Abstract
Intravascular lymphoma (IVL) is difficult to diagnose because its clinical presentation and laboratory and imaging findings are nonspecific. Herein, we report a case of IVL presenting as a lesion in the splenium of the corpus callosum. A 52-year-old man attended the emergency department with a 2-week history of progressively worsening abnormal behavior and gait disturbance. Magnetic resonance imaging on admission revealed an oval lesion in the splenium of the corpus callosum. The follow-up magnetic resonance imaging performed 2 months after disease onset revealed multiple high-signal areas in the bilateral cerebral white matter on T2-weighted images and diffusion-weighted images. The blood test results showed an elevated level of lactate dehydrogenase and serum-soluble interleukin-2 receptor. These findings were compatible with the diagnosis of IVL. IVL is often difficult to diagnose due to a wide variety of clinical presentations and imaging findings.
Keywords: Lymphoma, Intravascular large B-cell lymphoma, Splenium of the corpus callosum, Magnetic resonance imaging
Introduction
Intravascular lymphoma (IVL) is a rare form of large B-cell non-Hodgkin's lymphoma that is prone to the central nervous system (CNS) vascular invasion and occlusion, often resulting in non-localized neuropathy and altered mental status [1]. The diagnosis of IVL is challenging because its clinical presentation and laboratory and imaging findings are nonspecific [2], [3], [4], [5]. We report a case of IVL presenting as a lesion in the splenium of the corpus callosum at the onset.
Case report
A previously healthy 52-year-old Asian man attended the emergency department with a 2-week history of progressively worsening abnormal behavior and gait disturbance. Nonenhanced brain magnetic resonance imaging (MRI) performed on admission revealed an oval lesion in the splenium of the corpus callosum. It showed hyperintense on T2-weighted and diffusion-weighted images, with a decreased apparent diffusion coefficient (Fig. 1). A small high-signal lesion was also found in the subcortical region of the left frontal lobe on the diffusion-weighted images. The blood test showed an elevated lactate dehydrogenase level of 650 U/L and erythrocyte sedimentation rate of 45 mm/h. On the basis of the cerebrospinal fluid examination results that showed an elevated cell count of 9/μL and a total protein level of 125 mg/dL, encephalitis was suspected. Thus, antiviral, antibacterial, and antiepileptic drugs were administered. The patient's symptoms once improved, but fever, impaired consciousness and hypercalcemia appeared, and the patient went into disseminated intravascular coagulation. A possibility of paraneoplastic neurological syndrome was suspected, but no tumor was found on imaging studies or endoscopy. The follow-up MRIs 2 weeks and 1 month after the onset of the disease showed a reduction in the size and signal of the oval lesion in the splenium of the corpus callosum. The follow-up MRI performed 2 months after disease onset revealed mottled multiple high-signal areas in the bilateral cerebral white matter on T2-weighted images, fluid-attenuated inversion recovery (FLAIR) images and diffusion-weighted images. The lesions were distributed predominantly in the watershed area (Fig. 2). A reduction in the size and signal of the oval lesion in the splenium of the corpus callosum was observed. T1-weighted images showed diffuse low-signal areas in the bone marrow. On gadolinium-enhanced imaging, the dura mater was thick and enhanced. Although these findings might be interpreted as hyperplastic bone marrow and CSF hypovolemia after lumbar puncture, respectively, they could also indicate the possibility of tumor invasion.
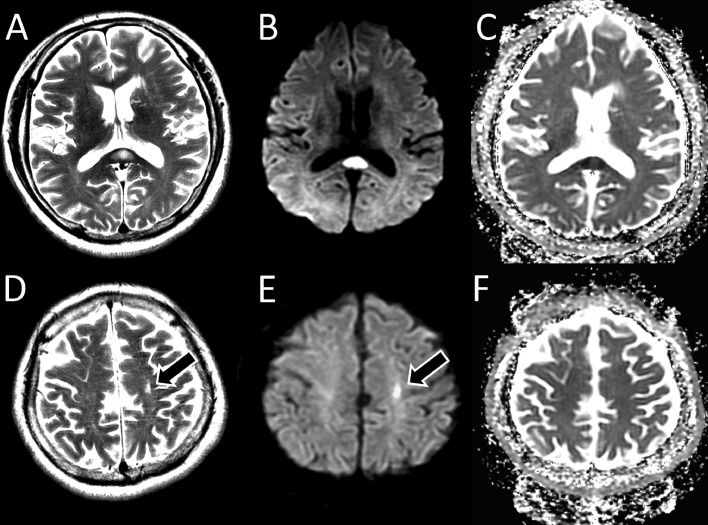
Fig. 1.
Axial magnetic resonance images obtained on admission showed a high-signal-intensity lesion in the splenium of the corpus callosum on T2-weighted images (A) and diffusion-weighted images (B), with a decreased apparent diffusion coefficient (C). On retrospection, a small area of high signal intensity was observed in the subcortical region of the left frontal lobe on the T2-weighted images (D, arrow) and diffusion-weighted images (E, arrow). Apparent diffusion coefficient map showed no diffusion restriction of the lesion (F).
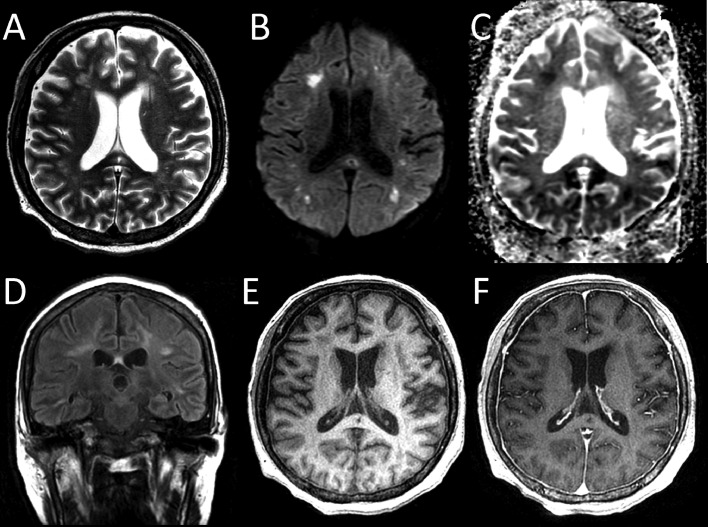
Fig. 2.
Follow-up magnetic resonance images taken 2 months after disease onset, revealed mottled multiple high-signal areas in the bilateral cerebral white matter on T2-weighted images (A) and diffusion-weighted images (B), and reduced signal on an apparent diffusion coefficient map (C, arrows). The lesions showed high signal intensity on fluid-attenuated inversion recovery images (D). The lesions were distributed predominantly in the watershed area. Reductions in the size and signal of the oval lesion in the splenium of the corpus callosum were observed. T1-weighted images showed diffuse low-signal intensity in the bone marrow (E). The dura mater was thick and enhanced with the gadolinium-based contrast agent (F).
The blood count indicated anemia, thrombocytopenia, and mild leukocytosis, with a hemoglobin concentration of 7.1 g/dL, platelet count of 19.2 × 10⁴/µL, and white blood cell count of 16.3 × 10³/µL. Other laboratory results were as follows: lactate dehydrogenase, 506 U/L; albumin, 2.2 g/dL; C-reactive protein, 10.05 mg/dL; and serum-soluble interleukin-2 receptor (sIL2R), 2956 U/mL. On the basis of the elevated sIL2R level and imaging findings of multiple micro cerebral infarcts in the deep white matter, IVL was suspected. Skin and bone marrow biopsy showed large atypical lymphocytes aggregating within small vessels in the dermis and subcutaneous adipose tissue and infiltrating the bone marrow. Immunohistochemically, these atypical lymphocytes were positive for CD20, CD79a, bcl-2, and bcl-6 and negative for CD3 and CD5. Pathological diagnosis was intravascular large B-cell lymphoma. Two days after the diagnosis was confirmed, the patient died of respiratory failure.
Discussion
Lesions in the splenium of the corpus callosum are seen in various diseases [6,7]. Diseases that present with lesions in the splenium of the corpus callosum include traumatic lesions (diffuse axonal injury), ischemic lesions (infarction, hypoxic-Ischemic encephalopathy, hypoglycemic encephalopathy), tumors (lymphoma, glioma), degenerative and demyelinating disease (Wallerian degeneration, multiple sclerosis, acute disseminated encephalomyelitis, posterior reversible encephalopathy syndrome), toxic encephalopathy (Marchiafava-Bignami disease, metronidazole-induced encephalopathy, carbon monoxide intoxication), and miscellaneous lesions (clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS), white matter injury in neonatal viral infection) [8]. In this case, the patient was elderly with a subacute course of gait disturbance and encephalopathy. Immediately after the onset, the lesions were almost confined to the splenium of the corpus callosum and this lesion remained 1 month later. Although the initial imaging findings were similar to those of MERS, the adult onset is atypical for MERS, and the subsequent course of the disease is also different [9].
The early lesions observed are atypical for multiple sclerosis (MS) as they are almost confined in the splenium of the corpus callosum and not prominently distributed in the vicinity of the lateral ventricles [10]. The absence of gray matter lesions, lack of prior infection, and the age of the patient also make ADEM somewhat atypical [11]. The possibility of trauma or poisoning as the cause of the symptoms is also ruled out by the patient's history. Infarcts are rare in the splenium of the corpus callosum due to the presence of an abundant blood supply [12]. A chronic lesion involving the splenium of the corpus callosum may be caused by a tumor, such as lymphoma [12]. Glioma is also difficult to rule out, although the sporadic nature of the lesions is atypical [12]. Although the noncircular shape of the lesions is somewhat atypical, it is difficult to rule out metastasis [13]. However, spontaneous regression is atypical for these kinds of tumors. In this case, the laboratory and imaging findings suggestive of ILV became apparent as the course of the disease progressed. IVL presents with varied imaging findings, including infarct-like lesions, nonspecific white matter lesions, meningeal enhancement, mass formation, and T2-weighted high signal areas of pons [2]. A dynamic pattern of MRI findings with a resolution of some lesions and new appearance of other lesions on diffusion-weighted or T2-weighted images can be indicators of the disease [3]. Infarct-like lesions are suggestive of microvascular tumor invasion and appear as multifocal high-signal areas on T2-weighted images with diffusion restriction in the white matter [2,3]. It is reported to occur in 36% of patients with IVL [14].
In the present case, the MRI 2 months after disease onset showed multiple cerebral infarcts in the deep white matter predominantly involving the watershed area. The small-high signal lesion seen in the left frontal lobe detected on the diffusion-weighted imaging on admission was also indicative of multifocal vessel disease, providing a clue to the diagnosis. Although differential diagnosis based on imaging findings included the diseases listed above, IVL was suspected due to the elevation of the sIL2R and LDH.
A few cases of IVL mainly involving the splenium of the corpus callosum in the first place have been reported [15,16]. In one case, fever up to 40.5°C with ongoing delirium and mental status changes appeared along with a lesion of the splenium of the corpus callosum [15]. This case was somewhat similar to the present case in both the clinical presentation and imaging findings. In the other case, the main complaint was hearing loss without fever or psychiatric symptoms, and there were lesions of the corpus callosum, pons and left middle cerebellar peduncle [16]. IVL is often difficult to diagnose due to a variety of clinical and imaging findings.
Patient consent
A consent was obtained from the patient's family for publication of this report.
Footnotes
Competing Interests: The authors declare that they have no conflicts of interests.
References
- 1.Ponzoni M, Campo E, Shigeo N. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132(15):1561–1567. doi: 10.1182/blood-2017-04-737445. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto A, Kikuchi Y, Homma K, O’uchi T. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. AJNR Am J Neuroradiol. 2012;33(2):292–296. doi: 10.3174/ajnr.A2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baehring JM, Henchcliffe C, Ledezma CJ, Fulbright R, Hochberg FH. Intravascular lymphoma: magnetic resonance imaging correlates of disease dynamics within the central nervous system. J Neurol Neurosurg Psychiatry. 2005;76(4):540–544. doi: 10.1136/jnnp.2003.033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Duverneuil N, Mokhtari K, Behin A, Lafitte F, Hoang-Xuan K, Chiras J. Intravascular malignant lymphomatosis. Neuroradiology. 2002;44(9):749–754. doi: 10.1007/s00234-002-0808-9. [DOI] [PubMed] [Google Scholar]
- 5.Voultsinou D, Mantatzis M, Gerukis T, Heva A, Birbilis T, Prassopoulos P. Magnetic resonance imaging patterns in central nervous system lymphomas: a pictorial review. Clin Imag. 2021;78:1–7. doi: 10.1016/j.clinimag.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Park MK, Hwang SH, Jung S, Hong SS, Kwon SB. Lesions in the splenium of the corpus callosum: clinical and radiological implications. Neurol Asia. 2014;19(1):79–88. [Google Scholar]
- 7.Michael JD, Sumie J, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005;62(3):433–437. doi: 10.1001/archneur.62.3.433. [DOI] [PubMed] [Google Scholar]
- 8.Park SE, Choi DS, Shin HS, Baek HJ, Choi HC, Kim JE, et al. Splenial lesions of the corpus callosum: disease spectrum and MRI findings. Korean J Radiol. 2017;18(4):710–721. doi: 10.3348/kjr.2017.18.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takanashi J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev. 2009;31(7):521–528. doi: 10.1016/j.braindev.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Etemadifar M, Ashourizadeh H, Nouri H, Kargaran PK, Salari M, Rayani M, et al. MRI signs of CNS demyelinating diseases. Mult Scler Relat Disord. 2021;47:102665. doi: 10.1016/j.msard.2020.102665. [DOI] [PubMed] [Google Scholar]
- 11.Kesselring J, Miller DH, Robb SA, Kendall BE, Moseley IF, Kingsley D, et al. Acute disseminated encephalomyelitis. MRI findings and the distinction from multiple sclerosis. Brain. 1990;113(Pt 2):291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 12.Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW, et al. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002;179(1):251–257. doi: 10.2214/ajr.179.1.1790251. [DOI] [PubMed] [Google Scholar]
- 13.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl. 4):S209–S219. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams RL, Meltzer CC, Smirniotopoulos JG, Fukui MB, Inman M. Cerebral MR imaging in intravascular lymphomatosis. AJNR Am J Neuroradiol. 1998;19(3):427–431. [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelo CR, Ku K, Gulliver J, Chang J. Intravascular large B-cell lymphoma presenting with altered mental status: a case report. World J Clin Oncol. 2019;10(12):402–408. doi: 10.5306/wjco.v10.i12.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger JR, Jones R, Wilson D. Intravascular lymphomatosis presenting with sudden hearing loss. J Neurol Sci. 2005;232(1–2):105–109. doi: 10.1016/j.jns.2005.01.001. [DOI] [PubMed] [Google Scholar]