Graphical abstract
Keywords: Post-acute SARS-CoV-2 symptoms, Thymosin alpha 1, Immune regulation, Anti-inflammatory response
Abstract
The complex alterations of the immune system and the immune-mediated multiorgan injury plays a key role in host response to SARS-CoV-2 infection and in the pathogenesis of COVID-19, being also associated with adverse outcomes. Thymosin alpha 1 (Tα1) is one of the molecules used in the treatment of COVID-19, as it is known to restore the homeostasis of the immune system during infections and cancer. The use of Tα1 in COVID-19 patients had been widely used in China and in COVID-19 patients, it has been shown to decrease hospitalization rate, especially in those with greater disease severity, and reduce mortality by restoring lymphocytopenia and more specifically, depleted T cells. Persistent dysregulation with depletion of naive B and T cell subpopulations and expansion of memory T cells suggest a chronic stimulation of the immune response in individuals with post-acute sequelae of SARS-CoV-2 infection (PASC). Our data obtained from an ex vivo study, showed that in PASC individuals with a chronically altered immune response, Tα1 improve the restoration of an appropriate response, most evident in those with more severe illness and who need respiratory support during acute phase, and in those with specific systemic and psychiatric symptoms of PASC, confirming Tα1 treatment being more effective in compromised patients. The results obtained, along with promising reports on recent trials on Tα1 administration in patients with COVID-19, offer new insights into intervention also for those patients with long-lasting inflammation with post-infectious symptoms, some of which have a delayed onset.
1. Introduction
SARS-CoV-2 infection is able to activate macrophages and dendritic cells that trigger an initial immune response, including lymphocytosis and cytokine release, often leading to an uncontrolled cytokine storm that results in respiratory stress syndrome (ARDS) [1], [2], [3]. In this context, actors such as myeloid cells have been described as responsible for the pathophysiology of the disease by contributing to local tissue damage and acting as potential producers of cytokines that lead to the hyper-inflammatory state observed in severe COVID-19 [4], [5], [6], [7]. In agreement, the marker of viral disease CD169 [8] was shown to be strongly increased in circulating monocytes from COVID-19 patients compared to those from healthy donors [9]. The complex alterations of the immune system and the immune-mediated multiorgan injury plays a key role in host response to SARS-CoV-2 infection and in the pathogenesis of COVID-19, and modification of total lymphocytes indicates a potential association between this cell subset and viral pathogenic mechanism. The function of natural killer and CD8 + T cells has been found to be affected during infection and restoration after therapy, highlighting the association of functional exhaustion of cytotoxic lymphocytes with COVID-19 and high circulation of cytokine, which have been associated with adverse outcomes [10], [11]. Hence, the viral sepsis observed in more severe COVID-19 is the systemic consequences causing the multi-organ dysfunction syndrome [12], [13].
For its pleiotropic activities, especially in restoring the homeostasis of the immune system during infections and cancer [14], [15], [16], Thymosin alpha 1 (Tα1) is one of the molecules used in the treatment of COVID-19, and several studies demonstrated its possible involvement in the control of the disease [17], [18]. In our previous work, we observed the upregulation of genes associated with cytokine signalling and production in blood cells from COVID-19 patients, and that ex vivo treatment with Tα1 was able mitigate cytokine storm [19]. Moreover, we captured the interconnected biological processes regulated by Tα1 in CD8 + T cells under inflammatory conditions, demonstrating the ability of Tα1 to inhibit lymphocyte activation in the CD8 + T cell subset specifically, highlighting the possible Tα1 mechanism of action in COVID-19.
After three years from the first cases, it is well known that SARS-CoV-2 infection causes several alterations that have an impact on various body districts, with important and persistent symptoms after infection. Complex symptoms lasting more weeks after infection have been defined by Centre for Disease Control and Prevention (CDC) as post-acute sequelae of SARS-CoV-2 infection (PASC) characterized by neurological, cardiorespiratory, and others physical problems, but also social and psychological impairments [20], [21], [22]. In PASC individuals, persistent immune dysfunction has been demonstrated to be caused by a chronic inflammatory state. Pro-inflammatory cytokines such as IL-1, IL-6, TNF-α persist at the serum level even after SARS-CoV-2 infection, probably defining the persistent inflammation present in individuals with PASC. Additionally, PASC with symptoms shown higher levels of IL-17 and IL-2, while subjects without alterations showed higher levels of modulatory cytokines such as IL-10 [23]. Furthermore, persistent immune dysregulation with depletion of naive B and T cells and expansion of PD-1 (Programmed cell death-1) + CD8 memory T cells was described in PASC individuals [24].
Based on previous data and promising achievements obtained from several trials of the treatment with Tα1 in acute COVID-19 patients [17], [18], aim of this work was to evaluate the possible role of Tα1 in modulating the expression of genes and proteins involved in immune response, cytokine storm and inflammation in post-COVID syndrome.
2. Materials and methods
2.1. Enrolment of patients and clinical data
Ten post-acute sequelae of SARS-CoV-2 infection (PASC) previously hospitalized during the acute Policlinic phase of Tor Vergata in the Infectious Diseases Clinic were enrolled in an open study by the Departments of System Medicine and Experimental Medicine of the University of Rome, ‘Tor Vergata’. Ethical approval for the collection and use of human samples was obtained from the ethics board of the Hospital 'Tor Vergata', COrona VIrus Disease: Safety and efficacy of experimental treatment (COVID_SEET prot.7562/2020, 9 April 2020, experimental register 46.20).
All subjects included in the study provided their written informed consent. Clinical data from PASC individuals were collected and reported in Table 1 . It should be noted that the specific biochemical alteration (high level of CRP, fibrinogen, and LDH) remains persistent even several weeks after acute infection. For certain molecular and immunological analysis, this cohort was compared with healthy donors (HD) and acute COVID-19 (a-COV) enrolled in a previous study [19].
Table 1.
Clinical data of PASC individuals (N = 10).
|
Number |
Percentage (%) |
|||
|---|---|---|---|---|
| Sex (F/M) | 3/7 | 30/70 | ||
| Severity in Acute Phase (yes/no) | 6/4 | 60/40 | ||
| Respiratory support (yes/no) | 6/4 | 60/40 | ||
| Comorbidities | 2/8 | 20/80 | ||
| PASC symptoms | ||||
| Sistemic | 5/5 | |||
| Cardiorespiratory | 5/5 | |||
| Cutaneous | 6/4 | |||
| Gastrointestinal | 7/3 | |||
| Neurological | 5/5 | |||
| Psichiatric | 5/5 | |||
| Interquartile Range (IQR) (25–50-75) | ||||
| Age | 32.75 | 55.00 | 75 | Years |
| Days of Hospitalization in Acute Phase | 0 | 10.50 | 68.65 | Days |
| Weeks after the-Acute Infection | 9.75 | 22 | 14.75 | Weeks |
| Biochemical Data | ||||
| Interquartile Range (IQR) (25–50-75) | Range values | |||
| RED BLOOD CELLS | 3.16 | 4.08 | 4.79 | 4.40–6 (106/μl) |
| HEMOGLOBIN | 11.77 | 12.70 | 13.47 | 13–18 g/dl |
| HEMATOCRIT | 34.52 | 38.30 | 41.17 | 36–51 (%) |
| PLATELETS | 131.75 | 217.50 | 246.75 | 150–450 (103/μl) |
| WHITE BLOOD CELLS | 5.24 | 6.11 | 8.81 | 4.30–10.8 (103/μl) |
| NEUTROabs | 3.21 | 4.58 | 5.735 | 103/μl L |
| LYMPHOabs | 0.82 | 1.285 | 2.0125 | 103/μl |
| MONOabs | 0.28 | 0.45 | 0.64 | 103/μl |
| EOabs | 0.00 | 0.015 | 0.05 | 103/uL |
| BASOabs | 0.01 | 0.01 | 0.015 | 103/uL |
| NEUTROperc | 60.60 | 66.35 | 82.45 | 40–75 (%) |
| LYMPHOperc | 13.30 | 22.90 | 32.10 | 20–45 (%) |
| MONOperc | 6.10 | 6.95 | 0.775 | 3,4–11 (%) |
| EOperc | 0.00 | 0.30 | 0.80 | 0–7 (%) |
| BASOperc | 0.175 | 0.20 | 0.30 | 0–1,50 (%) |
| PTper | 73.50 | 88.50 | 95.50 | 70–130 (%) |
| PTINR | 1.02 | 1.08 | 1.20 | 0,80–1,20 |
| PTSec | 11.92 | 12.95 | 14.25 | sec |
| APTTRATIO | 0.90 | 0.925 | 1.07 | 0,80–1,20 |
| APTTSec | 26.50 | 27.10 | 30.57 | 25–38,50 (sec) |
| Fibrinogen | 420.00 | 614.50 | 682.50 | 200–400 (mg/dl) |
| D-DIMER | 333.50 | 470.00 | 982.75 | 0–500 (ng/ml) |
| GLYCEMIA | 102.50 | 112.50 | 131.00 | 83–100 (mg/dl) |
| AZOTEMIA | 29.25 | 34.50 | 62.50 | 18–55 (mg/dl) |
| ALBUMIN | 3.50 | 3.90 | 4.00 | 3,20–4,60 (gr/dl) |
| AST | 19.25 | 21.50 | 48.00 | 5–34 (U/l) |
| ALT | 11.00 | 24.00 | 47.50 | 0–55 (U/l) |
| LDH | 207.00 | 230.00 | 363.00 | 125–220 (U/l) |
| Reactive C Protein (RCP) | 10.42 | 34.45 | 68.72 | 0–5 mg/l |
| Red colour for values out of Normal Range | ||||
2.2. Ex vivo treatment
Blood samples were diluted (1:2) in Roswell Park Memorial Institute (RPMI) 1640 medium enriched with 2 mM L-glutamine, 100 U / ml of penicillin, 0.1 mg/ml of streptomycin, 10% fetal bovine serum. Blood samples were exposed to 50 μg/ml Tα1 for 48 h at 37° C in 5% CO2 (SciClone, Pharmaceutical). After incubation, samples were collected and analyzed by flow cytometry and real-time PCR. Each treatment condition was done in duplicate.
2.3. Real-time analysis
Blood samples were centrifuged and treated twice with red blood lysing buffer to remove red cells. After extraction, 100 ng of DNase-treated RNA (blood from the total RNA extraction kit, Grisp) was reverse transcribed into cDNA according to the manufacturer’s protocol (ImProm-IITM reverse transcription system, Promega). Gene expression was evaluated by real-time PCR on the Bio-Rad instrument CFX96Real-Time System, using SYBR Green (SMOBIO) chemistry and primers pairs as previously described [19].
2.4. Flow cytometry analysis
Blood samples (100 μL) were vortexed for 5 min and incubated for 15 min in the dark with 1.5 ml of VersaLyse Lysing Solution (Beckman Coulter, BC) to lyse red blood cells and select the total leukocyte population. Samples were incubated with antibodies of interest for 15 min in the dark: anti-human CD38-ECD, CD4-APC, CD8-BV605, CD3-BV510 (Biolegend). The stained cells were then washed with Dulbecco’s phosphate buffered saline, permeabilized with the IntraPrep Permeabilization Reagent kit and stained with anti-human IL-6 FITC (Immunotool), anti IFN-γ BV650 (BD Bioscences) and anti-IL-10 AF700 (BC). For certain analyses, the DuraClone IM T-cell subset tube (B53328, BC) was used and gated according to the Manufacturer’ instructions.
All stained cells were analyzed using CytoFLEX software (Beckman Coulter) and CytExpert 2.3 (BC). Results were expressed as percentage of positive cells.
2.5. Statistics
Statistical analysis of group-wise expression levels and response to treatment was performed using the nonparametric Kruskal-Wallis tests in the case of independent samples and through the Friedman test in the case of dependent samples. Pairwise associations between continuous variables were tested and the Spearman correlation coefficient was calculated. Significant differences are shown as *p < 0.050, **p < 0.010 and *** p < 0.001. Data analysis were performed using the SPSS statistical software system (version 23.0 for Windows, USA).
3. Results
3.1. Persistent deregulation of differentiation and exhaustion T cells markers in PASC individuals
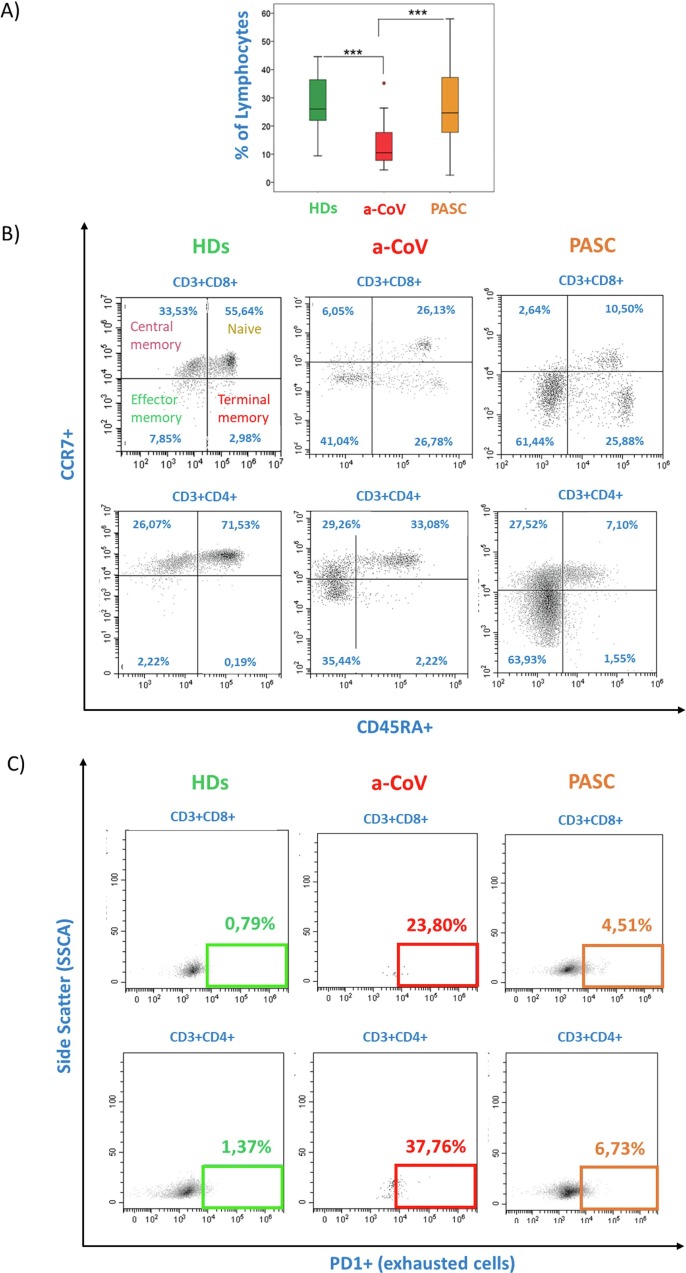
By means of flow cytometry analysis, a restoration in the percentage of lymphocytes was observed in PASC individuals with respect to a-COV, with results comparable to those observed in HD (Fig. 1 A). A deep analysis of markers associated with the differentiation process of CD8+ and CD4+ T cells (Fig. 1 B), as well as exhaustion markers (Fig. 1 C), demonstrated that a-COV had a significant alteration in T-cell differentiation markers with a relevant decrease in naive cells and an expansion of the effector memory (EM) compartment. Concomitantly, a significant increase in terminal effector memory (TEM) associated with a major loss of function occurred, particularly in CD8+ T cells. In PASC individuals, this situation remained unchanged compared to a-COV with a persistent loss of naive cells and a higher percentage of TEM in both the CD4+ and CD8+ T cell compartments, confirming the failure to recover the normal immune homeostasis in these subjects.
Fig. 1.
Flow cytometry analysis of differentiation and exhaustion markers in individuals with a-COV, PASC, and HD. The A) box plot represented the percentage of lymphocytes in HD (green), a-COV (red), and PASC (orange) individuals. Statistically significant values were obtained by Kruskal-Wallis test and considered when p < 0.001 (***). B) Representative dot plots of differentiation markers (CD45RA and CCR7) and C) Exhaustion PD-1 marker in the subsets of CD3+ CD4+ and CD3+ CD8+ lymphocytes. All data are reported in Table S1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The expression of the exhaustion markers PD-1 was significantly increased in the T cells of a-COV patients and remained altered in the PASC state, suggesting chronic functional exhaustion in these individuals (Fig. 1 and Table S1).
3.2. Treatment with Tα1 determines a reduction in central memory cells, an increase in effector Memory, and a mitigation of PD1 expression in PASC individuals
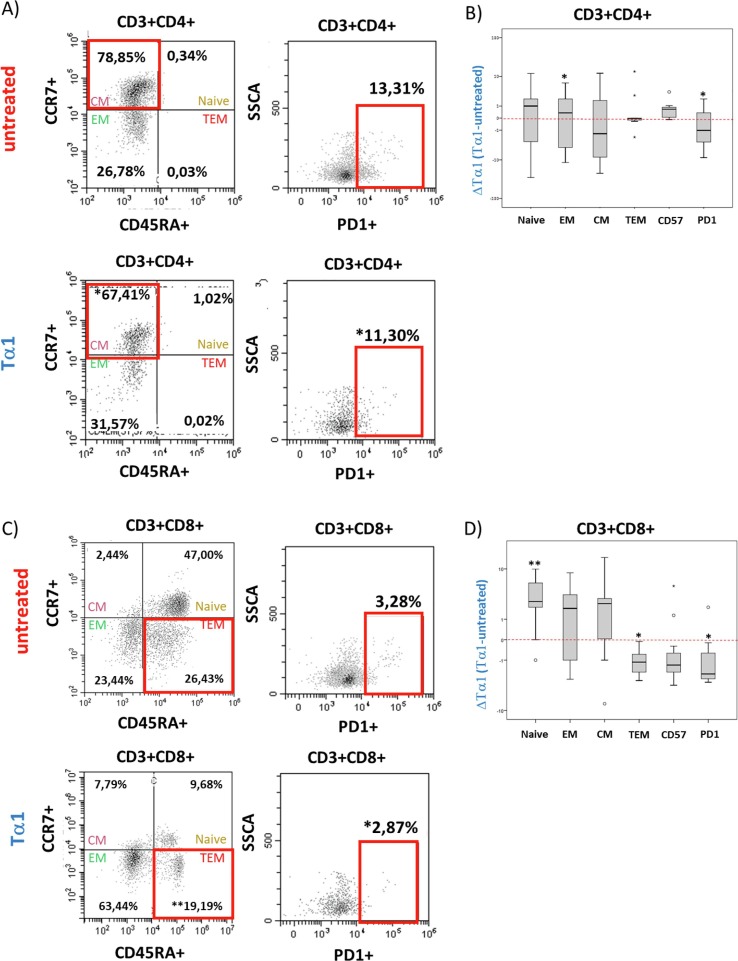
Taking into account the persistent immune dysfunction in individuals with PASC several weeks after the acute phase, we investigated the effects of Tα1 ex vivo treatment on blood cells of these individuals. Treatment with CD4+ T cells resulted in a decrease in central memory (CM) cells toward an EM profile and a significant decrease in cells expressing PD-1. Moreover, in CD8+ T cells a significant decrease in PD-1 and terminally differentiated cells was observed, suggesting that also in PASC individuals Tα1 modulated important processes favouring a restoration of a correct immune response (Fig. 2 and Table 2 ).
Fig. 2.
Flow cytometry analysis of differentiation and exhaustion markers in PASC individuals treated with Tα1. A) Representative dot plots of differentiation markers (CD45RA and PD-1 CCR7) and exhaustion marker in CD3+CD4+ (A) and CD3+CD8+ (C) lymphocytes in presence or not of Tα1. B) Data are represented as Histogram (Delta of Tα1 vs untreated) of differentiation and exhaustion markers in CD3+CD4+ (B) and CD3+CD8+ (D) T cells. The nonparametric Kruskal-Wallis test for independent samples and the Friedman test for dependent samples were used. Statistically significant values were considered when p < 0.001 (***).
Table 2.
Interquartile range and Friedman test of differentiation and exhaustion markers in blood samples from PASC individuals after Tα1 treatment (N = 10).
| CD3 | CD4CD8 | CD3CD8 | CD3CD4 | ||
|---|---|---|---|---|---|
| IQR | 25 | 34,72 | 1,15 | 22,52 | 49,60 |
| 50 | 50,26 | 2,66 | 36,85 | 56,05 | |
| 75 | 60,77 | 4,92 | 43,48 | 62,00 | |
| CD3 +Tα1 |
CD4CD8 +Tα1 |
CD3CD8 +Tα1** |
CD3CD4 +Tα1** |
||
| IQR | 25 | 36,25 | 1,49 | 22,50 | 48,92 |
| 50 | 51,00 | 2,67 | 37,35 | 54,96 | |
| 75 | 61,25 | 3,66 | 43,72 | 62,00 |
| CD4naive | CD4CM | CD4EM | CD4TEM | CD4PD1 | CD4CD57 | ||
|---|---|---|---|---|---|---|---|
| IQR | 25 | 24.38 | 16.62 | 45.78 | 0.00 | 3.58 | 1.34 |
| 50 | 32.20 | 25.02 | 59.51 | 0.68 | 8.93 | 2.72 | |
| 75 | 37.50 | 30.60 | 66.91 | 5.14 | 15.33 | 3.52 | |
| CD4naive + Tα1 |
CD4CM + Tα1** |
CD4EM + Tα1** |
CD4TEM + Tα1 |
CD4PD1 + Tα1** |
CD4CD57 + Tα1 |
||
| IQR | 25 | 16.64 | 10.56 | 44.06 | 0.00 | 2.88 | 1.63 |
| 50 | 32.20 | 23.12 | 61.50 | 0.63 | 7.19 | 3.15 | |
| 75 | 38.02 | 33.93 | 67.40 | 4.82 | 13.50 | 3.73 | |
| CD8naive | CD8CM | CD8EM | CD8TEM | CD8PD1 | CD8CD57 | ||
| IQR | 25 | 2.25 | 38.45 | 6.50 | 0.40 | 2.39 | 16.04 |
| 50 | 23.47 | 43.00 | 39.00 | 9.50 | 3.84 | 25.49 | |
| 75 | 32.96 | 53.98 | 45.61 | 12.75 | 5.28 | 31.75 | |
| CD8naive + Tα1** |
CD8CM +Tα1** |
CD8EM + Tα1 |
CD8TEM +Tα1** |
CD8PD1 +Tα1** |
CD8CD57 +Tα1 |
||
| IQR | 25 | 4.50 | 37.83 | 7.31 | 0.15 | 1.91 | 17.73 |
| 50 | 25.16 | 47.24 | 39.00 | 8.50 | 2.42 | 24.50 | |
| 75 | 35.81 | 53.93 | 47.60 | 10.77 | 3.38 | 31.50 | |
| ** p<0.01 Friedman test for dependent samples were used | |||||||
3.3 Tα1 treatment decreases intracellular IL-6 and IFN-γ protein expression and increases IL-10 protein expression in T cell compartments and in activated CD8 + T lymphocytes of PASC individuals.
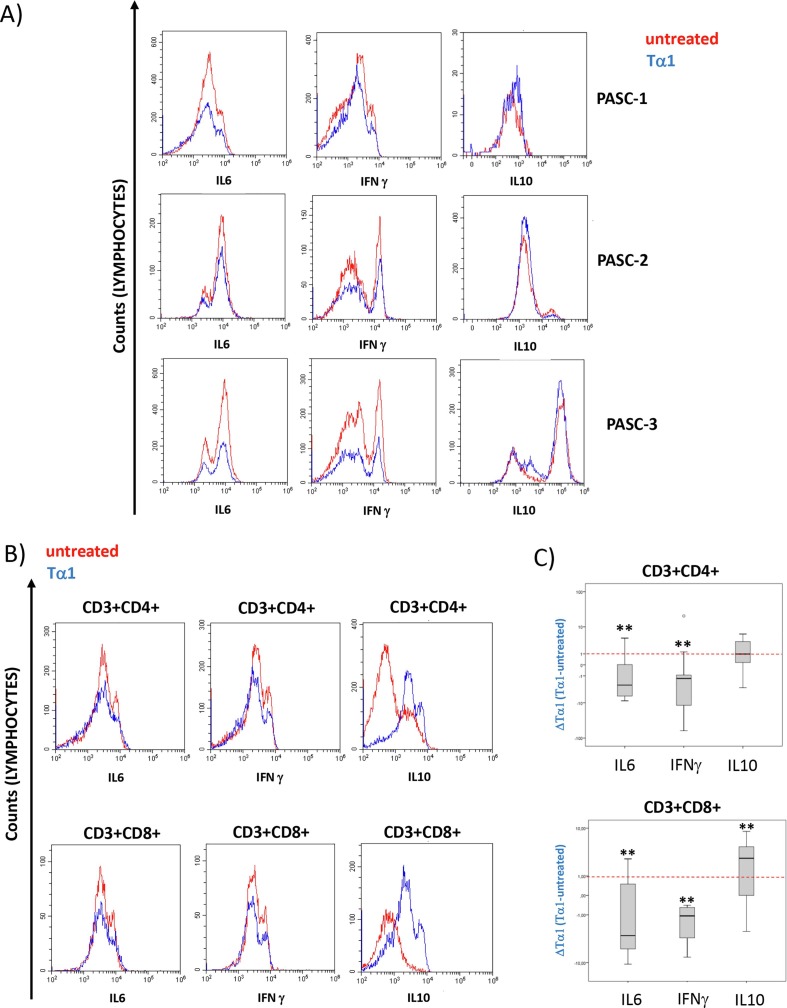
To better understand the impact of Tα1 treatment on inflammation, the analysis of intracellular expression of some cytokines was analyzed by flow cytometry. Ex vivo treatment with Tα1 determined a significant decrease in cells expressing IL-6 and IFN-γ in the CD8 + and CD4+ T compartments and a significant increase in cells expressing IL-10 (Fig. 3 A-C).
Fig. 3.
Flow cytometry analysis of intracellular cytokine expression in PASC individuals treated with Tα1. A) representative histogram overlay (N = 3 individuals) of IL-6, IFN and IL-10 in lymphocytes in presence or absence of Tα1 (red line untreated, blue line treated cells). B) representative histogram overlay (N = 1 individuals) of IL-6, IFN and IL-10 in CD3+CD4+ and CD3+CD8+ T cells in presence or absence of Tα1. C) Box plot of changes in Tα1 treatment (delta of Tα1 vs. untreated) of cytokines protein changes in both the CD3+CD4 + and CD3+CD8+ subsets. Statistically significant values were considered when p < 0.01 (**). The nonparametric Kruskal-Wallis test was used for independent samples and the Friedman test for dependent samples. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
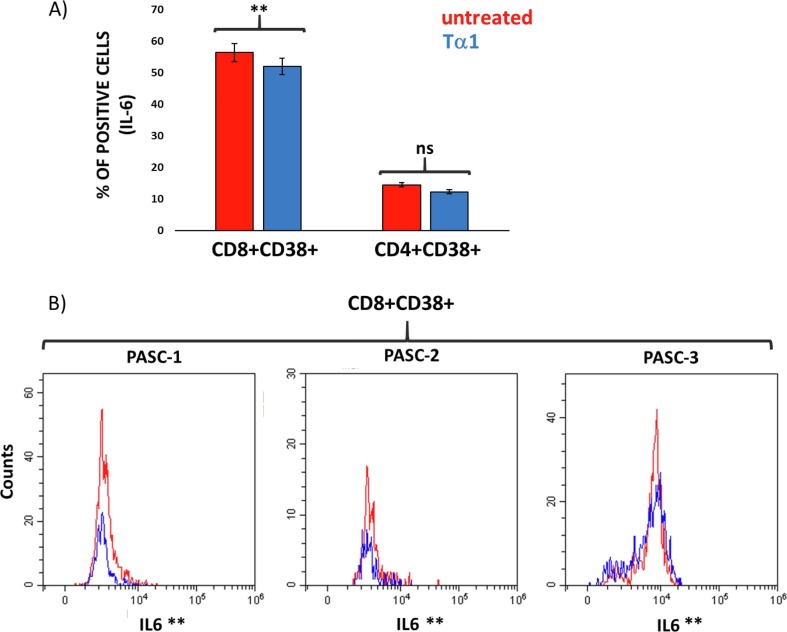
Furthermore, treatment with Tα1 significantly reduced the number of cells expressing IL-6 specifically in activated CD8+ T cells expressing the CD38 (Fig. 4 A and 4B), confirming its ability to regulate a context-dependent response in specific cells.
Fig. 4.
Flow cytometry analysis of intracellular IL-6 expression in activated CD8 + and CD4 + expressing CD38 in PASC individuals treated with Tα1. A) Histogram of the percentage of cells expressing IL-6 in the CD8+CD38+ and CD4+CD38+ cell subsets. B) representative histogram overlay (N = 3 individuals) of IL-6 in CD8+CD38+T cells in presence or not of Tα1 (red line untreated, blue line treated cells). Statistically significant values were considered when p < 0.01 (**). The Friedman test was used. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Tα1 immunomodulation is associated with severity and respiratory support in the acute phase and with some PASC symptoms
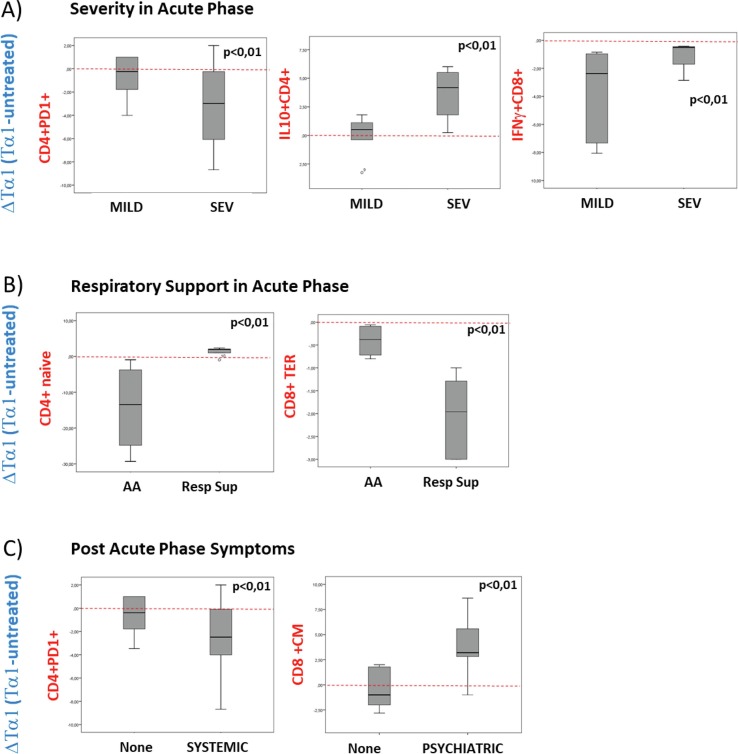
Post-COVID-19 infection is associated with long-term systemic symptoms, such as cardiologic and neurological symptoms. We analysed the ex-vivo impact of Tα1 treatment in the immune regulation of some specific parameters associated with the acute phase of the infection (severity and respiratory support) and with PASC specific symptoms. In PASC individuals with an history of severe disease during the acute phase of the infection (SEV), compared to PASC with mild symptoms during the acute phase (MILD), Tα1 treatment resulted in a significant decrease in PD-1 expression in CD4+ T cells and a significant increase in IL-10, while in CD8+ T cells Tα1 increased the expression of IFN-γ positive cells (Fig. 5 A). Furthermore, dividing the PASC into two groups, based on the need for respiratory support during the acute phase, Ambient Air (AA) versus any Respiratory Support (Resp Sup), Tα1 treatment resulted in a significant increase in naive CD4+ T cell percentage and a decrease in CD8+ TEM T cell percentage in PASC individuals of res Sup group (Fig. 5 B). Hence, these data underline how Tα1 treatment seems to be more effective in patients who had a more severe condition in the acute phase. Analysing post-acute infection symptoms, in blood cells from PASC subjects with systemic symptoms such as asthenia, myalgia, fever and joint pain, Tα1 treatment significantly reduced the percentage of PD-1 expressing CD4+ T cells. Moreover, the treatment induced a significant increase in CD8+ CM T cells from people with psychiatric symptoms (anxiety, depression, and emotional lability (Fig. 5 C).
Fig. 5.
Tα1 immune modification in association with acute and PASC symptoms. Effects of Tα1 treatment (Delta = Tα1-untreated, red line divided down with respect to up modulated proteins) in PASC individuals. A) Box plot of the percentage of CD4+ T cells expressing PD-1 and IL-10 and of CD8+ cells expressing IFN-γ in group divided respect the stage of severity of the acute phase (MILD vs SEV). B) Box plot of the percentage of CD4 + naive and in CD8+ TEM cells respecting the respiratory support (AA vs Resp Sup). C) Box plot of the percentage of CD4+ cells expressing PD-1 respecting the Systemic PASC symptoms, left panel, and CD8+CM cells expressing respect the psychiatric symptoms, right panel. Statistically significant values were considered when p < 0.01, the Friedman test was used. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Tα1 reduced pro-inflammatory cytokine mRNA expression and increased IL-10 expression in PASC individuals.
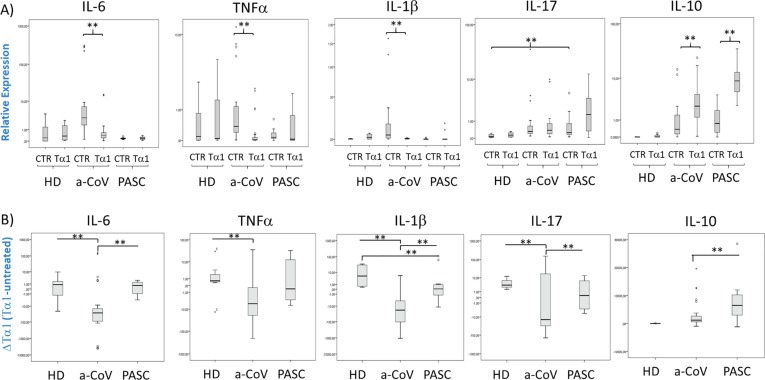
To further verify the ability of Tα1 in modulating cytokine storm in blood cells from PASC individuals, we have analysed the cytokines expression also at the transcriptional level. Patients with acute COVID-19 (a-COV) had higher expression of IL-6, TNF-α and IL-1β with respect to HD and also to PASC at basal level (Fig. 6 A and B). The ex-vivo treatment with Tα1 of blood samples of a-COV patients reduced the expression of these pro-inflammatory and conversely induced the expression of anti-inflammatory cytokine IL-10. In PASC individuals, Tα1 treatment determined a difference in the expression of IL-1β and IL-17 with respect to HD, accompanied to an increased expression of IL-10 (Fig. 6B).
Fig. 6.
Effects on immune regulation by Tα1 treatment in blood cells from Acute COVID-19 and PASC individuals. Transcriptional levels in human blood samples from individuals with acute COVID-19 (a-COV, N = 15), PASC (N = 10) and healthy donors (HD, N = 5). Data are represented as a box plot, showing mild (grey) and extreme (point) outliers. Expression levels were analyzed by real-time PCR and represented in logarithmic scale. A) Relative expression in the presence or absence of Tα1 and (B) Tα1 treatment changes (delta of Tα1 vs untreated) of IL-6, TNF-α, IL-1β, IL-17 and IL-10. Statistically significant values were considered when p < 0.010 (**). The nonparametric Kruskal-Wallis tests in the case of independent samples and the Friedman test for dependent samples were used.
4. Discussion
For its ability to restore immune system homeostasis during infections from different types of pathogens [25], [26], Tα1 has been proposed for immunomodulation in COVID-19 [17], [18], [27], [28]. Indeed, Tα1 has already been used in China during the SARS-CoV-2 outbreak [17]. Moreover, Tα1 has already been shown to reduce mortality and improve immune responses in patients with sepsis [29]. Immunophenotyping analyses revealed a strong effect of Tα1 in T cell subsets increasing lymphocyte counts, representing a potential approach to protect effector T cells during COVID-19 [30]. We have previously demonstrated that Tα1 attenuated transcriptional expression levels of the major cytokines and factors responsible for the cytokine storm in COVID-19 patients. Indeed, Tα1 can contribute to inhibit IL-6 at the protein level and reduce lymphocyte activation, particularly in the CD8+ T-cell subset, which has been associated with disease severity [19]. Hyper-inflammation and immune-mediated injury characterizing COVID-19 disease have been associated with poor outcomes in patients and suggested the potential advantage of anti-inflammatory drugs [20]. Indeed, a sepsis-like conditions has been found in COVID-19 [31], with complex alterations of the immune system ranging from inhibition to activation and exhaustion [32]. The immunopathological condition of patients with COVID-19 is characterized by lymphopenia, a relative increase in neutrophils, depletion of CD8 + T cells, and a rise in Th17 and a decrease in regulatory T cell responses, often associated with hyper inflammation and subsequent cytokine storm [33]. The inefficient and uncontrolled initial response is then reflected at the systemic level. At a cellular level, severe lymphopenia was observed specifically in T cells, and was associated with disease severity. The causes of lymphopenia could be found in the excessive inflammation, in particular due to high levels of the cytokine IL-6 and down-regulation of genes involved in T cell expansion [34]. In the context of viral infections, CD8+ T lymphocytes block infected cells producing effector molecules such as granzyme A / B and perforin, or through CD95/Fas-mediated apoptosis; CD4+ follicular helper T lymphocytes stimulate B lymphocytes to produce specific antibodies [35], but lymphopenia does not allow proper antiviral T cell activity function. Indeed, a significant change in the differentiation phenotype with an increase in terminal effector memory T cells and a decrease in naïve and early memory T cells, as well as with elevated expression of the senescence marker CD57 could explain this dysfunction of the immunological response [36], [37]. Furthermore, an increase in PD-1, which regulates cell exhaustion, was found in CD4+ and CD8+ T cells [38], [39], [40], [41]. In this context, several molecules with inhibitory and immunomodulatory activities have been found to inhibit SARS-CoV-2 infection [42], [43]. However, the controversy in the scientific community regarding their effectiveness is still open[44].
Unfortunately, the persistent inflammatory state and inadequate immune response, characteristic of the acute phase of COVID-19 disease, can persist over time and cause post-acute COVID syndrome. Individuals with Long-COVID, also defined as PASC, showed significant immune activation and a reduced proportion of naive T and B cells and high expression of IFN-β and IFN-λ1 [45]. The alterations that appear post-infection could be caused by a persistent chronic inflammatory state. It has been recently demonstrated that cytokines such as IL-1β, IL-6, TNF-α and the S100A8/A9 protein persist at serum level even after SARS-CoV-2 infection and could cause persistent inflammation present in PASC individuals [46]. Our results showed that in this cohort, as previously seen in another group of acute patients [18], treatment with Tα1 resulted in a significant decrease in IL-6 and IFN-γ expressing T cells in both CD4 + and CD8 + T lymphocyte subsets. Furthermore, in CD8 + T cells there was also a significant increase in the percentage of cells expressing IL-10, a key regulator of the immunologic response, specifically suggesting the need to have a high expression of this regulatory cytokine to counteract ongoing chronic inflammation, and the ability of Tα1 treatment to further increase it.
It is noteworthy that IL-6 was significantly decreased in Tα1-treated activated CD38 + CD8 + T cells, highlighting the ability of Tα1 in the attenuation of inflammation specifically in the CD8 + T cell compartment, as previously demonstrated in both COVID-19 and HIV infection [19], [25], [47].
Several recent studies profiling the immune system in individuals recovering from COVID-19 using multi-parametric flow cytometry, bulk and single-cell transcriptomic, and other approaches [48], [49], [50], [51], [52], [53] demonstrated persistent dysregulation in immune cell subtypes in COVID-19 convalescents, depletion of naive B and T cell subpopulations, and expansion of memory PD-1+CD8+ T cells. These evidences suggested persistent conversion of naive T cells into activated states resulting in chronic stimulation of the immune response in individuals with PASC [46].
In the PASC patients enrolled for this study, despite the recovery of the percentage of lymphocyte subsets, which were comparable to those observed in HD, the analysis of cell phenotype and differentiation, revealed a condition surprisingly similar to that observed in the a-COV group, confirming data from the literature that emphasize the failure of PASC to recover immune homeostasis, even several weeks/months after acute infection [46]. Our data demonstrated that in PASC individuals with a chronically altered immune response, Tα1 appeared to promote the restoration of an appropriate immune response. Indeed, treatment with Tα1 changed the profile of T cell differentiation in both CD4+ and CD8+ subsets from PASCs by increasing central memory cells in CD4+ T subset, while in the CD8+ T subset there was a significant increase in naive and an equally important decrease in TEM. Furthermore, the percentage of cells that express the PD-1 exhaustion marker decreased in both subsets after treatment. Interestingly, the decrease in CD4+ T cells expressing PD-1 and the increase in IL-10 CD4+ expressing cells due to Tα1 treatment, were most evident in PASC individuals with a history of a more severe form during the acute phase of COVID-19. Furthermore, with respect to the need for respiratory support in the acute phase, ex vivo treatment increased the percentage of CD4+ naive cells and decreased CD8+ TEM, confirming how the use of Tα1 treatment is most effective in more severe patients.
Recovery from SARS-CoV-2 infection is often associated with other persistent symptoms months after infection, including fatigue, muscle weakness, sleep disruption, and anxiety or depression that were associated with persistence of chronic inflammatory and immune dysfunction [54], [55], [56].
In PASC individuals with systemic symptoms such as asthenia, fever, myalgia and join pain, Tα1 treatment determined a significant decrease of exhausted cells more than in individuals without these symptoms. Moreover, Tα1 increased the percentages of CD8+ CM T cells in PASC with psychiatric symptoms such as anxiety, depression, and emotional lability. These aspects suggested how the immune restoration and anti-inflammatory effect of Tα1 could be useful in the management of complex symptoms distinctive of PASC individuals. A limitation of the study is the limited number of patients included in this cohort. Further studies on wider cohorts are needed to confirm these preliminary data. These results, along with the promising reports on recent trials on Tα1 administration in COVID-19 patients, offer new insights into treatment options for COVID-19 after the acute phase of the disease, specifically for those patients with long-lasting inflammation, experiencing disabling post-infectious symptoms, some of which appear with delayed onset.
Funding
VP and MF were supported by the HERVCOV project funded by the HORIZONHLTH- 2021-DISEASE project (Personalized medicine and infectious disease: Understanding the individual host response to virus) of the European Commission under the Horizon Europe Framework Program. G.A.101057302.
CRediT authorship contribution statement
Antonella Minutolo: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft. Vita Petrone: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft. Marialaura Fanelli: Data curation, Investigation, Validation, Visualization, Writing – original draft. Christian Maracchioni: Data curation, Investigation, Methodology, Supervision, Validation, Validation, Writing – original draft, Writing – review & editing. Martina Giudice: Data curation, Validation. Elisabetta Teti: Data curation. Luigi Coppola: Data curation, Investigation, Writing – review & editing. Chiara Sorace: Data curation. Marco Iannetta: Investigation, Writing – review & editing. Martino Tony Miele: Writing – review & editing. Sergio Bernardini: Data curation. Antonio Mastino: Writing – review & editing. Paola Sinibaldi Vallebona: Writing – review & editing. Emanuela Balestrieri: Formal analysis, Validation. Massimo Andreoni: Writing – review & editing. Loredana Sarmati: Investigation. Sandro Grelli: Data curation, Investigation, Writing – review & editing. Enrico Garaci: Writing – review & editing. Claudia Matteucci: Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2023.110055.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Darif D., Hammi I., Kihel A., El Idrissi Saik I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond M.S., Kanneganti T.-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frederico B., Chao B., Lawler C., May J.S., Stevenson P.G. Subcapsular sinus macrophages limit acute gammaherpesvirus dissemination. J. Gen. Virol. 2015;96:2314–2327. doi: 10.1099/vir.0.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKechnie J.L., Blish C.A. The Innate Immune System: Fighting on the Front Lines or Fanning the Flames of COVID-19? Cell Host Microbe. 2020;27:863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N. Vabret, G.J. Britton, C. Gruber, S. Hegde, J. Kim, M. Kuksin, R. Levantovsky, L. Malle, A. Moreira, M.D. Park, L. Pia, E. Risson, M. Saffern, B. Salomé, M. Esai Selvan, M.P. Spindler, J. Tan, V. van der Heide, J.K. Gregory, K. Alexandropoulos, N. Bhardwaj, B.D. Brown, B. Greenbaum, Z.H. Gümüş, D. Homann, A. Horowitz, A.O. Kamphorst, M.A. Curotto de Lafaille, S. Mehandru, M. Merad, R.M. Samstein, M. Agrawal, M. Aleynick, M. Belabed, M. Brown, M. Casanova-Acebes, J. Catalan, M. Centa, A. Charap, A. Chan, S.T. Chen, J. Chung, C.C. Bozkus, E. Cody, F. Cossarini, E. Dalla, N. Fernandez, J. Grout, D.F. Ruan, P. Hamon, E. Humblin, D. Jha, J. Kodysh, A. Leader, M. Lin, K. Lindblad, D. Lozano-Ojalvo, G. Lubitz, A. Magen, Z. Mahmood, G. Martinez-Delgado, J. Mateus-Tique, E. Meritt, C. Moon, J. Noel, T. O’Donnell, M. Ota, T. Plitt, V. Pothula, J. Redes, I. Reyes Torres, M. Roberto, A.R. Sanchez-Paulete, J. Shang, A.S. Schanoski, M. Suprun, M. Tran, N. Vaninov, C.M. Wilk, J. Aguirre-Ghiso, D. Bogunovic, J. Cho, J. Faith, E. Grasset, P. Heeger, E. Kenigsberg, F. Krammer, U. Laserson, Immunology of COVID-19: Current State of the Science, Immunity. 52 (2020) 910–941. 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed]
- 8.Herzog S., Fragkou P.C., Arneth B.M., Mkhlof S., Skevaki C. Myeloid CD169/Siglec1: An immunoregulatory biomarker in viral disease. Front. Med. 2022;9 doi: 10.3389/fmed.2022.979373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minutolo A., Petrone V., Fanelli M., Iannetta M., Giudice M., Ait Belkacem I., Zordan M., Vitale P., Rasi G., Sinibaldi-Vallebona P., Sarmati L., Andreoni M., Malergue F., Balestrieri E., Grelli S., Matteucci C. High CD169 Monocyte/Lymphocyte Ratio Reflects Immunophenotype Disruption and Oxygen Need in COVID-19 Patients. Pathogens. 2021;10:1639. doi: 10.3390/pathogens10121639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koçak Tufan Z., Kayaaslan B., Mer M. COVID-19 and Sepsis. Turk. J Med. Sci. 2021;51:3301–3311. doi: 10.3906/sag-2108-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camacho V., Kuznetsova V., Welner R.S. Inflammatory Cytokines Shape an Altered Immune Response During Myeloid Malignancies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.772408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matteucci C., Minutolo A., Sinibaldi-Vallebona P., Palamara A.T., Rasi G., Mastino A., Garaci E. Transcription profile of human lymphocytes following in vitro treatment with thymosin alpha-1: Transcription profile of Tα1-treated PBMCs. Ann. N. Y. Acad. Sci. 2010;1194:6–19. doi: 10.1111/j.1749-6632.2010.05484.x. [DOI] [PubMed] [Google Scholar]
- 15.Matteucci C., Argaw-Denboba A., Balestrieri E., Giovinazzo A., Miele M., D’Agostini C., Pica F., Grelli S., Paci M., Mastino A., Sinibaldi Vallebona P., Garaci E., Tomino C. Deciphering cellular biological processes to clinical application: a new perspective for Tα1 treatment targeting multiple diseases. Expert Opin. Biol. Ther. 2018;18:23–31. doi: 10.1080/14712598.2018.1474198. [DOI] [PubMed] [Google Scholar]
- 16.Costantini C., Bellet M.M., Pariano M., Renga G., Stincardini C., Goldstein A.L., Garaci E., Romani L. A Reappraisal of Thymosin Alpha1 in Cancer Therapy. Front. Oncol. 2019;9:873. doi: 10.3389/fonc.2019.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Y. Liu, Y. Pan, Z. Hu, M. Wu, C. Wang, Z. Feng, C. Mao, Y. Tan, Y. Liu, L. Chen, M. Li, G. Wang, Z. Yuan, B. Diao, Y. Wu, Y. Chen, Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 71 (2020) 2150–2157. 10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed]
- 18.Bersanelli M., Giannarelli D., Leonetti A., Buti S., Tiseo M., Nouvenne A., Ticinesi A., Meschi T., Procopio G., Danielli R. The right immune-modulation at the right time: thymosin α1 for prevention of severe COVID-19 in cancer patients. Future Oncol. 2021;17:1097–1104. doi: 10.2217/fon-2020-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.C. Matteucci, A. Minutolo, E. Balestrieri, V. Petrone, M. Fanelli, V. Malagnino, M. Ianetta, A. Giovinazzo, F. Barreca, S. Di Cesare, P. De Marco, M.T. Miele, N. Toschi, A. Mastino, P. Sinibaldi Vallebona, S. Bernardini, P. Rogliani, L. Sarmati, M. Andreoni, S. Grelli, E. Garaci, Thymosin Alpha 1 Mitigates Cytokine Storm in Blood Cells From Coronavirus Disease 2019 Patients, Open Forum Infect. Dis. 8 (2021) ofaa588. 10.1093/ofid/ofaa588. [DOI] [PMC free article] [PubMed]
- 20.CDC, Healthcare Workers, Cent. Dis. Control Prev. (2020). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed January 8, 2023).
- 21.J.R. Chevinsky, G. Tao, A.M. Lavery, E.A. Kukielka, E.S. Click, D. Malec, L. Kompaniyets, B.B. Bruce, H. Yusuf, A.B. Goodman, M.G. Dixon, J.H. Nakao, S.D. Datta, W.R. MacKenzie, S.S. Kadri, S. Saydah, J.E. Giovanni, A.V. Gundlapalli, Late Conditions Diagnosed 1–4 Months Following an Initial Coronavirus Disease 2019 (COVID-19) Encounter: A Matched-Cohort Study Using Inpatient and Outpatient Administrative Data—United States, 1 March–30 June 2020, Clin. Infect. Dis. 73 (2021) S5–S16. 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed]
- 22.Fanelli M., Petrone V., Buonifacio M., Delibato E., Balestrieri E., Grelli S., Minutolo A., Matteucci C. Multidistrict Host-Pathogen Interaction during COVID-19 and the Development Post-Infection Chronic Inflammation. Pathogens. 2022;11:1198. doi: 10.3390/pathogens11101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Laughlin K.N., Thompson M., Hota B., Gottlieb M., Plumb I.D., Chang A.M., Wisk L.E., Hall A.J., Wang R.C., Spatz E.S., Stephens K.A., Huebinger R.M., McDonald S.A., Venkatesh A., Gentile N., Slovis B.H., Hill M., Saydah S., Idris A.H., Rodriguez R., Krumholz H.M., Elmore J.G., Weinstein R.A., Nichol G. INSPIRE Investigators, Study protocol for the Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE): A longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection. PloS One. 2022;17:e0264260. doi: 10.1371/journal.pone.0264260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holms R.D. Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy. Immuno. 2022;2:512–533. doi: 10.3390/immuno2030033. [DOI] [Google Scholar]
- 25.Matteucci C., Grelli S., Balestrieri E., Minutolo A., Argaw-Denboba A., Macchi B., Sinibaldi-Vallebona P., Perno C.F., Mastino A., Garaci E. Thymosin alpha 1 and HIV-1: recent advances and future perspectives. Future Microbiol. 2017;12:141–155. doi: 10.2217/fmb-2016-0125. [DOI] [PubMed] [Google Scholar]
- 26.Camerini R., Garaci E. Historical review of thymosin α 1 in infectious diseases. Expert Opin. Biol. Ther. 2015;15(Suppl 1):S117–S127. doi: 10.1517/14712598.2015.1033393. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Li R., Wu Z., Yang X., Zhao M., Liu J., Chen D. Therapeutic strategies for critically ill patients with COVID-19. Ann. Intensive Care. 2020;10:45. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romani L., Tomino C., Puccetti P., Garaci E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020;6:49. doi: 10.1038/s41420-020-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Zhou L., Liu J., Ma G., Kou Q., He Z., Chen J., Ou-Yang B., Chen M., Li Y., Wu X., Gu B., Chen L., Zou Z., Qiang X., Chen Y., Lin A., Zhang G., Guan X. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit. Care Lond. Engl. 2013;17:R8. doi: 10.1186/cc11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu K., He J., Wu Y., Xie B., Liu X., Wei B., Zhou H., Lin B., Zuo Z., Wen W., Xu W., Zou B., Wei L., Huang X., Zhou P. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020;30:814–816. doi: 10.1038/s41422-020-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M.A.F. Queiroz, P.F.M. das Neves, S.S. Lima, J. da C. Lopes, M.K. da S. Torres, I.M.V.C. Vallinoto, C.D.A. Bichara, E.F. Dos Santos, M.T.F.M. de Brito, A.L.S. da Silva, M. de M. Leite, F.P. da Costa, M. de N. do S. de A. Viana, F.B.B. Rodrigues, K.M.L. de Sarges, M.H.D. Cantanhede, R. da Silva, C.N.C. Bichara, A.V.S. van den Berg, A. de O.L. Veríssimo, M. da S. Carvalho, D.F. Henriques, C.P. Dos Santos, J.A.L. Nunes, I.B. Costa, G.M.R. Viana, F.R.O. Carneiro, V.R. da C.M. Palacios, J.A.S. Quaresma, I. Brasil-Costa, E.J.M. Dos Santos, L.F.M. Falcão, A.C.R. Vallinoto, Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome, Front. Cell. Infect. Microbiol. 12 (2022) 922422. 10.3389/fcimb.2022.922422. [DOI] [PMC free article] [PubMed]
- 32.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond. Engl. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Signore A., Lauri C., Colandrea M., Di Girolamo M., Chiodo E., Grana C.M., Campagna G., Aceti A. Lymphopenia in patients affected by SARS-CoV-2 infection is caused by margination of lymphocytes in large bowel: an [18F]FDG PET/CT study. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:3419–3429. doi: 10.1007/s00259-022-05801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Biasi S., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L., Gozzi L., Iannone A., Lo Tartaro D., Mattioli M., Paolini A., Menozzi M., Milić J., Franceschi G., Fantini R., Tonelli R., Sita M., Sarti M., Trenti T., Brugioni L., Cicchetti L., Facchinetti F., Pietrangelo A., Clini E., Girardis M., Guaraldi G., Mussini C., Cossarizza A. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., Nazemiyeh M., Aghebati-Maleki L., Jadidi-Niaragh F., Abbaspour-Aghdam S., Roshangar L., Mikaeili H., Ahmadi M. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236:2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 37.Westmeier J., Paniskaki K., Karaköse Z., Werner T., Sutter K., Dolff S., Overbeck M., Limmer A., Liu J., Zheng X., Brenner T., Berger M.M., Witzke O., Trilling M., Lu M., Yang D., Babel N., Westhoff T., Dittmer U., Zelinskyy G. Impaired Cytotoxic CD8+ T Cell Response in Elderly COVID-19 Patients. MBio. 2020;11:e02243–e10320. doi: 10.1128/mBio.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellesi S., Metafuni E., Hohaus S., Maiolo E., Marchionni F., D’Innocenzo S., La Sorda M., Ferraironi M., Ramundo F., Fantoni M., Murri R., Cingolani A., Sica S., Gasbarrini A., Sanguinetti M., Chiusolo P., De Stefano V. Increased CD95 (Fas) and PD-1 expression in peripheral blood T lymphocytes in COVID-19 patients. Br. J. Haematol. 2020;191:207–211. doi: 10.1111/bjh.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobcakova A., Petriskova J., Vysehradsky R., Kocan I., Kapustova L., Barnova M., Diamant Z., Jesenak M. Immune Profile in Patients With COVID-19: Lymphocytes Exhaustion Markers in Relationship to Clinical Outcome. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.646688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn E., Araki K., Hashimoto M., Li W., Riley J.L., Cheung J., Sharpe A.H., Freeman G.J., Irving B.A., Ahmed R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 2018;115:4749–4754. doi: 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A., Di Gaetano M., Puzzolante C., Carli F., Bedini A., Corradi L., Fantini R., Castaniere I., Tabbì L., Girardis M., Tedeschi S., Giannella M., Bartoletti M., Pascale R., Dolci G., Brugioni L., Pietrangelo A., Cossarizza A., Pea F., Clini E., Salvarani C., Massari M., Viale P.L., Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study, Lancet. Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., Kelleher A.D., Matthews G.V. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 46.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., Garcia-Valtanen P., Al-Delfi Z., Gummow J., Ferguson C., O’Connor S., Reddi B.A.J., Hissaria P., Shaw D., Kok-Lim C., Gleadle J.M., Beard M.R., Barry S.C., Grubor-Bauk B., Lynn D.J. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matteucci C., Minutolo A., Pollicita M., Balestrieri E., Grelli S., D’Ettorre G., Vullo V., Bucci I., Luchini A., Aquaro S., Sinibaldi-Vallebona P., Macchi B., Perno C.F., Mastino A., Garaci E. Thymosin α 1 potentiates the release by CD8 + cells of soluble factors able to inhibit HIV-1 and human T lymphotropic virus 1 infection in vitro. Expert Opin. Biol. Ther. 2015;15:83–100. doi: 10.1517/14712598.2015.1021677. [DOI] [PubMed] [Google Scholar]
- 48.Koutsakos M., Rowntree L.C., Hensen L., Chua B.Y., van de Sandt C.E., Habel J.R., Zhang W., Jia X., Kedzierski L., Ashhurst T.M., Putri G.H., Marsh-Wakefield F., Read M.N., Edwards D.N., Clemens E.B., Wong C.Y., Mordant F.L., Juno J.A., Amanat F., Audsley J., Holmes N.E., Gordon C.L., Smibert O.C., Trubiano J.A., Hughes C.M., Catton M., Denholm J.T., Tong S.Y.C., Doolan D.L., Kotsimbos T.C., Jackson D.C., Krammer F., Godfrey D.I., Chung A.W., King N.J.C., Lewin S.R., Wheatley A.K., Kent S.J., Subbarao K., McMahon J., Thevarajan I., Nguyen T.H.O., Cheng A.C., Kedzierska K. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Yang X., Wang H., Li Z., Deng H., Liu J., Xiong S., He J., Feng X., Guo C., Wang W., Zelinskyy G., Trilling M., Sutter K., Senff T., Menne C., Timm J., Zhang Y., Deng F., Lu Y., Wu J., Lu M., Yang D., Dittmer U., Wang B., Zheng X. Analysis of the Long-Term Impact on Cellular Immunity in COVID-19-Recovered Individuals Reveals a Profound NKT Cell Impairment. MBio. 2021;12:e00085–e121. doi: 10.1128/mBio.00085-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.A.J. Wilk, M.J. Lee, B. Wei, B. Parks, R. Pi, G.J. Martínez-Colón, T. Ranganath, N.Q. Zhao, S. Taylor, W. Becker, Stanford COVID-19 Biobank, T. Ranganath, N.Q. Zhao, A.J. Wilk, R. Vergara, J.L. McKechnie, L. de la Parte, K. Whittle Dantzler, M. Ty, N. Kathale, G.J. Martinez-Colon, A. Rustagi, G. Ivison, R. Pi, M.J. Lee, R. Brewer, T. Hollis, A. Baird, M. Ugur, M. Tal, D. Bogusch, G. Nahass, K. Haider, K.Q. Thi Tran, L. Simpson, H. Din, J. Roque, R. Mann, I. Chang, E. Do, A. Fernandes, S.-C. Lyu, W. Zhang, M. Manohar, J. Krempski, A. Visweswaran, E.J. Zudock, K. Jee, K. Kumar, J.A. Newberry, J.V. Quinn, D. Schreiber, E.A. Ashley, C.A. Blish, A.L. Blomkalns, K.C. Nadeau, R. O’Hara, A.J. Rogers, S. Yang, D. Jimenez-Morales, A.L. Blomkalns, R. O’Hara, E.A. Ashley, K.C. Nadeau, S. Yang, S. Holmes, M. Rabinovitch, A.J. Rogers, W.J. Greenleaf, C.A. Blish, Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19, J. Exp. Med. 218 (2021) e20210582. 10.1084/jem.20210582. [DOI] [PMC free article] [PubMed]
- 51.Kratzer B., Trapin D., Ettel P., Körmöczi U., Rottal A., Tuppy F., Feichter M., Gattinger P., Borochova K., Dorofeeva Y., Tulaeva I., Weber M., Grabmeier-Pfistershammer K., Tauber P.A., Gerdov M., Mühl B., Perkmann T., Fae I., Wenda S., Führer H., Henning R., Valenta R., Pickl W.F. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy. 2021;76:751–765. doi: 10.1111/all.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peluso M.J., Deitchman A.N., Torres L., Iyer N.S., Munter S.E., Nixon C.C., Donatelli J., Thanh C., Takahashi S., Hakim J., Turcios K., Janson O., Hoh R., Tai V., Hernandez Y., Fehrman E.A., Spinelli M.A., Gandhi M., Trinh L., Wrin T., Petropoulos C.J., Aweeka F.T., Rodriguez-Barraquer I., Kelly J.D., Martin J.N., Deeks S.G., Greenhouse B., Rutishauser R.L., Henrich T.J. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheon I.S., Li C., Son Y.M., Goplen N.P., Wu Y., Cassmann T., Wang Z., Wei X., Tang J., Li Y., Marlow H., Hughes S., Hammel L., Cox T.M., Goddery E., Ayasoufi K., Weiskopf D., Boonyaratanakornkit J., Dong H., Li H., Chakraborty R., Johnson A.J., Edell E., Taylor J.J., Kaplan M.H., Sette A., Bartholmai B.J., Kern R., Vassallo R., Sun J. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci. Immunol. 2021;6:eabk1741. doi: 10.1126/sciimmunol.abk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran V.-T., Riveros C., Clepier B., Desvarieux M., Collet C., Yordanov Y., Ravaud P. Development and Validation of the Long Coronavirus Disease (COVID) Symptom and Impact Tools: A Set of Patient-Reported Instruments Constructed From Patients’ Lived Experience. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022;74:278–287. doi: 10.1093/cid/ciab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carfì A., Bernabei R., Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group, Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.