Abstract
Purpose
The energy density (ED) of a diet can be leveraged to prevent weight gain or treat overweight and obesity. By lowering the ED of the diet, energy intake can be reduced while maintaining portion size. However, a reliable meta-analysis of data from randomized controlled trials (RCTs) is missing. Therefore, this meta-analysis synthesized the evidence of ED manipulation on energy intake in RCTs.
Methods
The systematic literature search of multiple databases according to PRISMA criteria considered RCTs investigating the objectively measured energy intake from meals with different ED (lower ED (median 1.1 kcal/g) versus higher ED (median 1.5 kcal/g)) under controlled conditions. Subgroup analyses for age (children versus adults), meal type (preload versus entrée design), and intervention length (1 meal versus > 1 meal) were performed to achieve the most homogeneous result.
Results
The meta-analysis of 38 included studies demonstrated that lowering ED considerably reduced energy intake – 223 kcal (95% CI: – 259.7, – 186.0) in comparison to the higher ED interventions. As heterogeneity was high among studies, subgroup analyses were conducted. Heterogeneity decreased in subgroup analyses for age and meal type combined, strengthening the results. An extended analysis showed a positive linear relationship between ED and energy intake. Dietary ED did not affect the amount of food intake.
Conclusion
Manipulating ED substantially affects energy intake whereas food intake remains constant. Thus, this approach can be regarded as a powerful tool for weight management through nutrition therapy. Registration on 08/08/2021: CRD42021266653.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-03054-z.
Keywords: Energy density, Energy intake, Manipulation, Nutrition, Diet, Obesity
Introduction
Body weight maintenance is based on the balance between energy intake and energy expenditure. Consequently, the principles of weight management focus on reducing energy intake by changing diet and eating patterns while increasing energy expenditure through higher levels of physical activity and reduced sedentary behavior [1].
Food portion size and energy density (ED; calories per gram) are critical determinants of energy intake [1, 2], which is why their manipulation is the basis of many weight management interventions [3–6]. Thus, foods with high water content have a low ED, whereas foods containing high proportions of macronutrients, especially carbohydrates and fats, have a higher ED [7]. Many laboratory studies have shown that the reduction of meal ED allows for consumption of a satiating amount of food while simultaneously reducing energy intake [8, 9] due to relatively constant amounts of food consumed across conditions [10, 11]. In response, the World Health Organization has highlighted energy-dense foods as a key contributing factor to the increasing prevalence of overweight and obesity [12], noticing the role of either low- or high-energy-dense food selection in the diets has on body weight [13–16]. In practice, the methods that lower ED are not only flexible and adaptive, but also allow for application in a great variety of dietary patterns, aligning with individual food preferences as well as personal and cultural backgrounds [2, 17, 18].
Although, data on the impact of an ED intervention on energy intake in both children [19] and adults [20] clearly point towards the same outcome, study designs are highly heterogeneous in relation to intervention and duration approaches. First, differing satiation mechanisms are triggered based on either an entrée or a preload design. Preload interventions trigger between-meal-satiety at the end of the preload inhibiting further food intake, whereas, in an ad libitum entrée design, intra-meal satiation is initiated, triggering meal termination and determining the size of the meal [21]. A systematic review and meta-analysis of clinical trials was performed to assess the effect of preload ED on energy intake in subsequent meals. The analysis revealed that compared to a high ED preload, a low ED preload resulted in higher subsequent energy intake, but the total energy intake (preload + subsequent meal) was still lower in the latter condition [22]. Similarly, participants showed a trend toward some compensation for reduced energy intake after consuming preloads, but without reaching statistical significance [23]. Conversely, when subjects were satiated consuming either high or low ED ad libitum entrées for 5 days, the subjects in the low ED group halved their energy intake without any compensation [24]. Second, differences in intervention length contribute to more heterogeneity since the effects of ED manipulation differ in short-to-medium term studies versus longer term interventions [25]. A recent meta-analysis confirmed that manipulating ED for at least one day results in significantly altered energy intake. The meta-analysis of 41 studies with human participants examined randomized and non-randomized experimental studies that took place either in the laboratory or in a free-living setting [19]. Moreover, it has been suggested in an experimental study, that over longer experimental settings the amount of food consumed would gradually increase to compensate for reduced ED and therefore the daily energy intake would be maintained [10].
Currently no reliable meta-analysis of data regarding the impact of ED manipulation on energy intake in humans from exclusively randomized controlled trials (RCTs) considering data of objectively measured food intake exists. Additionally, the relationship between the offered ED of foods and energy intake has not been mathematically described and remains unclear. This systematic review and meta-analysis aimed to close these gaps by synthesizing the best available evidence on the effectiveness of influencing the energy intake by manipulating ED in children and adults. In addition, different meal types and intervention lengths were considered. The secondary objective was to analyze the impact of the various ED conditions on the amount of food intake. The following hypotheses were tested qualitatively and quantitatively:
1. Energy intake in kcal is lower in the meal conditions with lower ED in comparison with the meal conditions with higher ED.
2. The amount of food in g consumed is similar across the meal conditions with different ED.
Materials and methods
This review was developed and executed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. To identify all relevant studies examining the effect of ED manipulation on the consumed energy in humans the databases PubMed, Web of Science, Cochrane Library (Wiley) and EBM-Reviews (Ovid) Cochrane Library were searched on December 14th 2020 and updated at January 6th 2021. The protocol of this systematic review is registered on the PROSPERO platform with the registration number CRD42021266653. The full search strategy is documented in the supplementary information (Text S1) and consists of the four modules manipulated ED, group comparison, energy intake and the exclusion of animals.
Eligibility criteria
Inclusion criteria were established based on the five PICOS dimensions, i.e., participants, interventions, comparator, outcome and study design [27].
Participants
Human adults, adolescents and children aged older than one year, without any restrictions on sex and weight status were included. Participants unable to eat solid foods were excluded (e.g. breast, complementary or tube feeding). Studies exclusively conducted in specific patient groups with e.g. type 2 diabetes/ insulin resistance, cardiovascular disease, metabolic syndrome, cancer, immunodeficiency diseases, malnutrition/anorexia, renal disease, diarrhea or after any kind of surgical intervention were excluded to avoid selection bias of specific groups. In addition, articles examining participants with food intolerances or food allergies were not considered.
Interventions
A controlled environment (such as a laboratory or a researcher manipulated group setting) was required. Participants were required to be served at least one test meal per day with a manipulated ED, resulting in meals with a lower and a higher ED. Studies were included that had either (i) a compulsory manipulated preload (all participants had to consume the same amount) followed by an unmanipulated ad libitum test meal, or (ii) solely an ad libitum entrée manipulated in the ED, or (iii) a manipulated ad libitum entrée and unmanipulated compulsory side dishes (all participants were required to eat the offered side dishes), or (iv) a manipulated ad libitum entrée and unmanipulated ad libitum side dishes. Studies that achieved manipulation of ED either by modifying the proportions of macronutrients in % (e.g., low-fat versus high-fat products) or by changing the water content (e.g. adding water or vegetables), or by substituting foods of a lower or a higher ED (e.g. commercially available food products) were eligible. Studies that altered the ED of meals by increasing the amount of water (ED 0.0 kcal/g) consumed during meals in the form of a beverage were excluded since it was found that water had a greater impact on satiety when included in food than when consumed as beverage along with food [28], as water blended into foods has been shown to slow stomach emptying more than consuming the water separately [29]. Studies focusing on portion size manipulation, conditioning periods for becoming familiar with a certain food or energy intake, studies that allowed alcohol consumption within the intervention or that focused on physical activity were excluded.
Comparator
A comparison among intervention arms was required either between or within subjects.
Outcome
The primary outcome was energy intake in kcal resulting from the corresponding ED manipulation. Therefore, food intake in g had to be measured by a calibrated scale and the foods’ caloric value had to be derived from validated sources, either bomb calorimetry or internationally known food databases. Data from FFQs, 24-h recalls or similar sources were excluded. The secondary outcome was food intake in g after the meals.
Study design
The systematic data analysis referred exclusively to randomized trials as parallel and crossover designs.
Study selection
To identify eligible studies, the search results of the databases were combined and the duplicates removed. Two authors (B.K. and J.C.) independently screened titles and abstracts to identify relevant randomized trials. Full-text articles were evaluated regarding their eligibility with uncertainties being discussed between the authors (< 3% cases). In case of discrepancies, a third author was involved (I.M.). In case of missing data, the authors of the RCTs were contacted by email with a response rate of 85%.
Data extraction
The following information was extracted from each included article: year of publication, country of origin, study design and intervention length, sample characteristics (including age, sex, body mass index (BMI) or BMI percentile curves) and sample size, details of the intervention (modified ED per meal, manipulated meal and the type of the manipulated meal, time of day to which the meal belongs (breakfast, lunch, dinner), possible other meal manipulations), outcomes including energy intake in kcal and food intake in g and information for assessment of the risk of bias were recorded.
Characteristics across studies are presented as median [interquartile range], minimum and maximum for sample size, age, BMI or BMI percentile curves, study length, washout period and provided ED. The results across studies are presented as per cent (%) for origin and sex distribution.
Data analyses
For energy and food intake, data were evaluated qualitatively and quantitatively (meta-analysis). The qualitative analyses allowed all findings to be summarized regarding their direction of change between the intervention groups, as not all studies provided sufficient data. For the meta-analysis, a random-effect model was applied [30] using the software package Review Manager, version 5.4.1 [31] and sample size, energy intake in kcal, food intake in g presented as mean and SD are reported separately for lower ED and higher ED intervention. The difference is expressed as mean difference and 95% confidence interval, and graphically presented in forest plots. To eliminate underweighting and double-counting errors, factorial crossover designs were included in the analysis once per ED intervention according to their factor (2 × 2 or 3 × 2) as recommended by the Cochrane Handbook [32]. Thus, multifactorial studies were recorded as single effects in the quantitative analyses and were separated by a unique study ID. Statistical heterogeneity was examined by visual inspection of forest plots and using the I2-statistics to quantify inconsistency between the studies. I2-heterogeneity below 40% was considered as low, whereas heterogeneity up to 60% was classified as moderate and from 75% onwards as considerably high [32].
Subgroups were developed to provide a more homogenous summary of findings. In both the qualitative and quantitative analyses, studies were classified according to age of the study population. Hence, the subgroups of children and adolescents (< 18 years, BMI given by percentiles) and adults (> 18 years, BMI given in kg/m2) were identified (subgroup analysis 1). For the quantitative meta-analysis, additionally the subgroups regarding meal type (preload vs. entrée; subgroup analysis 2) were formed. In preload studies, study participants are served a mandatory preload followed by an ad libitum test meal. The time between preload and test meal had to be at least 15 min. Only the combined data of preload and subsequent test meal was used for the analysis. In contrast, entrée studies included meals with different ED served ad libitum. Side dishes were possible and were considered as well. Finally, the data were analyzed according to intervention length (energy intake after 1 meal is assessed versus energy intake after > 1 meal is assessed; subgroup analysis 3). A statistically significant subgroup effect was considered at a p -value of less than 0.05 [32]. No subgroup analysis was performed in the context of weight status as the sample was too small (n = 3).
To analyze the relationship between consumed delta ED (lower versus higher ED condition per study) and delta energy intake, linear regression analyses were conducted. Thereby, the consumed ED was calculated from objectively measured energy [kcal] and food intake [g]. The ED consumed was not calculated in situations where data on food intake was absent. Regression analyses were only performed when more than 5 cases were available to avoid any bias, allowing for analyses of the following subgroups: (i) children, entrée design, 1 meal; (ii) adults, entrée design, 1 meal, (iii) adults, entrée design, > 1 meal, (iv) preload design. The coefficient of determination R2 was used as a criterion for linearity. Generally, higher coefficients indicate a better prediction of the dependent variable, where coefficients of 0.5 or more are considered to indicate a linear relationship [33].
Risk of bias
For the eligible studies, a risk of bias assessment was conducted using the Cochrane risk-of-bias tool for randomized trials (RoB2) [34]. The tool consists of 5 domains addressing different types of bias: randomization, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. In any domain, appropriate questions had to be answered for each study. Next, the RoB2 algorithm is applied which evaluates the risks of the individual domains. Finally, an overall risk is calculated and expressed as “low” or “high” risk of bias, or the judgment can be expressed with “some concerns”.
Since blinding is very difficult in nutritional studies, it was also investigated which studies concealed ED manipulation from their participants to the highest possible extent. Secrecy was achieved by reducing visual, sensory and taste differences between the meals to a minimum.
Results
Study selection and categorization
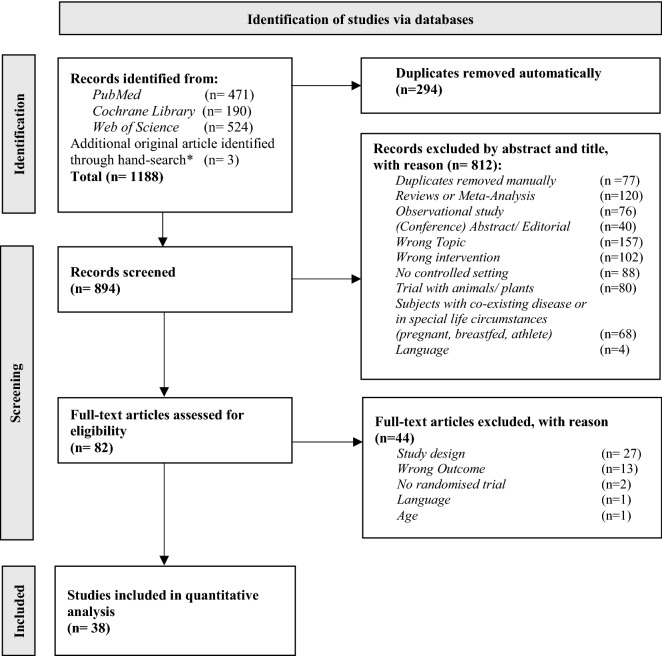
The literature search process for identification of eligible studies is shown in Fig. 1. Out of 1188 identified studies, 38 RCTs remained for analysis.
Fig. 1.
PRISMA flowchart for study inclusion. *Hand-search via database Ovid representative for Cochrane Library Search Strategy
Summary of study characteristics
A detailed overview of the characteristics for the single trials is presented in Table 1. The characteristics across the studies are given in the text and summarized in Fig. 2.
Table 1.
Summarized study characteristics for crossover trials
| Author (year) |
Country | Intake length (i), washout length (w) |
Participants N (A); sex (f %); age [y] mean (SD) |
Manipulated ED [kcal/g] of total meal (manipulated + if available unmanipulated items) | Consumed ED [kcal/g] of total meal (manipulated + if available unmanipulated items) | Outcomes |
|---|---|---|---|---|---|---|
| Studies with entrée design (adults) | ||||||
| Bell and Rolls [46] | US | i: 6 × 1 meal | N: 46 (36); f: 100%; | LED: 1.1 | LED: 1.1 | EI [LED]: ↓ |
| w: 5 x ≥ 5 days | age: S1: 26.9 (7.9), S2: 25.8 (1.3) | HED: 1.4 | HED: 1.5 | FI [LED]: ↔ | ||
| Blatt et al. [47] | US | i: 3 × 1 day | N: 48 (41); f: 51.2%; | LED: 2.0 [+ side dishes; ED N.A.] | N.A. | EI [LED]: ↓ |
| w: 2 × 7 days | age: m: 24.4 (4.5), f: 23.9 (5.5) | HED: 2.6 [+ side dishes; ED N.A.] | FI [LED]: ↔ | |||
| Cheskin et al. [49] | US | i: 2 × 4 meals | N: 76 (54); f: 66.7%; age: 35.5 (N.R.) | LED: 2.0 | N.A. | EI [LED]: ↓ |
| w: 1 × 3 days | HED: 4.6 | FI [LED]: ↔ | ||||
| Devitt and Mattes [50] | US | i: 4 days | N: 26 (20); f: 45.0%; age: 22.6 (5.8) | LED: 1.5 | LED: 1.5 | EI [LED]: ↓ |
| w: N.R. | HED: 2.6 | HED: 2.6 | FI [LED]: ↔ /↑ | |||
| *Hogenkamp et al. [52] | NL | i: 4 × 5 meals | N: 118 (105); f: 56.2%; age: 22.0 (3.0) | LED: 0.5 | LED: 0.5 | EI [LED]: ↓ |
| w: 3 × 2 days | HED: 1.4 | HED: 1.4 | FI [LED]: ↑ | |||
| Hogenkamp et al. [53] | NL | i: 4 × 4 days | N: 38 (27); f: 66.7%; age: 21.0 (2.4) | LED: 0.3 | N.A. | EI [LED]: ↓ |
| w: 3 × 3 days | HED: 1.3 | FI [LED]: ↑ | ||||
| Karl et al. [54] | US | i: 4 × 1 meal | N: 20 (20); f: 40.0%; age: 30.0 (11.0) | LED: 1.2 | LED: 1.2 | EI [LED]: ↓ |
| w: yes. but N.R. | HED: 1.6 | HED: 1.6 | FI [LED]: ↔ | |||
| Kral et al. [55] | US | i: 3 × 1 day | N: 40 (40); f: 100%; | LED: 1.3 [+ side dishes; ED N.A.] | LED: 1.1 | EI [LED]: ↓ |
| w: 2 × 6 days | age: S1: 20.5 (3.1), S2: 21.8 (2.7) | HED: 1.8 [+ side dishes; ED N.A.] | HED: 1.4 | FI [LED]: ↑ | ||
| Kral et al. [56] | US | i: 6 × 1 day | N: 45 (39); f: 100%; age: 23.4 (1.0) | LED: 1.3 | LED: 1.1 | EI [LED]: ↓ |
| w: 5 × 7 days | HED: 1.8 | HED: 1.6 | FI [LED]: ↑ | |||
| McCrickerd et al. [57] | SGP | i: 4 × 1 meal | N: 61 (58); f: 53.5%; | LED: 0.6 | N.A. | EI [LED]: ↓ |
| w: 3 x ≥ 3 days | age: m: 25.6 (5.3), f: 23.5 (3.5) | HED: 1.0 | FI [LED]: ↔ | |||
| Poppitt and Swann [58] | UK | i: 2 × 12 days | N: 6 (5); f: 0%; age: 35.0 (8.9) | LED: 0.9 | N.A. | EI [LED]: ↓ |
| w: 1 × 3 days | HED: 2.0 | FI [LED]: ↔ | ||||
| Rolls et al. [61] | US | i: 4 × 2 days | N: 25 (24); f: 100%; age: 21.9 (3.4) | LED: 1.6 | LED: 1.6 | EI [LED]: ↓ |
| w: 3 × 7 days | HED: 2.1 | HED: 2.1 | FI [LED]: ↔ | |||
| Rolls et al. [62] | US | E1: i: 6 × 1 meal (w: 5 × 7 days) | N: 100 (97); f: 49.5%; | LED: 1.2 | LED: 1.2 | EI [LED]: ↓ |
| age E1:m: 26.8 (6.0), f: 26.7 (7.8); | ||||||
| E2: i: 6 × 1 meal (w: 5 × 7 days) | age E2:m: 24.8 (6.4), f: 28.5 (7.4) | HED: 1.3 | HED: 1.3 | FI [LED]: ↔ | ||
| Silver et al. [63] | US | i: 6 meals/wk over 7 months | N: 52 (45); f: 68.9%; age: 84.4 (1.0) | LED: 1.1 | N.A. | EI [LED]: ↓ |
| w: N.R. | HED: 2.2 | FI [LED]: ↔ | ||||
| Stubbs, Harbron et al. [65] | UK | i: 3 × 9 days | N: 6 (6); f: 0%; age: 41.8 (10.6) | LED: 1.2 | LED: 1.0 | EI [LED]: ↓ |
| w: NR | HED: 1.7 | HED: 1.5 | FI [LED]: ↑ | |||
| Stubbs, Ritz et al. [67] | UK | i: 3 × 16 days | N: 7 (7); f: 0%; age: 36.9 (7.6) | LED: 1.2 | N.A. | EI [LED]: ↓ |
| w: N.R. | HED: 1.7 | FI [LED]: ↔ | ||||
| Stubbs, Johnstone, Harbron et al. [64] | UK | i: 2 × 16 days | N: 6 (6); f: 0%; age: 32.2 (5.3) | LED: 0.9 | LED: 0.8 | EI [LED]: ↓ |
| w: 1 x ≥ 7 days | HED: 1.5 | HED: 1.6 | FI [LED]: ↑ | |||
| Stubbs, Johnstone, O’Reilly et al. [66] | UK | i: 3 × 16 days | N: 6 (6); f: 0%; age: 30.0 (12.8) | LED: 0.9 | LED: 0.9 | EI [LED]: ↓ |
| w: 2 x ≥ 7 days | HED: 1.8 | HED: 1.7 | FI [LED]: ↑ | |||
| Williams et al. [69] | US | i: 4 × 1 day | N: 62 (59); f: 49.2%; | LED: 1.4 [+ side dishes; ED N.A.] | LED: 1.5 | EI [LED]: ↓ |
| w: 3 × 7 days | age: m: 26.1 (5.4), f: 25.6 (5.9) | HED: 1.8 [+ side dishes; ED N.A.] | HED: 1.7 | FI [LED]: ↔ | ||
| Williams et al. [68] | US | i: 6 × 1 meal | N: 53 (46); f: 100%; age: 25.4 (5.4) | LED: 1.3 | LED: 1.3 | EI [LED]: ↓ |
| w: 5 × 7 days | HED: 1.7 | HED: 1.7 | FI [LED]: ↔ | |||
| Yeomans et al. [70] | UK | E1: i: 4 × 1 meal (w: 3×) | N: 16(16); f: 0%; age: 21.4 (1.6) | LED: 0.9 | N.A. | EI [LED]: ↓ |
| E2: i: 8 × 1 meal (w: 7×) | HED: 2.0 | FI [LED]: ↔ /↑ | ||||
| Yeomans et al. [71] | UK | i: 8 × 1 meal | N: 32 (32); f: 0%; | LED: 0.6 | N.A. | EI [LED]: ↓ |
| w: 7× | age: S1: 22.3 (2.4), S2: 22.9 (3.8), | HED: 1.7 | FI [LED]: ↔ | |||
| S3: 22.6 (2.8), S4: 22.5 (2.1) | ||||||
| Studies with entrée design (children) | ||||||
| Fisher et al. [35] | US | i: 4 × 1 meal | N: 53 (53); f: 52.8%; age: 5–6 (N.R.) | LED: 1.3 + 0.8 | LED: 1.1 | EI [LED]: ↓ |
| w: 3 × 7 days | HED: 1.8 + 0.8 | HED: 1.3 | FI [LED]: ↔ | |||
| Johnson et al. [36] | US | i: 1–2 meals/ wk | N: N.R. (21); f: 47.6%; | LEDE1: 1.1, HEDE1: 2.2 | LEDE1: 1.1, HEDE1: 2.2 | EI [LED]: ↓ |
| w: N.R. | age: E1: 4.0 (0.7). E2: 2.8 (0.3) | LEDE2: 1.1, HEDE2: 2.3 | LEDE2: 1.1, HEDE2: 2.3 | FI [LED]: ↔ | ||
| Kling, Roe, Keller et al. [37] | US | i: 6 × 1 meal | N: 131 (120); f: 49.2%; age: 4.4 (1.1) | LED: 0.8 | LED: 0.8 | EI [LED]: ↓ |
| w: 5 × 7 days | HED: 1.1 | HED: 1.1 | FI [LED]: ↔ | |||
| Kling, Roe, Sanchez et al. [38] | US | i: 4 × 1 meal | N: 143 (125); f: 46.4%; age: 4.2 (1.1) | LED: 0.4 + 1.3 | LED: 0.9 | EI [LED]: ↔ |
| w: 3 × 7 days | HED: 0.6 + 1.3 | HED: 1.0 | FI [LED]: ↔ | |||
| Leahy, Birch, Fisher et al. [42] | US | i: 4 × 1 meal | N: 75 (61); f: 50.8%; | LED: 0.8 | LED: 0.7 | EI [LED]: ↓ |
| w: 3 × 7 days | age: m: 4.5 (0.6). f: 4.3 (0.6) | HED: 0.9 | HED: 0.9 | FI [LED]: ↔ | ||
| Leahy, Birch & Rolls [41] | US | i: 2 × 2 days | N: 29 (26); f: 61.5%; age: 4.2 (0.5) | LED: 0.9 | LED: 1.0 | EI [LED]: ↓ |
| w: 1 × 12 days | HED: 1.1 | HED: 1.2 | FI [LED]: ↔ | |||
| Olsen et al. [43] | DK | i: 2 × 1 meal | N: N.R. (74); f: 60.8%; age: 5.6 (0.8) | LED: 0.9 | LED: 0.9 | EI [LED]: ↓ |
| w: N.R. | HED: 1.4 | HED: 1.5 | FI [LED]: ↔ | |||
| Smethers et al. [44] | US | i: 3 × 5 days | N: 56 (49); f: 46.9%; age: 4.3 (0.7) | LED: 0.9 | LED: 0.9 | EI [LED]: ↓ |
| w: 2 × 7 days | HED: 1.0 | HED: 1.1 | FI [LED]: ↔ | |||
| Spill et al. [45] | US | i: 3 × 1 day | N: 49 (39); f: 53.8%; age: 4.7 (0.6) | LED: 1.6 | LED: 1.3 | EI [LED]: ↓ |
| w: 2 × 6 days | HED: 2.0 | HED: 1.5 | FI [LED]: ↔ | |||
| Studies with preload design (adults) | ||||||
| Blatt et al. [48] | US | i: 4 × 1 day | N: 73 (68); f: 58.8%; | LED: 1.0 + 1.7 | LED: 1.2 | EI [LED]: ↓ |
| w: 3 × 7 days | age: m: 26.8 (5.8); f: 27.6 (7.0) | HED: 1.6 + 1.7 | HED: 1.6 | FI [LED]: ↑ | ||
| Gray et al. [51] | UK | i: 5 × 1 meal | N: 18 (18); f: 0%; age: 26.0 (5.2) | LED: 0.3 + N.A. | N.A. | EI [LED]: ↓ |
| w: 4 × 2–14 days | HED: 1.0 + N.A. | FI [LED]: N.R. | ||||
| *Hogenkamp et al. [52] | NL | i: 2 × 5 meals | N: 118 (105); f: 56.2%; age: 22.0 (3.0) | LED: 0.5 + 2.7 | LED: 1.2 | EI [LED]: ↓ |
| w: 1 × 28 days | HED: 1.4 + 2.7 | HED: 1.8 | FI [LED]: ↑ | |||
| Rolls et al. [59] | US | i: 2 × 1 meals | N: 24 (24); f: 100%; age: 21.8 (4.4) | LED: 0.1 + 4.5 | LED: 1.5 | EI [LED]: ↓ |
| w: 1 × 7 days | HED: 0.5 + 4.5 | HED: 1.6 | FI [LED]: ↔ | |||
| Rolls et al. [60] | US | i: 7 × 1 meal | N: 50 (42); f: 100%; age: 26.3 (7.8) | LED: 0.3 + 2.0 | N.A. | EI [LED]: ↓ |
| w: 6 × 7 days | HED: 1.3 + 2.0 | FI [LED]: ↔ | ||||
| Yeomans et al. [72] | UK | i: 6 × 1 meal | N: NR (36); f: 50.0%; age: 21.9 (3.2) | LED: 0.3 + N.A. | N.A. | EI [LED]: ↓ |
| w: 5 x | HED: 0.9 + N.A. | FI [LED]: N.R. | ||||
| Studies with preload design (children) | ||||||
| Kral et al. [39] | US | i: 3 × 1 meal | N: 94 (94); f: 53.2%; age: 8.9 (2.3) | LED: 0.6 + 0.6 | N.A. | EI [LED]: ↓ |
| w: 2 × 7 days | HED: 1.0 + 0.6 | FI [LED]: N.R. | ||||
| Kral et al. [40] | US | i: 2 × 1 meal | N: 212 (212); f: 54.7%; | LED: 1.0 + 2.1 | N.A. | EI [LED]: ↓ |
| w: 1 × 7 days | age: S1: 8.3 (0.7), S2/S3: 8.3 (0.8) | HED: 1.6 + 2.1 | FI [LED]: N.R. | |||
Consumed ED was calculated with energy intake (EI) and food intake (FI), from total meal (manipulated + unmanipulated food components); EI and FI after lower ED intervention compared to the higher ED diet: ↑: increase, ↓: decrease, ↔ : no difference, *: study with > 1 assignment
Abbreviations: A analyzed sample size, DK Denmark, E trial, ED energy density, f female, HED higher energy density condition, i intervention length, LED lower energy density condition, m male, N number of participants, N.A. not available/not calculable, NL Netherlands, N.R. not reported, S subgroup, SD standard deviation, SGP Singapore, UK United Kingdom, US United States of America, w washout period, wk week, y years
Fig. 2.
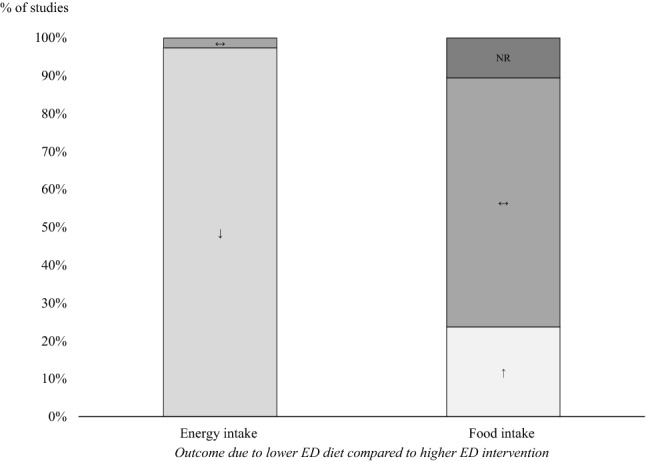
Changes in energy intake and food intake after lower energy density (ED) in comparison to higher ED diet across studies. Energy intake, food intake: ↑ intake is higher with lower ED than with higher ED intervention; ↓ intake is lower with lower ED than with higher ED intervention, ↔ no significant differences between lower ED and higher ED intervention; NR not reported
Among the 38 trials, most were conducted in America (n = 25; 65.8%), followed by Europe (n = 12; 31.6%) and Asia (n = 1; 2.6%). These were RCTs published between 1988 and 2020.
Total participants from the eligible trials for quantitative analysis of RCTs were 1831 participants; of which 874 were children or adolescents, whereas 957 were adults.
For the children studies (n = 11, [35–45]), the median age was 4.6 [4.3–8.3] years, covering the ages between 2 and 12 years. Girls represented 53% of the participants. The median BMI percentile was 59 [56.1–68.8], with a range of 42.5–94.5. The majority of the trials investigated the effects on children of normal weight and only two trials included children with overweight in their research. Two studies offered the children a manipulated preload and analyzed the subsequent ad libitum meal, whereas nine studies provided children with a manipulated entrée. All of the preload studies manipulated only one meal per day, in contrast to 33% (n = 3) of entrée studies lasting longer than one meal.
In the studies with focus on adults (n = 27, [46–72]), the median age was 25 [22.5–26.9] years, covering the ages between 21 and 84 years. Women represented 52% of the participants. The general median BMI for adults was 23.2 [22.4–24.0] kg/m2, with a range of 21–34.7 kg/m2. One study examined the effects of ED manipulation on adults with overweight, whereas the remaining studies focused on the effects on participants of normal weight. Five of the 27 studies offered a preload and analysed the subsequent ad libitum meal, whereas 78% of the studies (n = 21) served a manipulated entrée. One study applied both preload and entrée design [52].
As a result, a total of eight studies [39, 40, 48, 51, 52, 59, 60, 72] served a compulsory manipulated preload and measured the food intake of the following unmanipulated ad libitum test meal. A total of 31 studies modified an entrée and measured the ad libitum intake of this entrée. The length of all interventions ranged from 2 to 48 days with a median length of 6 [4–8] days. A washout period was performed between the dietary interventions in each of the 38 crossover studies. The washout periods lasted minimum 2 and maximum 28 days with a median length of 6 [6–7] days. When the effects of the ED manipulation were investigated for only a single and not multiple meals (n = 15), lunch was mostly used as the intervention meal (n = 14), followed by breakfast (n = 8) and dinner (n = 1). There were test foods with a median ED of 1.1 kcal/g [0.80–1.2], ranging from 0.1 to 2.0 kcal/g in the lower ED intervention and a median ED of 1.5 kcal/g [1.10–1.80] with a range of 0.5–4.6 kcal/g in the higher ED intervention. The ED consumed correlates linearly with the ED served (R2lowerED = 0.9181, R2higherED = 0.9494). In 22 studies, in addition to ED manipulation, portion size (n = 12), sensory quality (e.g. viscosity, taste, color or palatability, (n = 7)), other macronutrient compositions (n = 2) or information regarding a manipulation (n = 1) were varied. This resulted in 2 × 2 or 3 × 2 factorial crossover designs, with the ED manipulation supplemented by one or two of the aforementioned manipulations in each case. In most trials, energy intake was the primary endpoint, only 2 studies (5%) considered energy intake as secondary endpoint.
Summary of study outcomes
Overall, the heterogeneity of studies was high with respect to study design, sample size and research question.
Energy intake
Energy intake was compared between the lower ED and higher ED interventions at qualitative and quantitative levels for all 38 studies. The results of the qualitative analysis are presented as an overview in Table 1 and across studies in Fig. 2.
Thirty-seven studies (97%) indicated that energy intake was lower with lower ED than with higher ED intervention. Only one study [38] showed no change in energy intake in children after the lower ED intervention, indicating that the same amount of energy was consumed via both the lower ED and higher ED diets. There were also no differences between participants with normal-weight or overweight/obesity.
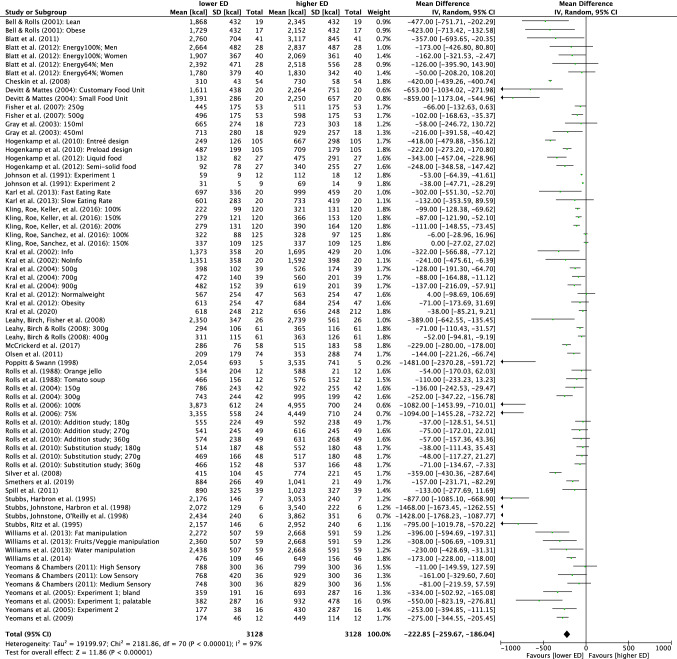
For quantitative analysis, the 38 multifactorial crossover studies were split according to their study conditions [32] resulting in 71 effects. The result of the quantitative analysis is presented as a forest plot in Fig. 3. Energy intake was reduced in the lower ED relative to higher ED conditions (mean energy intake difference – 223 kcal (95% CI: – 259.7, – 186.0); p < 0.001). However, the heterogeneity was high with I2 = 97% despite the applied random effect model.
Fig. 3.
Quantitative analysis of energy intake of all randomized controlled trials receiving either lower energy density (ED) or higher ED meals. The forest plot displays effect estimates and 95% confidence intervals (CI) for individual studies and the summary of findings. Additionally, for each study mean energy intake [kcal], standard deviation (SD) [kcal] and the number of total participants of both lower ED and higher ED conditions are presented. IV inverse-variance
To investigate the sources of heterogeneity, subgroup analyses were performed according to participants’ age (subgroup analysis 1), meal type (subgroup analysis 2) and intervention length per day (subgroup analysis 3).
Subgroup analysis 1: effects of participants’ age (children versus adults) on energy intake
This analysis aimed at reducing heterogeneity by dividing the studies according to the age of the participants (Figure S1). Although heterogeneity of adult studies remained high (I2 = 94%), was still reduced in the lower ED relative to higher ED interventions (mean energy intake difference – 302 kcal (95% CI: – 358.9, – 246.4); p < 0.001). In contrast, for trials with children heterogeneity was reduced (I2 = 80%) and accompanied by a drop in efficacy (mean energy intake difference – 65 kcal (95% CI: – 83.5, – 47.0); p < 0.001).
Subgroup analysis 2: effects of meal type (preload versus entrée design) on energy intake
In the analysis examining preload versus entrée studies (Figure S2), lower ED conditions were associated with a reduction in energy intake (mean energy intake difference – 111 kcal (95% CI: – 159.2, – 62.5); p < 0.001) and manipulated entrées (mean energy intake difference – 261 kcal (95% CI: – 304.6, – 217.9); p < 0.001), although treatment effects were significantly greater for manipulated entrées than manipulated preloads (p < 0.001). Heterogeneity decreased when analyzing preload studies (I2 = 66%), but remained high for entrée studies (I2 = 98%).
Subgroup analysis 3: effects of intervention length (1 meal versus > 1 meal) on energy intake
Lastly, this analysis distinguished according to length of intervention period (Figure S3). Effectiveness of the multiple meal interventions was superior to single meal interventions (p < 0.001), indicating a persistence effect of the ED manipulation. However, heterogeneity remained high for both single interventions (I2 = 97%) and multiple interventions (I2 = 92%).
Combination of subgroup analysis 1 + 2: effects of age and meal type on energy intake
Heterogeneity decreased when subgroups 1 and 2 were combined for analysis (Fig. 4). Here, heterogeneity decreased slightly when analyzing only children/entrée studies (I2 = 83%), but dropped strongly for children/preload interventions (I2 = 0%). Nevertheless, no significant subgroup effect for preloads was found (p = 0.07). A reduced heterogeneity was found when analyzing adult/preload studies (I2 = 42%) but adult/entrée studies remained high in their heterogeneity (I2 = 95%). Subgroup differences were significant (p < 0.001). Energy intake was lower in all subgroups in the lower ED relative to the higher ED intervention with mean energy intake differences of – 69, – 37, – 374 and – 139 kcal for children/entrée, children/preload, adults/entrée, and adults/preload studies, respectively. No further subgroup analysis could reduce heterogeneity, which is why no further analyses are mentioned.
Fig. 4.
Quantitative analysis of age (children versus adults) and meal type (preload versus entrée) on energy intake of randomized controlled crossover trials in humans receiving either lower energy density (ED) or higher ED diets. The forest plot displays effect estimates and 95% confidence intervals (CI) for individual studies and the summary of findings. Additionally, for each study mean energy intake [kcal], standard deviation (SD) [kcal] and the number of total participants of both lower ED and higher ED conditions are presented. IV inverse-variance
Overall, the subgroup analyses were able to explain the heterogeneity of studies in the full analyses. The data of the RCTs clearly demonstrated that the lower ED intervention reduced the energy intake compared to the higher ED intervention.
Food intake
To improve understanding of the findings regarding the outcome of energy intake in lower ED versus higher ED interventions, the amount of food intake in both conditions is presented in the following. The results regarding food intake for the single studies are presented at qualitative level for 38 studies as an overview in Table 1 and across studies in Fig. 2.
Twenty-five studies showed no significant difference in food intake between the two interventions (66%), nine studies reported an increase in food intake after a lower ED meal in comparison to a higher ED meal (24%) and the remaining studies (n = 4, 10%) did not report on food intake. Except for one study [28], all of the studies with higher food intake in the lower ED than in the higher ED diet were studies in which adult study participants received a manipulated entrée.
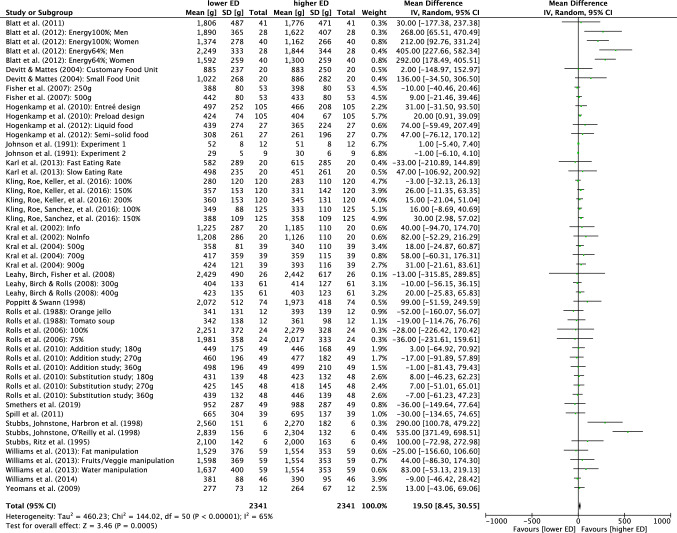
For quantitative analysis, 26 studies were included and the results are presented as a forest plot in Fig. 5. Independent of ED manipulation, the amount of food consumed was rather similar between the intervention groups, although in some cases food intake was slightly increased in the lower ED test meals (mean food intake difference 20 g (95% CI: 8.5, 30.6); p < 0.001), meaning marginally more food was eaten in lower ED interventions. The heterogeneity from the trials (I2 = 65%) required no further exploration.
Fig. 5.
Quantitative analysis of food intake of randomized controlled trials receiving either lower energy density (ED) or higher ED meals. The forest plot displays effect estimates and 95% confidence intervals (CI) for individual studies and the summary of findings. Additionally, for each study mean energy intake [kcal], standard deviation (SD) [kcal] and the number of total participants of both lower ED and higher ED conditions are presented. IV inverse-variance
Relationship between delta ED consumed and delta energy intake
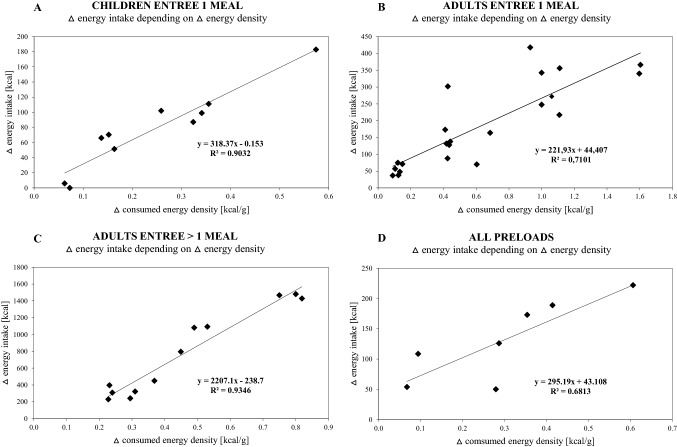
Substantial linear relationships between △ consumed ED (lower versus higher ED condition) and △ energy intake were found across different meal types and age (Fig. 6). The linear relationship in children with entrée design and 1 meal per day (A) was stronger (R2 = 0.90) than in adults (R2 = 0.71, B). In adults, the linear relationship became very strong when analyzing the entrée design including more than 1 intervention meal per day (R2 = 0.93; C). Studies with preload design (D) also showed a clear linear relationship (R2 = 0.68; separation between adults and children was not possible due to the small sample size).
Fig. 6.
Relationship between △ energy density (ED) and △ energy intake. Data of △ ED (lower versus higher ED condition of each single study; △ kcal/g) with the corresponding △ energy intake (lower versus higher ED condition of each single study, kcal) are displayed. A: Entrée studies in children, 1 meal interventions. B: Entrée studies in adults, 1 meal interventions. C: Entrée studies in adults, > 1 meal interventions. D: Preload studies in children and adults
Risk of bias
Figure 7 is a risk of bias summary showing the review authors judgements about each risk of bias item for each included study. The overall risk of bias was low in 37 studies and with some concerns in 1 study. None of the studies were identified with a high risk. All of the 38 trials were analyzed per protocol rather than intention-to-treat.
Fig. 7.
Risk of bias. D1 Randomization process, D2 Deviations from the intended interventions, D3 Missing outcome data, D4 Measurement of the outcome, D5 Selection of the reported results. + : Low risk of bias, ! : Some concerns in risk of bias, − : High risk of bias
Only in 4 out of 38 studies (11%) the ED condition was evident to the participant. All other studies tried to conceal the ED condition to the highest possible extent. However, it is unclear if this goal was achieved in the single studies. Excluding the 4 studies (n = 430, [49, 55, 62, 63]) with overt manipulation did not influence the findings and no sub-group differences were observed between overt and covert manipulation (data not shown). Hence, all studies were included in this meta-analysis.
Discussion
This systematic review and meta-analysis summarized the data of RCT studies investigating the influence of dietary ED on energy and food intake. Both hypotheses were qualitatively and quantitatively confirmed, showing that the lower ED reduced energy intake compared to the higher ED intervention, while food intake was unaffected. Meta-analysis data clearly indicated decreased energy intake with lower ED interventions than with higher ED interventions, as supported by another meta-analysis that included both RCTs and non-randomized controlled trials [19]. Moreover, a clear linear relationship was demonstrated between delta ED and delta energy intake, resulting in a lower energy intake with lower ED food. In contrast, food intake exhibits a non-linear relationship in regard to portion size [73] and rather a person’s consumption approaches an asymptote after exceeding a certain portion size [74, 75]. In comparison to the meta-analysis by Robinson et al. [19], our impacts were less strong but still substantial. Reasons for this may be due to different approaches taken, as the presented analysis only incorporated RCTs, where each participant always served as their own control and food intake had to be measured objectively by the investigators. Moreover, this review also comprised studies including ≥ 1 manipulated meal, resulting in other foci of analysis (meal type, 1 meal versus > 1 meal).
Subgroup analyzes were performed to produce the most homogeneous results possible, with highest homogeneity of the data found by combining the subgroups age (children versus adults) and meal type (preload versus entrée). The division of the subgroups ‘preload’ versus ‘entrée’ appeared essential due to their differing mechanism (inter-meal-satiety in preload versus intra-meal-satiation in ad libitum entrées) [76]. Preload studies with low ED foods such as salad [60], fruit [77] or soup [78] reported a reduction in energy intake in the following ad libitum meal. Nevertheless, the participants showed a non-significant trend of compensation for the reduction in energy intake [23]. In line, our data suggests that some kind of compensation must have taken place for the ED manipulation in preload studies, because the differences in energy intake between lower versus higher ED conditions were smaller in preload (– 118 kcal) than in ad libitum entrée studies (– 261 kcal). However, it should be noted that the differences between lower and higher ED conditions were generally smaller in preload than in ad libitum entrée studies. In regards to the entrée design, we observed a strong linear relationship between delta ED and delta energy intake in children and adults, indicating a lack of compensation in energy intake in this study design.
As hypothesized, food intake remained constant which removes the possibility of a compensation via the amount of food. As reported earlier [79, 80], satiety/satiation processes are not only dependent on the caloric content of the food but also on gastric capacity. Studies in which subjects received intragastric infused preloads bypassing sensory stimuli showed that the volume was more decisive for the feeling of satiety than the energy content [80, 81]. It has also been shown that the decreasing hedonic response to foods during consumption depends more on the volume of the food than on its energy content [82]. In addition, the duration of oral exposure is at least as important for the reduction of energy intake as the gastric filling volume [83, 84]. Similarly, it is possible that individuals use prior learning experiences to consume similar portion sizes under controlled conditions, regardless of ED [2]. In total, a complex interplay of cognitive, sensory, neural, gastrointestinal and hormonal influences [11] is involved and the above-described mechanisms should be seen in the context of the complex regulation of hunger and satiety. The topic has already been excellently reviewed elsewhere [76, 85].
Overall, the results of this conservative meta-analysis are substantial and, together with the findings of the linear relationship between ED and caloric intake, of high relevance for body weight management. In practice, this means that reducing the ED of food allows individuals to eat satiating quantities while at the same time consuming less energy. Additionally, ED lowering strategies are flexible and diverse and can be adapted to different dietary patterns, food preferences and cultural characteristics [2, 17, 18]. In line, two randomized controlled trials have shown that already primary school children can understand and are able to apply ED knowledge to their daily routine, even at six months follow-up [86, 87]. Taken together, these are strong arguments for increase integration of ED manipulation as dietary strategy in prevention and clinical practice.
Strengths and weaknesses of the systematic review and meta-analysis
Overall, this systematic review and meta-analysis has several limitations and strengths. A clear strength is the methodological approach taken according to PRISMA [26] and Cochrane criteria [32]. To provide homogeneity of the trials, the search was limited to RCTs and therefore, the probability of comparability between groups and the validity of the results was very high. In each study, participants served as their own control of the intervention regarding the ED manipulation, which further increased comparability. A limiting factor is the possibility for performance bias, as a common problem with nutritional studies in a controlled environment is that blinding of the participants and research personnel is impossible [88]. Nevertheless, most of the trials were covertly performed and a part of the meal was substituted with a similar lower ED alternative. Studies indicate that a covert incorporation of puréed vegetables into entrées significantly reduced energy intake in children [45] and adults [47]. Since only RCT studies were summarized and participants blinded to the best of the ability, it can be expected that the results are due to the nature of the ED manipulation. Despite clear eligibility criteria, the heterogeneity of the studies was high at the descriptive and meta-analytical levels, and therefore, subgroup analyses were performed, reducing heterogeneity to some degree. Finally, this meta-analysis had not yet been conducted in a comparable setting, meaning that it was unprecedented and provided new results, especially in the context of the impact of ED manipulation in different age groups and different manipulated meal types. By using exclusion criteria and introducing a moderator variable to code the studies according to their methodological quality, very strong methodological studies without a risk of bias were included. The subgrouping of consistent dependent variables enabled an interpretation of the resulting mean effect size. The clear tendency of all hypotheses was confirmed with a sufficient degree of certainty, which is why the importance of this systematic review and meta-analysis is very high and of interest for future preventive and therapeutic approaches.
Conclusion
In conclusion, energy intake in humans, irrespective of age, meal type and intervention length is determined by the ED of a meal. The magnitude of the effect is substantial and the relationship between consumed ED and energy intake linear. Thus, manipulating the ED of foods has the potential to be a powerful tool for body weight management in prevention and therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: IM, BK and JC; methodology: BK; software: BK; validation: BK and IM; formal analysis: BK and LC; writing—original draft preparation: BK and IM; writing—review and editing: IM, JC, and BK; visualization: BK; supervision: IM and SZ; all authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The current project is funded by the Innovation Committee of the German Joint Federal Committee (G-BA) with the funding number 01NVF18013 (STARKIDS).
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Ethical approval
Not applicable, but the protocol of this systematic review was registered at PROSPERO (registration number: CRD42021266653).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Raynor HA, Champagne CM. Position of the academy of nutrition and dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet. 2016;116:129–147. doi: 10.1016/j.jand.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Rolls BJ. Dietary energy density: applying behavioral science to weight management. Nutr Bull. 2017;42:246–253. doi: 10.1111/nbu.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie WS, Taylor R, Harris L, Lean MEJ. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis. Int J Obes. 2017;41:96–101. doi: 10.1038/ijo.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer K, Lau T, Schwille-Kiuntke J, et al. Conventional weight loss interventions across the different bmi obesity classes: a systematic review and quantitative comparative analysis. Eur Eat Disord Rev. 2020;28:492–512. doi: 10.1002/erv.2741. [DOI] [PubMed] [Google Scholar]
- 5.Willems AEM, Sura-de Jong M, van Beek AP, et al. Effects of macronutrient intake in obesity: a meta-analysis of low-carbohydrate and low-fat diets on markers of the metabolic syndrome. Nutr Rev. 2021;79:429–444. doi: 10.1093/nutrit/nuaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18:309–321. doi: 10.1038/s41574-022-00638-x. [DOI] [PubMed] [Google Scholar]
- 7.Rolls BJ, Hermann MG. The ultimate volumetrics diet: smart, simple, science-based strategies for losing weight and keeping it off. 1. New York: William Morrow; 2012. [Google Scholar]
- 8.Pérez-Escamilla R, Obbagy JE, Altman JM, et al. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Diet. 2012;112:671–684. doi: 10.1016/j.jand.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Rouhani MH, Haghighatdoost F, Surkan PJ, Azadbakht L. Associations between dietary energy density and obesity: a systematic review and meta-analysis of observational studies. Nutrition. 2016;32:1037–1047. doi: 10.1016/j.nut.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Kendall A, Levitsky DA, Strupp BJ, Lissner L. Weight loss on a low-fat diet: consequence of the imprecision of the control of food intake in humans. Am J Clin Nutr. 1991;53:1124–1129. doi: 10.1093/ajcn/53.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Kral TVE, Rolls BJ. Energy density and portion size: their independent and combined effects on energy intake. Physiol Behav. 2004;82:131–138. doi: 10.1016/j.physbeh.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (2021) Obesity and overweight. In: World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 30 Aug 2021
- 13.Karl JP, Roberts SB. Energy density, energy intake, and body weight regulation in adults. Adv Nutr. 2014;5:835–850. doi: 10.3945/an.114.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. Nutr Rev. 2009;50:283–290. doi: 10.1111/j.1753-4887.1992.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 15.Ello-Martin JA, Roe LS, Ledikwe JH, et al. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465–1477. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005;13:1052–1060. doi: 10.1038/oby.2005.123. [DOI] [PubMed] [Google Scholar]
- 17.Smethers AD, Rolls BJ. Dietary management of obesity. Med Clin North Am. 2018;102:107–124. doi: 10.1016/j.mcna.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schusdziarra V, Hausmann M, Wiedemann C, et al. Successful weight loss and maintenance in everyday clinical practice with an individually tailored change of eating habits on the basis of food energy density. Eur J Nutr. 2011;50:351–361. doi: 10.1007/s00394-010-0143-6. [DOI] [PubMed] [Google Scholar]
- 19.Robinson E, Khuttan M, McFarland-Lesser I, et al. Calorie reformulation: a systematic review and meta-analysis examining the effect of manipulating food energy density on daily energy intake. Int J Behav Nutr Phys Act. 2022;19:48. doi: 10.1186/s12966-022-01287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stelmach-Mardas M, Rodacki T, Dobrowolska-Iwanek J, et al. Link between food energy density and body weight changes in obese adults. Nutrients. 2016;8:229. doi: 10.3390/nu8040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin T, Mercer JG. Hunger and satiety mechanisms and their potential exploitation in the regulation of food intake. Curr Obes Rep. 2016;5:106–112. doi: 10.1007/s13679-015-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouhani MH, Surkan PJ, Azadbakht L. The effect of preload/meal energy density on energy intake in a subsequent meal: a systematic review and meta-analysis. Eat Behav. 2017;26:6–15. doi: 10.1016/j.eatbeh.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Rolls BJ, Laster LJ, Summerfelt A. Hunger and food intake following consumption of low-calorie foods. Appetite. 1989;13:115–127. doi: 10.1016/0195-6663(89)90109-8. [DOI] [PubMed] [Google Scholar]
- 24.Duncan KH, Bacon JA, Weinsier RL. The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am J Clin Nutr. 1983;37:763–767. doi: 10.1093/ajcn/37.5.763. [DOI] [PubMed] [Google Scholar]
- 25.Stubbs J, Ferres S, Horgan G. Energy density of foods: effects on energy intake. Crit Rev Food Sci Nutr. 2000;40:481–515. doi: 10.1080/10408690091189248. [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr. 1999;70:448–455. doi: 10.1093/ajcn/70.4.448. [DOI] [PubMed] [Google Scholar]
- 29.Marciani L, Hall N, Pritchard SE, et al. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr. 2012;142:1253–1258. doi: 10.3945/jn.112.159830. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.(2020) Review Manager (RevMan)
- 32.Higgins J, Thomas J (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). In: Cochrane. https://training.cochrane.org/handbook/current. Accessed 23 Aug 2021
- 33.Frost J (2019) Interpret the Pearson’ correlation coefficient. In: Regression analysis: an intuitive guide for using and interpreting linear models. p 7
- 34.Sterne J, Savović J, Page M, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 35.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children’s intake at a meal. Am J Clin Nutr. 2007;86:174–179. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson SL, McPhee L, Birch LL. Conditioned preferences: young children prefer flavors associated with high dietary fat. Physiol Behav. 1991;50:1245–1251. doi: 10.1016/0031-9384(91)90590-K. [DOI] [PubMed] [Google Scholar]
- 37.Kling SMR, Roe LS, Keller KL, Rolls BJ. Double trouble: portion size and energy density combine to increase preschool children’s lunch intake. Physiol Behav. 2016;162:18–26. doi: 10.1016/j.physbeh.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kling SMR, Roe LS, Sanchez CE, Rolls BJ. Does milk matter: Is children’s intake affected by the type or amount of milk served at a meal? Appetite. 2016;105:509–518. doi: 10.1016/j.appet.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kral TVE, Allison DB, Birch LL, et al. Caloric compensation and eating in the absence of hunger in 5- to 12-y-old weight-discordant siblings. Am J Clin Nutr. 2012;96:574–583. doi: 10.3945/ajcn.112.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kral TVE, Moore RH, Chittams J, et al. Caloric compensation and appetite control in children of different weight status and predisposition to obesity. Appetite. 2020;151:104701. doi: 10.1016/j.appet.2020.104701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leahy KE, Birch LL, Rolls BJ. Reducing the energy density of multiple meals decreases the energy intake of preschool-age children. Am J Clin Nutr. 2008;88:1459–1468. doi: 10.3945/ajcn.2008.26522. [DOI] [PubMed] [Google Scholar]
- 42.Leahy KE, Birch LL, Fisher JO, Rolls BJ. Reductions in entrée energy density increase children’s vegetable intake and reduce energy intake. Obesity. 2008;16:1559–1565. doi: 10.1038/oby.2008.257. [DOI] [PubMed] [Google Scholar]
- 43.Olsen A, van Belle C, Meyermann K, Keller KL. Manipulating fat content of familiar foods at test-meals does not affect intake and liking of these foods among children. Appetite. 2011;57:573–577. doi: 10.1016/j.appet.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smethers AD, Roe LS, Sanchez CE, et al. Both increases and decreases in energy density lead to sustained changes in preschool children’s energy intake over 5 days. Physiol Behav. 2019;204:210–218. doi: 10.1016/j.physbeh.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spill MK, Birch LL, Roe LS, Rolls BJ. Hiding vegetables to reduce energy density: an effective strategy to increase children’s vegetable intake and reduce energy intake. Am J Clin Nutr. 2011;94:735–741. doi: 10.3945/ajcn.111.015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73:1010–1018. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 47.Blatt AD, Roe LS, Rolls BJ. Hidden vegetables: an effective strategy to reduce energy intake and increase vegetable intake in adults. Am J Clin Nutr. 2011;93:756–763. doi: 10.3945/ajcn.110.009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blatt AD, Williams RA, Roe LS, Rolls BJ. Effects of energy content and energy density of pre-portioned entrées on energy intake. Obesity. 2012;20:2010–2018. doi: 10.1038/oby.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheskin LJ, Davis LM, Lipsky LM, et al. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite. 2008;51:50–57. doi: 10.1016/j.appet.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Devitt AA, Mattes RD. Effects of food unit size and energy density on intake in humans. Appetite. 2004;42:213–220. doi: 10.1016/j.appet.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Gray RW, French SJ, Robinson TM, Yeomans MR. Increasing preload volume with water reduces rated appetite but not food intake in healthy men even with minimum delay between preload and test meal. Nutr Neurosci. 2003;6:29–37. doi: 10.1080/1028415021000056032. [DOI] [PubMed] [Google Scholar]
- 52.Hogenkamp PS, Mars M, Stafleu A, de Graaf C. Intake during repeated exposure to low- and high-energy-dense yogurts by different means of consumption. Am J Clin Nutr. 2010;91:841–847. doi: 10.3945/ajcn.2009.28360. [DOI] [PubMed] [Google Scholar]
- 53.Hogenkamp PS, Stafleu A, Mars M, de Graaf C. Learning about the energy density of liquid and semi-solid foods. Int J Obes. 2012;36:1229–1235. doi: 10.1038/ijo.2011.231. [DOI] [PubMed] [Google Scholar]
- 54.Karl JP, Young AJ, Rood JC, Montain SJ. Independent and combined effects of eating rate and energy density on energy intake, appetite, and gut hormones: eating rate, energy density, and appetite. Obesity. 2013;21:E244–E252. doi: 10.1002/oby.20075. [DOI] [PubMed] [Google Scholar]
- 55.Kral TVE, Roe LS, Rolls BJ. Does nutrition information about the energy density of meals affect food intake in normal-weight women? Appetite. 2002;39:137–145. doi: 10.1006/appe.2002.0498. [DOI] [PubMed] [Google Scholar]
- 56.Kral TVE, Roe LS, Rolls BJ. Combined effects of energy density and portion size on energy intake in women. Am J Clin Nutr. 2004;79:962–968. doi: 10.1093/ajcn/79.6.962. [DOI] [PubMed] [Google Scholar]
- 57.McCrickerd K, Forde CG. Sensory influences on food intake control: moving beyond palatability. Obes Rev. 2016;17:18–29. doi: 10.1111/obr.12340. [DOI] [PubMed] [Google Scholar]
- 58.Poppitt S, Swann D. Dietary manipulation and energy compensation: does the intermittent use of low-fat items in the diet reduce total energy intake in free-feeding lean men? Int J Obes. 1998;22:1024–1031. doi: 10.1038/sj.ijo.0800726. [DOI] [PubMed] [Google Scholar]
- 59.Rolls BJ, Hetherington M, Burley VJ. Sensory stimulation and energy density in the development of satiety. Physiol Behav. 1988;44:727–733. doi: 10.1016/0031-9384(88)90053-4. [DOI] [PubMed] [Google Scholar]
- 60.Rolls BJ, Roe LS, Meengs JS. Salad and satiety: energy density and portion size of a first-course salad affect energy intake at lunch. J Am Diet Assoc. 2004;104:1570–1576. doi: 10.1016/j.jada.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Rolls BJ, Roe LS, Meengs JS. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. Am J Clin Nutr. 2006;83:11–17. doi: 10.1093/ajcn/83.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rolls BJ, Roe LS, Meengs JS. Portion size can be used strategically to increase vegetable consumption in adults. Am J Clin Nutr. 2010;91:913–922. doi: 10.3945/ajcn.2009.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silver HJ, Dietrich MS, Castellanos VH. Increased energy density of the home-delivered lunch meal improves 24-hour nutrient intakes in older adults. J Am Diet Assoc. 2008;108:2084–2089. doi: 10.1016/j.jada.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Stubbs R, Johnstone A, Harbron C, Reid C. Covert manipulation of energy density of high carbohydrate diets in ‘pseudo free-living’ humans. Int J Obes. 1998;22:885–892. doi: 10.1038/sj.ijo.0800676. [DOI] [PubMed] [Google Scholar]
- 65.Stubbs RJ, Harbron CG, Murgatroyd PR, Prentice AM. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr. 1995;62:316–329. doi: 10.1093/ajcn/62.2.316. [DOI] [PubMed] [Google Scholar]
- 66.Stubbs R, Johnstone A, O’Reilly L, et al. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in ‘pseudo free-living’ humans. Int J Obes. 1998;22:980–987. doi: 10.1038/sj.ijo.0800715. [DOI] [PubMed] [Google Scholar]
- 67.Stubbs RJ, Ritz P, Coward WA, Prentice AM. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr. 1995;62:330–337. doi: 10.1093/ajcn/62.2.330. [DOI] [PubMed] [Google Scholar]
- 68.Williams RA, Roe LS, Rolls BJ. Assessment of satiety depends on the energy density and portion size of the test meal: test meal affects satiety assessment. Obesity. 2014;22:318–324. doi: 10.1002/oby.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams RA, Roe LS, Rolls BJ. Comparison of three methods to reduce energy density. Effects on daily energy intake. Appetite. 2013;66:75–83. doi: 10.1016/j.appet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeomans M, Weinberg L, James S. Effects of palatability and learned satiety on energy density influences on breakfast intake in humans. Physiol Behav. 2005;86:487–499. doi: 10.1016/j.physbeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Yeomans MR, Gould NJ, Leitch M, Mobini S. Effects of energy density and portion size on development of acquired flavor liking and learned satiety. Appetite. 2009;52:469–478. doi: 10.1016/j.appet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Yeomans MR, Chambers L. Satiety-relevant sensory qualities enhance the satiating effects of mixed carbohydrate-protein preloads. Am J Clin Nutr. 2011;94:1410–1417. doi: 10.3945/ajcn.111.011650. [DOI] [PubMed] [Google Scholar]
- 73.Cahayadi J, Geng X, Mirosa M, Peng M. Expectancy versus experience – comparing portion-size-effect during pre-meal planning and actual intake. Appetite. 2019;135:108–114. doi: 10.1016/j.appet.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Roe LS, Kling SMR, Rolls BJ. What is eaten when all of the foods at a meal are served in large portions? Appetite. 2016;99:1–9. doi: 10.1016/j.appet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zlatevska N, Dubelaar C, Holden SS. Sizing up the effect of portion size on consumption: a meta-analytic review. J Mark. 2014;78:140–154. doi: 10.1509/jm.12.0303. [DOI] [Google Scholar]
- 76.Blundell J, de Graaf C, Hulshof T, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flood-Obbagy JE, Rolls BJ. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite. 2009;52:416–422. doi: 10.1016/j.appet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flood JE, Rolls BJ. Soup preloads in a variety of forms reduce meal energy intake. Appetite. 2007;49:626–634. doi: 10.1016/j.appet.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mack I, Sauer H, Weimer K, et al. Obese children and adolescents need increased gastric volumes in order to perceive satiety: perception of satiety in obese children. Obesity. 2014;22:2123–2125. doi: 10.1002/oby.20850. [DOI] [PubMed] [Google Scholar]
- 80.Rolls BJ, Roe LS. Effect of the volume of liquid food infused intragastrically on satiety in women. Physiol Behav. 2002;76:623–631. doi: 10.1016/S0031-9384(02)00801-6. [DOI] [PubMed] [Google Scholar]
- 81.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell EA, Roe LS, Rolls BJ. Sensory-specific satiety is affected more by volume than by energy content of a liquid food. Physiol Behav. 2003;78:593–600. doi: 10.1016/S0031-9384(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 83.Wijlens AGM, Erkner A, Alexander E, et al. Effects of oral and gastric stimulation on appetite and energy intake. Obesity. 2012;20:2226–2232. doi: 10.1038/oby.2012.131. [DOI] [PubMed] [Google Scholar]
- 84.Camps G, Mars M, de Graaf C, Smeets PA. Empty calories and phantom fullness: a randomized trial studying the relative effects of energy density and viscosity on gastric emptying determined by MRI and satiety. Am J Clin Nutr. 2016;104:73–80. doi: 10.3945/ajcn.115.129064. [DOI] [PubMed] [Google Scholar]
- 85.Halford JCG, Harrold JA. Satiety-enhancing products for appetite control: science and regulation of functional foods for weight management. Proc Nutr Soc. 2012;71:350–362. doi: 10.1017/S0029665112000134. [DOI] [PubMed] [Google Scholar]
- 86.Mack I, Reiband N, Etges C, et al. The kids obesity prevention program: cluster randomized controlled trial to evaluate a serious game for the prevention and treatment of childhood obesity. J Med Internet Res. 2020;22:e15725. doi: 10.2196/15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weiland A, Reiband N, Schäffeler N, et al. A serious game for the prevention of obesity in school children-impact of parent’s involvement: a randomized controlled trial. Life. 2022;12:779. doi: 10.3390/life12060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weaver CM, Miller JW. Challenges in conducting clinical nutrition research. Nutr Rev. 2017;75:491–499. doi: 10.1093/nutrit/nux026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.