Abstract
Orexins are excitatory neuropeptides, which are predominantly associated with feeding behavior, sleep-wake cycle and energy homeostasis. The orexinergic system comprises of HCRTR1 and HCRTR2, G-protein-coupled receptors of rhodopsin family and the endogenous ligands processed from HCRT pro-hormone, Orexin A and Orexin B. These neuropeptides are biosynthesized by the orexin neurons present in the lateral hypothalamus area, with dense projections to other brain regions. The orexin-receptor signaling is implicated in various metabolic as well as neurological disorders, making it a promising target for pharmacological interventions. However, there is limited information available on the collective representation of the signal transduction pathways pertaining to the orexin-orexin receptor signaling system. Here, we depict a compendium of the Orexin A/B stimulated reactions in the form of a basic signaling pathway map. This map catalogs the reactions into five categories: molecular association, activation/inhibition, catalysis, transport, and gene regulation. A total of 318 downstream molecules were annotated adhering to the guidelines of NetPath curation. This pathway map can be utilized for further assessment of signaling events associated with orexin-mediated physiological functions and is freely available on WikiPathways, an open-source pathway database (https://www.wikipathways.org/index.php/Pathway:WP5094).
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-022-00700-3.
Keywords: Orexin, PathVisio, Sleep, Feeding, Hypothalamus, Signaling pathway
Introduction
Orexins are excitatory neuropeptides, which have a primary role in the regulation of feeding behavior, sleep or wakefulness and energy homeostasis (Yamanaka et al. 1999; Sutcliffe and de Lecea 2000; Willie et al. 2001). In 1998, the presence of orexins was independently identified by two groups; Sakurai et al. 1998 who named it “orexin” after the Greek word orexis, which means appetite and de Lecea et al. 1998 who called it “hypocretin” due to its expression in the hypothalamus as well as sequence homology with gut peptide hormone secretin. Orexins, also known as hypocretins (Hcrt), are produced by the neurons of the dorsolateral and perifornical area of the hypothalamus (de Lecea et al. 1998; Sakurai et al. 1998). While the cell bodies of orexinergic neurons are confined to the hypothalamus, dense projections are found all over the brain in regions such as the limbic system, thalamus and brain stem, implying complex functions (Peyron et al. 1998). Monoaminergic neurons are innervated by orexin neurons, they specifically activate noradrenergic neurons in the locus coeruleus, dopaminergic neurons in the ventral tegmental region, and histaminergic neurons in the tuberomammillary nucleus (Hagan et al. 1999; Yamanaka et al. 2002; Kohlmeier et al. 2008).
The HCRT gene, which has two exons and one intron encoding a 131-amino-acid precursor polypeptide, is located on chromosome 17q21-q24 (Sakurai et al. 1999). The two subtypes of orexin which are synthesized from this single pro-hormone preprohypocretin/prepro-orexin, include orexin A/hypocretin 1 and orexin B/hypocretin 2 (Ebrahim et al. 2003). Pre-prohypocretin is cleaved at unique sites to yield the two mature peptides upon removal of the N-terminal secretory signal sequence (Chieffi et al. 2017). Orexin A (Ox-A) is a 33 amino acid long peptide with two intrachain disulfide bonds, which has N-terminal proglutamyl residue and C-terminal amidation. Orexin B (Ox-B) is a 28 amino acid long peptide and the C-terminal is almost similar to Ox-A (Tsujino and Sakurai 2009). Orexins exert their function by binding to its cognate G-protein–coupled receptors (GPCRs), orexin receptor type 1 (OX1R, also named as Hcrtr-1) and type 2 (OX2R, or Hcrtr-2) that exhibit 64% amino acid identity (Sakurai 2005). OX1R has a higher affinity (~ 5–100-fold greater) towards Ox-A than Ox-B, whereas, OX2R has similar affinities to both Ox-A and Ox-B (Sakurai et al. 1998; Ammoun et al. 2003). The existence of two receptors and selective binding of agonists may be responsible for the cellular diversity of the orexin-orexin receptor functions.
The binding of orexins to the respective receptors activates at least three subtypes of G-proteins (Gq/11, Gi/0, and Gs) or other proteins such as β-arrestins. This subsequently controls the activation of downstream signaling cascades such as phospholipases, ion channels, and protein kinases (Leonard and Kukkonen 2014). As described in previous studies, the elevation of intracellular calcium (Ca2+) is a hallmark of orexin action in expression systems (Lund et al. 2000). Orexin receptors activate PKC in CNS neurons, which possibly necessitates PLC activity and may thus correlate with IP3-triggered Ca2+ release (Ammoun et al. 2006; Johansson et al. 2008). OX1R couples to Gq/11 class of G-proteins, whereas, OX2R couples to Gq and Gi class of G-proteins. Additionally, stimulation of OX1R and OX2R is associated with the activation of the p38-MAPK signaling pathway and increases the level of phosphorylated ERK1/2 via Gq/PLC/PKC cascade, but not the PKA pathway (Milasta et al. 2005; Wenzel et al. 2009). The binding of orexins and the receptors also results in the stimulation of phospholipase D (PLD)/phosphatidic acid (PA), phospholipase A (PLA)/arachidonic acid (AA), PI3K/Akt and MTORC1 (ERK1/2-Akt-mediated) pathways (Johansson et al. 2008; Sokołowska et al. 2012; Turunen et al. 2012; Wang et al. 2014). Orexin signaling involves the activation of classical GPCR signaling as well as other intracellular signal transduction mechanisms.
Orexin signaling is critical in the regulation of feeding, energy metabolism (Haynes et al. 1999; Edwards et al. 1999; Moriguchi et al. 1999), gastrointestinal system (Kirchgessner and Liu 1999) and modulation of sense of pain (van den Pol 1999). They are also postulated to have roles in regulating neuroendocrine functions by altering the levels of corticotropin, gonadotropin, glucocorticoids and insulin (van den Pol et al. 1998; Ida et al. 2000; Sutcliffe and de Lecea 2000; Ziolkowska et al. 2005). Further, orexins also play an essential role in neuroprotection, regulation of apoptosis and inflammation (Butterick et al. 2012; Xiong et al. 2013; Sokołowska et al. 2014). Of the two ligands, Ox-A has potent neuroprotective and immuno-regulatory actions (Yuan et al. 2011; Duffy et al. 2019). The orexin/receptor system is ectopically expressed in a number of neurological disorders, implying that it plays a role in their occurrence and pathogenesis. Defects in the orexin/receptor system have been linked to human narcolepsy in multiple studies (Lin et al. 1999; Burgess et al. 2010; Hasegawa et al. 2014). It is also strongly correlated to drug addiction, especially to alcohol, nicotine, and cocaine (Smith et al. 2009; Moorman et al. 2017), Alzheimer’s disease (Fronczek et al. 2012; Liguori et al. 2016; Gabelle et al. 2017), schizophrenia (Nishino et al. 2002; Dalal et al. 2003; Huang et al. 2014) and depression (Salomon et al. 2003).
Orexins, with both neuroprotective and immunomodulatory properties, have emerged as a promising new class of biological agents for the treatment of immune-mediated CNS disorders such as narcolepsy, metabolic and neurological disorders. However, a comprehensive understanding of the signaling mechanisms underlying the orexin receptor is missing. The development of a consolidated map of orexinergic signaling could be appreciated for the definitive assignment of mechanisms underlying orexin/receptor-promoted functions. This will aid in the generation of orexin/receptor-targeted pharmacological therapies for the successful treatment of disorders.
Methodology
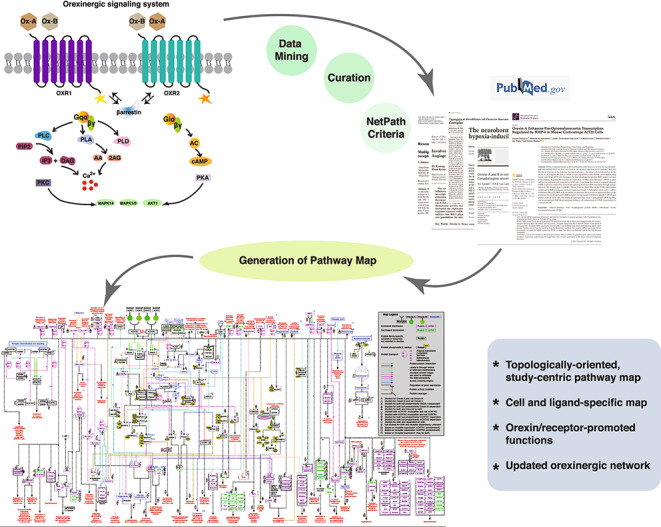
A literature search was executed in PubMed with key terms including ‘orexin, its aliases’ and ‘signaling’. Research articles excluding reviews were selected with information pertaining to the stimulation of orexin receptors. Following NetPath criteria, we have classified the annotated reactions into five categories; molecular association, (protein-protein interactions), catalysis (post-translational modifications, binding and cleavage), transport (translocation/transport of proteins between subcellular compartments), activation/inhibition, and, finally, gene regulation at the mRNA and/or protein level (both up and downregulation). The reactions pertaining to the orexin signaling pathway were filtered using NetPath criteria (Kandasamy et al. 2009, 2010) as described previously; Galanin receptor signaling (Gopalakrishnan et al. 2021), Serotonin (Sahu et al. 2018), oxytocin receptor (Chatterjee et al. 2016), Oncostatin M (Dey et al. 2013), AGE/RAGE signaling (Soman et al. 2013), MIF signaling (Subbannayya et al. 2015), IL33 signaling (Pinto et al. 2018), prolactin signaling (Radhakrishnan et al. 2012), FGF-1/FGFR (Raju et al. 2014), and VEGF-A/VEGFR2 (Sunitha et al. 2019) signaling pathways. The reactions were then represented in the form of a map with relevant information about orexin signaling using PathVisio, an open-source, free pathway depiction tool (van Iersel et al. 2008). The reactions were exported to WikiPathways, an open-source biological pathway database for the scientific community (Pico et al. 2008; Kelder et al. 2009). In addition, the pathway reactions and the map were subjected to manual review by internal curation experts and also by a pathway authority.
Results and discussion
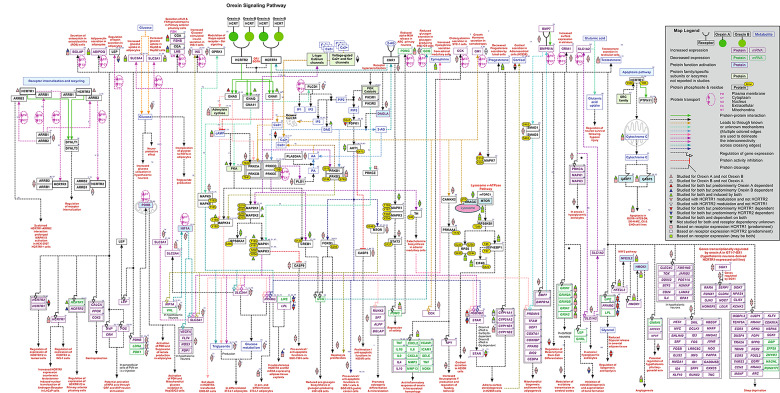
We designed a literature search term to identify research articles that would provide information on orexin receptor signaling. The search term “OREXIN” OR “HYPOCRETIN” OR “HCRT” OR “OX” OR “NRCLP1” OR “PPOX” OR “HCRT1” OR “HCRT2” OR “HYPOCRETIN1” OR “HYPOCRETIN2” OR “OX1” OR “OX2” OR “OREXIN1” OR “OREXIN2” OR “OX1R” OR “OX2R” OR “HCRTR1” OR “HCRTR2” OR “OREXIN 1 RECEPTOR” OR “OREXIN 2 RECEPTOR” OR"HYPOCRETIN 1 RECEPTOR” OR “HYPOCRETIN 2 RECEPTOR” AND (“SIGNALING” OR “PATHWAY” OR “SIGNALLING”) yielded 5,548 PubMed articles, among which 125 articles were selected for manual curation of reactions based on the NetPath annotation criteria described in (Kandasamy et al. 2009, 2010). We identified a total of 318 downstream signaling reactions, which include, 23 transport, 31 catalysis events (post-translational modifications), 15 molecular associations, 23 activation/inhibition reactions and a total of 226 gene regulation events comprising 56 reactions reported at protein and 170 reactions at mRNA level. A compendium of these annotated reactions is represented as a systematic pathway map in Fig. 1. This pathway map was generated on the standalone free pathway drawing tool, PathVisio (van Iersel et al. 2008) and is made freely accessible on https://www.wikipathways.org/index.php/Pathway:WP5094. The data pertaining to the pathway is presented in a standard community exchange format, Biological PAthway eXchange (BioPAX) (Demir et al. 2010) Level 3 (OWL). The entire data can be downloaded in .png, .pdf and .svg image formats as well as in .txt format from the WikiPathways (Pico et al. 2008; Kelder et al. 2009). A supplementary table (Supplementary Table S1) provides all the annotated reactions with a brief description of each.
Fig. 1.
A schematic representation of the reactions induced by orexin-orexin receptor
The orexinergic neuropeptides (Ox-A and Ox-B) are known to be evolutionarily conserved across several mammalian species and are thus predicted to have important implications in several physiological conditions (Wong et al. 2011). The initiation of signaling by the orexin neuropeptides (Ox-A and Ox-B) ensues with the ligand binding to orexin receptors (OXRs) and the subsequent activation of G-protein subtypes. Initial studies point towards the strong association of OXRs with Gq (Sakurai et al. 1998; Smart et al. 1999) coupled with PLC-mediated calcium elevation (Kukkonen 2016) in the regulation of feeding behavior. However, over the years several studies have also indicated an orexin stimulated differential association of OXRs with other G-protein sub-types, such as Gi/0 and Gs in tissues other than the hypothalamus (Randeva et al. 2001; Holmqvist et al. 2005; Karteris et al. 2005). This differential G-protein activation downstream of OX1R and OX2R can also be attributed to the ligand used for receptor stimulation. OX1R stimulated by Ox-A/B and OX2R stimulated Ox-B activated a Gq mediated PLC/PKC cascade ultimately leading to phosphorylation-based activation of the ERK pathway. Whereas, Ox-A stimulated OX2R achieved the same through a Gs/AC mediated PKA cascade. The activation of the JNK or p38 pathways, however, was mediated via Gi-coupled OX1R or OX2R, respectively (Tang et al. 2008; Ramanjaneya et al. 2009).
The orexinergic signaling also comprises of the receptor association with GPCR kinases such as GRK2 and GRK5, and recruitment of the β-arrestin proteins, which aid not only in the receptor trafficking but also act as signaling scaffolds (Dalrymple et al. 2011; Kukkonen and Leonard 2014; Cai et al. 2020). Another established component of the OXR signaling is the elevation of intracellular Ca2+. Studies on neuronal as well as non-neuronal cells have indicated a surge of Ca2+ ions on OXR stimulation by either Ox-A or Ox-B. This elevation of Ca2+ levels can be attributed to both, an influx of extracellular Ca2+ through L-type or DAG mediated channels, as well as IP3 mediated release from the intracellular Ca2+ stores in the sarcoplasmic reticulum (Ishibashi et al. 2005; Näsman et al. 2006; Xia et al. 2009; Peltonen et al. 2009; Wang et al. 2014). Interestingly, a study performed on Odora cells (derived from olfactory sensory neurons) revealed the contribution of both extracellular Ca2+ influx and intracellular Ca2+ release for the overall elevation of Ca2+ levels downstream of OX1R (Gorojankina et al. 2007). However, in the same cell line, the stimulation of OX2R led to an elevation of Ca2+ levels only through release from intracellular stores. Additionally, the stimulation of both the receptors in the rat arcuate nucleus in an agonist-dependent manner regulated the calcium signaling reciprocally to modulate feeding behavior (Muroya et al. 2004). Whilst a significant rise in Ca2+ levels was observed in rat NPY neurons in a Gq/PLC/IP3/PKC dependent manner upon stimulation of OXIR by Ox-A, a Gi/0 mediated decrease in the intracellular Ca2+ levels was elicited by Ox-A/B stimulated OX2R in rat POMC neurons (Muroya et al. 2004). Another interesting study performed on rat INS-1E cells revealed a TRPV calcium channel-mediated Ca2+ influx on OX1R stimulation, leading to a rise in insulin secretion and cell proliferation (Skrzypski et al. 2016). Taken together, the calcium signaling downstream of OXRs is implicated in several physiological functions including regulation of feeding behavior, synthesis and release of hormones like catecholamines, nociception and integration of the neural systems involved in maintaining wakefulness, energy homeostasis, sensory processing and other autonomic functions (Xia et al. 2009; Ozcan et al. 2010; Nakamura et al. 2010; Nemoto et al. 2013).
Orexin neurons in the hypothalamus have a role in postsynaptic neurotransmitter release (Peltonen et al. 2009), which include the release of glutamate, GABA as well as acetylcholine (Kodama and Kimura 2002; Davis et al. 2003; Bernard et al. 2006; Dong et al. 2006). The release of glutamate and acetylcholine downstream of OXRs in rat locus coeruleus and somatosensory cortex, respectively, are implicated in the promotion of wakefulness or arousal thus affecting the sleep-wake cycle (Kodama and Kimura 2002; Dong et al. 2006). The release of these neurotransmitters from the pre-synaptic nerve terminal can also be mediated by a secondary metabolite, 2-arachidonoyl glycerol (2-AG), which facilitates retrograde paracrine signaling as an effect of OXR stimulation. The interaction between orexin and cannabinoid receptors (CB1), upon OX1R activation, is attributed to the release of secondary metabolites. For instance, a PLC or PLD-activated DAGL mediated 2-AG release followed by arachidonic acid (AA) release was observed in HEK293 and Neuro 2 A cells expressing the human OX1R gene (Turunen et al. 2012). Interestingly, a similar PLC/DAGL mediated increase in 2-AG leads to the generation of inhibitory inputs on dopaminergic neurons in rat brain regions such as the nucleus accumbens (NAc) and the ventral tegmental area (VTA). This rise in 2-AG in turn induces an association of the dopamine receptor D2 (DRD2) and β-arrestin 2 (ARRB2) leading to the desensitization of DRD2 resulting in the regulation of the mesolimbic dopaminergic circuitry (Tunisi et al. 2021). Additionally, the release of AA is also involved in providing feedback to Ca2+ influx under Ox-A stimulation of OX1R in CHO-hOX1 cells (Turunen et al. 2010) as depicted in the map (Fig. 1). This release of AA coincided with the release of other high potency lipid messengers in this cell system, suggesting a role of orexin in the regulation of the lipid signaling system.
Under OXR stimulation we observed the adenylyl cyclase (AC) mediated cyclic AMP (cAMP) cascade to be seemingly less prominent when compared with Ca2+ cascades. As depicted in the map we curated Gs-mediated increase in cAMP, as well as Gi-mediated decrease in cAMP downstream to OX2R. Also, a Gs-dependent increase in cAMP accumulation was observed under OX1R stimulation in the central nervous system (Gorojankina et al. 2007; Tang et al. 2008; Woldan-Tambor et al. 2011; Urbańska et al. 2012). Additionally, studies on human as well as rat adrenocortical cells have implicated the role of AC/PKA mediated cortisol secretion as well as autocrine-paracrine regulation of adrenal glucocorticoid release (Spinazzi et al. 2005; Ziolkowska et al. 2005). Apart from glucocorticoid and cortisol signaling, orexins also play an important role in the synthesis and secretion of several other endocrine hormones aiding the modulation of the hypothalamic-pituitary axis (HPA) (Kuru et al. 2000; Al-Barazanji et al. 2001; Xu et al. 2003; Ramanjaneya et al. 2009). The differential activation of AC downstream of OX1R resulted in an increase of cAMP accumulation in the adrenocortical cells led to a rise in cortisol and aldosterone secretion, however, the decrease in cAMP accumulation in the medullary cells was associated with an increase in epinephrine and norepinephrine release (Nanmoku et al. 2002). Some of the enzymes secreted downstream of OXRs include amylase and cholecystokinin (Harris et al. 2002; Larsson et al. 2003). Among other hormones, we curated and mapped the release of gonadotropin hormones (LH and FSH) (Cataldi et al. 2014), serotonin (Tao et al. 2006), and testosterone (Barreiro et al. 2004; Liguori et al. 2017), as well as a decrease in progesterone (Cataldi et al. 2012) under both the receptors. In addition, we identified an increase of leptin secretion (Pruszynska-Oszmalek et al. 2018) in porcine adipocytes and an increase in the release of growth hormone in ovine somatotrophs (Xu et al. 2003) under the stimulation of OX1R and OX2R, respectively. However, the exact mechanism involved in the secretion of these hormones is still poorly understood.
A very unique and intriguing phenomenon identified among the reactions curated under OXR stimulation is that of its dual role in regulating apoptosis. There is a PI3K-AKT mediated anti-apoptotic function induced through OX1R in INS1 cells and rat hepatocytes (Chen et al. 2013; Ju et al. 2014), nevertheless, in cancer cell types like HT29-D4 cells or rat C6 glioma OX1R stimulation are reported to induce apoptosis (Rouet-Benzineb et al. 2004; Biegańska et al. 2012). This dynamic modulation of the apoptotic pathway by the OX1R is arbitrated by the activation/inhibition of caspases such as CASP3 and CASP7 (Rouet-Benzineb et al. 2004; Liu et al. 2015; Wen et al. 2015). This facet of apoptotic signaling by the orexinergic systems needs to be explored further to enable the use of orexin in cancer therapeutics. Apart from its potential anti-tumorigenic role, orexinergic neuropeptides are also implicated in neuroprotection from hypoxic injury and anti-inflammatory actions. OXR signaling essentially exerts an anti-inflammatory effect by reducing the pro-inflammatory markers such as TNFα, IL-1β, IL-6, CXCL8, CXCL2, and NOX4 in a MAPK14 and NFκB dependent manner (Xiong et al. 2013; Zhang et al. 2018; Sun et al. 2018; Wang et al. 2019; Li et al. 2020), in cells/tissue insulted with either oxidative or ischemic stress. On the other hand, in normal rat paraventricular neurons stimulation of OX1R resulted in the upregulation of IL6, IL1B, TNF, FOSL1 and AVP mRNA leading to the development of hypertension (Huber et al. 2017; Fan et al. 2018). Thus, depending on the physiological state, the orexinergic system is seen to adapt its downstream signaling to either protect the cell or facilitate programmed cell death. Yet, the exact cascade or the signaling modalities remains unknown.
The regulation of glucose metabolism is a widely studied and well-illustrated physiological significance attributed to orexinergic signaling. In this regard, we curated two equally important cascades, both mediated by PI3K/AKT activation. One of these includes the AKT/MTOR mediated upregulation as well as activation of HIF1A protein which then instigates the upregulation of GLUT1 protein, which eventually leads to glucose uptake (Wan et al. 2017). This study performed on HepG2 cells also revealed the activation of PDP1, upregulation of PDHB and downregulation of LDHA and PDK1 thus, enabling the shift from glycolysis to TCA cycle of glucose metabolism. The other cascade, however, functions downstream of both receptors and proceeds via a series of phosphorylation-based activation of PI3K, PDK1, AKT, and AS-160, ultimately causing the translocation of GLUT4 receptor to the plasma membrane and subsequent rise in glucose uptake (Skrzypski et al. 2011). Another study performed on HEK293 cells and rat hypothalamic tissue revealed several other genes upregulated downstream of OX1R stimulated HIF1A upregulation (Sikder and Kodadek 2007). These include VEGFA, FLT4, SLC2A1, and NOS3.
Furthermore, we curated a predominantly upregulated set of genes associated with sleep deprivation, osteoblast differentiation, adipogenesis, mitochondrial biogenesis and modulation of excitatory transmission (Fig. 1) (Yamada et al. 2008; Sellayah et al. 2011; Koesema and Kodadek 2017; Han et al. 2018).
Conclusion
We attempted to consolidate the reactions relevant to the stimulation of orexin receptors by Ox-A and Ox-B, thus generate an extensive signaling map. With the relative representation of the Ox-A or Ox-B stimulation and their associations with the two orexin receptors for the signaling pathway reactions, this collective depiction of the signal transduction mechanisms and the signaling cascades associated with orexins will help scientists improve the current knowledge of the orexinergic system. This pathway illustration provides the downstream effectors and the varied physiological outcomes associated with differential stimulation of orexin receptors. Moreover, this would also serve as a reference for up-to-date inclusion of information on orexin signaling and thereby a tool for pathway enrichment analysis. Overall, this information could be further utilized for the advancement of orexin research in health and disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). We thank the Department of Biotechnology, Government of India for research support to the Institute of Bioinformatics (IOB), Bangalore. Oishi Chatterjee and Lathika Gopalakrishnan are recipients of the DST-INSPIRE Fellowship (SRF) from the Department of Science and Technology (DST), Government of India. Rajesh Raju is a recipient of the Young Scientist Award (YSS/2014/000607) from the Science and Engineering Research Board, Department of Science and Technology (DST), Government of India.
List of abbreviations
- CNS
Central Nervous System
- HCRT
Hypocretin
- GPCR
G protein-coupled receptor
- HCRTR1
Hypocretin receptor 1
- HCRTR2
Hypocretin receptor 2
- OX1R
Orexin receptor 1
- OX2R
Orexin receptor 2
- Ox-A
Orexin A
- Ox-B
Orexin B
- OXR
Orexin receptors
- PPIs
Protein-protein interactions
- BioPAX
Biological Pathway Exchange
- SBML
Systems Biology Markup Language
- PKA
Protein kinase A
- PKC
Protein kinase C
- PLC
Phospholipase C
- PLD
Phospholipase D
- IP3
Inositol-3-phosphate
- MAPK
Mitogen activated protein kinases
- GRK
G Protein-Coupled Receptor Kinase
Declarations
Conflict of interest
The author(s) declare no conflicts of interest.
Footnotes
Oishi Chatterjee and Lathika Gopalakrishnan authors contributed equally
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Oishi Chatterjee, Email: oishichatterjee21@gmail.com.
Lathika Gopalakrishnan, Email: lathika.gnair@gmail.com.
Deepshika Pullimamidi, Email: deepshikag.puli@gmail.com.
Chinmayi Raj, Email: chinmayigraj@gmail.com.
Soujanya Yelamanchi, Email: souji.bio@gmail.com.
Bhavya Somaplara Gangadharappa, Email: bhavyasg09@gmail.com.
Bipin Nair, Email: bipin@am.amrita.edu.
Anita Mahadevan, Email: mahadevananita@gmail.com.
Rajesh Raju, Email: rajrrnbt@gmail.com.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in
References
- Al-Barazanji KA, Wilson S, Baker J, et al. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol. 2001;13:421–424. doi: 10.1046/j.1365-2826.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Johansson L, Ekholm ME, et al. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2 + influx in OX1 receptor signaling. Mol Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Pineda R, Navarro VM, et al. Orexin 1 receptor messenger ribonucleic acid expression and stimulation of testosterone secretion by orexin-A in rat testis. Endocrinology. 2004;145:2297–2306. doi: 10.1210/en.2003-1405. [DOI] [PubMed] [Google Scholar]
- Bernard R, Lydic R, Baghdoyan HA. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate g protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–171. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- Biegańska K, Sokołowska P, Jöhren O, Zawilska JB. Orexin A suppresses the growth of rat C6 glioma cells via a caspase-dependent mechanism. J Mol Neurosci. 2012;48:706–712. doi: 10.1007/s12031-012-9799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Tse G, Gillis L, Peever JH. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33:1295–1304. doi: 10.1093/sleep/33.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012;524:30–34. doi: 10.1016/j.neulet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang H, Wang M, et al. A novel phosphorylation site on orexin receptor 1 regulating orexinA-induced GRK2-biased signaling. Cell Signal. 2020;75:109743. doi: 10.1016/j.cellsig.2020.109743. [DOI] [PubMed] [Google Scholar]
- Cataldi NI, Lux-Lantos VAR, Libertun C. Effects of orexins A and B on expression of orexin receptors and progesterone release in luteal and granulosa ovarian cells. Regul Pept. 2012;178:56–63. doi: 10.1016/j.regpep.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Cataldi NI, Lux Lantos VAR, Libertun C. Orexin A and B in vitro modify orexins receptors expression and gonadotropins secretion of anterior pituitary cells of proestrous rats. Regul Pept. 2014;188:25–30. doi: 10.1016/j.regpep.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Chatterjee O, Patil K, Sahu A, et al. An overview of the oxytocin-oxytocin receptor signaling network. J Cell Commun Signal. 2016;10:355–360. doi: 10.1007/s12079-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhao Y, Zheng D, et al. Orexin A Affects INS-1 Rat Insulinoma Cell Proliferation via Orexin Receptor 1 and the AKT Signaling Pathway. Int J Endocrinol. 2013;2013:854623. doi: 10.1155/2013/854623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieffi S, Carotenuto M, Monda V, et al. Orexin System: The Key for a Healthy Life. Front Physiol. 2017;8:357. doi: 10.3389/fphys.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal MA, Schuld A, Pollmächer T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol Psychiatry. 2003;8:836–837. doi: 10.1038/sj.mp.4001363. [DOI] [PubMed] [Google Scholar]
- Dalrymple MB, Jaeger WC, Eidne KA, Pfleger KDG. Temporal profiling of orexin receptor-arrestin-ubiquitin complexes reveals differences between receptor subtypes. J Biol Chem. 2011;286:16726–16733. doi: 10.1074/jbc.M111.223537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SF, Williams KW, Xu W, et al. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, et al. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Radhakrishnan A, Syed N, et al. Signaling network of Oncostatin M pathway. J Cell Commun Signal. 2013;7:103–108. doi: 10.1007/s12079-012-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Fukuda S, Murata E, et al. Orexins increase cortical acetylcholine release and electroencephalographic activation through orexin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology. 2006;104:1023–1032. doi: 10.1097/00000542-200605000-00019. [DOI] [PubMed] [Google Scholar]
- Duffy CM, Hofmeister JJ, Nixon JP, Butterick TA. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem. 2019;157:41–47. doi: 10.1016/j.nlm.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim IO, Sharief MK, de Lacy S, et al. Hypocretin (orexin) deficiency in narcolepsy and primary hypersomnia. J Neurol Neurosurg Psychiatry. 2003;74:127–130. doi: 10.1136/jnnp.74.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- Fan Y, Jiang E, Hahka T et al (2018) Orexin A increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague Dawley rats. Acta Physiol (Oxf) 222. 10.1111/apha.12963 [DOI] [PMC free article] [PubMed]
- Fronczek R, van Geest S, Frölich M, et al. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Gabelle A, Jaussent I, Hirtz C, et al. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging. 2017;53:59–66. doi: 10.1016/j.neurobiolaging.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan L, Chatterjee O, Raj C, et al. An assembly of galanin-galanin receptor signaling network. J Cell Commun Signal. 2021;15:269–275. doi: 10.1007/s12079-020-00590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorojankina T, Grébert D, Salesse R, et al. Study of orexins signal transduction pathways in rat olfactory mucosa and in olfactory sensory neurons-derived cell line Odora: multiple orexin signalling pathways. Regul Pept. 2007;141:73–85. doi: 10.1016/j.regpep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhou J, Peng W. Orexins Facilitates Osteogenic Differentiation of MC3T3-E1 Cells. IUBMB Life. 2018;70:633–641. doi: 10.1002/iub.1757. [DOI] [PubMed] [Google Scholar]
- Harris DM, Go VLW, Reeve JR, Wu SV. Stimulation of amylase release by Orexin is mediated by Orexin 2 receptor in AR42J cells. Pancreas. 2002;25:405–410. doi: 10.1097/00006676-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Ostman M, et al. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Guilleminault C, Chen C-H, et al. Narcolepsy-cataplexy and schizophrenia in adolescents. Sleep Med. 2014;15:15–22. doi: 10.1016/j.sleep.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Huber MJ, Fan Y, Jiang E, et al. Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2017;313:H1075–H1086. doi: 10.1152/ajpheart.00822.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Kuroiwa T, et al. Both corticotropin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci Lett. 2000;293:119–122. doi: 10.1016/s0304-3940(00)01498-1. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Takano S, Yanagida H, et al. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Johansson L, Ekholm ME, Kukkonen JP. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol Life Sci. 2008;65:1948–1956. doi: 10.1007/s00018-008-8206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S-J, Zhao Y, Chang X, Guo L. Orexin A protects cells from apoptosis by regulating FoxO1 and mTORC1 through the OX1R/PI3K/AKT signaling pathway in hepatocytes. Int J Mol Med. 2014;34:153–159. doi: 10.3892/ijmm.2014.1769. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, et al. PathBuilder–open source software for annotating and developing pathway resources. Bioinformatics. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Machado RJ, Chen J, et al. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- Kelder T, Pico AR, Hanspers K, et al. Mining biological pathways using WikiPathways web services. PLoS ONE. 2009;4:e6447. doi: 10.1371/journal.pone.0006447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951. doi: 10.1016/s0896-6273(00)81041-7. [DOI] [PubMed] [Google Scholar]
- Kodama T, Kimura M. Arousal effects of orexin-A correlate with GLU release from the locus coeruleus in rats. Peptides. 2002;23:1673–1681. doi: 10.1016/s0196-9781(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Koesema E, Kodadek T. Global analysis of gene expression mediated by OX1 orexin receptor signaling in a hypothalamic cell line. PLoS ONE. 2017;12:e0188082. doi: 10.1371/journal.pone.0188082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier KA, Watanabe S, Tyler CJ, et al. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2 + transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–2281. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP. G-protein-dependency of orexin/hypocretin receptor signalling in recombinant Chinese hamster ovary cells. Biochem Biophys Res Commun. 2016;476:379–385. doi: 10.1016/j.bbrc.2016.05.130. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, et al. Centrally administered orexin/hypocretin activates HPA axis in rats. NeuroReport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Larsson KP, Akerman KE, Magga J, et al. The STC-1 cells express functional orexin-A receptors coupled to CCK release. Biochem Biophys Res Commun. 2003;309:209–216. doi: 10.1016/s0006-291x(03)01563-8. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kukkonen JP. Orexin/hypocretin receptor signalling: a functional perspective. Br J Pharmacol. 2014;171:294–313. doi: 10.1111/bph.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Xu W, Ouyang J, et al. Orexin A alleviates neuroinflammation via OXR2/CaMKKβ/AMPK signaling pathway after ICH in mice. J Neuroinflammation. 2020;17:187. doi: 10.1186/s12974-020-01841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Nuccetelli M, Izzi F, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol Aging. 2016;40:120–126. doi: 10.1016/j.neurobiolaging.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Liguori G, Pavone LM, Assisi L, et al. Expression of orexin B and its receptor 2 in rat testis. Gen Comp Endocrinol. 2017;242:66–73. doi: 10.1016/j.ygcen.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Ju S, Guo L. Orexin A upregulates the protein expression of OX1R and enhances the proliferation of SGC-7901 gastric cancer cells through the ERK signaling pathway. Int J Mol Med. 2015;35:539–545. doi: 10.3892/ijmm.2014.2038. [DOI] [PubMed] [Google Scholar]
- Lund PE, Shariatmadari R, Uustare A, et al. The orexin OX1 receptor activates a novel Ca2 + influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- Milasta S, Evans NA, Ormiston L, et al. The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J. 2005;387:573–584. doi: 10.1042/BJ20041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2017;1654:34–42. doi: 10.1016/j.brainres.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Sakurai T, Nambu T, et al. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264:101–104. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2 + signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Miura S, Yoshida T, et al. Cytosolic calcium elevation induced by orexin/hypocretin in granule cell domain cells of the rat cochlear nucleus in vitro. Peptides. 2010;31:1579–1588. doi: 10.1016/j.peptides.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Nanmoku T, Isobe K, Sakurai T, et al. Effects of orexin on cultured porcine adrenal medullary and cortex cells. Regul Pept. 2002;104:125–130. doi: 10.1016/s0167-0115(01)00356-1. [DOI] [PubMed] [Google Scholar]
- Näsman J, Bart G, Larsson K, et al. The orexin OX1 receptor regulates Ca2 + entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto T, Toyoshima-Aoyama F, Ueda Y, et al. Involvement of the orexin system in adrenal sympathetic regulation. Pharmacology. 2013;91:250–258. doi: 10.1159/000350391. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Mignot E, et al. CSF hypocretin-1 levels in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res. 2002;110:1–7. doi: 10.1016/s0165-1781(02)00032-x. [DOI] [PubMed] [Google Scholar]
- Ozcan M, Ayar A, Serhatlioglu I, et al. Orexins activates protein kinase C-mediated Ca(2+) signaling in isolated rat primary sensory neurons. Physiol Res. 2010;59:255–262. doi: 10.33549/physiolres.931739. [DOI] [PubMed] [Google Scholar]
- Peltonen HM, Magga JM, Bart G, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem Biophys Res Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico AR, Kelder T, van Iersel MP, et al. WikiPathways: pathway editing for the people. PLoS Biol. 2008;6:e184. doi: 10.1371/journal.pbio.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SM, Subbannayya Y, Rex DAB, et al. A network map of IL-33 signaling pathway. J Cell Commun Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynska-Oszmalek E, Kolodziejski PA, Kaczmarek P, et al. Orexin A but not orexin B regulates lipid metabolism and leptin secretion in isolated porcine adipocytes. Domest Anim Endocrinol. 2018;63:59–68. doi: 10.1016/j.domaniend.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Raju R, Tuladhar N, et al. A pathway map of prolactin signaling. J Cell Commun Signal. 2012;6:169–173. doi: 10.1007/s12079-012-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Palapetta SM, Sandhya VK, et al. A Network Map of FGF-1/FGFR Signaling System. J Signal Transduct. 2014;2014:962962. doi: 10.1155/2014/962962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanjaneya M, Conner AC, Chen J, et al. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–261. doi: 10.1677/JOE-08-0536. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, et al. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- Sahu A, Gopalakrishnan L, Gaur N, et al. The 5-Hydroxytryptamine signaling map: an overview of serotonin-serotonin receptor mediated signaling network. J Cell Commun Signal. 2018;12:731–735. doi: 10.1007/s12079-018-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Moriguchi T, Furuya K, et al. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–17776. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sikder D, Kodadek T. The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 2007;21:2995–3005. doi: 10.1101/gad.1584307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypski M, Khajavi N, Mergler S, et al. Orexin A modulates INS-1E cell proliferation and insulin secretion via extracellular signal-regulated kinase and transient receptor potential channels. J Physiol Pharmacol. 2016;67:643–652. [PubMed] [Google Scholar]
- Skrzypski M, Le T, Kaczmarek T, et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3-L1 adipocytes and isolated primary rat adipocytes. Diabetologia. 2011;54:1841–1852. doi: 10.1007/s00125-011-2152-2. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokołowska P, Urbańska A, Biegańska K, et al. Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J Mol Neurosci. 2014;52:48–55. doi: 10.1007/s12031-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokołowska P, Urbańska A, Namiecińska M, et al. Orexins promote survival of rat cortical neurons. Neurosci Lett. 2012;506:303–306. doi: 10.1016/j.neulet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Soman S, Raju R, Sandhya VK, et al. A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal. 2013;7:19–23. doi: 10.1007/s12079-012-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi R, Rucinski M, Neri G, et al. Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab. 2005;90:3544–3549. doi: 10.1210/jc.2004-2385. [DOI] [PubMed] [Google Scholar]
- Subbannayya T, Leal-Rojas P, Barbhuiya MA, et al. Macrophage migration inhibitory factor - a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843. doi: 10.1186/s12885-015-1855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Wang W, Li Q, et al. Orexin A may suppress inflammatory response in fibroblast-like synoviocytes. Biomed Pharmacother. 2018;107:763–768. doi: 10.1016/j.biopha.2018.07.159. [DOI] [PubMed] [Google Scholar]
- Sunitha P, Raju R, Sajil CK, et al. Temporal VEGFA responsive genes in HUVECs: Gene signatures and potential ligands/receptors fine-tuning angiogenesis. J Cell Commun Signal. 2019;13:561–571. doi: 10.1007/s12079-019-00541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Chen J, Ramanjaneya M, et al. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, McKenna JT, et al. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101–1105. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Tunisi L, D’Angelo L, Fernández-Rilo AC, et al. Orexin-A/Hypocretin-1 Controls the VTA-NAc Mesolimbic Pathway via Endocannabinoid-Mediated Disinhibition of Dopaminergic Neurons in Obese Mice. Front Synaptic Neurosci. 2021;13:622405. doi: 10.3389/fnsyn.2021.622405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen PM, Ekholm ME, Somerharju P, Kukkonen JP. Arachidonic acid release mediated by OX1 orexin receptors. Br J Pharmacol. 2010;159:212–221. doi: 10.1111/j.1476-5381.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen PM, Jäntti MH, Kukkonen JP. OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release. Mol Pharmacol. 2012;82:156–167. doi: 10.1124/mol.112.078063. [DOI] [PubMed] [Google Scholar]
- Urbańska A, Sokołowska P, Woldan-Tambor A, et al. Orexins/hypocretins acting at Gi protein-coupled OX 2 receptors inhibit cyclic AMP synthesis in the primary neuronal cultures. J Mol Neurosci. 2012;46:10–17. doi: 10.1007/s12031-011-9526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, et al. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iersel MP, Kelder T, Pico AR, et al. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Liu Y, Zhao Y, et al. Orexin A affects HepG2 human hepatocellular carcinoma cells glucose metabolism via HIF-1α-dependent and -independent mechanism. PLoS ONE. 2017;12:e0184213. doi: 10.1371/journal.pone.0184213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, He T, Wan B, et al. Orexin A ameliorates HBV X protein-induced cytotoxicity and inflammatory response in human hepatocytes. Artif cells, nanomedicine. Biotechnol. 2019;47:2003–2009. doi: 10.1080/21691401.2019.1614014. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu S, Kakizaki M, et al. Orexin/hypocretin activates mTOR complex 1 (mTORC1) via an Erk/Akt-independent and calcium-stimulated lysosome v-ATPase pathway. J Biol Chem. 2014;289:31950–31959. doi: 10.1074/jbc.M114.600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Zhao Y, Shen Y, Guo L. Effect of orexin A on apoptosis in BGC-823 gastric cancer cells via OX1R through the AKT signaling pathway. Mol Med Rep. 2015;11:3439–3444. doi: 10.3892/mmr.2015.3190. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Grabinski N, Knopp CA, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1601–R1609. doi: 10.1152/ajpregu.91034.2008. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Woldan-Tambor A, Biegańska K, Wiktorowska-Owczarek A, Zawilska JB. Activation of orexin/hypocretin type 1 receptors stimulates cAMP synthesis in primary cultures of rat astrocytes. Pharmacol Rep. 2011;63:717–723. doi: 10.1016/s1734-1140(11)70583-7. [DOI] [PubMed] [Google Scholar]
- Wong KKY, Ng SYL, Lee LTO, et al. Orexins and their receptors from fish to mammals: a comparative approach. Gen Comp Endocrinol. 2011;171:124–130. doi: 10.1016/j.ygcen.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Xia JX, Fan SY, Yan J, et al. Orexin A-induced extracellular calcium influx in prefrontal cortex neurons involves L-type calcium channels. J Physiol Biochem. 2009;65:125–136. doi: 10.1007/BF03179063. [DOI] [PubMed] [Google Scholar]
- Xiong X, White RE, Xu L, et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke. 2013;44:764–770. doi: 10.1161/STROKEAHA.112.681700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Roh S-G, Gong C, et al. Orexin-B augments voltage-gated L-type Ca(2+) current via protein kinase C-mediated signalling pathway in ovine somatotropes. Neuroendocrinology. 2003;77:141–152. doi: 10.1159/000069507. [DOI] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Tatsuno I, et al. Orexin decreases mRNA expressions of NMDA and AMPA receptor subunits in rat primary neuron cultures. Peptides. 2008;29:1582–1587. doi: 10.1016/j.peptides.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, et al. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Tsujino N, Funahashi H, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- Yuan L-B, Dong H-L, Zhang H-P, et al. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology. 2011;114:340–354. doi: 10.1097/ALN.0b013e318206ff6f. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liang B, Li T, et al. Orexin A Suppresses Oxidized LDL Induced Endothelial Cell Inflammation via MAPK p38 and NF-κB Signaling Pathway. IUBMB Life. 2018;70:961–968. doi: 10.1002/iub.1890. [DOI] [PubMed] [Google Scholar]
- Ziolkowska A, Spinazzi R, Albertin G, et al. Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the OX1 receptor. J Steroid Biochem Mol Biol. 2005;96:423–429. doi: 10.1016/j.jsbmb.2005.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.