Abstract
Introduction
Early-onset fetal growth restriction (FGR) requires timely, often preterm, delivery to prevent fetal hypoxia causing stillbirth or neurologic impairment. Antenatal corticosteroids (CCS) administration reduces neonatal morbidity and mortality following preterm birth, most effectively when administered within 1 week preceding delivery. Optimal timing of CCS administration is challenging in early-onset FGR, as the exact onset and course of fetal hypoxia are unpredictable. International guidelines do not provide a directive on this topic. In the Netherlands, two timing strategies are commonly practiced: administration of CCS when the umbilical artery shows (A) a pulsatility index above the 95thh centile and (B) absent or reversed end-diastolic velocity (a more progressed disease state). This study aims to (1) use practice variation to compare CCS timing strategies in early-onset FGR on fetal and neonatal outcomes and (2) develop a dynamic tool to predict the time interval in days until delivery, as a novel timing strategy for antenatal CCS in early-onset FGR.
Methods and analysis
A multicentre, retrospective cohort study will be performed including pregnancies complicated by early-onset FGR in six tertiary hospitals in the Netherlands in the period between 2012 and 2021 (estimated sample size n=1800). Main exclusion criteria are multiple pregnancies and fetal congenital or genetic abnormalities. Routinely collected data will be extracted from medical charts. Primary outcome for the comparison of the two CCS timing strategies is a composite of perinatal, neonatal and in-hospital mortality. Secondary outcomes include the COSGROVE core outcome set for FGR. A multivariable, mixed-effects model will be used to compare timing strategies on study outcomes. Primary outcome for the dynamic prediction tool is ‘days until birth’.
Ethics and dissemination
The need for ethical approval was waived by the Ethics Committee (University Medical Center Utrecht). Results will be published in open-access, peer-reviewed journals and disseminated by presentations at scientific conferences.
Trial registration number
ClinicalTrials.gov: NCT05606497
Keywords: NEONATOLOGY, EPIDEMIOLOGY, OBSTETRICS
Strengths and limitations of this study
This study includes a large sample of early-onset fetal growth restriction (FGR) patients using a consensus-based and internationally accepted FGR definition.
This study uses novel techniques in prediction research to develop a dynamic prediction tool to forecast the time interval in days until birth.
The outcomes of our study are in line with landmark trials in FGR and a core outcome set for this specific patient population (COSGROVE).
Residual confounding could be a possible limitation of our observational study, caused by other (unaccounted) differences in obstetric and neonatal routine care (other than antenatal CCS timing strategies) between participating hospitals that might influence study outcome measures.
Follow-up on secondary outcomes of the offspring, including long-term follow-up, might not be complete in all patients.
Background
Early-onset fetal growth restriction (FGR) is defined as failure of a fetus to meet its growth potential, with its detection before 32 weeks of pregnancy. Early-onset FGR occurs in approximately 0.5%–1% of all pregnancies and is a notable cause of stillbirth (2%), neonatal morbidity (24%) and mortality (8%–19%).1–5 In developed countries, early-onset FGR is most commonly caused by placental dysfunction leading to unmet fetal metabolic and gaseous demands.6 7 In a prolonged and increasing hypoxic state, the anticipated risks of stillbirth rise. Active fetal surveillance of early-onset FGR pregnancies is, therefore, warranted and consists of ultrasound (fetal Doppler sonography) and analysis of the fetal heart rate pattern (cardiotocography (CTG)) to detect critical fetal hypoxia and instigate timely, often preterm delivery. Alternatively, maternal health issues can necessitate preterm delivery as early-onset FGR frequently coincides with (pre-)eclampsia.8
Antenatal corticosteroids (CCS) lower the risks of neonatal morbidity and mortality following spontaneous preterm birth.9 10 Literature suggests that antenatal CCS treatment may be most beneficial in reducing adverse neonatal outcome when a completed course of CCS (ie, two doses of betamethasone or dexamethasone at an 24-hour interval) is administered 1 to 7 days prior to birth (adjusted OR 1.46, 95% CI 1.20 to 1.77 in comparison to a time span longer than 7 days prior to birth).11 Although the clinical benefit and possible harms of antenatal CCS therapy are subject of debate in early-onset FGR, it is one of the very few antenatal treatments that can possibly improve neonatal health. Repeated courses of CCS should be avoided, as they have been associated with decreased birth weight, length, head circumference and higher rates of cerebral palsy.12 13 Therefore, adequate timing of CCS administration is likely to be important, also in the setting of early-onset FGR pregnancies when preterm birth is anticipated.
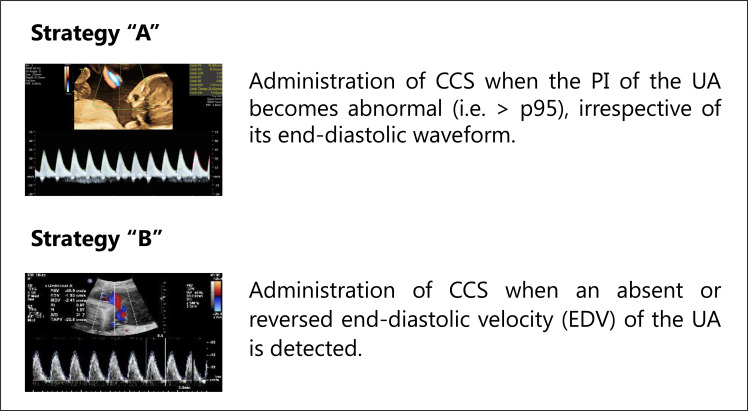
There is consensus that repetitive decelerations on the CTG-registration reflect fetal distress and an increased risk of fetal death.13 They are, thus, important triggers to initiate birth. Unfortunately, it is difficult to predict when these repetitive decelerations will occur during the period of active fetal surveillance, which makes it challenging to administer CCS within the ideal timeframe of 7 days prior to birth. International guidelines do not provide a clear directive regarding the timing of CCS treatment in early-onset FGR.1 14–16 In the Netherlands, two timing strategies regarding antenatal CCS administration in early-onset FGR are currently being practiced (figure 117):
Figure 1.
Timing strategies regarding antenatal CCS administration in early-onset FGR in the Netherlands. CCS, corticosteroids; PI, pulsatility index; UA, umbilical artery. Reference image strategy ‘B’.17
Strategy ‘A’: administration of CCS when the pulsatility index of the umbilical artery (UA) becomes abnormal (ie, >95th percentile), irrespective of its end-diastolic waveform.
Strategy ‘B’: administration of CCS when absent or reversed end-diastolic velocity of the UA is detected, thus in a more progressed disease state as compared with strategy A.
This study aims to compare these two timing strategies of antenatal CCS administration in early-onset FGR on a composite outcome of perinatal, neonatal and in-hospital mortality (definitions listed in the Methods section). With that, we aim to inform clinicians about the optimal timing management of antenatal CCS administration to improve outcomes of pregnancies complicated by early-onset FGR. In addition, we aim to develop a dynamic, prediction tool to regularly determine the time interval until birth in days during the period of active fetal surveillance. Ultimately, the use of such a dynamic risk tool could be used as an additional timing strategy for CCS treatment in early-onset FGR with the aim to improve neonatal outcome.
Methods
Study design and setting
This multicentre, retrospective cohort study is designed to mimic a cluster randomised controlled trial (RCT). The study will be performed in six tertiary teaching hospitals in the Netherlands, all equipped with a level III neonatal intensive care unit. These hospitals were selected based on their local guidelines for FGR management (ie, CCS timing strategy in early-onset FGR). The selection of these six hospitals resulted in an even distribution of the hospitals over the two CCS timing strategies (as is custom in a cluster-RCT) and a sufficient sample size of our study (see power calculation). To add, hospitals have a high adherence rate regarding the guidelines for the management of FGR pregnancies and, therefore, there is no within-hospital variation between physicians on this matter.
Patients will be included when diagnosed with early-onset FGR between 2012 and 2021. Neonates were actively managed at 24 weeks of gestational age since 2010 in the Netherlands. Therefore, and considering the learning curve neonatologists experienced in the first 2 years of this new policy, patients will be included from 2012 onwards. This study protocol was assessed by the Ethics Committee of the University Medical Center Utrecht (METC NedMec, registration number 22/613), which confirmed that the Medical Research Involving Human Subjects Act (WMO) does not apply to this study.18 In addition, the need for informed consent was waived as an exception was made in accordance with the General Data Protection Regulation as (A) processing the data is necessary with a view to scientific research; (B) the research is of public interest; (C) requesting consent requires disproportionate effort (ie, the number of patients is too high); (D) the research embodies such assurances that the privacy of the data subject will not be disproportionally harmed.19 A Data Management Plan has been drawn up and participating centres had to be rewarded with a ISO27001/NEN7510 certificate to meet the General Data Protection Regulation requirements.19 Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Study population
To be eligible for inclusion, a patient must meet all of the following criteria: (1) early-onset FGR in accordance with the consensus-based definition of Gordijn et al20; (2) Singleton pregnancy; (3) age ≥18 years; (4) consented active, neonatal management after counselling (thus having an indication for CCS administration in case of birth <34 weeks of gestational age). Exclusion criteria are (1) multiple pregnancies; (2) fetal congenital abnormalities or antenatal diagnosed genetic disorders; (3) patients who stated that their patient or offspring data may not be used for scientific research.
Data collection
Patients will be identified using parturition records. Data will be captured in a Castor electronic case report form, a Good Clinical Practice compliant Electronic Data Capture system.21
Medical records will be scrutinized to extract the patient characteristics of mothers as well as the offspring. The offspring is often transferred to a level II neonatology unit after being treated in the level III neonatal intensive care unit of the participating hospitals. To complete information on neonatal study outcomes, admission and discharge letters of these patient transfers will be used to ensure complete follow-up assessment. In addition, data collection regarding the primary outcome is safeguarded by a national registration on pregnancy outcomes (PERIDOS). Information on neurodevelopment will be obtained from follow-up assessments in the participating perinatal centres or from letters of the local paediatricians. All variables and outcomes that will be collected are summarised in table 1.
Table 1.
Patient characteristics of the mother and the offspring
| Maternal characteristics | Pregnancy characteristics | Characteristics of the offspring |
| Age | Gestational age at time of diagnosis | Sex |
| Ethnic background | Gravidity | Gestational age at birth |
| Smoking status | Parity | Birth weight |
| Drug use | Time between corticosteroid administration and birth (days) | Birth weight centile (Hoftiezer) |
| Body mass index | Mode of delivery (caesarean or vaginal) | Apgar scores at 5 min |
| Transfer to other perinatal centres before delivery | Obstetric history Previous pregnancy affected by fetal growth restriction, pre-eclampsia, (iatrogenic) preterm birth or diabetes gravidarum. |
Arterial and venous pH with base excess |
| Pre-existent disorders; chronic kidney disease; systemic lupus erythematosus; inflammatory bowel disease; antiphospholipid syndrome; diabetes; chronic hypertension; other medical disease affecting maternal or neonatal outcome |
Hypertensive disorders of pregnancy Pregnancy-induced hypertension; pre-eclampsia |
Mechanical ventilation Need for mechanical ventilation during admission, whether this was <72 hours after birth and the duration (days). |
| Other pregnancy-related disorders pregnancy cholestasis; gestational diabetes |
Perinatal, neonatal and in-hospital mortality | |
| Ultrasound-based markers (of each performed ultrasound examination) pulsatility index of umbilical artery; end-diastolic velocity waveform umbilical artery; estimated fetal weight; pulsatility index of middle cerebral artery; cerebroplacental ratio; pulsatility index of veins ductus venosus; atrial systolic velocity of ductus venosus; presence of echodense fetal bowel |
Adverse outcome measures Respiratory distress syndrome; necrotizing enterocolitis ≥2 according to the Bell’s stages; bronchopulmonary dysplasia, moderate and severe; intraventricular haemorrhage grade 3, venous infarction, posthemorrhagic ventricular dilatation; cystic periventricular leukomalacia; retinopathy of prematurity with plus disease to which treatment is needed; early and delayed neonatal sepsis, culture-proven or clinically suspected; persistent pulmonary hypertension of the newborn |
|
| Cardiotocography registration short-term variation (if available); presence of repetitive decelerations |
Duration supplemental oxygen during admission | |
| Fetal death | Long-term follow-up |
Outcomes
Objective (1) comparison of two main timing strategies of CCS in early-onset FGR
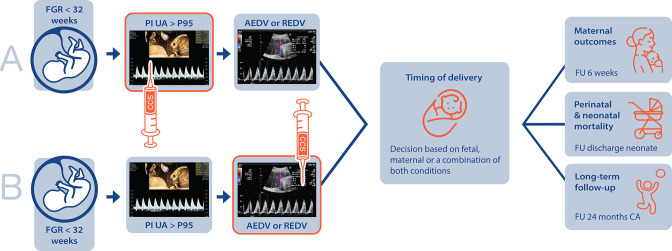
The primary outcome is defined as a composite of perinatal, neonatal and in-hospital mortality. Follow-up for this endpoint is defined as time between diagnosis of early-onset FGR and perinatal, neonatal and in-hospital mortality, or to discharge to home. Perinatal mortality will be defined as death from 22 completed weeks of gestation up to 7 days following birth, neonatal mortality as death within 28 days following birth and in-hospital mortality as death from birth up to hospital discharge of the infant.22 Secondary outcomes for this study objective are defined in accordance with the Core Outcomes Set for FGR (COSGROVE) study supplemented with other relevant maternal outcomes,23 see online supplemental file 1.24–36 Follow-up on secondary maternal outcomes ends after 6 weeks postpartum. Follow-up on offspring outcomes is extended until 2 years of corrected age (figure 217). Data regarding the long-term follow-up will be collected if available (ie, at least for children born before 30 weeks of gestational age or with a birth weight <1000 g). Follow-up management for children born after a longer pregnancy duration varies between hospitals.
Figure 2.
Study design and duration of follow-up. AEDV, absent end-diastolic velocity; CA, corrected age; CCS, corticosteroids; FGR, fetal growth restriction; FU, follow-up; PI, pulsatility index; REDV, reversed end-diastolic velocity; UA, umbilical artery. Reference image strategy ‘B’.17
bmjopen-2022-070729supp001.xlsx (14.9KB, xlsx)
Objective (2) development of a dynamic prediction tool of days until birth
We will develop a dynamic prediction model to regularly determine the time interval until birth during the period of active fetal surveillance. Such a dynamic prediction model could alert physicians about the upcoming preterm delivery and can, therefore, serve as a trigger for CCS administration. Traditionally, prediction models are based on ‘static’ information, not considering the vast amount of new information that becomes available on a daily basis. To better align with clinical care, dynamic prediction could be used, a novel technique in the risk assessment research field.37 Daily updates can be generated on the outcome of ‘days until birth’ by adding new information about maternal or fetal health, for example, retrieved by ultrasonography and CTG-registration routinely used in FGR pregnancies, to the dynamic model. This provides the physician with an up-to-date time interval assessment.
Statistical analyses
Objective (1) comparison of two main timing strategies of CCS in early-onset FGR
As our study design mimics a cluster-RCT, we will align our statistical analysis with the methods adopted by such trials. Intracluster correlation should, thus, be considered. Primary and secondary outcomes will be compared between the two timing strategies by use of the practice variation between the participating centres using a multivariable, mixed-effects model, taking hierarchy of the data into account. Important differences in routine care between the participating centres, other than the timing strategy, and between participants across the timing strategies are considered to be important confounding variables and will be adjusted for in the analyses. These differences in routine care will be identified by studying local, management protocols and by scheduling research meetings to discuss routine care in the participating centres. Adjusted ORs with 95% CIs will be calculated for dichotomous outcome measures and mean with SD will be calculated for continuous outcome measures (and median with IQR for continuous non-parametric outcomes). Timing strategy ‘A’ will be held as reference group. For secondary outcome measures, similar analyses will be performed. Exploratory subgroup analyses will be performed based on gestational age at birth (below vs above 34 weeks). The decision for this subgroup analysis was due to the fact that antenatal CCS are administered up to 34 weeks of gestation in the Netherlands. Heterogeneous treatment effects will be assessed by introducing an interaction term between the subgroup variable and the CCS treatment timing strategy to the mixed-effects model for the primary outcome. A formal test of interaction will be performed. Afterwards, the primary analysis will be repeated within each stratum of the subgroup.
Objective (2) development of a dynamic prediction tool of days until birth
To allow for dynamic prediction, information known at baseline as well as subsequent clinical and ultrasonographic information that becomes available will be used in a proportional baseline landmark supermodel, with days until birth as the outcome.38 Candidate predictors were selected based on the literature and clinical practice, summarised in table 2. For these candidate predictors, repeated measures will be gathered on the day of every follow-up ultrasonography (ie, once or two times a week). Missing data regarding possible predictors will be imputed by multiple imputation. The final set of predictors will be selected using backward stepwise elimination based on the Akaike Information Criterion. Internal validation using bootstrapping and subsequent shrinkage will be performed to account for potential overfitting. Model performance will be reported by assessing discrimination based on the c-statistic, and the calibration both visually using calibration plots and quantitatively using the calibration-in-the-large and calibration slope. The c-statistic, calibration-in-the-large and calibration slope will be determined at each time point and will be reported in a graph as a series. Statistical analyses will be conducted using the latest version of R at the time of analysis (current V.4.0.3.1.32).39
Table 2.
Candidate predictors dynamic, prediction tool
| Fetal | Maternal |
| Estimated fetal weight | Presence of hypertensive disorders of pregnancy |
| Gestational age | Use of anti-hypertensive drugs |
| Pulsatility index umbilical artery | Use of intravenous anti-hypertensive medication |
| Pulsatility index cerebral middle artery | Use of magnesium sulphate |
| Cerebroplacental ratio | Number of hypertensive crises |
| Pulsatility index of veins ductus venosus | Presence of lung oedema |
| Absence of interval growth | Progression of organ dysfunction |
| Repetitive decelerations on CTG | |
| Short-term variability | |
| Subjective fetal movements |
CTG, cardiotocography.
Sample size calculation
Objective (1) comparison of two main timing strategies of CCS in early-onset FGR
We performed a power calculation based on the fact that our study design mimics a cluster-RCT. In a cluster-RCT, the statistical power of a study is determined by among others the amount of clusters (ie, hospitals) to be included (not patients), the intracluster correlation of study outcomes and expected incidence of the primary outcome. We performed a power calculation using three clusters (ie, hospitals) per CCS timing strategy, an expected incidence of 6.8% on our primary outcome (based on the Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE)) and an intracluster correlation coefficient varying between 0.001 and 0.0091.3 40 Including patient data from six participating hospitals (three per timing strategy) will allow us to detect a range in minimal difference on the primary outcome of 1.7%–4.6% with an alpha (α) of 5% and a power (1–β) of 80%.3 41 We expect that inclusion in six hospitals over a 10-year time period will result in a total sample of approximately 1800 patients, based on the birth rates at the hospitals.
Objective (2) development of a dynamic prediction tool of days until birth
Currently, no formal sample size calculation requirements are available for dynamic prediction model development. We will use the same sample size as for objective 1. The number of candidate predictors will be based on Riley et al, using a margin of error of 5%, expected shrinkage factor of 0.9 and Cox-Snell R2 statistic of 0.099.42
Discussion
The OPtimal TIming of antenatal COrticosteroids in early-onset fetal growth REstriction study will provide a large cohort of early-onset FGR pregnancies, including patient data of six participating, tertiary hospitals in the Netherlands. The results derived from this study will likely provide the clinician with guidance on the optimal time frame for antenatal CCS administration in this patient population. With that, we aim to improve the neonatal and overall outcome for future early-onset FGR pregnancies.
There is an abundance of literature about the efficacy of antenatal CCS administration in women undergoing spontaneous preterm labour. Optimal timing of antenatal CCS administration—with a completed course between 1 and 7 days before delivery—shows the largest risk reduction for infant mortality compared with no administration of antenatal CCS (adjusted risk ratio 0.5, 95% CI 0.4 to 0.6) versus a time interval of more than 7 days till birth (adjusted risk ratio 0.7, 95% CI 0.6 to 0.9).43 Similar results were found for the outcome of severe neonatal brain injury and a composite outcome measure of mortality and/or severe neonatal morbidity.43 In addition, in a meta-analysis of 16 observational studies including mainly small-for-gestational age infants (ie, birthweight <10th centile), a significant lower neonatal mortality rate was found for infants exposed to antenatal CCS versus unexposed infants (pooled OR 0.63, 95% CI 0.46 to 0.86).44 However, strong evidence for the efficacy (or the absence of it) of antenatal CCS treatment in the setting of early-onset FGR is lacking, as no subgroup analysis has been performed on this specific population in previously performed RCTs, which would provide more robust information.9 The relative hypoxic and starved intrauterine environment in early-onset FGR likely results in higher levels of fetal endogenous steroids. It remains uncertain whether antenatal CCS administration on top of this increased fetal endogenous corticosteroid release is still of benefit.45 Nevertheless, international guidelines on FGR advise to administer antenatal CCS in pregnancies at risk for preterm birth.
Adequate timing of CCS treatment is challenging as the time interval until delivery in early-onset FGR pregnancies is difficult to predict. Risks of stillbirth or neurological impairment due to acute, on top of chronic, hypoxia have to be balanced against the risks of neonatal morbidity and mortality due to prematurity. The landmark Growth Restriction Intervention Trial (GRIT) and TRUFFLE trial, that assessed CTG and ultrasound parameters as triggers for timely delivery in FGR pregnancies, have not resulted in clear uniform recommendations on how to time delivery.3 46 In an observational study, Hecher et al described the time sequence pattern in the development of abnormalities in fetal Doppler patterns and CTG-registration.13 They included 110 cases of FGR in a prospective, longitudinal study. However, not all pregnancies complicated by early-onset FGR follow this pattern in daily practice and notably, the time line of changes in Doppler patterns until delivery especially varies between patients. Additionally, maternal factors (such as concomitant (pre-)eclampsia warranting birth) were ignored in the time sequence monitoring management summary. Consequently, due to the heterogeneity in time sequence patterns and the continuous trade-off between fetal, neonatal and maternal health, the optimal timing of delivery remains a major clinical challenge in early-onset FGR.
The ideal design to compare the two strategies for CCS administration would be a RCT. However, gathering a large enough sample for such a trial would be challenging given the low incidence of both early onset-FGR and our primary outcome. We, thus, chose to perform a retrospective cohort study over a timespan of a decade, using practice variation as an instrument to mimic a cluster-RCT. Follow-up on our primary outcome is safeguarded by a national registration on pregnancy outcomes (PERIDOS). However, achieving complete follow-up on the various other neonatal outcomes can be challenging, especially for the secondary outcome of bronchopulmonary dysplasia, as infants will be transferred from a level III neonatal intensive care unit to a level II neonatology unit when they are well enough to be discharged from the neonatal intensive care unit. To overcome this limitation, we will use discharge letters from the level II referral hospitals to complete follow-up information. Another challenge will be the patient transfers between tertiary care centres for delivery (eg, because of unavailability of capacity on the neonatal intensive care unit), as patients in our study are allocated to the centre where they give birth while their CCS were administered elsewhere. This results in cross-over between the treatment strategies in our intention-to-treat analysis. Other differences in obstetric and neonatal routine care (other than antenatal CCS timing strategies) might influence the primary and secondary outcome measures namely perinatal and neonatal mortality and morbidity. Analyses will be corrected for confounding factors, yet residual confounding could remain an issue of our study design.
Strengths of this study comprise the large sample size that will be included in the study, the use of a consensus-based definition of early-onset FGR and the collection of outcome measures according to the COSGROVE study with core outcomes for FGR.23 Also, we will use a novel and promising technique in prediction research, namely dynamic prediction.37 38 A multivariable and dynamic tool for initiation of CCS therapy might very well be superior to the use of a single-variable trigger (as used by strategies A and B) in terms of predicting the interval until birth. We will use this technique to develop an additional strategy to define the optimal time window for antenatal CCS therapy.
In summary, this large cohort of early-onset FGR pregnancies will provide important insights into the timing of antenatal CCS in pregnancies complicated by early-onset FGR. With that, we aim to reduce perinatal, neonatal and in-hospital mortality.
Ethics and dissemination
This study was submitted to the Ethics Committee of the University Medical Center Utrecht (METC NedMec, registration number 22/613), which confirmed that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study. Therefore, an official approval was not required under the WMO.18 In addition, the need for informed consent was waived as an exception was made in accordance with the General Data Protection Regulation.19 A Data Management Plan has been drawn up and participating centres had to be rewarded with a ISO27001/NEN7510 certificate to meet General Data Protection Regulation requirements.19 Results will be published in open-access, peer-reviewed journals and disseminated by presentations at scientific conferences. Data will be made available by requesting the corresponding author.
Supplementary Material
Footnotes
Contributors: JK, TL, WG, SG, FG, WO, EK, ES and MM contributed to the overall design of the study and JK is the principal investigator of the study. All authors read and approved the final version for submission.
Funding: This work was supported by Fonds Stichting Gezondheidszorg Spaarneland grant number 2021381 to J. Kooiman. This funding body was not involved in the development of the study design and will not be involved in conducting or reporting the results of the study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Melamed N, Baschat A, Yinon Y, et al. FIGO (international federation of gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet 2021;152 Suppl 1(Suppl 1):3–57. 10.1002/ijgo.13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pels A, Beune IM, van Wassenaer-Leemhuis AG, et al. Early-Onset fetal growth restriction: a systematic review on mortality and morbidity. Acta Obstet Gynecol Scand 2020;99:153–66. 10.1111/aogs.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lees C, Marlow N, Arabin B, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013;42:400–8. 10.1002/uog.13190 [DOI] [PubMed] [Google Scholar]
- 4.Lees CC, Marlow N, van Wassenaer-Leemhuis A, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015;385:2162–72. 10.1016/S0140-6736(14)62049-3 [DOI] [PubMed] [Google Scholar]
- 5.Crovetto F, Crispi F, Scazzocchio E, et al. First-trimester screening for early and late small-for-gestational-age neonates using maternal serum biochemistry, blood pressure and uterine artery doppler. Ultrasound Obstet Gynecol 2014;43:34–40. 10.1002/uog.12537 [DOI] [PubMed] [Google Scholar]
- 6.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018;218:S745–61. 10.1016/j.ajog.2017.11.577 [DOI] [PubMed] [Google Scholar]
- 7.Zur RL, Kingdom JC, Parks WT, et al. The placental basis of fetal growth restriction. Obstet Gynecol Clin North Am 2020;47:81–98. 10.1016/j.ogc.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 8.Maulik D. Fetal growth restriction: the etiology. Clin Obstet Gynecol 2006;49:228–35. 10.1097/00003081-200606000-00006 [DOI] [PubMed] [Google Scholar]
- 9.McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2020;12:CD004454. 10.1002/14651858.CD004454.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995;273:413–8. 10.1001/jama.1995.03520290065031 [DOI] [PubMed] [Google Scholar]
- 11.Melamed N, Shah J, Soraisham A, et al. Association between antenatal corticosteroid administration-to-birth interval and outcomes of preterm neonates. Obstet Gynecol 2015;125:1377–84. 10.1097/AOG.0000000000000840 [DOI] [PubMed] [Google Scholar]
- 12.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med 2007;357:1190–8. 10.1056/NEJMoa071453 [DOI] [PubMed] [Google Scholar]
- 13.Hecher K, Bilardo CM, Stigter RH, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol 2001;18:564–70. 10.1046/j.0960-7692.2001.00590.x [DOI] [PubMed] [Google Scholar]
- 14.Fetal growth restriction: ACOG practice bulletin, number 227. Obstet Gynecol 2021;137:e16–28. 10.1097/AOG.0000000000004251 Available: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/02/fetal-growth-restriction [DOI] [PubMed] [Google Scholar]
- 15.Royal College of Obstetricians and Gynaecologists . Green-top Guideline 31: the investigation and manangement of the small-for-gestational-age fetus. 2014: 1–34. Available: www.rcog.org.uk/globalassets/documents/guidelines/gtg_31.pdf [Google Scholar]
- 16.Lees CC, Stampalija T, Baschat A, et al. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020;56:298–312. 10.1002/uog.22134 [DOI] [PubMed] [Google Scholar]
- 17.Stanislavsky A. Severe IUGR with critical dopplers, Available: www.radiopaedia.org [Accessed 11 Aug 2022].
- 18.Wet medisch-wetenschappelijk onderzoek met mensen . Wettenbank Nederlandse Overheid. 2022. Available: https://wetten.overheid.nl/jci1.3:c:BWBR0009408&z=2022-07-01&g=2022-07-01 [accessed 9 Nov 2022]. [Google Scholar]
- 19.General Data Protection Regulation (GDPR),. 2018Available: https://gdpr-info.eu/ [Accessed 9 Nov 2022]. [PubMed]
- 20.Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. 10.1002/uog.15884 [DOI] [PubMed] [Google Scholar]
- 21.Castor Electronic Data Capture (EDC), Available: www.castoredc.com/electronic-data-capture-system/ [Accessed 11 Jun 2022].
- 22.World Health Organization . WHO Libr; Neonatal and Perinatal Mortality: country, regional and global estimates,. 2006Available: http://apps.who.int/iris/bitstream/handle/10665/43444/9241563206_eng.pdf;jsessionid=0C6676F5892402D63D4B6B1ECBE546C1?sequence=1 [Accessed 11 Jun 2022]. [Google Scholar]
- 23.Healy P, Gordijn SJ, Ganzevoort W, et al. A core outcome set for the prevention and treatment of fetal growth restriction: developing endpoints: the COSGROVE study. Am J Obstet Gynecol 2019;221:339. 10.1016/j.ajog.2019.05.039 [DOI] [PubMed] [Google Scholar]
- 24.Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018;72:24–43. 10.1161/HYPERTENSIONAHA.117.10803 [DOI] [PubMed] [Google Scholar]
- 25.Knight M, UKOSS . Eclampsia in the united kingdom 2005. BJOG 2007;114:1072–8. 10.1111/j.1471-0528.2007.01423.x [DOI] [PubMed] [Google Scholar]
- 26.Hoftiezer L, Hof MHP, Dijs-Elsinga J, et al. From population reference to national standard: new and improved birthweight charts. Am J Obstet Gynecol 2019;220:383.S0002-9378(18)32233-6. 10.1016/j.ajog.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 27.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- 28.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrin S, Munoz FM, Padula M, et al. Neonatal seizures: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2019;37:7596–609. 10.1016/j.vaccine.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopagondanahalli KR, Li J, Fahey MC, et al. Preterm hypoxic-ischemic encephalopathy. Front Pediatr 2016;4(OCT):114. 10.3389/fped.2016.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luca D, van Kaam AH, Tingay DG, et al. The montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med 2017;5:657–66. 10.1016/S2213-2600(17)30214-X [DOI] [PubMed] [Google Scholar]
- 32.Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 GM. J Pediatr 1978;92:529–34. 10.1016/s0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Biarge M, Groenendaal F, Kersbergen KJ, et al. Neurodevelopmental outcomes in preterm infants with white matter injury using a new MRI classification. Neonatology 2019;116:227–35. 10.1159/000499346 [DOI] [PubMed] [Google Scholar]
- 34.Bayley N. The Bayley scales of infant and toddler development. San Antonio, TX: Harcourt Assessment, Inc, 2006. [Google Scholar]
- 35.van Baar A, Steenis L, Verhoeven M, et al. Bayley-III-NL; technische handleiding. Amsterdam, the Netherlands: Pearson Assessment and Information B.V, 2014. [Google Scholar]
- 36.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 2005;47:571–6. 10.1017/s001216220500112x [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DA, Sperrin M, Martin GP, et al. Dynamic models to predict health outcomes: current status and methodological challenges. Diagn Progn Res 2018;2:23. 10.1186/s41512-018-0045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontein DBY, Klinten Grand M, Nortier JWR, et al. Dynamic prediction in breast cancer: proving feasibility in clinical practice using the team trial. Ann Oncol 2015;26:1254–62. 10.1093/annonc/mdv146 [DOI] [PubMed] [Google Scholar]
- 39.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. Available: https://www.r-project.org [Google Scholar]
- 40.Gulliford MC, Adams G, Ukoumunne OC, et al. Intraclass correlation coefficient and outcome prevalence are associated in clustered binary data. J Clin Epidemiol 2005;58:246–51. 10.1016/j.jclinepi.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 41.Hemming K, Girling AJ, Sitch AJ, et al. Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol 2017;17:8. 10.1186/s12874-017-0292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model [BMJ [online]]. BMJ 2020;368:m441. 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 43.Norman M, Piedvache A, Børch K, et al. Association of short antenatal corticosteroid administration-to-birth intervals with survival and morbidity among very preterm infants: results from the EPICE cohort. JAMA Pediatr 2017;171:678–86. 10.1001/jamapediatrics.2017.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blankenship SA, Brown KE, Simon LE, et al. Antenatal corticosteroids in preterm small-for-gestational age infants: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100215. 10.1016/j.ajogmf.2020.100215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. Am J Obstet Gynecol 2018;218:S818–28. 10.1016/j.ajog.2017.12.227 [DOI] [PubMed] [Google Scholar]
- 46.Walker D-M, Marlow N, Upstone L, et al. The growth restriction intervention trial: long-term outcomes in a randomized trial of timing of delivery in fetal growth restriction. Am J Obstet Gynecol 2011;204:34. 10.1016/j.ajog.2010.09.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070729supp001.xlsx (14.9KB, xlsx)