Abstract
Background
Evaluate the impact of sex on tofacitinib efficacy, safety and persistence (time to discontinuation) in patients with psoriatic arthritis (PsA).
Methods
Data were pooled from two phase 3 randomised controlled trials. Patients were randomised to tofacitinib 5 mg or 10 mg two times per day, adalimumab 40 mg every 2 weeks or placebo. Efficacy outcomes to month 12 included American College of Rheumatology (ACR)20/50/70, minimal disease activity (MDA), Psoriasis Area Severity Index (PASI)75, change from baseline (∆) in Health Assessment Questionnaire-Disability Index (HAQ-DI) and ∆Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Safety was assessed to month 12 and persistence was assessed to month 42 of a long-term extension study.
Results
Overall, 816 patients were included (54.3% females). At baseline, higher tender joint counts, enthesitis scores and worse HAQ-DI and FACIT-F were reported in females versus males; presence of dactylitis and PASI were greater in males versus females. At month 3, tofacitinib efficacy generally exceeded placebo in both sexes. Overall, similar ACR20/50/70, PASI75, ∆HAQ-DI and ∆FACIT-F were observed for tofacitinib between sexes; females were less likely to achieve MDA. Similar proportions of males/females receiving tofacitinib (both doses) experienced treatment-emergent adverse events (AEs). Serious AEs occurred in 3.4%/6.6% and 4.0%/5.9% males/females with tofacitinib 5 mg and 10 mg two times per day. Persistence was generally similar between sexes.
Conclusion
Tofacitinib efficacy exceeded placebo in both sexes and was comparable between sexes. Consistent with previous studies of PsA treatments, females were less likely to achieve MDA, likely due to baseline differences. Safety and time to discontinuation were generally similar between sexes.
Trial registration number
Keywords: arthritis, psoriatic; therapeutics; outcome assessment, health care
WHAT IS ALREADY KNOWN ON THIS TOPIC
Differences in clinical features and response to therapy have been demonstrated in males and females with psoriatic arthritis (PsA); compared with males, females have lower American College of Rheumatology (ACR) responses, higher rates of discontinuation due to lack of efficacy and adverse events following treatment, and they achieve minimal disease activity (MDA) less frequently.
The impact of sex on the efficacy, safety and persistence of tofacitinib treatment has not been previously reported in patients with PsA.
WHAT THIS STUDY ADDS
In this post-hoc analysis of phase 3 randomised controlled trial data with tofacitinib in PsA, ACR20/50/70, Psoriasis Area and Severity Index 75, improvements in musculoskeletal manifestations, physical function and fatigue scores were similar in males and females treated with tofacitinib; however, males were more likely to achieve MDA, likely due to sex differences in disease expression at baseline. Treatment-emergent adverse events were generally similar across sexes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Exploratory analyses of clinical trials by sex allow an assessment of potential sex differences in disease expression and responses to advanced therapies. This work underscores the need for further investigation of how sex influences disease phenotype and treatment response, which should result in more personalised and equitable management across men and women with PsA.
Introduction
Psoriatic arthritis (PsA) is an inflammatory musculoskeletal disease affecting up to one-third of patients with psoriasis, which can lead to significant joint damage and physical impairment.1 2 Response to advanced therapies varies between patients with PsA, and it has been associated with demographic factors, comorbidities and prior exposure to therapies. Sex (biological) and gender (sociocultural) differences are emerging as important additional determinants of response to therapy.3–9
PsA affects males and females equally, but differs across sexes in terms of clinical features, disease course and responses to therapy. Consistently, female patients are found to have higher disease activity scores at presentation, particularly for predominantly subjective measures (eg, tender joints, entheses and pain), whereas more objective measures (eg, swollen joint counts (SJCs) and dactylitis) are comparable between sexes.10 11 Females also report greater loss of function and affected health-related quality of life (HRQoL).10–12 In addition, despite generally having less severe Psoriasis Area and Severity Index (PASI) scores, females tend to report a higher burden of skin disease in terms of their HRQoL.13 14
Several observational studies in patients with PsA have shown that female sex predicts less favourable outcomes among patients initiating advanced therapies.3–9 Female patients were less likely to achieve treatment responses and low disease activity and were more likely to discontinue treatment early due to less therapeutic benefit and adverse effects.6 15 16 However, most studies focused on identifying predictors for composite outcome responses, without investigating which disease domains contributed to inferior responses in females. Additionally, observational data on treatment effectiveness are subject to multiple biases and confounders. Randomised controlled trials (RCTs) are the gold standard to assess treatment efficacy compared with placebo (PBO); however, sex disparities in responses to advanced therapies are infrequently reported in RCTs. A post-hoc analysis of the SPIRIT-P1 and SPIRIT-P2 trials in patients with PsA receiving ixekizumab showed that females had lower American College of Rheumatology (ACR) response rates versus males through week 156.17 Similarly, a post-hoc analysis of the EXCEED trial in patients with PsA receiving secukinumab demonstrated that males had numerically greater treatment responses than females.18 It is important to study sex differences across advanced therapies, as some modes of action may work differently for males and females, perhaps due to differences in biological mechanisms driving the disease. Specifically, there is a lack of studies assessing sex disparities in response to Janus kinase inhibitors. Such data are critical to compare treatment responses in males and females, to understand factors that contribute to observed disparities and to inform healthcare providers of these differences.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. The efficacy and safety of tofacitinib 5 mg two times per day and 10 mg two times per day have been demonstrated in two global, phase 3 RCTs of patients with active PsA with inadequate responses to either conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or tumour necrosis factor inhibitors (TNFi),19 20 and an open-label, long-term extension (LTE) study.21
Methods
Study design
This post-hoc analysis of pooled phase 3 RCTs (OPAL Broaden (NCT01877668), OPAL Beyond (NCT01882439)) and an LTE study (OPAL Balance (NCT01976364)) assessed the efficacy, safety and persistence (time to discontinuation) of tofacitinib in male and female patients. Full study details have been published previously.19–21 OPAL Broaden and OPAL Beyond were randomised, double-blind, PBO-controlled studies that enrolled patients aged ≥18 years diagnosed with PsA (≥6 months, based on the ClASsification criteria for Psoriatic ARthritis (CASPAR)).22 Patients were required to have active arthritis (≥3 swollen joints and ≥3 tender/painful joints) at screening and baseline and active plaque psoriasis at screening. Patients were randomised to receive tofacitinib 5 mg two times per day, tofacitinib 10 mg two times per day, adalimumab (ADA) 40 mg subcutaneous injection every other week (Q2W) (OPAL Broaden only) or PBO with a blinded switch to tofacitinib 5 mg or 10 mg two times per day at month 3. Patients participating in phase 3 studies received a stable dose of a single csDMARD. Upon entry into OPAL Balance, patients received open-label tofacitinib 5 mg two times per day, with increases to 10 mg two times per day for inadequate symptom control allowed from month 1 and reductions to 5 mg two times per day thereafter for safety. Selected csDMARDs (eg, methotrexate (MTX), sulfasalazine or leflunomide) could be continued concomitantly. Patients completing ≥24 months in OPAL Balance who received a stable dose of tofacitinib 5 mg two times per day for ≥3 months and a stable dose of oral MTX (7.5–20 mg weekly for at least 4 weeks) were eligible to participate in a substudy where they would be randomised to either continue or withdraw background MTX for an additional 12 months.21 23
All studies were conducted in accordance with the Declaration of Helsinki and the International Council on Harmonisation Guidelines for Good Clinical Practice, and all patients provided informed consent.
Post-hoc analysis
This post-hoc analysis assessed efficacy and safety in males versus females randomised at baseline to tofacitinib 5 mg two times per day, 10 mg two times per day, ADA 40 mg Q2W and PBO in OPAL Broaden, and tofacitinib 5 mg two times per day, 10 mg two times per day and PBO in OPAL Beyond. For patients randomised to receive tofacitinib, efficacy analyses were performed using pooled data from both RCTs up to month 12, and tofacitinib was analysed as combined doses (hereafter, tofacitinib) and separate doses (tofacitinib 5 mg and 10 mg two times per day). Safety analyses used pooled data from both trials, and tofacitinib was analysed as separate doses. Efficacy and safety data for tofacitinib past month 6 reflect patients from OPAL Broaden only. For patients who received ADA 40 mg Q2W, efficacy and safety were assessed up to month 12 of OPAL Broaden. For patients randomised at baseline to PBO, data were pooled from both studies, and efficacy and safety were assessed up to the end of the PBO-controlled period of month 3. Time to discontinuation of tofacitinib was analysed by sex up to the maximum half-year increment of follow-up (month 42) of the main OPAL Balance LTE study and substudy. Patients were categorised as ‘average tofacitinib 5 mg two times per day’ (<15 mg) or ‘average tofacitinib 10 mg two times per day’ (≥15 mg), based on average daily dose over the course of the study.
Efficacy endpoints included ACR20/50/70 responses, minimal disease activity (MDA), MDA components, very low disease activity (VLDA), 75% improvement in PASI (PASI75, in patients with baseline body surface area ≥3 and PASI >0), changes from baseline in SJCs (of 66 joints) and tender joint counts (TJCs, of 68 joints), Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index score (in patients with baseline SPARCC >0), Leeds Enthesitis Index (LEI, in patients with baseline LEI >0), Dactylitis Severity Score (DSS, in patients with baseline DSS >0), PASI, C-reactive protein (CRP, in mg/L), physician global assessment of PsA (PGA-PsA) by a Visual Analogue Scale (VAS, 0–100 mm) and PGA of psoriasis (PGA-PsO, in patients with baseline PGA-PsO >0).
Patient-reported outcomes included change from baseline in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Health Assessment Questionnaire-Disability Index (HAQ-DI), pain (VAS, 0–100 mm), Patient Global Assessment of Disease Activity (PtGA, VAS, 0–100 mm), Short Form-36 (SF-36) Physical (PCS) and Mental (MCS) Component Summary scores, FACIT-F ≥minimum clinically important difference (MCID; ≥4) and HAQ-DI ≥MCID (≥0.35).
Safety outcomes included treatment-emergent adverse events (TEAEs) (all causality), serious adverse events (SAEs), discontinuation due to adverse events (AEs), death, AEs of special interest (AESIs; serious infections, herpes zoster (HZ), venous thromboembolic events (VTEs)), adjudicated opportunistic infections, tuberculosis (TB), major adverse cardiovascular events (MACE), malignancies excluding non-melanoma skin cancer (NMSC), NMSC and gastrointestinal (GI) perforations.
Statistical analyses
Efficacy analyses were based on observed data from the full analysis set, which included all patients who received at least one dose of study medication and had at least one post-baseline assessment. Differences in response between males and females were assessed by generalised linear models with repeated measures and mixed effects: logistic regression models for binary endpoints and linear models for continuous, change-from-baseline endpoints. Models were fit using PROC GLIMMIX (with restricted pseudo-likelihood) and PROC MIXED (with restricted maximum likelihood) in SAS software V.9.4, respectively. Independent variables for both kinds of models included self-reported sex; RCT (OPAL Broaden or OPAL Beyond); geographical location; treatment group; study visit (in months); baseline value (linear model only); and all-way interactions among sex, treatment group and study visit. For patients randomised to PBO who were blindly switched to tofacitinib 5 mg or 10 mg two times per day at month 3, observations past month 3 were omitted from the models. PBO sequences were combined into a single PBO group. Tofacitinib 5 mg and 10 mg two times per day were analysed as a combined group and as separate groups. Additional information on statistical modelling is included in the online supplemental material. For all analyses, p values were not adjusted for multiplicity and are considered descriptive. Comparisons resulting in p<0.05 are considered nominally significant and are highlighted for the reader. Comparisons resulting in p≥0.05 with non-overlapping SEs or CIs are described as numerical differences.
rmdopen-2022-002718supp001.pdf (599.8KB, pdf)
Safety analyses included all patients who received at least one dose of study medication. Safety data by sex were summarised descriptively.
Persistence, as measured by all-cause discontinuation, discontinuation due to AEs and discontinuation due to lack of efficacy, was analysed using a Kaplan-Meier approach of time to discontinuation. Time to discontinuation was defined as the difference between the end-of-study date and first tofacitinib dose date+1 day; data from the LTE study, as well as the substudy, were analysed. Completers were censored at the end-of-study date. Kaplan-Meier analyses were performed within each treatment group, using sex as strata. Percentages and CIs for males and females are presented for 1-year, 2-year and 3-year survival. Analyses were also performed subsetting on patients who entered the LTE study.
Results
Patients
Overall, 816 patients were included in the analysis, consisting of 373 (45.7%) males and 443 (54.3%) females. Among treatment groups, 474 patients received tofacitinib 5 mg or 10 mg two times per day (217 (45.8%) males and 257 (54.2%) females), 106 patients received ADA (56 (52.8%) males and 50 (47.2%) females) and 236 patients received PBO (100 (42.4%) males and 136 (57.6%) females) (online supplemental table 1). In the overall study population at baseline (table 1), females had higher TJC, higher enthesitis score (by SPARCC and LEI), worse physical function and HRQoL (higher HAQ-DI and lower SF-36 PCS and MCS scores) and worse fatigue (by FACIT-F) versus males. The prevalence of depression was higher in females compared with males at baseline. Males were more likely to have dactylitis, higher CRP and higher PASI scores versus females. Baseline pain VAS was similar between males and females. Baseline demographic and clinical characteristics, stratified by treatment group and sex, reflect the overall study population (online supplemental table 1).
Table 1.
Demographic and clinical characteristics of the overall study population, stratified by sex; pooled data from OPAL Broaden and OPAL Beyond
| Variable | All treatment groups | |
| Sex, N (%) | Male: 373 (45.7) | Female: 443 (54.3) |
| Age (years), mean (SD) | 48.0 (12.1) | 49.6 (12.1) |
| Race, white, n (%) | 351 (94.1) | 421 (95.0) |
| BMI (kg/m2), mean (SD) | 29.5 (5.1) | 29.8 (6.7) |
| PsA duration | ||
| <2 years | 88 (23.6) | 86 (19.4) |
| ≥2 years | 285 (76.4) | 357 (80.6) |
| SJC (66), mean (SD) | 11.0 (9.0) | 12.2 (9.8) |
| TJC (68), mean (SD) | 19.4 (13.8) | 22.0 (14.4)* |
| SPARCC, patients included† | ||
| n (%) | 290 (77.7) | 337 (76.1) |
| Mean (SD) | 4.7 (3.5) | 6.1 (3.9)*** |
| LEI, patients included‡ | ||
| n (%) | 245 (65.7) | 310 (70.0) |
| Mean (SD) | 2.6 (1.6) | 3.0 (1.6)* |
| DSS, patients included§ | ||
| n (%) | 218 (58.4) | 213 (48.1)* |
| Mean (SD) | 8.9 (7.9) | 8.1 (8.3) |
| CRP (mg/L), mean (SD) | 14.6 (26.1) | 10.2 (16.2)* |
| PASI, patients included¶ | ||
| n (%) | 273 (73.2) | 285 (64.3) |
| Mean (SD) | 11.4 (9.6) | 8.3 (7.3)*** |
| PGA-PsA (VAS in mm), mean (SD) | 61.3 (22.6) (N=372) | 64.2 (22.5) |
| PGA-PsO**, n (%) | ||
| 0 | 11 (2.9) | 20 (4.5) |
| 1 | 100 (26.8) | 136 (30.7) |
| 2 | 154 (41.3) | 190 (42.9) |
| 3 | 92 (24.7) | 80 (18.1) |
| 4 | 14 (3.8) | 14 (3.2) |
| HAQ-DI, mean (SD) | 1.1 (0.6) (N=372) | 1.3 (0.7)*** |
| SF-36 PCS, mean (SD) | 35.4 (8.9) (N=370) | 34.1 (8.2)* (N=442) |
| SF-36 MCS, mean (SD) | 42.3 (11.8) (N=370) | 38.9 (11.7)*** (N=442) |
| FACIT-F, mean (SD) | 30.2 (10.7) (N=372) | 25.5 (10.7)*** |
| Patient Global Assessment of Arthritis and Skin (VAS in mm), mean (SD) | 53.6 (19.9) (N=371) | 54.1 (20.3) (N=436) |
| Patient Assessment of Arthritis Pain (VAS in mm), mean (SD) | 53.9 (23.7) (N=371) | 56.2 (22.8) |
| Presence of depression††, yes, n (%) | 21 (5.6) | 67 (15.1)*** |
*p<0.05, **p<0.001, ***p<0.0001 for females versus males.
†Patients with baseline SPARCC >0.
‡Patients with baseline LEI >0.
§Patients with baseline DSS >0.
¶PASI was assessed in patients with baseline BSA ≥3% and PASI >0.
**PGA-PsO assessed in patients with baseline PGA-PsO >0.
††Ascertained from medical history.
BMI, body mass index; BSA, body surface area; CRP, C-reactive protein; DSS, Dactylitis Severity Score; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; LEI, Leeds Enthesitis Index; n, number of patients with demographic/clinical characteristic at baseline; N, number of evaluable patients; PASI, Psoriasis Area and Severity Index; PGA-PsA, Physician Global Assessment of PsA; PGA-PsO, Physician Global Assessment of Psoriasis; PsA, psoriatic arthritis; SF-36 PCS, Short Form-36 Physical Component Summary; SJC, swollen joint count; SPARCC, Spondyloarthritis Research Consortium of Canada; TJC, tender joint count; VAS, Visual Analogue Scale.
Clinical efficacy
At month 3, males and females receiving tofacitinib achieved higher rates of ACR20/50, MDA and PASI75 responses, compared with patients of the same sex receiving PBO. Males receiving tofacitinib achieved higher rates of ACR70 compared with PBO, while a numerical difference in rates of ACR70 was observed for females compared with PBO. ACR20/50/70 and VLDA scores were comparable between males and females receiving tofacitinib at month 3, whereas males were more likely to achieve MDA and females PASI75 responses (figure 1 and online supplemental figure 1).
Figure 1.
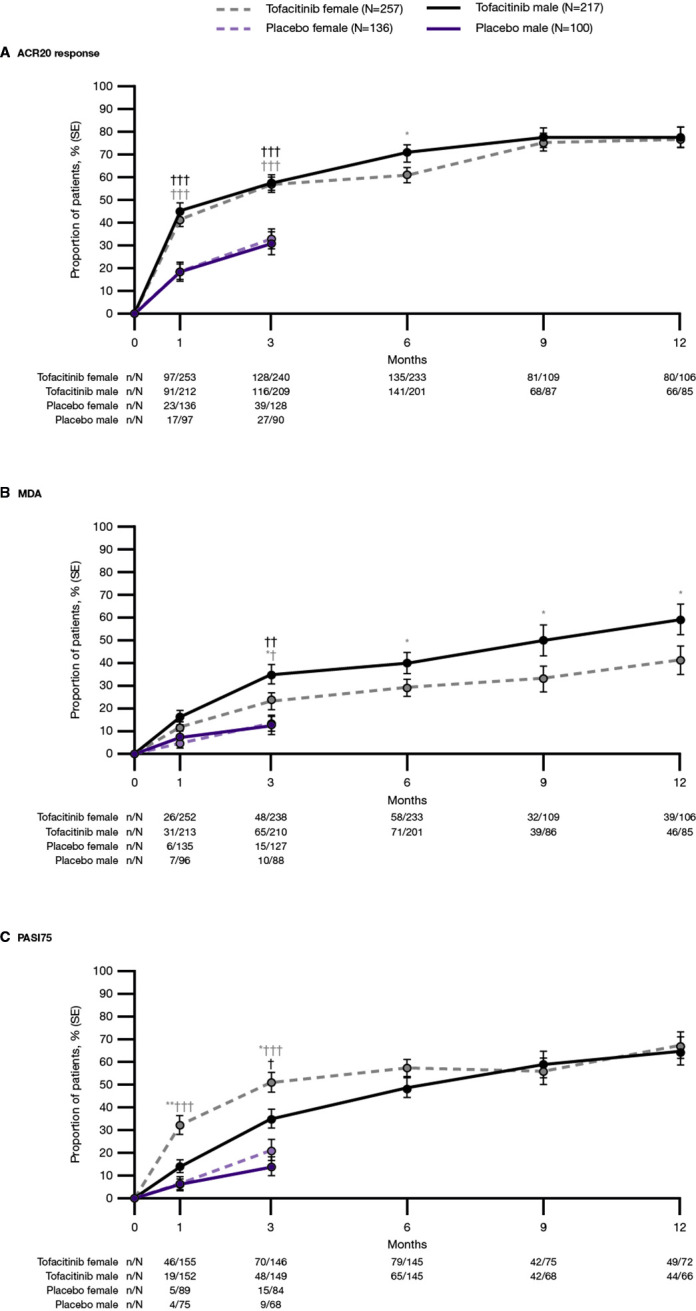
Proportion of patients achieving composite outcomes: (A) ACR20 response, (B) MDA and (C) PASI75, by treatment group and sex; pooled data from OPAL Broaden and OPAL Beyond. *p<0.05, **p<0.001 for females vs males, †p<0.05, ††p<0.001, †††p<0.0001 for tofacitinib versus placebo. ACR20, American College of Rheumatology≥20% response criteria; MDA, minimal disease activity; N, number of patients included in the analysis; n, number of patients achieving outcome; PASI75, 75% improvement in Psoriasis Area and Severity Index; Tofacitinib, tofacitinib 5 mg and 10 mg two times per day.
Past month 3, ACR responses were generally similar in males and females receiving tofacitinib, except ACR20 responses at month 6 and ACR70 responses at month 9, where males attained higher response rates compared with females (figure 1A and online supplemental figure 1A, B). Males also achieved higher rates of MDA at months 6, 9 and 12, and VLDA at month 6, compared with females (figure 1B and online supplemental figure 1C). PASI75 responses were similar between sexes from months 6 through 12 (figure 1C). Similar trends for ACR20/50/70, MDA/VLDA and PASI75 scores were evident across tofacitinib 5 mg and 10 mg two times per day, and ADA, when evaluated separately (online supplemental figure 2).
The proportion of patients attaining the MDA component thresholds of SJC ≤1, TJC ≤1, HAQ-DI ≤0.5, LEI ≤1, PtGA ≤20 and pain VAS ≤15 was generally higher in males compared with females receiving tofacitinib. Conversely, females were more likely to achieve PASI ≤1 or body surface area ≤3% (online supplemental figure 3).
Males and females receiving tofacitinib achieved greater improvements, compared with PBO, in SJC, TJC, SPARCC, DSS, PGA-PsO, PGA-PsA, CRP and PASI at month 3. Improvements from baseline in SJC, TJC, SPARCC, DSS, PGA-PsA and PASI were comparable between males and females receiving tofacitinib, while females achieved greater improvements in PGA-PsO at months 3 and 6, and CRP at month 3, compared with males. Similar trends were observed for tofacitinib 5 mg two times per day and 10 mg two times per day across most outcomes, with improvements with ADA also shown (online supplemental table 2).
Patient-reported outcomes
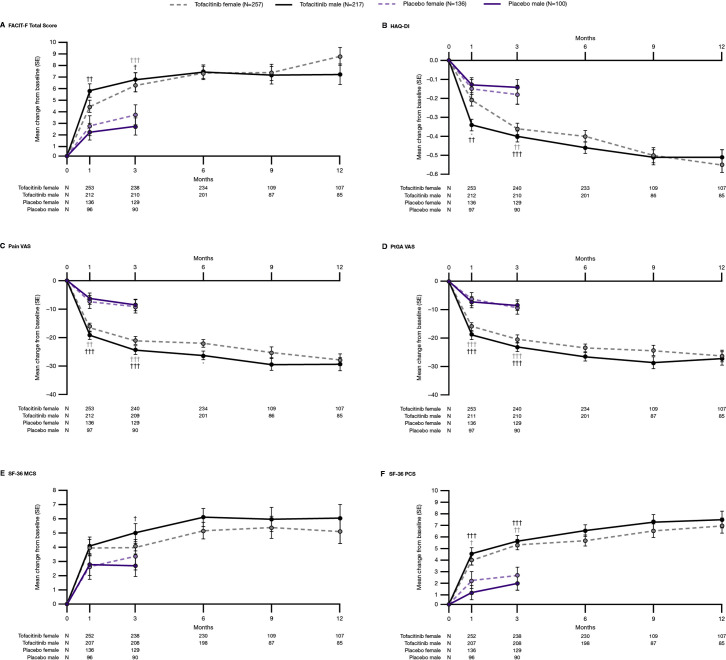
At month 3, both males and females receiving tofacitinib reported greater improvements in FACIT-F, HAQ-DI, pain VAS, PtGA VAS and SF-36 PCS, compared with patients of the same sex receiving PBO (figure 2). Males receiving tofacitinib reported greater improvements in SF-36 MCS, compared with PBO, at month 3. Between males and females receiving tofacitinib, improvements in FACIT-F, HAQ-DI, pain VAS, PtGA VAS, SF-36 MCS and SF-36 PCS were comparable at month 3.
Figure 2.
Change from baseline in patient-reported outcomes: (A) FACIT-F Total Score, (B) HAQ-DI, (C) Pain VAS, (D) PtGA VAS, (E) SF-36 MCS score, (F) SF-36 PCS score, by treatment group and sex; pooled data from OPAL Broaden and OPAL Beyond. *p<0.05, **p<0.001 for females versus males. †p<0.05, ††p<0.001, †††p<0.0001 for tofacitinib versus placebo. FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; N, number of patients included in the analysis; n, number of patients achieving outcome; SF-36 MCS, Short Form-36 Mental Component Summary; SF-36 PCS, Short Form-36 Physical Component Summary; Tofacitinib, tofacitinib 5 mg and 10 mg two times per day; VAS, Visual Analogue Scale.
Past month 3, improvements in FACIT-F, HAQ-DI, pain VAS, PtGA VAS, SF-36 PCS and MCS were generally similar in males and females receiving tofacitinib, with the exception of males achieving greater improvements in pain VAS at month 6, compared with females (figure 2). Results for improvements ≥MCID in FACIT-F and HAQ-DI are shown in online supplemental figure 4. Overall, similar results were reported by males and females receiving tofacitinib 5 mg two times per day, tofacitinib 10 mg two times per day and ADA up to month 12 (online supplemental figure 5).
Safety
During the PBO-controlled phase up to month 3, the proportions of patients experiencing TEAEs were similar among females and males receiving tofacitinib 5 mg two times per day and numerically higher among females, compared with males, receiving tofacitinib 10 mg two times per day and PBO. In patients receiving tofacitinib 5 mg two times per day, tofacitinib 10 mg two times per day and PBO, SAEs occurred in 1.7%/1.7%, 3.0%/0.7% and 0%/2.9% of males/females, respectively, and discontinuation due to AEs occurred in 0.9%/1.7%, 2.0%/3.7% and 3.0%/0.7% of males/females, respectively.
During the PBO-controlled phase, two patients experienced serious infections: one male (pyelonephritis) and one female (parotitis), both receiving tofacitinib 10 mg two times per day. Non-serious cases of HZ were experienced by three patients: one male receiving tofacitinib 5 mg two times per day and two females receiving tofacitinib 5 mg two times per day and 10 mg two times per day. One adjudicated opportunistic infection (moderate HZ affecting two dermatomes) was experienced by a male receiving tofacitinib 5 mg two times per day, and there were two adjudicated malignancies (excluding NMSC): one male (bladder transitional cell carcinoma) and one female (squamous cell carcinoma of the vulva), both receiving tofacitinib 5 mg two times per day. No deaths, TB, MACE, NMSC, GI perforations or VTEs were reported in either sex up to month 3 (table 2).
Table 2.
Summary of safety events (all causality) up to months 3 and 12 for patients receiving tofacitinib 5 mg two times per day, 10 mg two times per day and placebo, by sex*; pooled data from OPAL Broaden and OPAL Beyond
| Up to month 3 | Tofacitinib 5 mg two times per day | Tofacitinib 10 mg two times per day | Placebo | |||
| Patients with events, n (%) |
Male (N=117) | Female (N=121) | Male (N=100) | Female (N=136) | Male (N=100) | Female (N=136) |
| TEAEs | 55 (47.0) | 59 (48.8) | 44 (44.0) | 73 (53.7) | 30 (30.0) | 65 (47.8) |
| SAEs | 2 (1.7) | 2 (1.7) | 3 (3.0) | 1 (0.7) | 0 | 4 (2.9) |
| Discontinuation due to AEs | 1 (0.9) | 2 (1.7) | 2 (2.0) | 5 (3.7) | 3 (3.0) | 1 (0.7) |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious infections | 0 | 0 | 1 (1.0) | 1 (0.7) | 0 | 0 |
| HZ (serious and non-serious)† | 1 (0.9) | 1 (0.8) | 0 | 1 (0.7) | 0 | 0 |
| OIs (excluding TB) | 1 (0.9)‡ | 0 | 0 | 0 | 0 | 0 |
| TB | 0 | 0 | 0 | 0 | 0 | 0 |
| MACE | 0 | 0 | 0 | 0 | 0 | 0 |
| Malignancies (excluding NMSC) | 1 (0.9) | 1 (0.8) | 0 | 0 | 0 | 0 |
| NMSC | 0 | 0 | 0 | 0 | 0 | 0 |
| GI perforations | 0 | 0 | 0 | 0 | 0 | 0 |
| VTEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Up to month 12 | Tofacitinib 5 mg two times per day | Tofacitinib 10 mg two times per day | ||||
|
Patients with events,
n (%) |
Male (N=117) | Female (N=121) | Male (N=100) | Female (N=136) | ||
| TEAEs | 79 (67.5) | 85 (70.2) | 70 (70.0) | 99 (72.8) | ||
| SAEs | 4 (3.4) | 8 (6.6) | 4 (4.0) | 8 (5.9) | ||
| Discontinuation due to AEs | 3 (2.6) | 8 (6.6) | 4 (4.0) | 7 (5.1) | ||
| Deaths | 0 | 0 | 0 | 0 | ||
| Serious infections | 0 | 2 (1.7) | 1 (1.0) | 2 (1.5) | ||
| HZ (serious and non-serious)† | 1 (0.9) | 2 (1.7) | 2 (2.0) | 2 (1.5) | ||
| OIs (excluding TB)§ | 1 (0.9)‡ | 0 | 0 | 0 | ||
| TB | 0 | 0 | 0 | 0 | ||
| MACE¶ | 0 | 0 | 0 | 1 (0.7) | ||
| Malignancies (excluding NMSC) | 1 (0.9) | 2 (1.7) | 0 | 0 | ||
| NMSC | 0 | 0 | 1 (1.0) | 0 | ||
| GI perforations | 0 | 0 | 0 | 0 | ||
| VTEs | 0 | 0 | 0 | 0 | ||
*Includes all patients from OPAL Broaden and OPAL Beyond who were randomised at baseline to tofacitinib 5 mg two times per day, 10 mg two times per day or PBO.
†No serious events of HZ were reported in males or females receiving tofacitinib 5 mg or 10 mg two times per day.
‡Moderate HZ (three dermatomes affected).
§One adjudicated OI (multidermatomal HZ, two adjacent dermatomes) occurring in a female patient receiving tofacitinib 5 mg two times per day in OPAL Broaden was reclassified as a ‘Special Interest Infection’ and included in HZ (serious and non-serious).
¶One MACE was reported in a male patient receiving tofacitinib 5 mg two times per day outside the 28-day risk period.
AEs, adverse events; GI, gastrointestinal; HZ, herpes zoster; MACE, major adverse cardiovascular events; N, number of patients included in the analysis; n, number of patients with the event (events are counted up to 28 days beyond the last dose or the end of month 3 or month 12); NMSC, non-melanoma skin cancer; OIs, opportunistic infections; PBO, placebo; SAEs, serious adverse events; TB, tuberculosis; TEAEs, treatment-emergent adverse events; VTEs, venous thromboembolic events.
Through month 12, similar proportions of females and males receiving tofacitinib 5 mg two times per day and tofacitinib 10 mg two times per day experienced TEAEs. In patients receiving tofacitinib 5 mg two times per day and tofacitinib 10 mg two times per day, SAEs occurred in 3.4%/6.6% and 4.0%/5.9% of males/females, respectively, and 2.6%/6.6% and 4.0%/5.1% of males/females discontinued due to AEs, respectively.
Through month 12, three additional serious infections were experienced by females: two receiving tofacitinib 5 mg two times per day (oral candidiasis and pneumonia) and one receiving 10 mg two times per day (influenza). Non-serious cases of HZ were experienced by four additional patients: two females receiving tofacitinib 5 mg and 10 mg two times per day and two males both receiving tofacitinib 10 mg two times per day. One adjudicated MACE (ischaemic stroke) was experienced by a female receiving tofacitinib 10 mg two times per day, one additional malignancy (excluding NMSC) (invasive ductal breast carcinoma) was experienced by a female receiving tofacitinib 5 mg two times per day and one NMSC (basal cell carcinoma) was experienced by a male receiving tofacitinib 10 mg two times per day. No deaths, TB, GI perforations or VTEs were reported in either sex up to month 12 (table 2).
TEAEs occurred in 39.3%/54.0% of males/females, respectively, receiving ADA during the PBO-controlled phase up to month 3 of OPAL Broaden and in 66.1%/76.0% of males/females receiving ADA through month 12. Through both observation periods, SAEs and discontinuation due to AEs were similar between the sexes. No AESIs were reported up to month 3 in either sex. Through month 12, one serious infection (herpes simplex and pyoderma streptococcal) and one MACE (retinal artery occlusion) were experienced by female patients receiving ADA (online supplemental table 3).
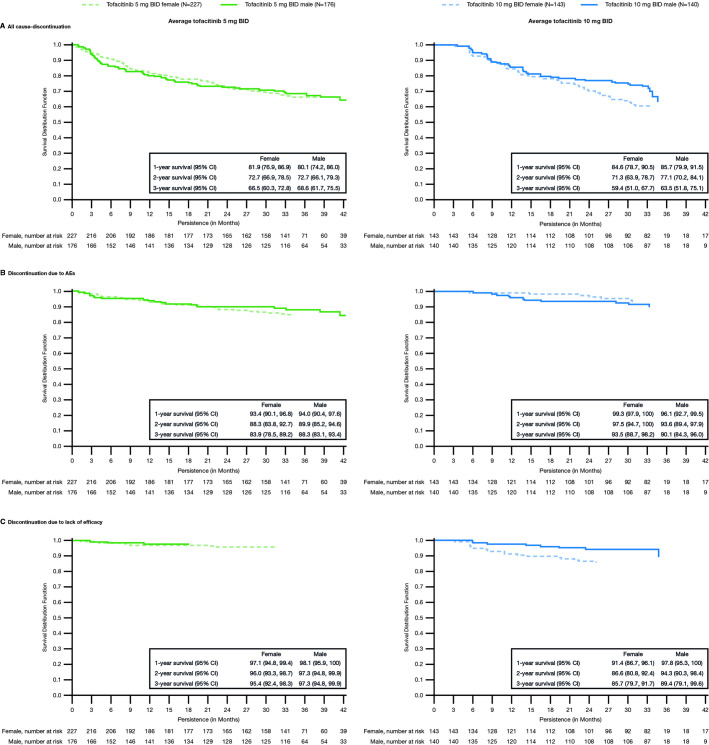
Persistence
In the average tofacitinib 5 mg two times per day group, all-cause discontinuation, discontinuation due to AEs and discontinuation due to lack of efficacy were similar overall in males and females up to month 42 (figure 3A–C). Some differences between the sexes were observed in the average tofacitinib 10 mg two times per day group. For all-cause discontinuation, the unadjusted Kaplan-Meier curves appear to separate after 21 months, suggesting potentially greater persistence in men after 21 months (figure 3A). Similar results were observed for discontinuation due to lack of efficacy past month 6 (figure 3C). However, the estimated 1-year, 2-year and 3-year survival rates were similar between sexes with overlapping CIs.
Figure 3.
Treatment persistence by sex for patients receiving average doses of tofacitinib 5 mg and 10 mg BID in the OPAL Balance LTE study: (A) time to all-cause discontinuation, (B) discontinuation due to AEs, (C) discontinuation due to lack of efficacy; data from OPAL Balance and the OPAL Balance substudy. Time to discontinuation was defined as the difference between the end-of-study date and first tofacitinib dose date+1 day. End-of-study date was the end of OPAL Balance; for patients enrolled in the substudy, it was the end of the OPAL Balance substudy. Patients were censored at the end-of-study date. Data included all patients who entered the LTE study and substudy. AEs, adverse events; BID, two times per day; LTE, long-term extension; N, number of patients included in the analysis.
Discussion
Sex and gender are important health determinants; however, potential differences in drug efficacy and safety in males and females are not typically reported in clinical trials. In this post-hoc analysis of pooled data from two RCTs of tofacitinib in patients with active PsA, treatment with tofacitinib resulted in greater efficacy in both sexes than PBO at month 3 across most of the endpoints evaluated. While males and females entered the studies with differences in several PsA disease activity measures, similar response rates and improvements from baseline following tofacitinib treatment were observed between sexes, although females were less likely to achieve MDA, a desirable clinical treatment target. Regarding safety, females receiving tofacitinib 10 mg two times per day and PBO were more likely to experience TEAEs up to month 3, while TEAEs were similar across sexes up to month 12. These data complement a previous post-hoc analysis of tofacitinib RCT data in male and female patients with rheumatoid arthritis, where efficacy outcomes were generally comparable across sexes, although males were more likely to achieve stringent disease activity thresholds and improvement in physical function compared with females. Safety findings with tofacitinib did not reveal a consistent pattern between sexes.24
In the present analysis, at baseline, females had worse TJC, enthesitis, physical function, fatigue and HRQoL, and males had more dactylitis, worse psoriasis and higher levels of the inflammatory marker CRP. These differences between males and females with PsA are consistent with previous observational studies,10–12 25 showing a tendency for more severe scores in ‘pain-sensitive’ PsA disease characteristics in females, including higher TJC and tender entheseal count, but similar SJC, higher CRP levels and more dactylitis in males. Thus, female patients tend to score higher in disease measures that are affected by patient response to local pressure26 and are more subjective in nature, while similar or even worse scores in males are observed in clinical features that rely on physician assessment or laboratory measurement. These differences highlight that males and females may be affected differently by certain PsA domains; for example, pain and physical function have greater impact in females, versus psoriasis in males, which may inform their respective treatment strategies. Sex-related differences may also have an impact on clinical trial results.25 Reasons for the observed baseline differences between males and females may relate to intrinsic biological factors, such as increased central sensitisation in females, sex dysmorphism in immune profile, environmental factors and/or the measures of disease activity and patient-reported outcomes themselves, which may be subject to sex-related and gender-related biases.27 28
Despite baseline differences observed in the present study, predominantly similar improvements in musculoskeletal measures and patient-reported outcomes were observed between males and females receiving tofacitinib. However, at early time points, females had higher PASI75 rates and greater improvements in PGA-PsO. Højgaard et al reported significantly higher ACR responses in male versus female patients with PsA initiating TNFi. Males were also approximately 50% more likely to achieve ACR20 responses at 3 months and twice as likely at 6 months.3 A recent analysis found that female patients receiving ixekizumab had lower ACR20/50/70 response rates than males.17 Another analysis showed that ACR responses with secukinumab were higher in female patients, compared with ADA18; responses within both therapeutic classes in that trial were numerically higher in males compared with females. Due to the lack of studies, it is difficult to determine whether a favourable response in males with PsA is more pronounced with some classes of advanced therapies than for others. Reporting of additional RCT data and head-to-head analyses by sex is required to address this important issue.
A lower proportion of females versus males achieved MDA across multiple time points. While females more commonly met psoriasis criteria, fewer met the other MDA cut-off points. These findings are consistent with those from previous studies showing that females with PsA are less likely to achieve MDA irrespective of therapy.11 18 29–33 Worse scores at baseline likely explain the lower percentage of females achieving MDA states despite the similar efficacy of tofacitinib in males and females. Females are more likely to have multimorbidity, including depression and fibromyalgia, which may strongly influence pain and function.26 34–36 Although a known diagnosis of fibromyalgia was excluded in these RCTs, central sensitisation may have influenced symptom burden and outcomes.27 Similarly, depression may have also contributed, as females were almost three times more likely to suffer from depression.
Increased discontinuation due to AEs in females compared with males with PsA receiving TNFi has been reported in analyses of Swedish healthcare registers,9 which may indicate biases towards AE reporting and perceptions of higher disease severity in females.13 A prospective, observational study of patients with psoriasis on systemic therapies found that females experience more frequent SAEs, which are less likely to be over-reported, pointing to a potential biological basis for the observed differences in safety outcomes between sexes.13 In the present study, through month 3, TEAEs were numerically higher in females compared with males receiving tofacitinib 10 mg two times per day; however, similar results for TEAEs were also reported with PBO, suggesting the trends may be attributed to the aforementioned gender-based or sex-based factors. To month 12, TEAEs in tofacitinib-treated patients were similar between sexes. The observed number of SAEs and discontinuation due to AEs after stratifying by sex becomes too low to interpret, and no notable trends in the type or frequency of AESIs were apparent with tofacitinib.
Persistence of tofacitinib in PsA has not been reported to date. In the analysis of open-label LTE data, persistence was similar between male and female patients in the average tofacitinib 5 mg two times per day group through 42 months. A trend towards greater persistence was observed in males, compared with females, in the average tofacitinib 10 mg two times per day group, for all-cause discontinuation and discontinuation due to lack of efficacy, although CIs of the 1-year, 2-year and 3-year survival rates overlapped. It should be noted that treatment persistence tends to be higher in clinical trials than in real-life settings, and this lack of sex differences in tofacitinib persistence awaits further confirmation in observational studies. Observational studies have shown differences in persistence between males and females with other advanced therapies in PsA. A study from Denmark showed that females had shorter drug persistence than males with PsA who were initiating their first TNFi.3 Other observational studies showed that female sex is an independent predictor of early discontinuation of biological therapies.6–8 37 38
We acknowledge that this study had several limitations. First, this was a post-hoc analysis of data pooled from two patient populations with PsA: TNF-naïve and TNF-inadequate responder (IR) patients. TNF-IR patients are represented up to month 6, and data could only be compared with PBO up to 3 months. The small number of patients in the ADA treatment arm limited the ability to draw firm conclusions about sex differences in treatment responses due to limited power. Sex differences in safety outcomes, particularly AESIs, could not be comprehensively assessed due to the limited follow-up period in this study and the limited number of patients. Additionally, because patients were eligible to enter the LTE study if they had completed the phase 3 RCTs or had discontinued them due to non-study drug-related AEs, analysis of time to discontinuation was performed in patients who were known to be responsive to, and had tolerated tofacitinib. Furthermore, flexibility of dosing in the LTE study and the use of an ‘average’ dosing algorithm precluded the ability to make dose comparisons. The main strength of these analyses was the ability to avoid inherent biases that affect observational studies, such as gender biases in treatment allocation and clinical management, based on a blinded RCT design. Completeness of data and availability of LTE data allowed us to perform an in-depth analysis of sex differences of tofacitinib efficacy considering all core PsA domains, as well as assess safety and treatment persistence across both male and female patients.
In summary, we reported differences in baseline disease characteristics between males and females participating in the phase 3 RCTs of tofacitinib in PsA. Regardless of sex, treatment with tofacitinib resulted in greater efficacy, compared with PBO, at month 3. Males and females achieved similar ACR responses and similar improvements from baseline in musculoskeletal, skin and patient-reported outcomes, but females were less likely to achieve MDA, likely due to baseline differences in disease expression. Overall, safety and time to discontinuation were generally similar between sexes. Our results are consistent with previous studies in PsA showing sex differences in disease activity and impact, as well as lower chances of achieving MDA states in female patients with other treatments. Sex (biological)-related and gender (sociocultural)-related mechanisms may underlie these differences and require additional research. Exploratory analyses of clinical trials by sex will allow future assessment of whether sex differences in drug efficacy and safety differ across classes of advanced therapies and will help to inform personalised and equitable clinical management across men and women with PsA.
Acknowledgments
Manuscript formatting/editorial support was provided by Justine Juana, Lewis C Rodgers and Gillian Becker, at CMC Connect, a division of IPG Health Medical Communications, and funded by Pfizer; no contribution was made to intellectual content.
Footnotes
Contributors: Conceptualisation of study and design—LE, RAP, RL, MJC, CK and VS. Acquisition of data—PM. Analysis of data—DDG, MJC and VS. Interpretation of data—LE, DDG, PM, RAP, RL, SZA, AO, AP, DG, MJC, CK and VS. Guarantor of study—LE. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication.
Funding: This study was sponsored by Pfizer.
Competing interests: LE has served as a consultant for AbbVie, Eli Lilly, Janssen, Novartis, Pfizer and UCB, and has received grant and/or research support from AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz and UCB. DDG has served as a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead Sciences, Janssen, Novartis, Pfizer and UCB, and has received grant and/or research support from AbbVie, Amgen, Celgene, Eli Lilly, Novartis, Pfizer and UCB. PM has served as a consultant for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Sciences, Inmagene, Janssen, Novartis, Pfizer, Sun and UCB, and has received grant and/or research support from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Sciences, Janssen, Novartis, Pfizer, Sun and UC. RAP, RL, DG, MJC and CK are employees and shareholders of Pfizer. SZA has served as a consultant for AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB, has received grant and/or research support from AbbVie, Celgene, Eli Lilly, Novartis, Pfizer and Sanofi, and has received speakers’ fees from AbbVie, Novartis and Pfizer. AO has received grant and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Eli Lilly, Novartis and Pfizer, and is a shareholder of Amgen, Novartis and Pfizer. VS has served as a consultant for AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Inmedix, Janssen, Kiniksa, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sofusa, Spherix and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This was a post-hoc analysis of two randomised controlled trials and one long-term extension study. All studies were conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Guidelines for Good Clinical Practice. The study protocols were approved by the Institutional Review Board and/or Independent Ethics Committee for each study centre. All patients provided written informed consent.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD. Recent advances in understanding and managing psoriatic arthritis. F1000Res 2016;5:2670. 10.12688/f1000research.9592.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Højgaard P, Ballegaard C, Cordtz R, et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology (Oxford) 2018;57:1651–60. 10.1093/rheumatology/key140 [DOI] [PubMed] [Google Scholar]
- 4.Vieira-Sousa E, Eusébio M, Ávila-Ribeiro P, et al. Real-world longterm effectiveness of tumor necrosis factor inhibitors in psoriatic arthritis patients from the Rheumatic Diseases Portuguese Register. J Rheumatol 2020;47:690–700. 10.3899/jrheum.181272 [DOI] [PubMed] [Google Scholar]
- 5.Chimenti MS, Triggianese P, Conigliaro P, et al. A 2-year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin Rheumatol 2017;36:2253–60. 10.1007/s10067-017-3769-4 [DOI] [PubMed] [Google Scholar]
- 6.Stober C, Ye W, Guruparan T, et al. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology (Oxford) 2018;57:158–63. 10.1093/rheumatology/kex387 [DOI] [PubMed] [Google Scholar]
- 7.Navarini L, Costa L, Tasso M, et al. Retention rates and identification of factors associated with anti-TNFα, anti-IL17, and anti-IL12/23R agents discontinuation in psoriatic arthritis patients: results from a real-world clinical setting. Clin Rheumatol 2020;39:2663–70. 10.1007/s10067-020-05027-1 [DOI] [PubMed] [Google Scholar]
- 8.Ramonda R, Lorenzin M, Carriero A, et al. Effectiveness and safety of secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study. RMD Open 2021;7:e001519. 10.1136/rmdopen-2020-001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geale K, Lindberg I, Paulsson EC, et al. Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract 2020;4:rkaa070. 10.1093/rap/rkaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eder L, Thavaneswaran A, Chandran V, et al. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis 2013;72:578–82. 10.1136/annrheumdis-2012-201357 [DOI] [PubMed] [Google Scholar]
- 11.Orbai A-M, Perin J, Gorlier C, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res (Hoboken) 2020;72:1772–9. 10.1002/acr.24090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Queiro R, Tejón P, Coto P, et al. Clinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. Clin Dev Immunol 2013;2013:482691. 10.1155/2013/482691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Fernández CP, Carretero G, Rivera R, et al. Effect of sex in systemic psoriasis therapy: differences in prescription, effectiveness and safety in the BIOBADADERM prospective cohort. Acta Derm Venereol 2021;101:1479. 10.2340/00015555-3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol 2020;45:705–11. 10.1111/ced.14218 [DOI] [PubMed] [Google Scholar]
- 15.Saad AA, Ashcroft DM, Watson KD, et al. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther 2009;11:R52. 10.1186/ar2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Bosch F, Manger B, Goupille P, et al. Effectiveness of adalimumab in treating patients with active psoriatic arthritis and predictors of good clinical responses for arthritis, skin and nail lesions. Ann Rheum Dis 2010;69:394–9. 10.1136/ard.2009.111856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eder L, Tony H-P, Odhav S, et al. Responses to ixekizumab in male and female patients with psoriatic arthritis: results from two randomized, phase 3 clinical trials. Rheumatol Ther 2022;9:919–33. 10.1007/s40744-022-00445-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright G, Nash P, Coates L, et al. Comparison of secukinumab versus adalimumab efficacy by sex in psoriatic arthritis from a phase 3b, double-blinded, randomized, active-controlled study. Arthritis Rheumatol 2020;72:0507. [Google Scholar]
- 19.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 20.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. 10.1056/NEJMoa1615977 [DOI] [PubMed] [Google Scholar]
- 21.Nash P, Coates LC, Fleishaker D, et al. Safety and efficacy of tofacitinib up to 48 months in patients with active psoriatic arthritis: final analysis of the OPAL Balance long-term extension study. Lancet Rheumatol 2021;3:e270–83. 10.1016/S2665-9913(21)00010-2 [DOI] [PubMed] [Google Scholar]
- 22.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 23.Nash P, Mease PJ, Fleishaker D, et al. Tofacitinib as monotherapy following methotrexate withdrawal in patients with psoriatic arthritis previously treated with open-label tofacitinib plus methotrexate: a randomised, placebo-controlled substudy of OPAL Balance. Lancet Rheumatol 2021;3:e28–39. 10.1016/S2665-9913(20)30339-8 [DOI] [PubMed] [Google Scholar]
- 24.Jones HN, Strand V, Schulze-Koops H, et al. POS0652 Sex differences in the efficacy and safety of tofacitinib in rheumatoid arthritis patients: a post hoc analysis of phase 3 and long-term extension trials. Ann Rheum Dis 2021;80:565–6. 10.1136/annrheumdis-2021-eular.359 [DOI] [Google Scholar]
- 25.Gladman DD. Sex effect in psoriatic arthritis. J Rheumatol 2023;50:159–60. 10.3899/jrheum.220948 [DOI] [PubMed] [Google Scholar]
- 26.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017;29:304–10. 10.1097/BOR.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 28.Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 2009;11:R7. 10.1186/ar2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease PJ, Gladman DD, Merola JF, et al. Potential impact of sex and BMI on response to therapy in psoriatic arthritis: post hoc analysis of results from the SEAM-PsA trial. J Rheumatol 2022;49:885–93. 10.3899/jrheum.211037 [DOI] [PubMed] [Google Scholar]
- 30.Perrotta FM, Marchesoni A, Lubrano E. Minimal disease activity and remission in psoriatic arthritis patients treated with anti-TNF-α drugs. J Rheumatol 2016;43:350–5. 10.3899/jrheum.150805 [DOI] [PubMed] [Google Scholar]
- 31.Haddad A, Thavaneswaran A, Ruiz-Arruza I, et al. Minimal disease activity and anti-tumor necrosis factor therapy in psoriatic arthritis. Arthritis Care Res (Hoboken) 2015;67:842–7. 10.1002/acr.22529 [DOI] [PubMed] [Google Scholar]
- 32.Queiro R, Cañete JD, Montilla C, et al. Minimal disease activity and impact of disease in psoriatic arthritis: a Spanish cross-sectional multicenter study. Arthritis Res Ther 2017;19:72. 10.1186/s13075-017-1277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchlin CT, Mease PJ, Boehncke W-H, et al. Sustained and improved guselkumab response in patients with active psoriatic arthritis regardless of baseline demographic and disease characteristics: pooled results through week 52 of two phase III, randomised, placebo-controlled studies. RMD Open 2022;8:e002195. 10.1136/rmdopen-2022-002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brikman S, Furer V, Wollman J, et al. The effect of the presence of fibromyalgia on common clinical disease activity indices in patients with psoriatic arthritis: a cross-sectional study. J Rheumatol 2016;43:1749–54. 10.3899/jrheum.151491 [DOI] [PubMed] [Google Scholar]
- 35.Wong A, Ye JY, Cook RJ, et al. Depression and anxiety reduce the probability of achieving a state of sustained minimal disease activity in patients with psoriatic arthritis. Arthritis Care Res (Hoboken) 2022;74:1430–4. 10.1002/acr.24593 [DOI] [PubMed] [Google Scholar]
- 36.McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol 2014;41:887–96. 10.3899/jrheum.130797 [DOI] [PubMed] [Google Scholar]
- 37.Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. 10.1002/art.30117 [DOI] [PubMed] [Google Scholar]
- 38.Sewerin P, Borchert K, Meise D, et al. Real-world treatment persistence with biologic disease-modifying antirheumatic drugs among german patients with psoriatic arthritis—a retrospective database study. Rheumatol Ther 2021;8:483–97. 10.1007/s40744-021-00286-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002718supp001.pdf (599.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.