Abstract
Objectives
To assess the characteristics of the global death burden imposed by chronic kidney disease (CKD) and the attributable risk factors from 1990 to 2019 to help inform a framework for policy discussions, resource allocation and research priorities.
Design
A population-based observational study.
Setting
The death data and relative risk factors were obtained from the Global Burden of Disease (GBD) Study 2019 database.
Main outcome measures
Based on the GBD database, we estimated the death burden attributable to CKD stratified by sociodemographic index (SDI), geographic location, sex, age group, time period and risk factors from 1990 to 2019.
Results
Over three decade study period, the global number of CKD-related deaths increased from 0.60 million (95% uncertainty interval (UI): 0.57–0.63 million) in 1990 to 1.43 million (95% UI: 1.31–1.52 million) in 2019. The age-standardised death rate (ASDR) of CKD, among all causes, increased from 15th in 1990 to 10th in 2019. Globally, the ASDR in males was higher than that in females. CKD-related deaths mainly occurred in those aged over 50 years, especially in regions with higher SDIs. The ASDR was negatively related to SDI (ρ=−0.603, p<0.0001). Among risk factors, metabolic risk factors, especially systolic blood pressure, fasting plasma glucose and body mass index, were the main contributors to CKD-related deaths. Although the high-temperature-related death burden was low, the trend increased sharply in lower SDI regions.
Conclusions
CKD-related deaths continue to increase, with the majority occurring in elderly adults. The CKD-related death burden is higher in males than in females. Additionally, the increasing high-temperature-related death burdens in lower SDI regions should receive social attention.
Keywords: public health, epidemiology, chronic renal failure
Strengths and limitations of this study.
This study evaluated the disease burden changing trends of chronic kidney disease (CKD) over the past three decades.
The disease burden changing trends and the relative risk factors of CKD were quantified to reveal the extent of the epidemic and suggest a framework for policy discussions, resource allocation and research priorities.
Data on the impact of metabolic factors other than blood pressure and blood glucose, such as triglycerides, cholesterol or uric acid, on the global burden of CKD are lacking.
Introduction
Chronic kidney disease (CKD) refers to chronic renal dysfunction caused by various factors for more than 3 months.1 2 According to relevant statistics, there were an estimated 697.5 million cases of CKD worldwide in 2017.1 The prevalence of CKD has increased with the ageing of the population and has become a global public health problem.3 The incidence rate increased by 29.3% compared with that in 1990.1 Once diagnosed with CKD, the kidney function would gradually decline until the end-stage renal disease or even death, with little chance of reversal, imposing a heavy economic burden on society.
Studies have found that the sociodemographic index (SDI), human development level, economic situation, and health access and quality index are closely related to the CKD incidence.4–7 Complex influencing factors make the diagnosis and treatment of CKD difficult, which seriously increases the public health burden.8 9 Although several studies on CKD have been conducted,1 10 detailed death burden analyses of recent three-decade period are scarce and need to be further studied.
In the context of current global Big Data, deep quantitative analyses of CKD morbidity and mortality and their influencing factors are crucial in guiding decisions by policymakers and healthcare providers. However, the details of CKD-related mortality have been poorly studied worldwide. Considering these factors, we carried out research on the global CKD-related death burden and related risk factors from 1990 to 2019. We also analysed the numbers of deaths caused by CKD and the age-standardised death rates (ASDRs) by time period, region, age, risk factors and sex.
Materials and methods
Study data
Data analysed in the present study were obtained from the online database the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool), which were based on literature studies, survey data, surveillance data, medical records, health insurance claims and statistical model analysis.11 The database provides epidemiological data about 369 diseases and 87 attributable risk factors in 204 countries and territories from 1 January 1990 to 31 December, 2019, disaggregated by time, location and sex and is constantly updated by ongoing multinational cooperative organisations.12 13 Detailed research methods followed the Global Burden of Disease (GBD) Study 2019.12 13 The SDI, based on fertility, income per capita and educational attainment, was used as a composite indicator of the degree of health-related development at the national or regional level. The SDI value ranges from 0 to 1, and all the countries were classified into five SDI regions: low-SDI, low-middle-SDI, middle-SDI, high-middle-SDI and high-SDI regions, according to the SDI quintiles. Regions with a lower value indicated a lower degree of development associated with health outcomes. In addition, countries were divided into 21 regions according to geographical location. Age-standardised rates (ASRs) based on the updated standard population were also available in the GBD 2019, allowing the analysis of CKD according to age structure and population size.
Statistical analysis
Numbers and ASDRs, and associated 95% uncertainty intervals (UIs) provided by GBD 2019, were analysed to quantify the CKD-related death burden attributed to risk factors. Ninety-five per cent UI values fell between the 2.5th and 97.5th percentiles of 1000 samples that were extracted from the posterior distribution in the modelling process.14 The time trend of an ASR over a specified time period was represented by the estimated annual percentage change (EAPC), which detailed calculation method has been reported in many previously published GBD studies.15 16 In brief, the natural logarithm of the ASR is assumed to be linear over time; therefore, ln(ASR) = α + β × (calendar year) + error term. The EAPC=100 × (exp(β) − 1), and the 95% CI can be obtained from the linear regression model. To assess the trends of data over time from 1990 to 2019, Joinpoint trend analyses were performed by fitting several different line segments on a logarithmic scale with the simplest model. Significant changes were identified by permutation tests and estimated by the Monte Carlo method. A p value <0.05 was used to denote statistical significance.
All statistical and graphical analyses were carried out using R V.4.1.2 (Vienna, Austria), Joinpoint software V.4.9.0.1 (National Cancer Institute, USA) and GraphPad Prism V.8 (California, USA).
Patient and public involvement
Patients or the public were not involved in this study.
Results
Global burden of CKD
Changing trend of global CKD-related burden from 1990 to 2019
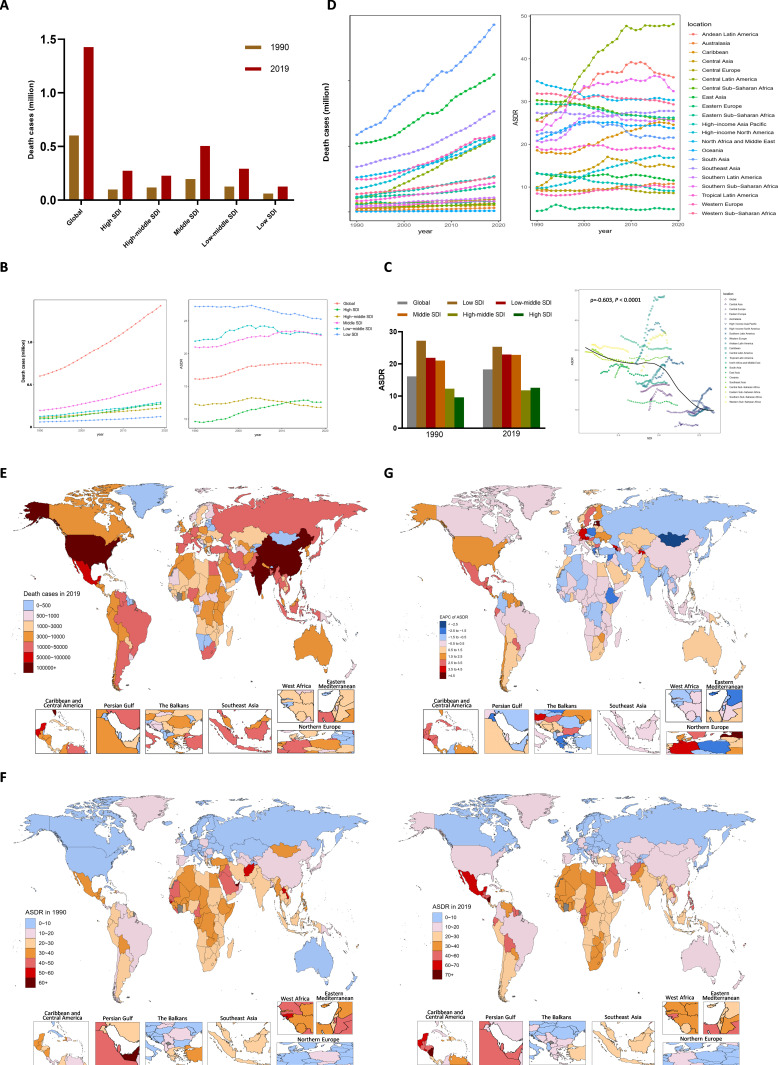
Globally, there were an estimated 697.3 million CKD cases and 19.0 million incident cases in 2019, both of which were more than twice those in 1990 (online supplemental table 1 and 2 and figure 1A, B). The number of deaths caused by CKD doubled from 0.6 million (95% UI: 0.57–0.63 million) in 1990 to 1.4 million (95% UI: 1.31–1.52 million) in 2019 (table 1; figure 1A). Among all the causes of death, the percentage of deaths due to CKD doubled from 1.3% to 2.5%. The numbers of deaths caused by CKD increased from 19th in 1990 to 11th in 2019 (online supplemental figure 2A), and the ASDR increased from 15th to 10th (online supplemental figure 2B). Global disability-adjusted life-years (DALYs), consisted of years of life lost prematurely (YLLs) and years lived with disability (YLDs), also doubled from 21.5 million in 1990 to 41.5 million in 2019 (online supplemental table 3 and figure 1C). During this period, the age-standardised incidence rate (ASIR) increased over time, with an EAPC of 3.2 (95% CI: 3.11 to 3.19) (online supplemental table 2 and figure 3). The ASDR, DALYs, YLLs and YLDs showed upward trends in the first half of the study period but downward trends in the latter years according to the Joinpoint analyses (figure 1B right, online supplemental figure 3). These trends were consistent in both the male and female (online supplemental figure 3).
Table 1.
Death cases and ASDRs of chronic kidney disease in 1990 and 2019, and the temporal trends from 1990 to 2019
| Characteristics | 1990 | 2019 | 1990–2019 | ||
| Death cases (×103) (95% UI) | ASDR per 100 000 population (95% UI) | Death cases (×103) (95% UI) | ASDR per 100 000 population (95% UI) | EAPC of ASDR (95% CI) | |
| Global | 601.31 (567.6–633.79) | 16.14 (15.13–17.13) | 1427.23 (1313.73–1524.55) | 18.29 (16.72–19.55) | 0.55 (0.46 to 0.64) |
| Gender | |||||
| Males | 313.94 (293.38–338.57) | 19.55 (18.19–21.18) | 741.38 (685.84–801.7) | 21.59 (19.93–23.27) | 0.55 (0.46 to 0.64) |
| Females | 287.37 (268.8–305.44) | 13.89 (12.91–14.79) | 685.85 (614.92–741.57) | 15.8 (14.17–17.09) | 0.55 (0.46 to 0.64) |
| SDI regions | |||||
| Low | 62.02 (55.89–68.54) | 27.21 (24.08–30.65) | 125.07 (112.78–137.96) | 25.28 (22.9–27.68) | −0.27 (−0.31 to -0.22) |
| Low-middle | 126.51 (114.96–138.84) | 21.9 (19.73–24.25) | 293.49 (265.9–320.13) | 22.95 (20.77–25.04) | 0.14 (0.01 to 0.26) |
| Middle | 196.59 (183.72–209.89) | 21.01 (19.54–22.84) | 506.13 (464.49–547.47) | 22.8 (20.79–24.75) | 0.43 (0.36 to 0.5) |
| High-middle | 116.33 (109.59–124.18) | 12.25 (11.42–13.2) | 227.99 (207.02–244.66) | 11.78 (10.65–12.65) | −0.08 (−0.22 to 0.06) |
| High | 99.47 (92.56–103.08) | 9.61 (8.92–9.98) | 273.59 (236.92–294.76) | 12.58 (11.09–13.47) | 1.21 (1.09 to 1.32) |
| 21 GBD regions | |||||
| Central Asia | 5.04 (4.73–5.75) | 10.01 (9.33–11.63) | 10.38 (9.43–11.49) | 14.75 (13.48–16.17) | 1.11 (0.73 to 1.49) |
| Central Europe | 13.14 (12.7–13.56) | 9.62 (9.22–9.93) | 18.76 (16.33–21.22) | 8.61 (7.5–9.74) | −0.23 (−0.36 to −0.11) |
| Eastern Europe | 11.06 (10.74–11.35) | 4.41 (4.25–4.53) | 15.93 (14.23–17.56) | 4.85 (4.34–5.34) | −0.16 (−0.42 to 0.09) |
| Australasia | 2.02 (1.85–2.14) | 9.27 (8.39–9.87) | 6.12 (5.14–6.85) | 10.77 (9.17–12) | 0.7 (0.62 to 0.78) |
| High-income Asia Pacific | 22.94 (21.27–23.86) | 13.12 (12.01–13.73) | 54.14 (42.82–60.58) | 9.13 (7.54–10.06) | −1.35 (−1.43 to −1.28) |
| High-income North America | 35.75 (32.89–37.2) | 9.79 (9.03–10.18) | 114.46 (102.38–122.48) | 16.88 (15.26–17.98) | 2.26 (2.07 to 2.45) |
| Southern Latin America | 8.99 (8.51–9.42) | 20.79 (19.61–21.83) | 21.69 (19.81–23.33) | 25.45 (23.27–27.34) | 0.72 (0.43 to 1.02) |
| Western Europe | 49.78 (46.2–51.89) | 8.49 (7.86–8.87) | 116.66 (99.12–126.16) | 9.97 (8.58–10.73) | 0.98 (0.86 to 1.1) |
| Andean Latin America | 5.24 (4.78–5.74) | 25.51 (23.13–28.1) | 19.38 (16.14–22.96) | 35.68 (29.73–42.21) | 1.37 (1.07 to 1.67) |
| Caribbean | 4.79 (4.47–5.28) | 18.59 (17.28–20.52) | 12.66 (10.85–14.78) | 24.58 (21.08–28.71) | 1.44 (1.31 to 1.58) |
| Central Latin America | 20.7 (19.77–21.36) | 25.77 (24.22–26.76) | 111.71 (98.57–125.86) | 48.11 (42.52–54.16) | 2.42 (2.09 to 2.74) |
| Tropical Latin America | 16.59 (15.87–17.14) | 19.36 (18.16–20.14) | 44.22 (40.46–46.73) | 18.96 (17.25–20.06) | 0.01 (−0.06 to 0.09) |
| North Africa and Middle East | 52.6 (47.64–61.84) | 34.74 (30.67–44.08) | 111.81 (96.42–130.85) | 30.37 (26.34–35.39) | −0.43 (−0.51 to −0.34) |
| South Asia | 117.5 (100.02–134.35) | 22.21 (18.57–25.75) | 285.34 (248.21–324.6) | 21.56 (18.82–24.36) | −0.3 (−0.51 to −0.08) |
| East Asia | 104.32 (92.79–116.88) | 13.28 (11.9–14.75) | 209.29 (179.98–237.7) | 11.55 (9.99–13.05) | −0.21 (−0.32 to −0.1) |
| Oceania | 0.62 (0.53–0.72) | 20.82 (18.22–23.98) | 1.6 (1.32–1.93) | 23.8 (20.26–27.96) | 0.31 (0.12 to 0.49) |
| Southeast Asia | 68.7 (63.3–74.86) | 27.38 (24.96–30.14) | 153.37 (137.32–169.64) | 27.79 (24.63–30.68) | 0.06 (0 to 0.12) |
| Central Sub-Saharan Africa | 6.45 (5.47–7.54) | 30.32 (25.81–34.98) | 12.36 (9.7–15.19) | 25.87 (19.78–31.73) | −0.64 (−0.68 to −0.61) |
| Eastern Sub-Saharan Africa | 21.64 (19.01–24.17) | 29.43 (25.56–33.33) | 38.6 (34.23–43.26) | 26.24 (23.43–29.23) | −0.5 (−0.55 to −0.46) |
| Southern Sub-Saharan Africa | 6.18 (5.58–7.05) | 23.04 (20.68–26.41) | 16.27 (14.95–17.65) | 32.44 (29.77–35.1) | 1.31 (0.95 to 1.66) |
| Western Sub-Saharan Africa | 27.27 (23.36–32.18) | 31.88 (27.56–37.76) | 52.47 (44.11–61.28) | 29.35 (25.21–33.71) | −0.21 (−0.25 to −0.17) |
ASDR, age-standardised death rate; EAPC, estimated annual percentage change; GBD, Global Burden of Disease; SDI, sociodemographic index; UI, uncertainty interval.
Figure 1.
Global death cases and death rate of CKD from 1990 to 2019. (A) Death cases in global and SDI areas in 1990 and 2019. (B) Death cases and ASDRs in global and SDI areas from 1990 to 2019. (C) Relationship between ASDRs and SDI from 1990 to 2019. (D) Death cases and ASDRs in 21 geographic regions from 1990 to 2019. (E) Death cases in 204 countries and territories in 2019. (F) The ASDRs in 204 countries and territories in 1990 and 2019. (G) The EAPCs of ASDRs in 204 countries and territories from 1990 to 2019. ASDR, age-standardised death rate (per 100 000 population); CKD, chronic kidney disease; EAPCs, estimated annual percentage change; SDI, sociodemographic index.
bmjopen-2022-064540supp001.pdf (4.6MB, pdf)
Changing trend of CKD-related death burden in each SDI region
Of the total DALYs caused by CKD, YLLs accounted for 78.9%, while YLDs accounted for 21.1% in 2019. Therefore, we focused subsequent analyses mainly on the CKD-related death burden. During the three decades, all five SDI regions showed increases in the numbers of deaths over time (figure 1B, left). Among them, the middle-SDI region showed the highest death numbers, reaching 0.5 million, and the low-SDI region had the lowest, with 0.1 million, in 2019 (figure 1A, B left). The ASDR in the high-SDI region increased dramatically, with an EAPC of 1.2 (95% CI: 1.09 to 1.32), and exceeded that of the high-middle-SDI from 2011 onward (figure 1B, right). In contrast, the low-SDI region still had the highest ASDR in 2019, at 25.3 per 100 000 population (95% UI: 22.90–27.68), although it had the most significant declining trend (EAPC: −0.27, 95% CI: −0.31 to −0.22) (figure 1B, right). The ASDR was negatively correlated with SDI level worldwide (ρ=−0.603, p<0.0001, figure 1C), while the ASIR was positively correlated with SDI level (ρ=0.54, p<0.0001, online supplemental figure 4).
The ASDR increased in most regions and countries
At the regional level, death numbers in all 21 regions and the ASDRs in most regions showed increasing trends during this period (figure 1D). At the country level, CKD-related deaths increased by more than 500% in the United Arab Emirates, Venezuela, Estonia, Armenia, El Salvador and Ecuador over the three-decade study period (online supplemental table 4). India (0.22 (95% UI: 0.19–0.26) million) surpassed inland China (0.2 (95% UI: 0.17–0.23) million) to be the country with largest number of CKD-related deaths in 2019. The number of deaths in the USA (0.11 (95% UI: 0.10–0.11) million) was second to only those in India and China. The total number of deaths for these three countries accounted for 36.9% of the worldwide number of deaths in 2019 (figure 1E, online supplemental figure 5). Nicaragua had the largest ASDR, at 80.2 (95% UI: 69.57–83.24) per 100 000 population (figure 1F, online supplemental table 4). Estonia showed the fastest increase in the ASDR with an EAPC of 5.4 (95% CI: 4.91 to 5.81) during this period (figure 1G, online supplemental table 4). While Mongolia had the fastest decrease in the ASDR of CKD (EAPC: −3.6, 95% UI: −4.07 to −3.12) (figure 1G, online supplemental table 4).
The CKD-related death burden in males was higher than that in females in relatively high-SDI regions
The CKD-related death burden was unbalanced between sexes at the SDI level
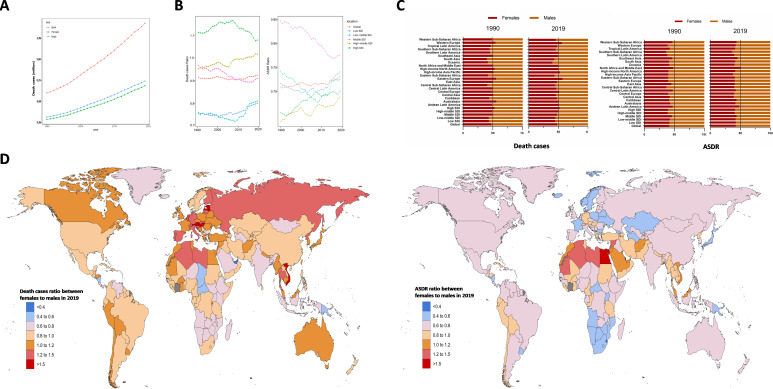
Generally, the number of CKD-related deaths was larger in males than in females over the past three decades (figure 2A), both of which showed synchronous upward trends, with the ratio of female to male deaths changing from 0.915 in 1990 to 0.925 in 2019 (figure 2B). In the high-SDI region, the ratio remained above 1 during this period. In 2019, the high-SDI and high-middle-SDI regions had higher female death burdens, with ratios of female deaths to male deaths of 1.1 and 1.0, respectively, while the ratios in low-SDI and low-middle-SDI regions were 0.802 and 0.812, which were the lowest (figure 2B, left). Similar to the number of deaths, the CKD-related ASDR in males was higher than that in females over this period. In 2019, the female to male ASDR ratios ranged from 0.6 to 0.8 in all the SDI regions (figure 2B right), suggesting that the CKD-related death burden was unbalanced between sexes.
Figure 2.
Sex differences and trends of CKD deaths and ASDR from 1990 to 2019. (A) The ASDRs in males and females globally from 1990 to 2019. (B) Death cases ratio between females to males (left) and ASDR ratio between females to males (right), in global and SDI areas in 1990 and 2019. (C) Gender composition of death cases and ASDR in global and SDI areas in 1990 and 2019. (D) Death cases ratio between females to males (left) and ASDR ratio between females to males (right) in 204 countries and territories in 2019. ASDR, age-standardised death rate (per 100 000 population); CKD, chronic kidney disease; SDI, sociodemographic index.
The ASDR in males increased in most regions and countries
At the regional level, the female to male deaths ratios in Eastern Europe was the highest (1.3), and that in Oceania was the lowest (0.7) in 2019. Death cases of both sexes were mainly concentrated in Asia. Both male and female deaths in Latin America had the fastest increase over time. In contrast, female death numbers in Eastern Europe and male death numbers in Central Europe had the slowest increases (figure 2C, online supplemental table 5). Unlike the number of deaths, the female to male ASDR ratios in most regions were less than 1.0, except in North Africa and Middle East (1.0). The ratio in Southern sub-Saharan Africa was the lowest, at 0.6 (online supplemental table 5).
At the country level, the numbers of male deaths in almost all the countries showed increasing trends, except in Slovakia and Poland. The death ratios in Estonia, Hungary, Latvia, Austria and Vietnam were over 1.5, which suggested that the CKD-related death burden was much higher in females than in males in these countries. In contrast, the ratio was less than 0.5 in Papua New Guinea and the United Arab Emirates, indicating a higher death burden in males (figure 2D, online supplemental table 6). The ASDRs in both females and males in Mongolia showed the largest decreases, with decreases of 43.2% in females and 57.1% in males.
The age-related CKD-related death burden is more serious in high-SDI regions
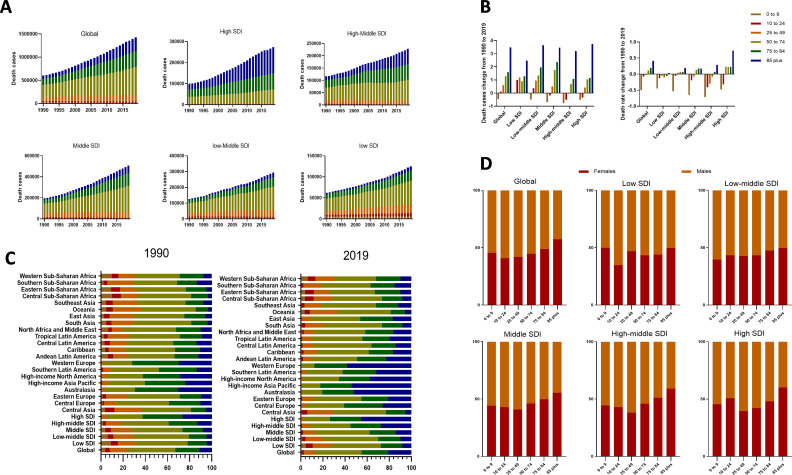
The numbers of CKD-related death increased with age in 2019
In 2019, global CKD-related deaths were mainly distributed in middle-aged and elderly people over 50 years old, specifically 50–74 years old, who accounted for 41.9% (figure 3A). Those aged 50–74 years had the largest death numbers in all the SDI regions except high-SDI region. In high-SDI region, the numbers of CKD-related death were increased with age, with the largest in 85 plus age group. The numbers of deaths in the 85 plus age group had the greatest increases in all SDI regions (figure 3A). Those aged 0–9 years showed decreases in the numbers of deaths in all the SDI regions except the low-SDI region. Those aged 10–24 years had increased numbers of deaths in the low- and low-middle-SDI regions (figure 3B). Therefore, premature deaths were mainly concentrated in the relatively lower SDI regions in 2019.
Figure 3.
The distribution of CKD death cases and rates in age groups, SDI areas and 21 geographic regions from 1990 to 2019. (A) Death cases in age groups in global and SDI areas from 1990 to 2019. (B) The change percentage of death cases (right) and rate (left) in age groups from 1990 to 2019. (C) Six age groups as percentages of total deaths in global, SDI areas and 21 geographic regions in 1990 and 2019. (D) Gender composition of death in global and SDI areas in 2019. CKD, chronic kidney disease; SDI, sociodemographic index.
The changing trend of CKD-related death burden from 1990 to 2019 in each SDI regions
During this period, the age distribution of CKD-related deaths in the relatively young population, including the 0–9, 10–24 and 25–49, and 50–74 age groups, showed a declining trend (figure 3C). In contrast, deaths in elderly individuals, particularly those aged 85 years and older, showed an upward trend. Central Asia was the only region that saw a decline composition of 85 plus age group (figure 3C). As to SDI level, the proportion of deaths the 85 plus age group showed an upward trend with increasing SDI level, with the highest value of 58.2% in the Western Europe region in 2019. Eastern sub-Saharan Africa and Western sub-Saharan Africa, with lower SDI levels, had the highest proportions of the 10–24 and 0–9 age groups, respectively. The high-income Asia Pacific region had the largest increase in the proportion among the 85 plus age group and the largest decrease among the 50–74 age group (figure 3C). These results revealed that the CKD-related death burden gradually shifted to elderly individuals in higher SDI regions. In addition, the proportion of females increased with increasing age was found in the further analysis of sex compositions among different age groups (figure 3D).
CKD burden attributable to risk factors
The main risk factors for death from CKD
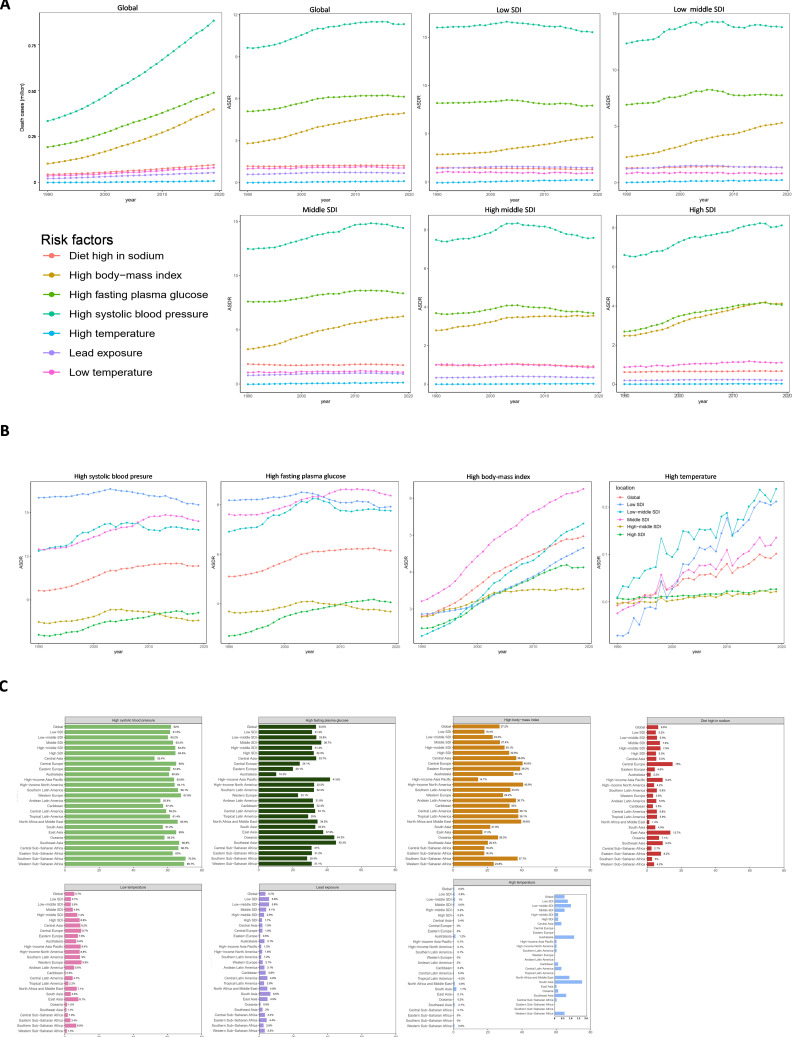
Globally, metabolic disorders (such as diabetes, obesity and hypertension) were the main risk factors for death from CKD. Over this period, the numbers of deaths due to CKD attributable to high systolic blood pressure (SBP), high fasting plasma glucose (FPG) and high body mass index (BMI) sharply increased (figure 4A), which were the main risk factors contributing to the ASDR of CKD, accounting for 62.0%, 33.6% and 27.2%, respectively in 2019. Even though the ASDR of CKD attributable to high BMI had the largest increase, at 77.1%, compared with 17.6% for high SBP and 20.4% for high FPG, the ranks of these risk factors did not change (figure 4A).
Figure 4.
Predominant contribution of risk factors to CKD-related deaths in global and SDI areas. (A) Death cases and ASDRs attributed to main risk factors by SDI region from 1990 to 2019. (B) The ASDRs trends in SDI regions attributed to each main risk factor. (C) Proportions of deaths in global and SDI regions attributed to each risk factors in 2019. ASDR, age-standardised death rate (per 100 000 population); CKD, chronic kidney disease; SDI, sociodemographic index.
The high SBP-attributed ASDRs in all five SDI regions
CKD attributable to high SBP was associated with the highest ASDRs in all the SDI regions over this period (figure 4A). In 2019, high SBP-attributed death rate in the low-SDI region was higher than in other SDI regions (figure 4B), even though it showed the greatest reduction during the 30-year study period (decrease of 3.0%). The high SBP-attributed death rate in the high-SDI region saw the fastest increase (figure 4B). In 2019, ASDR attributed to high SBP in the high-middle-SDI region was the lowest. At the regional level, the rank of the high SBP-attributed ASDR in Central Latin America increased from sixth in 1990 to first among all 21 regions in 2019, with the greatest increase.
Other metabolic disorders factors for CKD-related ASDR in each SDI regions
The highest FPG-attributed ASDRs were mainly concentrated in the low-middle and low-SDI regions during this period (figure 4B). In 2019, high FPG was the second most prevalent risk factor, followed by high BMI, in all regions except the high-SDI region (figure 4A–C). In contrast, BMI was ranked as the second most prevalent contributor, exceeding FPG, to the ASDR in the high-SDI region in 2019, even though the FPG-attributed death rate in this region showed a larger increase than those in other regions (figure 4B, C). In 2019, the FPG-attributed ASDR in the middle-SDI region was higher than those in the other SDI regions, while that in the high-middle-SDI region was the lowest (figure 4B). The BMI-attributed ASDR in the middle-SDI was the highest, while in the high-middle SDI, it was the lowest. It is worth noting that the BMI-attributed ASDRs in all five SDI regions had steeply increasing trends over this period (figure 4B). A diet high in sodium was the fourth-ranked contributor to the CKD ASDR in the high-middle-SDI, middle-SDI and low-middle-SDI regions and the fifth-ranked contributor in the high- and low-SDI regions (figure 4A).
The changing trends of ASDRs caused by high or low temperature in different areas
Notably, although the high temperature-related death burden was low (figure 4C), the high temperature-attributed ASDRs increased steeply in the low-middle-SDI and low-SDI regions over this period (figure 4B). These regions had the highest high temperature-attributed ASDRs in 2019 (figure 4C). In sharp contrast, the high temperature-attributed ASDRs in the high-middle-SDI and high-SDI regions decreased by 7.6 and 6.6 times, respectively, compared with those in 1990 (figure 4B). The high-middle-SDI region had the lowest high temperature-related death rate in 2019. At the regional level, East Asia, High-income North America, South Asia and Western Europe showed the fastest increases in the high temperature-attributed ASDRs, and South Asia had the highest high temperature-attributed death rate in 2019. Central Latin America and Southeast Asia had the fastest decreases in the high temperature-attributed ASDR. Tropical Latin America, Southern sub-Saharan Africa and Eastern sub-Saharan Africa had the lowest values. In addition, the risk factors of low temperature and lead exposure were much larger than those of high temperature, and low temperature exposure was the first of the three in high- and high-middle-SDI regions (figure 4A).
Discussion
Despite advancements in scientific research that have led to a better understanding of the pathogenesis of diseases and significant improvements in disease management, no significant improvement was observed in the burden of CKD-related deaths, in contrast with other non-communicable diseases. CKD is characterised by a gradual decline in kidney function that eventually results in end-stage renal disease, with little chance of reversal. CKD is therefore an important cause of morbidity and mortality among non-communicable diseases.17
Our results showed significant regional variations in the CKD-related death burdens. The ASDR of CKD was associated with SDI, with higher CKD ASDRs in lower SDI regions. This may be related to a lack of effective health interventions caused by inadequate healthcare funding and weak health systems in lower SDI regions.18 If these trends continue, differences in the CKD death burden among regions will increase further. In terms of geographical regions and countries, the CKD death burden in Mesoamerica (especially Nicaragua, El Salvador and Mexico) was more serious than those in other geographical locations. This may be due to the megathermal climate or the rates of diabetes and hypertension, which are major risk factors globally.19 A recent study revealed that agricultural workers who currently or used to work in the sugarcane industry may be at high-risk of CKD.19 20 In addition, climate change in this region may be a risk factor for CKD-related death.21 These findings call for increased government planning and interventions and the development of new and more appropriate solutions to address the CKD death burden in this area.
Globally, the number of CKD-related deaths and ASDR were larger and higher in males than in females, although the difference appearing to be slightly decreasing trend recent years. It is worth noting that the sex ratio of deaths in the high-SDI region remained above 1 during the study period, but the ASDR ratio did not, which may be attributed to the increase in the population and the ageing of the population in this region, especially among females. In contrast, the sex ratio of the ASDR was higher than the ratio of deaths in several countries in the Middle East, such as Egypt (1.60 vs 1.13 in Egypt). This result coincided with the lower disability-free life expectancy among females total lifetime in this region.22
Over the past 30 years, the age distribution of CKD-related deaths showed a gradual increasing trend with age, and people in the middle-aged and elderly groups (>50 years old) accounted for the main proportion of CKD deaths. This trend may be closely related to improvements in healthcare as well as population ageing worldwide. However, in regions with relatively low SDIs, such as Africa, premature deaths are still prevalent, with increasing numbers, which may be explained by the low levels of medical and healthcare in these regions. Patients diagnosed with CKD may not receive timely and adequate medical care to slow the progression of renal deterioration, resulting in premature death, which reflects the impact of socioeconomic development on premature CKD-related deaths.23 Therefore, policymakers and the healthcare sector in these regions should implement urgent measures to reverse this negative trend.
The most cost-effective strategy to reduce the number of CKD-related deaths is preventing the onset of CKD.24 Therefore, screening high-risk groups and developing interventions targeting a variety of risk factors are the most effective approaches.24 25 High SBP, FPG and BMI were the main risk factors for CKD-related death.1 7 26 Hence, implementing measures to improve blood pressure, plasma glucose and body weight is critical for reducing the CKD burden.27
Although the CKD-related death burden attributed to high temperature exposure was low, the rapid upward trend, especially in the low-SDI and low-middle-SDI regions, should be noted. In contrast, this burden in the high-SDI and high-middle-SDI regions tended to remain stable or even decreased. Some studies recently reported large numbers of young sugarcane workers with end-stage of CKD, who did not have any known risk factors for CKD, such as high blood pressure or diabetes before.19 Subsequent reports found that CKD of unknown origin also occurred in people who worked in agriculture or construction in other low-socioeconomic areas.28 It has already been the leading cause of hospitalisation and death among patients with CKD in Central America.29 Even though the exact pathogenesis of CKD in these individuals is currently unknown, it is undoubtedly related to heat exposure and dehydration caused by rapidly changing environmental conditions.28 30 31 Compared with the higher SDI regions, the ability to provide healthcare and adapt to climate change in relative lower regions were limited; therefore, the situation is severely affected by high temperature exposure and is getting worse.31 Local governments should formulate effective measures to cope with the high-temperature environment and formulate strategies to mitigate climate change. Moreover, cooperation among clinicians, public health personnel, government agencies and medical researchers should be promoted to raise public awareness of CKD, actively explore the specific mechanism of the influence of high temperature exposure on CKD and minimise the adverse effects of climate change on kidney health.
Additionally, it has been shown that the association between cold exposure promoting CKD-related hospital visits is stronger than at moderate and high temperatures.32 And acute cold exposure can lead to a significant increase in arterial blood pressure in patients with or without comorbid CKD.33 Therefore, avoiding acute cold exposure and enhancing warmth measures in cold exposure are particularly important. Environmental lead exposure increases the risk of CKD.34 35 Due to the prevalence of lead in the environment, human exposure to this metal is almost inevitable. Therefore, public measures to reduce environmental contamination and reduce the food chain transfer of lead are essential, as are measures to reduce risk by setting maximum allowable concentrations of lead in staple foods at the lowest achievable levels.
Limitations
The GBD database provides extensive data and estimates of risk factors for the global burden of CKD, but there are some limitations, which have been detailed in many previously published studies.13 First, more detailed information about the included countries and regions could not be obtained from the GBD database. In some countries with large territory spans or with multinational populations, the impact of regional and ethnic differences within countries on the global burden of CKD could not be further analysed. In addition, the CKD burden may be underestimated in less developed countries and areas due to limited access to healthcare. Moreover, data on the impact of metabolic factors other than blood pressure and blood glucose, such as triglycerides, cholesterol or uric acid, on the global burden of CKD are lacking.
Conclusions
In summary, CKD remains a critical global public health concern. CKD-related deaths continue to increase, with the majority now occurring in elderly adults, mainly due to the increase in and the ageing of the global population. However, the numbers of premature deaths in regions with low-SDI levels remain high, and the trend is increasing. In addition, the burden of CKD-related deaths is more severe in males than in females, though this difference is likely to narrow over time. Hypertension, diabetes and obesity remain critical risk factors for CKD. Moreover, in the low-SDI region, the rapidly increasing number of CKD-related deaths attributed to high temperature exposure should not be ignored, and national-level measures should be taken to reverse this trend.
Supplementary Material
Acknowledgments
Authors appreciate all the collaborators involved in the GBD study 2019.
Footnotes
Contributors: Idea and conceptual design: YL and XS; Data acquisition: ZC and NH; Literature retrieval and data collation: XL and XF; Data analysis: ZC and JL; Visualisation: XL and YL; Preparation of the original draft: ZC and NH; Manuscript editing and revising: YL, XF and XS. Authors confirm that all of them have full access to all the data of the present study and are responsible for submitting for publication. YL is responsible for the overall content as guarantor, who accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 82000755) and the Nature Science Foundation of Shandong Province (Grant No. ZR2020QH086).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available in a public, open access repository. Data used from the online database the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hockham C, Schanschieff F, Woodward M. Sex differences in CKD-associated mortality from 1990 to 2019: data from the global burden of disease study. Kidney Med 2022;4:100535. 10.1016/j.xkme.2022.100535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives-a position statement from kidney disease improving global outcomes. Kidney Int 2007;72:247–59. 10.1038/sj.ki.5002343 [DOI] [PubMed] [Google Scholar]
- 4.Dai H, Alsalhe TA, Chalghaf N, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: an analysis of the global burden of disease study. PLoS Med 2020;17:e1003198. 10.1371/journal.pmed.1003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovács N, Nagy A, Dombrádi V, et al. Inequalities in the global burden of chronic kidney disease due to type 2 diabetes mellitus: an analysis of trends from 1990 to 2019. Int J Environ Res Public Health 2021;18:4723. 10.3390/ijerph18094723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JR, Johnston JC, Ronald LA, et al. Screening for latent tuberculosis infection in migrants with CKD: a cost-effectiveness analysis. Am J Kidney Dis 2019;73:39–50. 10.1053/j.ajkd.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Zou M, Young CA, et al. Disease burden of chronic kidney disease due to hypertension from 1990 to 2019: a global analysis. Front Med (Lausanne) 2021;8:690487. 10.3389/fmed.2021.690487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y, Bowe B, Mokdad AH, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–81. 10.1016/j.kint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Norhammar A, Bodegard J, Eriksson JW, et al. Cost of healthcare utilization associated with incident cardiovascular and renal disease in individuals with type 2 diabetes: a multinational, observational study across 12 countries. Diabetes Obes Metab 2022;24:1277–87. 10.1111/dom.14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo C, Borrelli S, Provenzano M, et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients 2018;10:732. 10.3390/nu10060732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaboration GCKD . Data from the online database the global health data exchange (ghdx) query tool. Global Health Data Exchange; 2019. Available: http://ghdx.healthdata.org/gbd-results-tool [Google Scholar]
- 12.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1223–49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2019 Demographics Collaborators . Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet 2020;396:1160–203. 10.1016/S0140-6736(20)30977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol 2019;70:674–83. 10.1016/j.jhep.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Hu M, Liu H, et al. Global burden of disease study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab 2021;33:1943–56. 10.1016/j.cmet.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Stanifer JW, Von Isenburg M, Chertow GM, et al. Chronic kidney disease care models in low- and middle-income countries: a systematic review. BMJ Glob Health 2018;3:e000728. 10.1136/bmjgh-2018-000728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ameh OI, Ekrikpo UE, Kengne AP. Preventing CKD in low- and middle-income countries: a call for urgent action. Kidney Int Rep 2020;5:255–62. 10.1016/j.ekir.2019.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson R, Leatherman S, Fiore M, et al. Prevalence and risk factors for CKD in the general population of southwestern Nicaragua. J Am Soc Nephrol 2020;31:1585–93. 10.1681/ASN.2019050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson E, Mansourian A, Farnaghi M, et al. An ecological study of chronic kidney disease in five Mesoamerican countries: associations with crop and heat. BMC Public Health 2021;21:840. 10.1186/s12889-021-10822-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasai F, Roncal-Jimenez C, Rogers K, et al. Climate change and nephrology. Nephrol Dial Transplant 2023;38:41–8. 10.1093/ndt/gfab258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metwally S. Disability-free life expectancy at old ages in Egypt. J Biosoc Sci 2021;53:290–304. 10.1017/S0021932020000218 [DOI] [PubMed] [Google Scholar]
- 23.Bello AK, Levin A, Tonelli M, et al. Assessment of global kidney health care status. JAMA 2017;317:1864–81. 10.1001/jama.2017.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 2014;63:789–97. 10.1053/j.ajkd.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 25.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration . Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2014;2:634–47. 10.1016/S2213-8587(14)70102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Lu W, Wang A, et al. Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from global burden of disease 2017. J Diabetes Investig 2021;12:346–56. 10.1111/jdi.13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016;4:e307–19. 10.1016/S2214-109X(16)00071-1 [DOI] [PubMed] [Google Scholar]
- 28.Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med 2019;380:1843–52. 10.1056/NEJMra1813869 [DOI] [PubMed] [Google Scholar]
- 29.Sorensen C, Garcia-Trabanino R. A new era of climate medicine-addressing heat-triggered renal disease. N Engl J Med 2019;381:693–6. 10.1056/NEJMp1907859 [DOI] [PubMed] [Google Scholar]
- 30.Harries AD. Chronic kidney disease, tuberculosis and climate change. Int J Tuberc Lung Dis 2020;24:132–3. 10.5588/ijtld.19.0556 [DOI] [PubMed] [Google Scholar]
- 31.Borg MA, Bi P. The impact of climate change on kidney health. Nat Rev Nephrol 2021;17:294–5. 10.1038/s41581-020-00365-4 [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Ye Q, Fang L, et al. Short-term association of NO2 with hospital visits for chronic kidney disease and effect modification by temperature in hefei, china: a time series study. Ecotoxicol Environ Saf 2022;237:113505. 10.1016/j.ecoenv.2022.113505 [DOI] [PubMed] [Google Scholar]
- 33.Masajtis-Zagajewska A, Pawłowicz E, Nowicki M. Effect of short-term cold exposure on central aortic blood pressure in patients with CKD. Nephron 2021;145:20–6. 10.1159/000510365 [DOI] [PubMed] [Google Scholar]
- 34.Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 2009;170:1156–64. 10.1093/aje/kwp248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int 2006;70:2074–84. 10.1038/sj.ki.5001809 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064540supp001.pdf (4.6MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available in a public, open access repository. Data used from the online database the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool).