Abstract
Background
The COVID-19 pandemic highlighted the fragmented nature of governmental policy decisions in Europe. However, the extent to which COVID-19 vaccination policies differed between European countries remains unclear. Here, we mapped the COVID-19 vaccination policies that were in effect in January 2022 as well as booster regulations in April 2022 in Austria, Denmark, England, France, Germany, Ireland, Italy, the Netherlands, Poland, and Spain.
Methods
National public health and health policy experts from these ten European nations developed and completed an electronic questionnaire. The questionnaire included a series of questions that addressed six critical components of vaccine implementation, including (1) authorization, (2) prioritization, (3) procurement and distribution, (4) data collection, (5) administration, and (6) mandate requirements.
Results
Our findings revealed significant variations in COVID-19 vaccination policies across Europe. We observed critical differences in COVID-19 vaccine formulations authorized for use, as well as the specific groups that were provided with priority access. We also identified discrepancies in how vaccination-related data were recorded in each country and what vaccination requirements were implemented.
Conclusion
Each of the ten European nations surveyed in this study reported different COVID-19 vaccination policies. These differences complicated efforts to provide a coordinated pandemic response. These findings might alert policymakers in Europe of the need to coordinate their efforts to avoid fostering divergent and socially disruptive policies.
Keywords: COVID-19, Policy, Policy monitoring, Policy analysis, Europe, Vaccines
1. Introduction
The COVID-19 pandemic has exposed the fragmented nature of governmental policy decisions implemented in Europe [1], [2]. Non-pharmaceutical interventions (e.g., lockdowns, school closures, curfews, and travel restrictions) were frequently linked to national priorities and cultural, economic, and sociodemographic factors rather than scientific evidence [3]. Although partially explained by the differences in timing and spread of the pandemic, policy responses often were not centrally coordinated and lacked uniformity [3], [4], [5], [6], [7]. Likewise, the effectiveness of individual policy decisions varied dramatically [8], [9], [10], [11], [12].
National vaccine responses to COVID-19 were also varied. All vaccines are reviewed centrally by the European Medicines Agency (EMA), which authorizes the use of new agents based on quality, safety, efficacy, and risk–benefit balance. Despite this central coordination, the specific vaccines accepted for EU COVID-19 certificates (otherwise known as vaccine passports) differed substantially across Europe after their implementation in the summer of 2021 [4], [13]. Furthermore, reports that emerged in the spring of 2021 regarding the possible (albeit rare) risk of blood clots in individuals who received the AstraZeneca COVID-19 vaccine sparked divergent responses (eg, issuing a warning with no other restrictions, restricting use either completely or for certain population groups) [14]. Results from previous research studies have also observed variations in vaccine deployment within the European Union (EU) and on a global scale [15], [16]. As noted by Forman et al. [14], policy differences can fuel misinformation and confusion and may ultimately damage public trust and exacerbate vaccine hesitancy. A lack of international coordination could also lead to poorer public health outcomes and slower recoveries [15]. At this time, the divergence reflected by European COVID-19 vaccination policies and the overall impact of these policies remains unclear.
Travel medicine specialists have frequently been asked to consider the impact of divergent vaccine policies [17]. As early as the 1980s, physicians and policymakers within the European region recognized the challenges associated with attempts to harmonize childhood vaccinations [18]. Similarly, a 2017 analysis of migrant vaccination revealed significant differences between policies recommended for adults and those targeting children [19]. Legislation in the EU currently prohibits the adoption of regulations that enforce vaccine harmonization, seeing as the EU is not permitted to interfere with national health systems and can only “support Member States in their endeavours to combat cross-country health scourges” (Article 168(5) in the Treaty on the Functioning of the European Union) [20]. As a result, European countries retain sovereignty in this matter and can develop independent national vaccination policies. This policy can facilitate politically and culturally motivated decision-making and may lead to divergent vaccination strategies [21].
During the COVID-19 pandemic, several policy trackers were established to monitor the evaluation of COVID-19 policies across countries. For instance, the Oxford Government Response Tracker captures government policies related to closure and containment, as well as health and economic policy developments [22]. The COVID-19 Government Response Event Dataset (CoronaNet) is a daily-updated dataset that categorizes COVID-19 policies into over 100 sub-categories with detailed text descriptions [23]. Both datasets focus predominantly on the non-pharmaceutical interventions with only a few vaccine-specific measures being included. The included vaccine policy data comprises country vaccine allocation prioritisation lists, eligible groups, cost of vaccination to the individual, and the presence of a vaccine mandate for the Oxford Tracker and distribution, regulatory approval processes, purchasing, and resources spent on research and development for CoronaNet. While these datasets are useful for retrospective analyses to determine the effects of policies within national boundaries, they fall short when trying to understand how policy environments differ across countries and what the broader public health implications of such divergent policies can be.
In this manuscript, we aim to perform an in-depth analysis of the active COVID-19 vaccine policies in effect by February 1, 2022, across Europe and capture policy divergences between European countries. A follow-up analysis was performed for the booster vaccine policies that were in effect on April 30, 2022. We mapped and analyzed COVID-19 vaccine policy in Austria, Denmark, England,2 France, Germany, Ireland, Italy, the Netherlands, Poland, and Spain. We chose these ten countries based on convenience sampling [24]. However, the countries represent the majority of the EU population (74.44%; not including England) and a mix of different political and health systems [25], which allowed us to comparatively assess how a majority of EU citizens might be affected by divergent COVID-19 vaccine policies.
2. Methods
2.1. Questionnaire development and data collection
We developed a questionnaire designed to chart and analyze key vaccination policies across the aforementioned ten countries in Europe. This questionnaire was largely based on the framework established by the United States Operation Warp Speed (OWS) Strategy for Distributing a COVID-19 Vaccine [26], [27], [28]; four of the six critical components of our questionnaire came directly from the OWS Strategy. Three online meetings were held and several feedback rounds took place to consistently improve the form of the questionnaire. All co-authors were involved in the development of the questionnaire and the subsequent collection of materials using the questionnaire. The selection of questions in the questionnaire was intended to be broad enough to cover multiple dimensions of COVID-19 vaccine policy, yet remain focused enough to maintain feasibility. The full list of critical components and associated questions is included in the Supplementary Materials.
This effort was complemented by a rapid review of official national policy repositories (see eTable 1 in Supplementary Materials), PubMed, and Google (Scholar). The keywords used consisted of “COVID-19”, “coronavirus”, “policy”, “law”, “vaccine”, “vaccination” combined with the studied countries. These findings were ultimately merged into a single dataset based on categories and themes included in the questionnaire. The initial data collection took place between February 1 and February 28, 2022. The date of February 1, 2022 was chosen as the COVID-19 vaccine infrastructure had stabilised by this point and sufficient evidence was available on the effectiveness of COVID-19 vaccines [29], [30], [31]. Second, Europe had just experienced its largest COVID-19 wave so far [32], putting substantial pressure on healthcare systems. Booster vaccine policies were updated until April 30, 2022 (which still showed a sizeable daily incidence [32]), thus providing a complete overview of the COVID-19 vaccine policies in place following the largest wave of the pandemic. We ran the updated search for booster vaccine policies between May 1 and May 31, 2022. The updated search was performed by three authors (RVK, RF, RM) with support from all country contributors.
2.2. Data analysis
The data collected were open-ended, long-form responses to a series of questions focused on six critical components of vaccine implementation, including (1) authorization, (2) prioritization, (3) procurement and distribution, (4) data collection, (5) administration, and (6) mandate requirements. The scope of the data collected is shown in Table 1 . Because no standardized reporting guidelines exist for this type of research, we followed the Standards for Reporting Qualitative Research (SRQR) as closely as possible [33].
Table 1.
The scope of individual critical components of COVID-19 vaccine policy.
| Critical policy component | Scope |
|---|---|
| Vaccine authorization | Covers topics that include the specific vaccines that were chosen for use in each country and the individuals responsible for these choices. |
| Priority groups for vaccine eligibility | Captures topics that focus on the selection of groups that were provided with priority access to vaccines and which vaccine formulations were recommended for these individuals. |
| Vaccine procurement and distribution | Identifies the pathways used to make vaccines available to the general population. |
| Vaccine data collection | Captures the nature and type of data that were collected on vaccines and vaccination strategies. |
| Vaccine administration | Captures data on individuals tasked with administering the vaccines and how waste was minimized. |
| Vaccine mandate requirements | Covers topics including vaccine mandates, punitive measures and consequences for unvaccinated individuals, and the use of vaccine passports for international travel. |
3. Results
3.1. Vaccine authorization
By January 31, 2022, the Pfizer/BioNTech (Comirnaty®), Moderna (Spikevax®), and AstraZeneca (Vaxzeveria®) vaccines had been approved for use in all countries included in this study; the Johnson & Johnson and Novavax vaccines were authorized for use in all countries except England. The Pfizer/BioNTech vaccine was approved for use in individuals five years of age and older. The Moderna vaccine was approved for those 12 years of age and older, except in Ireland and Poland, where the lower age limit for this formulation was 18 years. The vaccines developed by AstraZeneca, Johnson & Johnson, and Novavax were approved for use for those 18 years of age and older in all ten studied countries.
In response to reports of the risk of developing vaccine-associated thrombosis, the AstraZeneca vaccine formulation was withheld by eight of the ten countries under study. England withheld the vaccine for individuals under 40 years of age and Austria ultimately stopped administration in June 2021 due to delivery problems and population wariness. France withheld the AstraZeneca vaccine from individuals under 30 years of age, while Ireland withheld the Johnson & Johnson formulation from anyone under age 50 after reports of a rare blood clotting condition following the vaccine administration. Over time, the AstraZeneca formulation was phased out in all ten countries that participated in this study in favor of the mRNA vaccines (Moderna and Pfizer/BioNTech).
3.2. Priority groups for vaccine eligibility
All countries initially prioritized vaccine distribution to healthcare workers, others at significant risk of contracting COVID-19, and individuals with significant pre-existing medical conditions. Among the policy differences reported in our data, England, Germany Ireland, and Italy included individuals aged 75 and older in their highest priority groups together with healthcare professionals. By contrast, Austria, France, the Netherlands, Poland, and Spain included this cohort in their second or third-highest priority groups. All children five years of age and older were eligible to be vaccinated in all countries under study, except in England where, as of January 2022, children were only eligible if they were members of a high-risk group (eg, children with auto-immune conditions, cardiovascular diseases, or respiratory conditions).
Our findings also revealed substantial heterogeneity in eligibility for a first booster vaccination. In Austria, Germany, Italy, and Poland, all individuals 12 years of age and older were eligible for a first booster vaccination, as were those who were 16 years of age and older in England and Ireland; by contrast, booster vaccinations for 12-to-15-year-olds in England were provided only to those who met strict conditions (e.g., were immunocompromised or members of specific clinical risk groups). Denmark, the Netherlands, and Spain considered individuals 18 years of age and older as eligible for a first booster vaccination. Similar heterogeneity was reported regarding eligibility for the second booster vaccination as of April 2022.
In terms of priority groups for second boosters, Austria recommended a second booster to those 80 years or older, and at-risk population groups between the ages of 65 and 79 years. Ireland advised a second booster for those 65 years or older and those who are immunocompromised. Denmark and Spain advised a second booster only for persons with severely compromised immune system. Germany advised a second booster for those 70 years and older, nursing home residents, those who are immunosuppressed, and healthcare workers working in medical facilities and nursing homes. England, France, Italy, the Netherlands, and Poland did not specifically designate any population groups as priority groups for a second booster.
3.3. Procurement and distribution of vaccines
Vaccine procurement was coordinated centrally in all ten countries under study. Vaccine procurement in England was coordinated by the United Kingdom; vaccines were then divided among the four member countries. While distribution efforts were also predominantly centralized in the countries under study, some differences were observed. For example, vaccine distribution in Germany was coordinated via a two-level process. Vaccines were first procured at the national level and distributed to constituent states according to their population size (until June 2021) or based on demand (after June 2021); the state authorities then distributed vaccines to various locales. Italy and the Netherlands used a similar approach and distributed the vaccines to their respective regions; the regional authorities were then tasked with delivering the vaccines to the people. Centralised distribution was based on factors such as the number of vaccines available on a given day and number of eligible people to be vaccinated in a designated region. Austria (similar to Germany) distributed vaccines to its constituent states according to population size, while Spain distributed its vaccines to each of its autonomous communities based on the population size of each priority group, as defined by the national vaccination strategy.
3.4. Collection of vaccination data
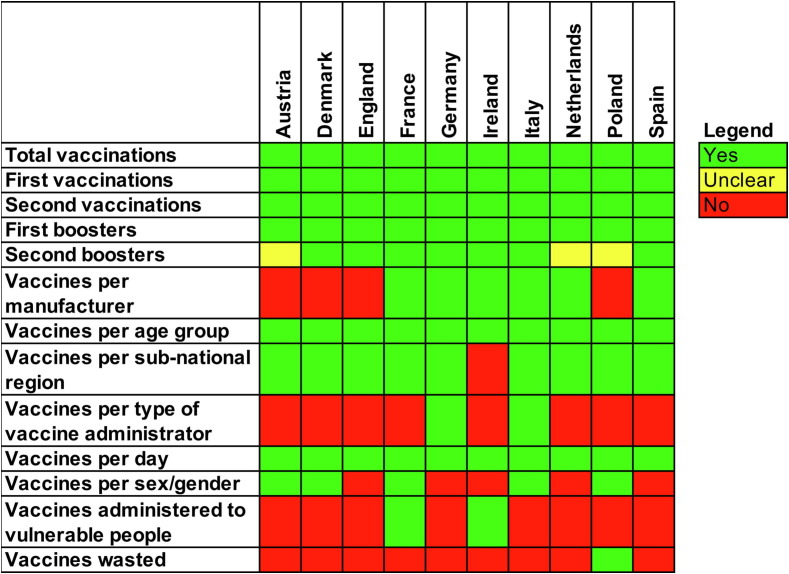
Authorities in each country collected different data related to COVID-19 vaccines, as shown in Fig. 1 . Poland was the only country that reported the number of vaccine doses wasted. All participating countries tracked data at both the national and sub-national levels and provided daily reports. All countries collected data on adverse events through their respective pharmacovigilance systems.
Fig. 1.
A heat map of the presence (green) or absence (red) of public data on COVID-19 vaccines and vaccination strategies collected from ten European countries and made available within days after vaccination. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Vaccine administration
All ten countries delivered vaccinations at centers or mega-centers at some point during the campaigns. Austria, England, France, Ireland, Italy, and Spain reported increased use of the outpatient healthcare sector over time as they became more experienced with the processes and in response to changes in the number of vaccine recipients. While all countries required physician oversight and physician approval of vaccine administration, the actual administration could be completed by other medical staff members under the supervision or management of a physician. In Italy, only healthcare workers trained in vaccination techniques were permitted to administer vaccines. Denmark, England,France, parts of Germany, and Spain mobilized retired healthcare professionals as additional support to administer vaccines. Several countries also recruited non-medical professionals who were trained to administer COVID-19 vaccines and remained under the supervision of medical staff. For example, France mobilized firefighters and England permitted paid volunteers to undergo training that would enable them to administer vaccines. Similarly, Poland recruited school hygienists who had at least six months of experience in administering preventive vaccinations to serve in this effort. All studied countries adopted strategies for reaching vulnerable populations, though the approaches diverged considerably (see Table 2 in Supplementary Materials pp11-12).
3.6. Vaccine mandate requirements
In most of the countries under study, vaccination was not legally required at the national level. However, many leisure activities (eg, local sports events, movie theaters, mid-sized gatherings) required proof of vaccination. In Austria, Denmark, Germany, the Netherlands, and Spain, proof of recovery was also accepted as a substitute for proof of vaccination. Among the studied countries, Austria was the only nation to announce a nationwide vaccine mandate; after March 2022, two fines of up to 600 euros per year would be issued to those failing to comply. In France, non-healthcare employers were permitted to issue vaccine requirements at their discretion. Similarly, a Danish law issued in November 2021 permitted employers to require documentation of vaccination or recovery status from their employees. By contrast, employers in the Netherlands were not permitted to request proof of vaccination as a prerequisite for employment. While policies in Spain differed across the autonomous communities, no national vaccine mandate was ratified. Instead, those who remained unvaccinated were required to quarantine themselves if they experienced close contact with anyone testing positive for COVID-19; this was not required for those who had been vaccinated. Germany also did not ratify a national vaccine mandate for the general population.
In contrast to rules established for the general public, healthcare workers in England, France, Germany, Italy, and Poland were subjected to vaccine mandates (with exemptions under specific conditions). Italy expanded this mandate to include school staff, the military, and prison personnel, while Poland expanded this mandate to include pharmacists and medical students.
Most countries adopted additional requirements for international visitors. Germany did not implement a vaccine mandate for those entering the country. However, depending on the country from which they entered (i.e., no risk, high risk, or COVID-19 variant of concern present), unvaccinated travelers were required to complete a digital entry registration, present a negative COVID-19 test upon arrival, and quarantine for up to 14 days; travelers who were fully vaccinated were not required to quarantine or undergo COVID-19 testing. By contrast, fully vaccinated passengers entering England were required to schedule a test on the second day of their stay but did not need to undergo quarantine; those who were not fully vaccinated were required to undergo COVID-19 testing on or before day 2 of their visit and on or after day 8 with polymerase chain reaction (PCR) tests, and quarantine for a full 10 days after their arrival. In France, fully vaccinated travelers were required to present a negative PCR test but were not quarantined upon arrival. Similarly, all travelers entering Denmark were required to present negative COVID-19 tests or proof of recovery during the period from December 27, 2021, to January 31, 2022. From February 1, 2022, onwards, travelers documenting full vaccination or recovery from COVID-19 were permitted to enter the country without restrictions. Persons arriving in Denmark from the EU and Schengen areas without this documentation were required to present a negative antigen or PCR test or to undergo testing within 24 hours of arrival. Travelers from high-risk countries outside the EU/Schengen were required to quarantine and undergo testing. Finally, Austria and Poland required unvaccinated travelers to quarantine for up to 10 days, although Poland provided exemptions for travelers that presented negative results from COVID-19 tests taken within 48 hours of entry.
Additional country-specific findings addressing all six categories are included in eTable 2 in the Supplementary Materials.
4. Discussion
The results of our study revealed substantial country-specific differences in COVID-19 vaccination policies throughout Europe. This was particularly evident in our findings related to the authorization of COVID-19 vaccines, recommendations for and prioritization of specific groups, recording of vaccination-related data, vaccination mandates, and requirements. Previous research has highlighted the impact of divergent COVID-19 policies that outline the vaccines that are accepted for COVID-19 certificates on travel capabilities within Europe [13]. Different vaccine mandates and requirements, as well as acceptance (or not) of proof of recovery, may further complicate a coordinated public health response across European countries. As shown in this study, the rules of entry were not harmonized at the European (or EU) level at the time this study was conducted. Results from previous studies revealed that divergent policies might also contribute to the persistence of infectious diseases in the population [18], [19].
However, our results revealed relatively less divergence among the COVID-19 vaccination policies compared to non-pharmaceutical interventions [3], [4], [5]. This may be attributed at least in part to EU-mediated intervention in the vaccine deployment process [15]. When our findings are compared to the values set out by the EU Strategy for COVID-19 Vaccines that was published in June 2020 during the first months of the pandemic [34], we observe that, although the ten countries under study ultimately followed the recommendations provided by the European Commission, these outcomes were reached via different strategies and on different timelines. The countries participating in our study also ensured incremental access to the vaccines for all those five years of age and older and followed the EU recommendations as much as possible despite initial supply-side vaccine shortages. In terms of equitable access, we found that population groups in these countries who were at high risk of developing severe COVID-19 (e.g., the elderly and immunocompromised individuals) were universally prioritized during the vaccine rollout. Many of the countries in our study also employed specific strategies or recommendations designed to reach underserved and minority communities (e.g., mobile/pop-up vaccination clinics, translations of vaccine advertisements), though the effects of these interventions remain unknown. Furthermore, if countries provide different recommendations based on safety concerns (i.e., rare thrombosis associated with the AstraZeneca and Johnson & Johnson vaccines), vaccine confidence issues may emerge, and individuals become concerned as to why a specific vaccine might be available in one country but not in another [14], [35], [36], [37].
Other issues reported across Europe include differences in the types of vaccines included in the vaccination schedule, the number of vaccine doses, the group for whom a specific vaccination is approved, and the ages at which vaccines and boosters are recommended [38], [39]. For instance, Sweden decided against vaccinating children aged 5–11 years who were in low-risk groups [10], [40]. Although Sweden was not included in our ten-nation dataset, this is yet another policy divergence that complicates the COVID-19 vaccination infrastructure within the European region. If left unaddressed, these policy differences may eventually impede public health outcomes and further erode the public trust in governmental institutions and vaccine-focused science [41].
Data collection processes, technology and the level of detail recorded all diverged across the ten countries under study. Several groups have called for efforts to harmonize data collection on policy responses to COVID-19 so that lessons can be learned and best practices identified [42]. Experts have also highlighted the need for interconnected and interoperable data networks based on common standards used in current disease surveillance and response efforts [1]. Our study highlights the divergences that remain common and reveals the points at which specific changes might be made. Our results provide important insights which may be used to improve pandemic responses and help European countries in their efforts to harmonize and coordinate vaccination efforts against existing and emerging diseases.
This study has several limitations. First, the findings represent a cross-section of the policy landscape in January 2022 (and April 2022 for data on second booster vaccine policies) and do not capture any temporal trends. Second, we did not delve deeply into potential regional differences. Third, our findings were based exclusively on publicly available data. We did not – where possible – initiate the process to gain access to internal governmental documentation which could contain richer COVID-19 vaccine data. Fourth, while our findings provide an overview of the policy measures established and announced in the ten countries under study, our data do not comment on how effectively these measures were implemented. Finally, this paper focused exclusively on COVID-19 vaccination policies; its findings cannot be directly applied to policies and strategies employed for other vaccinations.
Future work might focus on a deeper evaluation of processes that contributed to divergent COVID-19 vaccine policies that were instituted as the pandemic progressed. A follow-up study might provide insight into why countries were prioritizing different individuals and authorizing different vaccines, and why some countries began their vaccine campaigns before others. Additionally, further investigation might help those in charge to understand the problems that may have developed from inconsistent communication. Previous research reported varying levels of COVID-19 vaccine hesitancy for primary vaccinations and boosters across our studied countries, which results in a call for vaccination campaigns tailored to (sub-)national circumstances [43], [44]. While this article gives insights in the underlying vaccine policy landscape that underpins vaccination campaigns, it did not investigate the divergences between government or public health agencies’ communication policies across countries (aimed at tackling vaccine hesitancy). By extension, the effects of communication policies on vaccination rates and choices of vaccines might also be explored. Factors contributing to divergent vaccine policies should be explained more clearly to the general public in an effort to reduce misinformation and increase vaccine acceptance [14]. Our results may also serve as a cautionary signal to policymakers in Europe and beyond. Our findings highlight the potential negative impacts of operating in isolation and fostering additional and ongoing policy divergences, especially because these are not limited to Europe and are reported globally [16], [45], [46], [47]. Furthermore, this article encourages public and civic organizations to seek transparency from their governments, particularly with respect to vaccines and the rationales underlying specific policy divergences.
Overall, our findings revealed that COVID-19 vaccination policies in Europe diverged noticeably between the countries based on data collected through April 2022. While some vaccination policy differences may be justified by epidemiologic trends, cultural norms and practices, these differences may prevent governments and national public health and health security within Europe from providing a coordinated public health response to the current pandemic and illustrate an alarming precedent for future public health threats.
Funding
The authors declare that no funding was acquired for this research.
Data availability
All supporting data were acquired from publicly available databases. The list of databases is found in eTable 1 in the Supplementary Materials.
Ethical consideration
This article did not have any ethical implications or considerations.
Conflicts of interest.
ID-Z declares that she has received honoraria from Pfizer in the past for participating in advisory boards. These honoraria were not connected to this research project and Pfizer had no role in the development, execution, or presentation of this research. BR participated in this research independently of her role as an employee at MSD. MSD provided no funding or other support for this research. The views expressed are those of the authors and are not necessarily those of MSD.
CRediT authorship contribution statement
Robin van Kessel: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration. Rebecca Forman: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Project administration. Ricarda Milstein: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Alicja Mastylak: Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Katarzyna Czabanowska: Validation, Investigation, Writing – review & editing. Thomas Czypionka: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing. Isabelle Durand-Zaleski: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing. Anja Hirche: Validation, Investigation, Writing – review & editing. Magdalena Krysinska-Pisarek: Validation, Investigation, Writing – review & editing. Laia Maynou: Validation, Investigation, Writing – review & editing. Bjelle Roberts: Validation, Investigation, Writing – review & editing. Aleksandra Torbica: Validation, Investigation, Writing – review & editing. Karsten Vrangbæk: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing. Yuxi Wang: Validation, Investigation, Writing – review & editing. Olivier J. Wouters: Validation, Investigation, Writing – review & editing. Elias Mossialos: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Bjelle Roberts reports a relationship with Merck Sharp and Dohme that includes: employment. Isabelle Durand-Zaleski reports a relationship with Pfizer that includes: consulting or advisory.
Acknowledgements
We thank Katarzyna Kolasa for her input in discussions focused on this manuscript.
Footnotes
Although England is no longer an EU Member State, it has retained strong connections particularly with respect to work and travel. Thus, its conditions and policies remain relevant to those engaged in travel medicine, infectious disease control, and pandemic management within the European continent.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.036.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data is added in supplementary materials
References
- 1.Forman R., Azzopardi-Muscat N., Kirkby V., Lessof S., Nathan N.L., Pastorino G., et al. Drawing light from the pandemic: Rethinking strategies for health policy and beyond. Health Policy. 2021 doi: 10.1016/j.healthpol.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal N., Howlett M. “Measuring the Mix” of Policy Responses to COVID-19: Comparative Policy Analysis Using Topic Modelling. J Comp Policy Anal Res Pract. 2021;23:250–261. doi: 10.1080/13876988.2021.1880872. [DOI] [Google Scholar]
- 3.Baum F., Freeman T., Musolino C., Abramovitz M., Ceukelaire W.D., Flavel J., et al. Explaining covid-19 performance: what factors might predict national responses? BMJ. 2021;372 doi: 10.1136/bmj.n91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman R., Mossialos E. The EU Response to COVID-19: From Reactive Policies to Strategic Decision-Making. JCMS J Common Mark Stud. 2021;59:56–68. doi: 10.1111/jcms.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iftekhar E.N., Priesemann V., Balling R., Bauer S., Beutels P., Calero Valdez A., et al. A look into the future of the COVID-19 pandemic in Europe: an expert consultation. Lancet Reg Health - Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suau-Sanchez P., Voltes-Dorta A., Cugueró-Escofet N. An early assessment of the impact of COVID-19 on air transport: Just another crisis or the end of aviation as we know it? J Transp Geogr. 2020;86 doi: 10.1016/j.jtrangeo.2020.102749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque S., Hrzic R., Yassaee A., van Kessel R. European Public Health Alliance; Brussels: 2021. COVID-19 Response: Comparative Case Study between Italy, Sweden and South Korea. https://epha.org/wp-content/uploads/2021/12/hi-casestudy-covid19response.pdf. [Google Scholar]
- 8.Dergiades T., Milas C., Mossialos E., Panagiotidis T. Effectiveness of government policies in response to the first COVID-19 outbreak. PLOS Glob Public Health. 2022;2:e0000242. doi: 10.1371/journal.pgph.0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma M., Mindermann S., Rogers-Smith C., Leech G., Snodin B., Ahuja J., et al. Understanding the effectiveness of government interventions against the resurgence of COVID-19 in Europe. Nat Commun. 2021;12:5820. doi: 10.1038/s41467-021-26013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irfan F.B., Minetti R., Telford B., Ahmed F.S., Syed A.Y., Hollon N., et al. Coronavirus pandemic in the Nordic countries: Health policy and economy trade-off. J Glob Health. 2022;12:05017. doi: 10.7189/jogh.12.05017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timur L., Xie Y. Is border closure effective in containing COVID-19? Travel Med Infect Dis. 2021;44 doi: 10.1016/j.tmaid.2021.102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z., Weber E., Strohsal T., Serhan D. Sustainable border control policy in the COVID-19 pandemic: A math modeling study. Travel Med Infect Dis. 2021;41 doi: 10.1016/j.tmaid.2021.102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal-Ré R., Banzi R., Becker S.L., Launay O., Pavli A. High heterogeneity on the accepted vaccines for COVID-19 certificates in European countries. Travel Med Infect Dis. 2022;48 doi: 10.1016/j.tmaid.2022.102321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman R., Jit M., Mossialos E. Divergent vaccination policies could fuel mistrust and hesitancy. Lancet. 2021;397:2333. doi: 10.1016/S0140-6736(21)01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jit M., Ananthakrishnan A., McKee M., Wouters O.J., Beutels P., Teerawattananon Y. Multi-country collaboration in responding to global infectious disease threats: lessons for Europe from the COVID-19 pandemic. Lancet Reg Health - Eur. 2021:9. doi: 10.1016/j.lanepe.2021.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavli A., Maltezou H.C. Travel vaccines throughout history. Travel Med Infect Dis. 2022;46 doi: 10.1016/j.tmaid.2022.102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaack J.-C. Harmonization of Vaccination Policy. Biologicals. 1997;25:243–245. doi: 10.1006/biol.1997.0093. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves S, Nellums LB, Ravensbergen SJ, Friedland JS, Stienstra Y, on behalf of the ESGITM Working Group on Vaccination in Migrants. Divergent approaches in the vaccination of recently arrived migrants to Europe: a survey of national experts from 32 countries, 2017. Eurosurveillance 2018;23:1700772. Doi: 10.2807/1560-7917.ES.2018.23.41.1700772. [DOI] [PMC free article] [PubMed]
- 20.European Commission. Treaty on the Functioning of the European Union, Brussels. vol. 326. 2009.
- 21.Sabahelzain M.M., Hartigan-Go K., Larson H.J. The politics of Covid-19 vaccine confidence. Curr Opin Immunol. 2021;71:92–96. doi: 10.1016/j.coi.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale T., Angrist N., Goldszmidt R., Kira B., Petherick A., Phillips T., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 23.Cheng C., Barceló J., Hartnett A.S., Kubinec R., Messerschmidt L. COVID-19 Government Response Event Dataset (CoronaNet vol 1.0). Nat. Hum Behav. 2020;4:756–768. doi: 10.1038/s41562-020-0909-7. [DOI] [PubMed] [Google Scholar]
- 24.Marshall M.N. Sampling for qualitative research. Fam Pract. 1996;13:522–525. doi: 10.1093/fampra/13.6.522. [DOI] [PubMed] [Google Scholar]
- 25.Eurostat. Population size on 1 January 2022. https://ec.europa.eu/eurostat/databrowser/view/tps00001/default/table?lang=en (accessed February 14, 2023).
- 26.Kim J.H., Hotez P., Batista C., Ergonul O., Figueroa J.P., Gilbert S., et al. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017–e1021. doi: 10.1016/S2214-109X(21)00140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slaoui M., Hepburn M. Developing Safe and Effective Covid Vaccines — Operation Warp Speed’s Strategy and Approach. N Engl J Med. 2020;383:1701–1703. doi: 10.1056/NEJMp2027405. [DOI] [PubMed] [Google Scholar]
- 28.U. S. Government Accountability Office. Operation Warp Speed: Accelerated COVID-19 Vaccine Development Status and Efforts to Address Manufacturing Challenges 2021. https://www.gao.gov/products/gao-21-319 (accessed August 3, 2022).
- 29.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus Pandemic (COVID-19). Our World Data 2020.
- 33.O’Brien B.C., Harris I.B., Beckman T.J., Reed D.A., Cook D.A. Standards for Reporting Qualitative Research: A Synthesis of Recommendations. Acad Med. 2014;89:1245–1251. doi: 10.1097/ACM.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 34.European Commission. EU Strategy for COVID-19 vaccines, Brussels. 2020.
- 35.Lin C., Tu P., Beitsch L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines. 2021;9:16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Albuquerque Veloso Machado M, Roberts B, Wong BLH, van Kessel R, Mossialos E. The Relationship Between the COVID-19 Pandemic and Vaccine Hesitancy: A Scoping Review of Literature Until August 2021. Front Public Health 2021;9:747787. Doi: 10.3389/fpubh.2021.747787. [DOI] [PMC free article] [PubMed]
- 37.Ledford H. Which COVID boosters to take and when: a guide for the perplexed. Nature. 2022 doi: 10.1038/d41586-022-02221-w. [DOI] [PubMed] [Google Scholar]
- 38.Merck Sharp & Dohme (MSD). The value of paediatric vaccination: Protecting current and future generations across Europe. Brussels: Merck Sharp & Dohme; 2020.
- 39.Rechel B., Richardson E., McKee M. European Observatory on Health Systems and Policies; Brussels: 2019. The organization and delivery of vaccination services in the European Union. [Google Scholar]
- 40.Ahlander J., Kasolowsky R. Sweden decides against recommending COVID vaccines for kids aged 5–11. Reuters. 2022 [Google Scholar]
- 41.Lazarus J.V., Romero D., Kopka C.J., Karim S.A., Abu-Raddad L.J., Almeida G., et al. A multinational Delphi consensus to end the COVID-19 public health threat. Nature. 2022:1–14. doi: 10.1038/s41586-022-05398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson A., Forman R., Azzopardi-Muscat N., Battista R., Colombo F., Glassman A., et al. Effective post-pandemic governance must focus on shared challenges. Lancet. 2022;399:1999–2001. doi: 10.1016/S0140-6736(22)00891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinert J.I., Sternberg H., Prince H., Fasolo B., Galizzi M.M., Büthe T., et al. COVID-19 vaccine hesitancy in eight European countries: Prevalence, determinants, and heterogeneity. Sci Adv. 2022;8:eabm9825. doi: 10.1126/sciadv.abm9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarus J.V., Wyka K., White T.M., Picchio C.A., Gostin L.O., Larson H.J., et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. 2023;29:366–375. doi: 10.1038/s41591-022-02185-4. [DOI] [PubMed] [Google Scholar]
- 45.Itani R., Karout S., Khojah H.M.J., Rabah M., Kassab M.B., Welty F.K., et al. Diverging levels of COVID-19 governmental response satisfaction across middle eastern Arab countries: a multinational study. BMC Public Health. 2022;22:893. doi: 10.1186/s12889-022-13292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Brumer A., Hill D., Andrade-Romo Z., Solari K., Adams E., Logie C., et al. Vaccines for all? A rapid scoping review of COVID-19 vaccine access for Venezuelan migrants in Latin America. J Migr Health. 2021;4 doi: 10.1016/j.jmh.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bardosh K., de Figueiredo A., Gur-Arie R., Jamrozik E., Doidge J., Lemmens T., et al. The unintended consequences of COVID-19 vaccine policy: why mandates, passports and restrictions may cause more harm than good. BMJ Glob Health. 2022;7:e008684. doi: 10.1136/bmjgh-2022-008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data were acquired from publicly available databases. The list of databases is found in eTable 1 in the Supplementary Materials.
Data is added in supplementary materials