Abstract
Background
Cambodia has achieved great success in tuberculosis (TB) control in the past decade. Nevertheless, people with TB are missed by the health systems at different stages of the care pathway. This programme review corroborated the care-seeking behaviours of people with TB and TB services availability and estimated the number of people completing each step of the TB disease and TB preventive treatment (TPT) care cascade.
Methods
Patient pathways and the care cascades for TB disease and TPT were constructed using data from the latest national TB prevalence survey, routine surveillance and programme, the global TB database and published studies. We also randomly selected TB survivors in the 2019 cohort to assess recurrence-free survival 1-year post-treatment. TPT care cascade was constructed for people living with HIV (PLHIV) and household contacts (children <5 years and all ages) of persons with bacteriologically-confirmed TB in 2019 and 2020.
Results
Nationally, 54% of those who exhibited TB symptoms sought initial care in the private sector. Overall, 93% and 58% of people with presumptive TB did not access a facility with TB diagnostic and treatment services, respectively, at the first point of care-seeking. Approximately 56% (95% CI 52% to 57%) of the 47 000 (95% CI 31 000 to 68 000) estimated TB cases in 2019 achieved recurrence-free survival. Among the estimated PLHIV in Cambodia, <30% completed TPT. Among children <5 years, 53% (95% CI 29% to 65%) (2019) and 67% (95% CI 36% to 80%) (2020) of those eligible for TPT completed the regimen successfully. In 2019 and 2020, 23% (95% CI 22% to 25%) and 54% (95% CI 50% to 58%) of the estimated household contacts (all ages) eligible for TPT completed the regimen successfully.
Conclusion
There are significant gaps in care-seeking, coverage and access to TB services and TPT in Cambodia. Action plans to improve TB response have been co-developed with local stakeholders to address the gaps throughout the care cascades.
Keywords: tuberculosis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Empirical studies have been conducted to understand the care-seeking behaviours of people with presumptive tuberculosis (TB), but their interactions with TB services are unknown in Cambodia.
No study to date has estimated the number of people with TB who dropped out at each step of the care cascade in Cambodia.
Cambodia has implemented TB preventive treatment (TPT), but the care cascade among the target populations has not been examined.
WHAT THIS STUDY ADDS
This study, therefore, provides a comprehensive overview of TB care in Cambodia by corroborating the care-seeking behaviours of people with TB and TB services availability and estimating the number of people completing each step of the TB disease and TPT care cascade.
This is also the first study to investigate recurrence-free survival among TB survivors in Cambodia.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study identified gaps in care-seeking, coverage and access to TB services and TPT in Cambodia.
The gaps were deliberated with local stakeholders, and recommendations to improve TB response and action plans for implementation have been developed.
Introduction
Tuberculosis (TB) is a global public health concern. Although TB is preventable and treatable, approximately 10 million new TB cases and 1.3 million deaths were estimated to have occurred in 2020, making TB a leading infectious cause of morbidity and mortality worldwide.1 Of the 10 million cases, merely 62% were detected and notified to the national TB programmes globally, and a similar proportion (63%) was observed in Cambodia, a country with a considerable TB incidence (all-form TB; 274 per 100 000 population) in 2020.1
Cambodia’s pluralistic health system comprises public services and the private sector (including profit and non-profit organisations). The Cambodian National TB Programme (NTP) is coordinated by the National Centre for Tuberculosis and Leprosy Control (CENAT). It is supported by a comprehensive network of 9 national hospitals, 25 provincial hospitals, 68 district referral hospitals and 1143 health centres.2 The involvement of the private sector in TB care is less structured. The sales of TB drugs are prohibited in pharmacies.3 Most people with signs and symptoms of TB who visit private health practitioners are referred to public health facilities for investigation for TB.
Over the past two decades, improved socioeconomic status and concerted multifarious programmatic efforts through active case finding and community mobilisation saw TB incidence and mortality rates halved, and treatment success rates sustained at >90% in Cambodia.1 Nevertheless, people with TB are missed by the health systems at different stages of the care pathway and do not receive the care they need. Poor access to healthcare could be attributed to demographic factors such as age, rural residence, gender, financial insecurities, long distance to the health facilities, poor knowledge and awareness regarding TB and the absence of classic clinical features of TB.4 5 Systematic literature reviews from high TB burden countries highlighted organisational and policy factors such as poor referral mechanisms and inadequate resources, including trained staff and sensitive diagnostic tools.4 In Cambodia, several factors may hinder timely TB screening and diagnosis access. The factors include a strong preference for private healthcare and seeking multiple providers, particularly those not providing TB services, the lack of diagnostic tools in public facilities and awareness of TB symptoms among people with TB, and the presence of financial and geographical barriers.6 7 Furthermore, scaling up TB prevention activities, including using TB preventive treatment to disrupt transmission within households and communities, are key areas that have not been systematically evaluated locally. Therefore, it is critical to understand the gap and identify areas for improvement in the national TB response in Cambodia.
The patient pathway analysis (PPA) is a method to assess the gaps between the care-seeking behaviour of people with TB/presumptive TB and the availability of TB diagnosis and treatment services.8 Previous PPA studies conducted in five countries revealed three critical points to improve case detection (1) linkage with the private sector (66% initiated care in the private sector, but less than 10% were reported), (2) expansion of capacity in primary healthcare (>40% of initiated care at primary levels or lower) and (3) increased availability of TB diagnostic services (many facilities where care was initiated did not have TB diagnostic capabilities).8 Therefore, PPA renders valuable insights for programme planning and policymaking to improve TB services.
The care cascade is another valuable method for evaluating healthcare service delivery and identifying gaps in the stages of care that could be addressed towards a successful outcome. The analysis of the TB care cascade has been implemented in India and South Africa.9 10 It facilitated understanding gaps in the quality of care for people affected by TB. The utility of care cascade analyses was also instrumental in achieving the end TB goal.11
We reviewed the TB programme by corroborating the care-seeking behaviours of people with TB/presumptive TB and the availability of TB services at the facilities where care is first sought at the national and subnational levels. We constructed the care cascade to identify TB disease and infection programmatic gaps in the country and estimate the number of people completing each cascade step.
Methods
We completed the PPA and constructed the care cascade using the approaches described by Hanson et al, Seabrook et al and Subbaraman et al.12–14 Verbal consent was obtained to assess recurrence-free survival, but consent was not necessary for the other analyses involving existing anonymised data and records provided by the NTP or publicly available. We used Microsoft Excel 365 ProPlus (Microsoft, Redmond, Washington, USA) and R (R Foundation for Statistical Computing, Vienna) for the analyses.
PPA
PPA was conducted at the national and subnational levels (20 provinces and the capital city of Phnom Penh). It comprised seven indicators—place of initial care-seeking for TB symptoms, coverage of TB diagnostic services, access to TB diagnostic services at the care-seeking place, coverage of TB treatment services, access to TB treatment at the place of initial care-seeking, notification locations and treatment outcomes. Data were drawn from various sources, including the most recent national TB prevalence survey 2011, routine surveillance data at NTP and the WHO global TB databases (table 1).
Table 1.
Patient pathway analysis and care cascades: indicators and data sources
| Indicators | Descriptions and parameters | Sources |
| Patients pathway analysis (PPA) | ||
| 1. Place of initial care-seeking. | Place of initial care-seeking among those with cough ≥2 weeks or haemoptysis. | National TB prevalence survey (2011) |
| Urban population and per cent of total population in Cambodia. | United Nations Population Fund (2014)60 | |
| 2. Coverage of TB diagnostic services. | The proportion of health facilities (national hospital, provincial hospital, referral hospital and health centres) with TB diagnostic modalities (microscopes, GeneXpert instrument, culture). | NTP (2019) |
| 3. Access to TB diagnostic services at the place of initial care-seeking. | The proportion of those who sought care and accessed TB diagnostic services at their initial visit. | Product of core metric 1 and 2 |
| 4. Coverage of TB treatment services. | The proportion of health facilities (national hospital, provincial hospital, referral hospital and health centres) that provide treatment services. | NTP (2019) |
| 5. Access to TB treatment at the place of initial care-seeking. | The proportion of those who sought care and accessed a facility with TB treatment services at their initial visit. | Product of core metric 1 and 4 |
| 6. Notification location. | The estimated proportion of people with TB enrolled in treatment and notified to the national programme. | WHO and the NTP (2019) |
| 7. Treatment outcome. | The proportion of people with TB enrolled in treatment and completed TB treatment/were successfully treated. | WHO and the NTP (2019) |
| Care cascade for TB disease | ||
| 1. Estimated incidence of TB. | Total number of incident TB cases (all-forms) in 2019. | WHO global TB database 202016 |
| 2. Accessed TB tests. | ||
| The estimated number of people who reached TB diagnostic facilities and were evaluated for TB. Several key parameters (next column) were accounted for (by the different forms of TB) in estimating the total number. | Smear or GeneXpert positive: | |
| The proportion of individuals tested using smear microscopy (%). | NTP | |
| The proportion of individuals tested using GeneXpert (%). | NTP | |
| The proportion of individuals who failed to provide a second sputum specimen (%). | WHO policy statement 201117 | |
| The incremental yield of a second sputum smear (%). | Mase et al 200718 | |
| Smear negative and previously treated excluding relapse cases: | ||
| The ratio of smear-positive to negative. | NTP | |
| The ratio of GeneXpert-positive to negative. | NTP | |
| Sensitivity of smear microscopy (%). | National TB prevalence survey 2011 | |
| Sensitivity of GeneXpert (%). | Steingart et al 201419 | |
| Extrapulmonary TB: | ||
| The average proportion of undiagnosed smear-positive and smear-negative TB (%). | Average of the % of undiagnosed smear-positive and the % of undiagnosed smear-negative (online supplemental materials) | |
| 3. Diagnosed with TB. | ||
| The estimated number of individuals diagnosed with TB in 2019. Back calculated using the number of people registered in TB treatment by accounting for pre-treatment loss to follow-up rate. | Pre-treatment loss to follow-up rate (%). | Mao et al 201220 |
| 4. Registered in treatment. | Total number of people with TB (all-forms: new and relapse by smear status, previously treated excluding relapse cases, and extrapulmonary TB) registered in treatment in 2019. | WHO global TB database 202016 |
| 5. Treatment success. | Total number of people with TB who achieved treatment success in 2019. | NTP |
| 6. Recurrence-free survival. | The proportion of TB survivors who remained alive and TB-free 1 year after completing TB treatment (%). | Primary data |
| Care cascade for TPT (PLHIV) | ||
| 1. Estimated number of PLHIV. | PLHIV (all ages). | UNAIDS23 |
| 2. Number of PLHIV who know their HIV status. | PLHIV who know their status. | UNAIDS23 |
| 3. Number of PLHIV on antiretroviral therapy who are eligible for TPT. | PLHIV who are eligible for TPT. | National HIV Programme |
| 4. Number of PLHIV who initiated TPT. | PLHIV who initiated TPT (any course). | National HIV Programme |
| 5. Number of PLHIV who initiated TPT and completed the course. | PLHIV who initiated and completed TPT (any course). | National HIV Programme |
| Care cascade for TPT (household contacts <5 years) | ||
| 1. Estimated number of household contacts (children <5 years). | Point estimate of the annual contacts (children aged <5) needing evaluation. | Yuen et al 201624 |
| 2. Estimated number of household contacts (children <5 years) evaluated for TB disease and infection. | The proportion of household contact (children <5) who were evaluated for TB disease and infection* (%). | TPT programme database (COMMIT) |
| 3. Estimated number of children <5 years who were TB negative and eligible for TPT. | The proportion of household contact (children <5) who were eligible for and initiated TPT (%). | WHO global TB database 202016 |
| 4. Number of children <5 years who initiated TPT. | Number of children aged <5 who initiated TPT. | National TB Programme and WHO |
| 5. Estimated number of children <5 years who initiated TPT and completed the course. | The proportion of household contact (children <5) who completed TPT (%). | TPT programme database (COMMIT) |
| Care cascade for TPT (household contacts all ages) | ||
| 1. Estimated number of household contacts (all ages). | Number of persons with new and relapse bacteriologically-confirmed pulmonary TB. | WHO global TB database 202016 |
| Estimated average household size. | Demographic and health survey 201426 | |
| 2. Number of household contacts (all ages) evaluated for TB disease and infection. | Number of household contacts (all ages) evaluated for TB disease and infection.* | National TB Programme and WHO |
| 3. Estimated number of household contacts (all ages) who were TB negative and eligible for TPT. | The proportion of household contact (all ages) who were healthy and eligible for TPT (%). | National TB Programme |
| 4. Number of household contacts (all ages) who initiated TPT. | Number of household contacts (all ages) who initiated TPT. | National TB Programme |
| 5. Estimated number of household contacts (all ages) who initiated TPT and completed the course. | The proportion of household contact (children<5) who completed TPT (%). | TPT programme database (COMMIT) |
*Evaluation to rule of TB disease (clinical symptoms-based screening±chest radiography), and potential contraindications to TPT, such as liver disease and known allergies to TPT
COMMIT, Community Mobilization Initiatives to End TB (USAID funded project in Cambodia); NTP, National TB programme; PLHIV, people living with HIV; TB, tuberculosis; TPT, TB preventive treatment; UNAIDS, Joint United Nations Programme on HIV/AIDS.
bmjgh-2022-010994supp001.pdf (573.5KB, pdf)
We used the place of initial care-seeking among people with presumptive TB (those with cough for ≥2 weeks or haemoptysis) as how they were reported in the 2011 national TB prevalence survey.15 Four provinces—Koh Kong, Mondulkiri, Oddar Meanchey, and Stung Treng—were not included in the prevalence survey. Subsequently, we categorised and assigned the places of initial care-seeking to sectors and levels according to the levels of care provided—public: government hospitals (level 3) and health centres (level 1); private: private hospitals (level 3), private clinics (level 1) and pharmacies (level 0); non-medical (level 0): traditional healers, family members and self-medication (see description in online supplemental materials).12 The proportions of people with presumptive TB who initiated care-seeking were reported by sectors and levels of care.
Data on the types and number of health facilities included 1403 public facilities (hospitals at the national, provincial and district levels and health centres) in Cambodia. The number of private health facilities (private hospitals, clinics, pharmacies) and non-medical sectors (traditional healers) was unknown. We also lacked information on the types of government hospitals reported in the prevalence survey in indicator 1. Therefore, we grouped national, provincial and district referral hospitals into one single category of public hospitals to estimate access to TB diagnostic services at the place of initial care-seeking. Information on TB diagnostic services was unknown in private health facilities. However, TB services are mainly provided by the public sector in Cambodia. Hence, in the analysis of PPA, we assumed that the private and non-medical sectors did not provide TB diagnostic and treatment services.
The coverages of TB diagnostic services at the national and provincial levels were presented as the proportions of health facilities with at least one diagnostic modality, with the numerator being the number of health facilities equipped with at least one microscope, GeneXpert instruments or culture, and the denominator was the total number of health facilities. The breakdown of TB diagnostic modalities by facilities was also reported. Access to TB diagnosis at the point of initial care-seeking was estimated by multiplying indicator 1 (place of initial care-seeking) and indicator 2 (diagnostic coverage) at each sector and level.14
The NTP provided data on the availability of TB treatment services at different public health facilities. The coverage of TB treatment at the national and provincial levels was presented as the proportions of health facilities providing TB treatment. The numerator was the number of health facilities providing such services, and the denominator was the total number of health facilities. Access to TB treatment services at the point of initial care-seeking was estimated by multiplying indicator 1 (place of initial care-seeking) and indicator 4 (treatment coverage) at each sector and level.14
The health sector’s contribution to TB case notifications was estimated from the WHO data on notified new and relapse cases as a share of the estimated incidence of TB in Cambodia in 2020.1 We considered TB cases only reported by the public health facilities in our analyses. The final indicator showed the treatment outcome of notified cases for 2019.1
Cascade of care (TB disease)
The cascade of care for TB disease (excluding drug-resistance cases) was constructed at the national level using routine and existing data (details in online supplemental materials),13 and it included six steps. Data and parameters for constructing the care cascade were drawn from routine surveillance data at the NTP, WHO global TB databases and other relevant published studies (table 1).
Step 1 comprised the annual TB incidence in Cambodia estimated by WHO.16 The estimated number of people who reached TB diagnostic facilities and were evaluated for TB was presented in step 2. Step 2’s value was estimated by back-calculating from step 3 (the number of people diagnosed with TB). The calculations incorporated different sets of parameters and assumptions for the different types of TB to estimate the number of individuals who went undiagnosed despite being evaluated at the health facilities. For smear-positive TB, including retreatment cases (evaluated using smear microscopy), we assumed those who were evaluated yet remained undiagnosed were possibly due to a false-negative spot sample and the failure to submit a second/third sample the next day that would have resulted in a positive diagnosis. Hence, the proportion of individuals who failed to provide a second sputum specimen17 and the incremental yield of a second sputum sample in diagnosing TB18 was accounted for in the calculation.
The diagnosis of smear-negative TB was based on sputum specimens examinations, chest radiographs and a trial of broad-spectrum antibiotics in Cambodia.3 Similar to the challenges highlighted by Subbaraman et al in India,10 the attrition rate at each step before the diagnosis was unknown, and no formal evaluation was done on the robustness of the diagnostic algorithm against the gold standard (culture). Hence, the proportion of actual smear-negative TB that remain undiagnosed was undetermined. To estimate the number of individuals with smear-negative/clinically diagnosed TB in step 2, we used the ratio of smear-positive to smear-negative TB approximated using the yields of smear-negative and smear-positive from microscopy and GeneXpert instrument and the sensitivity of these diagnostic modalities.15 19 The final estimate for smear-negative/clinically diagnosed TB was extrapolated using the estimated number of smear-positive TB described above and the calculated ratio accordingly. The same approach was applied in estimating the number of retreatment smear-negative cases. We estimated the proportion of patients with extrapulmonary TB who remained undiagnosed despite presenting to a TB diagnostic facility by taking the average of the proportions of undiagnosed smear-positive TB because of failure to provide a second sputum sample (described above) and undiagnosed smear-negative TB (by comparing the number of smear-negative TB in step 2 and step 3). We summed the estimated number of individuals who reached TB diagnostic facilities and were evaluated by the different types of TB (smear and GeneXpert positive, smear-negative/clinically diagnosed TB, retreatment and extrapulmonary TB) as the final value for step 2 of the care cascade.
Subsequently, those successfully diagnosed with TB were presented as step 3 in the care cascade. We estimated step 3’s value by accounting for pretreatment loss to follow-up20 among those notified and registered in treatment (step 4). Steps 4 and 5 included the proportion of people with TB registered in treatment and successfully treated, respectively. We acquired the total number of people with TB (all forms) registered in TB treatment in 2019 (step 4) from the WHO global TB database 2020.16 We established step 5, representing the total number of people registered in treatment in 2019 who were successfully treated. Those who completed treatment and/or were cured of TB were considered successfully treated.
Finally, recurrence-free survival 1 year after completing TB treatment was presented in step 6. Recurrence-free survival was defined as individuals who remained alive and stayed TB-free 1 year after completing TB treatment.10 For this step, primary data were collected. The number of study participants was determined by assuming a design effect of 1, a 5% margin of error, a 10% prevalence of TB recurrence with 95% power and a 15% non-response rate. A sample of approximately 150 people with TB was required. To sample study participants, we used the cohort of people with TB notified in 2019 as the sampling frame. Given the possibility of participants being uncontactable due to invalid contact details, a random sample of 500 people who completed TB treatment (TB survivors) was first selected using the random number generator in Microsoft Excel 365 ProPlus (Microsoft, Redmond, Washington, USA). The interviewers went down the list of 500 randomly selected participants sequentially and contacted them by phone. We excluded those who refused to participate and were not contactable, and the data collection ceased when the target sample size was reached. We collected information on the current status (alive, deceased, date and cause of death, moved away, unknown) and TB diagnosis in the past year (if yes, the date and types of TB) from TB survivors or their next-of-kin. Verbal consent was obtained from all participants. Step 6 was estimated as the product of step 5’s value and the proportion of TB survivors who remained alive and TB-free.
Cascade of care (TPT)
The cascade of care for TB preventive treatment (TPT) was constructed at the national level using routine and existing data for three populations—people living with HIV (PLHIV) and household contacts (children <5 years and all ages) of persons with bacteriologically-confirmed TB (hereinafter children <5 and household contacts). The corresponding parameters and the calculations are presented in table 1 and online supplemental materials.
The key features of the care cascade for TPT included (1) the estimated size of the target population, (2) the number of individuals evaluated for active TB and their eligibility for TPT, (3) the number of eligible individuals who started TPT and (4) the number of individuals who completed TPT.21 In Cambodia, tests for TB infection (tuberculin skin test and interferon-gamma release assay) are not routinely conducted and are not a requirement for initiating TPT among PLHIV and children <5 years.21 22 While the cascade would have ideally included the number of people tested for TB infection, we lacked data on this indicator to incorporate it in the care cascade. Therefore, our analyses primarily focused on routinely available data to assess the adequacy of TPT services and data availability in Cambodia.
For PLHIV, we constructed the care cascade using data from 2019 and 2020. The cascade included five steps. We used data on the population size of PLHIV and the estimated number of PLHIV who knew their status from the Joint United Nations Programme on HIV/AIDS (UNAIDS).23 Data on the total number of PLHIV eligible for, initiated and completed TPT were obtained from the national HIV programme.
For household contacts (children <5 years) of persons with bacteriologically-confirmed TB, we adapted Yuen and colleagues’ point estimates of children contacts needed to be screened annually in Cambodia.24 The other information directly available from the NTP and WHO databases were the number of eligible children and children who initiated TPT. We estimated the number of children evaluated for TB disease and infection and the number of children who completed TPT using parameters from an ongoing project (Community Mobilization Initiatives to End Tuberculosis (COMMIT)) that integrates TB case finding and preventive treatment implemented in 10 operational districts (ODs) in Cambodia.25 The description of COMMIT and the derivation of parameters are presented in the online supplemental materials.
We estimated the number of household contacts (all ages) who needed to be screened using the number of persons with bacteriologically-confirmed TB (new and relapse) and the estimated household size in Cambodia.26 We obtained data on those evaluated for TB disease and infection and initiated TPT from NTP-led contact investigation activities conducted in 2019 and 2020 (step 1). The activities were held in 46 ODs in 2019 and extended to 76 ODs in 2020, representing a great bulk of the TPT service delivery in the country. The same data were submitted to the WHO’s annual TB data collection system.16 Subsequently, we estimated the number of eligible individuals for TPT by assuming that 60% and 80% of the household contacts were free of TB disease and eligible for TPT in 2019 and 2020, respectively. The difference in eligibility proportions could be attributed to the expansion of coverage and activities in 2020, which included eligible household contacts of all ages (beyond children <5) for TPT. However, due to the lack of precision in approximating the proportions of household contacts eligible for TPT, we assumed the lower and upper bounds to be within 50% of the estimates and that the resulting confidence intervals would likely cover a reasonable range. Lastly, as the TPT completion rate was not available from NTP, we used data from COMMIT to approximate the number of individuals who completed TPT treatment. The treatment completion rates of the 6-month isoniazid regimen were considered in both instances, as it was the predominant TPT regimen used in Cambodia in 2019 and most of 2020. It was also a more conservative and plausible assumption.
Results
PPA
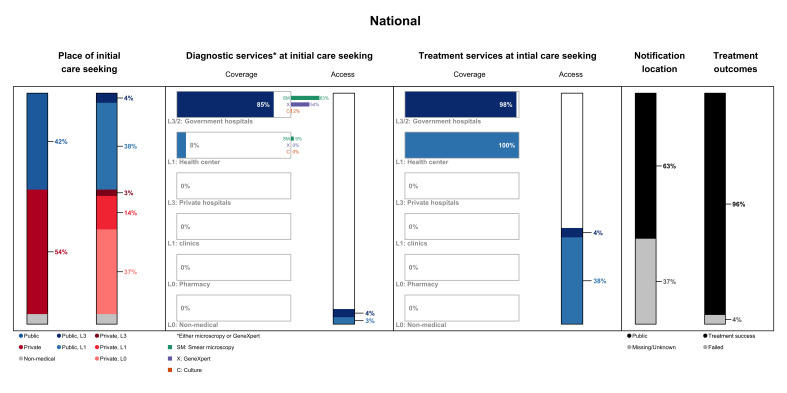
Nationally, 54% of those who exhibited symptoms (cough ≥2 weeks or haemoptysis) sought initial care in the private sector. Most visited private pharmacies, followed by private clinics and hospitals. For those who visited the public healthcare facilities, care-seeking for TB symptoms was mainly initiated at the health centres. Approximately 6% of individuals with presumptive TB sought non-medical care in the informal sector on falling ill (figure 1).
Figure 1.
Patient pathway analysis at the national level. The patient pathway describes the care-seeking patterns and their alignment with tuberculosis (TB) services. Column 1 represents the place of initial care-seeking reported by people with TB symptoms (cough ≥2 weeks and haemoptysis) in the national TB prevalence survey 2011. The second column shows the breakdown of the facilities visited at initial care-seeking by sector and facility levels. The third column represents the coverage of TB diagnostics in the different sectors and facilities using the proportion of facilities with at least one TB diagnostic platform (smear microscope, GeneXpert instrument or culture test). Column 4, which represents the access to diagnostic services at initial care-seeking, is the output of the product between the metrics in columns 2 and 3. The coverage (proportion of health facilities providing TB treatment) and access to treatment services are presented in columns 5 and 6. Column 7 shows which sector provided case notification, representing a share of the overall estimated TB incidence in 2019. The last column shows the treatment outcome among notified cases in 2019.
Most public hospitals (85%) were equipped with TB diagnostic modalities, 83% had at least one smear microscope, 54% were furnished with at least one GeneXpert system and 2% provided culture tests. A small fraction (9%) of the public health centres were equipped with at least one smear microscope, and none housed GeneXpert instruments or culture tests. The private and non-medical sectors did not provide TB diagnostic services. Overall, 7% of people with TB accessed a facility with TB diagnostic services at their first point of care.
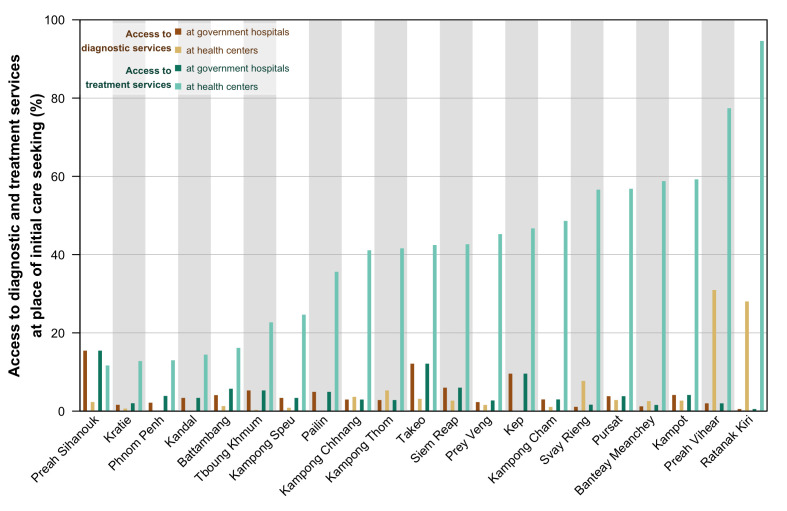
Care initiation and access to TB diagnostic services varied between provinces (figure 2). Twelve provinces with >50% of people with TB who sought initial care in the private sector had an average proportion of the urban population in these provinces of 30%. In nine provinces with >50% who initiated care in the public sector, the average proportion of the urban population in these provinces was lower at 21%. The difference, however, was not statistically significant (Wilcoxon rank-sum test; p=0.79). Access to TB diagnostics at the initial point of care-seeking was the highest in four provinces where most of its population live in rural areas—Svay Rieng, Preah Vihear, Ratanak Kiri and Takeo. Access to diagnostics was higher among those who initiated care at public hospitals (median: 3.4%) compared with public health centres (median: 1.6%) after experiencing TB symptoms, although not statistically significant (Wilcoxon rank-sum test; p=0.07).
Figure 2.
Access to tuberculosis (TB) diagnostic and treatment services at the provincial level. This figure shows the access to TB diagnostic and treatment services at initial care-seeking at the provincial level. Access (presented in percentage) was estimated by considering the place of initial care-seeking and diagnostic and treatment services coverage at the different sectors and facilities.
Notwithstanding the low coverage of diagnostic services at public health centres, TB treatment was available at all health centres and nearly all public hospitals in Cambodia. As TB treatment services are only available in the public health sector in Cambodia, none of the private health facilities administers TB treatment. Overall, 63% of the estimated 47 000 TB cases were notified to the NTP in 2019. The remaining 37% were presumably undiagnosed and unnotified, and it was possible that they were not duly managed and treated for TB. Of those who were notified and initiated TB treatment, the success rate was 96% in 2019.
Cascade of care (TB disease)
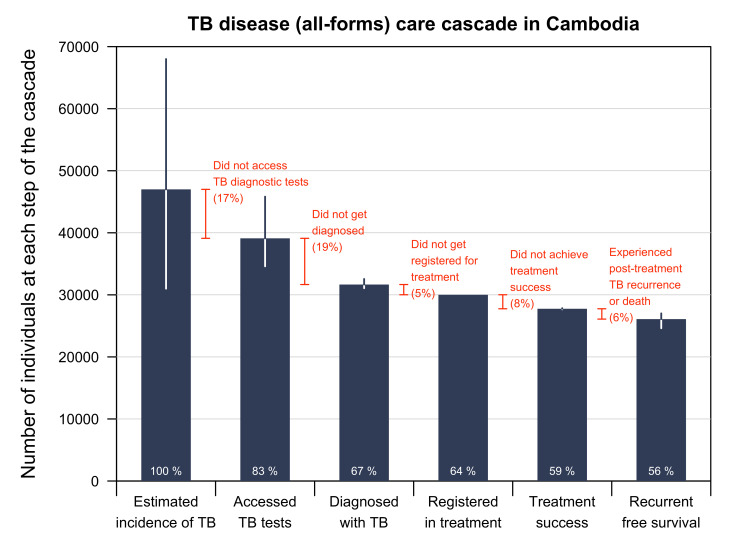
Of the 47 000 estimated incident TB (all forms) cases (95% CI 31 000 to 68 000) in Cambodia in 2019, we estimated that 39 110 (95% CI 34 603 to 45 827) people with TB (83%) accessed public health facilities and were evaluated for TB (figure 3). This suggested that 17% of people with TB never sought care or did not have access to public health facilities. Among 39 110 people with TB evaluated, we estimated that 31 664 (95% CI 31 073 to 32 556) (81% (95% CI 79% to 83%)) were diagnosed with TB. Among those diagnosed with TB, 30 017 (95%) were registered in treatment and notified to the NTP. A pretreatment loss to follow-up rate of 5% was estimated. A total of 27 758 (95% CI 27 667 to 27 847) people registered in treatment, and 92% (95% CI 92% to 93%) completed the regimen as intended and achieved treatment success. We estimated that 94% (95% CI 89% to 97%) of the TB survivors remained alive and TB-free 1 year after treatment (150 people surveyed, 143 were alive and 141 were TB-free). Of the estimated number of people who developed TB in 2019 in Cambodia, 56% (95% CI 52% to 57%) were identified, diagnosed, linked to care and successfully treated and remained alive and TB-free for 1 year post treatment.
Figure 3.
TB disease care cascade in Cambodia. Proportions (in white) refer to the percentage of people at each step of the cascade compared with the first step (estimated TB incidence). Proportions (in orange) refer to the percentage attritted at each step compared with the previous step (% difference). The treatment success rate presented in the care cascade differs from PPA despite representing 2019’s outcomes because of the denominator used. We used the preceding step to treatment outcome in the care cascade as the denominator resulting in a treatment success rate of 92%. We obtained the treatment success rate in patient pathway analysis (96%) from the WHO database, and it was based on the total number of persons evaluated in 2020 as the denominator. TB, tuberculosis.
Cascade of care (TPT)
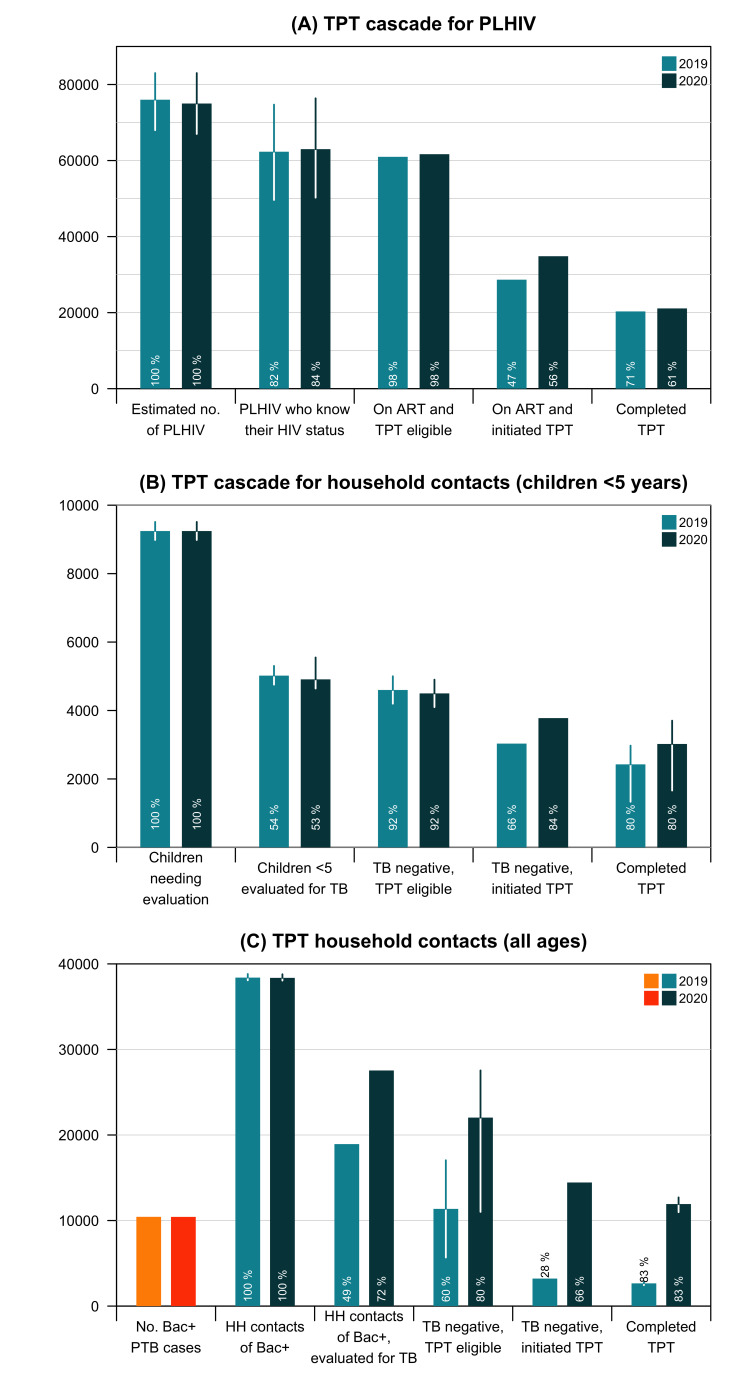
The estimated number of PLHIV in Cambodia was 76 000 (95% CI 68 000 to 83 000) and 75 000 (95% CI 67 000 to 83 000) in 2019 and 2020, respectively (figure 4A).23 Of the total, 82% (95% CI 73% to 90%) and 84% (95% CI 75% to 92%) knew their HIV status in the respective years.23 It was estimated that 98% of those who knew their HIV status were eligible for TPT in 2019 and 2020. The proportion of eligible PLHIV on antiretroviral therapy (ART) initiated by TPT was 47% in 2019 and rose to 56% in 2020. Among those who started TPT, 71% and 61% completed the treatment in 2019 and 2020, respectively. Among the estimated PLHIV in Cambodia, <30% initiated and completed TPT.
Figure 4.
TPT care cascades for (A) PLHIV, (B) household contacts <5 years and (C) household contacts of all ages. Proportions (in white) refer to the percentage of people at each step of the cascade compared with the preceding step. For household contacts (all ages), the first bars represent the estimated number of persons with bacteriologically-confirmed TB, used to estimate the number of household contacts that needed evaluation for TB disease and TPT. ART, antiretroviral therapy; BK+, bacteriologically-confirmed TB; PLHIV, people living with HIV; TB, tuberculosis; TPT, TB preventive treatment.
For the care cascade among children <5 years (figure 4B), of the 9245 (95% CI 8985 to 9510) children who needed evaluation annually in Cambodia,24 we estimated that 54% (95% CI 53% to 56%) and 53% (95% CI 52% to 58%) were evaluated for TB disease and infection in 2019 and 2020, respectively. In 2019, 4600 (95% CI 4200 to 5000) (92%) were estimated to be eligible for TPT; 66% initiated it. In 2020, of the 4500 (95% CI 4100 to 4900) (92%) eligible children, 84% initiated it. Overall, 53% (95% CI 29% to 65%) (2019) and 67% (95% CI 36 to 80%) (2020) of those eligible for TPT completed the recommended regimen.
The estimated number of TB household contacts was 38 397 (95% CI 38 084 to 38 814) in 2019 and 38 368 (95% CI 38 055 to 38 785) in 2020.26 In 2019, 18 938 all-age household contacts (49%) were evaluated for TB disease and infection (figure 4C). The number rose by 57% to 27 542 due to coverage expansion in 2020. In 2019, 28% of eligible individuals started TPT, and the figure quadrupled to 14 449 (66%) in 2020. The TPT completion rates among household contacts (children <5 years and all ages) were estimated to be approximately 80%. Overall, 23% (95% CI 22% to 25%) (2019) and 54% (95% CI 50% to 58%) (2020) of those eligible for TPT completed the recommended regimen.
Discussion
In Cambodia, TB services are predominantly provided by the public sector. The private sector does not provide direct TB care, and the notification rate remains poor.27 28 However, as in many high TB burden countries,8 most people with presumptive TB preferred to seek private healthcare. Such inclination was estimated to incur more than two-thirds of Cambodia’s out-of-pocket healthcare payments.29 In a 2015 systematic review, Lei and colleagues reported positive TB case detection rates and improved treatment outcomes through the involvement of the private health sector in referrals, diagnosis, treatment, awareness-raising and health education.30 While there have been efforts to improve referrals from the private to the public sector,27 31 inadequate funding, strong dependence on incentive-based referrals, challenges in persuading private medical practitioners to partake and maintaining seamless coordination between the sectors have hampered the scale-up and sustainability of public–private engagement in TB control.32 As the private sector plays a significant role in the care pathway, revitalising and contextualising the public–private engagement programmes will be critical in finding unreached and undiagnosed cases.33–35 Solutions should be tailored according to the country’s contexts for success and sustainability.
The availability of TB diagnostic modalities, such as smear microscopy and GeneXpert, in health facilities plays a crucial role in delivering TB services. TB work-up might not be initiated appropriately in places where such resources are scarce. The availability of GeneXpert systems in Cambodia has increased over the years (26 devices in 2015 and 75 in 64 sites in 2019).33 36 But the coverage will likely remain inadequate at 1.4 health facilities with GeneXpert assays per 1000 estimated incident TB cases.37 The requisite to ramp up the availability of rapid diagnostics is one of NTP’s primacies.33 TB diagnostic tools have a different turnaround and reporting time—GeneXpert assay (approximately 3 hours), smear microscopy (about 19 hours) and cultures (≥14 days)38 39—resulting in considerable impact on the time to diagnosis and treatment initiation.7 Nevertheless, the implementation of molecular diagnostics relies on a strong healthcare ecosystem comprising a sufficient and appropriately skilled workforce and robust specimen transportation, storage, communication and follow-up systems,40 which may be lacking in under-resourced settings.41 Furthermore, the quality of sputum specimens and laboratory processes are also critical in improving TB diagnosis.42 43 Therefore, these facets should be accounted for while considering the roll-out and scale-up of TB diagnostic modalities.
At the subnational level, access to TB diagnostics and treatment services at initial care-seeking was the highest among provinces with a higher proportion of the rural population. This finding could be attributed to the reliance of the rural population on public healthcare, which is more affordable, thereby rendering better access to TB services due to their availability in the public sector. However, this study could not account for the time taken for people with TB to reach such services. However, delay in TB diagnosis is more prominent among rural dwellers in Cambodia, suggesting other barriers to care-seeking among this population. A whole-of-society approach is warranted to address them.7 44 To improve the yield of TB detection, access to TB diagnostic services could be facilitated or provided directly by active case finding (ACF) interventions that bring TB diagnostic services closer to the communities.45 46 ACF interventions targeting urban and rural settings using various modus operandi have been shown to improve TB case detection in Cambodia.40 47 48 Therefore, despite being highly resource-intensive, ACF remains a critical mechanism in finding people with TB in Cambodia and other high-burden settings.49
In the 2011 Cambodia national TB prevalence survey, the prevalence of asymptomatic bacteriologically-confirmed TB was 71%.15 The lack of key TB symptoms, such as cough, haemoptysis and night sweats, has also been associated with delayed TB diagnosis in Cambodia.7 Nonetheless, symptom screening remains the mainstay in the initial screening of TB in both passive and active case-finding approaches. While we could not account for these populations in this study, about 50% of people with TB disease were subclinical/asymptomatic yet infectious, which may have contributed substantially to the ‘missing cases’.50 Apart from the possibility that people with TB were underdiagnosed or undiagnosed due to the lack of diagnostic resources and barriers to care-seeking and diagnosis, under-reporting is another reason TB cases could be missed. In Cambodia, TB notification was only done by the public health facilities to the NTP, and a surveillance system is currently in place to better manage data within the country. An evaluation conducted in 2010 exemplified that the TB data on case notification and treatment outcomes in Cambodia were complete and consistent.51 However, efforts to digitalise TB information systems should be routinely fortified, including research on data services,52 to enhance data management efficiency and policy decision-making. This includes addressing the gap in reporting TB cases in sectors outside NTP’s jurisdiction, such as private healthcare facilities. Notification data essentially include those diagnosed and registered for treatment. Pretreatment loss to follow-up, a key parameter reflecting those diagnosed and subsequently linked to treatment and care, should be periodically monitored with its associated factors understood.
Universal access to free TB treatment and expanding community-based care was pivotal in achieving high treatment success rates among people with TB in Cambodia.53 Among those who completed TB treatment, most remained alive and TB-free in the ensuing year. This estimate was higher than that in India, another high TB burden setting at 86%.10 Beyond TB, other lung diseases, poor mental health and well-being and excess mortality among TB survivors have been reported elsewhere.54 A 2021 systematic review estimated that post-treatment TB-related disabilities were frequently reported with the pooled prevalence of neurological impairments (40.9%), mental health disorders (19.8%) and respiratory disorders (19.3%).55 There is a scarcity of information on post-TB health and well-being in Cambodia. Further research should seek to understand this neglected area, and the current TB care package should be expanded to include support after TB.
Upstream in the spectrum of TB, before the development of active disease, we mapped the care cascade for TPT services among PLHIV and household contacts (children <5 years and all ages). The proportion of PLHIV enrolled in ART who initiated TPT in Cambodia (52%) was above the average of the Western Pacific region (41%) and just shy of the global coverage (68%) in 2019, and a similar trend was observed in 2020.1 In this study, the main losses occurred before treatment initiation among those eligible for TPT. While the barriers to starting and completing TPT have not been systematically understood in Cambodia, factors such as knowledge and information regarding TPT, proper referral and follow-up post evaluation, staff training about TPT, adverse events and pill burden have been reported in a recent systematic review.56 Therefore, efforts are needed to understand the gaps and develop contextualised interventions to improve the TPT care cascade among PLHIV locally.
A significant gap was observed among household contacts in the number evaluated for TPT, where only approximately half of those who needed evaluation were assessed. Contact investigation is effective in actively seeking people with TB, and it is typically done through TB registries and screening of household contacts of persons with bacteriologically-confirmed TB. However, contact investigation is financially and human resource-intensive and often implemented by non-governmental organisations with external donor funding. Nonetheless, ACF in Cambodia comes in many forms, including a strong presence in the community.57 58 Therefore, they could be capitalised by screening household contacts for TB disease, infection and TPT on identifying persons with TB through an integrated search-treat-prevent approach.59 Further down the cascade, the improvement in TPT initiation and estimated treatment success rates were encouraging. Nevertheless, remaining barriers should be addressed, and the usage of a shorter effective regimen should be scaled up, sustained and duly monitored.
This study has several limitations. We lacked several key local and timely data in conducting PPA and constructing the care cascades. Data on TB-specific care-seeking behaviours were collected in 2011 and lacked information on four provinces. The alternate option would be to use care-seeking data for general illnesses and injuries collected through the most recent demographic health survey (2014). However, we opined that the care-seeking patterns for respiratory symptoms might differ from general ailments; therefore, the former was analysed. We also lacked insights into the private sector. Despite assuming that the private sector was not providing TB services in our analyses, some larger and more equipped tertiary hospitals might have diagnostic capabilities. In 2019, 0.1% and 0.5% of the notified cases were referred from factories (ACF) and the private healthcare sector, respectively. But we lacked detailed information to include them in the analyses meaningfully. Hence, the visibility of the private sector’s involvement would be valuable.
Furthermore, we could not pinpoint the exact health facilities that a person visited and if diagnostics were available. We also did not know the proportion of people who did not submit a second/third specimen and the incremental yield of a second sample in Cambodia. However, the parameters used in the analyses were discussed with NTP for local relevance. One key element the PPA and care cascade for TB disease did not capture was the time delays to TB diagnosis and treatment. A previous study in Cambodia reported that the median time from the onset of TB symptoms to diagnosis of 49 days.7 Some people with TB might be eventually diagnosed, but long delays would continue to perpetuate TB transmission in the household and communities. For the TPT care cascade, the parameters inferred from the ongoing intervention (COMMIT) could have been overinflated as the project is adequately resourced and conducted in a controlled environment. Therefore, the estimated figures might not represent the outcomes observed outside the intervention settings. Nonetheless, the intervention is integrated within the public health system and strives to be scalable, translatable and sustainable within the country in the long run. They are the only available information, highlighting the need for better data. We also did not account for other scenarios (eg, people eligible for TPT who may not be offered TPT due to the lack of recommendation from clinicians) that could have contributed to the gaps in our calculations.
Despite the limitations, this study illustrated gaps in care-seeking preferences and access to diagnostic services, the coverage of TB diagnostics, laboratory processes and reaching those who do not access TB services. There were also noteworthy gaps in the evaluation, uptake and completion of TPT among PLHIV and household contacts in Cambodia. Systemic reforms such as engagement with the private sector, ramping up the availability of TB diagnostics, further decentralising diagnostic services to health centres and assessing the quality of sputum collection and laboratory procedures could improve TB care and control efforts in the country. It is also imperative to address the social, structural and systemic barriers to TB care by implementing multiprong strategies. The strategies may include taking a proactive approach in actively seeking people with TB in the health facilities and communities, ensuring people-centred TB services, community empowerment and mobilisation and implementing universal health coverage and other social protection schemes. TPT services should be integrated with routine case-finding interventions, and barriers to TPT uptake and completion should be understood and addressed. Efforts and investments should also be concerted in generating better data and facilitating data usage to inform policy and programmes (see online supplemental materials for action plan). Ultimately, optimising case finding, treatment and prevention of TB will curtail transmission, reduce the TB burden, and advance toward ending TB in Cambodia by 2030.
Acknowledgments
We would like to acknowledge tuberculosis (TB) survivors and their family members and communities affected by TB for contributing to this project. We would also like to thank the National Centre for Tuberculosis and Leprosy Control (CENAT) and The United States Agency for International Development (USAID) for supporting this work. We thank Dr Chansophal Ly, Mr Seyha Ong, Dr Chamroen Pall, Mr Taing Hangleang, Ms Yourk Chanleaksmy and the staff at KHANA and WHO Cambodia Representative Office for coordinating data collection and organising the dissemination workshop. We also thank Dr Christine Whalen, Dr Song Ngak and Dr Sotheara Nop for their feedback on the study.
Footnotes
Handling editor: Stephanie M Topp
AKJT, FM and KP contributed equally.
Contributors: AKJT (guarantor), FM, KP and SY conceptualised the study and wrote the grant application to obtain funding. AKJT, FM and KP contributed to the study design. AKJT, STu, YA, SE, CYH, KEK, SD and STi were involved in the data collection. AKJT and FM did the analyses. KP and AKJT made the figures. AKJT, FM and KP wrote the initial draft. All authors contributed equally to data interpretation, critically reviewed the manuscript and approved the final version.
Funding: This project was supported by the Saw Swee Hock School of Public Health Infectious Diseases Programme Research Grant, the National University of Singapore President’s Graduate Fellowship and the WHO Regional Office for the Western Pacific. This study was also partly funded by the Singapore Ministry of Education Academic Research Fund Tier 1 (Start-Up Grant) and the UHS-SSHSPH Integrated Research Programme, Saw Swee Hock School of Public Health, National University of Singapore.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. All data relevant to the study are included in the article or uploaded as supplementary information. No additional data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by National Ethics Committee for Health Research Cambodia (006/NECHR) and the National University of Singapore Institutional Review Board (NUS-IRB-2021-82). Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . Global tuberculosis report 2021. Geneva, 2021. Available: www.who.int/publications/i/item/9789240037021 [Google Scholar]
- 2.Department of Planning and Health Information . Health strategic plan 2016-2020. Phnom Penh: Department of Planning and Health Information, 2016. [Google Scholar]
- 3.National Center for Tuberculosis and Leprosy Control . Technical guideline on tuberculosis control. 2nd edn. Phnom Penh: National Center for Tuberculosis and Leprosy Control, Ministry of Health Cambodia, 2016. [Google Scholar]
- 4.Teo AKJ, Singh SR, Prem K, et al. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res 2021;22:251. 10.1186/s12931-021-01841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getnet F, Demissie M, Assefa N, et al. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med 2017;17:202. 10.1186/s12890-017-0551-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi S, Teo AKJ, Sok S, et al. Barriers in access to services and information gaps by genders and key populations in the National tuberculosis programme in Cambodia. Glob Public Health 2022;17:1743–56. 10.1080/17441692.2021.1954226 [DOI] [PubMed] [Google Scholar]
- 7.Teo AKJ, Ork C, Eng S, et al. Determinants of delayed diagnosis and treatment of tuberculosis in Cambodia: a mixed-methods study. Infect Dis Poverty 2020;9:49. 10.1186/s40249-020-00665-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson C, Osberg M, Brown J, et al. Finding the missing patients with tuberculosis: lessons learned from patient-pathway analyses in 5 countries. J Infect Dis 2017;216:S686–95. 10.1093/infdis/jix388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naidoo P, Theron G, Rangaka MX, et al. The south african tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis 2017;216:S702–13. 10.1093/infdis/jix335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbaraman R, Nathavitharana RR, Satyanarayana S, et al. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLOS Med 2016;13:e1002149. 10.1371/journal.pmed.1002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid MJA, Goosby E. Lessons learned from the HIV care cascade can help end TB. Int J Tuberc Lung Dis 2017;21:245–6. 10.5588/ijtld.17.0027 [DOI] [PubMed] [Google Scholar]
- 12.Hanson CL, Osberg M, Brown J, et al. Conducting patient-pathway analysis to inform programming of tuberculosis services: methods. J Infect Dis 2017;216:S679–85. 10.1093/infdis/jix387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subbaraman R, Nathavitharana RR, Mayer KH, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med 2019;16:e1002754. 10.1371/journal.pmed.1002754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seabrook D, Durham G, Brown J, et al. Patient pathway analysis: how to guide. assessing the alignment of TB patient care seeking and TB service delivery. Washington: Linksbridge and the Bill and Melinda Gates Foundation, 2017. Available: https://patientpathway.org/ [Google Scholar]
- 15.National Center for Tuberculosis and Leprosy Control . Second national tuberculosis prevalence survey Cambodia. Phnom Penh: National Center for Tuberculosis and Leprosy Control, Ministry of Health Cambodia, 2012. [Google Scholar]
- 16.World Health Organization . Tuberculosis data. 2020. Available: www.who.int/teams/global-tuberculosis-programme/data [Accessed 16 Feb 2022].
- 17.World Health Organization . Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
- 18.Mase SR, Ramsay A, Ng V, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2007;11:485–95. [PubMed] [Google Scholar]
- 19.Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;2014:CD009593. 10.1002/14651858.CD009593.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eang MT, Satha P, Yadav RP, et al. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health 2012;12:469. 10.1186/1471-2458-12-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO operational handbook on tuberculosis. module 1: prevention - tuberculosis preventive treatment. Geneva, 2020. Available: https://apps.who.int/iris/bitstream/handle/10665/331525/9789240002906-eng.pdf [PubMed] [Google Scholar]
- 22.Paton NI, Borand L, Benedicto J, et al. Diagnosis and management of latent tuberculosis infection in asia: review of current status and challenges. Int J Infect Dis 2019;87:21–9. 10.1016/j.ijid.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 23.Joint United Nations Programme on HIV/AIDS (UNAIDS) . AIDSinfo: global data on HIV epidemiology and response. 2022. Available: https://aidsinfo.unaids.org/ [Accessed 20 Feb 2022].
- 24.Yuen CM, Jenkins HE, Chang R, et al. Two methods for setting child-focused tuberculosis care targets. Public Health Action 2016;6:83–96. 10.5588/pha.16.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Agency for International Development (USAID) . Preventing tuberculosis transmission in Cambodia. 2021. Available: www.usaid.gov/global-health/health-areas/tuberculosis/resources/news-and-updates/preventing-transmission-cambodia [Accessed 20 Feb 2022].
- 26.National Institute of Statistics, Ministry of Planning Cambodia, Directorate General for Health Cambodia, ICF International . Cambodia demographic and health survey 2014. Dataset KHHR73DT. Phnom Penh: National Institute of Statistics Cambodia, Directorate General for Health Cambodia, and ICF International, 2015. [Google Scholar]
- 27.Bell CA, Eang MT, Dareth M, et al. Provider perceptions of pharmacy-initiated tuberculosis referral services in Cambodia, 2005-2010. Int J Tuberc Lung Dis 2012;16:1086–91. 10.5588/ijtld.11.0669 [DOI] [PubMed] [Google Scholar]
- 28.National Center for Tuberculosis and Leprosy Control (CENAT) . Technical guidelines on tuberculosis control. 2nd edn. Phnom Penh: Ministry of Health, 2016. [Google Scholar]
- 29.Ministry of Health Cambodia, World Health Organization Regional Office for the Western Pacific . Health service delivery profile. Cambodia, Manila: World Health Organization Western Pacific Region, 2012. Available: www.wpro.who.int/health_services/service_delivery_profile_cambodia.pdf [Google Scholar]
- 30.Lei X, Liu Q, Escobar E, et al. Public-private mix for tuberculosis care and control: a systematic review. Int J Infect Dis 2015;34:20–32. 10.1016/j.ijid.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 31.Bell CA, Ilomäki J, Pichenda K, et al. Referral of tuberculosis symptomatic clients from private pharmacies to public sector clinics for diagnosis and treatment in Cambodia. J Eval Clin Pract 2015;21:285–91. 10.1111/jep.12306 [DOI] [PubMed] [Google Scholar]
- 32.Mihalea H, Richardson D. Public-private mix involving pharmacies and other providers in TB control. A Cambodia case study. Phnom Penh: USAID, 2009. Available: https://path.azureedge.net/media/documents/CP_cambodia_ppm_tb_cs.pdf [Google Scholar]
- 33.World Health Organization Regional Office for the Western Pacific . Joint review of the national TB program 2019. 2020. [Google Scholar]
- 34.USAID . Cambodia tuberculosis roadmap overview, fiscal year 2021. Washington, DC: USAID, 2020. Available: www.usaid.gov/sites/default/files/documents/Cambodia_Narrative_TBRM21_TBDIAH_Version_Final.pdf [Google Scholar]
- 35.Uplekar M. Public-private mix for tuberculosis care and prevention. what progress? What prospects? Int J Tuberc Lung Dis 2016;20:1424–9. 10.5588/ijtld.15.0536 [DOI] [PubMed] [Google Scholar]
- 36.Médecins Sans Frontières, Stop TB Partnership . Out of step 2015: TB policies in 24 countries. A survey of diagnostic and treatment practices. Geneva: Médecins Sans Frontières and Stop TB Partnerships, 2015. [Google Scholar]
- 37.Médecins Sans Frontières, Stop TB Partnership . Step up for TB 2020: tuberculosis policies in 37 countries. A survey of prevention, testing, and treatment policies and practices. Geneva: Médecins Sans Frontières and Stop TB Partnership, 2020. [Google Scholar]
- 38.Pfyffer GE, Wittwer F. Incubation time of mycobacterial cultures: how long is long enough to issue a final negative report to the clinician? J Clin Microbiol 2012;50:4188–9. 10.1128/JCM.02283-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H-S, Kee S-J, Shin J-H, et al. Xpert MTB/RIF assay as a substitute for smear microscopy in an intermediate-burden setting. Am J Respir Crit Care Med 2019;199:784–94. 10.1164/rccm.201804-0654OC [DOI] [PubMed] [Google Scholar]
- 40.Camelique O, Scholtissen S, Dousset J-P, et al. Mobile community-based active case-finding for tuberculosis among older populations in rural Cambodia. Int J Tuberc Lung Dis 2019;23:1107–14. 10.5588/ijtld.18.0611 [DOI] [PubMed] [Google Scholar]
- 41.Parsons LM, Somoskövi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 2011;24:314–50. 10.1128/CMR.00059-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alisjahbana B, van Crevel R. Improved diagnosis of tuberculosis by better sputum quality. Lancet 2007;369:1908–9. 10.1016/S0140-6736(07)60893-9 [DOI] [PubMed] [Google Scholar]
- 43.Ho J, Marks GB, Fox GJ. The impact of sputum quality on tuberculosis diagnosis: a systematic review. Int J Tuberc Lung Dis 2015;19:537–44. 10.5588/ijtld.14.0798 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization Regional Office for the Western Pacific . Western pacific regional framework to end TB 2021-2030. Manila: World Health Organization Regional Office for the Western Pacific, 2022. Available: https://apps.who.int/iris/handle/10665/352278 [Google Scholar]
- 45.Nardell EA. Transmission and institutional infection control of tuberculosis. Cold Spring Harb Perspect Med 2015;6:a018192. 10.1101/cshperspect.a018192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morishita F, Eang MT, Nishikiori N, et al. Increased case notification through active case finding of tuberculosis among household and neighbourhood contacts in Cambodia. PLoS One 2016;11:e0150405. 10.1371/journal.pone.0150405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorent N, Choun K, Thai S, et al. Community-based active tuberculosis case finding in poor urban settlements of phnom penh, Cambodia: a feasible and effective strategy. PLoS ONE 2014;9:e92754. 10.1371/journal.pone.0092754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teo AKJ, Prem K, Tuot S, et al. Mobilising community networks for early identification of tuberculosis and treatment initiation in cambodia: an evaluation of a seed-and-recruit model. ERJ Open Res 2020;6:00368-2019. 10.1183/23120541.00368-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol 2016;5:374–8. 10.1016/j.ijmyco.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 50.Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease-a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis 2021;73:e830–41. 10.1093/cid/ciaa1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoa NB, Wei C, Sokun C, et al. Completeness and consistency in recording information in the tuberculosis case register, Cambodia, China and Viet Nam. Int J Tuberc Lung Dis 2010;14:1303–9. [PubMed] [Google Scholar]
- 52.Lee Y, Raviglione MC, Flahault A. Use of digital technology to enhance tuberculosis control: scoping review. J Med Internet Res 2020;22:e15727. 10.2196/15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill PS, Tan Eang M. Resistance and renewal: health sector reform and Cambodia’s national tuberculosis programme. Bull World Health Organ 2007;85:631–6. 10.2471/blt.06.036822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoeman I, Sifumba Z. Tuberculosis care does not end at treatment completion- a perspective from tuberculosis survivors. Lancet Infect Dis 2021;21:896–7. 10.1016/S1473-3099(20)30941-5 [DOI] [PubMed] [Google Scholar]
- 55.Alene KA, Wangdi K, Colquhoun S, et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med 2021;19:203. 10.1186/s12916-021-02063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastos ML, Melnychuk L, Campbell JR, et al. The latent tuberculosis cascade-of-care among people living with HIV: a systematic review and meta-analysis. PLoS Med 2021;18:e1003703. 10.1371/journal.pmed.1003703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ministry of Health Cambodia . Community participation policy for health. Phnom Penh: Ministry of Health, 2008. [Google Scholar]
- 58.National Center for Tuberculosis and Leprosy Control (CENAT) . National strategic plan for control of tuberculosis 2014-2020. Phnom Penh: National Center for Tuberculosis and Leprosy Control, 2014. [Google Scholar]
- 59.Zero TB Initiative . Getting to zero: a guide to the search, treat, prevent comprehensive approach to TB. North Carolina: Zero TB Initiative, 2017. Available: https://static1.squarespace.com/static/54e39cb6e4b096d5269be282/t/58eba0b53a0411706a599aff/1491837111138/Getting+to+Zero+STP+Pamphlet+-+March+2017.pdf [Google Scholar]
- 60.United Nations Population Fund (UNFPA) . Urbanization and its linkage to socio-economic and environmental issues. Geneva: United Nations Population Fund (UNFPA), 2014. Available: https://cambodia.unfpa.org/sites/default/files/pub-pdf/Urbanizationreport%282015%29.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-010994supp001.pdf (573.5KB, pdf)
Data Availability Statement
No data are available. All data relevant to the study are included in the article or uploaded as supplementary information. No additional data are available.