to obesity, hypertension and diabetes. This pattern was observed despite the relatively young age and high educational status of the sample but is consistent with findings suggesting the health of younger generations of Americans is worsening, as evidenced by comparisons to the health of their same-age counterparts from older generations. With the adulthood health measures now in place, the next steps for this work will entail leveraging the uniquely robust measures collected as a part of the original NICHD SECCYD to pinpoint specific early life risk and resilience factors as well as the correlates and potential mechanisms accounting for variability in trajectories of health and disease risk in the period of young adulthood. In addition, the work of the current study is discussed with an emphasis on lessons that were learnt conducting in-person, health focused research among participants living in distant locations throughout the USA and in a period overlapping with the COVID-19 pandemic.
Supplementary Material
Acknowledgments
We would like to recognise the important contributions of the large investigative team dedicated to the high-quality data collection of the Study of Early Child Care and Youth Development follow-up, Study of Health in Early and Adult Life. In addition to the lead investigators and coinvestigators identified as coauthors, the internal UW research team included Rebecca Christopfel, Madalyn Osbourne, Hailey Hagins and Linda Nzabamwita. The in-field data collectors and phlebotomists included Damara Acosta, Taylor Haase, Connie Showalter, Mary Koller, Khamia Powell, Rochelle Eskridge, Megan Buss, Kay Harrington, Amanda O’Brien, Angie Mondestin, Leah Welker, Karen Nowden, Emily Goodrum, Tracey Pinkney, Libby Shook, Kelsey Smith, Danielle Heusted and Kristen Roynesdal. The laboratory work was conducted by the UW Research Testing Services; the UW School of Nursing Biobehavioral Lab Testing and Education Core (Ernie Tolentino); the UVA Center for Research in Reproduction Ligand Assay and Analysis Core, supported by NIH/NICHD R24 HD102061; and the UW Center for Studies in Demography & Ecology (Eleanor Brindle, Melanie Martin, Carmen Hove and Maia Kent), supported by NIH/NICHD P2C HD042828. Questionnaire administration was conducted using REDCap, supported by the UW Institute of Translational Health Sciences, NIH/NCATS UL1 TR002319. Additional support was provided to George Slavich (#OPR21101) from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine.
Footnotes
Twitter: @uclastresslab
Contributors: MEB is responsible for the overall content as guarantor. MEB led the conceptualisation and writing of this manuscript with assistance with data cleaning and analysis from AST and WSY. MEB, GIR, SEG, BMA, RAH, RCP, ALM, GMS, AST, WSY, CB-L participated in the execution of the Study of Health in Early and Adult Life data collection and all authors collaborated on the conceptualisation, writing and critical review of this manuscript.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10HD025447, R01HD091132) and the National Heart, Lung, and Blood Institute (R01HL130103) at the National Institutes of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. Data and materials from the NICHD SECCYD are available online: icpsr.umich.edu/web/ICPSR/series/233. Researchers interested in working with the team of investigators who led the SHINE follow-up data collection are invited to contact MEB and GIR. Potential collaborative efforts will be considered under specific conditions, including, but not limited to, the proposed scope of work and assurances related to data security and integrity.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. Informed consent for the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development follow-up study, Study of Health in Early and Adult Life, was obtained from the target participants. The research was approved by the Human Subjects Division of the University of Washington (STUDY00001821). Participants gave informed consent to participate in the study before taking part.
References
- 1.NICHD Early Child Care Research Network . Child care and child development: results from the NICHD study of early child care and youth development. 2005: Guilford Press, [Google Scholar]
- 2.NICHD Early Child Care Research Network . The effects of infant child care on infant-mother attachment security: results of the NICHD study of early child care NICHD early child care research network. Child Dev 1997;68:860–79. 10.1111/j.1467-8624.1997.tb01967.x [DOI] [PubMed] [Google Scholar]
- 3.NICHD Early Child Care Research Network . Early child care and children’s development prior to school entry: results from the NICHD study of early child care. American Educational Research Journal 2002;39:133–64. 10.3102/00028312039001133 [DOI] [Google Scholar]
- 4.NICHD Early Child Care Research Network . Early child care and children’s development in the primary grades: follow-up results from the NICHD study of early child care. American Educational Research Journal 2005;42:537–70. 10.3102/00028312042003537 [DOI] [Google Scholar]
- 5.Belsky J, Vandell DL, Burchinal M, et al. Are there long-term effects of early child care? Child Dev 2007;78:681–701. 10.1111/j.1467-8624.2007.01021.x [DOI] [PubMed] [Google Scholar]
- 6.Vandell DL, Belsky J, Burchinal M, et al. Do effects of early child care extend to age 15 years? results from the NICHD study of early child care and youth development. Child Dev 2010;81:737–56. 10.1111/j.1467-8624.2010.01431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan O, Buisman RSM, Nivison MD, et al. Does secure base script knowledge mediate associations between observed parental caregiving during childhood and adult romantic relationship quality and health? Attach Hum Dev 2021;23:643–64. 10.1080/14616734.2020.1836858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunkel JJ, Magro SW, Bleil ME, et al. Early maternal sensitivity and markers of physical health: enduring or transient associations from childhood to adulthood? Dev Psychol 2022;58:2252–63. 10.1037/dev0001430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suglia SF, Koenen KC, Boynton-Jarrett R, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation 2018;137:e15–28. 10.1161/CIR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suglia SF, Campo RA, Brown AGM, et al. Social determinants of cardiovascular health: early life adversity as a contributor to disparities in cardiovascular diseases. J Pediatr 2020;219:267–73. 10.1016/j.jpeds.2019.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suglia SF, Appleton AA, Bleil ME, et al. Timing, duration, and differential susceptibility to early life adversities and cardiovascular disease risk across the lifespan: implications for future research. Prev Med 2021;153:106736. 10.1016/j.ypmed.2021.106736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berens AE, Jensen SKG, Nelson CA. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med 2017;15:135. 10.1186/s12916-017-0895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of Child and Family Health, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232–46. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- 14.Su S, Jimenez MP, Roberts CTF, et al. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep 2015;17:88. 10.1007/s11886-015-0645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev 2004;26:7–21. 10.1093/epirev/mxh008 [DOI] [PubMed] [Google Scholar]
- 16.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? update of a systematic review. J Epidemiol Community Health 2008;62:387–90. 10.1136/jech.2007.065508 [DOI] [PubMed] [Google Scholar]
- 17.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol 2006;16:91–104. 10.1016/j.annepidem.2005.06.053 [DOI] [PubMed] [Google Scholar]
- 18.Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obes Rev 2011;12:e159–72. 10.1111/j.1467-789X.2010.00823.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Midei AJ, Matthews KA, Chang Y-F, et al. Childhood physical abuse is associated with incident metabolic syndrome in mid-life women. Health Psychol 2013;32:121–7. 10.1037/a0027891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med 2009;71:805–12. 10.1097/PSY.0b013e3181bb2b46 [DOI] [PubMed] [Google Scholar]
- 21.Johnson WF, Huelsnitz CO, Carlson EA, et al. Childhood abuse and neglect and physical health at midlife: prospective, longitudinal evidence. Dev Psychopathol 2017;29:1935–46. 10.1017/S095457941700150X [DOI] [PubMed] [Google Scholar]
- 22.Farrell AK, Waters TEA, Young ES, et al. Early maternal sensitivity, attachment security in young adulthood, and cardiometabolic risk at midlife. Attach Hum Dev 2019;21:70–86. 10.1080/14616734.2018.1541517 [DOI] [PubMed] [Google Scholar]
- 23.Young ES, Farrell AK, Carlson EA, et al. The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: a prospective study. Psychol Sci 2019;30:739–47. 10.1177/0956797619833664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young ES, Doom JR, Farrell AK, et al. Life stress and cortisol reactivity: an exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Dev Psychopathol 2021;33:301–12. 10.1017/S0954579419001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson GF, Hurst J, Hussey PS, et al. Health spending and outcomes: trends in OECD countries, 1960-1998. Health Aff (Millwood) 2000;19:150–7. 10.1377/hlthaff.19.3.150 [DOI] [PubMed] [Google Scholar]
- 26.Anderson GF, Reinhardt UE, Hussey PS, et al. It’s the prices, stupid: why the United States is so different from other countries. Health Aff (Millwood) 2003;22:89–105. 10.1377/hlthaff.22.3.89 [DOI] [PubMed] [Google Scholar]
- 27.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018;319:1024–39. 10.1001/jama.2018.1150 [DOI] [PubMed] [Google Scholar]
- 28.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The nurses’ health study. Am J Epidemiol 1997;145:614–9. 10.1093/oxfordjournals.aje.a009158 [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk. Arch Intern Med 2002;162:2074. 10.1001/archinte.162.18.2074 [DOI] [PubMed] [Google Scholar]
- 30.Lapidus L, Bengtsson C, Larsson B, et al. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–61. 10.1136/bmj.289.6454.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieleman JL, Baral R, Birger M, et al. Us spending on personal health care and public health, 1996-2013. JAMA 2016;316:2627–46. 10.1001/jama.2016.16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin AB, Hartman M, Washington B, et al. National health spending: faster growth in 2015 as coverage expands and utilization increases. Health Aff (Millwood) 2017;36:166–76. 10.1377/hlthaff.2016.1330 [DOI] [PubMed] [Google Scholar]
- 33.Booth-LaForce C, Groh AM, Burchinal MR, et al. V. caregiving and contextual sources of continuity and change in attachment security from infancy to late adolescence. Monogr Soc Res Child Dev 2014;79:67–84. 10.1111/mono.12114 [DOI] [PubMed] [Google Scholar]
- 34.Ansari A, Hofkens TL, Pianta RC. Absenteeism in the first decade of education forecasts civic engagement and educational and socioeconomic prospects in young adulthood. J Youth Adolesc 2020;49:1835–48. 10.1007/s10964-020-01272-4 [DOI] [PubMed] [Google Scholar]
- 35.Nivison MD, Vandell DL, Booth-LaForce C, et al. Convergent and discriminant validity of retrospective assessments of the quality of childhood parenting: prospective evidence from infancy to age 26 years. Psychol Sci 2021;32:721–34. 10.1177/0956797620975775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bustamante AS, Dearing E, Zachrisson HD, et al. Adult outcomes of sustained high-quality early child care and education: do they vary by family income? Child Dev 2022;93:502–23. 10.1111/cdev.13696 [DOI] [PubMed] [Google Scholar]
- 37.University of Washington Policies Procedures and Guidance . Guidance on human subjects regulations. 2022. Available: www.washington.edu/research/policies/guidance-human-subjects-regulations-2/
- 38.National Cancer Institute . Division of Cancer Control & Population Sciences, Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool, Available: https://epi.grants.cancer.gov/asa24
- 39.US Department of Agriculture . Food and Nutrition Service, Healthy Eating Index, Available: www.fns.usda.gov/healthy-eating-index-hei
- 40.National Cancer Institute . The dietary screener questionnaire (DSQ) in the NHANES 2009-10: data processing and scoring procedure for earlier methods. epidemiology and genomics research program; Available: https://epi.grants.cancer.gov/diet/screeners/files [Accessed 2 Apr 2019].
- 41.Buysse DJ, Reynolds CF, Monk TH, et al. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 43.Slavich GM, Shields GS. Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult STRAIN): an overview and initial validation. Psychosom Med 2018;80:17–27. 10.1097/PSY.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977:385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 45.Achenbach TM, Rescorla LA. Manual for the ASEBA adult forms & profiles. Burlington, VT, USA: University of Vermont: Research Center for Children, Youth, & Families, 2003. [Google Scholar]
- 46.Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. Journal of Marriage and the Family 1976;38:15. 10.2307/350547 [DOI] [Google Scholar]
- 47.Fraley RC, Heffernan ME, Vicary AM, et al. The experiences in close relationships-relationship structures questionnaire: a method for assessing attachment orientations across relationships. Psychol Assess 2011;23:615–25. 10.1037/a0022898 [DOI] [PubMed] [Google Scholar]
- 48.U.S. Census Educational attainment in the united states table 3. detailed years of school completed by people 25 years and over by sex, age groups, race and hispanic origin. 2019. Available: www.census.gov/content/census/en/data/tables/2019/demo/educational-attainment/cps-detailed-tables.html
- 49.National Center for Health Statistics, National Health Interview Survey . Prevalence of obesity among adults aged 20 and over, by age group and sex: United States,. 2018Available: https://public.tableau.com/app/profile/nhis/viz/FIGURE6_2/Dashboard6_2
- 50.S. National Center for Health . National health and nutrition examination survey 2017–march 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes. Hyattsville, MD, 2021. 10.15620/cdc:106273 [DOI] [Google Scholar]
- 51.National Center for Health Statistics . National Health and Nutrition Examination Survey,. 2017Available: www.cdc.gov/nchs/about/factsheets/factsheet_nhanes.htm
- 52.Centers for Disease Control and Prevention . National diabetes statistics report. Atlanta, GA, Available: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [Google Scholar]
- 53.Cornelius ME, Loretan CG, Wang TW, et al. Tobacco product use among adults - united states, 2020. MMWR Morb Mortal Wkly Rep 2022;71:397–405. 10.15585/mmwr.mm7111a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations - united states, 2019. MMWR Morb Mortal Wkly Rep 2022;71:1–9. 10.15585/mmwr.mm7101a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.BlueCross BlueShield The Health Of America, The Health of Millennials . A focus on millennial health trends. n.d. Available: www.bcbs.com/sites/default/files/file-attachments/health-of-america-report/HOA-Millennial_Health_0.pdf
- 56.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol 1994;49:15–24. 10.1037//0003-066x.49.1.15 [DOI] [PubMed] [Google Scholar]
- 57.Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci 2003;12:119–23. 10.1111/1467-8721.01245 [DOI] [Google Scholar]
- 58.Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099–104. 10.1016/S0140-6736(05)71146-6 [DOI] [PubMed] [Google Scholar]
- 59.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991;337:1387–93. 10.1016/0140-6736(91)93068-k [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health (NIH) . Pathways Linking Education to Health, Available: https://grants.nih.gov/grants/guide/rfa-files/RFA-OB-03-001.html
- 61.Bleil ME, Adler NE, Appelhans BM, et al. Childhood adversity and pubertal timing: understanding the origins of adulthood cardiovascular risk. Biol Psychol 2013;93:213–9. 10.1016/j.biopsycho.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bleil ME, Appelhans BM, Gregorich SE, et al. Patterns of early life weight gain and female onset of puberty. J Endocr Soc 2021;5:bvab165. 10.1210/jendso/bvab165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bleil ME, Spieker SJ, Gregorich SE, et al. Early life adversity and pubertal timing: implications for cardiometabolic health. J Pediatr Psychol 2021;46:36–48. 10.1093/jpepsy/jsaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Institutes of Health (NIH) . Pathways and Mechanisms Linking Early Life Adversities (ELAs) and Adulthood Health Outcomes, Available: www.nia.nih.gov/research/dbsr/pathways-and-mechanisms-linking-early-life-adversities-elas-and-adult-health-outcomes
- 65.Hamlat EJ, Laraia B, Bleil ME, et al. Effects of early life adversity on pubertal timing and tempo in black and white girls: the National growth and health study. Psychosom Med 2022;84:297–305. 10.1097/PSY.0000000000001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belsky J, Houts RM, Fearon RMP. Infant attachment security and the timing of puberty: testing an evolutionary hypothesis. Psychol Sci 2010;21:1195–201. 10.1177/0956797610379867 [DOI] [PubMed] [Google Scholar]
- 67.Belsky J, Steinberg LD, Houts RM, et al. Family rearing antecedents of pubertal timing. Child Dev 2007;78:1302–21. 10.1111/j.1467-8624.2007.01067.x [DOI] [PubMed] [Google Scholar]
- 68.Deardorff J, Abrams B, Ekwaru JP, et al. Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol 2014;24:727–33. 10.1016/j.annepidem.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiatt RA, Stewart SL, Hoeft KS, et al. Childhood socioeconomic position and pubertal onset in a cohort of multiethnic girls: implications for breast cancer. Cancer Epidemiol Biomarkers Prev 2017;26:1714–21. 10.1158/1055-9965.EPI-17-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the bogalusa heart study. Int J Obes Relat Metab Disord 2003;27:1398–404. 10.1038/sj.ijo.0802422 [DOI] [PubMed] [Google Scholar]
- 71.He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol 2010;171:334–44. 10.1093/aje/kwp372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobsen BK, Heuch I, Kvåle G. Association of low age at menarche with increased all-cause mortality: A 37-year follow-up of 61,319 norwegian women. Am J Epidemiol 2007;166:1431–7. 10.1093/aje/kwm237 [DOI] [PubMed] [Google Scholar]
- 73.Jacobsen BK, Oda K, Knutsen SF, et al. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the adventist health study, 1976-88. Int J Epidemiol 2009;38:245–52. 10.1093/ije/dyn251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Remsberg KE, Demerath EW, Schubert CM, et al. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the fels longitudinal study. J Clin Endocrinol Metab 2005;90:2718–24. 10.1210/jc.2004-1991 [DOI] [PubMed] [Google Scholar]
- 75.Widén E, Silventoinen K, Sovio U, et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care 2012;35:850–6. 10.2337/dc11-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pirus C, Leridon H. Large child cohort studies across the world. Population (Paris) 2010;65:575. 10.3917/pope.1004.0575 [DOI] [Google Scholar]
- 77.The Inter-University Consortium for Political and Social Research (ICPSR) . NICHD Study of Early Child Care and Youth Development (SECCYD) Series, Available: www.icpsr.umich.edu/web/ICPSR/series/233
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request. Data and materials from the NICHD SECCYD are available online: icpsr.umich.edu/web/ICPSR/series/233. Researchers interested in working with the team of investigators who led the SHINE follow-up data collection are invited to contact MEB and GIR. Potential collaborative efforts will be considered under specific conditions, including, but not limited to, the proposed scope of work and assurances related to data security and integrity.