Highlights
-
•
Labeling effects in metabolites after oral intake of [1-13C]Glc in human brain.
-
•
High temporal and spatial resolution could be achieved using 1H-FID-MRSI at 9.4 t.
-
•
Determination of spatial dependence of glutamatergic metabolism.
-
•
enrichment curves of GluC4, GlxC3, GlxC2, GlnC4, AspC2,3, and NAAC6.
-
•
enrichment maps of GluC4.
Keywords: Glutamatergic metabolism, [1-13C]Glc labeling, Ultra-high field strengths, Proton magnetic resonance spectroscopy imaging, Human brain
Abbreviations: FID, free induction decay; TE, echo time, TR repetition time; MRSI, Magnetic Resonance Spectroscopic Imaging; Glc, glucose; Glu, glutamate; Gln, glutamine; Asp, Aspartate; PC, pyruvate carboxylase; TCA, tricarboxylic acid cycle; PDH, pyruvate dehydrogenase complex; SVS, single voxel spectroscopy
Abstract
Glutamate is the major excitatory transmitter in the brain and malfunction of the related metabolism is associated with various neurological diseases and disorders. The observation of labeling changes in the spectra after the administration of a 13C labelled tracer is a common tool to gain better insights into the function of the metabolic system. But so far, only a very few studies presenting the labeling effects in more than two voxels to show the spatial dependence of metabolism.
In the present work, the labeling effects were measured in a transversal plane in the human brain using ultra-short TE and TR 1H FID-MRSI. The measurement set-up was most simple: The [1-13C]Glc was administered orally instead of intravenous and the spectra were measured with a pure 1H technique without the need of a 13C channel (as Boumezbeur et al. demonstrated in 2004). Thus, metabolic maps and enrichment curves could be obtained for more metabolites and in more voxels than ever before in human brain. Labeling changes could be observed in [4–13C]glutamate, [3–13C]glutamate+glutamine, [2–13C]glutamate+glutamine, [4–13C]glutamine, and [3–13C]aspartate with a high temporal (3.6 min) and spatial resolution (32 × 32 grid with nominal voxel size of 0.33 µL) in five volunteers.
1. Introduction
Abnormal glutamate (Glu) brain levels and function of the glutamatergic system have been found in different psychiatric disorders (Yüksel and Öngür, 2010; Mitchell and Baker, 2010; Luykx et al., 2012; Sanacora et al., 2009) and neurological diseases including leukodystrophies and mitochondrial disorders (Blüml et al., 2001), epilepsy (Pfund et al., 2000; Bartnik-Olson et al., 2017)and Huntington's disease (Lustig et al., 2008) to just name a few. Carbon-13 Magnetic Resonance Spectroscopy (13C MRS) in combination with administering a (Rothman et al., 2019)C labelled substrate as tracer such as [1-13C]glucose (Glc) is a valuable tool to gain insight into brain metabolism. Different metabolic processes from glycolysis to the tricarboxylic acid (TCA) cycle and glutamatergic metabolism can thus be observed by following the incorporation of the 13C nucleus from Glc into different metabolites further downstream. The incorporation of the 13C nuclei into different metabolites was described in detail in previous publications (de Graaf et al., 2003; Moreno et al., 2001; Lanz et al., 2013; Choi and Gruetter, 2004).
So far, mainly two different approaches were used to investigate the incorporation of 13C label into downstream metabolites: direct 13C and indirect 1H-[13C] MRS. Due to the enormous amount of related studies the reader is referred to review articles on these methods and applications (Rothman et al., 2019; Morris and Bachelard, 2003; Rothman et al., 2011; de Graaf et al., 2011; Chen et al., 2017). Direct and indirect 13C methods both induce high specific absorption rates associated with heteronuclear decoupling and need special 13C hardware such as broadband amplifiers, multinuclear transmitter and receiver boards, dual-tune 1H/13C radiofrequency coils as well as non-standard sequences sending pulses on both 1H and 13C frequencies. Hence, Boumezbeur et al. (2004) proposed a new approach to measure metabolic rates. He showed the feasibility of conventional 1H MRS to measure metabolic rates without the use of any dedicated 13CMRS specific hardware. With this method, the incorporation of the 13C nuclei into different metabolites can be observed by the temporal changes in the spectral pattern in the 1H spectra due to heteronuclear scalar coupling of 1H and 13C, which leads to splitting of the resonance peaks, for instance of singlet resonances into doublets (Chen et al., 1998). To illustrate the effect: When 13C nuclei are incorporated into metabolites, the signal intensity of spectral peaks from the 12C-bonded protons decreases, while the peaks from 13C-bonded protons increase. The temporal changes of the spectra give insights into the speed of the corresponding metabolic processes.
So far, Boumezbeur's method was applied in only a few studies in animal brain (Xu et al., 2008; Valette et al., 2009) and recently also in human brain (Bartnik-Olson et al., 2017; An et al., 2015; Dehghani et al., 2020; Ziegs et al., 2022). These studies measured the temporal changes in the MR spectra in a maximum of two voxels. In addition, most other studies using 13C or 1H-[13C] MRS techniques showed only data from one or two voxels. Only four studies measured the temporal changes of the 13C label incorporation in multiple volumes or slices using multivolume 1H-[13C] MRS (De Graaf et al., 2004) or 1H-[13C] MRSI techniques in rat brain at 7T (Hyder et al., 1999) or 1H-[13C] MRSI techniques in human brain at 4T (Pan et al., 2000) and 2T (Watanabe et al., 2000). Nonetheless, the number of voxels from which data were measured were still rather small and/or the temporal resolution low: In the rat brain, de Graaf et al. (De Graaf et al., 2004) measured 3 volumes within 6.4 min, Hyder et al. (1999) at least a slice with a grid of 16 × 16, but with a low temporal resolution of 10.7 min. In human brain, Watanabe et al. (2000) acquired data from a slice with 4 voxels with a nominal 37 ml volume with 15 min time resolution in the occipital lobe. While Pan et al. (2000) measured data from 16 voxels in a row in a coronal slice in the occipital area with 4.5 min temporal resolution and a nominal voxel size of 6 ml. Both human MRSI studies acquired data from a small region in the occipital cortex. Pan et al. segmented the data and obtained rates of the tricarboxylic acid cycle in gray and white matter (GM, WM, respectively). Nonetheless, the investigated regions were rather small and hence no information about regional differences in the metabolism could be obtained. Especially, the frontal cortex would be of interest where MRS studies found more pronounced abnormalities in diseases than in the occipital lobe (Lin et al., 2003; Boumezbeur et al., 2010; Hasler et al., 2007).
The aim of this study was to show 13C label incorporation in different metabolites with regional and tissue type specificity using a substantially higher temporal and spatial resolution and spatial coverage than previous human studies. Moreover, metabolic maps as well as enrichment curves from a higher number of metabolites than the previous human spectroscopic imaging studies, which presented enrichment curves (Pan et al., 2000) and maps (Watanabe et al., 2000) for [4–13C]Glu only, was the aim. By applying ultra-short TE and TR single-slice 1H FID-MRSI at 9.4 T, data with high temporal (3.6 min) and spatial (0.7 ml) resolution could be obtained. Taking advantage of Boumezbeur's technique in addition to the simple set-up of an oral [1-13C]Glc administration, metabolic maps as well as enrichment curves for [4–13C]Glu, [4–13C]glutamine (Gln), [3–13C]glutamate+glutamine (Glx), [2–13C]Glx, and [3–13C]aspartate (Asp) could be determined. Due to the slice position in a transversal plane parallel to the corpus callosum, data from the frontal cortex could also be obtained, which was not done by the previous MRSI studies mentioned above.
2. Methods
The Glc preparation and the overall procedure of this experiment was very similar to a single-voxel spectroscopy (SVS) study published recently by the same group (Ziegs et al., 2022). For clarity, the methods parts, which are the same as before in Ziegs et al. (2022) were copied and not rephrased.
Data and code can be made available upon request due to the huge size of MRSI data
2.1. Human subjects
Labeling effects after the oral administration of [1-13C]Glc were measured in one slice above the corpus callosum in 5 healthy volunteers (2 female, 3 male, mean age 29±2 years). Before the measurement started, the volunteers gave their written informed consent according to the local research ethics regulations, the current version of the Declaration of Helsinki, DIN EN ISO 14 155 and were approved by the Institutional Review Board of the University of Tübingen.
2.2. [1-13C]Glc administration
The volunteers fasted for 9 h before the measurement started. Before and after the scan the blood sugar level was tested with a glucometer (Accu-Check, Roche Diabetes Care GmbH, Mannheim, Germany) to detect possible hyper- or hypoglycemia after the Glc administration. Hypoglycemia was not encountered for any subject. For each volunteer a solution containing 0.75 g of [1-13C]Glc (Aldrich Chemical Company, Miamisburg, Ohio, USA; API for clinical studies) per kilogram body weight was prepared by a pharmacy, who did analytic and microbiological tests as well as quality control to guaranty safety of the product for oral intake. The subjects drank the Glc solution after the acquisition of the first MRSI slice. If possible, the volunteers drank the solution while lying down to keep the head as still as possible. The volunteers drank with a straw; since the straw was flexible, the volunteer could adjust the speed of solution entering the mouth easily.
2.3. Data acquisition
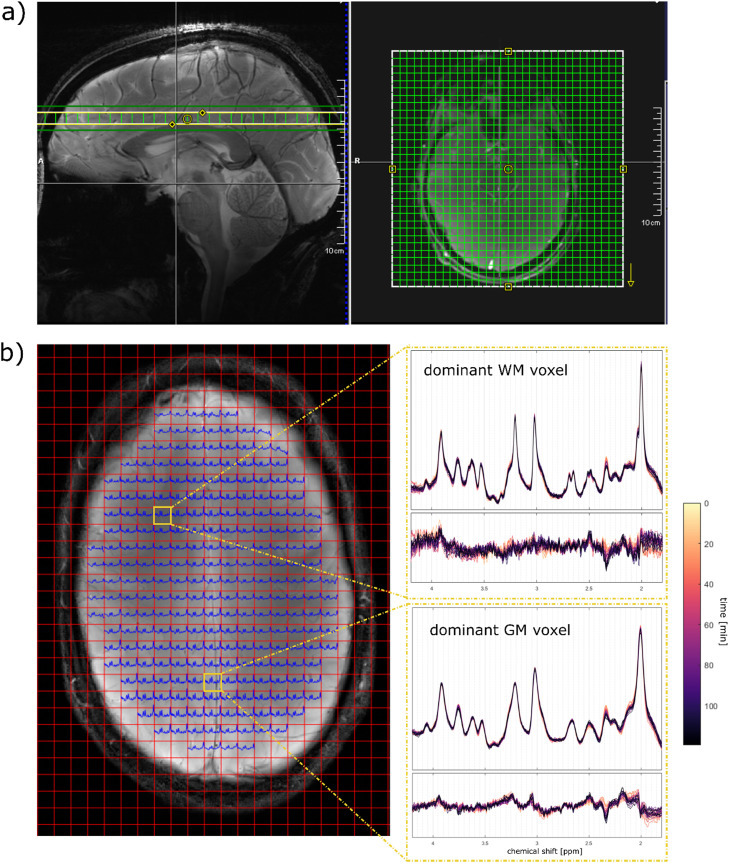
All measurements were performed using a 9.4T Magnetom whole-body MR scanner (Siemens Healthineers, Erlangen, Germany) with an in-house built radiofrequency (RF) array coil with 18 transmit and 32 receive channels (Avdievich et al., 2019). The subjects lay supine on the scanner table. Sagittal gradient-echo scout images were acquired for the positioning of the transversal slice (FOV 220 mm x 220 mm x 7 mm) just above the corpus callosum, see Fig. 1a. The slice was shimmed using the vendor implemented image-based second-order B0 shimming routine with a shim volume of 220 mm x 220 mm x 15 mm with the same center as the MRSI slice. A customized 1H FID MRSI sequence (Henning et al., 2009; Nassirpour et al., 2018) (resolution 32 × 32 with elliptical k-space shuttering, TR = 300 ms, TE*=1.5 ms, flip angle = 48°, spectral width= 8000 Hz, acquisition time = 128 ms) with optimized water suppression (Nassirpour et al., 2018) and without outer volume or lipid suppression was acquired. The nominal voxel size was nearly isotropic with 6.9 mm x 6.9 mm x 7 mm (0.33 µL). The effective spatial resolution (FWHM of the point spread function) was 8.4 mm. Subsequently, a non-water suppressed reference scan with the same resolutions and scan parameters as the 1H MRSI scan was measured. The scan time for one 1H MRSI acquisition was 3.6 min.
Fig. 1.
a) Position of the MRSI slice (220 mm x 220 mm x 7 mm; resolution 32 × 32) parallel to the corpus callosum (yellow box) and the corresponding shim volume (green box). b) Pre-Glc spectra for voxels within the brain (blue) and additionally, spectra and difference spectra for different time points from two voxel positions from the major gray and white matter area. Data from volunteer 1.
After the execution of these initial measurements, the scanner table was pulled out and the volunteers drank the Glc solution as fast as possible. After this short break, the scanner table was pushed back to its previous position inside the bore and a localizer was applied to verify the positioning of the slice. In case of a changed head position, the gradient-echo scout sequences to relocate the MRSI volume at the exact same position as prior to the Glc intake and B0 shimming was repeated before continuing to apply the 1H MRSI sequence. If no repositioning of the voxel was needed (4 of the 5 measurements), 1H MRSI data acquisition was continued immediately. The time after the Glc administration and the start of the 1H MRSI measurement was 4.5–12 min. As many as possible of the water suppressed 1H MRSI acquisitions were repeated to fill a maximum total scan time of 2 h (21-35 spectra were acquired within 70–120 min).
In a different session, an MP2RAGE from each volunteer was acquired for gray and white matter segmentation.
2.4. Data processing
1H MRSI raw data was processed with in-house-written code in Matlab (version 2016a; MathWorks, Natick, MA) as also described before in Nassirpour et al. (2018) including Haning filter of the MRSI and water reference data, spatial reconstruction by fast Fourier transformation, eddy current correction (Klose, 1990), coil combination using singular value decomposition (SVD), water removal with Hankel SVD and missing point prediction to remove the 1st phase due to the acquisition delay. The MP2RAGE images were reconstructed (Hagberg et al., 2017) and segmented using the SPM12 Matlab package.
2.5. Data fitting
The reconstructed MRSI data was fitted using LCModel (V6.3‐1L, the control file can be found in the Supplementary Material) (Provencher, 1993) with two basis set simulated with VeSPA (version 0.9.5 https://scion.duhs.duke.edu/vespa/ (Soher et al., 2011)) for the pre-Glc and the post-Glc administration spectra.
2.5.1. Pre-Glc-basis set
The first spectrum after the oral administration was used as baseline spectrum. In the following it will be called pre-Glc basis set, since the changes in the first minutes after the oral intake of labeled Glc are expected to be neglectable, while repositioning errors can be avoided. The pre-Glc basis set contained the following basis spectra: aspartate (Asp), γ-aminobutyric acid (GABA), glycine, glutathione (GSH), myo-Inositol (mI), NAAG, scyllo-inositol (scyllo), taurine (Tau), Glc, Glu, Gln, NAA, total CH3 creatine (CH3 of Cr and phosphocreatine (PCr)), CH2 peaks of Cr and PCr, phosphorylethanolamine (PE), total choline (tCho, glycerophosphocholine (GPC) + phosphocholine (PCho)), NAA and a simulated macromolecular baseline (MM).
The tCr basis set was splitted too into its CH3 moiety peak at approx. 3 ppm (tCr CH3) and the CH2 moiety. The temporal changes in the CH3 peak serves as a proof of spectral stability for the difference spectra.
J-coupling constants and chemical shifts were taken from Govindaraju et al. (2000); Govind et al. (2000) except for the J-coupling constants for GABA, which were taken from Near et al. (2013). The line broadening of the basis spectra was set to 3 Hz. The spectra were fitted between 1.8 and 4.2 ppm. The simulated MM baseline (MMAXIOM in Wright et al. (2021)) was used since the acquisition of a matching experimental macromolecular spectra was not possible with the same short TR due to SAR constraints of the required double inversion recovery sequence. The simulated MM spectrum used in this work considered T1- and T2-relaxation times of MM molecules as well as the linewidths of the MM peaks (Murali-Manohar et al., 2020; Murali-Manohar et al., 2020) and was successfully used in 1H MRSI data before (Ziegs et al., 2022; Wright et al., 2021).
2.5.2. Post-Glc basis set
This subsection is very close to what has been performed on the SVS at 9.4 in Ziegs et al. (2022) The sentences are copied and not rephrased: For fitting the changes caused by the incorporation of the 13C nuclei into downstream metabolites, difference spectra were used. No linewidth adjustments were applied. Before subtracting the pre-Glc administration baseline spectrum from the post-Glc administration spectra to obtain a time series of difference spectra, two changes were made: First, the fitted tCr CH3 peak at ∼3.0 ppm was subtracted from the pre-Glc spectrum to obtain a pre-Glc spectrum without the tCr CH3 peak. Thus, the tCr CH3 will not be subtracted when calculating the difference spectra so that the difference spectra still contained its tCr CH3 peak, which ensured better LCModel fitting stability since LCModel takes this Cr peak as reference for its first fit iteration.
Furthermore, the LCModel basis set for the post-Glc intake difference spectra contained metabolite peaks of Glu, Gln, Asp, and NAA, which were expected to change after the 13C incorporation with the heteronuclear J-coupling constants taken from de Graaf (2007). Which metabolites are labeled by the incorporation of the 13C nuclei after [1-13C]Glc intake is illustrated in previous publications (de Graaf et al., 2003; Moreno et al., 2001; Lanz et al., 2013). To account for the advantage to simultaneously detect the signal of 12C-bonded and 13C-bonded protons, a method described in Boumezbeur et al. (2004) was used, who fitted the difference spectra and combined the corresponding 13C uptake and 12C decrease in one basis set. The method shall be described shortly through the example of Glu labeled at the C4 position:
-
•
If the C4 position of Glu is labeled, the 13C nucleus couples to the 4H-Glu protons at 2.34 and 2.35 ppm with a coupling constant of 126.7 Hz. Glu spin systems with and without 13C at the C4 position were simulated with VeSPA. Both simulations were subsequently subtracted to account for the decrease of the unlabeled C4 peak and the increase of the labeled C4 peaks. The basis spectrum was called Glu4.
-
•
This was done for Gln labeled at the position C4 (Gln4), for Glu labeled at C3 and C2 (since Glu3 and Gln3 as well as Glu2 and Gln2 cannot be distinguished by LCModel, Glu3 and Glu2 changes represent the labeling of the mix of both and are thus called Glx3 and Glx2, respectively), NAA labeled at C6 (NAA C6) and Asp labeled at C2 and C3 (Asp C2 and Asp C3). The simulated basis spectra are shown the Supplementary Figure 1. Glu and Gln at the C3 and C2 positions were combined to Glx since the chemical shifts significantly overlap and cannot be distinguished at 9.4 T. GABA labeling was integrated in a first step, but the 13C satellite and difference peaks of GABA2 partly overlapped with those of Gln4, which lead to ambiguous results. GABA labeling was thus omitted in the present fitting results.
The spectra were fitted between 1.8 ppm and 4.1 ppm including water scaling and the LCModel parameter dkntmn was set to 999, which is a stiff baseline.
2.6. Concentration time courses and percent enrichment calculation
The concentration time courses for the labeled metabolites were fitted up to 200 min after the Glc administration to reach steady-state conditions for most metabolites using an asymmetric sigmoid (Gombertz) function. The percent enrichment (PE) was calculated from the concentration of the labeled metabolite in the post-Glc spectrum divided by the concentration of the unlabeled metabolite from the pre-Glc spectrum, e.g. [Glu4]/[Glu] for glutamate labeled at the 4th position. Sample metabolic and standard deviation maps for the pre-Glc spectrum for Glu, Gln, Asp and Cr are shown in Supplementary Figure 2. The concentrations are T1- and T2-corrected including tissue type information (Murali-Manohar et al., 2020; Murali-Manohar et al., 2020; Wright et al., 2022).
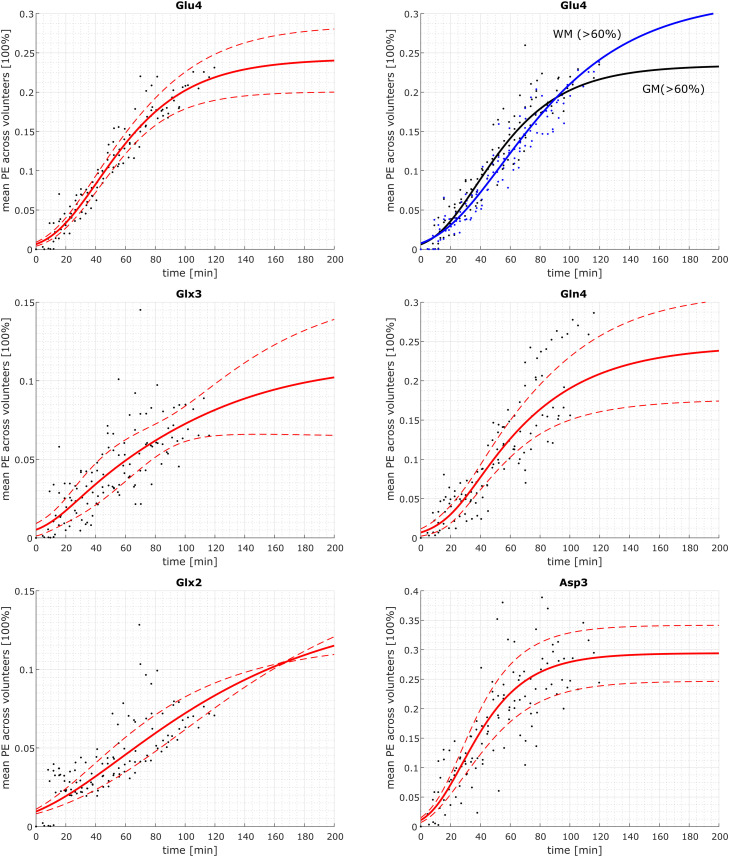
Additionally, the mean PE for each time point and volunteer was fitted with an asymmetric sigmoid function to yield mean final PE values across all volunteers. For Glu4, due to the best data quality, the mean PEs from voxels with high GM (> 60%) and voxels with high WM (> 60%) content was calculated for each volunteer separately to detect changes in different tissue types. A two-sided Wilcoxon rank sum test was performed to test for significant differences in the mean final PE values from voxels with high GM content to those with high WM content at the 5% significance level.
To prove the data and fitting quality, CRLB as reported from LCModel and the SNR of Cr and Glu4 is reported for each volunteer for the last spectrum. The FWHM of Cr was calculated from the pre-Glc spectrum. The SNR was calculated as before at 9.4 T data in Ziegs et al. (2022) and the specific sentences are copied here and are only slightly adapted: The SNR values were calculated as the ratio of the maximum peak for Cr CH3 peak or the absolute value of the [4–12C]Glu peak to the root-mean-square of the noise in the non-back-predicted 1H FID MRSI data (to avoid baseline distortions caused by the back-prediction). In addition, a 2nd order polynomial fit of the baseline was subtracted prior to the noise level estimation.
3. Results and discussion
3.1. Labeling effects on spectral pattern
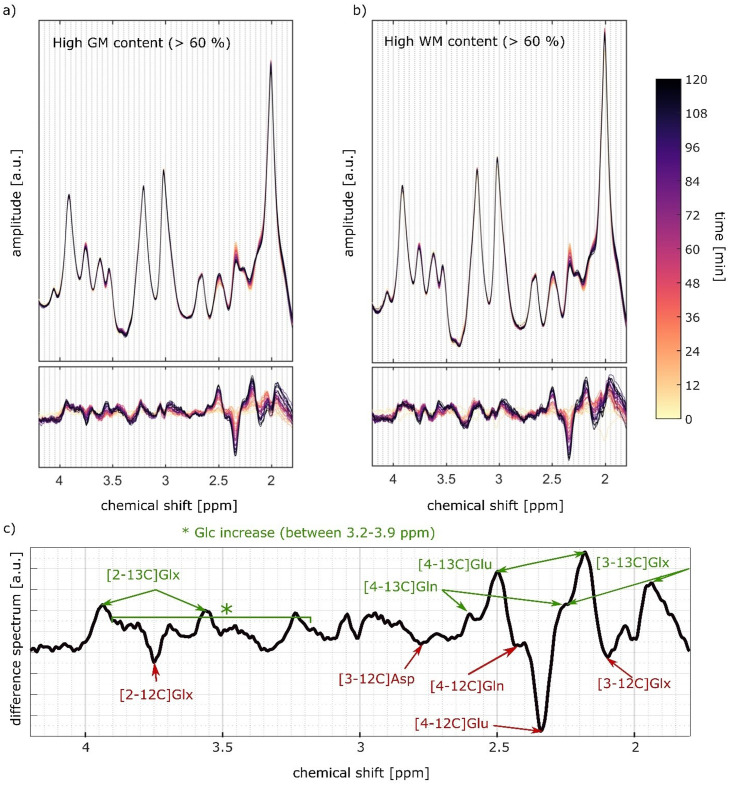
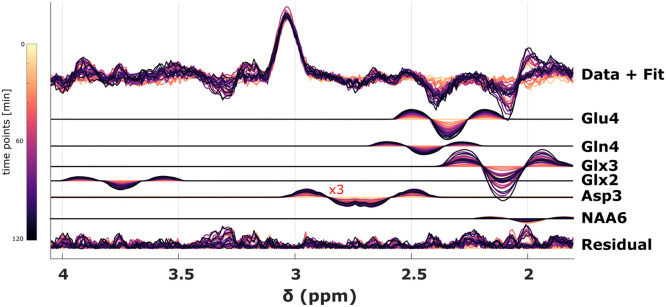
The averaged GM and WM spectra and respective difference spectra for all time points show systematic changes in the chemical shift region between 2 ppm – 4 ppm, see Figure 2a+b. Fig. 2c illustrates that these spectral pattern changes arise from 13C labeling of Glu, Gln, Asp and the increase of the sum of 12C and 13C labelled Glc concentration. These changes are consistent to results shown in 1H SVS data at 9.4T after oral Glc administration (Ziegs et al., 2022). Data from a sample voxel along with the metabolite spectra for Glu4, Glx3, Glx2, Gln4, and Asp3 is shown in Fig. 3. Asp2, and NAA6 cannot be reliably detected and are thus omitted from further analysis.
Fig. 2.
Temporal series of summed spectra for voxels with a) high gray matter and b) white matter content (>60%) with the corresponding difference spectra. Colors indicate the measured time points. C) Averaged last difference spectrum from high gray matter voxels with decreased metabolite peaks indicated in red, and increased metabolite peaks indicated in green as far as determination of the corresponding metabolites were possible. Data from volunteer V1.
Fig. 3.
Raw data and fitted spectra for Glu4, Glx3, Glx2, Gln4, Asp3 and GABA2 and fit residual from LCModel. Data from sample voxel from volunteer 1. Different timepoints are indicated with different colors.
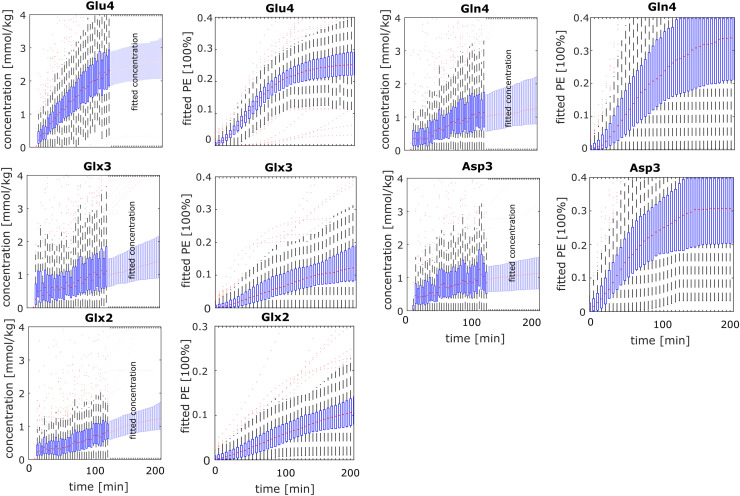
3.2. Time courses of labeled metabolites
The concentration time courses for those metabolite as well as the corresponding PE values are shown in Fig. 4 for volunteer 1 for all voxels and the PE for a mean across subject is presented in Fig. 5. The PE values after 2 h are collected in Table 1. Two hours after the Glc administration the Glu4 position is labeled to 24±4% (mean ± standard deviation across subjects); the Gln4 position to 23±7%; Glx3 by 10±5%; Glx2 by 11±2%; Asp3 by 30±2%. The single volunteer's time course for tCr CH3 can be found in the Supplementary Figure 3 and demonstrate reproducibly high data quality and consistency.
Fig. 4.
Boxplots of Glu4, Gln4, Glx3, Glx2, Asp3 and GABA2 with concentration [mmol/kg] and percent enrichment (PE) from volunteer 1. The first part of the concentration are raw data for all voxels within the brain. From 120 to 200 min, the fitted concentration is shown using an asymetric sigmoid function to fit the raw data up to 200 min. Boxplots indicate [25,75]-percentiles with the red line indicating the median.
Fig. 5.
Mean PE [100%] for Glu4, Gln4, Glx3, Glx2, Asp3, and GABA2 for each time point and volunteer (black dots) with mean of the individual fitted PE time courses (red solid line) and the 0.95*standard deviation interval. Fitted curves are asymmetric sigmoid functions. Mean PE for all time points and volunteers (dots) for voxels with high WM (blue) and high GM (black) content. Raw data (dots) and mean of the fitted time courses (solid lines). Fitted curves are asymmetric sigmoid functions.
Table 1.
Percent enrichment after 2 h of measurements averaged across subjects (mean, standard deviation) for all voxels within the brain slice.
| metabolite |
PE [%] |
|
|---|---|---|
| mean | std | |
| Glu4 | 24 | 4 |
| Gln4 | 23 | 7 |
| Glx3 | 10 | 5 |
| Glx2 | 11 | 2 |
| Asp3 | 30 | 2 |
3.2.1. Glu4 time-course and enrichment
Comparing the present results to the literature faces the following issues: There is not much MRSI data to compare, since it has only been rarely used to observe the spatial dependence of metabolism after the administration of a 13C labeled substrate either in animals (De Graaf et al., 2004; Hyder et al., 1999; Van Zijl et al., 1993; Morikawa and Inubushi, 2001) or humans (Pan et al., 2000; Watanabe et al., 2000). In human brain, Pan et al. (2000) showed PE curves for Glu4 with a saturated PE of approx. 40% in coronal slice with 16 volumes (6 mL each) after the infusion of [U-13C]Glc using indirect short spin echo single quantum 13C editing with a time resolution of 4.5 min. Watanabe et al. (2000) presented Glu4 peak height curves as well as temporal changes in the Glu4 maps but no PEs for 4 volumes (37 mL each) in a slice in the occipital lobe using also an indirect method (heteronuclear single quantum coherence, HSQC) by administering [1-13C]Glc orally with different doses with 15 min time resolution.
The PE of Glu4 from the present study is similar or higher than the values after 2 to 3 h from other oral studies administering the same amount of [1-13C]Glc (20–24% (Ziegs et al., 2022; Mason et al., 2003)) or less [1-13C]Glc (16% using 0.65 g Glc/kg body weight instead of 0.75 g/kg52). Most studies use i.v. administration and showed maximum Glu4 PEs of 11–40% (Dehghani et al., 2020; Pan et al., 2000; Mason et al., 2003; Moreno et al., 2001; Gruetter et al., 1998; Chhina et al., 2001; Mason et al., 1995; Gruetter et al., 2002; Mason et al., 1999), with our results being in the same range as most of these values (literature is collected in Supplementary Table S1). Results from studies using oral intake should be cautiously compared to studies using i.v. administration due to the following limitations: First, the maximum PE is obviously depending on the amount of Glc given. While the oral intake contains a defined amount of Glc, the i.v. Glc administration procedure includes often a maintenance dose, whose amount is in many cases not reported. Secondly, the same amount in an oral as well as an i.v. administration, does not lead to similar maximum enrichments (Moreno et al., 2001). Third, the oral administration leads to delayed enrichments curves due to the gastrointestinal Glc absorption (Blüml et al., 2001; Mason et al., 2003). Thus, the saturation plateau would only be reached after 3 h of measurement in contrast to the i.v. protocol, where the maximum PE is reached after 2 h of measurement (Mason et al., 2003). Unfortunately, 3 h of measurement could not be performed in the present study due to ethical regulations related to the maximum scan time. Additionally, such a long scan time would be uncomfortable for the volunteers and increase the risk of motion. An additional break taking the subject out of the scanner – as suggested from Mason et al. (2003) – would entail slice-repositioning problems and was thus not realized in this study. Due to the different Glc dose and the additional route through the body, the PE values from i.v. studies cannot be directly compared to the oral results, but are still included into this manuscript since it is the far more common procedure.
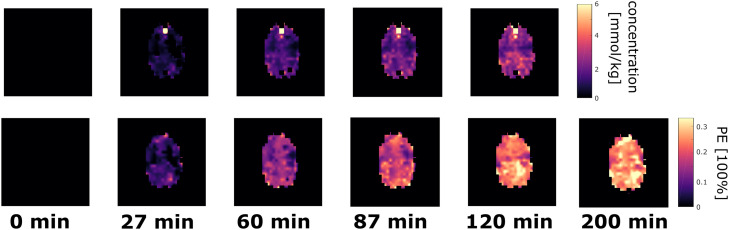
In addition to the averaged PE values, MRSI data offers the possibility to observe changes in different tissue types. These tissue dependence of the label effects in Glu4 are seen in the mean PEs averaged across all volunteers for voxels with high GM (>60%) or WM (>60) content in Fig. 5 for Glu4. The difference in WM and GM voxels are found to be significant. The results are very similar to the data in Mason et al.57: In the first hour, the labeling is dominated by the GM, the increase is faster and the plateau is reached earlier than in WM. The final PE in WM is higher than in GM. All of this can be seen in the present analysis in the averaged PE time course in Fig. 5 as well as in the concentration and PE maps in Fig. 6. In the latter, it is seen that the GM region is labeled first, before the PE values in the WM region becomes dominant. Unfortunately, most publications did not compare GM and WM regions or did not segment their voxels at all. Metabolic modeling should be used to further evaluate the WM/GM differences by calculating the corresponding rates and to verify that metabolism is faster in GM than in WM (De Graaf et al., 2004; Hyder et al., 1999; Pan et al., 2000; Mason et al., 1999). Due to the lack of time-course of labeled and unlabeled Glc, metabolic rates could not be determined in this study, metabolic modeling should be used.
Fig. 6.
Concentration [mmol/kg] and PE [100%] maps for different time points for Glu4 from volunteer 1. The PE map from 200 min results from the fitted data from each voxel.
3.2.2. Gln4 time-course and enrichment
In previous publications, it has been shown that Glu4 is labeled before Gln4 (Gruetter et al., 1998; Lapidot and Gopher, 1994). This is also the case in the present data for the concentration changes, where Glu4 increases much faster than Gln4, but due to the lower concentration of Gln-in comparison to Glu, both final PE values are similar after 2h: 23%. The enrichment of Gln4 is higher than the values reported previously from oral (approx. 15% in the occipital lobe (Ziegs et al., 2022; Mason et al., 2003)) and similar to the values reported in i.v. studies (10–25% (Mason et al., 2003; Chhina et al., 2001; Mason et al., 1995)).
3.2.3. Glx3, Glx2 time-course and enrichment
The herein presented Glx3 values are at the lower range of previous values for oral administration (17–19% after 2 h (Ziegs et al., 2022)) and more similar to the values reported in studies using i.v. protocols (5–10% (Dehghani et al., 2020) and 23% (Mason et al., 1995; Gruetter et al., 2002)). The Glx2 is in the range of previous values (10–15% after 1 h (Chhina et al., 2001) and 13–23% after 2 h (Ziegs et al., 2022)).
3.2.4. Asp3 time-course and enrichment
Asp enrichment is rarely reported in literature. In this study, Asp2 could not be reliably fitted as it was also the case in a previous SVS at 9.4 T. (Ziegs et al., 2022) Previous studies using oral administration reported significant higher values for Asp3 enrichment than the present data (Ziegs et al., 2022), but those values should be treated with caution due to artifacts mentioned in (Ziegs et al., 2022). I.v. studies observing Asp3 enrichment found values similar to the Glu4 enrichment (Gruetter et al., 1998) and values of approx. 27% (Gruetter et al., 2002), which is similar to herein reported Asp3 enrichment. 12% PE was found for Asp210, which is definitely lower than the herein presented values for Asp3 (Yüksel and Öngür, 2010; Sanacora et al., 2009; Blüml et al., 2001; de Graaf et al., 2003)
3.3. Data quality
The CRLB for all analyzed labeling positions were shown in Supplementary Figure S4. The FWHM and the SNR of Cr as shown in Supplementary Figure 5 + 6 are similar to other MRSI results published at 9.4 T (Ziegs et al., 2022) taking different voxel sizes into account.
4. Limitations
In the present study, it was not possible to calculate metabolic rates since the brain or blood 13C Glc enrichment was not accessible. While total Glc levels as well as unlabeled and labelled Glc fractions are in principle measurable in 1H SVS data (Ziegs et al., 2022; Pfeuffer et al., 1999), the line width in 1H MRSI data is too broad to measure (total) Glc levels in the spectra. A solution would be to sample the blood during the measurement as it was done in most other studies (Mason et al., 2003), which was not possible in the current study inside a non-clinical setting. Unfortunately, no control experiment could be conducted with the oral administration of nonlabelled Glc to confirm that the spectral changes observed are caused by 13C labeling and not by glucose administration or patient motion.
The sensitivity could be improved by the use of [1,6–13C]Glc or [U-13C]Glc instead of Glc labeled at a single position, which doubles the sensitivity since both compounds lead to labeling of two pyruvate molecules instead of only one. However, it would be (2–5 fold) more expensive to perform such a study in humans, which is not affordable since the costs were already approx. 5000 euro/person.
Another problem of the present study set-up was the short break for drinking the Glc solution after the baseline 1H MRSI measurement. This break causes uncertainties in the relocation of the 1H MRSI slice. Although the position of the post-Glc 1H MRSI slice was compared visually to the pre-Glc MRSI slice, the possibility of a mismatch between both slice positions remain. The error is of minor importance since the enrichment curves were calculated using an exponential fit using all time points; so, possible errors in the first value are negligible. Only the mismatch of the B0 shim volume could cause imperfect shim results, but since the shim volume was chosen to be larger than the slice, it is probably of minor importance, too. A possible solution would be automatized repositioning of the 1H MRSI slice, which was not available on the present scanner.
In further studies, it would be interesting to use this technique for the observation of different labeling effects in different brain regions, e.g. frontal cortex vs. occipital lobe.
5. Conclusion
The present study demonstrates for the first time that glutamatergic metabolism can be derived from 1H MRSI data with high temporal and spatial resolution without the need of specialized 13C hardware or scan software at 9.4 T. Enrichment curves of Glu4, Gln4, Glx3, Glx2, and Asp3 and enrichment maps of Glu4 have been obtained and revealed the possibility of this approach to investigate the dependence of the labeling effects on regions and tissue types across the brain.
Funding
The study was funded by European Research Council (ERC) (Grant No. 679927 to T.Z., L.R., A.M.W., and A.H.) and Cancer Prevention and Research Institute of Texas (CPRIT) (Grant No. RR180056 to A.H.)
CRediT authorship contribution statement
Theresia Ziegs: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft. Loreen Ruhm: Data curation. Andrew Wright: Methodology, Software. Anke Henning: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declarations of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2023.119940.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- An L., Li S., Murdoch J.B., Araneta M.F., Johnson C.S., Shen J. Detection of glutamate, glutamine, and glutathione by radiofrequency suppression and echo time optimization at 7 Tesla. Magn. Reson. Med. 2015;73(2):451–458. doi: 10.1002/mrm.25150. [DOI] [PubMed] [Google Scholar]
- Avdievich N.I., Giapitzakis I.A., Bause J., Shajan G., Scheffler K., Henning A. Double-row 18-loop transceive-32-loop receive tight-fit array provides for whole-brain coverage, high transmit performance, and SNR improvement near the brain center at 9.4T. Magn. Reson. Med. 2019;81(5):3392–3405. doi: 10.1002/mrm.27602. [DOI] [PubMed] [Google Scholar]
- Bartnik-Olson B.L., Ding D., Howe J., Shah A., Losey T. Glutamate metabolism in temporal lobe epilepsy as revealed by dynamic proton MRS following the infusion of [U-13C] glucose. Epilepsy Res. 2017;136:46–53. doi: 10.1016/j.eplepsyres.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Blüml S., Moreno A., Hwang J.H., Ross B.D. 1-13C Glucose Magnetic Resonance Spectroscopy of Pediatric and Adult Brain Disorders. NMR Biomed. 2001;14(1):19–32. doi: 10.1002/nbm.679. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F., Besret L., Valette J., et al. NMR measurement of brain oxidative metabolism in monkeys using13C-labeled glucose without a13C radiofrequency channel. Magn. Reson. Med. 2004;52(1):33–40. doi: 10.1002/mrm.20129. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F., Mason G.F., de Graaf R.A., et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cerebr. Blood Flow Metabol. 2010;30(1):211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., de Feyter H.M., Brown P.B., Rothman D.L., Cai S., de Graaf R.A. Comparison of direct 13 C and indirect 1 H-[13 C] MR detection methods for the study of dynamic metabolic turnover in the human brain. J. Magn. Reson. 2017;283:33–44. doi: 10.1016/j.jmr.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Chen W., Adriany G., Zhu X.H., Gruetter R., Ugurbil K. Detecting natural abundance carbon signal of NAA metabolite within 12-cm3 localized volume of human brain using 1H-13C NMR spectroscopy. Magn. Reson. Med. 1998;40(2):180–184. doi: 10.1002/mrm.1910400203. [DOI] [PubMed] [Google Scholar]
- Chhina N., Kuestermann E., Halliday J., et al. Measurement of human tricarboxylic acid cycle rates during visual activation by 13C magnetic resonance spectroscopy. J. Neurosci. Res. 2001;66(5):737–746. doi: 10.1002/jnr.10053. [DOI] [PubMed] [Google Scholar]
- Choi I.Y., Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1-13C]glucose in the rat brain in vivo. J. Neurochem. 2004;91(4):778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R.A. John Wiley & Sons, Ltd; 2007. In Vivo NMR Spectroscopy. [DOI] [Google Scholar]
- de Graaf R.A., Mason G.F., Patel A.B., Behar K.L., Rothman D.L. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16(67):339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- De Graaf R.A., Mason G.F., Patel A.B., Rothman D.L., Behar K.L. Regional glucose metabolism glutamatergic neurotransmission rat in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101(34):12700–12705. doi: 10.1073/pnas.0405065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R.A., Rothman D.L., Behar K.L. State-of-the-art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo: a practical guide. NMR Biomed. 2011;24(8):958–972. doi: 10.1002/nbm.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani M., Zhang S., Kumaragamage C., Rosa-Neto P., Near J. Dynamic 1H-MRS for detection of 13C-labeled glucose metabolism in the human brain at 3T. Magn. Reson. Med. 2020;84(3):1140–1151. doi: 10.1002/mrm.28188. [DOI] [PubMed] [Google Scholar]
- Govind V., Young K., Maudsley A.A. Corrigendum: proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(7):923–924. doi: 10.1002/nbm.3336. 129-153. NMR Biomed. 2015;28. [DOI] [PubMed] [Google Scholar]
- Govindaraju V., Karl Y., Maudsley A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Novotny E.J., Boulware S.D., et al. Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1-13C]Glucose. J. Neurochem. 2002;63(4):1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Seaquist E.R., Kim S., Ugurbil K. Localized in vivo 13C-NMR of glutamate metabolism in the human brain: initial results at 4 Tesla. Dev. Neurosci. 1998;20(4–5):380–388. doi: 10.1159/000017334. [DOI] [PubMed] [Google Scholar]
- Hagberg G., Bause J., Ethofer T., et al. Whole brain MP2RAGE-based mapping of the longitudinal relaxation time at 9.4T. Neuroimage. 2017;144:203–216. doi: 10.1016/j.neuroimage.2016.09.047. [DOI] [PubMed] [Google Scholar]
- Hasler G., van der Veen J.W., Tumonis T., Meyers N., Shen J., Drevets W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Henning A., Fuchs A., Murdoch J.B., Boesiger P. Slice-selective FID acquisition, localized by outer volume suppression (FIDLOVS) for 1H-MRSI of the human brain at 7 T with minimal signal loss. NMR Biomed. 2009;22(7):683–696. doi: 10.1002/nbm.1366. [DOI] [PubMed] [Google Scholar]
- Hyder F., Renken R., Rothman D.L. In vivo carbon-edited detection with proton echo-planar spectroscopic imaging (ICED PEPSI): [3,4-13CH2]glutamate/glutamine tomography in rat brain. Magn. Reson. Med. 1999;42(6):997–1003. doi: 10.1002/(SICI)1522-2594(199912)42:6<997::AID−MRM1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn. Reson. Med. 1990;14(1):26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Lanz B., Gruetter R., Duarte J.M.N. Metabolic flux and compartmentation analysis in the brain in vivo. Front. Endocrinol. 2013;4:1–18. doi: 10.3389/fendo.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot A., Gopher A. Cerebral metabolic compartmentation. J. Biol. Chem. 1994;269(44):27198–27208. doi: 10.1016/S0021-9258(18)46969-4. [DOI] [PubMed] [Google Scholar]
- Lin A.P., Shic F., Enriquez C., Ross B.D. Reduced glutamate neurotransmission in patients with Alzheimer's disease - An in vivo 13C magnetic resonance spectroscopy study. Magn. Reson. Mater. Phys. Biol. Med. 2003;16(1):29–42. doi: 10.1007/s10334-003-0004-x. [DOI] [PubMed] [Google Scholar]
- Lustig M., Donoho D.L., Santos J.M., Pauly J.M. Compressed sensing MRI. IEEE Signal Process. Mag. 2008;25:72–82. doi: 10.1109/Tit.2006.871582. (March 2008) [DOI] [Google Scholar]
- Luykx J.J., Laban K.G., van den Heuvel M.P., et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of 1H-MRS findings. Neurosci. Biobehav. Rev. 2012;36(1):198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Mason G.F., Falk Petersen K., de Graaf R.A., Kanamatsu T., Otsuki T., Rothman D.L. A comparison of 13C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-13C]glucose. Brain Res. Protocols. 2003;10(3):181–190. doi: 10.1016/S1385-299X(02)00217-9. [DOI] [PubMed] [Google Scholar]
- Mason G.F., Gruetter R., Rothman D.L., Behar K.L., Shulman R.G., Novotny E.J. Simultaneous determination of the rates of the TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cerebr. Blood Flow Metabol. 1995;15(1):12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- Mason G.F., Pan J.W., Chu W.J., et al. Measurement of the tricarboxylic acid cycle rate in human grey and white matter in vivo by 1H-[13C] magnetic resonance spectroscopy at 4.1T. J. Cerebr. Blood Flow Metabol. 1999;19(11):1179–1188. doi: 10.1097/00004647-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Mitchell N.D., Baker G.B. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr. Scand. 2010;122(3):192–210. doi: 10.1111/j.1600-0447.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- Moreno A., Blüml S., Hwang J.H., Ross B.D. Alternative 1-13C glucose infusion protocols for clinical 13C MRS examinations of the brain. Magn. Reson. Med. 2001;46(1):39–48. doi: 10.1002/mrm.1158. [DOI] [PubMed] [Google Scholar]
- Moreno A., Ross B.D., Blüml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by 13C MRS and [1-13C]glucose infusion. J. Neurochem. 2001;77(1):347–350. doi: 10.1046/j.1471-4159.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Inubushi T. Fast 13C-glucose metabolite mapping in rat brain using 1H echo planar spectroscopic imaging technique at 2T. J. Magn. Reson. Imaging. 2001;13(5):787–791. doi: 10.1002/jmri.1109. [DOI] [PubMed] [Google Scholar]
- Morris P., Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR Biomed. 2003;16(6–7):303–312. doi: 10.1002/nbm.844. [DOI] [PubMed] [Google Scholar]
- Murali-Manohar S., Borbath T., Wright A.M., Soher B., Mekle R., Henning A. T2 relaxation times of macromolecules and metabolites in the human brain at 9.4 T. Magn. Reson. Med. 2020;84(2):542–558. doi: 10.1002/mrm.28174. [DOI] [PubMed] [Google Scholar]
- Murali-Manohar S., Wright A.M., Borbath T., Avdievich N.I., Henning A. A novel method to measure T1-relaxation times of macromolecules and quantification of the macromolecular resonances. Magn. Reson. Med. 2020;85(2):601–614. doi: 10.1002/mrm.28484. [DOI] [PubMed] [Google Scholar]
- Nassirpour S., Chang P., Henning A. High and ultra-high resolution metabolite mapping of the human brain using 1 H FID MRSI at 9.4T. Neuroimage. 2018;168:211–221. doi: 10.1016/j.neuroimage.2016.12.065. [DOI] [PubMed] [Google Scholar]
- Near J., Evans C.J., Puts N.A.J., Barker P.B., Edden R.A.E. J -difference editing of gamma-aminobutyric acid (GABA): simulated and experimental multiplet patterns. Magn. Reson. Med. 2013;70(5):1183–1191. doi: 10.1002/mrm.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.W., Stein D.T., Telang F., et al. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn. Reson. Med. 2000;44(5):673–679. doi: 10.1002/1522-2594(200011)44:5<673::AID−MRM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J., Tkáč I., Choi I.Y., et al. Detection of [1,6-13 C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn. Reson. Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pfund Z., Chugani D.C., Juhász C., et al. Evidence for coupling between glucose metabolism and glutamate cycling using FDG PET and 1H magnetic resonance spectroscopy in patients with epilepsy. J. Cerebr. Blood Flow Metabol. 2000;20(5):871–878. doi: 10.1097/00004647-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localizedin vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rothman D.L., De Feyter H.M., de Graaf R.A., Mason G.F., Behar K.L. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24(8):943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D.L., de Graaf R.A., Hyder F., Mason G.F., Behar K.L., De Feyter H.M. In vivo 13C and 1H-[13C] MRS studies of neuroenergetics and neurotransmitter cycling, applications to neurological and psychiatric disease and brain cancer. NMR Biomed. 2019;32(10):1–21. doi: 10.1002/nbm.4172. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2009;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soher B.J., Semanchuk P., Todd D., Steinberg J., Young K. VeSPA: integrated applications for RF pulse design, spectral simulation and MRS data analysis. Proc. Intl. Soc. Mag. Reson. Med. 2011;19 doi: 10.1002/mrm.29686. https://cds.ismrm.org/protected/11MProceedings/PDFfiles/1410.pdf Accessed September 6, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valette J., Boumezbeur F., Hantraye P., Lebon V. Simplified 13C metabolic modeling for simplified measurements of cerebral TCA cycle rate in vivo. Magn. Reson. Med. 2009;62(6):1641–1645. doi: 10.1002/mrm.22160. [DOI] [PubMed] [Google Scholar]
- Van Zijl P.C.M., Scott Chesnick A., Despres D., Moonen C.T.W., Rhiz-Cabello J., Van Gelderen P. In Vivo proton spectroscopy and spectroscopic imaging of {1-13C}-g1ucose and its metabolic products. Magn. Reson. Med. 1993;30(5):544–551. doi: 10.1002/mrm.1910300504. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Umeda M., Ishihara Y., et al. Human brain glucose metabolism mapping using multislice 2D 1H-13C correlation HSQC spectroscopy. Magn. Reson. Med. 2000;43(4):525–533. doi: 10.1002/(SICI)1522-2594(200004)43:4<525::AID−MRM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wright A.M., Murali-Manohar S., Borbath T., Avdievich N.I., Henning A. Relaxation-corrected macromolecular model enables determination of 1H longitudinal T1-relaxation times and concentrations of human brain metabolites at 9.4T. Magn. Reson. Med. 2021;87(1):33–49. doi: 10.1002/mrm.28958. [DOI] [PubMed] [Google Scholar]
- Wright A.M., Murali-Manohar S., Henning A. Quantitative T1-relaxation corrected metabolite mapping of 12 metabolites in the human brain at 9.4 T. Neuroimage. 2022;263 doi: 10.1016/J.NEUROIMAGE.2022.119574. [DOI] [PubMed] [Google Scholar]
- Wright A.M., Murali-Manohar S., Henning A. Quantitative T1-relaxation corrected metabolite mapping of 12 metabolites in the human brain at 9.4 T. Biorxiv. January 1, 2021;12(24) doi: 10.1101/2021.12.24.474092. Published online. [DOI] [PubMed] [Google Scholar]
- Xu S., Yang J., Shen J. Measuring N-acetylaspartate synthesis in vivo using proton magnetic resonance spectroscopy. J. Neurosci. Methods. 2008;172(1):8–12. doi: 10.1016/j.jneumeth.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüksel C., Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol. Psychiatry. 2010;68(9):785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegs T., Dorst J., Ruhm L., Avdievitch N., Henning A. Measurement of glucose metabolism in the occipital lobe and frontal cortex after oral administration of [1-13C]glucose at 9.4 T. J. Cerebr. Blood Flow Metabol. 2022;42(10):1890–1904. doi: 10.1177/0271678X221104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegs T., Wright A.M., Henning A. Test–retest reproducibility of human brain multi-slice 1H FID-MRSI data at 9.4T after optimization of lipid regularization, macromolecular model, and spline baseline stiffness. Magn. Reson. Med. 2022 doi: 10.1002/mrm.29423. (Epub ahead) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.