Abstract
The rodent malaria Plasmodium berghei is one of a small number of species of Plasmodium that can currently be genetically transformed through experimentally controlled uptake of exogenous DNA by bloodstage parasites. Circular DNA containing a selectable marker replicates and is maintained under selection pressure in a randomly segregating episomal form during the first weeks after transformation. In this study, using pulsed field gel electrophoresis and ionising radiation, we show that in dividing asexual blood stage parasites the episomes are completely converted, within 2 weeks post-infection, into non-rearranged circular concatamers ranging in size between about 9 and 15 copies of the monomer. These occur as slow-moving aggregates held together by radiation-sensitive linkers consisting partly of single-stranded DNA. The process generating these complexes is not clear but 2D gel analysis showed that Cairns-type replication origins were absent and it seems most likely that the initial concatamerisation takes place using a rolling circle mechanism followed by circularisation through internal recombination. We propose a model in which continued rolling circle replication of the large circular concatamers and the recombinational activity of the tails of the rolling circles could lead to the formation of the large aggregates.
INTRODUCTION
The blood stages of several species of Plasmodium can be induced (or are able) to take up foreign plasmid DNA and express genes carried by those plasmids under the control of promoters derived from Plasmodium. Expression of mutant forms of dihydrofolate reductase/thymidylate synthase genes derived from various sources has been used to confer resistance to antifolate inhibitors, allowing selection of transformants from several species of Plasmodium on the basis of acquired drug resistance (1–8). Many circular bacterial or eukaryotic plasmids, either natural or chimeric, normally initiate replication bi-directionally at a single site (‘Cairns-type’ origin). The replication mechanism of plasmids transfected in malaria is uncertain, but the apparent copy number of the transfected plasmid DNA in Plasmodium is variable, being dependent on both the nature of the episome concerned and the duration and level of selection pressure (8). Moreover, in the absence of selection, transformed populations of both Plasmodium berghei and Plasmodium falciparum lose their episomes (1,2,4,8). These properties may be explained partly by the absence of any precise mechanism for segregation of circular molecules, but also by the structure adopted by the episomal DNA in vivo, since it has recently been shown that, at least in the case of P.falciparum, small transfected plasmids are rapidly converted into relatively large circular concatamers in complex aggregates (9). Segregation of such large entities may be intrinsically inefficient, and in the absence of selection they would be lost by default amongst a growing population. Formation of circular concatamers in this way may involve some element of recombination, and for Plasmodium species this possibility has been underlined in a recent study showing that two different plasmids in P.falciparum will recombine to generate chimeric hybrids. This recombination is thought to be homologous and mediated by common elements of Plasmodium DNA maintained in both recombining plasmids (10).
Against this background we have chosen to study the episomal DNA of P.berghei transfected with a non-integrating plasmid, using ionising radiation to dissect its topology, and Brewer/Fangman 2D gels to detect replication intermediates. We show that in dividing asexual blood stage parasites of the rodent malaria P.berghei, as in P.falciparum, a well-characterised episome is rapidly and completely converted into non-rearranged circular concatamers ranging in size between 9 and 15 copies of the monomer, and is maintained in large multi-circle aggregates by radiation-sensitive stretches of DNA. The Brewer/Fangman 2D gels revealed the absence of conventional Cairns-type replication origins, and replication most probably takes place through a rolling circle mechanism. The recombinational activities of the linear tails resulting from this replication may lead to the formation of the large multi-circle aggregates.
MATERIALS AND METHODS
Organisms, cultures and plasmid
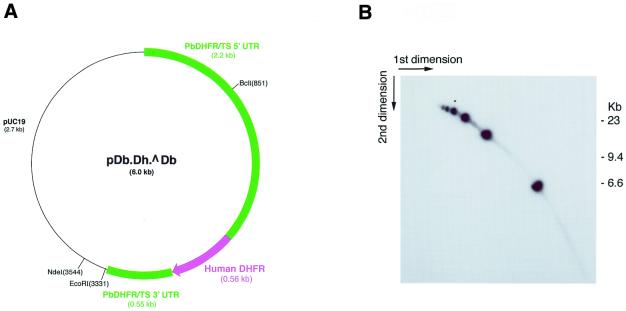
Clone 101.4 of P.berghei was used in this study. This parasite contains a 6 kb plasmid, pDb.Dh.^Db, which confers resistance to two antimalarials, pyrimethamine and WR99210, through expression of the human DHFR gene under control of a copy of the endogenous P.berghei DHFR/ts gene (8). Synchronous cultures for the isolation of the plasmid during replication were made as described (11), and the parasites harvested 20 h after onset of the asexual blood stage cycle, when the parasite nuclei were actively dividing. Other preparations were made from asynchronous parasite cultures. All preparations were routinely passed through Plasmodipur filters to remove contaminating host cells prior to DNA extraction (12). A partial restriction map of plasmid pDb.Dh.^Db, showing the restriction sites used in this work, is presented in Figure 1A.
Figure 1.
(A) Map and partial restriction sites of the plasmid used in these studies. (B) Brewer/Fangman 2D gel analysis of a partial NdeI digest, probed with pUC19. The blobs form an oligomeric series from 6 kb upwards.
DNA handling techniques
For analysis by Brewer/Fangman 2D gel electrophoresis, DNA was extracted from filter-purified infected erythrocytes and the crude extracts fractionated by isopycnic centrifugation in CsCl/DAPI gradients in a vertical rotor as described (13). The DAPI-labelled DNA fractions were visualised using 380 nm UV light and recovered by side puncture. The plasmid DNA was not resolved as a separate band in these gradients, but testing of various fractions by hybridisation revealed one that was plasmid enriched. This was diluted in sterile saline (NaCl, 0.09% w/v), pelleted at 48 000 r.p.m. (260 000 g) for 2.5 h in a Beckman SW55 rotor, and re-suspended in an appropriate volume of TE (0.01 M Tris–HCl pH 8.0, 0.001 M EDTA). DAPI was not removed from the isolated samples, experience showing that it does not interfere with subsequent restriction digestion or hybridisation. Whole cell DNA, for restriction analysis of certain samples from synchronised parasite cultures containing a majority of dividing cells (20 h after erythrocyte re-invasion), was prepared by standard methods (14).
Pulsed field gel electrophoresis and ionising irradiation
For pulsed field gel electrophoresis (PFGE) analysis and irradiation experiments, plugs of whole cells were made by standard methods in low melting agarose (Seaplaque, GCG) and extracted with sodium lauryl sarcosinate in the presence of proteinase K (15). PFGE was carried out in a custom-built CHEF apparatus (16) using gels containing 1.0% chromosomal grade agarose (BRL) in 0.25× TBE (0.025 M Tris, 0.025 M boric acid, 0.0625 M EDTA). The pulsing regime comprised a linear ramp of 90–300 s at 3.8 V/cm for 32 h, followed by a second linear ramp of 300–720 s at 3.4 V/cm for 32 h. Temperature was maintained at ∼13°C. This regime, slightly modified from Hinterberg and Scherf (17), provides an extensive spread of linear molecules up to at least the size of the largest malarial chromosomes. Markers used were the NO350 low range marker of New England Biolabs (a lambda ladder plus HindIII lambda fragments) and Saccharomyces cerevisiae strain YP148 (courtesy of P. Hieter, Centre for Molecular Medicine and Therapeutics, University of British Colombia, Vancouver, Canada). The latter had been engineered to separate chromosome 15 into two fragments of ∼120 and 1050 kb, respectively, both of which carry targets that hybridise to pUC19. On completion, the DNA in the gels was depurinated by acid treatment, transferred by alkali blotting to charged nylon membranes (Hybond N+; Amersham) and hybridised at high stringency using standard methods to 32P-labelled pUC19, which was prepared by random priming (18). Irradiation of either agarose plugs or tracks cut from pulsed field gels was carried out by exposure to γ-rays from a 60Co source for required times. During irradiation the samples were suspended in ice-cold water.
Electrophoretic analysis of replication intermediates
Neutral/neutral 2D gel electrophoresis for analysis of replication intermediates was carried out essentially as described (19), with minor adjustments to the times of running in each dimension. On completion of electrophoresis, the DNA was transferred in alkali to a Hybond N+ membrane (Amersham), and hybridised at high stringency to 32P-labelled pUC19 DNA as described above.
RESULTS
The topology of the transfected plasmid 2 weeks post-inoculation was explored using both conventional gel electrophoresis and PFGE augmented with exposure to ionising radiation. Neutral/neutral 2D gel electrophoresis was used to detect recombination and replication intermediates.
Restriction analysis
Agarose gel electrophoresis and Southern blotting of transfected plasmid DNA, digested to completion with either of the single-cutting restriction endonucleases EcoRI or BclI, revealed a single 6 kb band (data not shown). This suggested that the plasmid was non-rearranged and its copy number was known to be up to 20 per ring stage cell in the presence of drug (8). However, similar probing of undigested DNA revealed that the plasmid DNA hardly entered unidirectional agarose gels and, in particular, no bands corresponding to relaxed or twisted monomeric circles were detected (data not shown). A more detailed investigation was made by probing a partial NdeI digest of transfected plasmid DNA electrophoresed in a Brewer/Fangman 2D gel format. As shown in Figure 1B, the DNA comprised an oligomeric series of linear 6 kb molecules up to ∼6mers in size, but a heavier exposure (data not shown) revealed further oligomers up to at least 9mer in size. From this it is likely that all the plasmid DNA actually occurred in the form of large head-to-tail tandem arrays.
Pulsed field gel electrophoresis and irradiation
Pulsed field gel analysis in combination with ionising radiation (γ-rays) confirmed the overall large size of the transfected plasmid molecules and allowed analysis of the complex structures in which they occurred.
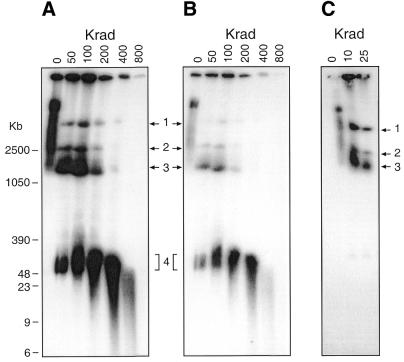
Figure 2 illustrates the results obtained with PFGE of the sample exposed to a range of γ-ray doses from 0 to 800 Krad. The non-irradiated plasmid molecules formed a long smear occupying roughly the top quarter of the gel, its leading edge migrating to a position between 1 and 2.5 Mb linear markers. This broad spread in mobility indicates a considerable heterogeneity in the molecular structure of the target molecules, the more so because the smear itself is not homogeneous, the major ‘blob’ at the top end (best seen in Fig. 2B) being distinguishable from the more evenly spread bulk of the majority.
Figure 2.
(A) PFGE analysis of the structure of transfected plasmid DNA following ionising radiation doses of 50–800 Krad. (B) Shorter exposure of the same autoradiograph. (C) Lower radiation doses: note the structure began to be disrupted after only 10 Krad. Bands numbered 1–4 are referred to in the text.
Exposure to moderate radiation doses resolved this smear into four major components (labelled 1–4 in Fig. 2). These are readily apparent at 50–100 Krad and above, but it is significant that, as shown in Figure 2C, this disruption started even at 10 Krad, a dose which normally has little measurable effect on the gel mobility of double-stranded DNA molecules, as demonstrated by the stability of the linear double-stranded products at band 4, which only showed significant fragmentation at doses of 200 Krad and above. This hypersensitivity of the plasmid complexes to irradiation suggests that single-stranded DNA molecules are intrinsic to their maintenance. From 200 Krad upwards, bands 1–3 were in sharp decline, whilst the blob of material in the band 4 position, which had increased throughout the dose range, showed increasing signs of random fragmentation.
Band 3 is accompanied by a distinct trailing smear (best seen in Fig. 2B). Bands 1 and 2 seem relatively discrete, but smears on these two slower moving bands may not have been resolvable on the gels. More importantly, the fact that no doses, even up to 400 Krad, generated forward-running smears contiguous with any of bands 1–3 shows conclusively that the latter did not consist of linear molecules. The products of their breakage presumably migrated in the position of the blob of evidently heterogeneous material at band 4 giving rise to the increased intensity and trailing smear of band 4 noted above. By contrast, a leading smear of band 4 was generated by exposure to irradiation doses of 200 Krad and above, yet throughout the dose range no discrete bands running faster than band 4 were detectable in the CHEF gels, even in heavily over-exposed films (data not shown). The lack of such small discrete bands is a clear indication that no circular 6 kb monomers remained in the plasmid population. It was noted that throughout the range of doses, some material remained in the wells, though the amount decreased sharply at 200 Krad and was trivial at 800 Krad.
2-Dimensional PFGE
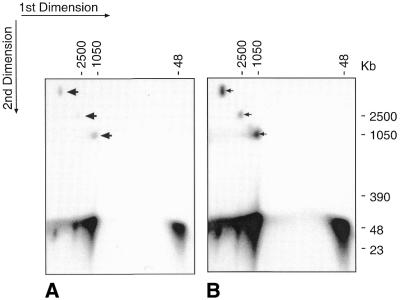
It has been established that in PFGE gels, circular molecules migrate anomalously as compared with linear ones, and their contour lengths can therefore only be determined from linearised forms. As already discussed, it was clear from the PFGE gels shown in Figure 2 that bands 1–3 represented circular forms of the original transfected plasmid, so any linear products generated by irradiation-induced double-strand breakage of these bands were presumably part of the mass of band 4. In order to directly visualise these linear fragments separately from that of band 4 and from each other, we ran 2D PFGE gels. A track cut from a CHEF gel of a sample previously exposed to 100 Krad was treated with a further 100 Krad before repeating the electrophoresis, using the same pulsing protocol, in a 90° orientation. The result is shown in Figure 3A. Bands 1, 2 and 3 each gave rise, as expected, to molecules running in about the position of band 4, seen to be linear by virtue of fragmentation following the second irradiation. The longer exposure of the same film (Fig. 3B) clearly shows there were no intermediates migrating between the residual circular bands and the linear products. Another PFGE run (not shown), using a protocol designed to expand the ‘band 4’ region, indicated that these linear molecules varied slightly in size, but all three fell within a size ranging between 9mers and 15mers.
Figure 3.
2D PFGE analysis. (A) A track cut from a PFGE gel of a sample that had been exposed to 100 Krad of γ-rays was treated with a further 100 Krad, before being subjected to PFGE in a 90° orientation. Arrows show residual portions of bands 1–3. (B) Longer photographic exposure of same gel, showing lack of intermediate forms between bands 1–3 and the linear radiation products.
Analysis of replication intermediates
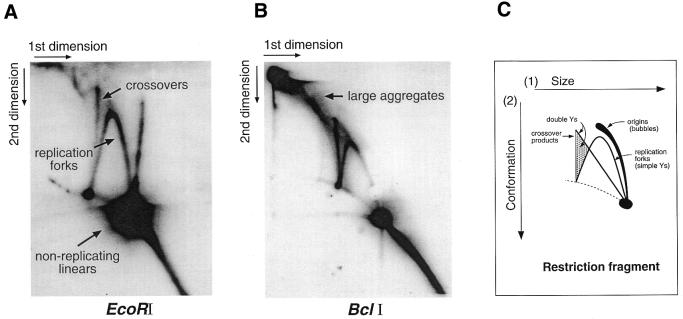
Neutral/neutral Brewer/Fangman 2D gel analysis, which permits detection of intermediates in DNA replication and recombination (19), was used in a preliminary attempt to explore the way the transfected plasmid replicates within the parasite. In this technique, using low voltage and agarose concentrations, DNA molecules are first separated exclusively on the basis of size. In the second dimension, using increased voltage and agarose concentration in the presence of ethidium bromide, DNA migration is influenced mainly by molecular topology. The net result in the subsequently blotted and hybridised gel is a selection of well-documented patterns such as those illustrated in Figure 4C.
Figure 4.
Brewer/Fangman 2D gels of transfected plasmid DNA (A) cut with EcoRI and (B) cut with BclI. Both were probed with pUC19. (C) Schematic diagram of the main autoradiographic patterns that can be generated by this technique. The gap in the replication fork arc in B is a blotting artefact. See text for further explanation.
Non-irradiated DNA isolated from late stage (replicating) parasites was restricted with either EcoRI or BclI, which cut at single sites on opposite sides of the plasmid (Fig. 1A). The cut DNA was separated using 2D gel electrophoresis and then hybridised to pUC19, a probe for the plasmid backbone. In both the EcoRI (Fig. 4A) and BclI (Fig. 4B) digests, a range of replication intermediates was detected. Both samples generated strong signals for replication forks, in addition to a prominent crossover spike resulting from recombination (19).
In the BclI digest (Fig. 4B) there is, in addition to these clearly identifiable products, a substantial amount of material smearing towards the left hand upper corner of the gel (labelled ‘large aggregates’). Large, structurally complex material like this can result from massive recombination interactions in multi-copy populations undergoing recombination-dependent replication (20). Alternatively, since the restriction digestion of this sample was evidently complete (compare with the partial digest of Fig. 1B), the large molecules comprising these aggregates may have resisted digestion because cutting sites were blocked, for instance if they were in a single-stranded conformation. In the EcoRI digest (Fig. 4A) the amount of this material was appreciably reduced. One striking feature of this digest, however, is the prominent spike initiating from the position of non-replicating linear molecules. This spike is completely absent from the BclI digest, and was significantly reduced when DNA from early (non-replicating) parasites was analysed (data not shown), indicating it may be a replication-related product. In none of the samples analysed was there any evidence for replication bubbles, even in heavily over-exposed filters (data not shown). The use of two restrictions enzymes cutting at sites widely separated on the plasmid avoided the risk of inadvertantly cutting a bubble, and it is abundantly clear that replication of the episomal DNA occurred by a mechanism independent of Cairns-type origins.
DISCUSSION
This study was initiated in the knowledge that the transfected plasmid we used is segregationally unstable in the absence of drug selection, and is even slow growing in its presence (2), the average cellular copy number of ∼16 obscuring a wide variation in the amount of plasmid DNA inherited by individual merozoites (4). We hoped that detailed scrutiny of the topology of the plasmid in vivo would illuminate this problem and also possibly lead to identification of an origin of replication derived from a chromosome. These aims have been partially successful, since by the time we took our first sample, 2 weeks post-infection, the plasmid molecules in vivo were represented entirely by oligomeric head-to-tail tandem arrays of 9–15mer size, held together in large complexes. This topology is entirely consistent with their instability, since in the absence of a precise regulated mechanism, segregation of the large entities we observed is likely to be intrinsically inefficient, causing their loss by default in a growing population. However, the search for a potential chromosomal origin of replication was unsuccessful. 2D gel examination of restriction-digested DNA revealed no trace of bubble patterns even after prolonged photographic exposure of the filters. This effectively ruled out the possibility that even a few of the copies in a tandem array might be carrying active Cairns origins; clearly the plasmid molecules were not simply replicating as free monomers in a conventional theta mode. Also, given the absence of any more than moderate recombinational activity, rolling circle replication is left as by far the most likely explanation of the generation of tandem arrays.
Disruption of the initial complex by exposure to low-level ionising radiation and subsequent PFGE analysis showed that its constituent tandem oligomers were in fact three different sized circular molecules, with contour lengths in a range between about 54 (9mer) and 90 kb (15mer), forming three discrete slow-moving bands in the pulsed field gels. The fact that they were liberated from the primary mass by relatively low radiation exposure indicated they were somehow linked together with DNA that was either single-stranded or had single-stranded stretches.
Any model to explain the topology of the plasmid DNA must account not only for the formation of the large circular concatamers and the involvement of single-stranded DNA in their linkage together, but should also embrace the results obtained with the Brewer/Fangman 2D gels of restriction-digested DNA. The crossover spike and the large aggregates observed in these gels provides clear evidence for recombination occurring in the transfected plasmid molecules, and recombination has been shown to occur between plasmids in multiple transfectants of the related malarial parasite P.falciparum (10). Recombination could in part contribute to the initial formation of larger oligomers, and while a recombination-dependent form of DNA replication has been proposed for the mitochondrial DNA of P.falciparum (20), this mechanism alone cannot account for the overall structure of the molecules we observed in P.berghei.
It has been suggested that in Leishmania tarentolae (21), and in a solitary example in Trypanosoma brucei (22), oligomerisation may have been a prerequisite for efficient episomal replication of otherwise weak replicons. However, our 2D gel analysis ruled out even the rare occurrence of Cairns-type origins in the P.berghei system, and in this light it seems much more likely that the observed oligomerisation was a consequence of rolling circle replication, rather than a prerequisite for stable maintenance.
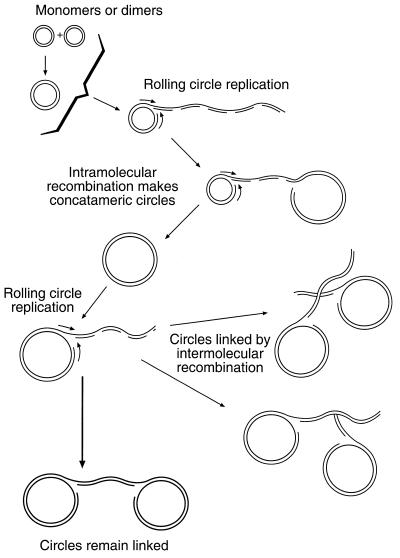
Linear tails generated by rolling circle activity are in all probability endowed with unprotected termini, which would be actively recombinogenic, and would be very likely to recombine homologously with themselves. Such internal homologous recombination within one molecule would normally be expected to give rise to large circles, topological or other constraints presumably acting, in this case, to restrict their size to within the 9mer to 15mer range. This process would, of course, explain the nearly complete absence of free linear oligomers. However, in a situation clearly foreign to the parasite, the generation of circles in this way might fail to be completed, leading to the formation of complex bi-circular molecules we have dubbed ‘handcuffs’. In addition, similar complex structures could be generated by recombinational interactions between large rolling circles. Various possibilities are illustrated in Figure 5.
Figure 5.
Schematic model, not drawn to scale, for the replication and structure of the transfected plasmids. Where co-transfection occurs, the plasmids may recombine, but replication from either monomers or higher forms (e.g. dimers) takes place by a rolling circle mechanism. The resulting linear tails enter into internal homologous recombination, sometimes generating free circular multimers, constrained in unknown ways to be within the observed 9mer to 15mer size range. In some cases the new circular entity fails to be separated, giving rise to an obvious ‘handcuff’ molecule. This may be the main product. Other variations on the handcuff theme may arise if the new circles start rolling circle replication and their linear tails recombine homologously with other lariats. The linear tails, undergoing discontinuous replication, bear transient single-stranded regions, represented only formally here, which account for the sensitivity of the handcuffs to low doses of ionising radiation, and may explain some aspects of the results of restriction digestion, as discussed in the text.
A common feature of these structures is that they all involve the large circular oligomers tied together by linear DNA molecules carrying heterogeneous single-stranded regions. By virtue of their conformation and size, these linked pairs would be extremely slow moving in gels, but readily disrupted by low exposure to ionising radiation. Consistent with this, both the circles themselves and the linear oligomers generated from them by higher radiation doses exhibited short trailing smears indicative of attached fragments of other DNA molecules. Finally, the single-stranded segments in the linking molecules would prevent restriction digestion of a proportion of the handcuffs, thus giving rise to the large complex aggregates especially notable in the 2D gel of BclI-cut DNA. An unexplained feature of the EcoRI-cut 2D gel (Fig. 4A) is the prominent vertical spike arising from the blob of uncut linear molecules. We suggest that this pattern, absent from the BclI digest (Fig. 4B), might reflect the occurrence of single-stranded regions traversing some BclI sites. In this case, flanking EcoRI sites would cut out fragments of nearly 6 kb size, but containing a small single-stranded region which would allow conformational changes and therefore variable migration of individual fragments in the second dimension of the 2D gels, thus giving rise to the spike pattern.
The origin of replication of the rolling circles is a matter of some interest, but cannot be detected using the 2D gel technique, since in this procedure rolling circle origins generate the same Y-form pattern as replication forks. Their identification by any other means would be beyond our capability, but in any case, such analysis may have limited value, as it has been shown that in the human malaria P.falciparum, replication of transfected plasmids does not require specific nucleotide sequences (9). Whether or not this is the case for P.berghei remains to be seen, but it may be a common feature of ancient microbial parasites, since it also seems to be the case in the kinetoplastid organisms L.tarentolae and Leptomonas (21,23). It may be relevant that in several kinetoplastids, (Leishmania mexicana, Leishmania donovani, T.brucei, Trypanosoma cruzi and Leptomonas seymouri) successful stable non-integrative transfection with small circular plasmids has, in several cases, led to the transfected molecules being converted into circular concatamers (22–24). To our knowledge the work we report here is the first time a 2D gel analysis for replication intermediates has been applied to an episome in a transfected protozoan parasite, and our direct evidence for the lack of a Cairns-type origin, coupled with the generation of concatamers, has implications for the replication processes in these same kinetoplastids, where concatameric circles were also observed. Given the demonstration in P.falciparum (9), Leishmania (21) and Leptomonas (23) that concatameric episomes in each of these protozoans are generated by replication processes requiring no organism-specific origins for initiation, a pattern begins to emerge. It is conceivable that the rolling circle mode of replication is a primitive mechanism, deep-rooted in evolution, and still available in these ancient protozoans when faced with a potentially useful exogenous invader.
REFERENCES
- 1.Waterkeyn J.G., Crabb,B.S. and Cowman,A.F. (1999) Transfection of the human malaria parasite Plasmodium falciparum. Int. J. Parasitol., 29, 945–955. [DOI] [PubMed] [Google Scholar]
- 2.van Dijk M.R., Waters,A.P. and Janse,C.J. (1995) Stable transfection of malaria parasite blood stages. Science, 268, 1358–1362. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Sifri,C.D., Lei,H.H., Su,X.Z. and Wellems,T.E. (1995) Transfection of Plasmodium falciparum within human red blood cells. Proc. Natl Acad. Sci. USA., 92, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dijk M.R., Vinkenoog,R., Ramesar,J., Vervenne,R.A., Waters,A.P. and Janse,C.J. (1997) Replication, expression and segregation of plasmid-borne DNA in genetically transformed malaria parasites. Mol. Biochem. Parasitol., 86, 155–162. [DOI] [PubMed] [Google Scholar]
- 5.van der Wel A.M., Tomas,A.M., Kocken,C.H., Malhotra,P., Janse,C.J., Waters,A.P. and Thomas,A.W. (1997) Transfection of the primate malaria parasite Plasmodium knowlesi using entirely heterologous constructs. J. Exp. Med., 185, 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocken C.H., van der Wel,A.M. and Thomas,A.W. (1999) Plasmodium cynomolgi: transfection of blood-stage parasites using heterologous DNA constructs. Exp. Parasitol., 93, 58–60. [DOI] [PubMed] [Google Scholar]
- 7.Mota M.M., Thathy,V., Nussenzweig,R.S. and Nussenzweig,V. (2001) Gene targeting in the rodent malaria parasite Plasmodium yoelii. Mol. Biochem. Parasitol., 113, 271–278. [DOI] [PubMed] [Google Scholar]
- 8.deKoning-Ward T.F., Fidock,D.A., Thathy,V., Menard,R., van Spaendonk,R.M., Waters,A.P. and Janse,C.J. (2000) The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome. Mol. Biochem. Parasitol., 106, 199–212. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell R.A., Preiser,P.R., Williamson,D.H., Moore,P.W., Cowman,A.F. and Crabb,B.S. (2001) An alteration in concatameric structure is associated with efficient segregation of plasmids in transfected Plasmodium falciparum parasites. Nucleic Acids Res., 29, 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadekoppala M., Cheresh,P., Catron,D., Ji,D., Deitsch,K., Wellems,T.E., Seifert,H.S. and Haldar,K. (2001) Rapid recombination among transfected plasmids, chimeric episome formation and trans gene expression in Plasmodium falciparum. Mol. Biochem. Parasitol., 112, 211–218. [DOI] [PubMed] [Google Scholar]
- 11.Janse C.J. and Waters,A.P. (1995) Plasmodium berghei: The application of cultivation and purification techniques to molecular studies of malaria parasites. Parasitol. Today, 11, 138–143. [DOI] [PubMed] [Google Scholar]
- 12.Janse C.J., Camargo,A., del Portillo,H., Herrera,S., Kumlien,S., Mons,B., Thomas,A. and Waters,A.P. (1994) Removal of leukocytes from Plasmodium vivax-infected blood. Ann. Trop. Med. Parasitol., 88, 213–216. [DOI] [PubMed] [Google Scholar]
- 13.Gardner M.J., Bates,P.A., Ling,I.T., Moore,D.J., McCready,S., Gunasekera,M.B.R., Wilson,R.J.M. and Williamson,D.H. (1988) Mitochondrial DNA of the human malarial parasite Plasmodium falciparum. Mol. Biochem. Parasitol., 31, 11–18. [DOI] [PubMed] [Google Scholar]
- 14.Paton M.G., Barker,G.C., Matsuoka,H., Ramesar,J., Janse,C.J., Waters,A.P. and Sinden,R.E. (1993) Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol., 59, 263–275. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989), Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 16.Chu G., Vollrath,D. and Davis,R.W. (1986) Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science, 234, 1582–1585. [DOI] [PubMed] [Google Scholar]
- 17.Hinterberg K. and Scherf,A. (1994) PFGE: Improved conditions for rapid and high-resolution separation of Plasmodium falciparum chromosomes. Parasitol. Today, 10, 225. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg A. and Vogelstein,B. (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem., 132, 6–13. [DOI] [PubMed] [Google Scholar]
- 19.Brewer B.J., Sena,E.P. and Fangman,W.L. (1988) Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Cancer Cells, 6, 229–234. [DOI] [PubMed] [Google Scholar]
- 20.Preiser P.R., Wilson,R.J.M., Moore,P.W., McCready,S., Hajibagheri,M.A.N., Blight,K.J., Strath,M. and Williamson,D.H. (1996) Recombination associated with replication of malarial mitochondrial DNA. EMBO J., 15, 684–693. [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou B., Roy,G. and Ouellette,M. (1994) Autonomous replication of bacterial DNA plasmid oligomers in Leishmania. Mol. Biochem. Parasitol., 65, 39–49. [DOI] [PubMed] [Google Scholar]
- 22.Ten Asbroek A.L., Mol,C.A., Kieft,R. and Borst,P. (1993) Stable transformation of Trypanosoma brucei. Mol. Biochem. Parasitol., 59, 133–142. [DOI] [PubMed] [Google Scholar]
- 23.Bellofatto V., Torres-Munoz,J.E. and Cross,G.A. (1991) Stable transformation of Leptomonas seymouri by circular extrachromosomal elements. Proc. Natl Acad. Sci. USA., 88, 6711–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly J.M., Ward,H.M., Miles,M.A. and Kendall,G. (1992) A shuttle vector which facilitates the expression of transfected genes in Trypanosoma cruzi and Leishmania. Nucleic Acids Res., 20, 3963–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]