Abstract
Mammary fibromatosis is a rare neoplastic proliferation of fibroblastic cells. Usually seen in abdominal and extra-abdominal sites, it is rarely seen in the breast. Patients with mammary fibromatosis usually present with a firm palpable mass with or without dimpling and skin retraction–often mimicking breast carcinoma. Here, we present a case of mammary fibromatosis in a 49-year-old woman who presented with a palpable lump in her right breast. Mammography tomosynthesis revealed architectural distortion which was seen on ultrasonography as a hypoechoic area. The patient underwent a wire-guided excision where the histology of this specimen showed irregular spindle cell proliferation with hemosiderin deposition, confirming mammary fibromatosis. Further re-excision of margins revealed no evidence of residual fibromatosis, and the patient underwent subsequent surveillance mammograms to ensure there was no recurrence.
Keywords: Extra-abdominal fibromatosis, Mammary fibromatosis, Desmoid tumor, Spindle cell tumor
Introduction
Breast fibromatosis; although lacking metastasizing potential, is an aggressive neoplastic proliferation of fibroblastic cells. This is seen in the abdominal wall and extra-abdominal sites but is rarely seen in the breast (1). Although breast fibromatosis may arise after surgical trauma or silicone implant insertion, the true etiology of breast fibromatosis remains unclear (2). This benign tumor accounts for 0.2% of all breast tumors, only 4% of which are bilateral and tumor recurrence rates are 18%-29% (3). The gold standard for treatment remains a surgical wide local excision with satisfactory margins. Radiation therapy and medical therapies such as the use of antiestrogen agents have been suggested for patients that are not viable surgical candidates (4). We report the case of a 49-year-old woman who presented with breast fibromatosis to our breast care unit in Letterkenny University Hospital.
Case report
A 49-year-old woman presented to the breast care unit of Letterkenny University Hospital with a palpable lump in her right breast which she had noticed 3 months earlier. Her previous visit to the breast care unit was 2 years prior for 2 separate lumps on her left breast–2-o'clock position, which were fibroadenomas–visible on mammography and ultrasound; confirmed on biopsy. Additionally, the patient also had several cysts bilaterally along with scattered benign calcification.
In the current presentation, clinical examination revealed 2 lumps on her right breast–Lump I was 40 mm in size, 12-o'clock position, 1 cm DPN, and scored S3 clinically. Lump II was 20 mm in size, 3-o'clock position, 2 cm DPN, and scored S3 clinically as well.
The patient's past medical history was significant only for hypothyroidism. The only significant breast cancer history was her paternal aunt who was diagnosed at 20 years and died at 75 years of age. The patient had no significant history of ovarian cancer and had been pregnant 3 times previous, having given birth 3 times as well. Medications that the patient was taking were Levothyroxine and the OCP.
Mammography; including bilateral tomosynthesis revealed dense breast tissue with scattered calcification bilaterally. A new smooth density in the medial right breast inferiority was seen–that wasn't present on her previous mammogram of 2018. In addition to this, an apparent slight architectural distortion in the upper outer left breast was noted that was more prominent than on the previous mammogram. This architectural distortion did not seem to involve either the chest wall or the pectoralis muscle (Fig. 1).
Fig. 1.
Left MLO tomosynthesis mammography revealing architectural distortion in the upper outer left breast.
Further ultrasound study showed multiple cysts bilaterally and a 1cm well-defined hypoechoic lesion on the medial right breast corresponding to the mammographic findings. On the left breast 2 previously biopsies lesions (2018) were shown again as well as multiple simple cysts. Importantly, at approximately 2-o'clock position, there was a 1.2 cm hypoechoic area of architectural distortion.
These sonography findings warranted stereotactic mammographic biopsy of this distortion. This biopsy was performed using a lateral approach and a metallic clip was inserted at the end of the procedure. The position of this clip was confirmed satisfactory using subsequent mammography. 3 samples were obtained. Sample 1 revealed breast tissue with stromal fibrosis, focal hemosiderin deposition, apocrine metaplasia and focal columnar cell change without atypia. Sample 2 revealed breast tissue with ductular structures showing focal mild epithelial hyperplasia set within a cellular stroma with focal sclerosis. This stromal component shows no evidence of cytological atypia or increased mitotic activity but did interdigitate with mature adipose tissue. Section 3 showed sections comprise fragments of breast tissue with stromal fibrosis, and a localized foreign body-type multinucleated giant cell reaction, focal columnar cell change without atypia and focally prominent sclerosing adenosis with benign microcalcification. The histological appearances in specimen B reflected origin in a fibroepithelial lesion with no sinister microscopic features apart from the fatty interdigitation described.
The patient subsequently came back 6 months later for a follow up ultrasound. A left breast ultrasound revealed architectural distortion seen in the upper left breast on mammography - visualized again at the 12-o'clock position. At the center of this was a 1.2 cm oval-shaped hypoechoic lesion (Fig. 2). It was unclear if this represents the FA biopsied in her left breast previously, and in the view of this, a core biopsy of this 1.2 cm lesion was performed.
Fig. 2.
Ultrasound revealing a 1.2 cm hypoechoic lesion.
Sections comprised cores of tissue with focally prominent hemosiderin deposition in association with a cytologically bland spindle cell proliferation as well as separate fragments comprising spindle cell proliferation with focal associated benign–appearing ductal structures. Within the spindle cell proliferation described, there was no evidence of cytological atypia or increased mitotic activity. One final fragment of breast tissue showed features of fibrocystic disease with associated benign calcification. There was no evidence of in-situ or invasive malignancy. The histological appearances on this specimen were most consistent with origin in a fibroepithelial lesion. While there were no sinister macroscopic features within current material apart from relative mild stromal overgrowth, which would facilitate a B2 designation, these findings were considered in the context of previous histological picture which suggested an irregular interface between spindle cells and adjacent fat, and the radiological report of breast parenchymal distortion. Under these circumstances, the possibility of a benign phyllodes tumor could not be excluded and a B3 designation would probably be more appropriate.
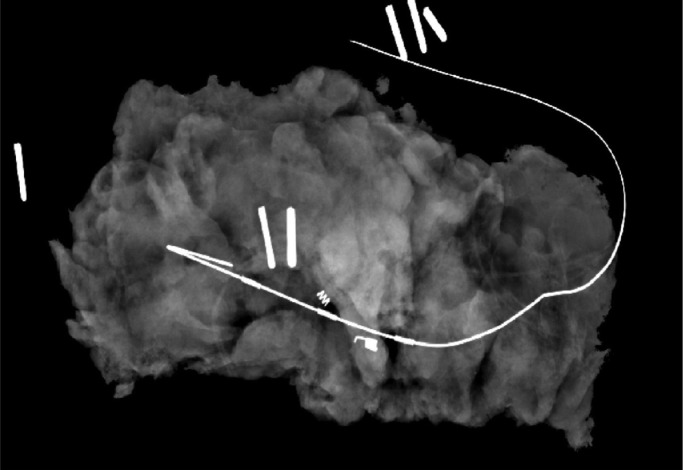
1 month later, the patient was scheduled for a wire-guided excision of this 12 mm lesion. Macroscopically, fibrofatty tissue measuring 7.5 × 4 × 2.5 cm and weighing 28.5 grams was excised. Sectioning demonstrated widespread irregular pale fibrous–type tissue with a well circumscribed nodule measuring 0.7 cm in diameter in proximity to the guidewire (Fig. 3).
Fig. 3.
Wire-guided excision specimen mammography revealing the nodule.
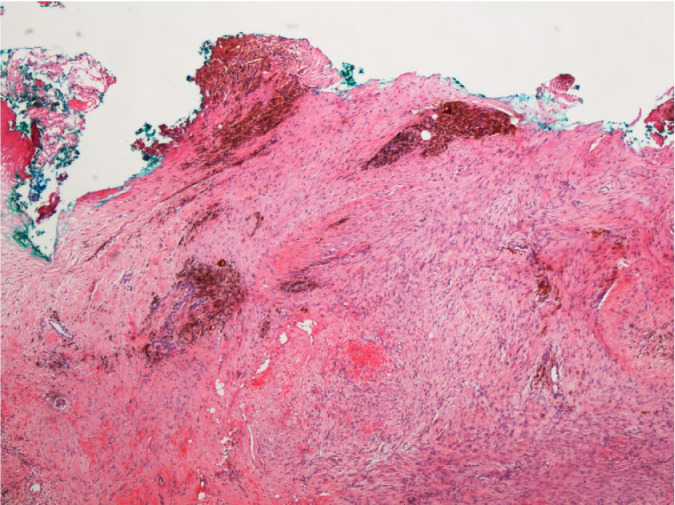
Microscopically, sections comprised breast tissue with an intracanalicular-type FA measuring 7.2 mm in maximum dimension microscopically adjacent to the inferior margin. Elsewhere, the breast tissue demonstrated columnar cell change with prominent adenosis and associated benign microcalcification as well as focal fibrosis, microcyst formation and apocrine metaplasia reflecting fibrocystic disease. A site of radiological clip insertion with surrounding palisading histocytes was situated at the center of an irregular, spindle cell proliferation measuring 14.1mm in maximum dimension microscopically. This proliferation was relatively cellular with focally prominent hemosiderin deposition, and an occasional mitotic figure but no associated cytological atypia (Fig. 4). It surrounded benign epithelial elements demonstrating an irregular interface with breast lobules and with the adjacent adipose tissue, focally extending to the anteroinferior margin of the specimen (Fig. 5). Immunohistochemical techniques demonstrated these spindle cells to be strongly positive for vimentin with negativity for muscle specific actin, S100 protein and pan-cytokeratins. The associated ki-67 proliferation index was 2%.
Fig. 4.
Irregular spindle cell proliferation with hemosiderin deposition.
Fig. 5.
Fibromatosis extending focally to the anteroinferior margin.
The appearances were those of an incidental fibroadenoma with a mammary parenchymal fibromatosis which focally extended to the excision margin of the specimen anteroinferiorly. There was no evidence of in-situ or invasive malignancy but the excision of fibromatoses with a clear margin was recommended to reduce the possibility of local recurrence. A week later, the patient underwent a re-excision of margins where sections were taken from the anteroinferior and inferomedial margin of the left breast lesion along with the skin (anterior margin). They revealed no evidence of residual fibromatosis or malignancy. The patient underwent surveillance mammograms after this procedure.
Discussion
Desmoid type fibromatosis is a rare locally infiltrative soft tissue tumor that lacks metastatic potential. Arising from fibroblasts or myofibroblasts; it is known to occur within the breast parenchyma but is more commonly found arising from the fascia of the pectoralis and extending into the breast [1]. It is usually found in the abdominal wall of women aged 25-35 years of age [5]. Desmoid-fibromatosis accounts for less than 3% of all soft tissue tumors [6] and only 0.2% of all primary breast neoplasms [3].
Although the etiology of breast fibromatosis is unclear; fibromatosis itself is broadly divided into 2 categories. The larger category being somatic mutations in the B-catenin gene; these are responsible for the sporadic cases [7]. The second category is associated with familial adenomatous polyposis (FAP), which is the result of the mutation in the APC gene which too indirectly leads to altered regulation of B-catenin [8]. Few cases have also been reported which are linked to previous trauma, surgery, and breast implant insertion [2].
Breast fibromatosis clinically presents as a mass that may mimic breast carcinoma [9]–it's usually a firm palpable mass and can even present with dimpling and skin retraction [10]. On mammography, these lesions usually appear as an irregular spiculated hyperdense mass and may contain low density in the center. They may also be described as an architectural or stromal distortion that is usually noncalcified with focal thickening of the surrounding skin and blurring of the adjacent subcutaneous tissue [11,12,13].
On ultrasound, mammary fibromatosis is visualized as an irregular shaped hypoechoic mass. The mass is usually poorly defined and heterogeneous is nature with spiculated and indistinct margins. Internal echoes may be present in the center of the mass and posterior acoustic shadowing can be appreciated as well; although there have been cases reported where there is a lack of this posterior sound attenuation [11,12,14].
MRI studies reveal the irregular mass to be isotense to muscle on T1 weighted images. This mass appears with low to high intense on T2 weighted images. The low intense is attributed to the fibromatosis containing dense collagenous tissue, whereas the high intense is attributed to myxoid changes within the tumor. Contrast-enhanced MRI can also be useful in differentiating breast fibromatosis from invasive breast cancers. While invasive breast cancers enhance with contrast administration–they tend to washout rapidly. On the contrary, kinetics of fibromatosis reveals a persistent enhancement pattern. Additionally, MRI can be key to determine the extent of pectoralis muscle involvement, especially in posterior tumors in the breast [11,15,16].
Microscopically, spindle cell proliferation containing uniform collagen deposition which has a keloidal appearance is characteristic of fibromatosis. Tumors may display tendency for collagenization either centrally or peripherally. The tumors have invasive stellate extensions into surrounding fat and glandular parenchyma. This infiltrative appearance at the margins mimic that of a benign phyllodes tumor. Cytological evaluation reveals uniform spindle cells containing minute amounts of cytoplasm that are present individually, or in groups of either clusters or relatively flat sheets. Nuclear reactivity for B-catenin favors the diagnosis of fibromatosis. In contrast, immunoreactivity for CK, p63, and CD10 is suggestive of metastatic carcinoma [7,17].
The treatment of choice remains surgical with wide local excision with negative margins being essential in obtaining a lower recurrence rate. If surrounding structures such as skin or chest wall show tumor involvement–resection of these structures must be performed to ensure clear surgical margins [15]. Local recurrence rates have been reported to be as high as 27% [18].
There have been cases reported where fibromatosis was associated with trauma to the breast and postsilicone implant insertion [17]. Only 4% of all breast fibromatoses were present bilaterally [3]. Differential diagnoses include malignant lesions such as metaplastic carcinoma, high-grade fibrosarcoma and fibrous histiocytoma. Benign lesions such as nodular fasciitis, benign phyllodes tumor and radial scar must also be taken into consideration [19].
Radiation therapy has been suggested where there is chest wall involvement, multiple recurrences, or surgery is not deemed appropriate due to patient factors such as age and multiple comorbidities [20]. There have also been cases reported where NSAID use in extra-abdominal fibromatoses resulted in regression of the tumor [21]. As most breast fibromatoses lack ER and PR positivity, the role of antiestrogen therapy remains unclear However, 1 study reported the cessation of recurrence of tumors in a patient with recurrent abdominal wall fibromatoses with Tamoxifen use [4]. More recently, the role of Imatinib is being studied for use in aggressive and recurrent fibromatoses [22]. It is important to note that there is a lack of evidence to support the use of these agents in breast fibromatoses specifically, however it is a field where further research could be promising for improved treatment and prognosis of breast fibromatosis.
Patient consent
Informed consent was obtained from the patient for the purposes of the publication of this case report.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Hicks DG, Lester SC. Diagnostic pathology: Breast. Amirsys, Inc; Letterkenny: 2016. Fibromatosis; p. 547. [Google Scholar]
- 2.Hill E, Merrill A, Korourian S, Bryant-Smith G, Henry-Tillman R, Ochoa D. Silicone breast implant associated fibromatosis. J Surg Case Rep. 2018;2018(9):rjy249. doi: 10.1093/jscr/rjy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor TV, Sosa J. Bilateral breast fibromatosis: case report and review of the literature. J Surg Educ. 2011;68(4):320–325. doi: 10.1016/j.jsurg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Procter H, Singh L, Baum M, Brinkley D. Response of multicentric desmoid tumors to tamoxifen. Br J Surg. 1987;74(5):401. doi: 10.1002/bjs.1800740527. [DOI] [PubMed] [Google Scholar]
- 5.McDonald ES, Yi ES, Wenger DE. Extraabdominal desmoid-type fibromatosis. RadioGraphics. 2008;28(3):901–906. doi: 10.1148/rg.283075169. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Ad-El D, Benjaminov O, Gutman H. Post-traumatic soft tissue tumors: case report and review of the literature a propos a post-traumatic paraspinal desmoid tumor. World J Surg Oncol. 2008;6(1):28. doi: 10.1186/1477-7819-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen PP, Hoda SA, Lerwill MF, Koerner FC. Rosen's breast pathology. Wolters Kluwer Health; Philadelphia: 2014. Benign mesenchymal neoplasms; p. 1020. [Google Scholar]
- 8.Barth AIM, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9(5):683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 9.Grimaldi MC, Trentin C, Lo Gullo R, Cassano E. Fibromatosis of the breast mimicking cancer: a case report. Radiol Case Rep. 2018;13(1):1–5. doi: 10.1016/j.radcr.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glazebrook KN, Reynolds CA. Mammary fibromatosis. Am J Roentgenol. 2009;193(3):856–860. doi: 10.2214/AJR.08.1892. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Liu P, Lu H, Zhang S, Zhu Y. Imaging manifestation of mammary fibromatosis. Breast J. 2013;19(6):673–675. doi: 10.1111/tbj.12188. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahim L, Parry J, Taylor DB. Fibromatosis of the breast: a pictorial review of the imaging and histopathology findings. Clin Radiol. 2014;69(10):1077–1083. doi: 10.1016/j.crad.2014.05.105. [DOI] [PubMed] [Google Scholar]
- 13.Ganau S, Frigola G, Bargalló X, Alonso I, Fernández PL. Breast fibromatosis: Variability among imaging methods. Breast J. 2019;25(4):750–752. doi: 10.1111/tbj.13320. [DOI] [PubMed] [Google Scholar]
- 14.Leibman AJ, Kossoff MB. Sonographic features of fibromatosis of the breast. J Ultrasound Med. 1991;10(1):43–45. doi: 10.7863/jum.1991.10.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Brodt JK, Rhodes DJ, Glazebrook KN, Hruska C, O'Connor M, Boughey JC. Radiologic and pathologic images of mammary fibromatosis. Breast J. 2011;17(2):207–209. doi: 10.1111/j.1524-4741.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzen J, Cramer M, Buck N, Friedrichs K, Graubner K, Lühr CS, et al. Desmoid type fibromatosis of the breast: ten-year institutional results of imaging, histopathology, and surgery. Breast Care. 2020;16(1):77–84. doi: 10.1159/000507842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benej R, Mečiarová I, Pohlodek K. Desmoid-type fibromatosis of the breast: a report of 2 cases. Oncol Lett. 2017;14(2):1433–1438. doi: 10.3892/ol.2017.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wargotz ES, Norris HJ, Austin RM, &NA CMDR, Enzinger FM. Fibromatosis of the breast: a clinical and pathological study of 28 cases. Am J Surg Pathol. 1987;11(1):38–45. doi: 10.1097/00000478-198701000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz GS, Drotman M, Rosenblatt R, Milner L, Shamonki J, Osborne MP. Fibromatosis of the breast: case report and current concepts in the management of an uncommon lesion. Breast J. 2006;12(1):66–71. doi: 10.1111/j.1075-122X.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Roman H, Caron P, Verspyck E, Vercoustre L, Bouleau-Desbordes O, Marpeau L. Fibromatose primitive du sein. Ann Chirurgie. 2001;126(6):561–564. doi: 10.1016/s0003-3944(01)00551-x. [DOI] [PubMed] [Google Scholar]
- 21.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and SULINDAC as first-line treatment for desmoid tumors. Cancer. 2004;100(3):612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 22.Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French sarcoma group phase II trial with a long-term follow-up. Ann Oncol. 2011;22(2):452–457. doi: 10.1093/annonc/mdq341. [DOI] [PubMed] [Google Scholar]