Abstract
Pathogenic infections have significant roles in the pathogenesis of colorectal cancer (CRC). These infections induce the secretion of various damage-associated molecular patterns (DAMPs) including interleukin-1 alpha (IL-1α) and high mobility group box-1 (HMGB1). Despite their implication in CRC pathogenesis, the mechanism(s) that modulate the secretion of IL-1α and HMGB1, along with their roles in promoting CRC tumourigenesis remain poorly understood. To understand the secretory mechanism, HT-29 and SW480 cells were stimulated with infectious mimetics; polyinosinic:polycytidylic acid [Poly(I:C)], lipopolysaccharide (LPS) and pro-inflammatory stimuli; tumour necrosis factor-alpha (TNF-α). IL-1α and HMGB1 secretion levels upon stimulation were determined via ELISA. Mechanism(s) mediating IL-1α and HMGB1 secretion in CRC cells were characterized using pharmacological inhibitors and CRISPR-Cas9 gene editing targeting relevant pathways. Recombinant IL-1α and HMGB1 were utilized to determine their impact in modulating pro-tumourigenic properties of CRC cells. Pharmacological inhibition showed that Poly(I:C)-induced IL-1α secretion was mediated through endoplasmic reticulum (ER) stress and RIPK1 signalling pathway. The secretion of HMGB1 was RIPK1-dependent but independent of ER stress. RIPK1-targeted CRC cell pools exhibited decreased cell viability upon Poly(I:C) stimulation, suggesting a potential role of RIPK1 in CRC cells survival. IL-1α has both growth-promoting capabilities and stimulates the production of pro-metastatic mediators, while HMGB1 only exhibits the latter; with its redox status having influence. We demonstrated a potential role of RIPK1-dependent signalling pathway in mediating the secretion of IL-1α and HMGB1 in CRC cells, which in turn enhances CRC tumorigenesis. RIPK1, IL-1α and HMGB1 may serve as potential therapeutic targets to mitigate CRC progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00681-3.
Keywords: Interleukin-1 alpha (IL-1α), High mobility group box-1 (HMGB1), Colorectal cancer, Inflammation, Damage-associated molecular patterns (DAMPs)
Introduction
Colorectal cancer (CRC) is responsible for over 1,000,000 new diagnoses and over 500,000 deaths annually worldwide (Sung et al. 2021). Although recent advances in diagnosis and surgical resection is curative for patients with localized disease, many CRC patients are diagnosed in advanced stages, whereby the cancer has metastasized to the secondary sites. Advanced stage CRC is associated with poor prognosis and high mortality rates (Brouwer et al. 2018). Thus, a better understanding of the underlying mechanisms that initiate and drive the progression of CRC is vital for the identification and development of novel diagnostic or therapeutic strategies to mitigate CRC.
A multitude of risk factors ranging from inflammatory syndromes, environmental and hereditary factors have been attributed to the development of CRC. Recently, various pathogens have also been identified as risk factors for a variety of epithelial cells-derived cancers including CRC (Antonic et al. 2013). Accumulating evidence has suggested a link between pathogenic infections (i.e. viruses and bacteria) with the onset of CRC (Antonic et al. 2013), yet, the mechanism(s) promoting carcinogenesis remains poorly understood. Notably, these pathogens mediate the release of endogenous molecules known as damage-associated molecular patterns (DAMPs) (Murao et al. 2021). The main function of DAMPs is to initiate the inflammatory and immune cascade to restore cellular homeostasis (Murao et al. 2021). Unresolved or chronic inflammation, however, can be mutagenic and has been shown to promote tumour initiation in various types of cancers (Greten and Grivennikov 2019).
Inflammation is also an important driver of cancer progression (Hanahan and Weinberg Robert 2011), and shapes the pathogenesis of CRC (Schmitt and Greten 2021). Interestingly, certain DAMPs such as interleukin 1 alpha (IL-1α) and high mobility group box 1 (HMGB1) have been shown to be significantly upregulated in tumour and serum samples of CRC patients (Hillenbrand et al. 2012; Wang et al. 2020), suggesting a possible link with the pathological processes of the disease. Indeed, IL-1α and HMGB1 have shown to exert critical functions in malignancies, influencing tumour microenvironments and promoting cancer initiation and progression in many cancers including CRC (Chiu et al. 2021; Tripathi et al. 2019). However, other studies on IL-1α and HMGB1 have shown conflicting results whereby these DAMPs were involved in enhancing innate immunity and promoting CRC cell death (Cheng et al. 2020, 2021). For HMGB1, emerging evidence has shown that the differential roles of HMGB1 in immune and inflammatory responses is dependent on the redox status of the protein, influenced by cysteine (C) residues located at C23, C45 and C106, which are sensitive to oxidation (Cheng et al. 2020). HMGB1 can be classified as fully reduced; all-thiol HMGB1, which acts as a chemoattractant, semi-oxidised; disulfide HMGB1, which has cytokine inducing activity and terminally oxidised HMGB1, which is inert and involved in the resolution of inflammation (Cheng et al. 2020). In contrast to HMGB1, the cause of the conflicting roles for IL-1α in inflammation are still uncertain. Note, HMGB1 may also form complexes with interleukin-1α, which enhances its pro-inflammatory capabilities (Wähämaa et al. 2011), but these aspects in cancer progression, and its relevance in CRC has not yet been explored.
CRC cells are capable of secreting IL-1α (Matsuo et al. 2009) and HMGB1 (Lee et al. 2012), though, the secretory pathway(s) are yet to be fully defined. Recent studies have reported that pathogen infections may induce HMGB1 and IL-1α secretion via endoplasmic reticulum (ER) stress (Choi and Song 2020; Kandel-Kfir et al. 2015). ER stress is a global stress response that occurs when there are perturbations in cellular homeostasis or misfolded/unfolded proteins which causes the activation of unfolded protein response (UPR) (Corazzari et al. 2017). ER stress is also an emerging driver of cancer invasion and metastasis, and activation of ER/UPR pathways have been linked with tumour progression (Bobrovnikova-Marjon et al. 2010; Zhang et al. 2020). Interestingly, ER stress induces inflammasome activation which also plays a crucial role in contributing to CRC progression (Wang et al. 2016), and this was reported to occur through the activation of kinase receptor-interacting protein 1 (RIPK1) (Tao et al. 2018). The NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) is the main inflammasome that has been shown to mediate IL-1α and HMGB1 secretion in various cell types (Di Paolo and Shayakhmetov 2016; Wang et al. 2018). However, the complete relationship between infectious agents and its downstream ER stress/RIPK1/NLRP3 inflammasome signalling pathway in promoting IL-1α/HMGB1 secretion in CRC cells is still undetermined.
In this study, we investigated the secretory mechanism(s) of IL-1α and HMGB1 in CRC cells and their functional roles in promoting tumorigenesis. We found that viral mimetic; Poly(I:C) induces the secretion of IL-1α and HMGB1 in CRC cells, and pharmacological inhibition of RIPK1 activity revealed that the secretory mechanism was dependent on this kinase. Targeting RIPK1 using CRISPR-Cas9 in HT-29 cell pools significantly decreased cell viability post Poly(I:C) stimulation, suggesting that RIPK1 may also have a role in cell survival. Upon investigating the activity and function of IL-1α and HMGB1, both DAMPs showed oncogenic roles but have differential impacts in regulating the process of tumorigenesis. Altogether, our data provides a molecular link between the secretory pathways of IL-1α and HMGB1, as well as demonstrating their mechanism of action as drivers of CRC pathogenesis.
Methods
Cell culture
Human CRC cell lines HT-29 and SW480 were obtained from the American Type Culture Collection (ATCC, USA). HEK293T cells, obtained from ATCC, were used for lentivirus production. The human CRC cell lines and HEK293T cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (Corning, USA) supplemented with 4.5 g/L of glucose, l-glutamine and sodium pyruvate containing 10% fetal bovine serum (FBS), penicillin (100 U mL−1) and streptomycin (100 μg mL−1).
CRISPR-Cas9-mediated RIPK1 targeting
To perform the RIPK1 CRC cells pool targeting, LentiCas9-Blast and sgRNA expression vector pKLV-U6-gRNA(BbsI)-PGKhygro2AeGFP were utilized. The LentiCas9-Blast was a gift from Feng Zhang (Addgene plasmid #52,962) (Sanjana et al. 2014) and the pKLV-U6-gRNA(BbsI)-PGKhygro2AeGFP was previously generated and characterized by Syafruddin et al., (Syafruddin et al. 2019). The lentivirus packaging plasmids psPAX2 (Addgene plasmid #12,260) and pMD2.G (Addgene plasmid #122,259) were gifts from Didier Trono. Lentivirus was produced by co-transfecting HEK293T cells with psPAX2, pMD2.G and sgRNA expression vector using Attractene transfection reagent (Qiagen, USA) according to the manufacturer’s recommendations. The media containing the lentivirus was collected 72 h post-transfection and transduced into previously established HT-29 stably expressing the Cas9 in the presence of 8 μg/mL Polybrene (Merck, USA). The positively transduced cells were selected using hygromycin (Nacalai Tesque, Japan).
For the sgRNA constructs cloning, the top and bottom strands were designed and purchased separately. Each of these strands were designed to harbour BbsI restriction site overhangs at their respective 5′ and 3′ end that were compatible for ligation into the sgRNA expression vectors. The sgRNA constructs used in this study were purchased for Integrated DNA Technologies (IDT). These sequences are listed in Supplementary Tables 1 and 2.
Cell stimulation
To investigate the effect of infectious mimetics and pro-inflammatory stimulus in mediating IL-1α and HMGB1 secretion, CRC cells were treated with polyinosinic:polycytidylic acid [Poly(I:C)], lipopolysaccharide (LPS) and tumour necrosis factor alpha (TNF-α) for 24 h. To study the mechanism(s) involved in mediating IL-1α and HMGB1 secretion, cells were pre-incubated with various pharmacological inhibitors or the appropriate vehicle for 2 h prior to stimulation with Poly(I:C) for a further 24 h.
To study the functional roles of IL-1α and the different redox states of HMGB1, cells were stimulated with human recombinant IL-1α, disulfide (DS) HMGB1 and non-oxidizable (NO) HMGB1, a mutant form of recombinant human HMGB1 that is resistant to oxidation. Complexes of HMGB1 proteins with IL-1α were prepared by mixing matching required concentrations of each protein, followed by a 16-h incubation at 4 °C as previously performed by others (Hreggvidsdóttir et al. 2012; Hreggvidsdottir et al. 2009). Details of all stimulus and pharmacological inhibitors used are provided in Supplementary Table 3.
ELISA
The levels of IL-1α, HMGB1, CXCL1, CXCL5 and CXCL8 were measured in the cell culture supernatants using commercially available ELISA kits. IL-1α, CXCL1, CXCL5 and CXCL8 were Duo-Set ELISAs purchased from R&D Systems, USA, while HMGB1 ELISA was purchased from Chondrex, USA. All assays were performed according to the manufacturer’s guidelines. Limit of detection are listed in Supplementary Table 4.
Caspase-1 assay kit
The activity of caspase-1 was measured in cell lysates using a commercially available Caspase-1 Colorimetric Assay Kit (Biovision, USA). All assays were performed according to the manufacturer’s guidelines.
MTT assay
Cells were seeded in 96-well plates at a density of 1 × 104 cells per well and treated with IL-1α and the different redox states of HMGB1 at four different time points: 6, 24, 48 and 72 h. At the end of the stimulation period, cells were incubated with 1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, USA), prepared in DMEM for 30 min at 37 °C in a 5% CO2 humidified environment to determine cell viability. The MTT solution was removed and 100 µl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) was added to solubilize the formazan product. Absorbance was measured at 570 nm using a plate reader (SpectraMax M3, Molecular Devices, USA).
Gap closure assay
Cells were seeded into a two-well chamber (IBIDI, USA) in a 24-well plate. Upon confluency, the chambers were removed, and the cells were stimulated with IL-1α and the different redox states of HMGB1. The proliferation rate of the cells was captured using live cell imaging microscope and its embedded software the Ti control (Nikon TI-E, Japan). The images captured were then analysed and the reduction in the gap area at 0, 12, 24, 36, 48, 60 and 72 h were measured using NIS Elements AR software. Cellular proliferation was then expressed as the percentage of gap closure using the formula below:
At = 0 h is the area of gap measured immediately after gap formed (t = 0 h), At = x h is the area of gap measured at x h after the gap was formed.
The gap closure rate was calculated with the formula below:
Western blotting
30 µg of protein were boiled at 100 °C with 2 × Laemmli concentrate sample buffer (Merck, USA) for 10 min. The proteins were then subjected to SDS–polyacrylamide gel electrophoresis (PAGE) at 175 V for 40 min and transferred onto polyvinylidene fluoride membranes via wet transfer at 110 V for 60 min. The membranes were blocked for 2 h at room temperate in 5% bovine serum albumin (BSA) diluted in Milli-Q water to prevent non-specific binding. Membranes were then incubated with primary antibodies; rabbit anti-RIPK1, rabbit anti-E-cadherin, mouse anti-PERK, mouse anti-IRE1α and mouse anti-ATF4. After washing with PBS 0.1%/Tween-20 (Sigma-Aldrich), the membranes were incubated for 2 h at room temperature with a secondary antibody; an ECL-anti-rabbit or ECL-anti-mouse IgG HRP-linked antibody (Cell Signaling Technology, USA) diluted 1:5000. The immunoreactive bands were detected using the enhanced chemiluminescence (ECL) system (MultiDoc-It Imaging System, UVP, USA). To ensure equal loading of proteins, membranes were re-probed with rabbit anti-β-actin or rabbit anti-GAPDH. The intensity of the bands was analysed using the ImageJ2 software. Details of primary antibodies and dilutions are listed in Supplementary Table 5.
Immunofluorescence staining and imaging
To study the distribution of E-cadherin, cells were seeded into 8 chamber µ-slides (IBIDI, USA) and grown to 60% confluence. Cells were stimulated with IL-1α and HMGB1 for 48 and 72 h. At the end of the stimulation period, cells were fixed with 4% paraformaldehyde at room temperature and permeabilized using 0.2% Triton X-100. To prevent non-specific binding, the cells were incubated in 5% BSA diluted in Milli-Q water for 2 h. Subsequently, cells were incubated overnight at 4 °C with 1:1000 dilution (52 ng/mL) of rabbit anti-E-cadherin (rabbit monoclonal to E-cadherin, 24E10, CST, USA) in 5% BSA. Normal rabbit IgG isotype control antibody corresponding to the final concentration of primary antibodies were used to serve as a staining control. Following this, the cells were stained with 1:1000 dilution (2 μg/mL) of Alexa Fluor® 488 Goat Anti-Rabbit IgG (SouthernBiotech, USA) for 1 h at room temperature. Cells were then washed 3 times with PBS. Finally, cells were mounted on slides using Fluoroshield™ with DAPI to stain for nuclei (Sigma, USA). 5 non-overlapping field views of each group were captured at 20 × magnification. DAPI (excitation 358 nm, emission 461 nm) and Alexa Fluor® 488 (excitation 495 nm, emission 519 nm) fluorescent images were visualized using an upright epifluorescence microscope (Nikon TI-E, Japan).
Spherogenic assay
To study spherogenic potential, 6 well plates were coated with a layer of 0.6% agar, to inhibit cellular adhesion to the culture plate surface. 1 × 104 cells were seeded for each well with 2 ml of complete media with human recombinant IL-1α, and the different redox states of HMGB1 added immediately. After 10 days of stimulation, pictures of eight non-overlapping fields for each well were captured using an inverted phase contrast microscope (Euromax, UK) at 4 × magnification. Spheroids from each picture were analysed, and the length of the major and minor axis of each spheroid was measured using ImageJ Software. Axis values below 70 μm were excluded as not corresponding to mature spheroids and the volume was calculated by applying the sphere adapted formula (major axis × minor axis)2/2 as previously performed by Gelfo et al. (2018).
Human EpCAM assay kit
At the end of the spherogenic assay, the spheroids were collected and lysed using a commercially available Human EpCAM Assay Kit (Abcam, USA). The total expression of EpCAM was measured in 5 μg/mL of spheroid lysates. All assays were performed according to the manufacturer’s guidelines.
Colony forming assay
To study clonogenic potential, 500 cells were seeded in 6-well plates and left to attach overnight. The cells were then stimulated with IL-1α and the different redox states of HMGB1 for 10 days. At the end of the stimulation period, the medium was removed, and the cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. After washing with PBS, the cells were stained with 0.5% crystal violet for 10 min, then washed with water to remove excess dye. A picture of each well was taken with a camera, and the number of colonies were measured using the ImageJ software, as performed by others (Gelfo et al. 2018; Huang et al. 2018).
Cancer metastasis multiplex assay
Pro-metastatic mediators were measured in cell culture supernatants and lysates using the MILLIPLEXMAP Human Cancer/ Metastasis Biomarker Magnetic Bead Panel (Merck, USA). All multiplex magnetic bead assays were performed according to manufacturer’s protocols. The Luminex xMAP system was used for detection and data acquisition.
Statistical analysis
For ELISA analysis, mean data are presented as absolute values ± standard error mean (SEM). Statistical analysis was performed using one-way with Dunnett’s post hoc test when comparing stimulants to control group. One/two-way ANOVA with Bonferroni’s correction post-hoc test was used when there are intragroup comparisons (i.e. stimulation in the presence/absence of inhibitors).
For western blotting, the expression of RIPK1, PERK and IRE1α were normalized to β-actin, while ATF4 and E-cadherin were normalized to GAPDH. The protein expression in treated cells were then expressed as fold change relative to controls. The protein expression in CRISPR transduced cells were expressed as fold change relative to the non-targeting control. For all analysis, P values ≤ 0.05 were considered statistically significant.
Results
Stimulation of CRC cells with infectious mimetics promotes active secretion of IL-1α and HMGB1
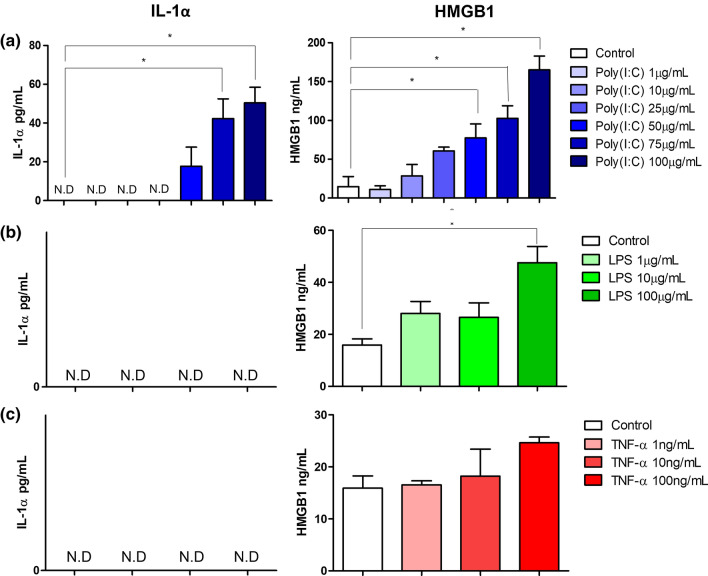
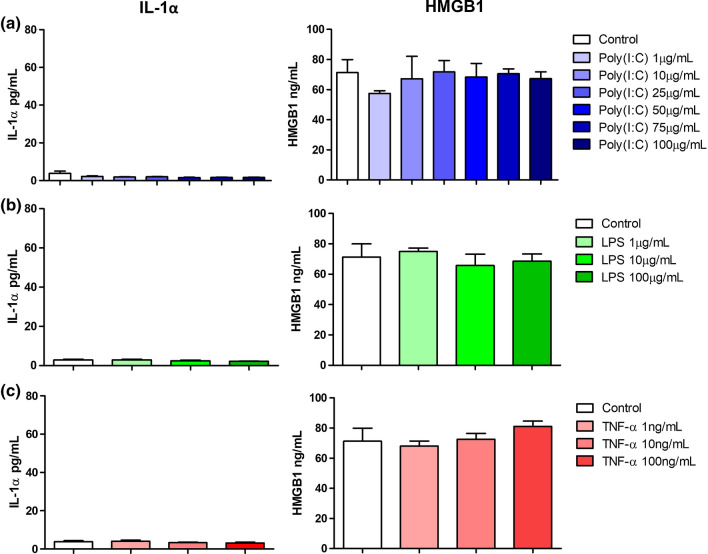
We first aimed to identify the factors that modulate IL-1α and HMGB1 secretion in CRC cells. HT-29 and SW480 CRC cells were stimulated with viral mimetic; Poly(I:C), bacterial mimetic; LPS and pro-inflammatory mediator; TNF-α for 24 h and the secretion of IL-1α and HMGB1 were measured. In HT-29 cells, Poly(I:C) significantly induced the secretion of both IL-1α and HMGB1 in a concentration-dependent manner (Fig. 1a). LPS significantly induced the secretion of HMGB1 at the highest concentration of 100 μg/mL, however, failed to induce any secretion of IL-1α (Fig. 1b). Surprisingly, TNF-α did not induce any significant secretion of neither IL-1α nor HMGB1 (Fig. 1c). In the SW480 cell lines, no upregulation in the secretion of IL-1α and HMGB1 were observed post-stimulation with Poly(I:C), LPS and TNF-α (Fig. 2).
Fig. 1.
Effect of infectious mimetics and pro-inflammatory stimulus on IL-1α and HMGB1 secretion by HT-29 cells. Cells were stimulated with a Poly(I:C), b LPS and c TNF-α for 24 h. IL-1α (left panel) and HMGB1 (right panel) secretion were determined by ELISA. Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. The levels of IL-1α and HMGB1 in cells treated with Poly(I:C), LPS and TNF-α were compared to unstimulated cells. *P < 0.05
Fig. 2.
Effect of infectious mimetics and pro-inflammatory stimulus on IL-1α and HMGB1 secretion by SW480 cells. Cells were stimulated with a Poly(I:C), b LPS and c TNF-α for 24 h. IL-1α (left panel) and HMGB1 (right panel) secretion were determined by ELISA. Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. The levels of IL-1α and HMGB1 in cells treated with Poly(I:C), LPS and TNF-α were compared to unstimulated cells. *P < 0.05
Given that DAMPs are also passively released upon cell death (Murao et al. 2021), the cell viability of both cell lines post stimulation were measured via MTT assay. All three stimulants did not compromise the cell viability of HT-29 and SW480 cells (Supplementary Fig. 1). These findings suggest that the secretion of these DAMPs was active, and differs between different CRC cell lines, which could be due to the mutation status or the Dukes’ staging of the cell.
Poly(I:C)-mediated IL-1α secretion is ER stress and RIPK1-dependent, while HMGB1 secretion is ER stress-independent
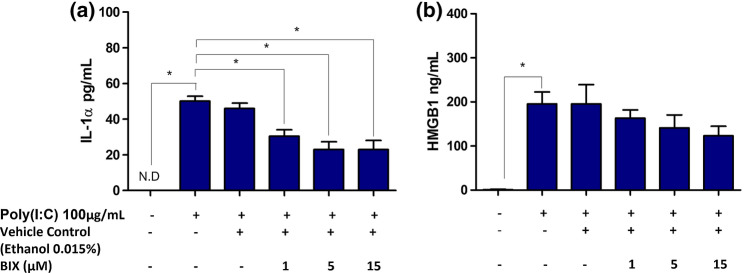
Having established that Poly(I:C) induced the secretion of both IL-1α and HMGB1 in HT-29 cells, the signalling pathway that regulates this secretory phenotype was further investigated. As described above, viral infection is an inducer of ER stress. Thus, to study the involvement of ER stress in mediating the secretion of IL-1α and HMGB1, HT-29 cells were pre-treated with BIX; a molecular chaperone that alleviates ER stress. BIX significantly reduced the levels of Poly(I:C)-induced IL-1α secretion, suggesting the involvement of ER stress (Fig. 3a). However, treatment with BIX did not significantly impair HMGB1 secretion in HT-29 cells even at the highest concentration (Fig. 3b).
Fig. 3.
Role of ER stress in mediating IL-1α and HMGB1 secretion in HT-29 cells. Cells were stimulated for 24 h with Poly(I:C) (100 μg/mL) in the presence or absence of molecular chaperone inducer; BIX. a IL-1α and b HMGB1 release were determined by ELISA. Bars represent mean data (± SEM). One-way ANOVA with Bonferroni’s correction was used to determine statistical differences. *P < 0.05
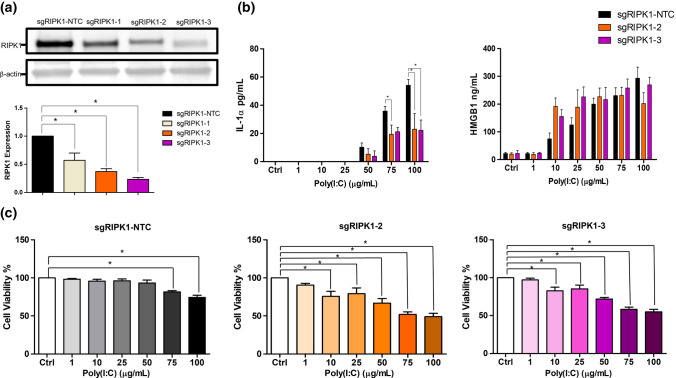
Previous studies have shown that ER stress activates RIPK1, which is an inflammatory-associated kinase in various cell lines (Estornes et al. 2014; Saveljeva et al. 2015). We showed that HT-29 but not SW480 were capable of secreting IL-1α and HMGB1 post Poly(I:C) stimulation (Figs. 1a and 2a). Of note, HT-29 expresses RIPK1, whereas SW480 does not, suggesting a possibility that RIPK1 played a role in regulating the secretion of these DAMPs (Moriwaki et al. 2015). To investigate whether RIPK1 was required in mediating the secretion of IL-1α and HMGB1 in HT-29 cells, we performed RIPK1 pool targeting in these cells using the CRISPR-Cas9 gene editing tool. Out of the three RIPK1-targeting sgRNAs designed, the sgRIPK1-2 and sgRIPK1-3 were efficient in targeting RIPK1. This was indicated by the significant reduction in the RIPK1 expression level in HT-29 cells transduced with these sgRIPK1 constructs (Fig. 4a).
Fig. 4.
Role of RIPK1 in mediating IL-1α and HMGB1 secretion in HT-29 cells. a The expression of RIPK1 in the pool targeted cells by western blot. Band densities were measured by densitometric analysis, and expression of RIPK1 were normalised to β-actin. Expression levels were expressed as fold change relative to RIPK1 non targeting control. b Cells were stimulated for 24 h with Poly(I:C) (100 μg/mL). IL-1α and HMGB1 concentrations were determined by ELISA. IL-1α and HMGB1 levels in sgRIPK1-2 and sgRIPK1-3 cells were compared to cells transduced with NTC. Two-way ANOVA with Bonferroni’s correction was used to determine statistical differences. c Cells transduced with sgRNAs were stimulated for 24 h with Poly(I:C). MTT absorbance was measured. Cell viability of stimulated cells were compared to unstimulated cells. Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. *P < 0.05
Next, the IL-1α and HMGB1 secretion level in response to Poly(I:C) were assessed in these RIPK1-targeted HT-29 cells pool. We found that IL-1α levels in RIPK1 targeted cell pools were significantly lower as compared to the control cells, but no significant changes were observed in HMGB1 secretion upon Poly(I:C) stimulation in these cells (Fig. 4b). However, the viability of RIPK1-targeted HT-29 cell pools was significantly compromised as Poly(I:C) concentration increased (Fig. 4c). Therefore, the reduction of IL-1α levels cannot be confirmed whether it was due to RIPK1 deficiency or reduction in cell number because of cell death. Since cell viability was compromised by approximately 50% in Poly(I:C) stimulated-RIPK1 depleted cells, this observation suggests that RIPK1 might have a role in promoting CRC cell survival in response to cellular stress.
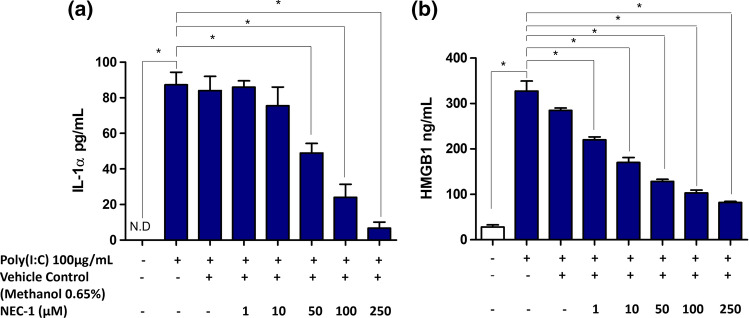
Alternatively, we used a small molecule inhibitor, NEC-1, which inhibits the kinase activity of RIPK1, to further determine the role of RIPK1 in mediating Poly(I:C)-induced secretion of IL-1α and HMGB1 in HT-29 cells. Surprisingly, a NEC-1 concentration-dependent inhibition of both IL-1α and HMGB1 secretion were observed, corroborating the role of RIPK1 in mediating the Poly(I:C)-induced secretion of these two DAMPs in HT-29 cells (Fig. 5).
Fig. 5.
Role of RIPK1 kinase activity in mediating IL-1α and HMGB1 secretion in HT-29 cells. Cells were stimulated for 24 h with Poly(I:C) (100 μg/mL) in the presence or absence of RIPK1 inhibitor; NEC-1. a IL-1α and b HMGB1 release were determined by ELISA. Bars represent mean data (± SEM). One-way ANOVA with Bonferroni’s correction was used to determine statistical differences. *P < 0.05
Subsequently, to identify the specific ER stress sensors involved in the signalling pathway and its relationship with RIPK1, we examined if Poly(I:C) regulates the total expression of ER stress-associated proteins; protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1 alpha (IRE1α) and activating transcription factor 4 (ATF4) along with RIPK1 via western blotting. Surprisingly, Poly(I:C) stimulation for 24 h did not change the total expression of PERK, IRE-1α, ATF4 nor RIPK1 (Supplementary Fig. 2).
Secretion of IL-1α and HMGB1 is independent of NLRP3 inflammasome/caspase-1
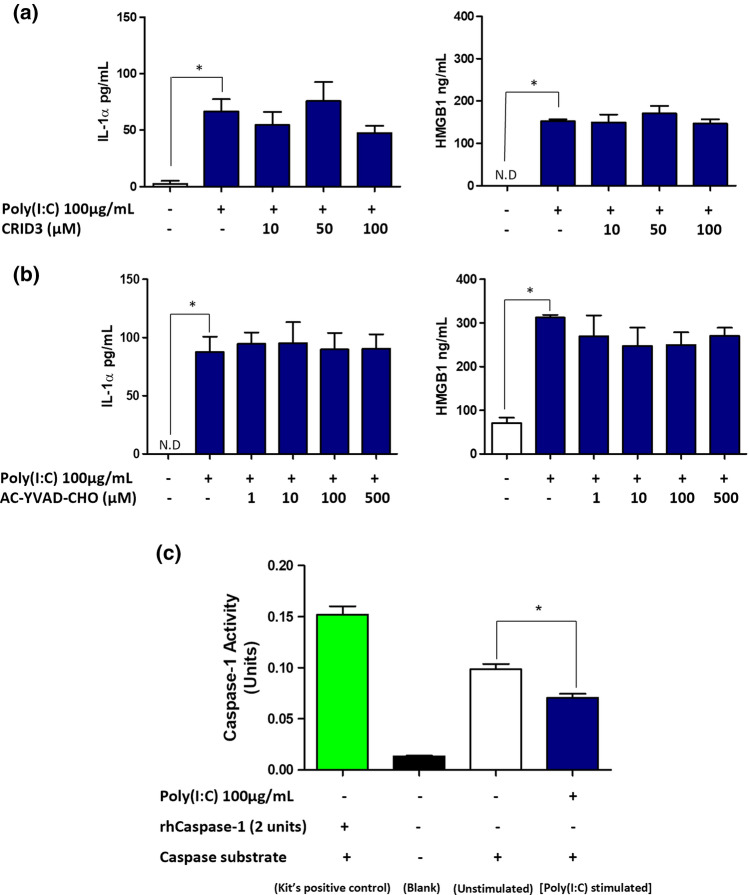
It has been previously reported that RNA viruses promoted the activation of the NLRP3 inflammasomes in macrophages via RIPK1-dependent signalling pathway (Wang et al. 2014). Thus, we next investigated whether NLRP3 inflammasome was involved in mediating Poly(I:C)-induced IL-1α and HMGB1 secretion in HT-29 cells. We observed that the inhibition of NLRP3 inflammasome by its specific inhibitor, CRID3, did not alter the secretion of IL-1α and HMGB1 in HT-29 cells (Fig. 6a).
Fig. 6.
Role of NLRP3 inflammasome and caspase-1 in mediating IL-1α and HMGB1 secretion in HT-29 cells. Cells were stimulated for 24 h with Poly(I:C) (100 μg/mL) in the presence or absence of a NLRP3 specific inhibitor; CRID3 and b caspase-1 inhibitor; AC-YVAD-CHO. IL-1α (left panel) and HMGB1 (right panel) concentration were determined by ELISA. c Adherent cells were lysed and the activity of caspase-1 in cell lysates were determined using caspase-1 activity kit. Bars represent mean data (± SEM). One-way ANOVA with Bonferroni’s correction was used to determine statistical differences. IL-1α and HMGB1 levels in cells treated with Poly(I:C) alone were compared to unstimulated cells, and cells treated with Poly(I:C) in the presence of CRID3 or AC-YVAD-CHO. *P < 0.05
In addition, the activation of NLRP3 inflammasomes is accompanied by caspase-1 activation (Fernandes-Alnemri et al. 2007). Since NLRP3 inflammasome signalling was not involved, we hypothesised that the secretion of IL-1α and HMGB1 would also be independent of caspase-1. To test this, we used AC-YVAD-CHO to inhibit the activation of caspase-1 and as hypothesised, the secretion levels of IL-1α and HMGB1 remained unchanged (Fig. 6b). We also measured caspase-1 activity in HT-29 cells post Poly(I:C) treatment. Rather than causing an increase in caspase-1 activity, Poly(I:C) significantly reduced the levels of the enzyme activity when compared to control (Fig. 6c). Taken together, these findings confirmed that the secretion of IL-1α and HMGB1 were independent of the NLRP3 inflammasome/caspase-1 signalling pathway.
IL-1α induces CRC cellular proliferation but not HMGB1
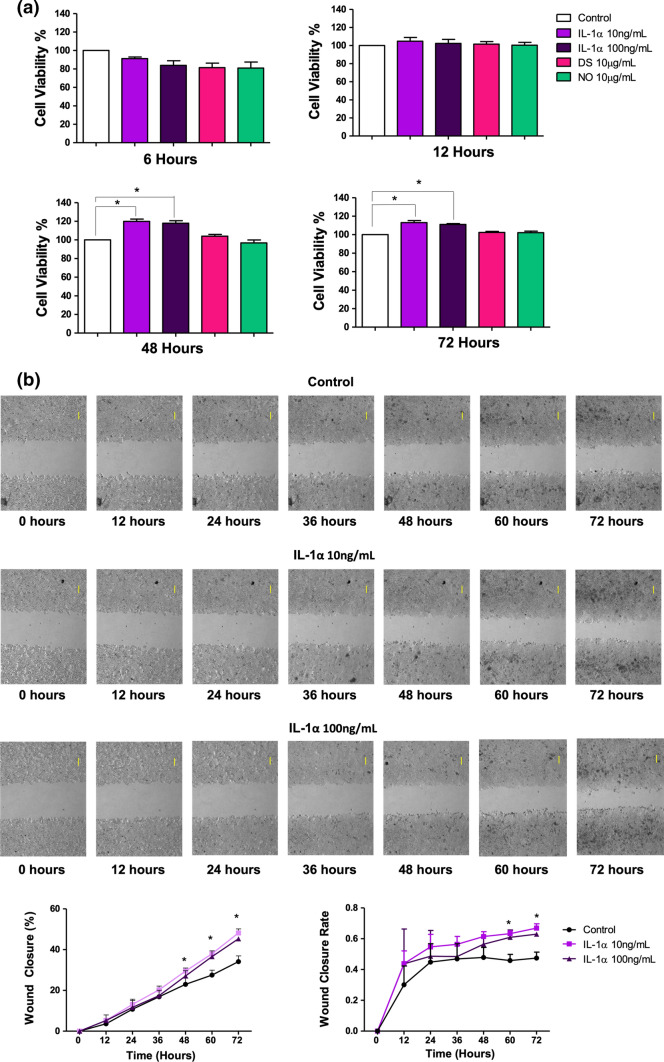
Having established the secretory mechanisms of IL-1α and HMGB1, we then investigated the roles of these DAMPs in promoting HT-29 cells’ growth. The HT-29 cells were treated with human recombinant IL-1α, disulfide (DS) or non-oxidizable (NO) HMGB1, followed by performing a series of in vitro functional assays. We first investigated the roles of these DAMPs in enhancing cellular proliferation. MTT data showed that stimulation with IL-1α for 48 and 72 h increased cellular proliferation by approximately 20%, whereas DS and NO HMGB1 did not induce any significant changes (Fig. 7a), pointing out the differential role between IL-1α and HMGB1.
Fig. 7.
Effect of IL-1α and the different redox states of HMGB1 on cellular proliferation of HT-29 cells. Cells were stimulated with IL-1α, DS and NO HMGB1. a The proliferation rate of HT-29 cells was measured via MTT at 6, 12, 48 and 72 h and b captured every hour via live imaging (top panel) and the gap closure percentage and rate were graphed (bottom panel). Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. Cell proliferation of stimulated cells were compared to unstimulated cells. Magnification 4 ×, scale bars, 100 µm (top right). *P < 0.05
Subsequently, we measured gap closure via live imaging and found that IL-1α induced the highest percentage of gap closure at the end of the 72-h timepoint (Fig. 7b). In contrast, the different redox states of HMGB1 had a similar gap closure percentage when compared to control (Supplementary Fig. 3). IL-1α significantly increased the gap closure rate at 60 and 72 h (Fig. 7b), whereas the different redox states of HMGB1 did not induce any significant changes (Supplementary Fig. 3). These results further strengthen the above findings from the MTT assay, that IL-1α enhanced the proliferation of HT-29 cells, while HMGB1 did not.
IL-1α and HMGB1 does not have any effect on E-cadherin in HT-29 cells
Studies have shown that DAMPs could directly induce epithelial barrier impairment in airway epithelial cell (Huang et al. 2016). In cancer, epithelial barrier impairment could result in the loss of cell integrity and reduced cohesion of structure, leading to invasion and metastasis of cancer cells. Because of this, we studied whether IL-1α and HMGB1 have any direct impact on epithelial barrier function in CRC cells by measuring the expression of E-cadherin, a key protein maintaining the integrity of cell–cell junction. Stimulation of IL-1α and HMGB1 did not have any effect on the total expression of E-cadherin (Supplementary Fig. 4a–c). We next prolonged the timepoint as 24 h might not be the ideal time-point to observe these changes. However, even at 48 and 72 h, no significant changes were observed (data not shown). This suggests that both IL-1α and HMGB1 did not have any direct impact on the expression of E-cadherin.
Previous studies have shown that HMGB1 could induce epithelial barrier impairment through the redistribution or internalization of E-cadherin on the cell membrane, although the total expression of the protein might not change (Huang et al. 2016). Hence, we employed an immunofluorescence technique to study the effects of IL-1α and HMGB1 on this. Results from the staining revealed that E-cadherin was localized on the plasma membrane of unstimulated HT-29 cells at both 48 and 72 h. This was retained in IL-1α and HMGB1-treated cells (Supplementary Fig. 4d and e). Since no diffused staining was observed, this suggests that the epithelial membrane was not disrupted.
Collectively, these findings indicated that IL-1α and HMGB1 may not induce epithelial barrier dysfunction as (i) the total expression of E-cadherin was unchanged and (ii) no redistribution/internalization of E-cadherin was observed.
IL-1α increases spheroid size, epithelial cell adhesion protein (EpCAM) and E-cadherin in HT-29 spheroids
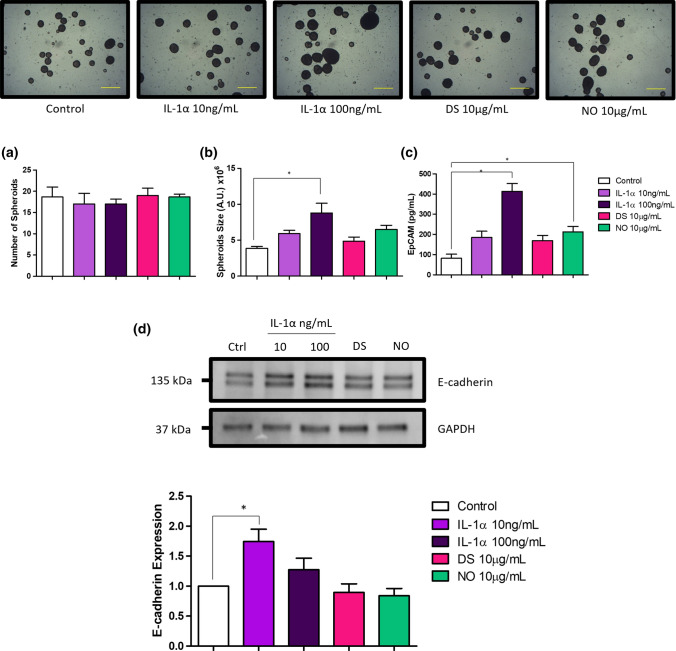
The ability to survive and grow in a non-adhesive environment is a hallmark of the neoplastic phenotype. We observed that HT-29 cells could survive and grow in suspensions by forming spheroids. Upon stimulation with IL-1α and the different redox states of HMGB1, no changes were observed in the number of spheroids formed (Fig. 8a). However, when the size of the spheroids was measured, stimulation with IL-1α at 100 ng/mL significantly increased mean spheroid size when compared to control (Fig. 8b), whereas no significant changes were observed in HMGB1 stimulated cells.
Fig. 8.
Effect of IL-1α and the different redox states of HMGB1 on spherogenic potential and expression of epithelial cell adhesion molecule (EpCAM) and E-cadherin in HT-29 spheroids. Suspension cells were stimulated for 10 days with IL-1α, DS and NO HMGB1. The a number and b volume of HT-29 spheroids were captured at the end of the stimulation period and graphed. Representative photomicrographs of 8 field views are shown (top panel). c HT-29 spheroids were then collected and lysed. Proteins were then standardized to 5 μg/mL and measured for the expression of EpCAM. d Expression of E-cadherin was determined by western blot. Band densities were measured by densitometric analysis, and data for the expression of E-cadherin were normalized to GAPDH. Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. Mean data and expression levels of stimulated spheroids were expressed as fold change relative to unstimulated spheroids. Magnification 4 ×, scale bars, 500 µm (bottom right)
Studies have shown that epithelial cell adhesion molecule (EpCAM) and E-cadherin are significantly increased in spheroids as compared to adherent CRC cells (Lin et al. 2012; Powan et al. 2017), and its expression has been positively correlated with better spheroid formation ability (Hu et al. 2021). Thus, to investigate the association between EpCAM and E-cadherin with spheroid size, the total EpCAM and E-cadherin expression in HT-29 spheroids in response to IL-1α and HMGB1 stimulation were determined via ELISA and western blotting, respectively. IL-1α increased the expression of EpCAM in a concentration dependent manner (Fig. 8c), while IL-1α at 10 ng/mL increased the expression levels of E-cadherin (Fig. 8d). These findings were in agreement with our postulation, where increased spheroid size was correlated with EpCAM and E-cadherin expression. Though, this could not be fully verified, as EpCAM expression in NO HMGB1 stimulated spheroids were also significantly increased despite no significant difference in size were observed in NO HMGB1. Although 100 ng/mL of IL-1α induced the largest change in mean spheroid size, increase of E-cadherin expression in these spheroids did not reach statistical significance.
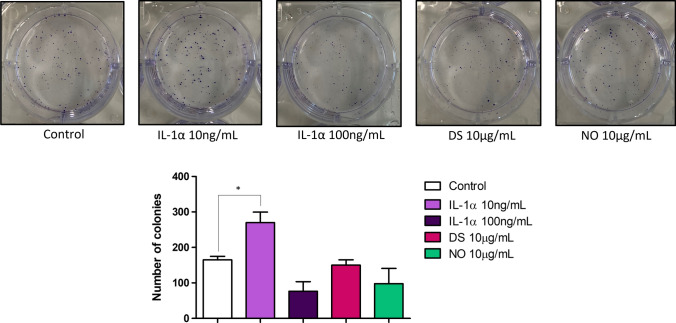
IL-1α induces clonogenic potential of HT-29 cells but not HMGB1
Next, we investigated the HT-29 cells tumour-initiating capability upon the stimulation with IL-1α and HMGB1 by performing clonogenic assay. We found that stimulation of HT-29 cells with 10 ng/mL IL-1α significantly increased the colony number when compared to control (Fig. 9). However, DS and NO HMGB1 did not have any significant impact on this (Fig. 9), once again highlighting the differential effects of these two DAMPs. These findings also reflect our observations above (Fig. 7), whereby IL-1α but not the different redox states of HMGB1 have any growth-promoting capabilities on HT-29 cells.
Fig. 9.
Effect of IL-1α and the different redox states of HMGB1 on clonogenic potential of HT-29 cells. Cells were stimulated for 10 days with IL-1α, DS and NO HMGB1. The number of colonies of HT-29 cells was captured at the end of the stimulation period (top panel) and bars represent mean values (± SEM) (bottom panel). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. Cell colonies of stimulated cells were compared to unstimulated cells. *P < 0.05
IL-1α and HMGB1 distinctly modulates the production of pro-metastatic mediators and chemokines
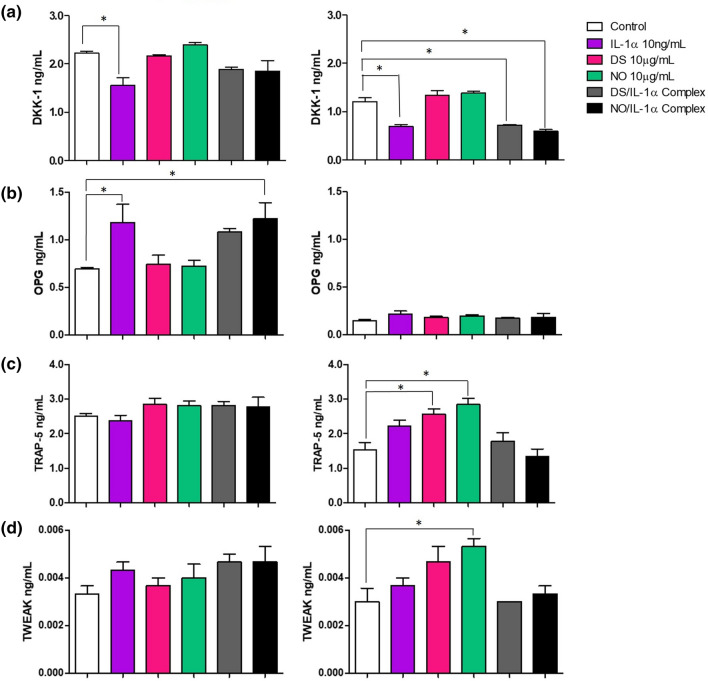
Tumour secreted factors such as pro-metastatic mediators, cytokines and chemokines play critical roles in promoting cancer metastasis (Liu et al. 2017). Hence, in this present study, we were interested to investigate whether IL-1α, HMGB1 and their ligand complexes; which has been shown to exacerbate inflammation (Wähämaa et al. 2011), regulated the production of pro-metastatic mediators which are associated with CRC progression, in the lysates and supernatant of HT-29 stimulated cells. By using the human cancer metastatic multiplex assay, we found that IL-1α significantly downregulated the intracellular expression and secretion levels of Dickkopf-related protein 1 (DKK-1) (Fig. 10a), while increasing the secretion levels of osteoprotegerin (OPG) (Fig. 10b). Both DS and NO HMGB1 increased the intracellular expression of TRAP-5 (Fig. 10c). Interestingly, it was observed that only NO HMGB1 increased the intracellular expression levels of TWEAK (Fig. 10d). Surprisingly, no significant changes were observed when IL-1α and HMGB1 were in complexes, and the modulatory effects were similar to stimulation with either IL-1α or HMGB1 alone (Fig. 10), suggesting that they may not synergistically act in modulating the expression and production of these mediators. Note, no changes were observed in the secretion and expression levels of YLK 40, periostin, GDF-15, and osteonectin, while the levels of osteonectin were undetectable in lysate samples (Supplementary Fig. 5).
Fig. 10.
Effect of IL-1α, the different states of HMGB1 and their ligand complexes on the secretion of pro-metastatic mediators in HT-29 cells. Cells were stimulated for 24 h with IL-1α, DS, NO HMGB1 and their ligand complexes. The expression of pro-metastatic mediators a DKK-1, b OPG, c TRAP-5 and d TWEAK, were measured via ELISA multiplex cancer metastasis panel in cell supernatant (left panel) and lysates (right panel). Bars represent mean data (± SEM). One-way ANOVA with Dunnett’s correction was used to determine statistical differences. The level of pro-metastatic mediators in cells treated with IL-1α, DS, NO HMGB1 their ligand complexes were compared to unstimulated cells. *P < 0.05
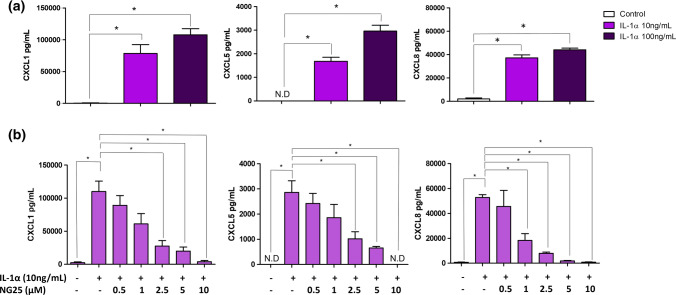
We then investigated if IL-1α and HMGB1 regulated the secretion of several pro-tumorigenic chemokines, specifically CXCL1 (le Rolle et al. 2015), CXCL5 (Zhao et al. 2017) and CXCL8 (Ning et al. 2011). Intriguingly, we observed that IL-1α stimulation significantly upregulated the secretion of these chemokines in HT-29 cells in a concentration-dependent manner (Fig. 11a). The different redox states of HMGB1 also significantly upregulated the secretion of CXCL8. Interestingly, when comparing between the different redox states of HMGB1, NO HMGB1 induced the secretion of CXCL8 by 1.5-fold higher than DS HMGB1, indicating that the different redox states of HMGB1 influences chemokine production (Supplementary Fig. 6). Collectively, these data also emphasizes that IL-1α and HMGB1 regulate different pro-metastatic mediators and chemokines, with the different redox states of HMGB1 distinctly influencing their modulatory effect.
Fig. 11.
Modulatory effects of IL-1α and the different redox states of HMGB1 on chemokine release in HT-29 cells. HT-29 cells were stimulated for 24 h with a IL-1α and b in the presence and absence of TAK1 inhibitor, NG25. CXCL1, CXCL5 and CXCL8 concentration were determined by ELISAs. One-way ANOVA with Bonferroni’s correction was used to determine statistical differences. CXCL1, CXCL5 and CXCL8 levels in cells stimulated with IL-1α alone were compared to unstimulated cells, and cells treated with IL-1α in the presence of NG25. Bars represent mean data (± SEM). *P < 0.05
IL-1α-mediated secretion of CXCL1, CXCL5 and CXCL8 is TAK1-dependent
A recent study has shown that inhibition of a central inflammatory signalosome known as transforming growth factor-β-activated kinase 1 (TAK1), ameliorates chemokine production in mouse astrocyte cultures (Soto-Díaz et al. 2020). Interestingly, TAK-1 is also a kinase that is involved in IL-1 receptor signalling (Jurida et al. 2015). Thus, we hypothesized that TAK1 might play a role in mediating IL-1α-induced chemokine secretion in HT-29 cells. We inhibited TAK1 activity using a small molecule inhibitor; NG25, and this significantly reduced the secretion levels of CXCL1, CXCL5 and CXCL8 in a concentration-dependent manner (Fig. 11b), demonstrating the role of TAK1 in IL-1α-mediated secretion of these chemokines.
Discussion
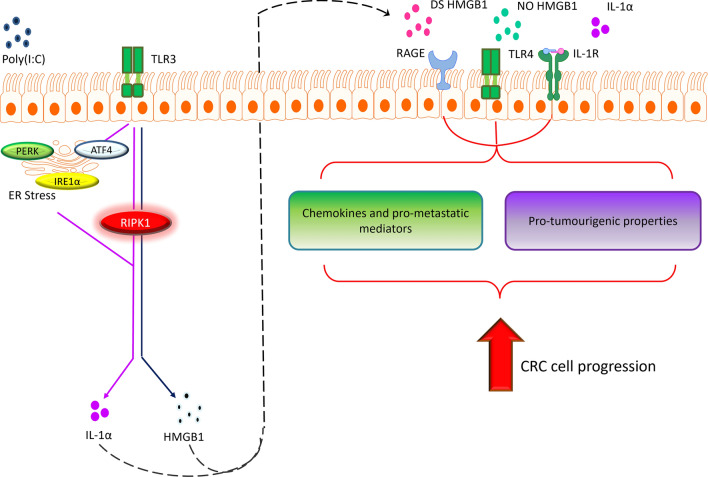
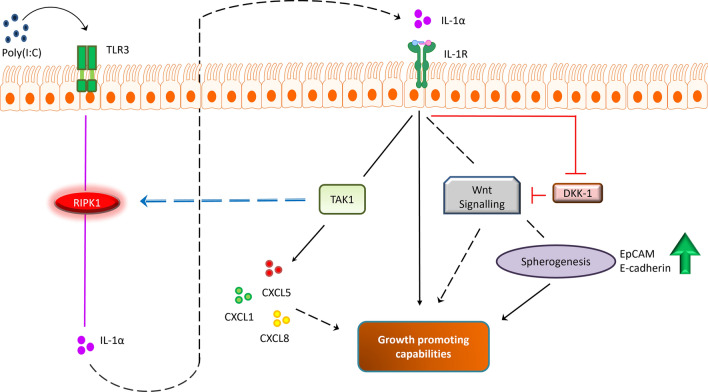
Here, we demonstrated a potential signalling pathway that regulates the secretion of IL-1α and HMGB1 in CRC cells in response to viral mimetic, Poly(I:C) (Fig. 12). Our findings suggests that Poly(I:C) induced secretion of IL-1α was ER stress and RIPK1-dependent, whereas HMGB1 was only dependent on RIPK1. We also performed CRISPR-Cas9 pooled targeting of RIPK1 in HT-29 cells. Reduced secretion levels of IL-1α were observed, but cell viability was also compromised, suggesting that RIPK1 plays a role in CRC cell survival upon cellular stress. Exploration into the roles of IL-1α and HMGB1 showed that these DAMPs have differential effects in regulating tumorigenesis in CRC cells. IL-1α directly promotes cellular proliferation, spherogenesis and clonogenesis as well as inducing the expression of various pro-metastatic mediators and chemokines. Notably, the different redox states of HMGB1 did not directly enhance cellular proliferation, spherogenesis and clonogenesis but induced the production of several pro-metastatic mediators with the redox status of HMGB1 having influence.
Fig. 12.
Potential signalling pathway of IL-1α and HMGB1 in promoting CRC cell progression. Poly(I:C) induces ER stress and RIPK1 activation, promoting the secretion of IL-1α while HMGB1 was independent of ER stress. Upon secretion, these DAMPs contribute to CRC progression by upregulating the expression/secretion of pro-metastatic mediators/chemokines and enhances pro-tumourigenic properties of CRC cells
In this study, we revealed that RIPK1 might have dual role in CRC cells; (i) mediating the secretion of IL-1α and HMGB1 and (ii) regulating cell survival. Using pharmacological inhibitor NEC-1, we showed that RIPK1 inhibition significantly reduced the levels of both IL-1α and HMGB1, highlighting the role of this kinase in modulating the secretion of these DAMPs. Although this could not be verified with the CRISPR-Cas9 gene editing tool, we do believe that RIPK1 plays a role, as NEC-1 significantly decreases intestinal inflammation (Günther et al. 2019) and reduces colitis-associated tumorigenesis in mice, which have been associated with IL-1α and HMGB1 production (Fan et al. 2018; Liu et al. 2015; Malik et al. 2016). Additionally, similar findings have also been observed in other cell types where Lukens et al., have showed that RIPK1 induces IL-1α production in haematopoietic cells and critically mediates chronic inflammation (Lukens et al. 2013). The inhibition of RIPK1 with NEC-1 markedly decreased the secretion of inflammatory mediators and inhibited the synthesis of IL1A transcripts (Lukens et al. 2013). Simpsons and colleagues have also shown that viral infection elevated the levels of HMGB1 in airway epithelial cells via RIPK1, and pharmacological inhibition with NEC-1 attenuated viral-induced HMGB1 translocation and release (Simpson et al. 2020). Taken together, these studies support our findings, that the secretion of IL-1α and HMGB1 are RIPK1-dependent. However, if this were to be verified via genetic alteration methods to exclude the chance of any off-target inhibition using NEC-1, future studies could opt to knockout endogenous RIPK1 and transduce the cells with a kinase-dead version of RIPK1. This had been shown possible by research, whereby genetic inactivation of RIPK1 activity using RIPK1 kinase-dead knock-in mice models resulted in reduced levels of HMGB1 release (Simpson et al. 2020).
We observed reduced viability in RIPK1 depleted cell pools post Poly(I:C) stimulation but not in the parental cells, suggesting that RIPK1 intracellular expression is crucial to maintain cell survival under stressful events. In line with our findings, previous studies have found that RIPK1 knockout potentiated cell death in macrophages and fibroblasts when stimulated with Poly(I:C) (Buchrieser et al. 2018; Dillon et al. 2014; Kearney et al. 2014). Intriguingly, Kearney et al., have previously shown that genetic manipulation of RIPK1 potentiates Poly(I:C)-induced necroptosis; a form of inflammatory cell death (Kearney et al. 2014). They clarified that (i) this kinase is critically required for cellular signalling interactions and activation of the NF-κB cell survival pathway (Kearney et al. 2014) and (ii) pharmacological inhibition with NEC-1 only hinders the kinase activity of RIPK1, whereas RIPK1 genetic alterations abolishes the expression of the protein. Several in vivo studies supported this notion and showed that RIPK1 deficient mice are not viable (Newton 2015; Suda et al. 2016), whereas RIPK1 kinase-dead (RIPK1K45A) mice which were viable and healthy (Berger et al. 2014). This confirms that the presence of RIPK1 protein itself is required to maintain cell survival, whereas its kinase activity is dispensable and gives insights to the decreased cell viability observed in our RIPK1 depleted cell pools stimulated with Poly(I:C).
The role of ER stress in HT-29 cells was demonstrated for IL-1α but not on HMGB1 secretion. Although a trend of reduction was observed in Poly(I:C)-mediated HMGB1 secretion in response to BIX, this did not reach statistical significance. These were in contrast with other studies, as HMGB1 release have been previously shown to be ER stress-dependent in human placental and breast cancer cells (Collett et al. 2018; Park et al. 2016). Poly(I:C) is an ER stress/UPR inducer (Ishaq and Natarajan 2016; Lenna et al. 2013) and other well-known ER stress activators, tunicamycin and tharpsigargin also promoted the release of HMGB1 (Park et al. 2016). Thus, there is a high possibility that the secretion of HMGB1 is ER-stress dependent. In this study, a BiP inducer (BIX) was used to alleviate ER stress. BiP is an ER chaperone that binds and represses the UPR sensors under non-stressed conditions but dissociates from these UPR sensors upon ER stress to assist in protein folding, allowing for UPR activation and its downstream signalling cascade. Supplementing cells undergoing ER stress with BIX reverses this effect, as it induces BiP mRNA and subsequently protein expression (Ha et al. 2019; Kudo et al. 2008). However, previous studies have shown that BiP is only capable of limiting the activation of UPR sensors and is not a principal determinant, but an adjustor for sensitivity to various stresses (Kimata et al. 2004). Thus, it is plausible that ER stress is still persistent in Poly(I:C) stimulated cells, despite the presence of BIX. Hence, future approaches using alternative ER stress/UPR inhibitors such as Sunitinib, Salubrinal and AZD 5582, could be used to validate if HMGB1 secretion is ER stress dependent.
We demonstrated that IL-1α and HMGB1 participates in tumourigenesis. From our findings, both DAMPs elicited differential impacts, with IL-1α exhibiting more versatile roles as compared to HMGB1. IL-1α had growth promoting capabilities; stimulating HT-29 cell proliferation, clonogenic, spherogenic potential, and induces the secretion of various pro-metastatic mediators, while HMGB1 only acquires the latter ability. Interestingly, there appears to be a link between the growth promoting potential of IL-1α and its regulation of pro-metastatic mediators. IL-1α decreases the production of DKK-1; a negative regulator of the Wnt signalling pathway, allowing for enhanced Wnt activation which crucially supports CRC cell proliferation, as well as the growth and maintenance of colonospheres (Kanwar et al. 2010; Nie et al. 2019). IL-1α also promotes TAK1-dependent secretion of pro-tumourigenic chemokines CXCL1, CXCL5 and CXCL8 which further supports CRC cell growth (Łukaszewicz-Zając et al. 2020). Interestingly, recent studies have suggested that TAK1 phosphorylation also regulates RIPK1 activation (Geng et al. 2017), which could result in a feedback loop, further upregulating the secretion of IL-1α, exacerbating CRC cell progression (Fig. 13). Additionally, IL-1α also increases OPG expression in CRC cells; a decoy receptor for TRAIL (cytokine released by immune cells to target cancer cells), which decreases immunosurveillance and prevents apoptosis of cancer cells (Smyth et al. 2003). In contrast, the different redox states of HMGB1 were incapable of directly promoting cellular proliferation, as HMGB1 did not induce similar mediators as IL-1α, apart from CXCL8. Alternatively, HMGB1 significantly increased the expression levels of TRAP-5 and TWEAK, which are mediators that are associated with cancer invasion and angiogenesis respectively (Liu et al. 2021; Reithmeier et al. 2017). Given that our experiments were performed in single cell culture models, the effects of HMGB1 could potentially be more prominent in complex in vitro co-culture models or in vivo, taking invasion and angiogenesis into account.
Fig. 13.
Growth promoting capability of IL-1α and hypothesis pathway. IL-1α interacts with Wnt to promote CRC cell proliferation and spherogenesis. IL-1α also decreases the production of DKK-1, a negative regulator of Wnt, exacerbating Wnt activation. IL-1α-mediated TAK1 activation induces the secretion of pro-tumourigenic chemokines, and this kinase have also been proposed to regulate RIPK1 activity, resulting in a feedback loop which collectively enhances the capability of IL-1α in promoting CRC cells’ growth
When further comparisons were made between the effects mediated by the different redox states of HMGB1, it was observed that NO and DS HMGB1, both generally have similar roles, inducing the production of pro-metastatic mediators. Surprisingly, NO HMGB1 which was previously thought to have chemotactic activity but not cytokine/chemokine inducing activity (Venereau et al. 2012), stimulated CXCL8 chemokine secretion in CRC cells. This contrasts with the previously reported findings, despite using the same formulation of DS and NO HMGB1 at similar concentrations (10 μg/mL) (Venereau et al. 2012; Yang et al. 2021). Venereau et al. have shown that NO HMGB1 was unable to induce cytokine/chemokine production including IL-6 and IL-8 in macrophages and was exclusively a chemoattractant in fibroblast cells (Venereau et al. 2012). Similar findings were observed by Yang et al., (Yang et al. 2021) where they have shown that only DS but not NO HMGB1 had cytokine inducing potential in macrophages. Interestingly, Di Maggio et al., have showed that none of the redox forms of HMGB1 induced proliferation and inflammatory mediators in human cardiac fibroblasts (Di Maggio et al. 2017). This suggests that the different redox states of HMGB1 could be cell-dependent or altered in different disease conditions or pathological settings. In our study, both NO and DS HMGB1 demonstrated CXCL8 chemokine and TRAP5-inducing ability. NO HMGB1 was also an independent inducer of TWEAK and increased the expression of EpCAM in spheroids, while DS HMGB1 did not. Though, it is important to note the limitations for our study, whereby unlike NO HMGB1, the DS HMGB1 used was not protected from oxidation, thus it is possible that this may have resulted in DS HMGB1 activity being altered.
It is important to acknowledge that unlike Poly(I:C), which mimics viral infections, induces the secretion of both IL-1α and HMGB1, other stimulus used in this study such as LPS only triggered the release of HMGB1, while TNF-α did not induce the secretion of both DAMPs. This suggests that the type of stimulus (i.e., viral/bacteria) may lead to the discrimination or synergistic effects of DAMPs in contributing towards the pathological processes of CRC. As LPS only induces the production of HMGB1 but not IL-1α, the role of HMGB1 could potentially be more prominent if the onset of CRC is associated with bacterial infection. Whether the pathological processes leading towards a HMGB1-centric pathway due to the absence of IL-1α, remains to be investigated. Since different inducers trigger the release of DAMPs through distinct signalling pathways, this could contribute towards divergent mechanism(s) in promoting CRC cell progression. Whether the different distinct pathways eventually converge at one target, which could be a rendezvous point is still unknown. Thus far, we have also showed in this study that IL-1α and HMGB1 when in complexes did not act in a synergistic manner in regulating the production of pro-metastatic mediators, however, these findings were limited to the analytes available in the multiplex assay. Therefore, further investigation into these proposed aspects is warranted to obtain a deeper understanding of how these DAMPs may interact and work together in promoting CRC tumourigenesis.
In conclusion, we described a molecular signalling pathway by which IL-1α and HMGB1 are secreted in CRC cells, and their functional roles in enhancing tumourigenesis. The proposed signalling pathway could serve as an attractive therapeutic target to combat CRC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Universiti Malaya Faculty Research Grant (Grant No: GPF006C-2019) and the Malaysia Toray Science Foundation, Science and Technology Research Grant (STRG0069).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kim Jun Cheng and Zaridatul Aini Ibrahim contributed equally to this work.
References
- Antonic V, Stojadinovic A, Kester KE, Weina PJ, Brücher BL, Protic M, Avital I, Izadjoo M. Significance of infectious agents in colorectal cancer development. J Cancer. 2013;4:227–240. doi: 10.7150/jca.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Cavener D, Diehl JA. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID, de Wilt JHW, Verhoeven RHA. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758–2766. doi: 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchrieser J, Oliva-Martin MJ, Moore MD, Long JCD, Cowley SA, Perez-Simón JA, James W, Venero JL. RIPK1 is a critical modulator of both tonic and TLR-responsive inflammatory and cell death pathways in human macrophage differentiation. Cell Death Dis. 2018;9:973. doi: 10.1038/s41419-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KJ, Alshawsh MA, Mejia Mohamed EH, Thavagnanam S, Sinniah A, Ibrahim ZA. HMGB1: an overview of its versatile roles in the pathogenesis of colorectal cancer. Cell Oncol (dordr) 2020;43:177–193. doi: 10.1007/s13402-019-00477-5. [DOI] [PubMed] [Google Scholar]
- Cheng KJ, Mejia Mohammed EH, Khong TL, Mohd Zain S, Thavagnanam S, Ibrahim ZA. IL-1α and colorectal cancer pathogenesis: enthralling candidate for anti-cancer therapy. Crit Rev Oncol Hematol. 2021;163:103398. doi: 10.1016/j.critrevonc.2021.103398. [DOI] [PubMed] [Google Scholar]
- Chiu JW, Binte Hanafi Z, Chew LCY, Mei Y, Liu H. IL-1α processing, signaling and its role in cancer progression. Cells. 2021;10:92. doi: 10.3390/cells10010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-A, Song C-H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front Immunol. 2020 doi: 10.3389/fimmu.2019.03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett GP, Redman CW, Sargent IL, Vatish M. Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget. 2018;9:6707–6717. doi: 10.18632/oncotarget.24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic reticulum stress unfolded protein response, and cancer cell fate. Front Oncol. 2017 doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, Shayakhmetov DM. Interleukin 1α and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maggio S, Milano G, De Marchis F, D’Ambrosio A, Bertolotti M, Palacios BS, Badi I, Sommariva E, Pompilio G, Capogrossi MC, Raucci A. Non-oxidizable HMGB1 induces cardiac fibroblasts migration via CXCR4 in a CXCL12-independent manner and worsens tissue remodeling after myocardial infarction. Biochim Biophys Acta (BBA) Mol Basis Dis. 2017;1863:2693–2704. doi: 10.1016/j.bbadis.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong Y-N, Janke LJ, Kelliher MA, Kanneganti T-D, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estornes Y, Aguileta MA, Dubuisson C, De Keyser J, Goossens V, Kersse K, Samali A, Vandenabeele P, Bertrand MJM. RIPK1 promotes death receptor-independent caspase-8-mediated apoptosis under unresolved ER stress conditions. Cell Death Dis. 2014;5:e1555–e1555. doi: 10.1038/cddis.2014.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Jiang C, Zhong B, Sheng J, Chen T, Chen Q, Li J, Zhao H. Matrine ameliorates colorectal cancer in rats via inhibition of HMGB1 signaling and downregulation of IL-6, TNF-α, and HMGB1. J Immunol Res. 2018;2018:5408324. doi: 10.1155/2018/5408324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfo V, Mazzeschi M, Grilli G, Lindzen M, Santi S, D'Uva G, Győrffy B, Ardizzoni A, Yarden Y, Lauriola M. A novel role for the interleukin-1 receptor axis in resistance to anti-EGFR therapy. Cancers (basel) 2018;10:355. doi: 10.3390/cancers10100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, Mookhtiar AK, Zhao H, Xu D, Shan B, Najafov A, Gao G, Akira S, Yuan J. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8:359. doi: 10.1038/s41467-017-00406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther C, Ruder B, Stolzer I, Dorner H, He GW, Chiriac MT, Aden K, Strigli A, Bittel M, Zeissig S, Rosenstiel P, Atreya R, Neurath MF, Wirtz S, Becker C. Interferon lambda promotes paneth cell death via STAT1 signaling in mice and is increased in inflamed ileal tissues of patients with Crohn's disease. Gastroenterology. 2019;157:1310–1322.e13. doi: 10.1053/j.gastro.2019.07.031. [DOI] [PubMed] [Google Scholar]
- Ha TK, Hansen AH, Kildegaard HF, Lee GM. BiP inducer X: An ER stress inhibitor for enhancing recombinant antibody production in CHO cell culture. Biotechnol J. 2019;14:e1900130. doi: 10.1002/biot.201900130. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hillenbrand A, Fassler J, Huber N, Xu P, Henne-Bruns D, Templin M, Schrezenmeier H, Wolf AM, Knippschild U. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer. 2012;12:545. doi: 10.1186/1471-2407-12-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- Hreggvidsdóttir HS, Lundberg AM, Aveberger A-C, Klevenvall L, Andersson U, Harris HE. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med. 2012;18:224–230. doi: 10.2119/molmed.2011.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-L, Zhang Y-J, Zhang X-F, Fei X, Zhang H, Li C-G, Sun B. 3D culture of circulating tumor cells for evaluating early recurrence and metastasis in patients with hepatocellular carcinoma. Onco Targets Ther. 2021;14:2673–2688. doi: 10.2147/OTT.S298427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhao H, Dong H, Wu Y, Yao L, Zou F, Cai S. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int J Mol Med. 2016;37:1189–1198. doi: 10.3892/ijmm.2016.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang W, Zeng H, Hu C, Zhang Y, Ding N, Fan G, Shao L, Kuang B. Droxinostat sensitizes human colon cancer cells to apoptotic cell death via induction of oxidative stress. Cell Mol Biol Lett. 2018;23:34–34. doi: 10.1186/s11658-018-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq M, Natarajan V. Integrated stress response signaling pathways induced by supraphysiological concentrations of thyroid hormone inhibit viral replication. Signal Transduct Insights. 2016 doi: 10.4137/STI.S39844. [DOI] [Google Scholar]
- Jurida L, Soelch J, Bartkuhn M, Handschick K, Müller H, Newel D, Weber A, Dittrich-Breiholz O, Schneider H, Bhuju S, Saul Vera V, Schmitz ML, Kracht M. The activation of IL-1-induced enhancers depends on TAK1 kinase activity and NF-κB p65. Cell Rep. 2015;10:726–739. doi: 10.1016/j.celrep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Kandel-Kfir M, Almog T, Shaish A, Shlomai G, Anafi L, Avivi C, Barshack I, Grosskopf I, Harats D, Kamari Y. Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice. J Hepatol. 2015;63:926–933. doi: 10.1016/j.jhep.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar APN. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212–212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney CJ, Cullen SP, Clancy D, Martin SJ. RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J. 2014;281:4921–4934. doi: 10.1111/febs.13034. [DOI] [PubMed] [Google Scholar]
- Kimata Y, Oikawa D, Shimizu Y, Ishiwata-Kimata Y, Kohno K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J Cell Biol. 2004;167:445–456. doi: 10.1083/jcb.200405153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- le Rolle A-F, Chiu TK, Fara M, Shia J, Zeng Z, Weiser MR, Paty PB, Chiu VK. The prognostic significance of CXCL1 hypersecretion by human colorectal cancer epithelia and myofibroblasts. J Transl Med. 2015;13:199. doi: 10.1186/s12967-015-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Song M, Shin N, Shin CH, Min BS, Kim HS, Yoo JS, Kim H. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS ONE. 2012;7:e34318. doi: 10.1371/journal.pone.0034318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenna S, Chrobak I, Farina GA, Rodriguez-Pascual F, Lamas S, Lafyatis R, Scorza R, Trojanowska M. HLA-B35 and dsRNA induce endothelin-1 via activation of ATF4 in human microvascular endothelial cells. PLoS ONE. 2013;8:e56123. doi: 10.1371/journal.pone.0056123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lin CW, Liao MY, Lin WW, Wang YP, Lu TY, Wu HC. Epithelial cell adhesion molecule regulates tumor initiation and tumorigenesis via activating reprogramming factors and epithelial-mesenchymal transition gene expression in colon cancer. J Biol Chem. 2012;287:39449–39459. doi: 10.1074/jbc.M112.386235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu BQ, Li JH, Jing L, Jiang JL, Tang J, Chen ZN. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5:3174–3185. [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D. Factors involved in cancer metastasis: a better understanding to "seed and soil" hypothesis. Mol Cancer. 2017;16:176–176. doi: 10.1186/s12943-017-0742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhang D, Luo M, Jia F, Peng L, Li X, Xia Y. TNF-like weak inducer of apoptosis promotes angiogenesis, thereby exacerbating cutaneous psoriatic disease. J Investig Dermatol. 2021;141:1356–1360.e8. doi: 10.1016/j.jid.2020.09.023. [DOI] [PubMed] [Google Scholar]
- Łukaszewicz-Zając M, Pączek S, Mroczko P, Kulczyńska-Przybik A. The significance of CXCL1 and CXCL8 as well as their specific receptors in colorectal cancer. Cancer Manag Res. 2020;12:8435–8443. doi: 10.2147/CMAR.S267176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, Kanneganti T-D. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti T-D. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469–4481. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Sawai H, Ma J, Xu D, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H. IL-1alpha secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1alpha release and tumor cells' potential for liver metastasis. J Surg Oncol. 2009;99:361–367. doi: 10.1002/jso.21245. [DOI] [PubMed] [Google Scholar]
- Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636–e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao A, Aziz M, Wang H, Brenner M, Wang P. Release mechanisms of major DAMPs. Apoptosis. 2021;26:152–162. doi: 10.1007/s10495-021-01663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347–353. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Nie X, Xia F, Liu Y, Zhou Y, Ye W, Hean P, Meng J, Liu H, Liu L, Wen J, Ren X, Chen W-D, Wang Y-D. Downregulation of Wnt3 suppresses colorectal cancer development through inhibiting cell proliferation and migration. Front Pharmacol. 2019 doi: 10.3389/fphar.2019.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, Ladner RD, Lenz H-J. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IA, Heo S-H, Song IH, Kim Y-A, Park HS, Bang WS, Park SY, Jo J-H, Lee HJ, Gong G. Endoplasmic reticulum stress induces secretion of high-mobility group proteins and is associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Oncotarget. 2016;7:59957–59964. doi: 10.18632/oncotarget.11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powan P, Luanpitpong S, He X, Rojanasakul Y, Chanvorachote P. Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. Am J Physiol Cell Physiol. 2017;313:C556–C566. doi: 10.1152/ajpcell.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier A, Panizza E, Krumpel M, Orre LM, Branca RMM, Lehtiö J, Ek-Rylander B, Andersson G. Tartrate-resistant acid phosphatase (TRAP/ACP5) promotes metastasis-related properties via TGFβ2/TβR and CD44 in MDA-MB-231 breast cancer cells. BMC Cancer. 2017;17:650–650. doi: 10.1186/s12885-017-3616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveljeva S, Mc Laughlin SL, Vandenabeele P, Samali A, Bertrand MJM. Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis. 2015;6:e1587–e1587. doi: 10.1038/cddis.2014.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–667. doi: 10.1038/s41577-021-00534-x. [DOI] [PubMed] [Google Scholar]
- Simpson J, Loh Z, Ullah MA, Lynch JP, Werder RB, Collinson N, Zhang V, Dondelinger Y, Bertrand MJM, Everard ML, Blyth CC, Hartel G, Van Oosterhout AJ, Gough PJ, Bertin J, Upham JW, Spann KM, Phipps S. Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells. Am J Respir Crit Care Med. 2020;201:1358–1371. doi: 10.1164/rccm.201906-1149OC. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MRM, Yagita H. Nature's TRAIL—on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/S1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- Soto-Díaz K, Juda MB, Blackmore S, Walsh C, Steelman AJ. TAK1 inhibition in mouse astrocyte cultures ameliorates cytokine-induced chemokine production and neutrophil migration. J Neurochem. 2020;152:697–709. doi: 10.1111/jnc.14930. [DOI] [PubMed] [Google Scholar]
- Suda J, Dara L, Yang L, Aghajan M, Song Y, Kaplowitz N, Liu ZX. Knockdown of RIPK1 markedly exacerbates murine immune-mediated liver injury through massive apoptosis of hepatocytes, independent of necroptosis and inhibition of NF-κB. J Immunol. 2016;197:3120–3129. doi: 10.4049/jimmunol.1600690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Syafruddin SE, Rodrigues P, Vojtasova E, Patel SA, Zaini MN, Burge J, Warren AY, Stewart GD, Eisen T, Bihary D, Samarajiwa SA, Vanharanta S. A KLF6-driven transcriptional network links lipid homeostasis and tumour growth in renal carcinoma. Nat Commun. 2019;10:1152. doi: 10.1038/s41467-019-09116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Lin H, Wen J, Sun Q, Gao Y, Xu X, Wang J, Zhang J, Weng D. The kinase receptor-interacting protein 1 is required for inflammasome activation induced by endoplasmic reticulum stress. Cell Death Dis. 2018;9:641–641. doi: 10.1038/s41419-018-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Shrinet K, Kumar A. HMGB1 protein as a novel target for cancer. Toxicol Rep. 2019;6:253–261. doi: 10.1016/j.toxrep.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wähämaa H, Schierbeck H, Hreggvidsdottir HS, Palmblad K, Aveberger AC, Andersson U, Harris HE. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther. 2011;13:R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15:1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang Y, Du Q, Lu P, Fan H, Lu J, Hu R. Inflammasome-independent NLRP3 is required for epithelial-mesenchymal transition in colon cancer cells. Exp Cell Res. 2016;342:184–192. doi: 10.1016/j.yexcr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Wang R, Wu W, Li W, Huang S, Li Z, Liu R, Shan Z, Zhang C, Li W, Wang S. Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. J Am Heart Assoc. 2018;7:e008596. doi: 10.1161/JAHA.118.008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Q, Huang B-F, Wang Y, Tang C-H, Jin H-C, Shao F, Shao J-K, Wang Q, Zeng Y. Subcellular localization of HMGB1 in colorectal cancer impacts on tumor grade and survival prognosis. Sci Rep. 2020;10:18587. doi: 10.1038/s41598-020-75783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lundbäck P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol Med. 2021;27:58. doi: 10.1186/s10020-021-00307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Liu H, Song Z, Jiang Y, Kim H, Samavati L, Nguyen HM, Yang Z-Q. The UPR transducer IRE1 promotes breast cancer malignancy by degrading tumor suppressor microRNAs. iScience. 2020 doi: 10.1016/j.isci.2020.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu C, Liu D, Zheng M, Sun J, Feng H, Lu A. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer. 2017;16:70. doi: 10.1186/s12943-017-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.