Abstract
Objectives
Multimorbidity is defined as the coexistence of two or more chronic physical or psychological conditions within an individual. The association between maternal multimorbidity and adverse perinatal outcomes such as preterm delivery and low birth weight has not been well studied. Therefore, this study aimed to investigate this association.
Methods
We conducted a prospective cohort study using data from the Japan Environment and Children’s Study of pregnant women between 2011 and 2014. Those with data on chronic maternal conditions were included in the study and categorised as having no chronic condition, one chronic condition or multimorbidities. The primary outcomes were the incidence of preterm birth (PTB), low birth weight (LBW) and small for gestational age (SGA). Adjusted logistic regression was performed to estimate ORs (aORs) and 95% CIs.
Results
Of the 104 062 fetal records, 86 885 singleton pregnant women were analysed. The median maternal age and body mass index were 31 years and 20.5 kg/m2, respectively. The prevalence of pregnant women with one or more chronic conditions was 40.2%. The prevalence of maternal multimorbidity was 6.3%, and that of PTB, LBW, and SGA were 4.6%, 8.1%, and 7.5%, respectively. Pre-pregnancy underweight women were the most common, observed in 15.6% of multimorbidity cases, followed by domestic violence from intimate partner in 13.0%. Maternal multimorbidity was significantly associated with PTB (aOR 1.50; 95% CI 1.33–1.69), LBW (aOR 1.49; 95% CI 1.35–1.63) and SGA (aOR 1.33; 95% CI 1.20–1.46).
Conclusion
Maternal multimorbidity was associated with adverse perinatal outcomes, including PTB, LBW and SGA. The risk of adverse perinatal outcomes tends to increase with a rise in the number of chronic maternal conditions. Multimorbidity becomes more prevalent among pregnant women, making our findings important for preconception counselling.
Keywords: epidemiology, obstetrics, fetal medicine, maternal medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Including a wide variety of chronic conditions concerning maternal health and well-being makes the study more comprehensive.
The study size is robust enough to investigate preterm birth, low birth weight and small gestational age; however, the numbers of secondary outcomes such as very preterm birth, very low birth weight and extremely low birth weight are too small to have enough statistical power.
Lack of information on the severity of maternal morbidity is a limitation.
Some self-reported maternal conditions, including domestic and substance abuse, may be under-reported.
Introduction
Multimorbidity is usually defined as the coexistence of two or more chronic physical or psychological conditions within an individual.1 2 Multimorbidity has attracted worldwide attention because of the increased complexity of treatment, consumption of medical care and healthcare costs compared with a single disease.1 3–5 Multimorbidity is also associated with high mortality, functional disability and diminished quality of life.6–8 For many countries, there is evidence that a significant proportion of adult population suffers from multiple chronic diseases, and the rates are increasing.2 9 However, the lack of uniform definitions and classifications of multimorbidity makes it difficult to ascertain their actual status. Consequently, the existing evidence is fragmented and often difficult to interpret.2
Although the prevalence of multimorbidity is highest in those aged 65 years or older, younger persons, including women of reproductive age, also represent a large proportion of those with multimorbidity, ranging from 8.7% to 18.8%.3 4 10 11 Regarding pregnant women, the prevalence of multimorbidity has varied from 0.83% to 24.2%.12–14 Although the prevalence of maternal multimorbidity in Japan has not been thoroughly studied, the prevalence of individual chronic conditions was 0.9% for chronic hypertension, 3.4% for diabetes mellitus, 10.6% for obesity and 18.2% for underweight.15 16 The number of pregnant women with multimorbidity is expected to increase as with maternal population ages.
The association between certain specific single maternal chronic diseases and related perinatal outcomes has been well studied; however, there are only a few studies on maternal multimorbidity and perinatal outcomes.12 13 17 The 2020 systematic review in low/middle-income countries by McCauley et al classified maternal multimorbidity into three categories: physical morbidity (such as medical, infectious and obstetrical conditions), psychological morbidity (such as depression and suicidal ideation) and social morbidity (such as domestic violence and substance abuse).17 The WHO Maternal Morbidity Working Group also considered physical, psychological and social morbidity to measure maternal morbidity.18 Domestic violence against pregnant women has also been a social issue in Japan,19 not only in low/middle-income countries.
It has been widely known that maternal physical morbidity such as hypertension, kidney disease and systemic lupus erythematosus increase the risk of preterm births (PTBs) and low birthweight (LBW) infants.20–23 Moreover, maternal psychological and social morbidity have been also associated with PTB and LBW.24–29 In their review, McCauley et al were trying to assess the impact of each type of morbidity on women’s health and well-being during pregnancy and after childbirth; however, a meta-analysis of the associations between multimorbidity and obstetrical complications could not be performed because of the heterogeneity of each study.17 The evidence of maternal multimorbidity has been insufficient because only a few studies reported the association between maternal multimorbidity and adverse perinatal outcomes.12 13 Additionally, a previous study in the USA by Admon et al underestimated the prevalence of maternal multimorbidity because only eight chronic conditions were included.13 There is a need to conduct a study covering a broad range of maternal chronic conditions to avoid overlooking pregnant women at risk of potentially adverse perinatal outcomes. Therefore, we hypothesised that the risk of adverse perinatal outcomes, such as PTB, LBW and small for gestational age (SGA), would increase with the number of chronic maternal conditions present in a woman, including physical, psychological and social conditions.
In Japan, the association between maternal multimorbidity and adverse perinatal outcomes has not yet been investigated. The present study aimed to determine the association between maternal multimorbidity and adverse perinatal outcomes, such as PTB, LBW and SGA, using a Japanese nationwide prospective cohort study.
Methods
Study design and participants
This prospective cohort study used the dataset jecs-ta-20190930 from the Japan Environment and Children’s Study (JECS). The JECS is an ongoing nationwide birth cohort study in Japan, the details of which have already been reported.30 31 The main aim of the JECS was to investigate the association between environmental factors and children’s health and development. The JECS recruited pregnant women from 15 regional centres selected to cover Japan’s geographical areas: Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka and South Kyusyu/Okinawa, between January 2011 and March 2014. The recruitment strategy in the JECS is shown in online supplemental appendix 1.30
bmjopen-2022-069281supp001.pdf (155.1KB, pdf)
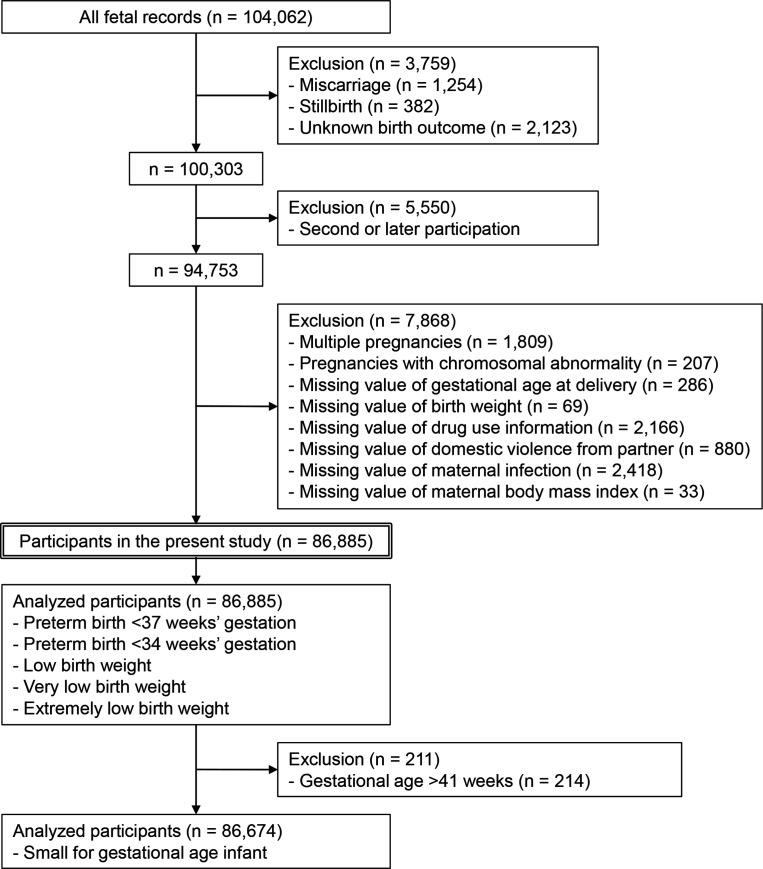
During the study period, 104 062 fetal records were included in the baseline survey of the JECS. Miscarriages (n=1254), stillbirths (n=382) and unknown birth outcomes (n=2123) were excluded, leaving 100 303 live births in the present study. When a mother had more than one pregnancy, only the first pregnancy was included in this study. After excluding pregnancies in the same mothers, there were 94 753 participants. Finally, patients with multiple pregnancies (n=1809), pregnancies with chromosomal abnormalities (n=207), missing values of gestational age at delivery (n=286), missing values of neonatal birth weight (n=69), missing values of medication information (n=2166), missing values of domestic violence from intimate partners (n=880), missing values of maternal infection (n=2418) and missing values of maternal pre-pregnancy body mass index (BMI) (n=33) were excluded, and the final number of study participants was 86 885 singleton pregnant women (figure 1). In the SGA analyses, the number of participants decreased to 86 674 because 211 deliveries at a gestational age of >41 weeks were excluded (figure 1).
Figure 1.
Flow diagram of the study participants.
Patient and public involvement
This study did not involve patients or the public.
Maternal and neonatal baseline information
Baseline information on the mothers, including educational level, smoking status and alcohol consumption, was collected from self-administered questionnaires applied to the enrolled pregnant women during the second or third trimesters. Maternal medical history was obtained using self-administered questionnaires and medical record transcripts during the first trimester of pregnancy. A history of domestic violence from an intimate partner was obtained from self-administered questionnaires applied to the enrolled pregnant women during the first trimester. The following maternal information was obtained from the medical record transcripts: maternal infection, neonate’s date of birth, parity and gestational period. Although maternal pre-pregnancy height and weight were collected from medical record transcripts, in instances where the above information was missing, the values were obtained from mothers’ self-reports. Maternal age at delivery was calculated from the birth dates of mothers and neonates. Parity was categorised as 0, 1, 2 or higher. The categories of maternal smoking status were defined as follows: never; previously did, but quit before recognising the current pregnancy; previously did, but quit after identifying the current pregnancy; and current smoker. The categories of maternal alcohol consumption were defined as follows: never consumed; previously consumed, but quit before identifying current pregnancy; previously consumed, but quit after identifying current pregnancy; and current drinker. The highest educational level of the mother was defined as follows: junior high school, high school, technical junior college, technical/vocational college or associate degree, bachelor’s degree or graduate degree (master/doctor). Annual household income was categorised as follows: <2 000 000; 2 000 000–3 990 000; 4 000 000–5 990 000; 6 000 000–7 990 000; 8 000 000–9 990 000 and >10 000 000 Japanese yen. Maternal and neonatal medical information, such as maternal age at delivery, gestational age at delivery, neonatal birth weight and neonatal sex, were collected from the medical record transcripts at birth.
Exposure: maternal multimorbidity
In our study, multimorbidity was defined as the coexistence of two or more physical, psychological or social conditions in an individual according to previous reports.13 14 17 18 Maternal chronic conditions included in multimorbidity were defined as the conditions with high prevalence among women of reproductive age or that had the potential to affect perinatal outcomes. The chronic conditions in this study were heterogeneous because the JECS lacked information regarding the disease severity. However, the definition of multimorbidity varies among studies.18 32 To identify pregnant women with chronic conditions more rigorously, a maternal chronic condition was defined as a condition that was medically treated at the time of pregnancy. This information was collected from self-reports, medical record transcripts and medication interviews. Maternal chronic conditions included allergic diseases such as asthma, anaemia, diabetes mellitus, dyslipidaemia, epilepsy, gastric or duodenal ulcer, heart disease, hepatitis, HIV infection, hypertension, inflammatory bowel disease, kidney disease, malignancy, migraine, neurological disease, other sexually transmitted diseases (Chlamydia trachomatis and syphilis), psychiatric disorders, rheumatic or collagen diseases, and thyroid disease.
Additionally, abnormal pre-pregnancy BMI, including underweight and obesity, physical or verbal domestic violence from intimate partners, and substance abuse were included in the maternal chronic conditions.18 33 Maternal pre-pregnancy BMI was calculated from the maternal pre-pregnancy weight divided by the square of maternal height collected from medical record transcripts or self-reports. Pregnant women were categorised according to their pre-pregnancy BMI as follows: underweight (BMI <18.5 kg/m2), normal weight (18.5 kg/m2≤BMI<25.0 kg/m2) and obesity (BMI >25.0 kg/m2).34 35 Information on domestic violence from intimate partners was obtained from a questionnaire administered during the first trimester: ‘Have you been verbally insulted or yelled at by your partner during pregnancy?’ and ‘Have you been physically abused, such as being slapped or beaten, resulting in injury because of a quarrel between you and your partner during pregnancy?’36 Each response was selected from one of four predefined categories: never, rarely, sometimes and often. If the response ‘rarely’ or ‘sometimes’ or ‘often’ was chosen, it was considered as presence of domestic violence. Medication information was obtained from interviews (online supplemental appendix 2).37 Medication was considered relevant from pregnancy diagnosis to 12 weeks of gestation to investigate the impact of maternal pre-pregnancy chronic conditions on perinatal outcomes. The types of medication in early pregnancy included antiallergic drugs, lipid-lowering drugs, antimigraine drugs, anti-parkinsonian drugs, antirheumatic drugs, antithyroid drugs, antiviral drugs, anticancer drugs, cardiovascular drugs, corticosteroids, gastrointestinal drugs, illegal drugs including marijuana, psychostimulant, ecstasy, thinner and toluene, insulin preparations, iron preparations, psychoactive drugs, respiratory drugs and thyroid hormone preparations.37
Outcomes
The primary outcome of this study was the incidence of adverse perinatal outcomes, including PTB, LBW and SGA. The secondary outcomes were the incidences of very preterm birth (VPTB), very low birth weight (VLBW) and extremely low birth weight (ELBW). PTB was defined as a gestational age of less than 37 weeks at delivery. VPTB was defined as a gestational age of less than 34 weeks at delivery. LBW, VLBW and ELBW were defined as neonatal birth weights of less than 2500 g, 1500 g and 1000 g, respectively. SGA was defined as birth weight below the 10th percentile, accounting for parity, gestational age and neonatal sex according to the Japan Pediatric Society guidelines,38 and percentiles were calculated using Excel-based clinical tools for growth evaluation of children distributed by the Japanese Society for Pediatric Endocrinology.39
Statistical analysis
In the main analyses, adjusted ORs (aORs) and 95% CIs for adverse perinatal outcomes were estimated using a multivariable logistic regression model adjusted for maternal age at delivery (<20, 20–24, 25–29, 30–34, 35–39 and >40 years), parity, maternal smoking status, maternal alcohol consumption, maternal educational level, household income and neonatal sex. In the SGA analyses, parity and neonatal sex were removed from the covariates. These covariates were selected based on previous studies on multimorbidity.13 18 33 Statistical analyses were used to compare pregnant women with multimorbidity or with one chronic condition, with those without chronic conditions as a reference group.
We used the k-nearest neighbour (kNN) imputation method in the R package ‘VIM’ (V.4.1.2; R Foundation for Statistical Computing, Vienna, Austria),40 introducing all outcomes and adjusted variables because the dataset had some missing values. The covariates, such as maternal age, parity, smoking status during pregnancy, drinking status during pregnancy, maternal education, household income and neonatal sex, were among the imputed missing data. kNN is a widely accepted single imputation method whose validity has been established.41 We performed an additional analysis to test the robustness of our findings using a complete dataset, which excluded all missing values. We also analytically evaluated the dose–response relationship between the number of chronic conditions and perinatal adverse outcomes using a detailed classification of the number of chronic conditions (0, 1, 2, 3 and >4) and a test for trend. Sensitivity analyses focusing on underweight, obesity, psychiatric disorders and domestic violence were performed. Each of these chronic conditions was categorised separately as included or not included in the multimorbidity category. The results of these analyses are shown in online supplemental tables 2-4. Statistical significance was defined as a two-tailed p<0.05. All analyses, except kNN imputation, were performed using STATA V.16.1, for Windows (Stata Corporation, College Station, Texas, USA). kNN imputation was conducted using R (V.4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 104 062 fetal records included in this study, 17 177 (16.5%) were excluded, leaving a final number of 86 885 singleton pregnant women (figure 1). The main and all maternal characteristics are shown in table 1 and online supplemental table 1, respectively. The median maternal age was 31 years (range, 14–48), and the median pre-pregnancy BMI was 20.5 kg/m2 (range, 13.2–52.8). In the present study, 40.2% of pregnant women had one or more chronic conditions (table 1). The prevalence of maternal multimorbidity was 6.3% (5462 of 86 885). Regarding perinatal outcomes, the prevalence of PTB and VPTB was 4.6% and 1.0%, respectively. The prevalence of LBW, VLBW, ELBW and SGA was 8.1%, 0.6%, 0.2%, and 7.5%, respectively (table 2). The details of the maternal chronic conditions are shown in table 3. Maternal underweight (15.6%) was the most frequently observed in chronic conditions, followed by domestic violence (13.0%). The prevalence of maternal obesity was 10.9%. The other most frequent chronic conditions were allergic diseases (3.1%), other sexually transmitted diseases (1.4%), anaemia (0.7%), psychiatric disorders (0.7%) and thyroid disease (0.7%).
Table 1.
Main maternal characteristics (n=86 885)
| Characteristics | Total | The number of chronic conditions | ||
| 0 (n=51 964) | 1 (n=29 459) | ≥2 (n=5462) | ||
| Maternal age (years) | ||||
| ≤24 | 8599 (9.9) | 4451 (8.6) | 3342 (11.3) | 806 (14.8) |
| 25–29 | 23 873 (27.5) | 14 205 (27.3) | 8232 (27.9) | 1436 (26.3) |
| 30–34 | 30 686 (35.3) | 18 776 (36.1) | 10 142 (34.4) | 1768 (32.4) |
| 35–39 | 19 703 (22.7) | 12 121 (23.3) | 6381 (21.7) | 1201 (22.0) |
| ≥40 | 4018 (4.6) | 2409 (4.6) | 1359 (4.6) | 250 (4.6) |
| Missing | 6 (0.01) | 2 (0.00) | 3 (0.01) | 1 (0.02) |
| Body mass index (kg/m2) | 20.5 (13.2–52.8) | 20.7 (18.5–24.9) | 20.0 (13.2–48.8) | 19.5 (13.3–52.8) |
| Parity | ||||
| 0 | 35 973 (41.4) | 21 952 (42.2) | 11 972 (40.6) | 2049 (37.5) |
| 1 | 31 810 (36.6) | 19 030 (36.6) | 10 750 (36.5) | 2030 (37.2) |
| ≥2 | 17 040 (19.6) | 9691 (18.7) | 6059 (20.6) | 1290 (23.6) |
| Missing | 2062 (2.4) | 1291 (2.5) | 678 (2.3) | 93 (1.7) |
| Smoking during pregnancy | ||||
| No | 49 414 (56.9) | 31 028 (59.7) | 15 937 (54.1) | 2449 (44.8) |
| Quit before pregnancy | 20 152 (23.2) | 12 094 (23.3) | 6777 (23.0) | 1281 (23.5) |
| Quit after pregnancy | 12 042 (13.9) | 6320 (12.2) | 4613 (15.7) | 1109 (20.3) |
| Yes | 3859 (4.4) | 1757 (3.4) | 1604 (5.4) | 498 (9.1) |
| Missing | 1418 (1.6) | 765 (1.5) | 528 (1.8) | 125 (2.3) |
| Drinking during pregnancy | ||||
| No | 28 652 (33.0) | 16 928 (32.6) | 9957 (33.8) | 1767 (32.4) |
| Quit before pregnancy | 14 108 (16.2) | 8321 (16.0) | 4832 (16.4) | 955 (17.5) |
| Quit after pregnancy | 40 354 (46.5) | 24 554 (47.3) | 13 378 (45.4) | 2422 (44.3) |
| Yes | 2370 (2.7) | 1386 (2.7) | 771 (2.6) | 213 (3.9) |
| Missing | 1401 (1.6) | 775 (1.5) | 521 (1.8) | 105 (1.9) |
Values are presented as n (%) or median (range: min–max).
Table 2.
Perinatal characteristics (n=86 885)
| Outcomes | Total | The number of chronic conditions | ||
| 0 (n=51 964) | 1 (n=29 459) | ≥2 (n=5462) | ||
| Gestational age at delivery (weeks) | 39 (22–43) | 39 (23–42) | 39 (22–42) | 39 (23–43) |
| Birth weight (g) | 3028 (312–5214) | 3038 (312–4700) | 3016 (350–5214) | 2998 (398–4568) |
| Neonatal sex | ||||
| Male | 44 628 (51.4) | 26 577 (51.2) | 15 225 (51.7) | 2826 (51.7) |
| Female | 42 252 (48.6) | 25 385 (48.8) | 14 233 (48.3) | 2634 (48.2) |
| Missing | 5 (0.01) | 2 (0.00) | 1 (0.00) | 2 (0.04) |
| Preterm birth (<37 weeks’ gestation) | 3963 (4.6) | 2125 (4.1) | 1498 (5.1) | 340 (6.2) |
| Very preterm birth (<34 weeks’ gestation) | 834 (1.0) | 441 (098) | 328 (1.1) | 65 (1.2) |
| Low birth weight (<2500 g) | 7013 (8.1) | 3775 (7.3) | 2661 (9.0) | 577 (10.6) |
| Very low birth weight (<1500 g) | 483 (0.6) | 248 (0.5) | 193 (0.7) | 42 (0.8) |
| Extremely low birth weight (<1000 g) | 205 (0.2) | 108 (0.2) | 76 (0.3) | 21 (0.4) |
| Small for gestational age | ||||
| No | 78 138 (89.9) | 46 975 (90.4) | 26 322 (89.4) | 4841 (88.6) |
| Yes | 6474 (7.5) | 3574 (6.9) | 2388 (8.1) | 512 (9.4) |
| Missing | 2273 (2.6) | 1415 (2.7) | 749 (2.5) | 109 (2.0) |
Values are presented as n (%) or median (range: min–max).
Table 3.
Prevalence of 23 maternal chronic conditions (n=86 885)
| Condition | n (%) |
| Abnormal pre-pregnancy BMI | |
| Underweight (BMI <18.5 kg/m2) | 13 533 (15.6) |
| Obesity (BMI >25.0 kg/m2) | 9461 (10.9) |
| Allergic disease | 2680 (3.1) |
| Anaemia | 628 (0.7) |
| Diabetes mellitus | 128 (0.2) |
| Domestic violence | 11 261 (13.0) |
| Dyslipidaemia | 6 (0.01) |
| Epilepsy | 133 (0.2) |
| Gastric or duodenal ulcer | 298 (0.3) |
| Heart disease | 7 (0.01) |
| Hepatitis | 5 (0.01) |
| HIV infection | 7 (0.01) |
| Hypertension | 96 (0.1) |
| Inflammatory bowel disease | 16 (0.02) |
| Kidney disease | 17 (0.02) |
| Malignancy | 0 (0) |
| Migraine | 44 (0.1) |
| Neurological disease | 0 (0) |
| Other sexually transmitted diseases | 1193 (1.4) |
| Psychiatric disorder | 604 (0.7) |
| Rheumatic or collagen disease | 94 (0.1) |
| Substance abuse | 1 (0.0) |
| Thyroid disease | 638 (0.7) |
BMI, body mass index.
Maternal multimorbidity was significantly associated with PTB (aOR 1.50; 95% CI 1.33–1.69), VPTB (aOR 1.34; 95% CI 1.03–1.74), LBW (aOR 1.49; 95% CI 1.35–1.63), VLBW (aOR 1.62; 95% CI 1.16–2.25), ELBW (aOR 1.81; 95% CI 1.12–2.90) and SGA (aOR 1.33; 95% CI 1.20–1.46) (table 4). aORs of PTB, LBW and SGA tended to increase with multimorbidity rather than with one chronic condition.
Table 4.
Crude and adjusted ORs of maternal chronic conditions for adverse perinatal outcomes (n=86 885)
| Outcome | The number of chronic conditions | ||
| 0 | 1 | ≥2 | |
| PTB | |||
| N (%) | 2125 (4.1) | 1498 (5.1) | 340 (6.2) |
| Crude OR (95% CI) | Reference | 1.26 (1.17–1.34) | 1.56 (1.38–1.75) |
| Adjusted OR (95% CI) | Reference | 1.24 (1.16–1.33) | 1.50 (1.33–1.69) |
| VPTB | |||
| N (%) | 441 (0.9) | 328 (1.1) | 65 (1.2) |
| Crude OR (95% CI) | Reference | 1.32 (1.14–1.52) | 1.41 (1.08–1.83) |
| Adjusted OR (95% CI) | Reference | 1.29 (1.12–1.49) | 1.34 (1.03–1.74) |
| LBW | |||
| N (%) | 3775 (7.3) | 2661 (9.0) | 577 (10.6) |
| Crude OR (95% CI) | Reference | 1.27 (1.20–1.33) | 1.51 (1.37–1.65) |
| Adjusted OR (95% CI) | Reference | 1.27 (1.20–1.34) | 1.49 (1.35–1.63) |
| VLBW | |||
| N (%) | 248 (0.5) | 193 (0.7) | 42 (0.8) |
| Crude OR (95% CI) | Reference | 1.38 (1.14–1.66) | 1.61 (1.16–2.24) |
| Adjusted OR (95% CI) | Reference | 1.39 (1.15–1.67) | 1.62 (1.16–2.25) |
| ELBW | |||
| N (%) | 108 (0.2) | 76 (0.3) | 21 (0.4) |
| Crude OR (95% CI) | Reference | 1.24 (0.93–1.67) | 1.85 (1.16–2.96) |
| Adjusted OR (95% CI) | Reference | 1.24 (0.92–1.67) | 1.81 (1.12–2.90) |
| SGA* | |||
| N (%) | 3685 (7.1) | 2443 (8.3) | 522 (9.6) |
| Crude OR (95% CI) | Reference | 1.18 (1.12–1.25) | 1.39 (1.26–1.52) |
| Adjusted OR (95% CI) | Reference | 1.17 (1.11–1.23) | 1.33 (1.20–1.46) |
OR, odds ratio compared with that of infants of mothers without chronic conditions, adjusted for maternal age at delivery, parity (except for SGA analysis), maternal smoking status, maternal alcohol consumption, maternal educational background and neonatal sex (except for SGA analysis).
Adjusted ORs with statistical significance are indicated in bold font.
*The total number of participants was 86 674.
ELBW, extremely low birth weight (<1000 g); LBW, low birth weight (<2500 g); PTB, preterm birth before 37 weeks of gestation; SGA, small for gestational age; VLBW, very low birth weight (<1500 g); VPTB, preterm birth before 34 weeks of gestation.
The association between maternal multimorbidity and adverse perinatal outcomes, using the complete dataset, is shown in online supplemental table 2. Maternal multimorbidity was also significantly associated with PTB, LBW and SGA; however, it was not associated with VPTB, VLBW and ELBW. Additionally, the aORs of PTB, LBW and SGA also tended to increase with multimorbidity rather than with one chronic condition.
Online supplemental table 3 shows the additional analysis for PTB, LBW and SGA using exposure as the number of chronic conditions (0, 1, 2, 3 and >4). All of the trend p values were statistically significant.
Online supplemental table 4 demonstrates the aOR for adverse perinatal outcomes, focusing on each disease, including underweight, obesity, psychiatric disorders and domestic violence. Regarding PTB and LBW, significant differences were found between maternal multimorbidity and no chronic conditions, regardless of the presence or absence of specific chronic conditions (online supplemental table 4A–D). For SGA, maternal multimorbidity with underweight, without obesity, without psychiatric disorder, and with and without domestic violence showed significant differences compared with no chronic conditions (online supplemental table 4A–D).
Discussion
In this study, one-third of pregnant women had one or more chronic conditions, and the prevalence of maternal multimorbidity was lower than that in previous studies.3 4 Among maternal chronic conditions, pre-pregnancy underweight was the most common, followed by domestic violence and pre-pregnancy obesity. This study showed that maternal multimorbidity was significantly associated with PTB, LBW and SGA. The number of chronic conditions in the mother tends to increase the risk of adverse perinatal outcomes.
To our knowledge, the present study is the first to investigate the association between maternal multimorbidity, including physical, psychological and social morbidities, with perinatal outcomes. In the present study, the use of data from the JECS, including 100 000 Japanese mothers and neonates, made it possible to study multimorbidities. The incidence of adverse perinatal outcomes was sufficient to investigate the influence of maternal multimorbidity on PTB, LBW and SGA.
However, this study has several limitations. First, the prevalence of maternal multimorbidity in this study was 6.3%, which was lower than the prevalence noted in reproductive-aged women in previous studies.3 4 14 The strict definition of a chronic condition in our study may be responsible for the lower prevalence of maternal multimorbidity than in previous studies. However, there is no consensus on the definition of maternal multimorbidity or classification system for reporting.2 32 Second, the details of the differences in risk by the combination of chronic conditions were not identified. The risk of adverse perinatal outcomes may vary depending on the combination of the chronic conditions. Although sensitivity analyses focusing on underweight, obesity, psychiatric disorders and domestic violence demonstrated the association of adverse perinatal outcomes with the presence or absence of each condition, no further detailed studies were conducted because the main aim of this study was not to examine the impact of each chronic condition. Third, some self-reported biases may exist. Self-reported body weight may be underestimated for underweight and overestimated for obesity.42 43 The prevalence of self-reported domestic violence may be underestimated due to social desirability bias.44 Fourth, the numbers of VPTB, VLBW and ELBW cases were small. This study was insufficient to investigate these severe adverse outcomes.
Conducting studies on maternal multimorbidity has been challenging because there is no agreed definition or uniform measurement tool for multimorbidity.32 A systematic review of multimorbidity in 2012 by Fortin et al 3 reported that the prevalence of multimorbidity seemed to be influenced by the operational definition of chronic conditions. Moreover, most studies on multimorbidity conducted in the general population predominantly used questionnaires. As this method was based on self-report, it might present the disadvantage of assigning equal weight to both major and minor chronic conditions.3 Although the present study also used self-report questionnaires, multimorbidity was defined as chronic conditions treated with medication to reduce the variability in the severity of chronic conditions in multimorbidity. However, our method may have decreased the prevalence of multimorbidities.
This study comprised 23 chronic conditions, which included not only physical morbidity, but also psychological and social morbidity. A systematic review of multimorbidity by Fortin et al 3 suggested using a list of at least the 12 most prevalent chronic conditions to conduct studies on multimorbidity. In a previous study by Admon et al 13 on maternal multimorbidity, only seven physical and one social morbidity were defined as chronic conditions, and the prevalence of one chronic condition and multimorbidity was 8.4% and 0.83%, respectively. These values are much lower than those of our results. Although it has been controversial which chronic conditions should be included in maternal multimorbidity, our study may have reduced the chance of missing pregnant women at a potential risk of adverse perinatal outcomes.
The present study confirmed that an increase in the number of chronic maternal conditions increases the risk of PTB, LBW and SGA. In a study on chronic diseases in pregnant women in Germany, pregnant women with at least one chronic condition had an increased risk of PTB.12 A study on maternal multimorbidity in the USA reported that the incidence of PTB less than 37 weeks of gestation, caesarean delivery, severe maternal morbidity and mortality in pregnant women with multimorbidity was higher than those without chronic conditions.13 These results were consistent with the present study, although the definition of maternal chronic conditions was different from our study.
In maternal multimorbidity, medication during pregnancy may affect perinatal outcomes. The present study defined a physical or psychological condition as one that required medical attention during pregnancy. The study on the exposure to medication for hypertension, diabetes and autoimmune disease during pregnancy reported that the ORs of PTB, LBW and SGA were higher in the antihypertensive and corticosteroid-exposed group compared with those in the unexposed group.45 However, it was also reported that chronic conditions, with or without medication exposure, may have affected perinatal outcomes.
Chronic physical conditions such as hypertension, kidney disease, systemic lupus erythematosus and abnormal pre-pregnancy BMI are associated with adverse perinatal outcomes.20–23 46 47 Our findings also indicated that multimorbidity might alter the risk of PTB, LBW and SGA depending on whether multimorbidity included or did not include abnormal BMI. Additionally, maternal infections, such as HIV, malaria, syphilis and tuberculosis, have been reported to be associated with adverse perinatal outcomes.48–51 However, the influence of these infections may be small in the present study because the prevalence of these maternal infections was very low in Japan.
Although the prevalence of psychiatric disorders was 0.7% in the present study, which was very low compared with a previous systematic review of adult women,52 maternal psychological morbidity was also associated with adverse perinatal outcomes.25 26 52 The present study also showed that the risk of PTB and SGA might vary depending on the presence or absence of psychiatric disorders. In previous studies on depression during pregnancy, depression was found to be associated with PTB, LBW and SGA.25 26 In addition, common antenatal mental disorders also increased the risk of PTB and LBW.53
In the present study, domestic violence from intimate partners was the second most frequent chronic condition at 13.0%. Multimorbidity with and without domestic violence was significantly associated with PTB, LBW and SGA, although the point estimate of the aOR for multimorbidity with domestic violence was slightly smaller than the aOR for those without domestic violence in this study. Social morbidity, including domestic violence, has been highlighted as a risk factor associated with adverse perinatal outcomes.17 28 29 A cross-sectional study in Iran found a significant association between intimate partner violence during pregnancy and PTB.28 A prospective cohort study in Tanzania reported that physical violence during pregnancy significantly increased the risk of PTB and LBW.29 However, a population-based study in Canada reported no association between domestic violence before and during pregnancy and adverse perinatal outcomes, including PTB and SGA.54 This previous study had the limitation that the prevalence of domestic violence was 10.9% and only 3.3% occurred during pregnancy.54
The mechanism by which maternal multimorbidity affected perinatal outcomes was not clear in the present study. However, maternal multimorbidity appears to affect perinatal outcomes as both an intermediate and direct factor. For example, abnormal maternal BMI, such as underweight and obesity, is known to be an independent risk factor for PTB.55 Maternal obesity is also a risk factor for pre-eclampsia.56 Furthermore, pre-eclampsia promotes the development of PTB. After all, both maternal obesity and pre-eclampsia are regarded as risk factors for PTB. PTB is considered a syndrome initiated by multiple mechanisms, including infection or inflammation, uteroplacental ischaemia or haemorrhage, uterine overdistension, stress and other immunologically mediated processes.57 In addition, the associations between other chronic diseases such as hypertension, kidney disease, and autoimmune disease and PTB are similar to the association between obesity and PTB.20–23 Therefore, we hypothesised that each chronic condition that composes multimorbidity, such as underweight, obesity, psychiatric disorder and domestic violence, played an intermediate or direct role in perinatal outcomes, and that the combination of these chronic conditions might further increase the risk of adverse perinatal outcomes.2
Conclusions
The present study reported an association between maternal multimorbidity and adverse perinatal outcomes including PTB, LBW and SGA. The risk of adverse perinatal outcomes tends to increase as the number of chronic maternal conditions increases. Since the number of reproductive-aged women with multimorbidity has been increasing as the maternal population ages, preconception care for maternal multimorbidity is becoming increasingly important. Our findings provide essential information for preconception counselling in women with multimorbidities.
Supplementary Material
Acknowledgments
We would like to express our gratitude to all the JECS participants and all staff members involved in data collection. Members of the JECS Group as of 2021: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (Kumamoto University, Kumamoto, Japan). We would like to thank Editage (www.editage.com) for the English language editing.
Footnotes
Contributors: KNakanishi and YSaijo designed the study. YSaijo, EY, KNagaya, ST, YI, SK, CM, AA, RK and the JECS Group collected data. KNakanishi and YSaijo conducted the data analysis. KNakanishi, YSaijo, EY, YSato, YK, KNagaya, ST, YI, SK, CM, AA and RK contributed to the data interpretation. YSaijo, EY, YSato, YK, KNagaya, ST, YI, SK, CM, AA, RK and the JECS Group conducted the critical reviews. KNakanishi drafted the manuscript. YSaijo made critical revisions. YSaijo is responsible for the overall content as guarantor. All authors reviewed and commented on the manuscript. All authors approved the final manuscript.
Funding: This study was funded by the Ministry of Environment of Japan.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data are unsuitable for public deposition because of ethical restrictions and Japan’s legal framework. The Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amended on 9 September 2015) prohibits making data containing personal information publicly available. The Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiological data. All inquiries about access to data were sent to jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this email address is Dr Shoji F Nakayama, JECS Program Office, National Institute for Environmental Studies.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study protocol was approved by the Ministry of Environment’s Institutional Review Board on Epidemiological Studies and by the Ethics Committees of all participating institutions (no. 100910001).30 The JECS was performed in accordance with the Declaration of Helsinki. All the participants provided written informed consent.
References
- 1. van den Akker M, Buntinx F, Metsemakers JF, et al. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol 1998;51:367–75. 10.1016/s0895-4356(97)00306-5 [DOI] [PubMed] [Google Scholar]
- 2. The Academy of Medical Science . Multimorbidity: a priority for global health challenge 2018, Available: https://acmedsci.ac.uk/file-download/82222577 [Accessed 21 Jul 2022].
- 3. Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–51. 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 5. Brown HK, McKnight A, Aker A. Association between pre-pregnancy multimorbidity and adverse maternal outcomes: a systematic review. J Multimorb Comorb 2022;12:26335565221096584. 10.1177/26335565221096584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gijsen R, Hoeymans N, Schellevis FG, et al. Causes and consequences of comorbidity: a review. J Clin Epidemiol 2001;54:661–74. 10.1016/s0895-4356(00)00363-2 [DOI] [PubMed] [Google Scholar]
- 7. Kadam UT, Croft PR, North Staffordshire GP Consortium Group . Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract 2007;24:412–9. 10.1093/fampra/cmm049 [DOI] [PubMed] [Google Scholar]
- 8. Fortin M, Lapointe L, Hudon C, et al. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2004;2:51. 10.1186/1477-7525-2-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract 2008;14 Suppl 1:28–32. 10.1080/13814780802436093 [DOI] [PubMed] [Google Scholar]
- 10. Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined american population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc 2014;89:1336–49. 10.1016/j.mayocp.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maciejewski ML, Hammill BG. Measuring the burden of multimorbidity among medicare beneficiaries via condition counts and cumulative duration. Health Serv Res 2019;54:484–91. 10.1111/1475-6773.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kersten I, Lange AE, Haas JP, et al. Chronic diseases in pregnant women: prevalence and birth outcomes based on the snip-study. BMC Pregnancy Childbirth 2014;14:75. 10.1186/1471-2393-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Admon LK, Winkelman TNA, Heisler M, et al. Obstetric outcomes and delivery-related health care utilization and costs among pregnant women with multiple chronic conditions. Prev Chronic Dis 2018;15:E21. 10.5888/pcd15.170397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SI, Azcoaga-Lorenzo A, Agrawal U, et al. Epidemiology of pre-existing multimorbidity in pregnant women in the UK in 2018: a population-based cross-sectional study. BMC Pregnancy Childbirth 2022;22:120. 10.1186/s12884-022-04442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Japanese Ministry of Health, Labour and Welfare . The 2nd study group on the health and medical care system for expectant and nursing mothers. 2019. Available: www.mhlw.go.jp/stf/newpage_03949.html [Accessed 24 Jan 2023].
- 16. Enomoto K, Aoki S, Toma R, et al. Pregnancy outcomes based on pre-pregnancy body mass index in Japanese women. PLoS One 2016;11:e0157081. 10.1371/journal.pone.0157081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCauley M, Zafar S, van den Broek N. Maternal multimorbidity during pregnancy and after childbirth in women in low- and middle-income countries: a systematic literature review. BMC Pregnancy Childbirth 2020;20:637. 10.1186/s12884-020-03303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barreix M, Barbour K, McCaw-Binns A, et al. Standardizing the measurement of maternal morbidity: pilot study results. Int J Gynaecol Obstet 2018;141 Suppl 1:10–9. 10.1002/ijgo.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miura A, Fujiwara T. Intimate partner violence during pregnancy and postpartum depression in Japan: a cross-sectional study. Front Public Health 2017;5:81. 10.3389/fpubh.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Khalaf S, Bodunde E, Maher GM, et al. Chronic kidney disease and adverse pregnancy outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol 2022;226:656–70. 10.1016/j.ajog.2021.10.037 [DOI] [PubMed] [Google Scholar]
- 21. Al Khalaf SY, O’Reilly ÉJ, McCarthy FP, et al. Pregnancy outcomes in women with chronic kidney disease and chronic hypertension: a national cohort study. Am J Obstet Gynecol 2021;225:298. 10.1016/j.ajog.2021.03.045 [DOI] [PubMed] [Google Scholar]
- 22. Agrawal A, Wenger NK. Hypertension during pregnancy. Curr Hypertens Rep 2020;22:64. 10.1007/s11906-020-01070-0 [DOI] [PubMed] [Google Scholar]
- 23. Polić A, Običan SG. Pregnancy in systemic lupus erythematosus. Birth Defects Res 2020;112:1115–25. 10.1002/bdr2.1790 [DOI] [PubMed] [Google Scholar]
- 24. Paarlberg KM, Vingerhoets AJ, Passchier J, et al. Psychosocial factors and pregnancy outcome: a review with emphasis on methodological issues. J Psychosom Res 1995;39:563–95. 10.1016/0022-3999(95)00018-6 [DOI] [PubMed] [Google Scholar]
- 25. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49:726–35. 10.1177/070674370404901103 [DOI] [PubMed] [Google Scholar]
- 26. Rahman A, Bunn J, Lovel H, et al. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand 2007;115:481–6. 10.1111/j.1600-0447.2006.00950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wado YD, Afework MF, Hindin MJ. Effects of maternal pregnancy intention, depressive symptoms and social support on risk of low birth weight: a prospective study from Southwestern Ethiopia. PLOS ONE 2014;9:e96304. 10.1371/journal.pone.0096304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hassan M, Kashanian M, Hassan M, et al. Maternal outcomes of intimate partner violence during pregnancy: study in Iran. Public Health 2014;128:410–5. 10.1016/j.puhe.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 29. Sigalla GN, Mushi D, Meyrowitsch DW, et al. Intimate partner violence during pregnancy and its association with preterm birth and low birth weight in tanzania: a prospective cohort study. PloS One 2017;12:e0172540. 10.1371/journal.pone.0172540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawamoto T, Nitta H, Murata K, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014;14:25. 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michikawa T, Nitta H, Nakayama SF, et al. Baseline profile of participants in the Japan environment and children’s study (JECS). J Epidemiol 2018;28:99–104. 10.2188/jea.JE20170018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stirland LE, González-Saavedra L, Mullin DS, et al. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ 2020;368:m160. 10.1136/bmj.m160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCauley M, Madaj B, White SA, et al. Burden of physical, psychological and social ill-health during and after pregnancy among women in India, Pakistan, Kenya and Malawi. BMJ Glob Health 2018;3:e000625. 10.1136/bmjgh-2017-000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki S. Optimal pre-pregnancy body mass index cut-offs for obesity in Japan. J Clin Med Res 2017;9:180–1. 10.14740/jocmr2883w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nomura K, Minamizono S, Nagashima K, et al. Maternal body mass index and breastfeeding non-initiation and cessation: a quantitative review of the literature. Nutrients 2020;12:2684. 10.3390/nu12092684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komori K, Komori M, Eitoku M, et al. Verbal abuse during pregnancy increases frequency of newborn hearing screening referral: the Japan environment and children’s study. Child Abuse Negl 2019;90:193–201. 10.1016/j.chiabu.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 37. Nishigori H, Obara T, Nishigori T, et al. Drug use before and during pregnancy in Japan: the Japan environment and children’s study. Pharmacy (Basel) 2017;5:21. 10.3390/pharmacy5020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Itabashi K, Fujimura M, Kusuda S. Introduction of the new standard for birth size by gestational ages. J Jpn Pediatr Soc 2010:1271–93. [Google Scholar]
- 39. Endocrinology TJSfP . Excel-based clinical tools for growth evaluation of children, Available: http://jspe.umin.jp/medical/chart_dl.html [Accessed 30 Jul 2021].
- 40. Kowarik A, Templ M. Imputation with the R package VIM. J Stat Soft 2016;74:1–16. 10.18637/jss.v074.i07 [DOI] [Google Scholar]
- 41. Jerez JM, Molina I, García-Laencina PJ, et al. Missing data imputation using statistical and machine learning methods in a real breast cancer problem. Artif Intell Med 2010;50:105–15. 10.1016/j.artmed.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 42. Seijo M, Minckas N, Cormick G, et al. Comparison of self-reported and directly measured weight and height among women of reproductive age: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2018;97:429–39. 10.1111/aogs.13326 [DOI] [PubMed] [Google Scholar]
- 43. Okamoto N, Hosono A, Shibata K, et al. Accuracy of self-reported height, weight and waist circumference in a Japanese sample. Obes Sci Pract 2017;3:417–24. 10.1002/osp4.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Köksal S, Pesando LM, Rotondi V, et al. Harnessing the potential of Google searches for understanding dynamics of intimate partner violence before and after the COVID-19 outbreak. Eur J Popul 2022;38:517–45. 10.1007/s10680-022-09619-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sato R, Ikuma M, Takagi K, et al. Exposure of drugs for hypertension, diabetes, and autoimmune disease during pregnancy and perinatal outcomes: an investigation of the regulator in Japan. Medicine (Baltimore) 2015;94:e386. 10.1097/MD.0000000000000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019;126:984–95. 10.1111/1471-0528.15661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vats H, Saxena R, Sachdeva MP, et al. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: a systematic review and meta-analysis. Obes Res Clin Pract 2021;15:536–45. 10.1016/j.orcp.2021.10.005 [DOI] [PubMed] [Google Scholar]
- 48. Shinar S, Agrawal S, Ryu M, et al. Perinatal outcomes in women living with HIV-1 and receiving antiretroviral therapy-a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2022;101:168–82. 10.1111/aogs.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogerson SJ, Desai M, Mayor A, et al. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018;18:e107–18. 10.1016/S1473-3099(18)30066-5 [DOI] [PubMed] [Google Scholar]
- 50. Schlueter A, Doshi U, Garg B, et al. Adverse pregnancy outcomes associated with maternal syphilis infection. J Matern Fetal Neonatal Med 2022;35:5828–33. 10.1080/14767058.2021.1895740 [DOI] [PubMed] [Google Scholar]
- 51. Orazulike N, Sharma JB, Sharma S, et al. Tuberculosis (TB) in pregnancy - a review. Eur J Obstet Gynecol Reprod Biol 2021;259:167–77. 10.1016/j.ejogrb.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 52. Bezerra H de S, Alves RM, Nunes ADD, et al. Prevalence and associated factors of common mental disorders in women: a systematic review. Public Health Rev 2021;42:1604234. 10.3389/phrs.2021.1604234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niemi M, Falkenberg T, Petzold M, et al. Symptoms of antenatal common mental disorders, preterm birth and low birthweight: a prospective cohort study in a semi-rural district of Vietnam. Trop Med Int Health 2013;18:687–95. 10.1111/tmi.12101 [DOI] [PubMed] [Google Scholar]
- 54. Urquia ML, O’Campo PJ, Heaman MI, et al. Experiences of violence before and during pregnancy and adverse pregnancy outcomes: an analysis of the Canadian maternity experiences survey. BMC Pregnancy Childbirth 2011;11:42. 10.1186/1471-2393-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shaw GM, Wise PH, Mayo J, et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr Perinat Epidemiol 2014;28:302–11. 10.1111/ppe.12125 [DOI] [PubMed] [Google Scholar]
- 56. Paré E, Parry S, McElrath TF, et al. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol 2014;124:763–70. 10.1097/AOG.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 57. Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069281supp001.pdf (155.1KB, pdf)
Data Availability Statement
No data are available. Data are unsuitable for public deposition because of ethical restrictions and Japan’s legal framework. The Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amended on 9 September 2015) prohibits making data containing personal information publicly available. The Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiological data. All inquiries about access to data were sent to jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this email address is Dr Shoji F Nakayama, JECS Program Office, National Institute for Environmental Studies.