Abstract
Introduction
Chronic pain of various origin is known to be associated with selective cognitive impairment. Osteoarthritis (OA) of the hip is one of the leading causes of chronic pain in the adult population, but its association with cognitive performance has not been evaluated. Here, we investigate the effect of chronic pain due to unilateral OA of one hip and no further source of chronic pain on cognitive performance.
Materials and methods
A neuropsychological test battery, consisting of the Mini-Mental State Examination, Rey–Osterrieth complex figure test, Rivermead behavioural memory test, d2 test of attention, and F-A-S test was applied in 148 patients and 82 healthy pain-free control individuals. The influence of potentially confounding factors such as depression and anxiety was examined.
Results
Patients with OA of the hip showed decreased performance in specific neuropsychological tests. Performance in verbal and visual short-term and long-term memory and selective attention tests was significantly poorer compared to healthy controls. Whereas the executive functions “updating”, “set shifting”, “response inhibition” and “reflection” appear intact, “problem solving” and “planning” were impaired. None of the confounders showed any influence on cognitive performance in both study groups.
Conclusion
We conclude that chronic pain secondary to end-stage hip OA is associated with selective cognitive impairment. Future studies are required to investigate the effect of total hip arthroplasty on cognitive performance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00402-022-04445-x.
Keywords: Chronic pain, Hip osteoarthritis, Hip replacement, Cognitive impairment
Introduction
Chronic pain represents a crucial factor of direct, indirect, and intangible costs to society, economy, and affected patients with a reported prevalence of 19% in the European population [1]. It is well known that chronic pain has a negative impact on cognitive performance. Jones [2] first described the impairment of episodic memory in patients with chronic pain in 1957. Since then, several studies have shown that chronic pain of various aetiologies, including musculoskeletal pain in conditions such as chronic back pain, rheumatoid arthritis, and others [3–10] can affect cognitive abilities, in particular working memory, attention, and executive functions. Many of these studies did not consider the influence of potential confounders such as depression and anxiety. Furthermore, the individual functional areas of cognition were investigated separately.
To the best of our knowledge, there are no studies examining all cognitive functional areas and considering potential confounders for a specific cause of chronic pain. Prakash et al. [11] showed that memory and pain activate the same central nervous pathways including the anterior cingulate cortex, prefrontal cortex, hypothalamus, island, hippocampus, and amygdala. This finding supports the relationship between the decrease of gray matter in different brain regions and chronic pain of various aetiologies [12–19].
Regarding OA of the hip, we have previously demonstrated that gray matter is reduced in patients with chronic pain in the anterior cingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, island, and operculum [13, 17]. Interestingly, despite these findings, the cognitive performance in patients with hip OA has not been examined in detail yet. One single study in the literature analyzed the physical limitation in hip OA and its interactions with body function, comorbidities, and cognition [8]. This study primarily focused on physical limitation and the results were not compared to standard values or to a control group (CG).
Although the prevalence of hip OA is > 7% within the age group of 60–90 years [20], and thus represents one of the leading causes of chronic pain [21], further studies focusing on cognition in patients with hip OA are lacking.
Since depression is associated with cognitive impairment [22], and the rate of depression is increased in patients with chronic pain, we hypothesize, that these two factors (depression and pain) may both be associated with cognitive impairment and may reinforce each other regarding the impact on cognition. We further hypothesize that also anxiety may add to cognitive impairment in patients with chronic hip OA pain, since there is an association of anxiety disorders and cognitive impairment, especially in the elderly [23].
The primary objective of the present study was to investigate the cognitive performance in chronic pain patients due to unilateral hip OA in comparison to a healthy CG. The secondary goal was to evaluate the effect of depression and anxiety on the relationship between pain and cognition.
Methods
Population and study design
This study was performed as a single institution prospective clinical trial. The inclusion criterion for the chronic pain group (CPG) was chronic pain caused by end-stage unilateral hip OA, scheduled for elective total hip replacement surgery. Based on patient´s symptoms, orthopedic examination, and radiograph images the diagnosis of end-stage OA was confirmed. The severity of the OA was described by the Kellgren–Lawrence grade. Exclusion criteria were chronic pain of further origin, bilateral hip osteoarthritis, dementia, substance-related addiction (including alcohol, psychotropic intoxicants, or medication), current psychiatric treatment, untreated visual or hearing impairment or lack of German language skills. The same criteria were applied to the healthy CG, with the only difference being chronic pain of any origin as an exclusion criterion. The CG consisted of healthy volunteers recruited by word-of-mouth, e-mail announcements, flyers placed in general practitioners' offices and through invitations to residents of senior citizen housing facilities. All study participants were informed about data protection regulations before the start of the study. Prior to study participation, written declaration of consent of all participants was signed and archived. The study conforms to the principles of the Declaration of Helsinki, was approved by the local research ethics committee (PV5016) and registered with ClinicalTrials.gov (NCT02997891).
Demographic and clinical data
Demographic and clinical data including age, gender, employment, comorbidities, pain medication and pain intensity in the last 7 days using the visual analog scale (VAS) were collected. Hip function was recorded by the Harris Hip Score (HHS) only in the CPG.
Neuropsychological tests
The Mini-Mental State Examination (MMSE) was performed to detect dementia and exclude patients and controls with a score of 24 and below. The distinct cognitive dimensions were investigated by the following neuropsychological tests:
Rey–Osterrieth complex figure test (ROCFT) [24]
The participant is requested to draw a complex figure with a total of 18 items, afterwards the figure must be drawn from memory and after another 30 min again. The ROCFT measures the visual construction and the visual memory performance.
Rivermead behavioral memory test (RBMT) [25]
The RBMT is a diagnostic test consisting of eleven tasks to identify memory problems. We used the sub-test “story” to examine verbal short- and long-term memory. A short story is read aloud, and the test person is asked to give a verbatim report of it (immediate recall). After 30 min, the participant is asked to repeat the story again (delayed recall). Each reproduced item was awarded with points for verbatim or analogous items reproduction. Conclusions about the executive functions “problem solving” and “planning” were drawn of the results.
d2 test of attention [26]
The participant must mark any letter "d" in 14 lines with two marks around above or below it in any order. The patient has 20 s per line to mark all items between wrong items. From the correct, incorrect, and omitted markings, scores can be formed, of which the “concentration performance” (CONC) is derived. CONC describes the number of correctly detected target items minus the number of errors of commission. The test is suitable for the examination of selective attention. Furthermore, the evaluation of “wrong marking errors” allows conclusions about the executive function "response inhibition".
Trail making test (TMT) [27]
The TMT part A comprises numbers from 1 to 25, which must be connected in ascending order as quickly as possible. In part B, letters are mixed in between, and the participant must alternately combine numbers and letters in sequence (1–A–2–B–3–C… 13–J). The time required for the two parts represents a measure of attention and executive function "updating" and "set shifting". Psychomotor speed and visual perception exert a significant influence. By subtracting A from B, the influence of psychomotor speed can be reduced [28].
Verbal fluency F-A-S test [29]
The task of the F-A-S test is to enumerate as many words as possible with the initial letter "s" in 60 s. Personal names and words with the same word stem are not validated. The total number of words was not only used for the short-term memory, but also for the evaluation of the executive function [30]. “Set shifting” and “updating” play a decisive role completing this task.
Confounding factors
The potentially confounding factors depression and anxiety were determined by means of questionnaires. The Patients Health Questionnaire (PHQ-9) [31] and Generalized Anxiety Disorder (GAD-7) [32] questionnaire include items about depression and anxiety, covering core DSM-IV criteria of depressions and generalized anxiety disorders. Both scores differentiate between four levels from mild to severe depression/anxiety disorder.
Statistical analysis
The primary outcome, differences in cognitive performance, was evaluated using independent t-tests for continuous data to determine whether significant differences exist between CPG and CG. To analyze whether these differences are also clinically relevant effect sizes according to Cohen (d-values between 0.2 and 0.5 represent a small effect without practical significance, d-values between 0.5 and 0.8 represent a medium effect with moderate practical significance, all values above 0.8 have a large effect with high practical importance) were calculated for each t-test [33]. The effect size illustrates the practical relevance of statistically significant results. To evaluate the secondary goal, confounding effect of depression and anxiety, mediator analyses with multiple regressions were conducted for each neuropsychological test. This method can be used to examine potentially significant indirect effects of depression and anxiety on the relationship between pain and cognition. This relationship is described by the Pearson correlation (r). Since age has a possible influence on neuropsychological test results partial correlations (rpartial) were calculated to exclude the influence of this variable if necessary. Additionally, descriptive statistics were calculated to present demographic data by means, standard deviations, and percentages. Any differences in sociodemographic parameters were analyzed by t-tests for continuous data and Chi-square tests for categorical data. Data analyses were performed with SPSS statistical software version 25 (IBM Corp., Armonk, NY, USA) and JASP (Version 0.14) [34]. P values ≤ 0.05 were considered statistically significant.
Results
The study enrolled 148 patients with unilateral OA of the hip into the CPG out of a total of 575 respondents who were screened for participation. In the CG, 83 participants out of a total 114 respondents were included. One participant of the CG had to be excluded from the study due to a MMSE result < 25. Demographic and clinical data, including outcomes of depression and anxiety scores, are listed in Table 1. There were no significant differences between the two groups in age, sex, occupation, and number of comorbidities. Pain assessment by the visual analog scale (VAS) showed a highly significant difference (p < 0.001) in pain between CPG (mean 6.1 ± 2.1) and CG (0.9 ± 1.3). No neurological comorbidities, such as brain trauma, stroke or epilepsy were reported. A synopsis of means and standard deviates of the neuropsychological test results are presented in supplementary Table 1.
Table 1.
Demographic and clinical characteristics of the study cohorts
| Chronic pain group | Control group | p value | |
|---|---|---|---|
| Age in years (mean, SD) | 68.0 (9.9) | 66.8 (7.8) | 0.306 |
| Sex (N, %) | 78 females (52.7%) | 44 females (53.7%) | 0.889 |
| Pain intensity (mean, SD) | |||
| 6.1 (2.1) | 0.9 (1.3) | < 0.001 | |
| Pain medication (N, %) | |||
| Opiates + non-opiates | 11 (7.8%) | 2 (2.9%) | |
| Non-opiates | 61 (43.6%) | 6 (8.7%) | < 0.001 |
| No pain medication | 68 (48.6%) | 61 (88.4%) | |
| (8 missings) | (13 missings) | ||
| Depression (N, %) | |||
| Minimal | 50 (38.5%) | 63 (81.8%) | |
| Mild | 62 (47.7%) | 12 (15.6%) | |
| Moderate | 13 (10.0%) | 1 (1.3%) | < 0.001 |
| Severe | 5 (3.8%) | 1 (1.3%) | |
| (18 missings) | (5 missings) | ||
| Anxiety (N, %) | |||
| Minimal | 91 (70.0%) | 66 (85.7%) | |
| Mild | 33 (25.4%) | 10 (13.0%) | |
| Moderate | 4 (3.1%) | 1 (1.3%) | 0.01 |
| Severe | 2 (1.5%) | 0 (0%) | |
| (18 missings) | (5 missings) | ||
| Occupation (N, %) | |||
| Full time | 25 (19.4%) | 20 (26.0%) | |
| Half time | 18 (14.0%) | 6 (7.8%) | 0.282 |
| Unemployed/pension | 86 (66.6%) | 51 (66.2%) | |
| (19 missing) | (5 missing) | ||
| Number of comorbidities (mean, SD) | |||
| 1.0 (± 0.9) | 1.0 (± 1.2) | 0.957 | |
| HHS (mean, SD) | 58.2 (± 13.9) | – | – |
| Kellgren–Lawrence score | |||
| 0 | 0 (0.0%) | – | – |
| 1 | 0 (0.0%) | ||
| 2 | 10 (6.8%) | ||
| 3 | 95 (64.2%) | ||
| 4 | 43 (29.1%) |
HHS Harris hip score, SD standard deviation
Memory
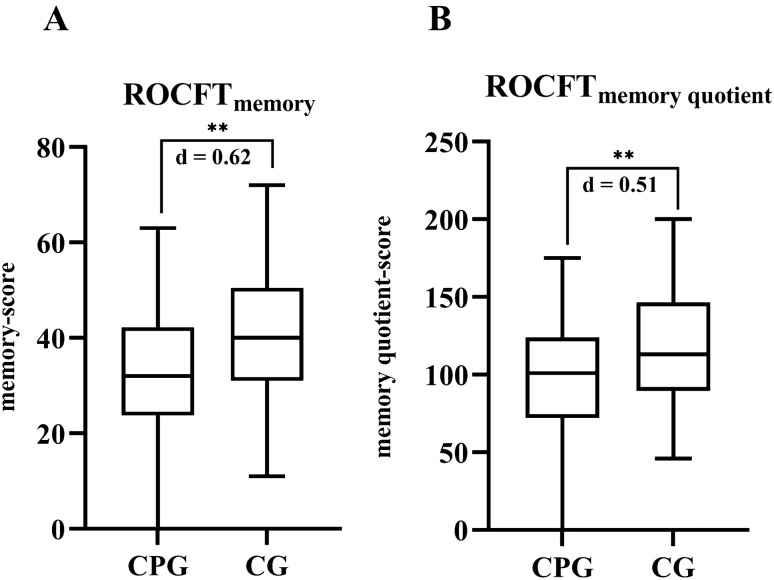
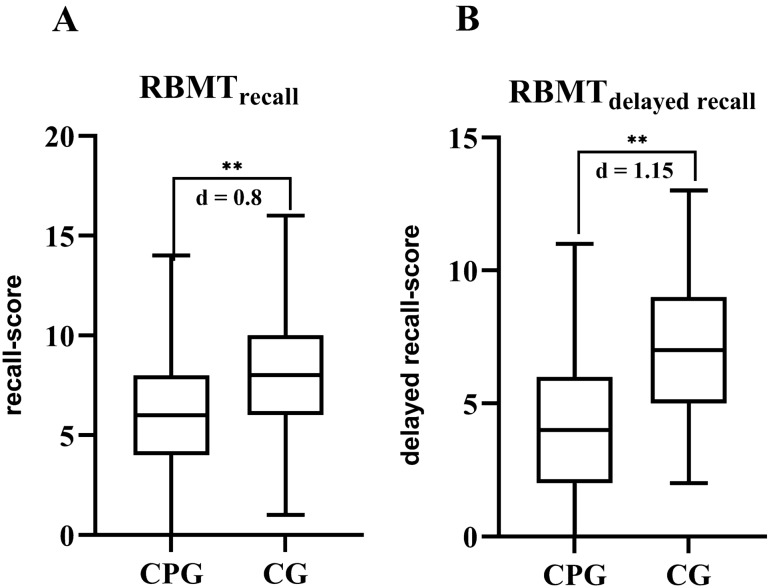
The impairment of the visual, spatial, and verbal short-term and long-term memory was tested by ROCFT and RBMT. In both tests, the results of the CPG were significantly lower with medium to large effect sizes (“ROCFTmemory” p < 0.001, d = 0.62; “ROCFTmemory quotient” p = 0.001, d = 0.51; “RBMTrecall” p < 0.001, d = 0.80; “RBMTdelayed recall” p < 0.001, d = 1.15; (Figs. 1 and 2).
Fig. 1.
The memory A and memory quotient B scores of the Rey–Osterrieth complex figure test (ROCFT) reveal a significant impairment of the memory in the chronic pain group (CPG) as compared to the control group (CG). **p < 0.001
Fig. 2.
The Rivermead behavioral memory test (RBMT) reveals a significant impairment of behavioral memory in the chronic pain group (CPG) as compared to the control group (CG) in both recall (A) and delayed recall (B) qualities. **p < 0.001
Attention
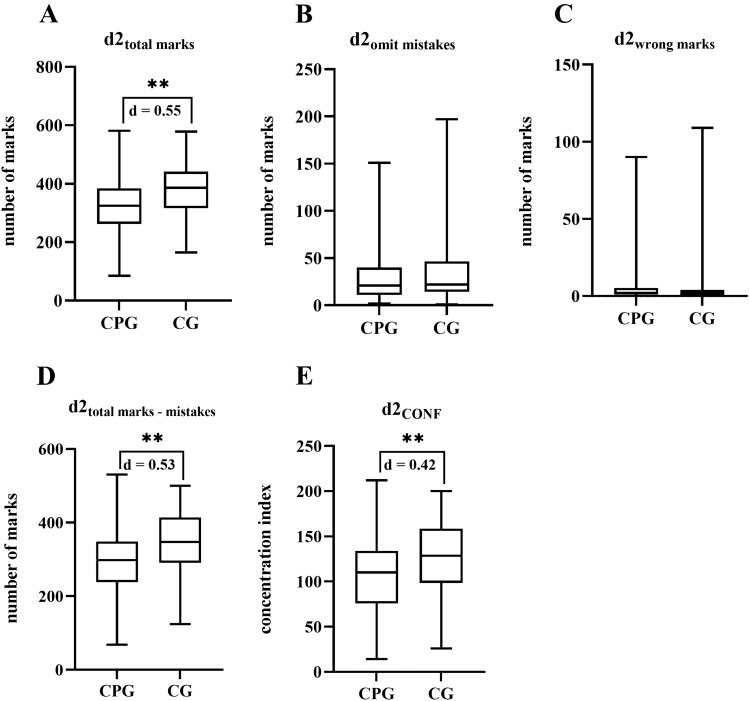
Selective attention, measured by d2 test, was significantly lower in the CPG with medium effect sizes (“total marks” p < 0.001, d = 0.55, “total marks – mistakes” p < 0.001, d = 0.53, “concentration performance” p = 0.004, d = 0.42). The investigation of split attention (TMTB) bordered on significance (p = 0.09; Figs. 3 and 4).
Fig. 3.
The d2 test reveals significant differences in the total marks (A), total marks minus mistakes (D) and concentration performance (E) indicating an impairment of selective attention and executive function in the chronic pain group (CPG) as compared to the control group (CG). Omit mistakes (B) and wrong marks (C) displayed no significant differences. **p < 0.001
Fig. 4.
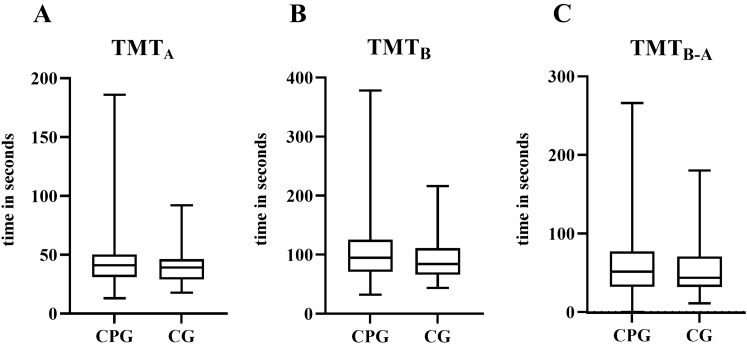
A, B, C The Trail Making Test (TMT) does not reveal differences in the executive functions set shifting and updating between the chronic pain group (CPG) and the control group (CG). These functions appear to be unaffected by hip OA
Executive function
Wrong markings from the d2 test were used as a measurement value for the executive function "response inhibition". There was no evidence for a significant deterioration of “response inhibition” in the CPG (p = 0.234). According to the results of TMTB and the.
F-A-S test (“TMTB” p = 0.09; “TMTB–A” p = 0.296; “F-A-S “ p = 0.129), “set shifting", "updating" and “complex executive functions” did not indicate significant differences between the groups (Figs. 4 and 5). On the contrary, considering the results of the ROCFT executive function such as “planning” and “problem solving” seem to be impaired.
Fig. 5.
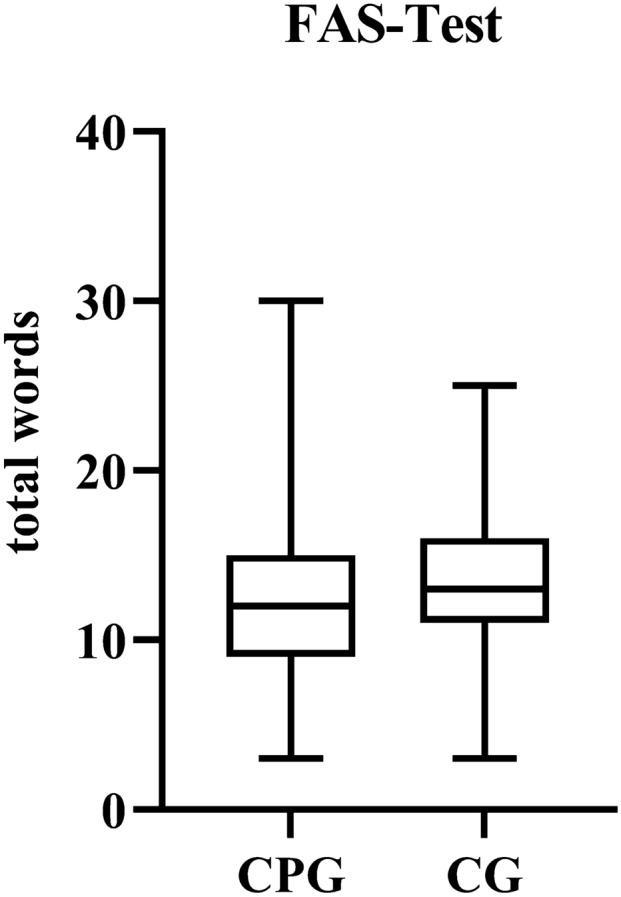
The verbal fluency test F-A-S does not reveal any differences in the executive functions set shifting and updating between the chronic pain group (CPG) and the control group (CG)
Influence of depression and anxiety on the relationship between pain and cognition
Significant small to moderate correlations (p < 0.001) between r = 0.225 and r = 0.446 were found between age and all but one neuropsychological test parameter. The sum of errors of the d2 test showed no significant correlation with age (p = 0.691). Taken the effect of age into account, partial correlations between VAS pain intensity and neuropsychological test parameters demonstrated significant correlations for selective attention (“d2total marks” rpartial = – 0.268, p < 0.001; “d2total marks – mistakes” rpartial = – 0.262, p < 0.001; “d2concentration performance” rpartial = – 0.194, p = 0.01), visual memory (“ROCFTmemory” rpartial = – 0.322, p < 0.001; “ ROCFTmemory quotient” rpartial = – 0.266, p < 0.001) and verbal memory (“RBMTrecall “ rpartial = – 0.268, p < 0.001; “RBMTdelayed recall “ rpartial = – 0.396, p < 0.001) but not for psychomotor speed (“TMTA” p = 0.066, “TMTB” p = 0.17, “TMTB–A” p = 0.497).
To evaluate the potential indirect effects of depression and anxiety as confounding variable on these significant relationships, a multivariate mediator analysis was performed. All analyses revealed no significant confounding influence of depression and anxiety (Table 2).
Table 2.
Multivariate mediator analysis for indirect effects of depression and anxiety on the relationship between pain and cognition
| Indirect effects of depression | Estimate | SD error | z-value | p value | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| VAS pain → PHQ → d2total marks | 0.275 | 1.426 | 0.193 | 0.847 | – 2.519 | 3.070 |
| VAS pain → PHQ → d2total marks-mistakes | 0.482 | 1.278 | 0.377 | 0.706 | – 2.024 | 2.988 |
| VAS pain → PHQ → d2concentration performance | 0.425 | 0.593 | 0.717 | 0.473 | – 0.737 | 1.588 |
| VAS pain → PHQ → ROCFTmemory | – 0.207 | 0.214 | – 0.966 | 0.334 | – 0.626 | 0.212 |
| VAS pain → PHQ → ROCFTmemory quotient | – 0.647 | 0.599 | – 1.081 | 0.280 | – 1.820 | 0.526 |
| VAS pain → PHQ → RBMTrecall | 0.010 | 0.044 | 0.223 | 0.823 | – 0.076 | 0.096 |
| VAS pain → PHQ → RBMTdelayed recall | 0.009 | 0.042 | 0.203 | 0.839 | – 0.074 | 0.092 |
| Indirect effects of anxiety | Estimate | SD error | z-value | p value | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| VAS pain → GAD → d2total marks | 0.527 | 0.687 | 0.768 | 0.443 | – 0.819 | 1.873 |
| VAS pain → GAD → d2total marks-mistakes | 0.597 | 0.619 | 0.964 | 0.335 | – 0.616 | 1.811 |
| VAS pain → GAD → d2concentration performance | 0.337 | 0.290 | 1.161 | 0.246 | – 0.232 | 0.906 |
| VAS pain → GAD → ROCFTmemory | 0.025 | 0.102 | 0.250 | 0.802 | – 0.174 | 0.225 |
| VAS pain → GAD → ROCFTmemory quotient | 0.003 | 0.286 | 0.011 | 0.991 | – 0.558 | 0.564 |
| VAS pain → GAD → RBMTrecall | – 0.003 | 0.021 | – 0.150 | 0.881 | – 0.045 | 0.038 |
| VAS pain → GAD → RBMTdelayed recall | 0.008 | 0.020 | 0.386 | 0.700 | – 0.032 | 0.047 |
SD error Delta method standard mistakes: Estimate = ML estimator, PHQ Patient Health Questionnaire, GAD generalized anxiety disorder scale
Discussion
To the best of our knowledge, this is the first study to investigate cognition in patients with chronic pain caused by OA of the hip. We analyzed various facets of cognitive performance (selective/shared attention, verbal/visual memory, and executive functions) and the impact of potential confounding factors (depression and anxiety) on cognitive performance in 148 patients with chronic pain due to end-stage unilateral hip OA. We compared the results to a CG of 82 free of chronic pain of any cause. The major finding is that chronic pain due to hip OA is associated with significantly worse test results with high effect sizes in neuropsychological tests measuring verbal/visual short-term, long-term memory and selective attention. Executive functions are only partially impaired, with lower scores for “problem solving” and “planning”, while “updating”, “set shifting”, “response inhibition”, and “reflection” appeared intact. In a follow up study of this patient collective, Strahl et al. [35] demonstrated the improvement of cognition, measured by the same neuropsychological test battery, six months after total hip arthroplasty. The potential confounders do not appear to have measurable impact on cognition in the hip OA population. Since the neuropsychological test battery used in this study is available in many languages, reproducing these tests and findings in an English-speaking population is possible.
Cognitive functions
Regarding memory and attention (measured by ROCFT and RBMT), the present findings do correspond to previously published results on cognitive impairment in patients with chronic pain of other etiology [3–10, 36–40].
Regarding executive function, the effect of chronic pain is more diverse. Executive functions are a set of cognitive processes that are necessary for cognitive control of behavior. Some executive functions are clearly impaired in hip OA such as “planning” and “problem solving” (measured by ROCFT), while others do not seem to be altered in patients with chronic hip OA pain. For example, “response inhibition” (measured by wrong markings in the d2 test), “reflecting” (measured by F-A-S), “set shifting” and “updating” (measured by TMTB and TMTB–A) did not show any difference.
Interestingly, some of these qualities, which are not affected by chronic hip OA, have been previously described to be altered in patients with other chronic pain conditions. Specifically, “set shifting” was shown to be impaired in patients with fibromyalgia [19, 41] and chronic pain conditions due to visceral, musculoskeletal, and neuropathic pain [42]. We believe that cognitive aging is the reason for this discrepancy [43]. The average age of patients in the present was 68.0 years while the average age of the previously published work ranged from 48.1 years to 53.6 years. Therefore, it is conceivable that a significantly younger study group with hip OA may also present impaired “set shifting” as compared to an CG of the same age.
Other qualities such as “planning” and “problem solving” have previously been shown to remain unaffected in patients with rheumatoid arthritis compared to a healthy CG [44].
Taken together, the present results and previously published work demonstrate that cognitive functions are subject to change in patients with chronic musculoskeletal pain in a complex, not a uniform, manner. The extent of cognitive impairment appears to depend on the cause of chronic musculoskeletal pain, pain intensity, pain progress and pain localization. Further delineating specific interactions of the various qualities of the CNS cognitive function with various chronic OA pain qualities from, e.g., knee, hip, and shoulder, may help counseling patients regarding the options and expected benefits of elective joint replacement surgery beyond pain control.
Potential confounders
In the present study, there was no significant influence of depression or anxiety on cognition (Table 2). This is consistent with published literature in that depression and anxiety secondary to chronic pain of different causes were not associated with impaired cognition [5, 42, 45, 46], while primary depression or anxiety disorders were related to cognitive impairment [22, 23, 41, 47–49]. Obviously, when depression or anxiety is secondary to pain, their effect on cognition is run over by a dominant effect of pain on cognition.
Pain severity
Increasing pain severity has been demonstrated to be associated with decreased cognitive performance in patients with chronic back pain, visceral, musculoskeletal, and neuropathic pain [46, 50, 51]. Our results also confirm these findings for pain due to hip OA. First, we found weak to moderate significant correlations between pain intensity and our neuropsychological parameter. Second, our CG with a low level of pain showed significantly higher neuropsychological test results as the CPG.
Limitation
Patients were generally tested a week or two before elective total hip arthroplasty. Awaiting surgery might have an influence on performance in neuropsychological tests. However, since anxiety had no influence on the test results, we consider it rather unlikely, that the prospect of surgery played a significant role.
Conclusions
Chronic pain due to end-stage hip OA is part of an emerging complex network of CNS interaction with the musculoskeletal system. It is associated with selective impairment of certain qualities of cognitive performance and this effect is independent of depression or anxiety. The current findings provide the rationale for future studies investigating to which extent total hip arthroplasty may improve cognition in elderly patients and, thus, provide benefits that may go well beyond pain control.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Maike Niemann, study nurse, Nils Kattwinkel and Wiebke Hauskeller, medical students, for their crucial role as contributors in data collection and documentation.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the “Stiftung Endoprothetik” [grant number S01/15].
Declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Ethical approval
The study conforms to the principles of the Declaration of Helsinki and was approved by the local research ethics committee (PV5016).
Informed consent
Prior to study participation, written declaration of consent of all participants was signed and archived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips CJ. The cost and burden of chronic pain. Rev Pain. 2009;3(1):2–5. doi: 10.1177/204946370900300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones E. Pain Int J Psychoanal. 1957;38:255–257. [PubMed] [Google Scholar]
- 3.Park DC, Glass JM, Minear M, Crofford LJ. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001;44(9):2125–2133. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Shin SY, Katz P, Wallhagen M, Julian L. Cognitive impairment in persons with rheumatoid arthritis. Arthritis Care Res. 2012;64(8):1144–1150. doi: 10.1002/acr.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira Kdos S, Oliver GZ, Thomaz DC, et al. Cognitive deficits in chronic pain patients, in a brief screening test, are independent of comorbidities and medication use. Arq Neuropsiquiatr. 2016;74(5):361–366. doi: 10.1590/0004-282X20160071. [DOI] [PubMed] [Google Scholar]
- 6.Karp JF, Reynolds CF, 3rd, Butters MA, et al. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006;7(5):444–452. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104(5):1223–1229. doi: 10.1213/01.ane.0000263280.49786.f5. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk GM, Veenhof C, Lankhorst GJ, Dekker J. Limitations in activities in patients with osteoarthritis of the hip or knee: the relationship with body functions, comorbidity and cognitive functioning. Disabil Rehabil. 2009;31(20):1685–1691. doi: 10.1080/09638280902736809. [DOI] [PubMed] [Google Scholar]
- 9.Oosterman JM, Derksen LC, van Wijck AJ, et al. Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain. 2011;27(1):70–75. doi: 10.1097/AJP.0b013e3181f15cf5. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Li L, Tang F, et al. Memory impairment in chronic pain patients and the related neuropsychological mechanisms: a review. Acta Neuropsychiatr. 2014;26(4):195–201. doi: 10.1017/neu.2013.47. [DOI] [PubMed] [Google Scholar]
- 11.Prakash S, Golwala P. Phantom headache: pain-memory-emotion hypothesis for chronic daily headache? J Headache Pain. 2011;12(3):281–286. doi: 10.1007/s10194-011-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz HC, McAuley JH, Wittfeld K, et al. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17(1):111–118. doi: 10.1016/j.jpain.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS ONE. 2013;8(2):e54475. doi: 10.1371/journal.pone.0054475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwilym SE, Filippini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 19.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131(12):3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 20.Quintana JM, Arostegui I, Escobar A, et al. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576–1584. doi: 10.1001/archinte.168.14.1576. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Hootman JM, Murphy L, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–1265. [PubMed] [Google Scholar]
- 22.Moritz S, Stöckert K, Hauschildt M, et al. Are we exaggerating neuropsychological impairment in depression? Reopening a closed chapter. Expert Rev Neurother. 2017;17(8):839–846. doi: 10.1080/14737175.2017.1347040. [DOI] [PubMed] [Google Scholar]
- 23.Mantella RC, Butters MA, Dew MA, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15(8):673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 24.Osterrieth PA. Le test de copie d’une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 25.Wilson B, Cockburn J, Baddeley AD. Rivermead Behavioral Memory Test. 1. Suffolk: Thames Valley; 1985. [Google Scholar]
- 26.Brickenkamp R, Zillmer E. D2 Test of Attention: Manual. 1. Oxford: Hogrefe and Huber; 1998. [Google Scholar]
- 27.Spreen S (1998) A compendium of neuropsychological tests. Administration, norms, and commentary. 2nd ed. New York: Oxford University Press; p. 447
- 28.Fals-Stewart W. An interrater reliability study of the Trail Making Test (parts A and B) Percept Mot Skills. 1992;74:39–42. doi: 10.2466/pms.1992.74.1.39. [DOI] [Google Scholar]
- 29.Muriel Deutsch Lezak . Neuropsychological Assessment. 3. New York: Oxford University Press; p; 1995. p. 545. [Google Scholar]
- 30.Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- 31.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81(1):61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioural sciences. 2. Erlbaum: Hillsdale; 1988. [Google Scholar]
- 34.JASP Team (2020). JASP (Version 0.14)[Computer software]
- 35.Strahl A, Kazim MA, Kattwinkel N, et al. Mid-term improvement of cognitive performance after total hip arthroplasty in patients with osteoarthritis of the hip: A prospective cohort study. Bone Joint J. 2022;104-B(3):331–340. doi: 10.1302/0301-620X.104B3.BJJ-2020-2021.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berryman C, Stanton TR, Jane Bowering K, et al. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain. 2013;154(8):1181–1196. doi: 10.1016/j.pain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Berryman C, Stanton TR, Bowering KJ, et al. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. doi: 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Moore DJ, Meints SM, Lazaridou A, et al. The effect of induced and chronic pain on attention. J Pain. 2019;20(11):1353–1361. doi: 10.1016/j.jpain.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Meade T, Manolios N, Cumming SR, et al. Cognitive impairment in rheumatoid arthritis: a systematic review. Arthrit Care Res. 2018;70(1):39–52. doi: 10.1002/acr.23243. [DOI] [PubMed] [Google Scholar]
- 40.Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):183–192. doi: 10.1016/j.pnpbp.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Suhr JA. Neuropsychological impairment in fibromyalgia: relation to depression, fatigue, and pain. J Psychosom Res. 2003;55:321–329. doi: 10.1016/S0022-3999(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 42.Oosterman J, Derksen LC, van Wijck A, et al. Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag. 2012;17(3):159–165. doi: 10.1155/2012/962786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson ND, Craik FI. 50 Years of Cognitive Aging Theory. J Gerontol B Psychol Sci Soc Sci. 2017;72(1):1–6. doi: 10.1093/geronb/gbw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roldán-Tapia L, Canovas-Lopez R, Cimadevilla J, et al. Cognition and perception deficits in fibromyalgia and rheumatoid arthritis. Rheumatol Clin. 2007;3:101–109. doi: 10.1016/S1699-258X(07)73676-8. [DOI] [PubMed] [Google Scholar]
- 45.Sjogren P, Thomsen AB, Olsen AK. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J Pain Symptom Manage. 2000;19(2):100–108. doi: 10.1016/S0885-3924(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 46.Weiner DK, Rudy TE, Morrow L, et al. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 47.Tempesta D, Mazza M, Serroni N, et al. Neuropsychological functioning in young subjects with generalized anxiety disorder with and without pharmacotherapy. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;45:236–241. doi: 10.1016/j.pnpbp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Roca M, Vives M, López-Navarro E, et al. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43(5):187–193. [PubMed] [Google Scholar]
- 49.Beekman ATF, Comijs HC, Deeg DJH, et al. A 13-year prospective cohort study on the effects of aging and frailty on the depression-pain relationship in older adults. Int J Geriatr Psychiatry. 2014;30(7):751–757. doi: 10.1002/gps.4224. [DOI] [PubMed] [Google Scholar]
- 50.Eccleston C. Chronic pain and distraction: An experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995;33(4):391–405. doi: 10.1016/0005-7967(94)00057-Q. [DOI] [PubMed] [Google Scholar]
- 51.Oosterman JM, Derksen LC, van Wijck AJ, et al. Memory functions in chronic pain: Examining contributions of attention and age to test performance. Clin J Pain. 2011;27:70–75. doi: 10.1097/AJP.0b013e3181f15cf5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.