Abstract
Background
Cardiac function of critically ill patients with COVID-19 generally has been reported from clinically obtained data. Echocardiographic deformation imaging can identify ventricular dysfunction missed by traditional echocardiographic assessment.
Research Question
What is the prevalence of ventricular dysfunction and what are its implications for the natural history of critical COVID-19?
Study Design and Methods
This is a multicenter prospective cohort of critically ill patients with COVID-19. We performed serial echocardiography and lower extremity vascular ultrasound on hospitalization days 1, 3, and 8. We defined left ventricular (LV) dysfunction as the absolute value of longitudinal strain of < 17% or left ventricle ejection fraction (LVEF) of < 50%. Primary clinical outcome was inpatient survival.
Results
We enrolled 110 patients. Thirty-nine (35.5%) died before hospital discharge. LV dysfunction was present at admission in 38 patients (34.5%) and in 21 patients (36.2%) on day 8 (P = .59). Median baseline LVEF was 62% (interquartile range [IQR], 52%-69%), whereas median absolute value of baseline LV strain was 16% (IQR, 14%-19%). Survivors and nonsurvivors did not differ statistically significantly with respect to day 1 LV strain (17.9% vs 14.4%; P = .12) or day 1 LVEF (60.5% vs 65%; P = .06). Nonsurvivors showed worse day 1 right ventricle (RV) strain than survivors (16.3% vs 21.2%; P = .04).
Interpretation
Among patients with critical COVID-19, LV and RV dysfunction is common, frequently identified only through deformation imaging, and early (day 1) RV dysfunction may be associated with clinical outcome.
Key Words: COVID-19, echocardiography, point-of-care ultrasound, strain, ultrasound
Take-home Points.
StudyQuestion: How does ventricular function change over time during the first week of critically ill COVID-19, and is it associated with clinical outcomes?
Results: Deformation imaging (ventricular strain) was abnormal during the first week of illness, while traditional assessments like ejection fraction were normal. One-third of patients with left ventricular dysfunction improved over the first week. Nonsurvivors had worse right ventricular strain.
Interpretation: Ventricular dysfunction is common in critically ill patients with COVID-19, and is often only identified using deformation imaging. Ventricular function often changes during the first week of COVID-19 illness.
The ongoing COVID-19 pandemic is the most lethal in recent history, with > 250 million people infected and > 5 million deaths.1 Cardiac complications reportedly are common among patients with COVID-19. The first case series of critically ill patients with COVID-19 in the United States documented probable cardiomyopathy in one-third of patients, consistent with early reports from China.2 , 3 Whether this cardiac dysfunction is the result of cytokine storm, viral myocarditis, or acute cor pulmonale resulting from elevated right ventricle (RV) afterload is unclear. Complicating interpretation of these data are reports in patients with COVID-19 of a high frequency of VTE, which also may affect RV afterload.4
A number of unanswered questions remain. Among critically ill patients with COVID-19, what are the frequency and implication of myocardial dysfunction identified with the more sensitive and specific method of deformation imaging?5 , 6 What is the time course of echocardiographic abnormalities? What is the frequency of DVT and the possible clinical role of screening for it? To address these questions, we performed a prospective, multicenter study of critically ill patients with COVID-19 in which serial echocardiographic and vascular ultrasound imaging were performed during the first week of ICU admission.
Study Design and Methods
Study Design
This was a multicenter, prospective, observational cohort study conducted within the Influenza and Other Viruses in the Acutely Ill Network. We enrolled patients at eight acute care medical centers in eight cities on hospitalization day 1 between September 2020 and January 2021. The protocol was approved by the Institutional Review Board (Identifier: 200973) and the Institutional Review Board (Identifier: 21-073). All participants (or their surrogates) provided informed consent.
Patients
We used a convenience sample of adult patients (aged ≥ 18 years) admitted with a confirmed diagnosis of COVID-19 who were critically ill, defined as receiving any of the following therapies: high-flow nasal oxygen, noninvasive ventilation for indication other than sleep apnea, invasive mechanical ventilation, continuous renal replacement therapy, or IV vasoactive medications (either vasopressor or inotrope). We excluded patients who were expected to die within 24 h or who had a contraindication to clinician-performed point-of-care ultrasound evaluation.
Transthoracic Echocardiography and Point-of-care Ultrasound
Patients underwent limited transthoracic echocardiography on study days 1, 3, and 8 while they remained hospitalized. This imaging protocol has been applied successfully in critically ill patients in prior studies (e-Appendix 1).7 Where capability existed, complete transthoracic echocardiography was performed. Imaging was performed by a cardiologist, cardiac sonographer, or intensivist or emergency physician trained in critical care echocardiography and point-of-care ultrasound. Lower extremity vascular ultrasound consisted of two-point compression ultrasonography, that is, imaging bilateral femoral and popliteal veins. All imaging protocols were in adherence to guidelines on imaging patients with COVID-19.8 If the patient was in prone position, assessment was performed when the patient was supine, if possible. Study images were anonymized before analysis.
Ultrasound Interpretation
All imaging data were interpreted by clinicians masked to clinical status. Cardiac images were interpreted offline by an advanced cardiac sonographer (T. D. O.) with physician overread (M. J. L.) or by direct physician read (S. P. D.). Vascular ultrasound images were interpreted directly by physicians (M. J. L. and S. P. D.). Both physician interpreters were board certified in critical care echocardiography and ultrasonography. Deformation imaging using speckle tracking to measure ventricular longitudinal strain (which assesses the extent to which adjacent portions of the myocardium move closer to each other during systole) was performed with Image Arena (TomTec). In cases where limited image quality prevented measurement of global ventricular longitudinal strain of all 16 segments of the heart, we substituted longitudinal strain from the apical four-chamber only, consistent with prior studies.9 , 10 We did not use strain assessments in echocardiograms demonstrating arrhythmia-inadequate image quality. RV free-wall strain was calculated from the RV-focused view, if available, and from standard apical four-chamber view if not. We defined left ventricle (LV) systolic dysfunction as either left ventricular ejection fraction (LVEF) of < 50% or absolute value of LV longitudinal strain of < 17%.11 We defined RV systolic dysfunction as RV fractional area change (FAC) of < 35%, tricuspid annulus systolic planar excursion (TAPSE) of < 1.7 cm, or absolute value of RV free-wall strain of < 22%. We selected RV FAC and TAPSE based on ease of measurement and ubiquity in clinical practice and chose thresholds based on published guidelines or reference studies.12, 13, 14 Vascular ultrasound was scored as thrombus absent or present depending on whether a thrombus was visualized.
Additional Clinical Data
We collected demographic data and presence of medical comorbidities. At the time of each ultrasound assessment, we recorded vital signs; ventilator settings; receipt of fluid and vasoactive medications; and adjunctive therapies for ARDS, such as prone positioning, neuromuscular blockade, or extracorporeal life support. We converted vasopressor infusion rates into norepinephrine-equivalent dosing, per previously described methods.15 We recorded clinically obtained arterial blood gas and serum lactate levels. We calculated Sequential Organ Failure Assessment scores.16 Patients were assessed daily for clinical evidence of atrial fibrillation, atrioventricular conduction block, or other arrhythmia. Patients underwent research laboratory assessments of troponin-I and B-type natriuretic peptide (BNP) on days 1, 3, and 8. We recorded in-hospital death and in-hospital clinical diagnosis of VTE.
Statistical Analysis
We compared clinical characteristics of survivors and nonsurvivors using χ2 tests for categorical variables and Wilcoxon tests for continuous variables. We compared patients with and without LV dysfunction on day 1 using a similar approach. Day 1 measurements were illustrated graphically using violin plots and scatterplots. We characterized echocardiographic changes over time using descriptive statistics and longitudinal spaghetti plots. We calculated the proportion of patients with thrombosis identified by research ultrasound examinations. The relationship between LV strain and LVEF was shown graphically using a scatterplot. We did not stratify analyses by treatments, such as fluid or vasoactive therapy, because of heterogeneity in treatments received and a moderate sample size that limited statistical power for subgroup comparisons. For longitudinal comparisons, we accounted for within-patient correlation by using the Friedman test (nonparametric repeated measure analysis of variance) and Wald χ2 test (generalized linear mixed-effects model) depending on the nature of the variable.
In an exploratory analysis, we predicted LV strain from LVEF by performing a linear regression. Residuals were compared using the Wilcoxon rank-sum test, stratifying by in-hospital mortality. In this analysis, a higher residual indicated a greater-than-anticipated difference between a patient’s actual strain and what the model predicted from ejection fraction data. For ease of graphic representation and reporting, LV strain and RV free-wall strain were converted to absolute values; troponin-I and BNP values were log transformed for graphic representation. P values of < .05 were considered statistically significant; no correction for multiple comparisons was used. The statistical analysis was conducted using R version 4.0.4 software (R Foundation for Statistical Computing).
Results
We enrolled 110 critically ill patients with COVID-19. Patient characteristics are presented in Table 1 .15 Median age was 63 years (interquartile range [IQR], 52-71 years), and 64% were men. Patients typically were overweight or obese (median BMI, 30 kg/m2; IQR, 26-33 kg/m2). Participants had a median day 1 Sequential Organ Failure Assessment score of 5 (IQR, 4-7) and initial Pao 2 to Fio 2 ratio of 104 (IQR, 71-158; normal, ≥ 400). Arrhythmias, including atrial fibrillation (18%), atrioventricular nodal block (9%), and other (21%) during the first 8 days of hospitalization were common. Thirty-nine patients (35%) died before hospital discharge. Nonsurvivors showed higher peak troponin levels than survivors (0.10 ng/mL vs 0.02 ng/mL; P = .002) and were more likely to receive invasive mechanical ventilation (64.1% vs 38.0%; P = .009) and vasopressors (56.4% vs 23.9%; P < .001) during the study period.
Table 1.
Characteristics of Critically Ill Study Patients With COVID-19 Stratified by Whether They Died in Hospital or Survived to Dischargea
| Variable | Total (N = 110) | Died (n = 39) | Alive (n = 71) | P Value |
|---|---|---|---|---|
| Age, y | 63 (52 to 71) | 69 (64 to 75) | 58 (43 to 67) | < .001 |
| Female sex | 40 (36.4) | 12 (30.8) | 28 (39.4) | .366 |
| Race | .093 | |||
| White | 52 (47.3) | 17 (43.6) | 35 (49.3) | . . . |
| Black | 18 (16.4) | 10 (25.6) | 8 (11.3) | . . . |
| Other | 18 (16.4) | 3 (7.7) | 15 (21.1) | . . . |
| Not documented | 22 (20.0) | 9 (23.1) | 13 (18.3) | . . . |
| Ethnicity | ||||
| Hispanic | 34 (30.9) | 12 (30.8) | 22 (31.0) | . . . |
| Not Hispanic | 74 (67.3) | 27 (69.2) | 47 (66.2) | . . . |
| Unknown | 2 (1.8) | 0 (0.0) | 2 (2.8) | . . . |
| BMI, kg/m2 | 30.0 (26.1 to 33.1) | 30.3 (26.1 to 32.8) | 29.9 (26.5 to 34.2) | .886 |
| Baseline comorbidities | ||||
| Cardiovascular disease, % | 66 (60.0) | 27 (69.2) | 39 (54.9) | .143 |
| Neurologic disease, % | 13 (11.8) | 7 (18.0) | 6 (8.5) | .140 |
| Pulmonary disease, % | 19 (17.3) | 6 (15.4) | 13 (18.3) | .698 |
| GI disease, % | 7 (6.4) | 2 (5.1) | 5 (7.0) | .694 |
| Endocrine disease, % | 57 (51.8) | 26 (66.7) | 31 (43.7) | .021 |
| Renal disease, % | 18 (16.4) | 8 (20.5) | 10 (14.1) | .383 |
| Hematologic disease, % | 4 (3.6) | 2 (5.1) | 2 (2.8) | .536 |
| Malignancy, % | 2 (1.8) | 2 (5.1) | 0 (0.0) | .054 |
| Other immunosuppression, % | 9.1 | 15.4 | 5.6 | .089 |
| Day 1 SOFA score | 5 (4 to 7) | 6 (4 to 9) | 4 (3 to 7) | .015 |
| Day 1 Pao2 to Fio2 ratio | 104 (71 to 158) | 79 (65 to 127) | 119 (81 to 167) | .020 |
| Day 1 highest PEEP, cm H2O | 5 (0 to 10) | 5 (0 to 10) | 0 (0 to 10) | .348 |
| Day 1 ventilation type | .198 | |||
| Invasive mechanical ventilation | 37 (33.6) | 15 (38.5) | 22 (31.0) | . . . |
| Noninvasive positive pressure ventilation | 10 (9.1) | 6 (15.4) | 4 (5.6) | . . . |
| High-flow nasal cannula | 58 (52.7) | 16 (41.0) | 42 (59.2) | . . . |
| Nasal cannula | 5 (4.6) | 2 (5.1) | 3 (4.2) | . . . |
| Room air | 0 (0) | 0 (0) | 0 (0) | . . . |
| Day 1 troponin I level, ng/mL | 0.02 (0.00 to 0.09) | 0.04 (0.01 to 0.14) | 0.01 (0.00 to 0.06) | .030 |
| Day 1 BNP level, pg/mL | 65 (0 to 150) | 71 (2 to 346) | 58 (0 to 117) | .284 |
| Day 1 receipt of vasopressors | 22 (20.0) | 10 (25.6) | 12 (16.9) | .273 |
| Vasopressor dosage (of those receiving vasopressors), μg/kg/min NEE15 | 0.08 (0.03 to 0.28) | 0.13 (0.03 to 0.41) | 0.08 (0.03 to 0.16) | .898 |
| Day 1 echocardiography and ultrasound measurements | . . . | |||
| LV systolic dysfunction, %b | 42.2 (38 of 90) | 50.0 (16 of 32) | 37.9 (22 of 58) | .267 |
| LVEF | 62 (52 to 69) | 65 (59 to 71) | 61 (51 to 66) | .060 |
| LV longitudinal strain, % | 16.0 (13.7 to 19.1) | 14.4 (12.8 to 17.9) | 17.9 (14.4 to 19.6) | .118 |
| RV dysfunction, %b | 64.2 (52 of 81) | 75.9 (22 of 29) | 57.7 (30 of 52) | .102 |
| RV fractional area change, % | 38.8 (32.8 to 47.5) | 37.5 (22.9 to 42.8) | 40.8 (34.4 to 49.1) | .077 |
| RV free-wall strain, % | 19.2 (12.3 to 24.9) | 16.3 (12.0 to 20.4) | 21.2 (15.0 to 25.6) | .042 |
| Tricuspid annulus systolic plane excursion, cm | 2.03 (1.69 to 2.33) | 1.85 (1.27 to 2.10) | 2.14 (1.90 to 2.45) | .110 |
| RV to LV end-diastolic diameter ratio | 0.90 (0.78 to 0.97) | 0.92 (0.85 to 1.01) | 0.89 (0.74 to 0.96) | .155 |
| IVC collapsibility index | 0.21 (0.12 to 0.51) | 0.21 (0.13 to 0.53) | 0.22 (0.11 to 0.48) | .812 |
| Presence of DVT on study ultrasound | 3 (2.7) | 2 (5.1) | 1 (1.4) | .252 |
| Hospital course | . . . | |||
| Atrial fibrillation, % | 20 (18.2) | 8 (20.5) | 12 (16.9) | .639 |
| AV-nodal blockade, % | 10 (9.1) | 5 (12.8) | 5 (7.0) | .313 |
| Other arrhythmia, % | 23 (20.9) | 10 (25.6) | 13 (18.3) | .366 |
| Highest BNP, pg/mL | 73 (1 to 201) | 92 (6 to 430) | 70 (0 to 131) | .189 |
| Highest troponin I, ng/mL | 0.03 (0.01 to 0.26) | 0.10 (0.02 to 0.70) | 0.02 (0.00 to 0.09) | .002 |
| Clinical diagnosis of DVT or PE, % | 14 (12.7) | 5 (12.8) | 9 (12.7) | .983 |
| Fluid balance over first 7 d, L | –6.4 (–10.5 to –2.0) | –7.5 (–10.5 to –2.6) | –5.9 (–10.2 to –2.0) | .749 |
| Received IMV during study, % | 53 (48.2) | 25 (64.1) | 28 (39.4) | .013 |
| Received vasopressors during study, % | 39 (35.5) | 22 (56.4) | 17 (23.9) | < .001 |
| Hospital length of stay, d | 14 (7-22) | 15 (9-21) | 13 (7-25) | .641 |
Data are presented as No. (%) or median (interquartile range), unless otherwise indicated. AV = atrioventricular; BNP = B-type natriuretic peptide; IMV = invasive mechanical ventilation; IVC = inferior vena cava; LV = left ventricle; LVEF = left ventricular ejection fraction; NEE = norepinephrine equivalent; PE = pulmonary embolism; PEEP = positive end-expiratory pressure; RV = right ventricle; SOFA = Sequential Organ Failure Assessment.
Tests of significance are unadjusted for multiple comparisons.
For left ventricular and right ventricular systolic dysfunction, when complete imaging was not available, the proportion followed by numerator and denominator of completed measurements are reported.
Frequency and Implications of Systolic Dysfunction
LV dysfunction was present at admission in 38 of 110 patients (34.5%). Nineteen of those patients (50.0%) with LV dysfunction received a diagnosis only via deformation imaging. Median LVEF was 62% (IQR, 52.4%-69.2%) at baseline, whereas median absolute value of LV strain was 16.0% (IQR, 13.7%-19.1%) at baseline. Day 1 LV function could not be determined in 20 patients (18.2%) because of limited image quality. In 22 patients, day 1 LV strain was measured using only the apical four-chamber view. Day 1 RV free-wall strain was measured in 56 patients. We noted no significant difference between survivors and nonsurvivors with respect to day 1 LV strain (17.9% [IQR, 14.4%-19.6%] vs 14.4% (IQR, 12.8%-17.9%]; P = .12) or day 1 LVEF (60.5% [IQR, 50.5%-66.5%] vs 65.0% [IQR, 58.9%-71.2%]; P = .06). Nonsurvivors showed worse day 1 absolute value of RV strain than survivors (16.3% [IQR, 12.02%-20.4%] vs 21.2% [IQR, 15.0%-25.6%]; P = .04) (Table 1).
Patients with LV dysfunction on day 1 were significantly more likely to have pre-existing pulmonary disease (31.6% vs 9.6%; P = .009), and rates of pre-existing cardiovascular disease were similar (63.2% vs 55.8%; P = .48). We observed no other differences between patients with and without LV dysfunction in frequency of arrhythmia, peak BNP or troponin values, or in-hospital mortality (Table 2 ). Additional comparisons between normal and abnormal LV function are displayed in Table 2.
Table 2.
Characteristics of Critically Ill Study Patients With COVID-19 in Whom Function Could Be Determined (n = 90), Stratified by Day 1 LV Functiona
| Variable | LV Normal Function (n = 52) | LV Dysfunction (n = 38) | P Value |
|---|---|---|---|
| Age, y | 60 (48-69) | 64 (57-71) | .257 |
| Female sex | 21 (40.4) | 11 (29.0) | .263 |
| Race | .833 | ||
| White | 24 (46.2) | 21 (55.3) | |
| Black | 8 (15.4) | 5 (13.2) | |
| Other | 8 (15.4) | 4 (10.5) | |
| Not documented | 12 (23.1) | 8 (21.1) | |
| Ethnicity | .962 | ||
| Hispanic | 16 (30.8) | 11 (29.0) | |
| Not Hispanic | 35 (67.3) | 26 (68.4) | |
| Unknown | 1 (1.9) | 1 (2.6) | |
| BMI, kg/m2 | 30.0 (25.1 to 32.4) | 30.7 (26.2 to 34.4) | .395 |
| Baseline comorbidities | |||
| Cardiovascular disease, % | 29 (55.8) | 24 (63.2) | .482 |
| Neurologic disease, % | 7 (13.5) | 4 (10.5) | .675 |
| Pulmonary disease, % | 5 (9.6) | 12 (31.6) | .009 |
| GI disease, % | 4 (7.7) | 3 (7.9) | .972 |
| Endocrine disease, % | 24 (46.2) | 22 (57.9) | .271 |
| Renal disease, % | 9 (17.3) | 8 (21.1) | .654 |
| Hematologic disease, % | 2 (3.9) | 1 (2.6) | .751 |
| Malignancy, % | 1 (1.9) | 1 (2.6) | .822 |
| Other immunosuppression, % | 6 (11.5) | 3 (7.9) | .569 |
| Day 1 SOFA score | 4 (3 to 6) | 7 (4 to 9) | .017 |
| Day 1 Pao2 to Fio2 ratio | 95 (67 to 134) | 108 (77 to 133) | .467 |
| Day 1 highest PEEP, cm H2O | 0 (0-8) | 0 (0-10) | .354 |
| Day 1 ventilation type | .689 | ||
| Invasive mechanical ventilation, % | 13 (25.0) | 12 (31.6) | |
| Noninvasive positive pressure ventilation, % | 6 (11.5) | 3 (7.9) | |
| High-flow nasal cannula, % | 31 (59.7) | 20 (52.6) | |
| Nasal cannula, % | 5 (5.6) | 2 (3.9) | |
| Room air, % | 0 (0) | 0 (0) | |
| Day 1 troponin I level, ng/mL | 0.01 (0.00 to 0.04) | 0.02 (0.01 to 0.06) | .188 |
| Day 1 BNP level | 40 (0 to 98) | 47 (2 to 113) | .282 |
| Day 1 receipt of vasopressors | 8 (15.4) | 8 (21.1) | .487 |
| Vasopressor dosage (of those receiving vasopressors), μ g/kg/min NEE | 0.04 (0.02 to 0.17) | 0.18 (0.06 to 0.33) | .545 |
| Day 1 echocardiography and ultrasound measurements | |||
| LVEF, % | 65 (60 to 70) | 48 (41 to 63) | < .001 |
| LV longitudinal strain, % | 19.6 (18.4 to 21.6) | 13.8 (11.3 to 14.8) | < .001 |
| RV dysfunction, %b | 55.3 (26 of 47) | 77.8 (21 of 27) | .053 |
| RV fractional area change, % | 42.9 (36.8 to 48.4) | 35.3 (17.4 to 40.7) | .005 |
| RV free-wall strain, % | 21.6 (18.3 to 26.3) | 13.8 (7.0 to 20.5) | < .001 |
| Tricuspid annulus systolic plane excursion, cm | 2.1 (1.9 to 2.3) | 1.9 (1.3 to 2.2) | .227 |
| RV to LV ratio | 0.89 (0.81 to 0.97) | 0.90 (0.77 to 0.96) | .819 |
| IVC collapsibility index | 0.21 (0.13 to 0.52) | 0.28 (0.09 to 0.51) | .858 |
| Presence of DVT on study ultrasound, % | 1 (1.9) | 2 (5.3) | .383 |
| Hospital course | |||
| Experienced atrial fibrillation, % | 7 (13.5) | 8 (21.1) | .340 |
| Experienced AV-nodal blockade, % | 4 (7.7) | 5 (13.2) | .393 |
| Experienced other arrhythmia, % | 13 (25.0) | 5 (13.2) | .165 |
| Highest BNP level, pg/mL | 42 (0 to 107) | 77 (3 to 150) | .181 |
| Highest troponin I level, ng/mL | 0.02 (0.00 to 0.09) | 0.03 (0.01 to 0.12) | .220 |
| Clinical diagnosis of DVT or PE, % | 8 (15.4) | 4 (10.5) | .503 |
| Fluid balance over first 7 d, L | –7.0 (–10.5 to –2.6) | –5.9 (–9.8 to –0.8) | .460 |
| Received IMV during study, % | 22 (42.3) | 17 (44.7) | .818 |
| Received vasopressors during study, % | 16 (30.8) | 14 (36.8) | .546 |
| Hospital length of stay, d | 13 (7-20) | 15 (7-22) | .844 |
Data are presented as No. (%) or median (interquartile range), unless otherwise indicated. AV = atrioventricular; BNP = B-type natriuretic peptide; IMV = invasive mechanical ventilation; IVC = inferior vena cava; LV = left ventricle; LVEF = left ventricular ejection fraction; NEE = norepinephrine equivalent; PE = pulmonary embolism; PEEP = positive end-expiratory pressure; RV = right ventricle; SOFA = Sequential Organ Failure Assessment.
Tests of significance were unadjusted for multiple comparisons.
For right ventricular systolic dysfunction, when complete imaging was not available, the proportion followed by numerator and denominator of completed measurements are reported.
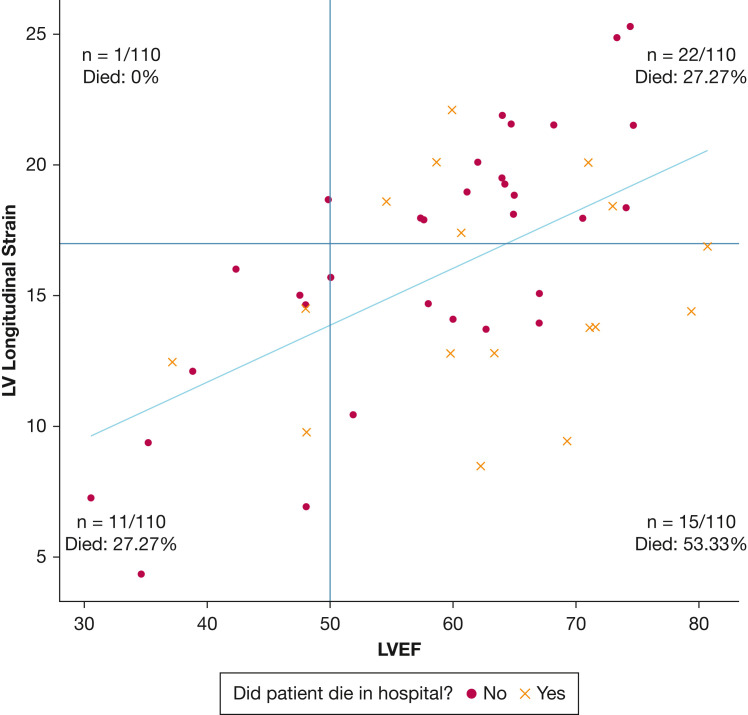
We noted that LV strain, when measurable, identified LV dysfunction in 41% of patients with normal LVEF (Fig 1 ). Patients with normal LVEF and normal LV strain showed a mortality of 27.3% (Fig 1, right upper quadrant), the same as patients with low LVEF and abnormal LV strain (Fig 1, left lower quadrant). Patients who showed high LVEF and abnormal strain demonstrated a mortality of 53.3% (Fig 1, right lower quadrant).
Figure 1.
Scatterplot showing LV global strain (y-axis) vs LVEF (x-axis) at day 1 in 49 patients in whom both were measured. Strain measurements were converted to absolute values. The plot area was divided into four quadrants by the red lines, which indicate the lower threshold for normal strain and ejection fraction: normal strain and low LVEF (one patient), normal strain and normal LVEF (22 patients), abnormal strain and normal LVEF (15 patients), and abnormal strain and low LVEF (11 patients). Patients who died are represented by an ‘×,’ whereas patients who survived are represented by a dot. The percentage of patients who died was highest among those with abnormal strain and normal LVEF (53.33% mortality). The blue line is the best fit linear regression line representing the relationship between strain and LVEF. Patients with values beneath the linear regression line (indicating lower strain than predicted based on LVEF) showed higher mortality than those with values higher than the line (indicating higher strain than predicted based on LVEF). LV = left ventricle; LVEF = left ventricle ejection fraction.
In our exploratory analysis comparing cardiac function in patients who died or survived to discharge, patients who died showed a lower residual than survivors off the best fit line in the association between LVEF and LV strain (median, –3.2 [IQR, –4.7 to 1.4] vs 1.7 [IQR, –1.4 to 2.9]; P = .03), indicating that, on average, patients who died showed worse actual strain than would be predicted by the ejection fraction.
Serial Echocardiographic Assessments
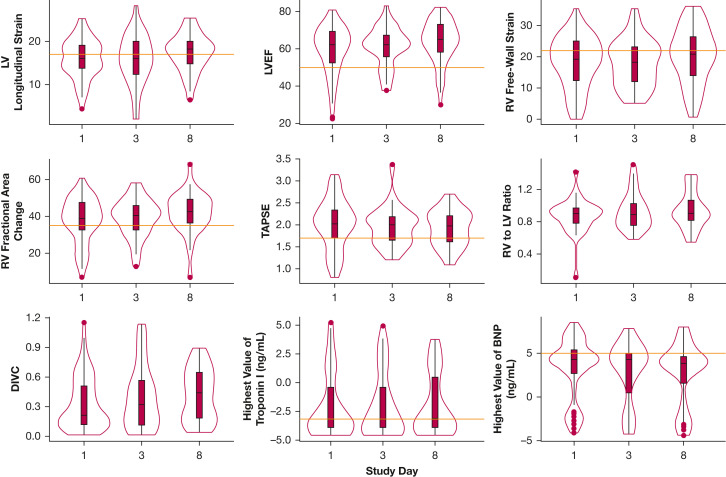
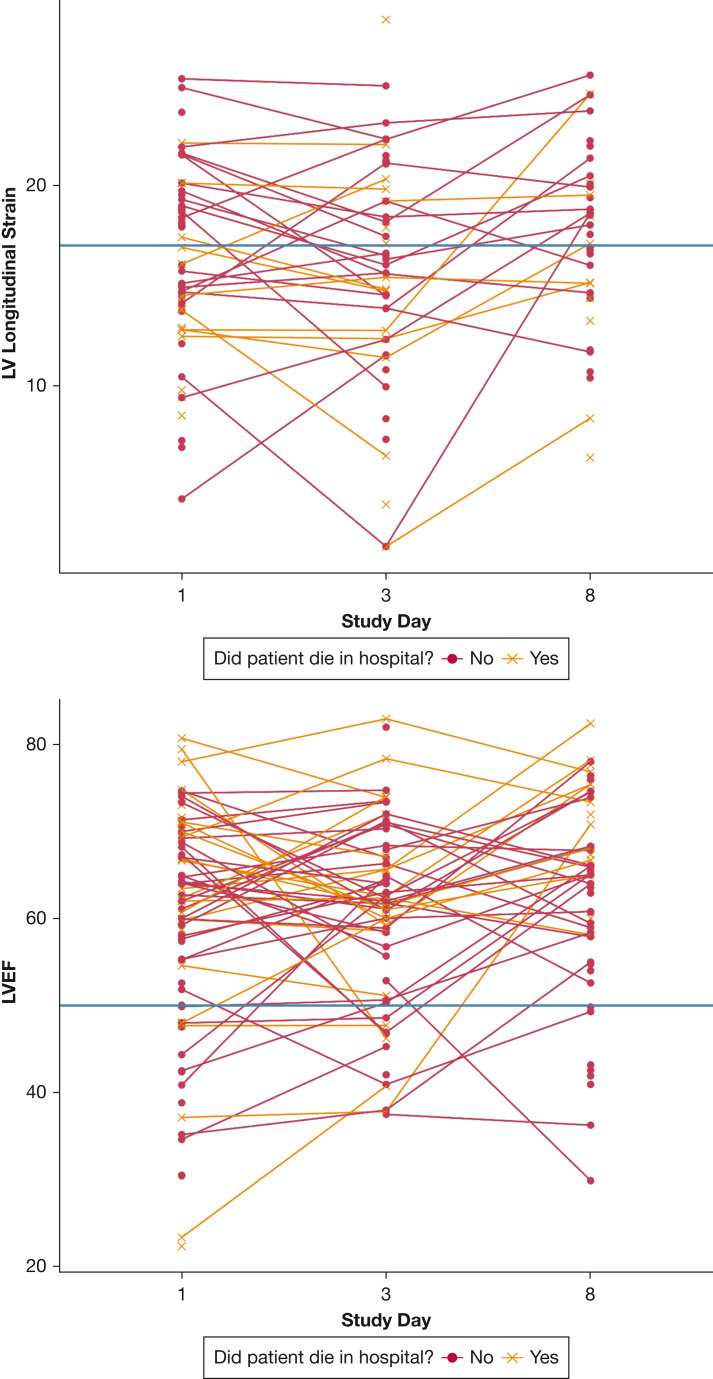
Characteristics of cardiac function over time are presented in Table 3 and Figures 2 and 3 . Median absolute value of LV strain was abnormal across study assessments on days 1, 3, and 8 (16.0%, 16.0%, and 18.2%, respectively), whereas median LVEF was normal (62%, 62%, and 65%, respectively) (Table 3, Fig 2). Additionally, median RV free-wall strain was mildly abnormal on days 1, 3, and 8 (19.2%, 18.3%, and 20.8%, respectively), whereas RV FAC (39%, 40%, and 43%, respectively) and TAPSE (2.0 cm, 2.0 cm, and 2.0 cm, respectively) consistently were normal. The RV to LV end-diastolic area consistently was 0.9 in all three assessments (Table 3). Of patients who demonstrated normal LV function on day 1, 11.5% progressed to LV dysfunction on the day 8 echocardiogram, whereas 34.2% of those with LV dysfunction improved to normal on the day 8 echocardiogram. Figure 3 demonstrates changes in individual participants over time. Although we noted improvements in in LVEF (P < .001) and changes in RV to LV ratio over time (P = .04), we observed no significant temporal trends in LV strain or in RV strain among the study cohort over the study period (Table 3).
Table 3.
Echocardiographic Assessments in Critically Ill Patients With COVID-19 Over Time (N = 110)a
| Variable | Day 1 (N = 110) | Day 3 (n = 109) | Day 8 (n = 102) | P Value |
|---|---|---|---|---|
| LV systolic dysfunction, %b | 42.2 (38 of 90) | 45.1 (32 of 71) | 36.2 (21 of 58) | .416 |
| LVEF, % | 62 (52-69) | 62 (56-67) | 65 (58-73) | < .001 |
| LV longitudinal strain, % | 16.0 (13.7-19.1) | 16.0 (12.3-20.1) | 18.2 (14.8-20.0) | .085 |
| RV dysfunction, %b | 64.2 (52 of 81) | 68.7 (46 of 67) | 67.4 (31 of 46) | .820 |
| RV fractional area change, % | 38.8 (32.8-47.5) | 40.3 (32.9-45.8) | 42.7 (36.6-49.1) | .368 |
| RV free-wall strain, % | 19.2 (12.3-24.9) | 18.3 (12.0-23.1) | 20.8 (14.0-26.4) | .327 |
| Tricuspid annulus systolic plane excursion | 2.0 (1.7-2.3) | 2.0 (1.7-2.2) | 2.0 (1.6-2.2) | .264 |
| RV to LV ratio | 0.90 (0.78-0.97) | 0.89 (0.75-1.03) | 0.90 (0.81-1.06) | .038 |
| IVC collapsibility index | 0.21 (0.12-0.51) | 0.32 (0.11-0.57) | 0.44 (0.18-0.65) | .167 |
| Presence of DVT on ultrasound, % | 2.1 | 2.4 | 3.1 | .999 |
Data are presented as median (interquartile range), unless otherwise indicated. IVC = inferior vena cava; LV = left ventricle; LVEF = left ventricular ejection fraction; RV = right ventricle.
Tests of significance are unadjusted for multiple comparisons. Strain is converted to absolute value.
Proportion of patients with right ventricular and with left ventricular dysfunction are reported, followed by the number of abnormal measurements among patients with interpretable function.
Figure 2.
Violin plots showing cardiac characteristics of 110 critically ill patients with COVID-19 over time. The violin plot is a hybrid of a boxplot and a kernel density plot, which depicts summary statistics and the density of each variable. Left ventricular longitudinal strain was converted to an absolute value. BNP and troponin I were log-transformed. Red horizontal lines indicate lower thresholds for normal. BNP = B-type natriuretic peptide; DIVC = distensibility index of the inferior vena cava; LV = left ventricle; LVEF = left ventricular ejection fraction; RV = right ventricle; TAPSE = tricuspid annular plane systolic excursion.
Figure 3.
A-B, Graphs showing changes in LV strain (A) and LVEF (B) over time among 110 critically ill patients with COVID-19. LV strain was converted to absolute values. Horizontal lines indicate lower thresholds for normal LV strain and LVEF. LV = left ventricle; LVEF = left ventricular ejection fraction.
The Role of VTE Screening
VTE was documented clinically in 14 of 110 patients (12.7%) during hospitalization, although only two patients (1.8%) showed lower extremity thrombus seen on research ultrasound during the first 8 days. One patient demonstrated DVT in the left popliteal vein on day 3, which was visualized again on day 8. The second patient showed DVT in the left popliteal and common femoral veins on day 1, which were revisualized on days 3 and 8. The remainder of patients showed no observed lower extremity DVT during the first 8 days of hospital stay.
Discussion
This was a prospective, multicenter, observational study of critically ill patients with COVID-19 using serial echocardiographic evaluations over the first week of hospitalization for severe illness. We identified a high proportion of ventricular dysfunction, much of which was identified only through deformation imaging. This proportion is comparable with other observations of similar cohorts that used clinically obtained echocardiograms.17 , 18 Deformation imaging revealed a much higher frequency of abnormal ventricular function compared with traditional imaging techniques such as LVEF, TAPSE, or RV FAC. This discrepancy was not characterized previously in a prior study that evaluated LVEF and strain.17 We did not observe an association between day 1 LV dysfunction and mortality, although we noted survivors showed better day 1 RV free-wall strain values than nonsurvivors. This association between RV dysfunction and mortality expands on prior work that used traditional RV imaging techniques.17 , 19 We also characterized the course of cardiac dysfunction over the first week of critical illness. Our data suggest limited usefulness of routine prospective screening for lower extremity DVT in this population in the first week of critical illness.
We found that ventricular strain more often was abnormal than traditional echocardiographic measurements in critically ill patients with COVID-19. LV strain is more sensitive at detecting myocardial injury compared with ejection fraction, because ejection fraction is more dependent on loading conditions than longitudinal strain.20 We observed that around 25% of interpretable echocardiograms showed ejection fractions of > 70% (Table 3), which can occur with reduced LV preload or afterload. RV free-wall longitudinal strain also similarly was abnormal more often compared with FAC and TAPSE, which generally were normal. The disparity between deformation imaging and traditional echocardiographic markers may reflect early cardiomyopathy, higher circulating catecholamine levels, lower ventricular preload or afterload, or shock severity. Patients with COVID-19 and respiratory failure frequently demonstrate shock consistent with this pattern.21 , 22 We observed that patients who died were more likely to have worse strain than would have been expected from ejection fraction values. We speculate that these patients have ventricular dysfunction with falsely reassuring ejection fraction values because of low loading conditions. Patients with hyperdynamic ejection fractions previously were reported with higher mortality rates in the ICU setting.23 Critically ill patients with low preload are more likely to show worse strain that improves on fluid resuscitation.24 The high mortality observed in patients with normal LVEF and abnormal strain is both intriguing and supported by these physiologic rationales, but inferences are limited because of smaller numbers.
We observed that some patients with LV and RV dysfunction improved over the 8-day study period, although we observed no significant change in the proportion of patients with LV dysfunction in LVEF, LV strain, or RV strain during this period. Additionally, we observed no significant trends in deformation imaging or traditional echocardiographic measures of cardiac function over the first week. Additional inferences regarding trends within individuals were limited because of small numbers. We did not observe a change in median values for troponin-I or BNP over the study period.
We observed that very few patients received a diagnosis of VTE during hospitalization, and even fewer during the first week. These rates seem substantially lower than those reported in other COVID-19 cohorts.25 Possible explanations for this discrepancy include the possibility that VTE may not develop until after the first week of critical illness or that these cohorts were different than those described early in the pandemic, where patients might have been more thrombophilic as a result of clinical management, differences in mobilization practices, or underlying disease process. It is also possible that a lower extremity clot might have been missed by two-point compression venous ultrasound, although evidence suggests that it is comparable with whole-leg ultrasound when performed by experienced clinicians.26 The diagnosis of VTE during hospitalization was made according to routine hospital care, and it is possible that routine hospital care may not have captured all instances of VTE.
Strengths of this study include its multicenter recruitment, its use of deformation imaging, and its incorporation of prospective serial imaging over the early course of disease. Prior convenience samples relying on clinically obtained biomarkers and traditional imaging have suggested that cardiac dysfunction is common among hospitalized patients with COVID-19.18 , 27 Abnormal strain also is common among noncritically ill hospitalized patients with COVID-19 and is associated with mortality and increased inflammatory cytokines.28, 29, 30, 31 Inferences regarding the early ICU stay from prior studies are limited, because most patients were not critically ill, and echocardiography was performed late in the hospital course, sometimes weeks after admission. One single-center study addressed the limitations of prior retrospective studies.32 As in our study, Doyen et al32 performed serial assessments in 42 consecutive patients with COVID-19 admitted to the ICU, demonstrating that cardiac injury was common and typically occurred within the first week of illness. Our study significantly expands on those findings by incorporating deformation imaging, more than doubling the sample size, and the improved generalizability based on multicenter enrollment.
Our study has limitations. Although performed by clinicians skilled in performing and interpreting point-of-care ultrasound in critically ill patients, imaging quality sometimes was limited in this cohort, which may have led to an underestimation of the incidence of cardiac dysfunction, including images that used strain from the apical four-chamber view only in lieu of global LV strain. This study may be susceptible to selection bias. The number of enrolled patients is much lower than the number of critically ill patients with COVID admitted to the study hospitals, many of whom could not provide written informed consent. As such, this study is probably classified more appropriately as a convenience sample, although we note a somewhat higher rate of enrollment than the one patient, site, or month typical of enrollment in critical care trials.33 , 34 Because many patients did not undergo prior imaging, the baseline cardiac function of these patients is not known. Because of limitations of patient exposures during the COVID-19 pandemic, interobserver variability was not assessable. Because of heterogeneity, this study did not account for treatments received over time at the point of serial assessments. Many of the study comparisons are univariate and do not account for other patient variables. Patients enrolled at participating centers may not be representative of US patients with severe COVID-19. Patients were enrolled between September 2020 and January 2021, which omits the newer Omicron variants of SARS-2-CoV. In addition, we may have missed cardiac injuries that occurred after the eighth ICU day. Specific thresholds for strain, including the lower limit of normal, are not well established, which could affect patient assignment into normal or abnormal function categories. Similarly, we chose a threshold of 50% for ejection fraction based on simplicity, although intersocietal guidelines use thresholds of 52% for male patients and 54% for female patients.13 We also acknowledge the risk of type I statistical error, with potential for spurious associations to be found by chance because of multiple comparisons.
Interpretation
Using sensitive methods and prospective ascertainment, we confirmed that cardiac dysfunction is common among critically ill patients with COVID-19. The most common abnormalities were abnormalities in RV free-wall strain and LV longitudinal strain. Nonsurvivors tended to have worse LV strain than expected for LVEF values and tended to have worse RV strain. We did not observe significant changes in cardiac function over the first week of critical illness, although inferences on temporal changes are limited. Early lower extremity VTE screening seems to have limited usefulness in this patient population.
Funding/Support
This work was funded by the US Centers for Disease Control and Prevention [Contract 75D30120C07637]. M. N. G. was supported by grants from US Centers for Disease Control and Prevention during the conduct of the study and National Institutes of Health funding for COVID-19 trials. W. H. S. reports grants from US Centers for Disease Control and Prevention during the conduct of the study.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST Critical Care the following: M. N. G. reports fees from Regeneron for efforts on data monitoring and safety board on their trials of monoclonal antibodies. S. M. B. reports other from Sedana, personal fees from Hamilton, grants from the US Centers for Disease Control and Prevention (CDC). A. D. reports personal fees from ALung Technologies. D. C. F. reports personal fees from Medpace, grants from the National Institutes of Health (NIH). K. W. G. reports grants from NIH, grants from the US Department of Defense (DOD), grants from CDC; A. K. reports grants from 4D Medical, grants from Ely Lilly, grants from Regeneron, grants from Dompe Pharmaceuticals, grants from United Theraputics, grants from Roche, and Dompe Pharmaceutical consulting for developing a clinical trial. C. J. L. reports other from CDC, during the conduct of the study; grants from NIH, other from CDC, grants from DOD, other from Endpoint Health, other from AbbVie, other from bioMerieux, other from AstraZeneca, other from Entegrion Inc, outside the submitted work; In addition, C. J. L. has a patent for risk stratification in sepsis and septic shock issued. T. W. R. reports personal fees from Cumberland Pharmaceuticals, Inc, personal fees from Cytovale, Inc, personal fees from Sanofi, Inc. W. H. S. reports grants from CDC, during the conduct of the study. None declared (M. J . L., S. P. D., H. L. P., J. S. B., J. D. R., N. Q., G. W. L., A. L. S., V. D., S. W. F., E. L. H., J. K., J. H. S., N. M., M. E. P., D. P.-M., M. B., T. D. O., D. B. K., M. M. C., O. M., M. W. T., M. M. P.).
Acknowledgments
Author contributions: M. J. L., S. P. D., J. S. B., J. D. R., N. Q., A. L. S., W. H. S., and S. M. B. participated in study design. M. J. L., S. P. D., J. S. B., J. D. R., N. Q., G. W. L., A. L. S., V. D., S. W. F., E. L. H., J. K., J. H. S., M. E. P., D. P. M., M. M. C., and O. M. participated in enrollment and image acquisition. M. J. L., S. P. D., and T. D. O. participated in image analysis. M. J. L., D. B. K., M. B., and H. L. P. participated in data extraction and abstraction. M. J. L., H. L. P., C. J. L., W. H. S., and S. M. B. participated in data analysis and drafting the main manuscript text. All authors reviewed and substantively revised the manuscript.
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
∗Collaborators for the Influenza and Other Viruses in the Acutely Ill Network: Nicole Calhoun, Judy Herrick, Eric Hoffman, Amanda McKillop, Kempapura Murthy, Michael Smith, Martha Zayed (Baylor Scott & White Health, Baylor, TX); Lesley De Souza, Ryan Kindle, Lori-Ann Kozikowski, Scott Ouellette, Sherell Thornton-Thompson (Baystate Medical Center, Springfield, MA); Michael Bolstad, Robert Ciottone, Brianna Coviello, Arnaldo Devilla, Ana Grafals, Conor Higgins, Carlo Ottanelli, Kimberly Redman, Douglas Scaffidi, Alexander Weingart (Beth Israel Deaconess Medical Center, Boston, MA); Nathaniel Lewis, Samantha Olson (Centers for Disease Control and Prevention, Atlanta, GA); Kiran Ashok, Connery Brennan, Omar Mehkri, Megan Mitchell, Bryan Poynter (Cleveland Clinic, Cleveland, OH); Nicholas Stanley, Caitlin ten Lohuis (Emory University, Atlanta, GA); Sean Caspers, Heidi Erikson, Audrey Hendrickson, Olivia Kaus, Ellen Maruggi, Tyler Scharber, Walker Tordsen (Hennepin County Medical Center, Minneapolis, MN); Valerie Aston, Robert Bowers, Jeffrey Jorgensen, Jennifer King (Intermountain Medical Center, Murray, UT); Harith Ali, Richard E. Rothman (Johns Hopkins University, Baltimore, MD); Rahul Nair, Jen-Ting Chen (Montefiore Medical Center, Bronx, NY); Sarah Karow, Emily Robart, Paulo Nunes Maldonado, Maryiam Khan, Preston So, Elizabeth Schwartz, Madison So, Michael Weigand (Ohio State University, Columbus, OH); Andrea Luong, Jesus Martinez, Bao Huynh, Habiba Ibrahim, Cynthia Villanueva-Vargas, Haeun Jung, Juliana Villanueva-Vargas, Suha Quadri (Oregon Health & Science University, Portland, OR); Alexandra Jun Gordon, Joe Levitt, Cynthia Perez, Anita Visweswaran, Jonasel Roque (Stanford University, Stanford, CA); Adreanne Rivera, Trevor Frankel (University of California, Los Angeles, Los Angeles, CA); Jennifer Goff, David Huynh, Kelly Jensen, Conner Driver, Ian Chambers (UCHealth University of Colorado Hospital, Aurora, CO); Paul Nassar, Lori Stout, Zita Sibenaller, Alicia Walter, Jasmine Mares, Logan Olson, Bradley Clinansmith, University of Iowa, Iowa City, IA; Hayley Gershengorn, Carolina Rivas (University of Miami, Coral Gables, FL); EJ McSpadden, Rachel Truscon, Anne Kaniclides, Lara Thomas, Ramsay Bielak, Weronika Damek Valvano, Rebecca Fong, William J. Fitzsimmons, Christopher Blair, Andrew Valesano, Leigh Baker, Julie Gilbert (University of Michigan, Ann Arbor, MI); Christine D. Crider, Kyle A. Steinbock, Thomas C. Paulson, Layla A. Anderson (University of Washington, Seattle, WA); Christy Kampe, Jakea Johnson, Laura L. Short, Lauren J. Ezzell, Margaret E. Whitsett, Rendie E. McHenry, Samarian J. Hargrave, Marica Blair, Jennifer L. Luther, Claudia Guevara Pulido, Bryan P. M. Peterson (Vanderbilt University Medical Center, Nashville, TN); Mary LaRose, Leigha Landreth, Madeline Hicks, Lisa Parks (Wake Forest University, Winston-Salem, NC); Jahnavi Bongu, David McDonald, Candice Cass, Sondra Seiler, David Park, Tiffany Hink, Meghan Wallace, Carey-Ann Burnham, Olivia G. Arter (Washington University, St Louis, MO).
Other contributions: The authors thank Kiran Ashok, BS, for their contributions toward the study.
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data.”
Contributor Information
Influenza and Other Viruses in the Acutely Ill Network:
Nicole Calhoun, Judy Herrick, Eric Hoffman, Amanda McKillop, Kempapura Murthy, Michael Smith, Martha Zayed, Lesley De Souza, Ryan Kindle, Lori-Ann Kozikowski, Scott Ouellette, Sherell Thornton-Thompson, Michael Bolstad, Robert Ciottone, Brianna Coviello, Arnaldo Devilla, Ana Grafals, Conor Higgins, Carlo Ottanelli, Kimberly Redman, Douglas Scaffidi, Alexander Weingart, Nathaniel Lewis, Samantha Olson, Kiran Ashok, Connery Brennan, Omar Mehkri, Megan Mitchell, Bryan Poynter, Nicholas Stanley, Caitlin ten Lohuis, Sean Caspers, Heidi Erikson, Audrey Hendrickson, Olivia Kaus, Ellen Maruggi, Tyler Scharber, Walker Tordsen, Valerie Aston, Robert Bowers, Jeffrey Jorgensen, Jennifer King, Harith Ali, Richard E. Rothman, Rahul Nair, Jen-Ting Chen, Sarah Karow, Emily Robart, Paulo Nunes Maldonado, Maryiam Khan, Preston So, Elizabeth Schwartz, Madison So, Michael Weigand, Andrea Luong, Jesus Martinez, Bao Huynh, Habiba Ibrahim, Cynthia Villanueva-Vargas, Haeun Jung, Juliana Villanueva-Vargas, Suha Quadri, Alexandra Jun Gordon, Joe Levitt, Cynthia Perez, Anita Visweswaran, Jonasel Roque, Adreanne Rivera, Trevor Frankel, Jennifer Goff, David Huynh, Kelly Jensen, Conner Driver, Ian Chambers, Paul Nassar, Lori Stout, Zita Sibenaller, Alicia Walter, Jasmine Mares, Logan Olson, Bradley Clinansmith, Hayley Gershengorn, Carolina Rivas, E.J. McSpadden, Rachel Truscon, Anne Kaniclides, Lara Thomas, Ramsay Bielak, Weronika Damek Valvano, Rebecca Fong, William J. Fitzsimmons, Christopher Blair, Andrew Valesano, Leigh Baker, Julie Gilbert, Christine D. Crider, Kyle A. Steinbock, Thomas C. Paulson, Layla A. Anderson, Christy Kampe, Jakea Johnson, Laura L. Short, Lauren J. Ezzell, Margaret E. Whitsett, Rendie E. McHenry, Samarian J. Hargrave, Marica Blair, Jennifer L. Luther, Claudia Guevara Pulido, Bryan P.M. Peterson, Mary LaRose, Leigha Landreth, Madeline Hicks, Lisa Parks, Jahnavi Bongu, David McDonald, Candice Cass, Sondra Seiler, David Park, Tiffany Hink, Meghan Wallace, Carey-Ann Burnham, and Olivia G. Arter
Supplementary Data
References
- 1.WHO coronavirus (COVID-19) dashboard. World Health Organization. Accessed November 23, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J.O., Shin D.H., Cho S.W., et al. Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography. 2008;25(8):873–879. doi: 10.1111/j.1540-8175.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- 6.Burns A.T., La Gerche A., D’Hooge J., MacIsaac A.I., Prior D.L. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010;11(3):283–289. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- 7.Jensen M.B., Sloth E., Larsen K.M., Schmidt M.B. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol. 2004;21(9):700–707. doi: 10.1017/s0265021504009068. [DOI] [PubMed] [Google Scholar]
- 8.Johri A.M., Galen B., Kirkpatrick J.N., Lanspa M., Mulvagh S., Thamman R. A.S.E. Statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020;33(6):670–673. doi: 10.1016/j.echo.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanspa M.J., Shahul S., Hersh A., et al. Associations among left ventricular systolic function, tachycardia, and cardiac preload in septic patients. Ann Intensive Care. 2017;7(1):17. doi: 10.1186/s13613-017-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanspa M.J., Pittman J.E., Hirshberg E.L., et al. Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock. Crit Care. 2015;19:304. doi: 10.1186/s13054-015-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto T., Dulgheru R., Bernard A., et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18(8):833–840. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 12.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Muraru D., Onciul S., Peluso D., et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking Echocardiography. Circ Cardiovasc Imaging. 2016;9(2) doi: 10.1161/CIRCIMAGING.115.003866. [DOI] [PubMed] [Google Scholar]
- 15.Brown S.M., Lanspa M.J., Jones J.P., et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–671. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Karagodin I., Carvalho Singulane C., Woodward G.M., et al. Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: the World Alliance Societies of Echocardiography (WASE-COVID) Study. J Am Soc Echocardiogr. 2021;34(8):819–830. doi: 10.1016/j.echo.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dweck M.R., Bularga A., Hahn R.T., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J., Volodarskiy A., Sultana R., et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965–1977. doi: 10.1016/j.jacc.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 21.Hollenberg S.M., Safi L., Parrillo J.E., et al. Hemodynamic profiles of shock in patients with COVID-19. Am J Cardiol. 2021;153:135–139. doi: 10.1016/j.amjcard.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evrard B., Goudelin M., Montmagnon N., Fedou A.L., Lafon T., Vignon P. Cardiovascular phenotypes in ventilated patients with COVID-19 acute respiratory distress syndrome. Crit Care. 2020;24(1):236. doi: 10.1186/s13054-020-02958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paonessa J.R., Brennan T., Pimentel M., Steinhaus D., Feng M., Celi L.A. Hyperdynamic left ventricular ejection fraction in the intensive care unit. Crit Care. 2015;19:288. doi: 10.1186/s13054-015-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nafati C., Gardette M., Leone M., et al. Use of speckle-tracking strain in preload-dependent patients, need for cautious interpretation. Ann Intensive Care. 2018;8(1):29. doi: 10.1186/s13613-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraaijpoel N., Carrier M., Le Gal G., et al. Diagnostic accuracy of three ultrasonography strategies for deep vein thrombosis of the lower extremity: a systematic review and meta-analysis. PloS One. 2020;15(2) doi: 10.1371/journal.pone.0228788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Li H., Zhu S., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R., Wang H., Ma F., et al. Widespread myocardial dysfunction in COVID-19 patients detected by myocardial strain imaging using 2-D speckle-tracking echocardiography. Acta Pharmacol Sin. 2021;42(10):1567–1574. doi: 10.1038/s41401-020-00595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janus S.E., Hajjari J., Karnib M., Tashtish N., Al-Kindi S.G., Hoit B.D. Prognostic value of left ventricular global longitudinal strain in COVID-19. Am J Cardiol. 2020;131:134–136. doi: 10.1016/j.amjcard.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockenhuber A., Vrettos A., Androschuck V., et al. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID-19 patients with evidence of cardiac involvement. Echocardiography. 2021;38(2):222–229. doi: 10.1111/echo.14966. [DOI] [PubMed] [Google Scholar]
- 32.Doyen D., Dupland P., Morand L., et al. Characteristics of cardiac injury in critically ill patients with coronavirus disease 2019. Chest. 2021;159(5):1974–1985. doi: 10.1016/j.chest.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalamalapu S.R., Needham D.M., Stapleton R.D. Patient recruitment rate in multicentered randomized trials in critical care. Crit Care Med. 2016;44(7):e588–e589. doi: 10.1097/CCM.0000000000001703. [DOI] [PubMed] [Google Scholar]
- 34.Krutsinger D.C., Yadav K.N., Harhay M.O., Bartels K., Courtright K.R. A systematic review and meta-analysis of enrollment into ARDS and sepsis trials published between 2009 and 2019 in major journals. Crit Care. 2021;25(1):392. doi: 10.1186/s13054-021-03804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.