Abstract
Inflammatory bowel disease (IBD) is considered a chronic inflammatory and multifactorial disease of the gastrointestinal tract. Crohn’s disease (CD) and ulcerative colitis (UC) are two types of chronic IBD. Although there is no accurate information about IBD pathophysiology, evidence suggests that various factors, including the gut microbiome, environment, genetics, lifestyle, and a dysregulated immune system, may increase susceptibility to IBD. Moreover, inflammatory mediators such as interleukin-6 (IL-6) are involved in the immunopathogenesis of IBDs. IL-6 contributes to T helper 17 (Th17) differentiation, mediating further destructive inflammatory responses in CD and UC. Moreover, Th1-mediated responses participate in IBD, and the antiapoptotic IL-6/IL-6 receptor (IL-6R)/signal transducer and activator of transcription 3 (STAT3) signals are responsible for preserving Th1 cells in the site of inflammation. It has been revealed that fecal bacteria isolated from UC-active and UC-remission patients stimulate the hyperproduction of several cytokines, such as IL-6, tumor necrosis factor-α (TNF-α), IL-10, and IL-12. Given the importance of the IL-6/IL-6R axis, various therapeutic options exist for controlling or treating IBD. Therefore, alternative therapeutic approaches such as modulating the gut microbiome could be beneficial due to the failure of the target therapies so far. This review article summarizes IBD immunopathogenesis focusing on the IL-6/IL-6R axis and discusses available therapeutic approaches based on the gut microbiome alteration and IL-6/IL-6R axis targeting and treatment failure.
Keywords: Inflammatory bowel disease, Interleukin 6, Crohn’s Disease, Ulcerative Colitis
Introduction
Inflammatory bowel disease (IBD) is known as a chronic inflammatory disorder of the gastrointestinal (GI) tract (Wallace et al. 2014). IBD is categorized into two main subtypes, including Crohn’s disease (CD) and ulcerative colitis (UC) (Xavier and Podolsky 2007; De Souza and Fiocchi 2016). The mechanisms involved in immunopathogenesis and IBD development have not been fully elucidated. However, dysregulated responses of the mucosal immune system against intestinal microorganisms play a significant role in developing the disease in genetically predisposed individuals (Xu et al. 2014). Because lifestyle and diet significantly change the gut microbiota pattern, these alterations can predispose people to IBD (Neuman and Nanau 2012). In IBD patients, it has been shown that the diversity of microorganisms that make up the gut microbiome has considerably decreased, and dysbiosis has occurred (Swidsinski et al. 2002; Manichanh et al. 2006; Round and Mazmanian 2009). Furthermore, genetics and environmental factors could be involved in the overall pathogenesis of IBD (De Souza and Fiocchi 2016).
Evidence revealed that inflammatory mediators such as cytokines and chemokines play a pivotal role in the pathogenesis of inflammatory-based disorders such as IBD (Marafini et al. 2019). In patients with IBD, mucosal dendritic cells (DCs) and macrophages (MQs) increase the expression of pattern recognition receptors (PRRs) such as toll-like receptor 2 (TLR2), TLR4 as well as CD40 and chemokine receptor C–C motif chemokine receptor 7 (CCR7) all of which initiate inflammatory responses by inducing the release of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and IL-18 (Ahluwalia et al. 2018). Among these cytokines, IL-6, as a pro-inflammatory cytokine, could mediate various physiologic and pathologic phenomena (Mihara et al. 2012). It has been well-documented that IL-6 contributes to the differentiation of T helper 17 (Th17) (Kimura and Kishimoto 2010). Th17, along with Th1, are involved in the pathogenesis of IBD (Lavoie et al. 2019; Kojima et al. 2022). In CD and UC, the persistence of CD4+ T cells at the site of inflammation is censoriously dependent on the antiapoptotic IL-6/IL-6R/STAT3 axis signals, inducing the expression of BCL2 and BCLxL. Accumulating apoptosis-resistance T cells in this milieu leads to chronic inflammation (Mudter and Neurath 2007).
Evidence revealed an association between the gut microbiome and the immune system (Smith et al. 2019). The fermenting bacteria metabolites such as short chain fatty acids (SCFA), butyrate, and acetate inhibit the release of inflammatory cytokines such as IL-6 and interact with regulatory B and T cells to ameliorate colitis (Smith et al. 2013).
On the other hand, the gut microbiota can stimulate macrophages and DCs to release IL-1β and IL-6, leading to Th17 differentiation (Wu et al. 2010; Rosser et al. 2014). Furthermore, indole, another bacterial metabolite, induces the expression of IL-22, producing anti-microbial peptides and protecting the intestine against pathogens damage (Zelante et al. 2013). Therefore, the gut microbiome is involved in proinflammatory and regulatory responses (Federico et al. 2009; Rosser et al. 2014). Consequently, manipulating the gut microbiome and its-induced IL-6 could be a potential therapeutic purpose for treating IBD (Ahluwalia et al. 2018; Kang et al. 2019).
This review highlights the role of the gut microbiome in regulating the IL-6/IL-6R axis in IBD. Additionally, the targeted therapy of the IL-6/IL-6R axis in IBD and the reasons for the failure of this therapeutic approach have been investigated.
Inflammatory bowel disease
IBD is mainly employed to define two long-range pathologic conditions, UC and DC, characterized by gut inflammation (Glassner et al. 2020). Basically, UC only affects the colon or large intestine, while CD can influence any part of the digestive system, from the anus to the mouth. People of all ages are usually susceptible to IBD, but it has been reported most often between the ages of 15 and 40. The symptoms of IBD include weight loss, abdominal pain, swelling or cramps in the tummy, bloody or recurring diarrhea, and tiredness (Inflammatory bowel disease2022). Furthermore, severe symptoms such as vomiting, anemia, and high temperature may occur in some patients. It has been reported that uveitis, arthritis, erythema nodosum and jaundice are less commonly IBD-associated symptoms. IBD symptoms can fluctuate; for example, these symptoms may be severe at times (flare-up) or may occur little or not at all over long periods (remission) (Inflammatory bowel disease 2022). It is unknown what causes IBD, but based on information from patients and their records, a combination of factors, including environmental factors, genetics, immune system disorders, and smoking, increases the risk of developing IBD (Molodecky and Kaplan 2010; Ananthakrishnan 2015).
To the best of our knowledge, there is currently no cure for UC and DC. Existing treatments include lifestyle changes, specific diets, surgery, and medications generally used to relieve IBD symptoms and prevent recurring symptoms (Nakase et al. 2021). Medications used to treat CD or UC include anti-inflammatory drugs (aminosalicylates or mesalazines), immunosuppressive drugs (steroids and azathioprine), biological and biosimilar medicines (monoclonal antibodies [mAb] and antagonist inhibitors). However, 1 in 5 UC patients do not respond to these treatments, and surgery should be performed to remove the inflamed tissue in the severe stages of the disease. These patients are also prone to gastrointestinal cancers, especially colorectal cancer (CRC), and should periodically undergo endoscopy and other screening tests (Clarke and Feuerstein 2019).
Evidence shows that the risk of CRC is 2- to 3-fold higher in IBD patients and the severity, extent and persistence of the inflammatory responses are principally responsible for CRC development in patients with IBD (Fantini and Guadagni 2021). Patients with IBD and CRC have been documented to have a worse prognosis than those without a history of IBD, so the effectiveness of existing treatments for IBD can reduce the chance of colitis-associated cancer (CAC). According to existing studies, chronic intestinal inflammation increases the risk of developing CAC in UC and CD, and controlling inflammation can be imperative in preventing CAC (Fantini and Guadagni 2021).
Biology and signaling of the IL-6/IL-6R axis
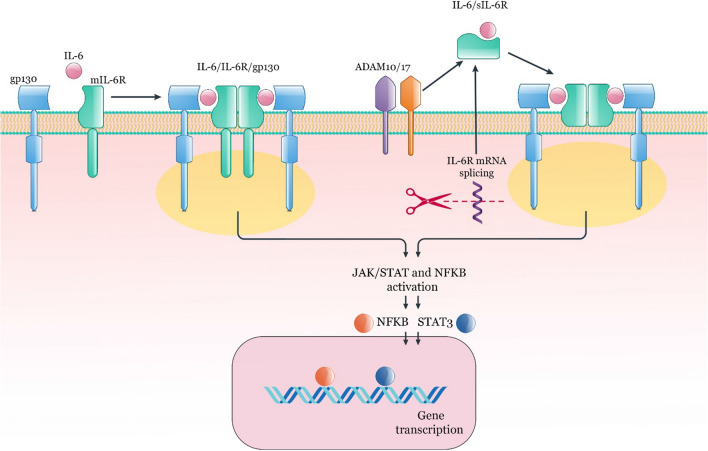
Interleukin-6 (IL-6), a glycosylated protein, comprises 184 amino acids. This cytokine can produce and release by various immune and non-immune cells such as T cells, monocytes, macrophages, endothelial cells, and fibroblasts (Schaper and Rose-John 2015). The receptor of IL-6 is IL-6R with a size of 80 kDa and belongs to the type I transmembrane proteins (Yamasaki et al. 1988). As a second transmembrane protein signal transducer, the dimerization of glycoprotein 130 (gp130, CD130) is essential for the IL-6/IL-6R binding and initiating various intracellular signaling pathways (Taga et al. 1989; Hibi et al. 1990). It has been revealed that in the IL-6 cytokine family, gp130 is a common subunit receptor (Kishimoto 2010). Almost all cells express gp130; nevertheless, IL-6R is expressed by CD4+ T cells, monocytes, neutrophils, and hepatocytes express (Atreya et al 2000; Borghini et al. 2021). Interestingly, IL-6 does not have a binding affinity for gp130 alone, and only in cells that express the IL-6R along with gp130 does effective binding occur and lead to signaling (Taga et al. 1989). The IL-6R can have two forms; attached to the cell membrane (mIL-6R) and the soluble (sIL-6R). The soluble form of IL-6R is created by mRNA splicing as well as the proteolytic cleavage of the mIL-6R (Lust et al. 1992; Mülberg et al. 1993; Dimitrov et al. 2006). In the IL-6R biostructure, the cytoplasmic part could be detached without any signaling disruption, and the transmembrane domain is not required for IL-6 activity (Taga et al. 1989). The two main signaling types are known depending on the type of IL-6R. Signals of sIL-6R are routed through trans-signaling, and mIL-6R signals are routed through classic signaling (Rose-John and Heinrich 1994; Garbers et al. 2012). The homodimerization of IL-6, IL-6R, and gp130 complex activates Janus kinases (JAKs), resulting in tyrosine residues phosphorylation of the cytoplasmic domain of gp130. Following these occurrences, various pathways, including mitogen-activated protein kinases (MAPK), signal transducer and transcription-3 (STAT3), phosphoinositol-3 kinase (PI3K)/AKT, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB) could trigger (Dolcet et al. 2005; Bradham and McClay 2006) (Fig. 1). Augmented IL-6 levels are detected in several human inflammatory disorders, such as rheumatoid arthritis (RA), systemic juvenile idiopathic arthritis, Castleman's disease, IBD, colorectal cancer and autoimmune diseases (Mihara et al. 2012).
Fig. 1.
The IL-6/IL-6R axis. Dimerization of gp130, IL-6R, and IL-6 is essential for the initiation of signal transduction and activation of various pathways such as NFKB and STAT3. IL-6R can be in two forms; mIL-6R and sIL-6R. The soluble form of IL-6R is created by mRNA splicing as well as the proteolytic cleavage of the mIL-6R by ADAM10 or ADAM17. STAT; signal transducer and activator of transcription, NFKB; nuclear factor-kappa B, ADAM; A disintegrin and metalloprotease, sIL-6R; soluble IL-6R, mIL-6R; membrane IL-6R
IL-6 supports the proliferation and regeneration of intestinal epithelial cells (IECs). It has been revealed that IL-6-deficient mice are highly sensitive to dextran sulfate sodium salt (DSS)-induced colitis (Grivennikov et al. 2009). Interestingly, following the DSS challenge, mucosal regeneration needs concomitant initiation of Yes-associated protein (YAP) and Notch (Okamoto et al. 2009; Cai et al. 2010).
As a transcriptional co-activator, YAP can be involved in tissue growth. However, in normal conditions, YAP is inactive in the cytoplasm via serine phosphorylation by the Hippo effector kinase LATS and is activated following tyrosine phosphorylation by Yes (a member of Src family kinase) or suppression of Hippo signaling (Rosenbluh et al. 2012; Yu and Guan 2013). Moreover, Jagged 1 (JAG-1), JAG-2 and delta-like 1 (DLL1), DLL2, DLL3, and DLL4 are ligands of Notch and responsible for Notch cleavage triggering by c-secretase, activating target gene transcription (Bray 2006). Nevertheless, how YAP and Notch are activated following intestinal injury is still not fully understood. In this context, it has been reported that gp130, with the support of Src family kinases, can activate YAP and Notch in a STAT3-independent mechanism following mucosal damage. Therefore, activating the gp130/YAP/Notch pathway is crucial for inflammation-mediated regeneration of intestinal epithelium in DSS-induced colitis and human IBD (Taniguchi et al. 2015).
Role of the IL-6/IL-6R axis in immune system
Innate immunity and acute phase response
Amplified levels of acute-phase proteins (APPs), including serum amyloid A, C-reactive protein (CRP), ferritin, fibrinogen, and haptoglobin, are considered the hallmark of inflammation, and measurement of these proteins could be helpful in the monitoring of inflammatory states (Pincus and Sokka 2009). In this context, released IL-6 by hepatocytes leads to instigating the acute phase response and release of APPs (Gabay and Kushner 1999). To prove the role of IL-6 in the initiation of acute-phase responses, tocilizumab, an anti-IL-6R mAb, was used, and the results showed that by inhibiting the IL-6/IL-6R axis signals, the CRP level was normalized (Genovese et al. 2008).
In innate immunity, it has been revealed that several immune and stromal cells are able to release and respond to IL-6 (Calabrese and Rose-John 2014; Mauer et al. 2014). Therefore, IL-6 can be essential in inducing innate immune responses and stromal cell interactions (West 2019). As the rate of inflammation increases in an autocrine manner, IL-6 can lead to the switching of acute to chronic inflammatory responses (Caiello et al. 2014). Endothelial cells, monocytes, and macrophages are responsible for releasing IL-6 throughout acute inflammation, resulting in the expression of some adhesion molecules and chemokines by smooth muscle cells, fibroblasts, and endothelial cells involved in neutrophils recruitment (Fielding et al. 2008; Mauer et al. 2014). Another important role of IL-6 in the innate immune system is to increase the survival of neutrophils by reducing their apoptosis, which leads to chronic inflammation (Asensi et al. 2004). Some stromal cells, despite expressing gp130, cannot respond to IL-6 due to the lack of IL-6R expression (Modur et al. 1997; Hurst et al. 2001). During chronic inflammation, the shedding of mIL-6R from neutrophils gives them the ability to respond to IL-6 and release chemokines involved in monocyte recruitment, such as CCL2, leading to monocyte infiltration in the site of inflammation (Hurst et al. 2001; Gabay 2006). Like numerous cells, human endothelial cells express gp130 in the absence of IL-6R, whereas sIL-6R-mediated trans-signaling upsurges the expression of adhesive molecules by endothelial cells, resulting in neutrophil rolling and adhesion to migrate into the inflamed tissue as well as vascular inflammation (Modur et al. 1997; Romano et al. 1997; Rose-John 2012).
Adaptive immunity
Based on available knowledge, activated T and B lymphocytes are the main arms of cellular and humoral immunity, involved in inflammation induction and antibody production (Park et al. 2014). In adaptive immunity, IL-6 plays a pivotal role in activating T and B cells (Boe et al. 1999).
IL-6 and transforming growth factor-beta (TGF-β) participate in the differentiation of Th17 cells, which are the main producer of IL-17 (Chonov et al. 2019). On the other hand, CD4+ CD25+ Foxp3+ regulatory T (Treg) cells are the controller of Th17 cells, especially in an autoimmune condition. Because TGF-β is essential for Tregs differentiation, the presence of IL-6 in the environment prevents TGF-dependent Tregs differentiation, which leads to an increase in Th17 destructive responses (Bettelli et al. 2006). Interestingly, in addition to IL-17, Th17 can also produce IL-6, which helps induce differentiation and enhance Th17 responses in an autocrine manner (Ogura et al. 2008). Moreover, IL-6 and IL-17 produced by Th17 affect fibroblasts to release IL-6 and further inflammatory responses (Ota et al. 2015). Produced IL-6 by T cells induces the B cell's maturation into plasma cells (Muraguchi et al. 1988). Furthermore, IL-21 producer T cell subsets induced by IL-6 are involved in B cell maturation (Diehl et al. 2012; Yang and Rincon 2016). As a result, IL-6 is indirectly involved in antibody production. In this regard, it has been shown that antibody-mediated responses in animals with IL-6 deficiency are significantly reduced, associated with increased susceptibility to infection (Kopf et al. 1994). On the other hand, IL-6 may also induce regulatory and anti-inflammatory responses because this cytokine can induce regulatory B cells (Bregs) (Rosser et al. 2014; Jansen et al. 2021). IL-6 can also link adaptive and innate immunity because IL-6-producing B cells stimulate stromal and innate immune cells to produce pro-inflammatory mediators, promoting inflammation (Hunter and Jones 2015).
Another imperative biological activity of IL-6 is participation in fibrosis processes, a common feature of chronic inflammatory diseases (Barnes et al. 2011; Chen et al. 2019). IL-6, during the initial phases of inflammation, induces epithelial and mesenchymal cells to stimulate polymorphonuclear leukocytes (PMNs) recruitment, which is important for wound healing (Hunter and Jones 2015). Activated M2 macrophages and fibroblasts have been shown to induce fibrosis by releasing IL-6 and TGF-β as profibrotic cytokines (O'Reilly et al. 2014; Maier et al. 2017). Trans-IL-6 signaling increases collagen 1 synthesis by activating STAT3 and SMAD3 pathways and producing Gremlin-1 (O’Reilly 2021).
Role of the IL-6/IL-6R axis in inflammatory bowel diseases
Evidence demonstrated that numerous pro-inflammatory cytokines and chemokines, including IL-1β, IL-4, IL-6, IL-13, IL-17, IL-18, IL-33, IL-10, TNF-α, CCL2, and TGF-β are involved in the pathogenesis and development of IBD (McAlindon et al. 1998; Dinarello 1999, 2009; Pizarro et al. 1999; Kanai et al. 2001; Maerten et al. 2004; Schmitz et al. 2005; Beltrán et al. 2010; Kobori et al. 2010). IL-6 activates STAT3 signal transducer and activator, inducing an inflammatory response. It has been reported that the expression of IL-6 and its sIL-6R are amplified in CD and UC patients and reduced in IBD remission (Gross et al. 1992; Mitsuyama et al. 1995; Reinisch et al. 1999; Atreya and Neurath 2008; Bouguen et al. 2011; Pawłowska-Kamieniak et al. 2021). In addition, cohort studies showed higher levels of IL-6-mediated high-sensitivity CRP in patients with IBD (Lochhead et al. 2016). In patients with CAC, IL-6 also plays a crucial role in the pathogenesis and progression of CRC (Li et al. 2010). The clinical severity of CD and UC is associated with serum levels of TNF-α in patients with IBD patients. Moreover, TNF-α inducing the expression of IL-6, IL-1β, and IL-33 plays a significant function in the pathogenesis of IBD (Murch et al. 1993; Sanchez-Muñoz et al. 2008). The IL-6 level is positively associated with the severity of histopathological and endoscopic manifestations, relapse frequency, and clinical disease activity in patients with CD (Reinisch et al. 1999; Van Kemseke et al. 2000; Atreya and Neurath 2008).
It appears that the major source of IL-6 in IBD are intestinal epithelial cells (IELs) and lamina propria mononuclear phagocytes (MNPs), mesenchymal cells, and T cells (Kusugami et al. 1995; Atreya and Neurath 2008). Another function of IL-6 is the regulation of intestinal epithelial tight junctions (Suzuki et al. 2011). In this context, IL-6, by inducing claudin-2 in intestinal epithelial cells and pore formation, upsurges paracellular permeability for cations in a selective manner. It has been reported that signals of downstream IL-6 pathways such as MEK/ ERK and PI3K/AKT are responsible for initiating the pore formation processes (Lee 2015).
Most cell types can respond to IL-6 by shedding the sIL-6R and trans-signaling (Hunter and Jones 2015). T cell-mediated responses (Th1, Th2, and Th17) are important in the pathogenesis of IBD. IL-6 can induce cytokine secretion and survival of T cells by inducing antiapoptotic signals and molecules (Atreya et al. 2000). Moreover, it has been reported that intestinal T cell-mediated inflammation and T cell expansion could be attenuated by IL-6 blocking (Atreya et al. 2000; Yamamoto et al. 2000).
Nonetheless, this is not the whole story because IL-6 can be involved in regulating the integrity of the intestinal epithelial barrier and mucus secretion (Zhou and Sonnenberg 2018). In this regard, it has been shown that innate lymphoid cells (ILCs), which are equivalent to Th17 in innate immunity, contribute to the integration of the intestinal epithelial barrier and homeostasis by secreting IL-6, IL-17, and IL-22 in response to commensal gut microbiota (Fung et al. 2016; Zhou and Sonnenberg 2018). The gut microbiome also can stimulate the secretion of IL-1β, IL-6, and IL-23 by MNPs, resulting in IL-22 secretion by ILC3s and IL-6 production by IELs (Kuhn et al. 2018). Furthermore, IL-6 controls the balance between Tregs and pro-inflammatory T cells (Ye et al. 2020). As a result, intervention through IL-6/IL-6R inhibitors may not always be beneficial to treatment.
It has been revealed that stress can trigger the hypothalamus-pituitary axis (HPA) and activate the autonomic nervous system. Additionally, stress elevates cortisol levels and proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, resulting in chronic inflammation and IBD development. Increased IL-6 following acute or chronic stress increases intestinal permeability by weakening tight junctions. After increasing the permeability of the intestinal epithelial barrier, the translocation of pathogenic bacteria to the colonic tissue increases and leads to infection, excessive cytokine release, and dysregulated inflammatory responses, which can be associated with the pathogenesis of IBD (Brzozowski et al. 2016). IL-6 also plays a role in the activation of colonic natural cytotoxicity receptor-positive (NCR+), CD4− ILC3s in TRUC mice (mice with chronic inflamed intestine) by upregulation of IL-1α-induced production of IL-17A and IL-22 as well as IL-23 and interferon-γ (IFN-γ). Therefore, this axis might be targeted for treating patients with IBD (Powell et al. 2015).
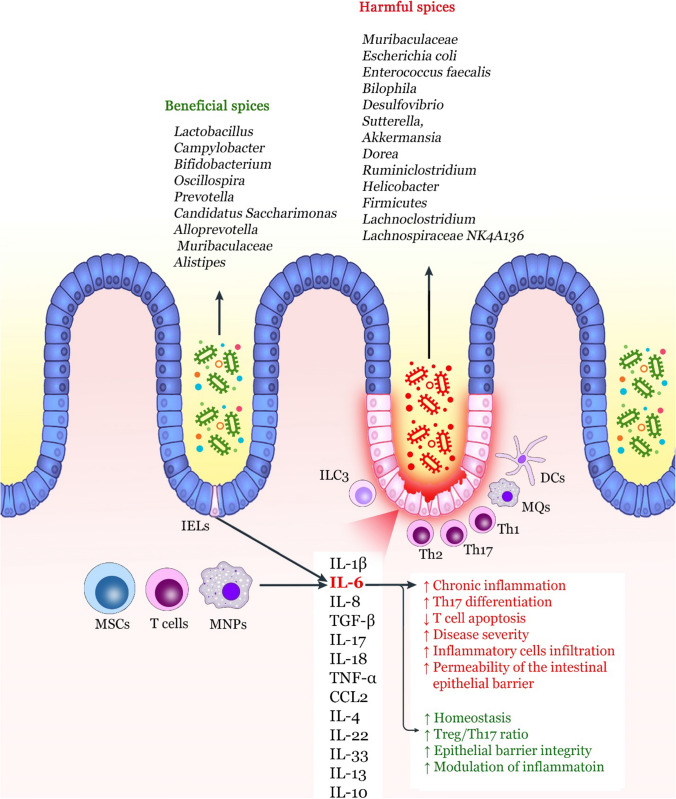
Collectively, the production and secretion of TNF-α, IL-1β, and IL-18 by IECs and MNPs can lead to chronic inflammation and the onset of colitis (Fig. 2). However, in the case of IL-6 and IL-33, due to the dual role of these cytokines depending on the environmental signals and the stage of the disease, they may not be definitively involved in the pathogenesis and progression of IBD. Although IL-6 prevents T cell apoptosis in colitis and contributes to chronic inflammation, it also mediates repair mechanisms through its positive effects on the epithelial barrier. This beneficial and harmful dual behavior of IL-6 in the gut poses challenges for targeting clinical therapeutic purposes. As a result, further structure–function analysis and understanding of cellular/signal interactions in the intestinal tract may effectively advance IL-6/IL-6R inhibition-based therapies (Friedrich et al. 2019).
Fig. 2.
Role of IL-6 in the pathogenesis of IBD. Several mediators are involved in causing chronic intestinal inflammation, the most important of which are IL-1β, IL-6, and IL-17. Among these cytokines, IL-6 is produced by T cells, MNPs, and MSCs. IL-6 plays a dual role in causing inflammation or intestinal homeostasis. Increasing differentiation to Th17, increasing survival of T cells, and increased intestinal epithelial barrier permeability are the most important functions of IL-6, resulting in chronic inflammation. The gut microbiome dysbiosis by increasing harmful bacteria spices and decreasing beneficial spices also can increase the expression of inflammatory cytokines such as IL-6. However, IL-6 can also improve the integrity of the intestinal epithelial barrier and create a balance between Tregs and Th17 to preserve intestinal homeostasis. MSC mesenchymal stem cell, MNP mononuclear phagocytes, IEL intestinal epithelial cell, ILC innate lymphoid cell, MQ macrophage, DC dendritic cell, TGF tumor growth factor, TNF tumor necrosis factor, Treg regulatory T cell
IL-6 and the gut microbiome
Mucosal immune cells are responsible for the release of IL-6. This cytokine could induce inflammatory responses, gastric homeostasis, and post-damage regeneration of the intestine. Studies also suggested an association between the content of the gut microbiome, dysbiosis, and the expression of IL-6 (Wu et al. 2022). A study on IL-6 gene knockout (KO) and wild-type (WT) C57BL/6 J mice demonstrated that gut microbiome diversity was considerably changed in IL-6 KO compared with the WT. It was observed that the frequency of Verrucomicrobia and Akkermansia increased while the number of Firmicutes and Lactobacillus spices decreased in the IL-6 KO group.
Moreover, in the absence of IL-6, intestinal expression of defensins α3 and α4, as well as the frequency of natural isolated TCRγδ+ intraepithelial lymphocytes (IELs), was remarkably increased, inducing mucosal immune responses. Consequently, it is possible that the absence of IL-6 remodels gut microbiome structure and changes the maintenance of IELs (Wu et al. 2022). As a pleiotropic cytokine, IL-6 is elevated in IBD. In parallel with these findings, a study on Il6−/− mice with UC showed that IL-6 deficiency leads to colitis development. Furthermore, IL-6 deficiency pointedly amplified the colon tissue expression of Ccl2/Ccr2, increasing the recruitment of Ly6Chigh monocytes, Il6−/− macrophages, and neutrophils to the inflamed colon of the studied mice (Cao et al. 2021). It has been reported that the intestine's various populations of Escherichia coli increased during DSS-mediated inflammation and induced strain-specific disease phenotypes and immunopathological alterations. Following treatment with DSS, the levels of the inflammatory mediators, including IFN-γ, IL-6, and granulocyte–macrophage colony-stimulating factor (GM-CSF), were increased, which is considered a characteristic of the severe inflammation induced by ST129 and ST375 strains of E. coli.
Interestingly, data mining and in vivo neutralization revealed that IL-6 plays an important role in causing inflammatory responses in different phases of the disease. This is despite the fact that colonization of DDS-treated mice with ST150 and ST468 E. coli strains led to a negligible increase in inflammatory responses (Kittana et al. 2018). It has been reported that the gut microbiota induced the differentiation of regulatory B cells (Breg cells) in the mesenteric lymph nodes and spleen. As mentioned, IL-6 is involved in gut homeostasis. A study reported that IL-6 could reduce excessive inflammation caused by microbiome alteration by inducing differentiation and expansion of Bregs (Rosser et al. 2014). In patients with type 2 diabetes (T2D), the prevalence of gram-negative bacteria such as Prevotella. copri and Bacteroides vulgatus species in the gut and the elevated plasma levels of IL-6 could be associated with insulin resistance and mild inflammation. Therefore, gut microbiota signature might accompany T2D development (Leite et al. 2017).
Collectively, IL-6 may have a dual role depending on the gut microbiota pattern. With the increase of bacterial pathogen species and the disturbance of the microbiome balance, increased inflammatory responses can be seen that IL-6 is involved in these destructive responses. On the other hand, when the normal intestinal flora and beneficial bacterial species are sufficiently present in the intestine, IL-6 is more involved in homeostasis and the reduction of pathologic inflammation.
Targeting the IL-6/IL-6R axis in inflammatory bowel diseases
According to the reviewed studies, local release of a wide range of non-specific inflammatory mediators, including leukotrienes, free radicals, cytokines, and chemokines, could cause IBD development. The production and secretion of the mentioned cytokines and chemokines such as TNF-α, IL-1β, IL-6, IL-12, IL-17, IL-18, IL-23, TGF-β, and CCL2 could lead to the recruitment of inflammatory cells into the intestinal tissue (Monteleone et al. 2006). Therefore, targeting these pro-inflammatory cytokines and chemokines or their receptors via various inhibitors could effectively treat IBD (Guan 2019). For instance, using mAbs against IL-12/23 p40 in morin models of colitis could reduce disease severity (Becker et al. 2006; Yen et al. 2006). Moreover, in DSS-induced colitis mice treated with anti-IL-21 antibody, colonic T cells' infiltration and release of IL-6 and IL-17A were significantly decreased in inflamed intestinal tissue (Stolfi et al. 2011). A major challenge in treating IBD via IL-6 targeting is the dual role of this cytokine in the pathogenesis of IBD. IL-6 can participate in both IBD progression and mucosal repair (Choi et al. 2015). To confirm this issue, previous studies have shown that IL-6-deficient mice had moderated inflammatory responses after the induction of colitis, while the mucosal healing phase in the animals was impaired (Naito et al. 2004; Gay et al. 2006). These findings suggest that targeting IL-6 may affect and disrupt the mucosal healing stage during recovery (Fattori et al. 1994; Tebbutt et al. 2002). As a result, optimizing therapeutic methods based on inhibition of IL-6 in the stages of hyperinflammation where IL-6 production is dysregulated, and selective induction of IL-6 in the mucosal healing stage can be useful in the treatment of IBD (Choi et al. 2015). However, the diagnosis of these stages and treatment optimization need more studies. In this section, according to the purpose of the study, IL-6/IL-6R axis inhibitors and their effect on the treatment of IBD and CAC are investigated (Table 1).
Table 1.
Effective probiotics and natural compounds in treating colitis mice models
| Compound | Outcomes | Ref. |
|---|---|---|
| Moringa oleifera |
Restoration of the number of Bifidobacteria and Lactobacilli ↓ IL-6 |
Elabd et al. (2018) |
| Ginger |
↓ colitis-associated pathological alterations ↓ IL-6 mRNA expression ↓ Muribaculaceae Regulating the gut microbiome structure in DDS-induced colitis mice |
Guo et al. (2021) |
| Water-soluble garlic polysaccharide (WSGP) |
↓ Colonic damage and levels of IL-6 in DSS-induced colitis mice models ↑ The production of SCFAs Enhancing the mucosal barriers and the gut microbiome composition |
Shao et al. (2020) |
| Butyrate |
↓ The signs of colitis and improved colonic histological injury ↓ The expression of inflammatory cytokines such as IL-6 and increasing the expression of CCR9 ↑ MDSCs recruitment |
Xiao et al. (2021) |
| Dehydroepiandrosterone (DHEA) |
↓ IL-1β, IL-6, and TNF-α Improving colon barrier integrity and modulated gut microbiota composition ↓ Inflammatory responses in the intestine |
Cao et al. (2020) |
| Evodiamine (EVO) |
↓ Tumor formation ↓ Tumor cell proliferation ↑ Tumor cell apoptosis ↓ Escherichia coli and Enterococcus faecalis ↑ Lactobacillus, Campylobacter, and Bifidobacterium ↓ The IL-6/STAT3/P65 pathway |
Zhu et al. (2021) |
| Shenling Baizhu San (SLBZS) |
Improving the function of the intestinal barrier and modulating the gut microbiome ↑ The number of Oscillospira and Prevotella, which produce SCFA ↓ The number of Bilophila and Desulfovibrio ↓ The expression of IL-6 and MPO |
Gu et al. (2020) |
| Resveratrol |
Balancing the gut microbiota composition ↓ The production of IL-1β, IL-2, IL-6, TNF-α, IFN-γ, GM-CSF, and CXCL1 ↓ The number of Bilophila, Sutterella, Akkermansia, and Dorea ↑ The abundance of Bifidobacterium |
Li et al. (2020) |
| Polysaccharide (SP) extracted from Gracilaria Lemaneiformis |
Recovering physical conditions of the studied mice ↓ Weight loss and suppressing appetite ↓ The release of IL-1β, IL-6, and TNF-α in inflamed colon tissue Improving the expression of tight junction proteins Enhancing the intestinal barrier |
Han et al. (2020a, b) |
| Egg white peptides (EWPs) |
Regulating the content of microbiota ↑ The number of Candidatus Saccharimonas and Lactobacillus ↓ The number of Akkermansia and Ruminiclostridium ↓ IL-1β, IL-6, and TNF-α |
Ge et al. (2021) |
| Food incidental NPs |
Modulating the composition of the gut microbiome ↑ The abundance of Alloprevotella, Muribaculaceae, and Alistipes ↓ The number of harmful spices such as Helicobacter Balancing the production of inflammatory cytokines such as IL-6 |
Wang et al. (2022) |
| Lactobacillus helveticus KLDS 1.8701, Lactobacillus plantarum KLDS 1.0318, and Lactobacillus acidophilus KLDS 1.0901 |
↓ The colon shortening ↓ DAI ↓ IL-1β, IL-6, PGE2, TNF-α ↓ MPO activity ↑ Production of SCFAs Improving intestinal barrier |
Shi et al. (2021) |
| Lactobacillus reuteri F-9–35 |
↓ MPO activity ↓ The expression of IL-6, COX-2, and TNF-α Modulating the gut microbiota dysbiosis |
Sun et al. (2018) |
| Bifidobacterium longum |
Producing CLA ↑ Goblet cells, mucin2, α-catenin, ZO-1, and claudin-3 ↓ The release of IL-6 and TNF-α ↑ The production of IL-10 |
(Chen et al. 2021) |
| SEL001 |
↓ IL-6 and TNF-α ↓ The number of Firmicutes |
Rather et al. (2020) |
| Desmethylbellidifolin |
Modulating the gut microbiome ↓ DAI was reduced, and colonic inflammation ↓ The expression of IL-6 and TNF-α |
Zelante et al (2013) |
| Saccharomyces boulardii |
Modulating the gut microbiome ↓ UC tumorigenesis ↓ Tumor load ↓ The IL-6 and TNF-α ↓ The number of Lachnoclostridium and Lachnospiraceae NK4A136 |
Wang et al. (2019) |
| Akkermansia muciniphila |
↓ The symptoms of DSS-induced acute colitis ↓ Colon histological inflammatory score, body weight loss, and colon length shortening ↑ The expression of the mucin family ↓ IL-1β, IL-6, TNF-α, and CCL2 |
Qu et al. (2021) |
Inflammatory bowel disease
Corticosteroids are anti-inflammatory agents used to induce remission in UC patients and can inhibit inflammatory transcription factors such as activator protein 1 (AP-1) and NFKB and repress the release of IL-1 and IL-6 (Oakley and Cidlowski 2013; Rezaie et al. 2015). A double-blind, randomized, parallel-group study used beclomethasone dipropionate and prednisone in patients with mild-to-moderate UC, and the findings demonstrated promising clinical outcomes with a respectable safety profile (Assche et al. 2015).
However, some patients become resistant to corticosteroid therapy, and the treatment fails. In this type of patient, administering intravenous infliximab (a chimeric, IgG-1 monoclonal anti-TNF-α antibody) can be useful in patients with moderate to severe DC and UC as well as patients with corticosteroid-refractory acute UC (Hanauer et al. 2002; Rutgeerts et al. 2005; Kevans et al. 2018). As mentioned, TNF-α induces the production of IL-6 by macrophages, and treatment with infliximab may indirectly control the overproduction of IL-6 (McGee et al. 1995). Fascinatingly, soluble gp130 may act as a natural inhibitor of IL-6 in IBD, which may be due to the IL-6 co-elution of IL-6 with soluble gp130. Moreover, soluble gp130, the serum levels are elevated in UC and to a lesser extent in CD than in healthy subjects (Mitsuyama et al. 2006).
Antibodies blocking the IL-6/IL-6R axis include tocilizumab, clazakizumab, olokizumab, PF-04236921, Clazakizumab (BMS-945429), C326, and other inhibitors may be effective in the treatment of IBD. Although some of these inhibitors have entered phases I and II of clinical trials, these studies were incomplete or terminated for various reasons. Among these drugs, tocilizumab and PF-04236921 are well-studied IL-6/IL-6R axis inhibitors in IBD (Coskun et al. 2017). Tocilizumab is a human anti-IL-6R antibody that was first used in 2004 in a phase I clinical trial on CD patients, but although patients well-tolerated tocilizumab, only 20% of patients attained clinical remission, and no difference was observed between the findings of endoscopy or histology in patients treated with tocilizumab and placebo (Ito et al. 2004). A study on a T cell transfer murine colitis model demonstrated that blocking the IL-6/IL-6R axis signals with tocilizumab reduced lamina propria T cells apoptosis, repressed the expression of vascular adhesion molecules, and effectively decreased intestinal inflammation (Ito 2005). To evaluate the safety and efficacy of tocilizumab, the researchers of this study performed a clinical trial in patients with CD. The outcomes showed that 12 weeks of administration of tocilizumab (dose of 8 mg/kg) could significantly increase the clinical response rate in 80% of undertreatment CD patients. This dose of tocilizumab was safe and well-tolerated by patients.
Moreover, remission was observed in 20% of CD patients treated with tocilizumab disease, and the acute-phase responses were regularized (Ito 2005). Despite the therapeutic benefits of tocilizumab in IBD, there are reports of challenges and limitations in using this drug. In this context, some investigations have reported that tocilizumab may exacerbate pre-existing UC or cause de novo drug-induced IBD, which may be due to the inability to suppress IL-6 production in the inflamed gut (Borghini et al. 2021). Additionally, tocilizumab can lead to severe colitis in patients with Takayasu arteritis. A recent study reported bacteremia, colonic perforation, and wide lesions in the sigmoid colon were detected in some patients with Takayasu arteritis under treatment with tocilizumab (Ishii et al. 2022). Since IL-6 plays a significant role in intestinal biological processes such as recovery from ischemic injury and infection control, inhibition of IL-6R with tocilizumab may be associated with adverse effects such as infection, and this is one of the major challenges in utilizing this inhibitor in the treatment of autoimmune/inflammatory diseases such as Takayasu arteritis and IBD.
A fully human anti-IL-6 mAb termed PF-04236921 is used in a phase I clinical trial conducted on patients with CD who failed anti-TNF therapy (NCT01345318). The outcomes of this study showed that PF-04236921 induced clinical response and remission in refractory patients with CD (Danese et al. 2019). Nevertheless, perforation and gastrointestinal abscesses were detected as adverse effects of PF-04236921 therapy, which may be due to the restorative role of IL-6/IL-6R signals in IEC. Clazakizumab is a fully-humanized mAb that is able to inhibit free IL-6 and IL-6/sIL-6R complex (Semerano et al. 2014; Rossi et al. 2015a, b). A phase II trial (NCT01545050) was designed to evaluate the efficacy of Clazakizumab in patients with moderate to severe CD who had an unsatisfactory response to conventional therapy or failed anti-TNF therapy (Rogler 2015). However, more information about this study is unavailable and has been terminated due to the sponsor's decision (CSL Behring).
Moreover, a study on IL-10−/− mice demonstrated that complete blockade of IL-6 significantly worsens gut inflammation. The exact mechanism of this outcome is not yet fully understood; nevertheless, intestinal inflammation may develop following Treg/CD152 inhibition and induction of the IL-1β/Th2 pathway. It has also been shown that more severe inflammatory conditions are seen in the intestines of double-mutant mice (IL-10−/−, IL-6−/−), indicating the importance of IL-6 in the homeostasis of mucosal immune responses. As a result, blockade of the IL-6/IL-6R axis components in IBD patients should be associated with greater caution as it may worsen the patient's condition (Ye et al. 2020).
Regarding the role of ILCs in IBD pathogenesis, these cells might be potential therapeutic targets for IBD treatment (Goldberg et al. 2015). For instance, depletion of ILCs as well as targeting IL-6, IL-23, and JAK signaling can lead to inhibition of ILCs and Th17 (Buonocore et al. 2010). Consequently, inhibiting Th17 and ILCs as IL-6 producers in the inflamed intestinal tissue can suppress the IL-6/IL-6R/STAT3 signals, reducing inflammatory responses and enhancing apoptosis of effector T cells. Filgotinib is a JAK inhibitor that impairs downstream signals of IL-6, IL-10, and interferon (IFN) family cytokines via hindering JAK1. Findings of a clinical trial study (NCT02048618) demonstrated that using this inhibitor in patients with CD had a promising clinical outcome. This study reported that following 20 weeks of treatment of CD patients with filgotinib (200 mg once a day), patients achieved significantly clinical remission, defined as Crohn's disease activity index (CDAI) of less than 150. Advantageous effects of filgotinib therapy were also realized on D'Haens histopathology scores, simple endoscopic score for Crohn's disease (SES-CD) responses, and inflammatory biomarkers activity (Vermeire et al. 2017).
Evidence shows that tryptanthrin (TRYP) with anti-inflammatory properties has been successful in DSS-induced colitis by improving the histopathological structure of the inflamed colon tissue and reducing IL-6, STAT3, and TNF-α. Additionally, the phosphorylation of STAT3 was repressed by TRYP. These findings demonstrated that TRYP could regulate the IL-6/STAT3 and TNF-α/NFKB p56 signaling pathways by hindering STAT3 phosphorylation and degradation of IκBα (Wang et al. 2018).
It has been shown that Sphaerophysa salsula, honey bee propolis, and Pterocarpus marsupium contain 3′-Hydroxypterostilbene (trans-3,5-dimethoxy-3′,4′-hydroxystilbene), which can efficiently ameliorate the number of tumors and colon shortening in AOM/DSS-induced colitis. Molecular analysis disclosed that 3′-hydroxypterostilbene has anti-inflammatory properties because this compound significantly decreases the levels of IL-6, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Furthermore, dietary 3′-hydroxypterostilbene could markedly reduce IL-6/STAT3 signals in the inflamed colonic tissue (Lai et al. 2017).
Sesbania grandiflora is a herbal plant containing polyphenol, flavonoid, and flavanone with antitumor and anti-inflammatory characteristics used in treating UC. In this regard, an experimental investigation reported that the IL-6 and TNF-α levels were reduced following treatment with hydroalcoholic extract of Sesbania grandiflora in an acetic acid-induced UC mouse model (Gupta et al. 2018). This study claimed that 200 mg of hydroalcoholic extract of Sesbania grandiflora could be as effective as 2 mg of prednisolone. The presence of highly polymerized polyphenols and flavonoids, particularly quercetin, in this plant may be the reason for its antioxidant and anti-inflammatory activities (Gupta et al. 2018).
Gegen Qinlian decoction (GQ) is a traditional Chinese herbal medicine broadly utilized to treat enteric typhoid fever and bacterial dysentery. Recently, clinical studies have revealed that GQ could be a potential therapeutic option for UC treatment. Experimental investigations demonstrated that GQ significantly alleviated UC symptoms in DSS-induced mice models of UC and repressed myeloperoxidase (MPO) activity. Additionally, the production of TNF-α, IL-1β, IL-6, IL-17, and TGF-β1, as well as infiltration of Th17 and Treg cells into the colons, was reduced following administration of GQ. The findings also showed that phosphorylation of STAT3 and JAK2 was decreased after treatment (Zhao et al. 2021). Therefore, GQ can reduce the dysregulated inflammatory responses in the inflamed tissue of the colon by inhibiting IL-6/IL-6r/STAT3 axis signaling, which can be beneficial for treatment.
Another anti-inflammatory alkaloid compound found in the Boldo tree is Boldine. It has been reported that Boldine can decrease inflammatory-associated colon injury. The mechanism of this anti-inflammatory action has not yet been fully elucidated, but there is evidence of decreased TNF-α, IL-6, IL-17, pSTAT3, and p65 NFKB levels, as well as decreased inflammatory cell infiltration into colon tissue (Pandurangan et al. 2016). Pien Tze Huang (PZH) is a famous traditional Chinese formula used in inflammatory diseases. An investigation reported that PZH could noticeably alleviate DSS-induced colitis symptoms, such as stool consistency, rectal bleeding, and body weight loss. Furthermore, PZH could prevent DSS-induced colon shortening and improve colonic histopathological alterations, including crypt distortion, infiltration of immune cells, hyperplastic epithelium, and mucosal ulceration.
Moreover, PZH treatment pointedly repressed the DSS-induced expression of IL-6 in colon tissues and inhibited the IL-6/IL-6r/STAT3 signals (Li et al. 2018). An open-label, prospective phase 2a trial was conducted on patients with active IBD undertreatment with the olamkicept (a trans-signaling inhibitor) to block IL-6 trans-signaling in vivo. Findings showed that olamkicept significantly reduced phosphorylated STAT3 and noticeable transcriptional alterations in the inflamed mucosa. These data proposed that blockade of IL-6 trans-signaling could be a promising and novel strategy for treating IBD (Schreiber et al. 2021).
Previous studies reported that oleuropein could reduce the production of IL-6 and increase the release of anti-inflammatory cytokines in colitis and colorectal cancer models (Giner et al. 2016; Larussa et al. 2017; Deiana et al. 2018). An investigation explored the effect of administration of germinated barley foodstuff (GBF) on inflammatory mediators, including IL-6, IL-8, and TNF-α. The findings showed that IL-6 and IL-8 were significantly reduced in the GBF-treated group compared with baseline (Faghfoori et al. 2011). Cladosiphon fucoidan, a dietary substance, also might help treat colitis. An investigation revealed that DAI, MPO, and IL-6 were significantly decreased following administration of Cladosiphon fucoidan in DDS-induced mice models (Matsumoto et al. 2004). A study on DDS-induced colitis mice models suggested that Lizhong Decoction (LZD) could be used to treat UC. After treatment, colonic inflammation was remarkably alleviated via inhibiting the production of inflammatory mediators, including IL-1, IL-6, IFN-γ, TNF-α, nitric oxide (NO) and suppressing the activity of MPO and superoxide dismutase (SOD). Fascinatingly, the levels of anti-inflammatory cytokines such as IL-4 and IL-10 were significantly decreased following LZD treatment. Furthermore, LZD significantly reduced the mRNA level of toll-like receptor 4 (TLR4) and NF-κB and increased the expression of claudin-1, zonula occluden-1, and occludin to reduce gut inflammation and improve the intestinal barrier (Shen et al. 2020). It has been reported that other compounds, such as tilapia head glycolipids (TH-GLs) and Jiangxiangru (JXR), had beneficial effects on reducing gut IL-6-mediated inflammation (Gu et al. 2021; Wang et al. 2021).
Colitis-associated cancer
One of the reasons that researchers have increasingly considered IBD is to provide the appropriate conditions for CRC and the development of this malignancy in humans. A study on mice models of colitis revealed that administration of Rho-associated protein kinase inhibitor (Y-27632) could pointedly improve colitis severity which was proved by histological damage, colon length, and the disease activity index (DAI) scores. Moreover, treatment by Y-27632 significantly reduced CD68 and pro-inflammatory mediators such as TNF-α, IL-1β, IL-6, and IL-17F. Y-27632 effectively repressed NFKB and STAT3 activation and prosurvival gene activity associated with these transcription factors. These outcomes indicated that Y-27632 inhibition of the NF-κB and IL-6/STAT3 pathways could ameliorate colonic inflammation and be an effective anti-inflammatory drug to treat UC and prevent CAC (Wang et al. 2020a, b).
Silibinin, an antineoplastic and anti-inflammatory used in a CAC mouse model to determine its impact on IL-6/IL-6R/STAT3 signaling, revealed that silibinin could reduce the size and amount of tumors in AOM/DSS mice. Moreover, scores of colitis and tumor were decreased. Silibinin also inhibited tumor cell proliferation and induced apoptosis of tumor cells. Furthermore, the production of IL-6, as well as STAT3 phosphorylation, were remarkably repressed by silibinin (Zheng et al. 2018).
Oroxylin A, an O-methylated flavone, is another IL-6 inhibitor that can be used to treat CAC. A study on human HCT-116 cells treated with oroxylin A showed that this compound effectively repressed IL-6/IL-6R/STAT3 signaling. Additionally, during the experimental period, dietary administration of oroxylin A pointedly inhibited tumor cell proliferation, decreased the tumor burden, and induced apoptosis. On the other hand, the expression of IL-6 and IL-1β was down-regulated in isolated tumor tissues from mice treated with oroxylin A. Moreover, the IL-6/IL-6R/STAT3 signaling was reduced in the treated animals. Accordingly, oroxylin A may be a potential option for treating CAC via regulating IL-6/IL-6R/STAT3 signals in HCT-116 cells and AOM/DSS mice models (Yang et al. 2013).
Embelin, a small molecule that inhibits X-linked inhibitors of apoptosis protein (XIAP), can also have anti-inflammatory, antitumor, and antioxidant properties. An investigation on CAC-bearing mice reported that embelin considerably reduced tumor size and incidence in the studied animals. Furthermore, embelin repressed the expression and release of colonic IL-6 and subsequently activated STAT3 in vivo. Notably, embelin reduced both the IL-6–induced and constitutive STAT3 activation in CRC cells via promoting Src homology domain 2-containing protein tyrosine phosphatase (SHP2) activity in vitro. Additionally, embelin down-regulated the expression of IL-1β, IL-17A, and IL-23, along with reducing the number of infiltrated macrophages and CD4+ T cells in the inflamed colonic tissues. Accordingly, these outcomes showed that embelin could suppress CAC tumorigenesis, and its antitumor impact is partly mediated via reducing Th17-mediated responses and the activation of IL-6/IL-6R/STAT3 signals and immune response (Dai et al. 2014).
Interestingly, cocoa as a compound enriched with polyphenols has anti-inflammatory, antitumor, and antioxidant activities. A study reported that the cocoa diet significantly reduced CAC-induced mice's tumor size and incidence by inhibiting tumor epithelial cell proliferation and inducing apoptosis. Cocoa repressed colonic IL-6 expression and STAT3 activation (Saadatdoust et al. 2015).
Tea polysaccharides (TPS) isolated from tea leaves are the main nutraceutical component with anti-inflammatory, antioxidant, and antitumor activities. A study on AOM/DSS mouse model and IL-6-induced CT26 CRC line demonstrated that TPS considerably condensed tumor size and incidence and noticeably repressed the infiltration of pro-inflammatory immune cells and release of IL-6 matrix metalloproteinase-2 (MMP-2), VEGF, cyclin Dl, and survivin in vitro and in vivo. Therefore, TPS can attenuate the CAC progression by suppressing the IL-6/IL-6R/STAT3 pathway and the expression of downstream genes (Liu et al. 2018).
Although the mentioned compounds have been studied in CAC, it appears that the involvement of colitis in the development of CRC and the effect of these compounds on the IL-6/IL-6R axis can be used in the treatment of IBD.
Modifying the gut microbiome to reduce IL-6-mediated inflammation
The gut microbiome's content has been considered one of the main factors associated with inflammatory-based diseases, diabetes, obesity, and metabolic disorders (Tilg and Kaser 2011; Devaraj et al. 2013; Taneja 2014; Halfvarson et al. 2017). According to the cross-sectional studies, dysregulated immune responses to the gut microbiome could be involved in the pathogenesis of IBD because the microbiome pattern of patients with UC, CD and ileal CD is altered more than in healthy individuals (Halfvarson et al. 2017). Therefore, therapies aimed at changing the microbiome content can help treat inflammatory diseases such as IBD (Table 1).
Herbal medicine and other natural compounds
An experimental study conducted a nutritional intervention using Moringa oleifera to modulate inflammation by affecting the number of caecal Bifidobacteria and Lactobacilli in mice under a high-fat diet. Finding demonstrated that in mice with a high-fat diet, intestinal levels of Bifidobacteria significantly decreased while the number of Lactobacilli pointedly increased compared with normal control mice. Furthermore, serum levels of IL-6 and body weight increased in mice fed a high-fat diet. Interestingly, following treatment with Moringa oleifera, the number of Bifidobacteria and Lactobacilli, as well as IL-6 levels and body weight, were meaningfully restored in the studied animals feeding a high-fat diet (Elabd et al. 2018).
Ginger also can affect the regulation of the gut microbiome (Wang et al. 2020a; b). In DDS-induced mice models of colitis administration, ginger improved colitis-associated pathological alterations and reduced the mRNA levels of IL-6. Lactobacillus murinus, Lachnospiraceae bacterium 615, and Ruminiclostridium KB18 as pathogenic spices were found in the gut microbiome of mice with colitis and treatment with ginger reduced Muribaculaceae, resulting in regulation of the gut microbiome structure in DDS-induced colitis mice models (Guo et al. 2021). It has been reported that treatment with water-soluble garlic polysaccharide (WSGP) reduces colonic damage and levels of IL-6 in DSS-induced colitis mice models. Furthermore, WSGP boosted the production of SCFAs and enhanced the mucosal barriers and the gut microbiome composition (Shao et al. 2020). Butyrate is a kind of SCFAs and is negatively associated with colitis development. An investigation demonstrated that the administration of butyrate pointedly reversed the signs of colitis and improved colonic histological injury by reducing the expression of inflammatory cytokines such as IL-6 and increasing the expression of CCR9 chemokine in DSS-induced colitis mice models. However, it was found that these therapeutic effects were performed in collaboration with CCR9+ myeloid-derived suppressor cells (MDSCs) as an immunosuppressive cell. Therefore, butyrate alone could not have a significant effect on the treatment of colitis (Xiao et al. 2021).
Dehydroepiandrosterone (DHEA) is another anti-inflammatory factor with promising effects in the treatment of colitis. It has been revealed that DHEA administration in DSS-induced colitis mice could reduce IL-1β, IL-6, and TNF-α. DHEA also improved colon barrier integrity and modulated gut microbiota composition to decrease inflammatory responses in the intestine (Cao et al. 2020).
One of the most important risk factors for colitis and CRC is high-fat diets, which change the gut microbiome composition (Zhu et al. 2021). An investigation explored the therapeutic effects of Evodiamine (EVO) in DDS-induced mice models. This study showed that the numbers of Escherichia coli and Enterococcus faecalis were amplified in patients with CRC, whereas Bifidobacterium, Campylobacter and Lactobacillus were reduced. Additionally, the levels of p-STAT3 were significantly elevated in these patients. Furthermore, treating mice with EVO and 5-aminosalicylic acid (ASA) could inhibit tumor formation, reduce tumor cell proliferation, and induce tumor cell apoptosis. The numbers of Escherichia coli and Enterococcus faecalis were reduced, while the frequency of Lactobacillus, Campylobacter, and Bifidobacterium was increased in treated mice. Furthermore, the IL-6/STAT3/P65 pathway was inhibited in treated mice with EVO (Zhu et al. 2021). Shenling Baizhu San (SLBZS) is another complementary medical therapy for UC by improving the function of the intestinal barrier and modulating the gut microbiome pattern by increasing the number of Oscillospira and Prevotella, which produce SCFA, and decreasing the number of pathogenic bacteria such as Bilophila and Desulfovibrio. Furthermore, the expression of IL-6 and MPO was significantly reduced after therapy (Gu et al. 2020). Orally administration of resveratrol also could have therapeutic effects in (DSS)-induced colitis mice model through balancing the gut microbiota composition and reducing the production of IL-1β, IL-2, IL-6, TNF-α, IFN-γ, GM-CSF, and CXCL1. Moreover, resveratrol can significantly reduce the number of Bilophila, Sutterella, Akkermansia, and Dorea. Additionally, resveratrol increases the abundance of Bifidobacterium in mice with colitis (Li et al. 2020).
The protective effects of an extracted polysaccharide (SP) from Gracilaria Lemaneiformis were investigated in DDS-induced mice models, and results disclosed that this extract could recover physical conditions of the studied mice by reducing weight loss and suppressing appetite. Furthermore, SP also repressed the release of IL-1β, IL-6, and TNF-α in inflamed colon tissue. The expression of tight junction proteins is also upregulated following treatment with SP to enhance the intestinal barrier (Han et al. 2020).
Egg white peptides (EWPs) can regulate the content of microbiota through their anti-inflammation and anti-oxidation properties. Treatment of mice with colitis with 200 mg/kg of EWPs showed an increased number of Candidatus Saccharimonas and Lactobacillus and decreased the number of Akkermansia and Ruminiclostridium. Moreover, IL-1β, IL-6, and TNF-α decreased following this treatment in DDS-induced colitis mice models (Ge et al. 2021).
Self-assembled nanoparticles (NPs) incidentally formed throughout food processing. The advantage of these food incidental NPs is that they can directly access the GI and modulate the composition of the gut microbiome. Food incidental NPs increased the abundance of beneficial bacterial spices such as Alloprevotella, Muribaculaceae, and Alistipes and decreased the number of harmful spices such as Helicobacter. Moreover, the correlation analysis showed that pro-inflammatory cytokines were negatively correlated with Muribaculaceae, Alloprevotella, and Alistipes but positively correlated with Helicobacter to balance the production of inflammatory cytokines such as IL-6 (Wang et al. 2022). Other studies have shown that compounds such as Berberine, Rhein, and algal oil rich in docosahexaenoic acid in a similar behavior reduce inflammation by inhibiting the production of inflammatory cytokines such as IL-6, as well as improving intestinal mucosal barrier and microbiome composition (Yang et al. 2021; Deng et al. 2022; Dong et al. 2022).
Probiotics
Some bacterial species include Lactobacillus helveticus KLDS 1.8701, Lactobacillus plantarum KLDS 1.0318, and Lactobacillus acidophilus KLDS 1.0901 could have immunomodulatory, antioxidant, and antibacterial properties in DDS-induced colitis mice. The mixture of these strains significantly reduced colon shortening, disease activity index (DAI), the levels of inflammatory mediators such as IL-1β, IL-6, PGE2, TNF-α, and the activity of MPO. In addition, following supplementation with the lactobacilli mixture, bacterial diversity, gut microbiota content, production of SCFAs, and the intestinal barrier significantly improved in DDS-induced colitis (Shi et al. 2021). Other probiotics such as Lactobacillus reuteri F-9-35 and its wild type have also been studied for the treatment of UC, and findings demonstrated that in the treated animals, MPO activity, and the expression of IL-6, cyclooxygenase-2 (COX-2), and TNF-α were decreased in inflamed colonic tissue compared with control. Additionally, Lactobacillus reuteri F-9-35 modulated the gut microbiota dysbiosis in a protective manner (Sun, Zhang et al. 2018). Therefore, supplementation with probiotics can be a decent way to reduce gut inflammation. Bifidobacterium longum is able to produce conjugated linoleic acid (CLA). Treating animals by Bifidobacterium longum remarkably amplified goblet cells, mucin2, α-catenin, ZO-1, and claudin-3. Additionally, the therapy can reduce the release of IL-6 and TNF-α, while the production of IL-10 was increased, and all of these events could be due to the effects of produced CLA (Chen et al. 2021). A bioactive product isolated from a probiotic strain Lactobacillus sakei probio65 termed SEL001 was administrated in TNBS-induced UC mice models. The findings showed that SEL001 significantly reduced IL-6 and TNF-α as well as the number of phylum Firmicutes, which is correlated to UC development (Rather et al. 2020). Desmethylbellidifolin or balsalazide sodium (isolated from Gentianella acuta) can have anti-colitis properties via modulation of the gut microbiome. It has been reported that upon9 days of treatment with desmethylbellidifolin, the DAI was reduced, and colonic inflammation was ameliorated via inhibiting the expression of IL-6 and TNF-α in DDS-induced mice models. Additionally, desmethylbellidifolin could modulate the gut dysbiosis induced by DDS (Wu et al. 2021).
It has been revealed that periodontal disease could be associated with IBD via unknown mechanisms. The number of Porphyromonadaceae significantly increased in fecal samples isolated from CD patients (Lee et al. 2022). Following intrarectal implantation of Porphyromonas gingivalis, the DAI, as well as infiltration of inflammatory cells, levels of IL-6 and TNF-α were intensified in DDS-induced colitis mice models. Therefore, concurrent treatment of periodontal disease should be considered for IBD patients (Lee et al. 2022). Saccharomyces boulardii is another beneficial bacterium for treating CAC with the capability to reduce inflammation via modulating the gut microbiome. An investigation of azoxymethane and DDS-induced colitis models demonstrated that Saccharomyces boulardii treatment condensed UC tumorigenesis in the studied mice, as specified by the decreased tumor load and reduced the levels of IL-6 and TNF-α. Moreover, the number of Lachnoclostridium and Lachnospiraceae NK4A136 was elevated in the gut microbiome (Wang et al. 2019).
It has been found that the fecal level of Akkermansia muciniphila was reduced in UC patients compared to healthy individuals. An experimental study demonstrated that oral administration of Akkermansia muciniphila BAA-835 strain pointedly amended the symptoms in DSS-induced acute colitis, proved by reduced colon histological inflammatory score, body weight loss, and colon length shortening. Additionally, the frequency of goblet cells and the expression of mucin family were improved upon Akkermansia muciniphila treatment. The levels of other inflammatory mediators such as IL-1β, IL-6, TNF-α, and CCL2 decreased after the treatment (Qu et al. 2021). Probiotic interventions also treat the major depressive disorder (MDD) to modulate the gut microbiome and inflammatory mechanisms. Results showed that the expression of the IL-6 gene was significantly decreased following administration of probiotics than in the placebo group (Reiter et al. 2020).
Concluding remarks
Based on the existing findings and reviewed studies, chronic inflammation underlies IBD, and the IL-6/IL-6R axis can be considered a therapeutic target as an important part of developing this chronic inflammation. On the other hand, this chronic inflammation can increase the development of CAC. A wide range of inhibitors with relatively promising outcomes, including natural compounds, anti-IL-6/IL-6R mAbs, as well as small molecules, have been used to inhibit the IL-6/IL-6R axis in IBD and CAC. However, considering the multiple roles of IL-6 in enhancing the integrity of the epithelial barrier and intestinal homeostasis and inhibiting the infection caused by various microorganisms in the intestinal mucosa, the question arises about whether inhibiting the IL-6/IL-6R axis is always in favor of treatment? The involvement of a wide range of inflammatory mediators in developing chronic inflammation complicates treatment, and monotherapy may not be as successful in the clinic.
For this reason, more studies in this field are needed to discover novel and optimized therapeutic methods such as modulation of the gut microbiome composition using probiotics or natural compounds with minimal side effects to preserve the physiological functions of IL-6. Several of these compounds and their action against intestinal inflammation and microbiome Alteration have been introduced in this study. It is possible that these compounds could be used as a therapeutic combination with other IL-6/IL-6R axis inhibitors to benefit from their synergistic effects. Because according to studies, the effect of microbiome-altering compounds is not limited to reducing IL-6 and can affect other inflammatory mediators and improve the mucosal barrier.
Acknowledgements
Mashhad University of Medical Sciences supported this study.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53(4):379–389. doi: 10.1080/00365521.2018.1447597. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- Asensi V, Valle E, Meana A, Fierer J, Celada A, Alvarez V, Paz J, Coto E, Carton JA, Maradona JA. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun. 2004;72(7):3823–3828. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6(5):583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Atreya R, Neurath M. New therapeutic strategies for treatment of inflammatory bowel disease. Mucosal Immunol. 2008;1(3):175–182. doi: 10.1038/mi.2008.7. [DOI] [PubMed] [Google Scholar]
- Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011 doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr H-A, Murphy AJ, Valenzuela DM. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177(5):2760–2764. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- Beltrán CJ, Núñez LE, Díaz-Jiménez D, Farfan N, Candia E, Heine C, López F, González MJ, Quera R, Hermoso MA. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(7):1097–1107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boe A, Baiocchi M, Carbonatto M, Papoian R, Serlupi-Crescenzi O. Interleukin 6 knock-out mice are resistant to antigen-induced experimental arthritis. Cytokine. 1999;11(12):1057–1064. doi: 10.1006/cyto.1999.0502. [DOI] [PubMed] [Google Scholar]
- Borghini R, Vescovo M, Giordano C, Donato G, Picarelli A. Onset of suspected ulcerative colitis after treatment with tocilizumab in patient with celiac disease and juvenile idiopathic arthritis. Inflamm Bowel Dis. 2021;27(6):e76–e78. doi: 10.1093/ibd/izab036. [DOI] [PubMed] [Google Scholar]
- Bouguen G, Chevaux J-B, Peyrin-Biroulet L. Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol: WJG. 2011;17(5):547. doi: 10.3748/wjg.v17.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5(8):824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, Mach T, Brzozowski T. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14(8):892–900. doi: 10.2174/1570159X14666160404124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, De Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24(21):2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiello I, Minnone G, Holzinger D, Vogl T, Prencipe G, Manzo A, De Benedetti F, Strippoli R. IL-6 amplifies TLR mediated cytokine and chemokine production: implications for the pathogenesis of rheumatic inflammatory diseases. PLoS ONE. 2014;9(10):e107886. doi: 10.1371/journal.pone.0107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–727. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhang H, Yang Z, Zhao J, Ma H. Effect of dehydroepiandrosterone on the immune response and gut microbiota in dextran sulfate sodium-induced colitis mice. Mol Immunol. 2020;118:60–72. doi: 10.1016/j.molimm.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Cao Q, Lin Y, Yue C, Wang Y, Quan F, Cui X, Bi R, Tang X, Yang Y, Wang C. IL-6 deficiency promotes colitis by recruiting Ly6Chi monocytes into inflamed colon tissues in a CCL2-CCR2-dependent manner. Eur J Pharmacol. 2021;904:174165. doi: 10.1016/j.ejphar.2021.174165. [DOI] [PubMed] [Google Scholar]
- Chen W, Yuan H, Cao W, Wang T, Chen W, Yu H, Fu Y, Jiang B, Zhou H, Guo H. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics. 2019;9(14):3980. doi: 10.7150/thno.32352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen H, Ding J, Stanton C, Ross RP, Zhao J, Zhang H, Yang B, Chen W. Bifidobacterium longum ameliorates dextran sulfate sodium-induced colitis by producing conjugated linoleic acid, protecting intestinal mechanical barrier, restoring unbalanced gut Microbiota, and regulating the toll-like receptor-4/Nuclear Factor-κB signaling pathway. J Agric Food Chem. 2021;69(48):14593–14608. doi: 10.1021/acs.jafc.1c06176. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim K-H, Lau LF. The matricellular protein CCN1 promotes mucosal healing in murine colitis through IL-6. Mucosal Immunol. 2015;8(6):1285–1296. doi: 10.1038/mi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonov DC, Ignatova MMK, Ananiev JR, Gulubova MV. IL-6 activities in the tumour microenvironment. Part 1. Open Access Macedonian J Med Sci. 2019;7(14):2391. doi: 10.3889/oamjms.2019.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: practice guidelines and recent developments. World J Gastroenterol. 2019;25(30):4148. doi: 10.3748/wjg.v25.i30.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017;38(2):127–142. doi: 10.1016/j.tips.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jiao H, Teng G, Wang W, Zhang R, Wang Y, Hebbard L, George J, Qiao L. Embelin reduces colitis-associated tumorigenesis through limiting IL-6/STAT3 signaling. Mol Cancer Ther. 2014;13(5):1206–1216. doi: 10.1158/1535-7163.MCT-13-0378. [DOI] [PubMed] [Google Scholar]
- Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, Kohn A, Desreumaux P, Leong RW, Comer GM. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II) Gut. 2019;68(1):40–48. doi: 10.1136/gutjnl-2017-314562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- Deiana M, Serra G, Corona G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018;9(8):4085–4099. doi: 10.1039/C8FO00354H. [DOI] [PubMed] [Google Scholar]
- Deng J, Zhao L, Yuan X, Li Y, Shi J, Zhang H, Zhao Y, Han L, Wang H, Yan Y. Pre-administration of berberine exerts chemopreventive effects in aom/dss-induced colitis-associated carcinogenesis mice via modulating inflammation and intestinal microbiota. Nutrients. 2022;14(4):726. doi: 10.3390/nu14040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59(4):617–628. doi: 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol Cell Biol. 2012;90(8):802–811. doi: 10.1038/icb.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J, Dimitrov S, Lange T. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20(12):2174–2176. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103(1):11–24. doi: 10.1016/S0091-6749(99)70518-X. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1β and the autoinflammatory diseases. Mass Medical Soc. 2009;360:2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- Dong L, Du H, Zhang M, Xu H, Pu X, Chen Q, Luo R, Hu Y, Wang Y, Tu H. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother Res. 2022;36(5):2081–2094. doi: 10.1002/ptr.7429. [DOI] [PubMed] [Google Scholar]
- Elabd EMY, Morsy SM, Elmalt HA. Investigating of Moringa oleifera role on gut microbiota composition and inflammation associated with obesity following high fat diet feeding. Open Access Macedonian J Med Sci. 2018;6(8):1359. doi: 10.3889/oamjms.2018.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-α, interleukin-6 and-8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48(3):233–237. doi: 10.1258/acb.2010.010093. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: pathogenesis and impact of current therapies. Dig Liver Dis. 2021;53(5):558–565. doi: 10.1016/j.dld.2021.01.012. [DOI] [PubMed] [Google Scholar]
- Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A, Tuccillo C, Grossi E, Abbiati R, Garbagna N, Romano M, Tiso A, Blanco CDV, Loguercio C. The effect of a new symbiotic formulation on plasma levels and peripheral blood mononuclear cell expression of some pro-inflammatory cytokines in patients with ulcerative colitis: a pilot study. Eur Rev Med Pharmacol Sci. 2009;13(4):285–293. [PubMed] [Google Scholar]
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Fung TC, Bessman NJ, Hepworth MR, Kumar N, Shibata N, Kobuley D, Wang K, Ziegler CG, Goc J, Shima T. Lymphoid-tissue-resident commensal bacteria promote members of the IL-10 cytokine family to establish mutualism. Immunity. 2016;44(3):634–646. doi: 10.1016/j.immuni.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(2):1–6. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23(3):85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gay J, Kokkotou E, O’Brien M, Pothoulakis C, Karalis KP. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. NeuroImmunoModulation. 2006;13(2):114–121. doi: 10.1159/000096656. [DOI] [PubMed] [Google Scholar]
- Ge H, Cai Z, Chai J, Liu J, Liu B, Yu Y, Liu J, Zhang T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021;360:129981. doi: 10.1016/j.foodchem.2021.129981. [DOI] [PubMed] [Google Scholar]
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis & Rheumatism: off J Am Coll Rheumatol. 2008;58(10):2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- Giner E, Recio MC, Ríos JL, Cerdá-Nicolás JM, Giner RM. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food Res. 2016;60(2):242–255. doi: 10.1002/mnfr.201500605. [DOI] [PubMed] [Google Scholar]
- Glassner KL, Abraham BP, Quigley EM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Goldberg R, Prescott N, Lord GM, MacDonald TT, Powell N. The unusual suspects—innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol. 2015;12(5):271–283. doi: 10.1038/nrgastro.2015.52. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu G-Y, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V, Andus T, Caesar I, Roth M, Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992;102(2):514–519. doi: 10.1016/0016-5085(92)90098-J. [DOI] [PubMed] [Google Scholar]
- Gu D, Zhou S, Yao L, Tan Y, Chi X, Shi D, Guo S , Liu C (2020) ShenLing BaiZhu San alleviates Ulcerative colitis in rats by regulating gut microbiota.10.21203/rs.3.rs-90760/v1
- Gu Z, Zhu Y, Mei F, Dong X, Xia G, Shen X. Tilapia head glycolipids protect mice against dextran sulfate sodium-induced colitis by ameliorating the gut barrier and suppressing NF-kappa B signaling pathway. Int Immunopharmacol. 2021;96:107802. doi: 10.1016/j.intimp.2021.107802. [DOI] [PubMed] [Google Scholar]
- Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Geng W, Chen S, Wang L, Rong X, Wang S, Wang T, Xiong L, Huang J, Pang X. Ginger alleviates DSS-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front Pharmacol. 2021;12:53. doi: 10.3389/fphar.2021.632569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Motiwala MN, Mahajan UN, Sabre SG. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol. 2018;219:222–232. doi: 10.1016/j.jep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D'amato M, Bonfiglio F, McDonald D, Gonzalez A. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):1–7. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Wang L, Zhao Z, You L, Pedisić S, Kulikouskaya V, Lin Z. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocolloids. 2020;109:106048. doi: 10.1016/j.foodhyd.2020.106048. [DOI] [Google Scholar]
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. The Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–714. doi: 10.1016/S1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Inflammatory bowel disease (2022) Retrieved 3.19.2022, from https://www.nhs.uk/conditions/inflammatory-bowel-disease/.
- Ishii K, Shirai T, Kakuta Y, Machiyama T, Sato H, Ishii T, Harigae H, Fujii H. Development of severe colitis in Takayasu arteritis treated with tocilizumab. Clin Rheumatol. 2022;41:1911–1918. doi: 10.1007/s10067-022-06108-z. [DOI] [PubMed] [Google Scholar]
- Ito H. Treatment of Crohn’s disease with anti-IL-6 receptor antibody. J Gastroenterol. 2005;40(16):32–34. doi: 10.1007/BF02990576. [DOI] [PubMed] [Google Scholar]
- Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126(4):989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. 2021;76(9):2699–2715. doi: 10.1111/all.14763. [DOI] [PubMed] [Google Scholar]
- Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Totsuka T, Iiyama R, Okamoto R. Macrophage-derived IL-18–mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121(4):875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- Kevans D, Murthy S, Mould DR, Silverberg MS. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohns Colitis. 2018;12(6):662–669. doi: 10.1093/ecco-jcc/jjy028. [DOI] [PubMed] [Google Scholar]
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- Kittana H, Gomes-Neto JC, Heck K, Geis AL, Segura Muñoz RR, Cody LA, Schmaltz RJ, Bindels LB, Sinha R, Hostetter JM. Commensal Escherichia coli strains can promote intestinal inflammation via differential interleukin-6 production. Front Immunol. 2018;9:2318. doi: 10.3389/fimmu.2018.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45(10):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- Kojima F, Sekiya H, Hioki Y, Kashiwagi H, Kubo M, Nakamura M, Maehana S, Imamichi Y, Yuhki K-I, Ushikubi F. Facilitation of colonic T cell immune responses is associated with an exacerbation of dextran sodium sulfate–induced colitis in mice lacking microsomal prostaglandin E synthase-1. Inflamm Regen. 2022;42(1):1–24. doi: 10.1186/s41232-021-00188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kuhn KA, Schulz HM, Regner EH, Severs EL, Hendrickson JD, Mehta G, Whitney AK, Ir D, Ohri N, Robertson CE. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018;11(2):357–368. doi: 10.1038/mi.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J-I, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage-and epithelial cell-dependent. Dig Dis Sci. 1995;40(5):949–959. doi: 10.1007/BF02064182. [DOI] [PubMed] [Google Scholar]
- Lai C-S, Yang G, Li S, Lee P-S, Wang BN, Chung M-C, Nagabhushanam K, Ho C-T, Pan M-H. 3′-Hydroxypterostilbene suppresses colitis-associated tumorigenesis by inhibition of IL-6/STAT3 signaling in mice. J Agric Food Chem. 2017;65(44):9655–9664. doi: 10.1021/acs.jafc.7b03712. [DOI] [PubMed] [Google Scholar]
- Larussa T, Oliverio M, Suraci E, Greco M, Placida R, Gervasi S, Marasco R, Imeneo M, Paolino D, Tucci L. Oleuropein decreases cyclooxygenase-2 and interleukin-17 expression and attenuates inflammatory damage in colonic samples from ulcerative colitis patients. Nutrients. 2017;9(4):391. doi: 10.3390/nu9040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Comeau CAG, Dreyfuss JM. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8:e39982. doi: 10.7554/eLife.39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13(1):11. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-C, Liu C-Y, Lee C-L, Zhang R-H, Huang C-J, Yen T-L. The periodontopathic pathogen, porphyromonas gingivalis, involves a gut inflammatory response and exacerbates inflammatory bowel disease. Pathogens. 2022;11(1):84. doi: 10.3390/pathogens11010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite AZ, Rodrigues NDC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Matheucci Junior E. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017;8:1107. doi: 10.3389/fimmu.2017.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Han Y, Cai X, Gu M, Sun J, Qi C, Goulette T, Song M, Li Z, Xiao H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020;11(1):1063–1073. doi: 10.1039/C9FO01519A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen A, Chu J, Sferra TJ, Sankararaman S, Ke X, Chen Y, Peng J. Pien Tze Huang ameliorates DSS-induced colonic inflammation in a mouse colitis model through inhibition of the IL-6/STAT3 pathway. Mol Med Rep. 2018;18(1):1113–1119. doi: 10.3892/mmr.2018.9051. [DOI] [PubMed] [Google Scholar]
- Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59(2):227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- Liu LQ, Nie SP, Shen MY, Hu JL, Yu Q, Gong D, Xie MY. Tea polysaccharides inhibit colitis-associated colorectal cancer via interleukin-6/STAT3 pathway. J Agric Food Chem. 2018;66(17):4384–4393. doi: 10.1021/acs.jafc.8b00710. [DOI] [PubMed] [Google Scholar]
- Lochhead P, Khalili H, Ananthakrishnan AN, Richter JM, Chan AT. Association between circulating levels of C-reactive protein and interleukin-6 and risk of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(6):818–824. doi: 10.1016/j.cgh.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4(2):96–100. doi: 10.1016/1043-4666(92)90043-Q. [DOI] [PubMed] [Google Scholar]
- Maerten P, Shen C, Colpaert S, Liu Z, Bullens D, Van Assche G, Penninckx F, Geboes K, Vanham G, Rutgeerts P. Involvement of interleukin 18 in Crohn's disease: evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135(2):310–317. doi: 10.1111/j.1365-2249.2004.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Ramming A, Bergmann C, Weinkam R, Kittan N, Schett G, Distler JH, Beyer C. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis. 2017;76(6):1133–1141. doi: 10.1136/annrheumdis-2016-210189. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafini I, Sedda S, Dinallo V, Monteleone G. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther. 2019;19(11):1207–1217. doi: 10.1080/14712598.2019.1652267. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Nagaoka M, Hara T, Kimura-Takagi I, Mistuyama K, Ueyama S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin Exp Immunol. 2004;136(3):432–439. doi: 10.1111/j.1365-2249.2004.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon M, Hawkey C, Mahida Y. Expression of interleukin 1β and interleukin 1β converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42(2):214–219. doi: 10.1136/gut.42.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee D, Bamberg T, Vitkus S, McGhee J. A synergistic relationship between TNF-alpha, IL-1 beta, and TGF-beta 1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86(1):6. [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- Mitsuyama K, Tomiyasu N, Suzuki A, Takaki K, Takedatsu H, Masuda J, Yamasaki H, Matsumoto S, Tsuruta O, Toyonaga A. A form of circulating interleukin-6 receptor component soluble gp130 as a potential interleukin-6 inhibitor in inflammatory bowel disease. Clin Exp Immunol. 2006;143(1):125–131. doi: 10.1111/j.1365-2249.2005.02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36(1):45–49. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modur V, Li Y, Zimmerman GA, Prescott SM, McIntyre TM. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. J Clin Investig. 1997;100(11):2752–2756. doi: 10.1172/JCI119821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol. 2010;6(5):339. [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22(4):361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13(8):1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- Mülberg J, Schooltink H, Stoyan T, Günther M, Graeve L, Buse G, Mackiewicz A, Heinrich PC, Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993;23(2):473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch S, Braegger C, Walker-Smith J, MacDonald T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34(12):1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, Yanagisawa R, Inoue K-I, Takano H, Satoh M. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med. 2004;14(2):191–196. [PubMed] [Google Scholar]
- Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, Hirai F, Hata K, Hiraoka S. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi: 10.1007/s00535-021-01784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160(1):29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- O'Reilly S, Ciechomska M, Cant R, van Laar JM. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem. 2014;289(14):9952–9960. doi: 10.1074/jbc.M113.545822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly S. Circulating Gremlin-1 is elevated in systemic sclerosis patients. J Scleroderma Relat Disorders. 2021;6(3):286–289. doi: 10.1177/23971983211036571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Tsuchiya K, Nemoto Y, Akiyama J, Nakamura T, Kanai T, Watanabe M. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol-Gastrointest Liver Physiol. 2009;296(1):G23–G35. doi: 10.1152/ajpgi.90225.2008. [DOI] [PubMed] [Google Scholar]
- Ota M, Yanagisawa M, Tachibana H, Yokota K, Araki Y, Sato K, Mimura T. A significant induction of neutrophilic chemoattractants but not RANKL in synoviocytes stimulated with interleukin 17. J Bone Miner Metab. 2015;33(1):40–47. doi: 10.1007/s00774-014-0565-y. [DOI] [PubMed] [Google Scholar]
- Pandurangan AK, Mohebali N, Hasanpourghadi M, Looi CY, Mustafa MR, Mohd Esa N. Boldine suppresses dextran sulfate sodium-induced mouse experimental colitis: NF-κB and IL-6/STAT3 as potential targets. BioFactors. 2016;42(3):247–258. doi: 10.1002/biof.1267. [DOI] [PubMed] [Google Scholar]
- Park H-J, Kim D-H, Lim S-H, Kim W-J, Youn J, Choi Y-S, Choi J-M. Insights into the role of follicular helper T cells in autoimmunity. Immune Network. 2014;14(1):21–29. doi: 10.4110/in.2014.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłowska-Kamieniak A, Krawiec P, Pac-Kożuchowska E. Interleukin 6: biological significance and role in inflammatory bowel diseases. Adv Clini Exp Med. 2021;30(4):465–469. doi: 10.17219/acem/130356. [DOI] [PubMed] [Google Scholar]
- Pincus T, Sokka T. Laboratory tests to assess patients with rheumatoid arthritis: advantages and limitations. Rheum Dis Clin. 2009;35(4):731–734. doi: 10.1016/j.rdc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162(11):6829–6835. doi: 10.4049/jimmunol.162.11.6829. [DOI] [PubMed] [Google Scholar]
- Powell N, Lo JW, Biancheri P, Vossenkämper A, Pantazi E, Walker AW, Stolarczyk E, Ammoscato F, Goldberg R, Scott P. Interleukin 6 increases production of cytokines by colonic innate lymphoid cells in mice and patients with chronic intestinal inflammation. Gastroenterology. 2015;149(2):456–467. doi: 10.1053/j.gastro.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, Liu W, Liu W, Si J. Akkermansia muciniphila Alleviates dextran sulfate sodium (DSS)-induced acute colitis by NLRP3 activation. Microbiol Spect. 2021;9(2):e00730–e1721. doi: 10.1128/Spectrum.00730-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather IA, Bajpai VK, Ching LL, Majumder R, Nam G-J, Indugu N, Singh P, Kumar S, Hajrah NH, Sabir JS. Effect of a bioactive product SEL001 from Lactobacillus sakei probio65 on gut microbiota and its anti-colitis effects in a TNBS-induced colitis mouse model. Saudi J Biol Sci. 2020;27(1):261–270. doi: 10.1016/j.sjbs.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch W, Gasché C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, Dejaco C, Waldhör T, Bakos S, Vogelsang H. Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94(8):2156–2164. doi: 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- Reiter A, Bengesser SA, Hauschild A-C, Birkl-Töglhofer A-M, Fellendorf FT, Platzer M, Färber T, Seidl M, Mendel L-M, Unterweger R. Interleukin-6 gene expression changes after a 4-week intake of a multispecies probiotic in major depressive disorder—Preliminary Results of the PROVIT Study. Nutrients. 2020;12(9):2575. doi: 10.3390/nu12092575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, Kaplan GG and Seow CH (2015) Budesonide for induction of remission in Crohn's disease. Cochrane Database of Syst Reviews 6 [DOI] [PMC free article] [PubMed]
- Rogler G. Where are we heading to in pharmacological IBD therapy? Pharmacol Res. 2015;100:220–227. doi: 10.1016/j.phrs.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, Van Hinsbergh V, Sozzani S. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–325. doi: 10.1016/S1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Pt 2):281. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20(11):1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- Rossi D, Modena V, Sciascia S, Roccatello D. Rheumatoid arthritis: biological therapy other than anti-TNF. Int Immunopharmacol. 2015;27(2):185–188. doi: 10.1016/j.intimp.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Rossi J-F, Lu Z-Y, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21(6):1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- Saadatdoust Z, Pandurangan AK, Sadagopan SKA, Esa NM, Ismail A, Mustafa MR. Dietary cocoa inhibits colitis associated cancer: a crucial involvement of the IL-6/STAT3 pathway. J Nutr Biochem. 2015;26(12):1547–1558. doi: 10.1016/j.jnutbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol: WJG. 2008;14(27):4280. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26(5):475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Aden K, Bernardes JP, Conrad C, Tran F, Höper H, Volk V, Mishra N, Blase JI, Nikolaus S. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. 2021;160(7):2354–2366. doi: 10.1053/j.gastro.2021.02.062. [DOI] [PubMed] [Google Scholar]
- Semerano L, Thiolat A, Minichiello E, Clavel G, Bessis N, Boissier M-C. Targeting IL-6 for the treatment of rheumatoid arthritis: phase II investigational drugs. Expert Opin Investig Drugs. 2014;23(7):979–999. doi: 10.1517/13543784.2014.912276. [DOI] [PubMed] [Google Scholar]
- Shao X, Sun C, Tang X, Zhang X, Han D, Liang S, Qu R, Hui X, Shan Y, Hu L. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis. J Agric Food Chem. 2020;68(44):12295–12309. doi: 10.1021/acs.jafc.0c04773. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zou J, Chen M, Zhang Z, Liu C, Jiang S, Qian D, Duan J-A. Protective effects of Lizhong decoction on ulcerative colitis in mice by suppressing inflammation and ameliorating gut barrier. J Ethnopharmacol. 2020;259:112919. doi: 10.1016/j.jep.2020.112919. [DOI] [PubMed] [Google Scholar]
- Shi J, Xie Q, Yue Y, Chen Q, Zhao L, Evivie SE, Li B, Huo G. Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice. Food Funct. 2021;12(11):5130–5143. doi: 10.1039/D1FO00317H. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E, Lopez JV, Tartar JL. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE. 2019;14(10):e0222394. doi: 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi C, Rizzo A, Franzè E, Rotondi A, Fantini MC, Sarra M, Caruso R, Monteleone I, Sileri P, Franceschilli L. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208(11):2279–2290. doi: 10.1084/jem.20111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MC, Zhang FC, Yin X, Cheng BJ, Zhao CH, Wang YL, Zhang ZZ, Hao HW, Zhang TH, Ye HQ. Lactobacillus reuteri F-9-35 prevents DSS-Induced colitis by inhibiting proinflammatory gene expression and restoring the gut microbiota in mice. J Food Sci. 2018;83(10):2645–2652. doi: 10.1111/1750-3841.14326. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286(36):31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122(1):44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014;588(22):4244–4249. doi: 10.1016/j.febslet.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Wu L-W, Grivennikov SI, De Jong PR, Lian I, Yu F-X, Wang K, Ho SB, Boland BS, Chang JT. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8(10):1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Investig. 2011;121(6):2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche G, Manguso F, Zibellini M, Nuño JLC, Goldis A, Tkachenko E, Varoli G, Kleczkowski D, Annese V, F. D'heygere, Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Off J Am Coll Gastroenterol ACG. 2015;110(5):708–715. doi: 10.1038/ajg.2015.114. [DOI] [PubMed] [Google Scholar]
- Van Kemseke C, Belaiche J, Louis E. Frequently relapsing Crohn's disease is characterized by persistent elevation in interleukin-6 and soluble interleukin-2 receptor serum levels during remission. Int J Colorectal Dis. 2000;15(4):206–210. doi: 10.1007/s003840000226. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. The Lancet. 2017;389(10066):266–275. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- Wallace KL, Zheng L-B, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol: WJG. 2014;20(1):6. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li W, Wang H, Ma Y, Zhao X, Zhang X, Yang H, Qian J, Li J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019;19(1):1–12. doi: 10.1186/s12866-019-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang J, Ding Y, Zhou J, Gao G, Han H, Zhou J, Ke L, Rao P and Chen T (2022) Nanoparticles isolated from porcine bone soup ameliorated dextran sulfate sodium-induced colitis and regulated gut microbiota in mice. Front Nutr 9 [DOI] [PMC free article] [PubMed]
- Wang J, Wang P, Li D, Hu X, Chen F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur J Nutr. 2020;59(2):699–718. doi: 10.1007/s00394-019-01938-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Cheng K, Liu Z, Sun Y, Zhou L, Xu M, Dai X, Xiong Y, Zhang H. Bioactive constituents of Mosla chinensis-cv. Jiangxiangru ameliorate inflammation through MAPK signaling pathways and modify intestinal microbiota in DSS-induced colitis mice. Phytomedicine. 2021;93:153804. doi: 10.1016/j.phymed.2021.153804. [DOI] [PubMed] [Google Scholar]
- Wang Y, Duan X, Liu X, Liu Y, Fan H, Xu M, Chen Q, Tang Q. Rho kinase blockade ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of the NF-κB and IL-6/STAT3 pathways. Inflammation. 2020;43(3):857–867. doi: 10.1007/s10753-019-01171-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu X, Wang C-L, Wang L, Sun C, Zhang D-B, Liu J-L, Liang Y-N, Tang D-X, Tang Z-S. Tryptanthrin protects mice against dextran sulfate sodium-induced colitis through inhibition of TNF-α/NF-κB and IL-6/STAT3 pathways. Molecules. 2018;23(5):1062. doi: 10.3390/molecules23051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NR. Coordination of immune-stroma crosstalk by IL-6 family cytokines. Front Immunol. 2019;10:1093. doi: 10.3389/fimmu.2019.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-J, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu Y, Chen Y, Liu M, Yu H, Zhang Y, Wang T. Desmethylbellidifolin attenuates dextran sulfate sodium-induced colitis: impact on intestinal barrier, intestinal inflammation and gut microbiota. Planta Med. 2021;88:559–569. doi: 10.1055/a-1506-3476. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhang Y, Ma J, Liu Y, Li W, Wang T, Xu X, Wang Y, Cheng K, Zhuang R. Interleukin-6 absence triggers intestinal microbiota dysbiosis and mucosal immunity in mice. Cytokine. 2022;153:155841. doi: 10.1016/j.cyto.2022.155841. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Xiao T, Zhang P, Feng T, Lu K, Wang X, Zhou S, Qiang Y. Butyrate functions in concert with myeloid-derived suppressor cells recruited by CCR9 to alleviate DSS-induced murine colitis. Int Immunopharmacol. 2021;99:108034. doi: 10.1016/j.intimp.2021.108034. [DOI] [PubMed] [Google Scholar]
- Xu X-R, Liu C-Q, Feng B-S, Liu Z-J. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol: WJG. 2014;20(12):3255. doi: 10.3748/wjg.v20.i12.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164(9):4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFNβ 2) receptor. Science. 1988;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yang C, Qiao Z, Xu Z, Wang X, Deng Q, Chen W, Huang F. Algal oil rich in docosahexaenoic acid alleviates intestinal inflammation induced by antibiotics associated with the modulation of the gut microbiome and metabolome. J Agric Food Chem. 2021;69(32):9124–9136. doi: 10.1021/acs.jafc.0c07323. [DOI] [PubMed] [Google Scholar]
- Yang R, Rincon M. IL-6 induces IL-21 and B cell helper function in CD8 cells. J Immunol. 2016;196(1):189.8. doi: 10.4049/jimmunol.196.Supp.189.8. [DOI] [Google Scholar]
- Yang X, Zhang F, Wang Y, Cai M, Wang Q, Guo Q, Li Z, Hu R. Oroxylin A inhibits colitis-associated carcinogenesis through modulating the IL-6/STAT3 signaling pathway. Inflamm Bowel Dis. 2013;19(9):1990–2000. doi: 10.1097/MIB.0b013e318293c5e0. [DOI] [PubMed] [Google Scholar]
- Ye M, Joosse ME, Liu L, Sun Y, Dong Y, Cai C, Song Z, Zhang J, Brant SR, Lazarev M. Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-deficient mice. J Crohns Colitis. 2020;14(6):831–840. doi: 10.1093/ecco-jcc/jjz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Investig. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F-X, Guan K-L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang Y, Li R. Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine. 2021;84:153519. doi: 10.1016/j.phymed.2021.153519. [DOI] [PubMed] [Google Scholar]
- Zheng R, Ma J, Wang D, Dong W, Wang S, Liu T, Xie R, Liu L, Wang B, Cao H. Chemopreventive effects of silibinin on colitis-associated tumorigenesis by inhibiting IL-6/STAT3 signaling pathway. Med Inflamm. 2018 doi: 10.1155/2018/1562010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sonnenberg GF. Essential immunologic orchestrators of intestinal homeostasis. Sci Immunol. 2018;3(20):1605. doi: 10.1126/sciimmunol.aao1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L-Q, Zhang L, Zhang J, Chang G-L, Liu G, Yu D-D, Yu X-M, Zhao M-S, Ye B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J Integr Med. 2021;19(1):56–65. doi: 10.1016/j.joim.2020.11.001. [DOI] [PubMed] [Google Scholar]