Abstract
Autologous chondrocyte implantation has shown optimal long-term outcomes in the treatment of cartilage lesions. The challenge for a single-stage approach lies in obtaining sufficient number of cells with high viability. The answer could lie in supplementing or replacing them with allogenic chondrocytes coming from cadaveric donors. In the present work, we aimed to compare the number of viable cells isolated from cartilage of live and cadaveric donors and to determine the suitable characteristics of the best donors. A total of 65 samples from donors aged from 17 to 55 years, either women or men, were enrolled in this study (33 living vs. 32 cadaveric). The mean time of hours from death to processing samples in cadaveric donors was higher compared to live donors (64.3 ± 17.7 vs. 4.6±6.4). The number of isolated chondrocytes per gram of cartilage was higher in cadaveric donors (5.389 × 106 compared to 3.067 × 106 in living donors), whereas the average of cell viability was comparable in both groups (84.16% cadaveric, 87.8% alive). It is possible to obtain viable chondrocytes from cartilage harvested from cadaveric donors, reaching a similar cell number and viability to that obtained from the cartilage of living donors.
Keywords: Allogeneic chondrocytes, Cadaveric donors, Live donors, Cartilage repair, Cell viability
Introduction
The clinical significance of articular cartilage lesions is a topic of considerable interest for tissue engineering purposes. Focal cartilage lesions may progress into early osteoarthritis (Flanigan et al. 2010; Houck et al. 2018; Huey et al. 2012). To restore the joint cartilage layer integrity with hyaline like cartilage is considered the ultimate goal when treating chondral lesions (De Windt et al. 2013; Minas et al. 2010). Autologous chondrocyte implantation (ACI) provides optimal outcomes in patients with lesions greater than 3–4 cm2 without involvement of subchondral bone (Bentley et al. 2003; Brittberg et al. 1994; Dekker et al. 2021; Mastbergen et al. 2013; Niemeyer et al. 2016). Although this surgical treatment is effective for many patients, current research for ACI improvement is focused on using stem cells and 3D printing (Humphries et al. 2022). Unfortunately this technology is currently only available for use in experimental ACI models (Liao et al. 2019; Zopf et al. 2018). All technologies that have evolved from the second generation of ACI (Brittberg 2010), which are now offered in the market for use in humans, are based on the use of chondrocytes obtained from adult donors (for example: Spherox®, Cartimax®, and DeNovo™). Therefore, through regenerative medicine and tissue engineering, we seek to find solutions to improve the treatment strategies applied today to repair damaged cartilage (Hulme et al. 2021). The challenge for a single-stage approach lies in obtaining sufficient number of cells with high viability. Due to the low cell number in native cartilage and the large surface area to volume ratio of chondral defects, the manufacturing of matrix-induced autologous chondrocyte implantation (MACI) requires expanded culture of chondrocytes, previously obtained from a biopsy, for approximately 4 weeks until obtaining 15–20 million cells (Brittberg 2010) This fact represents a problem because chondrocytes in culture tend to dedifferentiate to fibroblasts (Gosset et al. 2008), significantly reducing the quality of the implant. Therefore, the answer could lie in supplementing or replacing them with allogenic chondrocytes coming from cadaveric donors, completely eliminating the need to expand chondrocytes in culture. However, the number of cells and their viability obtained from cadaveric donors compared to live donors is unknown. The culture expansion of chondrocytes for clinical use is expensive, highly regulated, and requires a good manufacturing practice (GMP) facility to be able to receive and culture the cells between 3 and 6 weeks. The regulation and governance of cell therapy treatments (advanced therapeutic medicinal product, ATMP) are greater than for other pharmaceutical products (Gardner and Webster 2017). Although autologous chondrocyte implantation has been for a long time the gold standard for cartilage repair and has demonstrated to be a viable option in terms of cost–benefit analysis, a single-stage allogeneic cell therapy with large-scale cell manufactures from donors has potential clinical and economic advantages, high-success rate, and less complications (Alford and Cole 2005; Armoiry et al. 2019; Mistry et al. 2017). Allogenic cartilage is a possible solution for the limitations of autologous chondrocytes and for autologous minced cartilage techniques that use healthy cartilage from a cartilage defect edge (Frisbie et al. 2005; Lu et al. 2006; Mccormick et al. 2008; Schneider et al. 2021). Implantation of allogeneic chondrocytes could be a realistic and promising approach for the treatment of cartilage defects.
Materials and methods
Obtaining the samples
This is a comparative and descriptive research study. The processing of the cartilage samples was carried out in the laboratory of Novoinjertos, a Mexican Tissue Bank. The inclusion criteria were either live or cadaveric donors from both genders aged from 17 to 55 years. The cadaveric donors had no history of knee injury, rheumatoid disease, or knee surgery whereas the live donors were scheduled for knee arthroscopy surgery (Anterior cruciate ligament (ACL) or meniscal injury). Samples obtained from patients with knee osteoarthritis, macroscopic cartilage damage, crystal deposition, or sample contamination were excluded. In the case of cadaveric donors, macroscopic damage, crystal deposition, or positive serum serology tests were also exclusion criteria. The Institutional Ethics and Review Board approved this research.
Cartilage samples in live donors
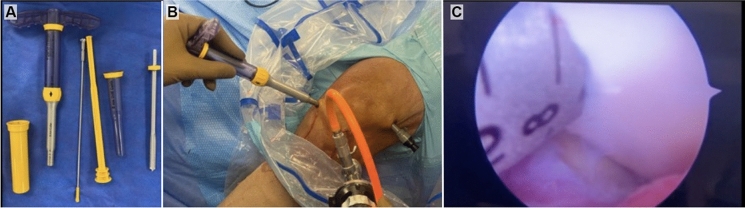
During arthroscopic procedures focused on joint repair, samples were obtained by taking a biopsy of articular cartilage from the non-load bearing areas of the femoral condyle. All patients signed an informed consent for this procedure. In most patients, the biopsies were obtained from the notch area, and in one it was obtained from the lateral femoral condyle. The biopsy obtained from the notch and lateral femoral condyle were harvested by arthroscopy with an osteochondral transfer system with a diameter of 4 mm and a depth of 10 mm using a COR® Precision Targeting System (J & J Medical Devices, Brunswick, NJ, US) (see Fig. 1). The biopsies were transported in 50-mL tubes, containing 10 mL of culture medium DMEM (Caisson Labs, Inc., Smithfield, UT, US), supplemented with 10% antibiotic/antimycotic (Thermo Fisher Scientific, Waltham, MA, US), in a cooler at 4 ºC. Afterward, in the laboratory facilities, inside a laminar flow hood and by mechanical/enzymatic digestion, first in a Petri dish, the subchondral bone was separated from the cartilaginous tissue and the bone tissue was discarded, the cartilage was weighed to obtain the amount in grams of tissue, then, the cartilage was finely minced with a scalpel blade (tissue storage times prior to chondrocyte isolation are shown in Table 1).
Fig. 1.
Obtaining cartilage tissue in living donors. a The biopsy obtained were harvested with an osteochondral transfer system with a diameter of 4 mm; b visualization of cartilage in the lateral femoral condyle was made by arthroscopy; and c donor cartilage before harvesting was observed smooth, shiny and bluish-white without evidence of lesion or crystal deposition
Table 1.
Demographic characteristics and analyzed variables of donors. The number of cadaveric donors was 4, from which 32 osteochondral samples of 4-mm diameter were obtained compared to the 33 cartilage samples obtained from the live donors
| Variable | Cadaveric donors | Live donors |
|---|---|---|
| Age | 33.5 ± 7.1 | 38.3 ± 11.8 |
| Gender (men/women) | 3/1 | 20/13 |
| Body Mass Index | 24.7 ± 2.3 | 26.1 ± 3.1 |
| Processing time (h) | 64.3 ± 17.7 | 4.6 ± 6.4 |
| Cell number | 5.38 × 106 ± 1.47 × 106 | 3.06 × 106 ± 2.18 × 106 |
| Cell viability (%) | 84.16 ± 7.4 | 87.8 ± 9.7 |
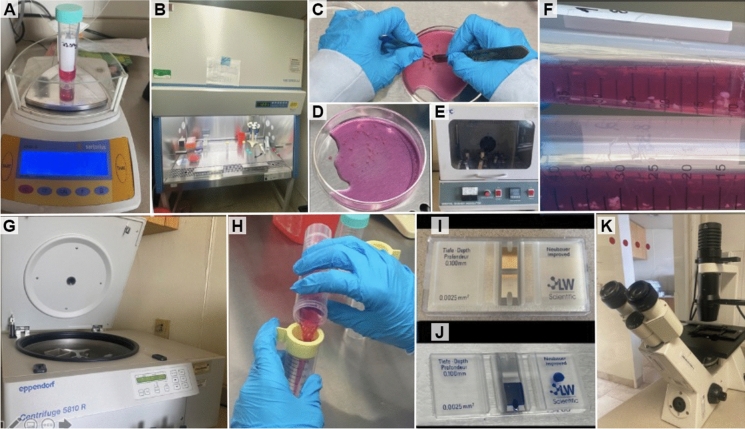
For the enzymatic digestion, the fragments of cartilage tissue were transferred into 50-mL tubes with 10 mL of culture medium and 100 µL of 0.3% type II collagenase (Thermo Fisher), and kept in digestion for 4 h at 37 °C at 150 rpm in an orbital shaker. After mechanical/enzymatic digestion, using a 100-µM pore size cell strainer, the cartilage fragments that were not digested were removed. The enzymatic digestion medium was removed by centrifugation, one time at 300 g, and the cells were suspended in 10 mL of culture medium (DMEM) supplemented with 10% antibiotic/antimycotic and 10% fetal bovine serum (Thermo Fisher Scientific). To establish the number and viability of the cells, the trypan blue stain and a Neubauer chamber were used (see Fig. 2). Finally, after establishing the cell number and viability, they were washed with PBS and centrifuged for 10 min at 300 g, then the PBS was removed and the number of cells was adjusted to 1 × 106 of cells per 1 mL of cryopreservation medium free of fetal bovine serum (MACS-Freezing Solution; Miltenyi Biotec, Bergisch Gladbach, Germany). The mixture of cells and cryopreservation medium was placed into 2-mL cryo-tubes and placed in ultra-freezing at −80 °C for 24 h, to be stored later in a liquid nitrogen-containing tank where the cryo-tubes remain submerged at −196 ºC until they are used.
Fig. 2.
Cartilage tissue processing. a Weighing the cartilage tissue before processing. b The entire process was carried out inside a laminar flow hood under sterile conditions. c and d Mechanical digestion process. e and f Enzymatic digestion with type II collagenase. g The mechanical and enzymatic digestion product was passed through a cell strainer to remove any remaining undigested tissue. h The medium used for enzymatic digestion was removed by centrifugation. i and j The cell pellet was resuspended in culture medium to establish the viability and cell number in a Neubauer chamber. k The viability and cell number were quantified using an Axiovert 40 CFL inverted microscope (Carl Zeiss, AG, Oberkochen, Germany)
Cartilage samples in cadaveric donors
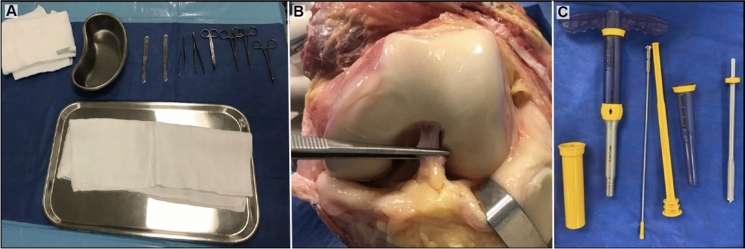
Cartilage procurement was performed taking cartilage biopsies in the same areas as in living donors (lateral wall of the notch and/or roof of the notch), under sterile conditions with the COR® system (8 mm width and a depth of 10 mm), in the hospital where the donor died. A biopsy was obtained in the form an osteochondral plug composed of a layer of cartilage followed by a layer of bone (Fig. 3). The transport of the samples to laboratory facility was carried out in a cooler with refrigerants (at 4 °C), in 50-mL tubes to which 10 mL of DMEM-F12 with 10% antibiotic/antimycotic had been added. The cadaveric tissue was processed the same way as described above for the procurement of chondrocytes in live donor patients.
Fig. 3.
Obtaining cartilage tissue in cadaveric donors. a Sterile material for sampling cadaveric donors. b Cadaveric donor sampling site with adequate macroscopic characteristics of cartilage. c Osteochondral transfer system with a diameter of 8 mm
Statistical analysis
The sample size of this study was adjusted to sampling at convenience due to the COVID-19 pandemic. A total sample size of n = 65 was achieved, 33 of them corresponded to samples from living donors and 32 to cadaveric samples. The power obtained with the sample size achieved in this study was 23.38%, with an alpha of 0.5, with two-tailed models to detect a difference of 0.33%. Shapiro–Wilk test was performed, which served to check the normality of the viability distribution along with the other continuous variables. If the variable was normally distributed, mean and standard deviation (SD) were used as descriptive statistic measures; if they were not normally distributed, median and interquartile range were used instead. To compare the continuous variables between groups, Student t test or Mann–Whitney U test were used if the variable was normally distributed or not, respectively.
Spearman rho correlation coefficient was estimated to evaluate the link between continuous variables and the number of chondrocytes per gram of tissue as well as with the percentage of viability. Those variables that showed a significant correlation were further analyzed by bivariate linear regression models to establish the mean estimated difference of number of chondrocytes and viability, stratified by origin of the sample.
To evaluate the possible impact of age, body mass index (BMI), and gender on the viability of chondrocytes, the latter was categorized in two strata: low viability (samples with viability lower than 80%), and high viability (samples that had viability of 80% or greater). Fisher´s exact test was used to compare the proportion of low and high viability samples between cadaveric and living donors, as well as between genders. Mann–Whitney U test was performed to compare age and BMI between origins of the sample stratified by viability.
Finally, bivariate and multivariate linear and logistic regression models, adjusted by variables of interest, were evaluated stratifying by the origin of the sample.
The statistical significance was set at 0.05 and all statistical analyses were performed in STATA v.14 (StataCorp, LLC; College Station, TX, USA).
Results
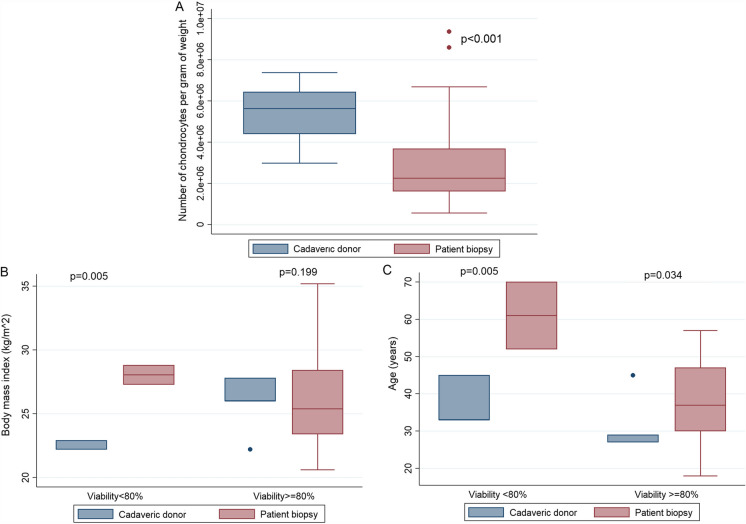
Viability depicted a normal distribution, whereas the rest of the variables showed a non-normal distribution. The age and BMI of the live donors were higher than those of the cadaveric donors (p = 0.0394, p = 0.0451, respectively). As expected, the time from sampling to processing was significantly higher in the samples of cadaveric donors than in the ones provided by live donors (p = 0.0001). Nonetheless, a greater number of chondrocytes per gram of tissue was observed in the cadaveric donors´ samples in comparison to the live donors´ samples (p = 0.000001) (Table 1, Fig. 4a).
Fig. 4.
Statistical analysis of parameters obtained. a Number of Chondrocytes per gram of weight obtained from cadaveric donors or live donor’s samples. b BMI of donors by the level of viability of the chondrocytes obtained from cadaveric or live donors. c Age of donors by the level of viability of the chondrocytes obtained from cadaveric or live donors
Characteristics of the donors
A total of 33 samples were obtained from living donors who underwent a knee arthroscopy scheduled for anterior cruciate ligament reconstruction. Samples were obtained from the non-loading zone of the knee in the femoral notch. Furthermore, 32 fresh cartilage samples were obtained from cadaveric donors (Novoinjertos, SC, a Mexican Tissue Bank) with the characteristics described in Table 1. To ensure safety, all cadaveric donors underwent a serological analysis for hepatitis B, hepatitis C, syphilis, HIV, and Chagas disease confirming their negativity.
Chondrocyte number per gram of cartilage
The mean number of chondrocytes per gram of tissue in cadaveric donors was 5.389197 × 106 (± 1.47 × 106), whereas in living donors an average of 3.06 × 106 (± 2.18 × 106) was obtained. The average viability of cadaveric samples was 84.16% (± 7.4), whereas in live donor samples it was 87.8% (± 9.7).
Cell viability by gender
The average viability of samples from male donors was 87.06%, whereas for women it was 81.71%, presenting a significant difference (p = 0.0409). However, when a stratified analysis was carried out per sample origin, no differences were found in percentage of viability between both genders (p = 0.074 cadaveric; p = 0.15 patient's biopsy). A Student's t test was performed (unadjusted, with two-tailed hypotheses, after checking the homoscedasticity of the groups) in which no differences were observed between the two populations (p = 0.22). However, as a high standard deviation due to the small sample size was observed and the estimated power (23.38%), there may be a type II error.
Cell viability by sampling processing time
A Spearman correlation analysis was performed for the non-parametric variables, revealing significant positive correlation only between the analysis of time from harvesting to processing and the number of chondrocytes (Rho 0.5118, p < 0.001). Linear regression stratified by origin showed that the time from sampling to processing decreases by 0.004863% the viability for each hour only among cadaveric donor samples.
Cell number by origin: cadaveric vs. live donor
A linear regression was performed for viability and sample origin that showed a higher viability for samples coming from living donors (0.033%). However, it was not statistically significant compared to cadaveric donors. In the linear regression, the number of chondrocytes were contrasted with the origin of the cartilage samples, observing that the living donor presented less cells (2.32 × 106) per gram of cartilage compared to those of cadaveric samples revealing a significant difference (p = 0.00001).
Percentage of high cell viability
A stratified analysis was performed with viability greater than or equal to 80% (high) and less than 80% (low). It was found that 20% of the samples presented a low viability and 80% presented a high viability. The analysis of cell viability, calculated by the origin of the samples, revealed that 65% of the samples from cadaveric donors showed high viability compared to 93.9% samples from the live donor (exact Fisher test, p = 0.005).
Sample viability vs. age of donor
When analyzing age with the two categories of viability, no significant difference was observed. However, when stratifying by origin of sample, there was a significant difference in age between high and low viability categories in the samples from cadaveric donor (p = 0.0054).
Body Mass Index (BMI) and cell viability
When performing a global analysis of BMI and stratified viability in high and low, a significant difference was found. However, when performing the stratification by origin of the samples no differences were found in those of live origin, whereas the cadaveric samples presented a significant difference (p < 0.00001). In the samples with low viability there was a significant difference in BMI between samples depending on their origin (the living donors showed higher BMI). In the samples with high viability, it was not possible to detect a significant difference in BMI between the groups. A statistical analysis was performed analyzing the cut-off points for viability greater than 80%, finding that the samples of cadaveric donors had some special characteristics (less than 29 years of age, BMI < 26 kg/m2, time of dead of the donor and processing time of the sample less than 60 h). A bivariate linear regression was performed for the variable viability with age, BMI and dead-sample processing time, stratified by sample origin. A significant association between viability, age, BMI and dead-sample processing time was observed in samples from cadaveric donors. A logistic regression was performed for the two categories of low viability with the variables of age, BMI, time, gender, and origin, finding no statistically significant association.
Influence of age and gender in cell viability and cell number
Multivariate linear regression models were performed to analyze the estimated difference in the viability and number of chondrocytes per gram of tissue according to the age and gender of the sample donor stratified by origin of the sample. The viability of cadaveric samples showed that for every year of age of the donor, the cell viability decreases 0.02% (p = 0.002). It also showed that cadaveric samples from female donors had a mean of 25% more viability than the samples from male donors (p = 0.0027). The linear regression analysis for viability with age and gender for the live donor samples was not conclusive due to insufficient power of the test to detect significant coefficients. The linear regression model for the number of chondrocytes per gram of tissue with age and gender in the cadaveric donor samples revealed that, with an increase for every year of age, there is an increase of 0.29 × 106 chondrocytes per gram (p = 0.014). It was also found that the cartilage samples from female live donors had 4.21 × 106 less chondrocytes per gram of tissue compared to male samples (p = 0.026), (see Fig. 4).
Discussion
The treatment of knee-focused chondral lesions with ACI has demonstrated good clinical and imaging results, and is one of the treatments with the highest long-term success rate (Brittberg et al. 2018). The main and most relevant limitations of this technique for large cartilage lesions are the limited amount of harvestable cartilage, the need for culture with the consequent cell dedifferentiation, as well as the need for two surgical times (Brittberg et al. 1994; Jones et al. 2019; Vonk et al. 2021). Due to these limitations and the promising results with the use of allogeneic chondrocytes in animal models (Olivos-Meza et al. 2017), it is important to consider the potential use of cadaveric donor chondrocytes as a cellular source for the treatment of cartilage lesions. The advantages would be to offer an unlimited source of donor tissue, avoid cell culture and expansion, less cell dedifferentiation, better quality of repair tissue, avoid donor site morbidity, and treatment in a single surgical event reducing costs and risks. It should be considered that the avascular nature of cartilage makes it a non-immunogenic tissue, so the next step would be to evaluate the safety of allogeneic chondrocyte transplantation in humans (Alford and Cole 2005).
Our study reports the viability and cell number chondrocytes obtained from samples of cadaveric donors, estimating the ideal time from donor's death to sample processing. These results were compared with cartilage samples obtained from live donors. We found a higher number of cells per gram of tissue (p = 0.000001) in cadaveric donor samples, which could correspond to the fact that the knees of the cadaveric donors could be considered healthier (none of the allogeneic donors had a history of knee injury) than those of living donors (all scheduled for knee surgery). In addition, the number of chondrocytes per gram of tissue in cadaveric donors was 2.32 × 106 higher than those of living donors (p = 0.00001), supporting the idea of using cartilage coming from a tissue bank as a cell source. No differences were observed between the percentage of viability analyzed by gender (p = 0.215), which allows us to consider harvesting of cartilage samples from both genders to treat chondral lesions with allogeneic chondrocyte transplantation.
The linear regression stratified by source of cartilage revealed that the dead-sample time processing decreased the viability by 0.0048% for each hour in the cadaveric donors. These results provide an acceptable margin for the procurement, processing, and use of this cellular source. When stratifying the samples between high (> 80%) and low (< 80%) viability, a significant difference in the high viability was found between cadaveric donor samples (65%) and live donor samples (93.9%) (p = 0.005). However, it was clear that in cadaveric donors with an increased age, the cell viability decreased 0.02% (p = 0.002). In this regard, the linear regression model found a decrease of 0.29 × 106 chondrocytes per gram of tissue for each year of age increase (p = 0.014). With these preliminary results, we can estimate the characteristics of the ideal cadaveric donor to set a source of allogenic chondrocytes, considering that the cadaveric donor should be less than 29-year-old, have a BMI below 26 kg/m2 and a dead-processing sample time less than 60 h to obtain a viability greater than 80%. We can consider two factors, which have been previously reported, to be influencing the average viability (84.16%) after 3 days of storage of samples from cadaveric donors in refrigeration at 4 ºC. First, the cartilage, when subjected to stress, actively induces reactive oxygen and nitrogen species (ROS and RNS) capable of causing cellular dysfunction and death (Grishko et al. 2009). Second, the cartilage after being subjected to mechanical stress, such as being injured by the insertion of an osteochondral graft, reduces 21% the viability of the surrounding cartilage at 1 h by an increase in caspase-3 activation (Borazjani et al. 2006). Therefore, it is to be expected that after the period of preservation in refrigeration, followed by the mechanical detachment of the cartilage, we will have a decrease in the viability of the harvested chondrocytes of around 20%.
Recently Acevedo et al. (2021) reported high viability values ranging from 73 to 100% in chondrocytes isolated from peripheral and central regions of the femoral condyle. Taking into account the above, we consider that the viability in both groups: cadaveric, 84.16%; and alive, 87.8%, is acceptable after subjecting the chondrocytes to the mechanical/enzyme process to obtain them (Acevedo et al. 2021). Also, Xia et al. (2008) reported about chondrocytes that were isolated from 15 patients undergoing total knee replacement and established viability immediately after enzymatic isolation from fresh articular cartilage, reporting an average viability of 74.7% (Xia et al. 2008), in comparison our results indicated higher averages for both groups.
On the other hand, It has been reported that osteochondral allograft from cadaveric donors, on average, are collected between 12 and 24 h and between ages ranging from 15 to 35 years (Ozenci et al. 2007), and its main application was as a salvage procedure in lesions larger than 2 cm2 where the previous treatment with cells had failed, thus seeking to return the normal biology of the knee, reducing pain and improving knee function, and delay a new future arthroplasty (Zouzias and Bugbee 2016). Unfortunately, it has been observed that the median survival of an osteochondral allograft after being grafted is 42 months, due to various reasons such as immunological rejection, loss of bone attachment, and breakdown of the cartilage matrix. Despite these results, it has also been shown that chondrocytes that manage to integrate into the native cartilage can remain viable for many years after being grafted (Williams et al. 2007). Trying to obtain better results, the use of mesenchymal stem cells (MSCs) together with allogeneic cartilage has been proposed, where it has been shown that the combined use of MSCs with allogeneic cartilage is accompanied by better clinical results (Kim et al. 2020). This improvement in the results could be due to a characteristic that chondrocytes possess, since when they are analyzed in primary culture, they do not present significant differences between the expression of phenotypic markers for MSCs such as: CD73, CCD90 and CD105 (Cournil-Henrionnet et al. 2008). This similarity could give chondrocytes the ability to immunomodulate the immune response when implanted in the same way as MSCs (Zhang et al. 2015). In this way, we propose the possibility of using chondrocytes recently disintegrated from the extracellular matrix of cartilage as a cell therapy that favors their incorporation into native cartilage while reducing rejection. In addition, cryopreserving the chondrocytes guarantees the availability of viable cells for many years (Gole et al. 2004). Also, we demonstrated that increasing the number of hours for the collection in cadaveric donors from 24 to 64 h does not decreases significantly affect the viability of the chondrocytes (84.16%). This fact is crucial for obtaining cells in countries with emerging economies where there are not enough personnel and infrastructure available to carry out the procurement of tissues within the first 24 h after the death of the donor.
Furthermore, besides the high viability of cells in young donors, there is strong evidence regarding the enhanced chondrogenic potential of juvenile chondrocytes versus adult chondrocytes. Data also suggest that juvenile chondrocytes may not be immunogenic in adult hosts, supporting the possibility that such cells may be used as allografts for cartilage regeneration in vivo (Adkisson et al. 2010; Ibrahim et al. 2021). In addition, use of young articular chondrocytes derived from allogeneic cadavers eliminates donor-site morbidity, the requirement for two surgical procedures associated with the therapeutic application of autologous chondrocyte implantation, and finally our work seeks to generate knowledge about the correct selection of allogeneic chondrocyte donors, and is aimed at providing knowledge to be useful for research groups that are working on the development of new techniques that are evolving from ACI.
Conclusions
It is possible to obtain viable chondrocytes from cartilage harvested from cadaveric donors, reaching a similar cell number and viability compared to the cartilage obtained from live donors.
The characteristics of the ideal cadaveric donor to for a high viability of chondrocytes are:
A donor younger than 29 years
A donor with a BMI below 26 kg/m2.
A processing time to obtain the chondrocytes of less than 60 h.
Acknowledgements
The authors wish specialty to thank to Novoinjertos, Musculoskeletal Tissue and Skin Bank. We also appreciate the support and collaboration of Ivonne Trigueros-Anaya, Gloria Gonzalez-Vellano, Montserrat Gabriela Romero Lobera, Ana Maria Godinez-Monroy, Carmen Gonzalez-Vellano, Yesica de Jesus Corona, and Angelica Arcadio Tinoco (Arthroscopy Surgical Nursing team). María del Rocío Aguilar Gaytán (Tissue engineering unit team). For their assistance in preparing and conducting this study.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anell Olivos-Meza, Email: aolivos_meza@hotmail.com.
Mats Brittberg, Email: mats.brittberg@telia.com.
Gabriela Martínez-Nava, Email: gamartinezn@inr.gob.mx.
Carlos Landa-Solis, Email: clanda@inr.gob.mx.
References
- Acevedo L, Iselin L, Berkelaar MHM, Salzmann GM, Wolf F, Feliciano S, Vogel N, Pagenstert G, Martin I, Pelttari K, Barbero A, Arnold MP. Comparison of human articular cartilage tissue and chondrocytes isolated from peripheral versus central regions of traumatic lesions. Cartilage. 2021;13:68s–81s. doi: 10.1177/1947603520958154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkisson HDt, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal KB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- Armoiry X, Cummins E, Connock M, Metcalfe A, Royle P, Johnston R, Rodrigues J, Waugh N, Mistry H. Autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects in the knee: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37:879–886. doi: 10.1007/s40273-018-0737-z. [DOI] [PubMed] [Google Scholar]
- Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–230. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- Borazjani BH, Chen AC, Bae WC, Patil S, Sah RL, Firestein GS, Bugbee WD. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am. 2006;88:1934–1943. doi: 10.2106/jbjs.E.00992. [DOI] [PubMed] [Google Scholar]
- Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/nejm199410063311401. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Recker D, Ilgenfritz J, Saris DBF. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46:1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]
- Cournil-Henrionnet C, Huselstein C, Wang Y, Galois L, Mainard D, Decot V, Netter P, Stoltz JF, Muller S, Gillet P, Watrin-Pinzano A. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45:513–526. doi: 10.3233/BIR-2008-0487. [DOI] [PubMed] [Google Scholar]
- de Windt TS, Vonk LA, Brittberg M, Saris DB. Treatment and prevention of (early) osteoarthritis using articular cartilage repair-fact or fiction? A systematic review. Cartilage. 2013;4:5s–12s. doi: 10.1177/1947603513486560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker TJ, Aman ZS, DePhillipo NN, Dickens JF, Anz AW, LaPrade RF. Chondral lesions of the knee: an evidence-based approach. J Bone Joint Surg Am. 2021;103:629–645. doi: 10.2106/jbjs.20.01161. [DOI] [PubMed] [Google Scholar]
- Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes' knees: a systematic review. Med Sci Sports Exerc. 2010;42:1795–1801. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- Frisbie D, Lu Y, Colhoun H, Kawcak C, Binette F, McIlwraith C (2005) In vivo evaluation of a one step autologous cartilage resurfacing technique in a long term equine model. In: Transactions of the 51st Annual Meeting of the Orthopaedic Research Society, Washington, DC.
- Gardner J, Webster A. Accelerating innovation in the creation of biovalue: the cell and gene therapy catapult. Sci Technol Human Values. 2017;42:925–946. doi: 10.1177/0162243917702720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gole MD, Poulsen D, Marzo JM, Ko SH, Ziv I. Chondrocyte viability in press-fit cryopreserved osteochondral allografts. J Orthop Res. 2004;22:781–787. doi: 10.1016/j.orthres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- Grishko VI, Ho R, Wilson GL, Pearsall AWt. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartil. 2009;17:107–113. doi: 10.1016/j.joca.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck DA, Kraeutler MJ, Belk JW, Frank RM, McCarty EC, Bravman JT. Do focal chondral defects of the knee increase the risk for progression to osteoarthritis? A review of the literature. Orthop J Sports Med. 2018;6:2325967118801931. doi: 10.1177/2325967118801931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme CH, Perry J, McCarthy HS, Wright KT, Snow M, Mennan C, Roberts S. Cell therapy for cartilage repair. Emerg Top Life Sci. 2021;5:575–589. doi: 10.1042/etls20210015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries S, Joshi A, Webb WR, Kanegaonkar R. Auricular reconstruction: where are we now? A critical literature review. Eur Arch Otorhinolaryngol. 2022;279:541–556. doi: 10.1007/s00405-021-06903-5. [DOI] [PubMed] [Google Scholar]
- Ibrahim S, Nagesh HY, Pandey V. Allogeneic chondrocyte implantation: What is stopping it from being a standard of care? J Arthrosc Surg Sports Med. 2021;3:34–39. doi: 10.25259/JASSM_8_2021. [DOI] [Google Scholar]
- Jones KJ, Kelley BV, Arshi A, McAllister DR, Fabricant PD. Comparative effectiveness of cartilage repair with respect to the minimal clinically important difference. Am J Sports Med. 2019;47:3284–3293. doi: 10.1177/0363546518824552. [DOI] [PubMed] [Google Scholar]
- Kim YS, Chung PK, Suh DS, Heo DB, Tak DH, Koh YG. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2020;28:544–554. doi: 10.1007/s00167-019-05729-3. [DOI] [PubMed] [Google Scholar]
- Liao J, Chen Y, Chen J, He B, Qian L, Xu J, Wang A, Li Q, Xie H, Zhou J. Auricle shaping using 3D printing and autologous diced cartilage. Laryngoscope. 2019;129:2467–2474. doi: 10.1002/lary.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- Mastbergen SC, Saris DB, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9:277–290. doi: 10.1038/nrrheum.2013.29. [DOI] [PubMed] [Google Scholar]
- McCormick F, Yanke A, Provencher MT, Cole BJ. Minced articular cartilage–basic science, surgical technique, and clinical application. Sports Med Arthrosc Rev. 2008;16:217–220. doi: 10.1097/JSA.0b013e31818e0e4a. [DOI] [PubMed] [Google Scholar]
- Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468:147–157. doi: 10.1007/s11999-009-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, Baumann M, Bosch U, Erggelet C, Fickert S, Gebhard H, Gelse K, Günther D, Hoburg A, Kasten P, Kolombe T, Madry H, Marlovits S, Meenen NM, Müller PE, Nöth U, Petersen JP, Pietschmann M, Richter W, Rolauffs B, Rhunau K, Schewe B, Steinert A, Steinwachs MR, Welsch GH, Zinser W, Fritz J. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Clinical Tissue Regeneration" of the German Society of Orthopaedics and Trauma (DGOU) Knee. 2016;23:426–435. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Olivos-Meza A, Velasquillo Martínez C, Olivos Díaz B, Landa-Solís C, Brittberg M, Pichardo Bahena R, Ortega Sanchez C, Martínez V, Alvarez Lara E, Ibarra-Ponce de León JC. Co-culture of dedifferentiated and primary human chondrocytes obtained from cadaveric donor enhance the histological quality of repair tissue: an in-vivo animal study. Cell Tissue Bank. 2017;18:369–381. doi: 10.1007/s10561-017-9635-4. [DOI] [PubMed] [Google Scholar]
- Ozenci AM, Gür S, Aydin AT. Osteochondral allograft transplantation in the knee. Acta Orthop Traumatol Turc. 2007;41(Suppl 2):87–92. [PubMed] [Google Scholar]
- Schneider S, Ossendorff R, Holz J, Salzmann GM. Arthroscopic Minced Cartilage Implantation (MCI): a technical note. Arthrosc Tech. 2021;10:e97–e101. doi: 10.1016/j.eats.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk LA, Roël G, Hernigou J, Kaps C, Hernigou P (2021) Role of matrix-associated autologous chondrocyte implantation with spheroids in the treatment of large chondral defects in the knee: a systematic review. Int J Mol Sci 22. 10.3390/ijms22137149 [DOI] [PMC free article] [PubMed]
- Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, Badlani NM, Emery SC, Haghighi P, Bugbee WD. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- Xia Z, Murray D, Hulley PA, Triffitt JT, Price AJ. The viability and proliferation of human chondrocytes following cryopreservation. J Bone Joint Surg Br. 2008;90:1245–1248. doi: 10.1302/0301-620x.90b9.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, Hu D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234. doi: 10.1186/s13287-015-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf DA, Flanagan CL, Mitsak AG, Brennan JR, Hollister SJ. Pore architecture effects on chondrogenic potential of patient-specific 3-dimensionally printed porous tissue bioscaffolds for auricular tissue engineering. Int J Pediatr Otorhinolaryngol. 2018;114:170–174. doi: 10.1016/j.ijporl.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias IC, Bugbee WD. Osteochondral allograft transplantation in the knee. Sports Med Arthrosc Rev. 2016;24:79–84. doi: 10.1097/jsa.0000000000000109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.