Abstract
Understanding and exploiting genetic diversity is a key factor for the productive and stable production of rice. Here, we utilize 73 high-quality genomes that encompass the subpopulation structure of Asian rice (Oryza sativa), plus the genomes of two wild relatives (O. rufipogon and O. punctata), to build a pan-genome inversion index of 1769 non-redundant inversions that span an average of ~29% of the O. sativa cv. Nipponbare reference genome sequence. Using this index, we estimate an inversion rate of ~700 inversions per million years in Asian rice, which is 16 to 50 times higher than previously estimated for plants. Detailed analyses of these inversions show evidence of their effects on gene expression, recombination rate, and linkage disequilibrium. Our study uncovers the prevalence and scale of large inversions (≥100 bp) across the pan-genome of Asian rice and hints at their largely unexplored role in functional biology and crop performance.
Subject terms: Comparative genomics, Evolutionary genetics, Agricultural genetics, Plant genetics
Pan-genomes provide useful resources for evolutionary studies, functional genomics and breeding of cultivated plants. Here, the authors report a new rice pan-genome including 73 Asian rice and two wild relatives (Oryza rufipogon and O. punctata), and reveal the prevalence and scale of large inversions across the pan-genome.
Introduction
Asian rice (Oryza sativa) is a staple cereal crop that has played an essential role in feeding much of the world for millennia1,2. Since the world population is expected to increase to approximately 10 billion by 2060–20703, the rice community is searching for novel ways to breed new varieties that are nutritious, sustainable, and climate resilient2. One source of the raw material required to meet this urgent demand is the standing natural variation that exists in the genomes of the more than 500,000 accessions of rice and its wild relatives deposited in germplasm banks around the world2, i.e., single nucleotide polymorphisms (SNPs), insertions/deletions (INSs/DELs), translocations (TRAs), and inversions (INVs).
Inversions have been reported as an important class of structural variation across all life forms, beginning with the first reported inversion in Drosophila in 19214, followed by discoveries in fungi5, bacterial6, plants7–12, and animals13–17. In many cases, inversions have been found to play important roles in genetic recombination, genome evolution, speciation, and phenotypic variation in these species. For example, in Drosophila subobscura, inversions have been hypothesized as a driver of genomic structural evolution due to suppressed recombination in inverted regions that contain adaptive genes18. In birds, the great tit (Parus major) has a 64 megabase (Mb) inversion that covers more than 90% of chromosome 1A (5% of a 1.2 Gb genome size), which was shown to be involved in speciation19. In humans, many INVs have been found to be associated with human diseases, e.g., hemophilia A20, neurodegenerative diseases21, autoimmune diseases22, mental disorders23, and gene expression abnormalities15,24. In plants, INVs have been reported to play roles in, for example - local adaptation10,25, genome-environment associations25, gene regulation10,26,27, flowering time26, seed germination26, and fruit shape27.
In rice and the genus Oryza, inversion studies have been limited to small and mid-size inversions (10 bp–500 Kb) as a consequence of the reliance on short-read data for their detection or larger inversions (100 bp–5.5 Mb) via single or a limited number of pairwise genome scans. For example, Wang et al. performed a genome scan of inversions in the O. sativa cv. Nipponbare genome (i.e., IRGSP RefSeq) using short-read resequencing data from 453 high-coverage genomes (>20×) from the 3K Rice Genome Project (3K-RGP), and detected 152 ± 62 inversions per genome with a size range of 127.1 ± 19.4 Kb28,29. A phylogenetic analysis of this dataset, including other SV data, demonstrated that SVs could be used to define the population structure of Asian rice28. Fuentes et al. investigated the entire 3K-RGP dataset similarly and identified 1,255,033 inversions, with the vast majority (85%) falling in a size range of 10 bp–100 Kb11. Using a genome scanning strategy, a scan of the IRGSP RefSeq, plus reciprocal genome alignments to nine Asian rice and two AA-genome wild relatives (i.e., O. rufipogon and O. longistaminata) confirmed the presence a previously detected ~5 Mb inversion spanning the centromere of chromosome 6 in four Xian-indica (XI) varieties, relative to four Geng-japonica (GJ) varieties, as well as two outgroup species30. A broader phylogenetic study interrogating two cultivated and 11 wild Oryza genomes using SVs resulted in the identification of 12 inversions (i.e., 60–300 Kb) that the authors inferred potentially led to the rapid diversification of the AA genome species within a 2.5 million years (MY) span31. Recently, a genome scan of 33 rice genome assemblies identified 954 inversions ranging in size from 100 bp to ~5 Mb12. This study identified larger inversions that included seven inversions greater than 1 Mb.
Although these studies combined contributed to a preliminary understanding of inversions in rice, a comprehensive analysis of inversions that utilize ultra-high-quality reference genome sequences that takes into account the population structure of Asian rice, remains uncharted. To reveal a comprehensive understanding of inversions (≥100 bp) and explore their evolutionary impacts in Asian rice, we interrogated a set of 73 high-quality genome assemblies - as a pan-genome proxy - that spanned the K = 1532 population structure of cultivated Asian rice, plus two additional high-quality de novo assembled genomes from a representative species of the progenitor of Asian rice (O. rufipogon) and the BB genome species - O. punctata, as outgroups.
Here, we describe and characterize a pan-genome inversion index (PGII) of 1769 non-redundant inversions, of which 1085 are identified in this study. Phylogenetic analysis of the PGII shows that 66 of the 73 genomes can be subdivided across the expected K = 15 subpopulation structure of Asian rice, while the remaining seven fall into two Xian/Indica clusters that have yet to be characterized. Clustering the index into 1426 inversion clusters shows that most clusters are O. sativa species-specific (n = 885), followed by O. rufipogon species-specific (n = 96), O. punctata species-specific or AA genome fixed (n = 322), and basal to O. sativa/O. rufipogon divergence, or introgression (n = 123). Characterization of subsets of the index shows evidence for both recombination suppression as well as trace patterns of linkage disequilibrium, both of which suggest that some inversions may be under positive selection. Finally, with this index we estimate a population-level inversion rate for Asian rice of 735–749 inversions per million years, which is 16–50 times higher than previous estimates for plants.
Results
The 18-genome data package
To investigate the genome inversion landscape of Asian rice from a population structure perspective, we first combined a set of 16 previously published high-quality genomes32–34 that represent the K = 15 population structure of O. sativa, plus the largest Xian/indica (XI) admixed subpopulation (XI-adm: Minghui 63 (MH63)) to create a “Rice Population Reference Panel” (RPRP - https://yongzhou2019.github.io/Rice-Population-Reference-Panel/). This panel was annotated with a uniform pipeline to minimize methodological artifacts using RNA-Seq and Iso-Seq data generated or collected from all 16 genomes as evidence (Table 1, Supplementary Tables 1–3, Supplementary Fig. 1, Supplementary Data 1, and Supplementary Note 1).
Table 1.
Assembly and annotation statistics of the 18-genome data package
| Acronyms | Variety name/Accession ID | Species/genome | Subpopulationa | GenBank accession | Assembly size (Mb) | Repeat | Annotated locib |
|---|---|---|---|---|---|---|---|
| IRGSP | IRGSP-1.0/NIPPONBARE | O. sativa-AA (Asian rice) | GJ-temp | GCA_001433935.1 | 373.25 | 51.79% | 37,140 |
| CMeo | CHAO MEO::IRGC 80273-1 | O. sativa-AA (Asian rice) | GJ-subtrp | GCA_009831315.1 | 376.86 | 47.41% | 36,601 |
| Azu | Azucena | O. sativa-AA (Asian rice) | GJ-trop1 | GCA_009830595.1 | 379.63 | 52.56% | 36,623 |
| KeNa | KETAN NANGKA::IRGC 19961-2 | O. sativa-AA (Asian rice) | GJ-trop2 | GCA_009831275.1 | 380.76 | 50.30% | 36,609 |
| ARC | ARC 10497::IRGC 12485-1 | O. sativa-AA (Asian rice) | cB | GCA_009831255.1 | 378.46 | 49.50% | 36,423 |
| PR106 | PR 106::IRGC 53418-1 | O. sativa-AA (Asian rice) | XI−1B2 | GCA_009831045.1 | 391.18 | 50.45% | 36,405 |
| MH63 | Minghui 63 | O. sativa-AA (Asian rice) | XI-adm | GCA_001623365.2 | 387.43 | 53.41% | 38,047 |
| IR64 | IR 64 | O. sativa-AA (Asian rice) | XI−1B1 | GCA_009914875.1 | 386.7 | 53.55% | 36,065 |
| ZS97 | Zhenshan 97 | O. sativa-AA (Asian rice) | XI−1A | GCA_001623345.2 | 387.33 | 53.41% | 37,651 |
| Lima | LIMA::IRGC 81487-1 | O. sativa-AA (Asian rice) | XI−3A | GCA_009829395.1 | 392.63 | 48.93% | 36,217 |
| KYG | KHAO YAI GUANG::IRGC 65972-1 | O. sativa-AA (Asian rice) | XI−3B1 | GCA_009831295.1 | 393.74 | 53.93% | 36,212 |
| GoSa | GOBOL SAIL (BALAM)::IRGC 26624-2 | O. sativa-AA (Asian rice) | XI−2A | GCA_009831025.1 | 391.77 | 51.01% | 36,222 |
| LiXu | LIU XU::IRGC 109232-1 | O. sativa-AA (Asian rice) | XI−3B2 | GCA_009829375.1 | 392.03 | 51.66% | 36,378 |
| LaMu | LARHA MUGAD::IRGC 52339-1 | O. sativa-AA (Asian rice) | XI−2B | GCA_009831355.1 | 390.2 | 51.25% | 36,299 |
| N22 | N22 (N 22::IRGC 19379-1) | O. sativa-AA (Asian rice) | cA1 | GCA_001952365.2 | 382.95 | 52.82% | 36,262 |
| NaBo | NATEL BORO::IRGC 34749-1 | O. sativa-AA (Asian rice) | cA2 | GCA_009831335.1 | 383.72 | 50.64% | 36,196 |
| O. ruf | O. rufipogon PNG91-7::IRGC 106523-1 | O. rufipogon-AA (Asian rice progenitor) | – | GCA_023541355.1 | 462.58 | 56.54% | – |
| O. pun | O. punctata:: IRGC 105690 | O. punctata-BB (Outgroup) | – | GCA_000573905.2 | 422.39 | 53.21% | – |
Previously published genomes and annotations are cited.
aSubpopulations: GJ = Geng/Japonica where trop = tropical, subtrp = subtropical; cB = circum/Basmati; XI = Xian/Indica; cA = circum-Aus.
bGene annotations can be obtained from Gramene (https://www.gramene.org/) or the RPRP website (https://yongzhou2019.github.io/Rice-Population-Reference-Panel/data/).
To anchor this panel within a phylogenetic context, we long-read sequenced, and de novo assembled two additional genomes from both a representative species of the progenitor of Asian rice - i.e., O. rufipogon (AA) and the African BB genome outgroup species - O. punctata (Table 1, Supplementary Table 4, and Supplementary Fig. 2). Both species are diploid and have similar genome sizes as Asian rice. These genomes were assembled to a similar quality as the RPRP (i.e., BUSCO > 95%, number of gaps <50, ContigN50 > 10 Mb) (Supplementary Table 4 and Supplementary Note 1).
All 18 genomes and their annotations are henceforth referred to as the “18-genome data package” (See “18-genome data package” in the Supplementary Note 1 section for a complete description of this data set).
Creation of a pan-genome inversion index for Asian rice
To ensure the detection of the majority of inversions present in cultivated Asian rice, we selected an additional 57 publicly available long-read genome assemblies (out of 94) for analyses that met similar quality standards present in the 18-genome data package, for a total of 75 genomes (Supplementary Fig. 3, Supplementary Data 2, and Supplementary Note 2).
To detect inversions, we compared pairwise all 74 reference genome assemblies with the IRGSP RefSeq34, following a workflow as detailed in Supplementary Note 3 (Supplementary Fig. 4 and Supplementary Table 5), and identified a total of 12,141 inversions (≥100 bp) (Supplementary Data 3), 1769 of which were non-redundant (Supplementary Data 4). Finally, since several inversions had overlapping coordinates (i.e., 80% of their length), they were clustered to obtain a set of 1426 clustered inversions (Supplementary Data 4). We then validated a subset of 264 inversions from four randomly selected genome assemblies (i.e., LM, NABO, CM, and MH63) using PacBio long-reads, and found that 97.7% of the inversion breakpoints could be detected (Supplementary Table 6), thereby confirming the high accuracy of our detection strategy.
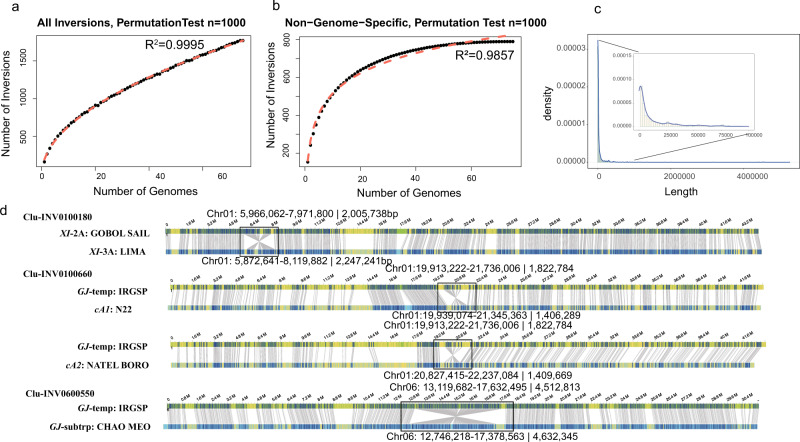
To determine if the use of 75 genomes was sufficient to call the majority of 100 bp or greater inversions in the pan-genome of Asian rice, we performed a permutation test (n = 1000), and found that the use of ~60 genomes was sufficient to capture the majority of inversions with allele frequencies greater than 2 out of 75 genomes (Fig. 1a, b). These results demonstrate that employing 75 genomes, that bridge the K = 15 population structure of cultivated rice, can yield a robust pan-genome inversion index for Asian rice.
Fig. 1. Rice inversion index summary.
a, b Resampling permutation test to identify the relationship between the number of genomes and all inversions and shared (non-genome specific) inversions, respectively; c Density of inversion lengths; d Bionano validation of inversions larger than 1 Mb, i.e., Clu-INV0100180, Clu-INV0100660, and Clu-INV0600550. In each panel, the top line shows the optical map used as a reference, the bottom line shows the genome assembly of the variety with the inversion. Gray lines connect restriction sites that are aligned (blue regions), while yellow segments show unaligned regions. Black boxes highlight the position each inversion. Source data are provided as a Source Data file.
Our inversion index was compared with previous studies in rice, i.e., 3K-RGP11 and the pan-genome study of 33 rice genomes12. Inversions were treated as identical if they matched the following two criteria: 1) the inversion length difference was smaller than 200 bp, and 2) the differences between the coordinates of breakpoints of inversions were smaller than 100 bp. In doing so, we found that of the 1769 nonredundant inversions identified, 38.6% have already been reported (Supplementary Data 4).
As expected, more inversions were observed when we compared the BB genome outgroup (O. punctata) than AA genomes to the IRGSP RefSeq: 337 (total length = 15.16 Mb) (Supplementary Data 3), followed by the AA genome outgroup (O. rufipogon): 230 (total length = 12.95 Mb). On average, each O. sativa genome was found to contain 164 inversions, ranging from 33 (TG22) to 230 (YX1) (Supplementary Data 3). We found a larger number of inversions (i.e.,164–230) when comparing the O. sativa XI-subgroup genomes to the GJ-temp: IRGSP RefSeq than when comparing the O. sativa GJ-subgroup genomes (33–201 inversions) to the same reference, which is consistent with the long divergence time estimate between the XI and GJ varietal groups (i.e., 360,000–400,000 years35).
The total length of inversions from one genome to another ranged from 14.95 Mb (cA2: NATEL BORO) to 255 Kb (GJ-temp: TG19) (Supplementary Data 3) and totaled 117.75 Mb across all 75 genomes tested. We identified 470 clustered inversions shorter than 1 Kb and 11 clustered inversions greater than 1 Mb relative to the IRGSP RefSeq (Fig. 1c and Supplementary Data 4). These extra-large inversions (>1 Mb) were found on seven chromosomes (i.e., 1, 4, 5, 6, 8, 10, and 11) and included seven of which were previously reported (i.e., Clu-INV0600550 (4.5 Mb)30, Clu-INV0800800 (1.1 Mb)36, Clu-INV0100660 (1.8 Mb)12, Clu-INV0500270 (3.1 Mb)12, Clu-INV0800450 (5.2 Mb)12, Clu-INV0800470 (1.2 Mb)12, Clu-INV110070 (1.1 Mb)12, and four were identified in this study (i.e., Clu-INV0100180 (2 Mb), Clu-INV0400050 (1.3 Mb), Clu-INV0400650 (4.3 Mb), and Clu-INV1000940 (1.3 Mb)). Three of the 11 clustered inversions (i.e., Clu-INV0100180, Clu-INV0100660, and INV0600550) were validated with available Bionano optical maps (Fig. 1d), while the remaining eight were not due to the lack of Bionano data.
Chromosomal distribution of the pan-genome inversions index
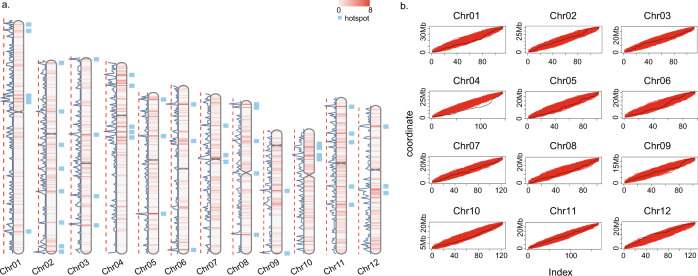
To determine if the inversions detected were uniformly distributed across all chromosomes, we tested for uniformity using the Kolmogorov–Smirnov (KS) test, and found that only one chromosome, chromosome 4, deviated significantly from uniformity (i.e., p = 3e−04; Fig. 2a, b, and Supplementary Data 5). To check if inversion size played a role in this chromosome 4 anomaly, we repeated the KS test by classifying inversions according to their lengths (<1 Kb, 1–5 Kb, 5–10 Kb, and >10 Kb). We found no significant deviation from uniformity except for the >10 Kb inversion class on chromosome 4 (Supplementary Fig. 5 and Supplementary Data 5), where inversions are mainly concentrated near the centromere on both the short and long arms of chromosome 4. These results demonstrate that the inversions detected appear evenly distributed genome-wide, with one exception.
Fig. 2. Genome-wide inversion distribution.
a Chromosome distribution of the pan-genome inversion index, and inversion hotspots. Chromosome heatmaps in the middle along the chromosome represent the density of inversions. The line on the left of each chromosome represents the number of inversions per 200 Kb window. The red dotted line cut off is the top 2% number of inversions in per 200 Kb window. The blue boxes on the right of each chromosome represents the inversion hotspot regions. b The Kolmogorov–Smirnov (KS) test for inversion uniformity distribution across the 12 rice chromosomes. The black line is actual inversion distribution, and the red line is the 10,000th uniformly distributed simulation. Source data are provided as a Source Data file.
To search for inversion hotspots, we performed a 200 Kb window-based analysis across all chromosomes. We defined the top 2% of all windows with the highest frequency of inversion start coordinates as hotspots. This analysis revealed 47 putative hotspots, including 239 independent inversions where 12 inversions overlapped with centromeres (on chromosomes 7 and 8) but none with telomeres (Fig. 2a and Supplementary Data 6).
Phylogenetic analysis of the pan-genome inversion index
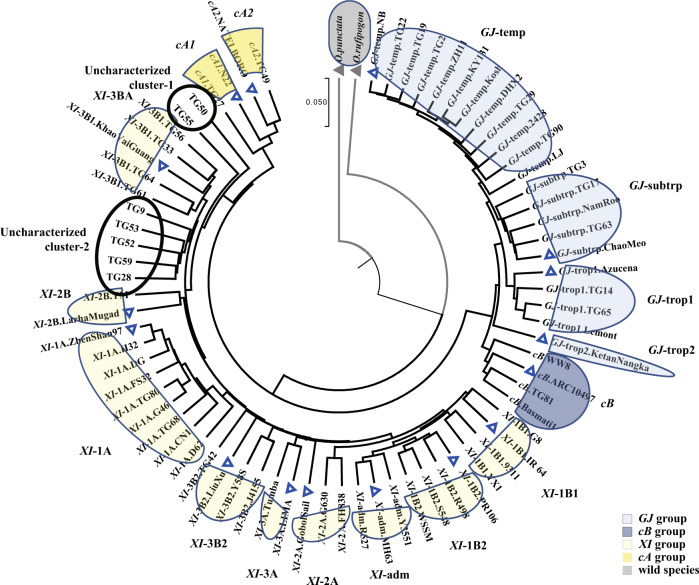
To determine if the pan-genome inversion index could be used to phylogenetically distinguish each of the 75 high-quality genomes into the expected K = 15 subpopulation structure, we used the unweighted pair group with arithmetic mean distance tree method, with the O. punctata (BB) and O. rufipogon (AA) genomes as outgroups (Fig. 3). As shown in Fig. 3, 66 of the 73 genomes could be subdivided across the expected subpopulation structure of Asian rice, while the remaining seven fell into two XI clusters that have yet to be characterized.
Fig. 3. Phylogenetic tree of 75 high-quality rice genomes using the pan-genome inversion index.
Phylogenetic relationships of the 75 high-quality genomes used to create the pan-genome inversion index, inferred using the UPGMA method (unweighted pair group method with arithmetic mean95). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Evolutionary analyses were conducted in MEGA796. Two wild relatives (O. rufipogon and O. punctata) were heighted with light gray arc. Subpopulations from GJ, cB, XI, and cA groups were highlighted with light blue, dark blue, light yellow and dark yellow, respectively. Two uncharacterized XI subpopulations are shown with a black circle. The distance matrix of SNP and INV polymorphisms was significantly correlated (Mantel test, simulation with n = 999,999, r = 0.79, p = 1e−6). Source data are provided as a Source Data file.
Inversion rate estimations for Asian rice
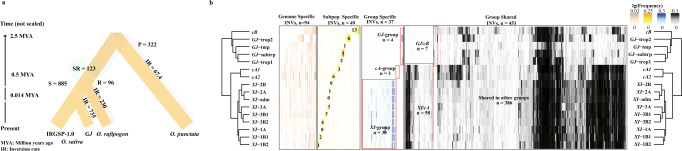
It was estimated that the AA genomes of the Oryza diverged from the BB genome type about 2.5 million years ago (MYA)2, which equates to an inversion rate of 67.4 inversions per million years (Table 2 and Fig. 4a). If we use the inferred AA genome diversification (i.e., O. sativa GJ-temp IRGSP vs. O. rufipogon) rate of ~0.50 net new species/million years2,31, then the estimate is 230 inversions per MY (Table 2 and Fig. 4a).
Table 2.
Inversion rate estimations
| Comparison | Time to MRCA (MY) | Number of inversion polymorphisms | Inversion mutation rate (IR) | |
|---|---|---|---|---|
| Raw | Usablea | Number of inversions per million years | ||
| O. punctata - NB | 2.5 | 323 | 323 | 64.6 |
| O. rufipogon - NB | 0.5 | 230 | 230 | 230 |
| GJ-temp vs other GJ (average) | 0.0142 | 82.96 | 21.27 | 749 |
| Average of pairs of GJ genomes | 0.0142 | 20.86 | 735 | |
MRCA most recent common ancestor, MY million years.
aInversions shared with subpopulations other than GJ were excluded to avoid counting regions that were possibly introgressed from populations with higher times to MRCA.
Fig. 4. Species-specific, population-specific and shared inversion analysis of the pan-genome inversion index of rice.
a A model showing species specific inversions and inversion rates in Asian rice and two wild relatives (O. punctata and O. rufipogon). b Frequency of genome-specific, subpopulation specific, group specific and group shared inversions across the population structure of Asian rice (n = 631). Source data are provided as a Source Data file.
Regarding O. sativa subpopulations, it was estimated that temperate japonica (GJ-temp) first diverged from proto-japonica about 14,200 years ago37, thus we used this population divergence time for comparisons within Geng/japonica. For the computation of time to a MRCA for a pair of genomes, we added the expected time to coalesce within the proto-japonica population38, which can be estimated to be 10,000 generations based on previous estimates of effective population size37. For a comparison involving tropical japonica, care should be taken to exclude inversions that possibly originated in Xian/Indica through introgression. Thus, out of the 431 clustered inversions that were segregated within GJ, we focused on 170 that were not segregated within non-GJ subpopulations. To deal with the possibility of inversions that originated in XI but did not appear in our data set, we also filtered out 26 inverted regions closer to XI than to GJ based on the 3K-RGP SNP data39. The remaining 144 inversions were used to compute the number of inversions between each pair of GJ genomes. The average number of (filtered) inversions between two GJ genomes is 20.86, which translates to an inversion rate of 735 inversions per MY. Calculations involving comparisons only between a temperate GJ and another GJ population lead to a similar estimate of 749 inversions per MY (Table 2 and Fig. 4a).
Species and population scale analysis
Of the 1426 clustered inversions detected (Supplementary Data 4), we classified them into four different groups: Inversions segregating in O. sativa (S), Inversions segregating in both O. sativa and O. rufipogon (SR), O. rufipogon specific (R), and O. punctata specific or AA-fixed (i.e., ancestral state not clear) (P) (Fig. 4a). As a result, 1303 (91.4%) appeared to be species-specific, i.e., O. sativa (S): 885 (totaling 54.07 Mb), O. rufipogon (R): 96 (totaling 4.66 Mb), and O. punctata specific or AA-fixed (P): 322 (totaling 10.06 Mb) (Fig. 4a). The O. sativa specific inversions (S) were further classified into three categories: i.e., the IRGSP-1.0 RefSeq represents ancestral state (S1), IRGSP-1.0 RefSeq represents the derived state (S2), all O. sativa represent the derived state (fixed inversions) (S3), in which 872 (48.7 Mb), 11 (5.2 Mb), and 2 (72.5 Kb) inversions for S1, S2 and S3 were observed, respectively (Supplementary Table 7). The remaining 123 non-specific inversions were found to segregate in both O. sativa and O. rufipogon (i.e., originated before O. sativa and O. rufipogon divergence, or introgression) and totaled to about 3.3 Mb in size, including 121 inversions with IRGSP-1.0 RefSeq as the ancestral state (SR1), and 2 inversions with IRGSP-1.0 RefSeq as the derived state (SR2) (Supplementary Table 7).
To gain deeper insight into the distribution of inversions in rice at the population scale, we studied the 872 O. sativa specific inversions (IRGSP-1.0 RefSeq has ancestral state) across the 3K-RGP data set28. Beginning with a manually curated set of 8720 inversion alignment patterns at their breakpoints (i.e. 872 inversion alignment patterns across ten accessions), we classified their short-read alignment patterns into four categories (i.e., presence of both breakpoints = inversion, absence of both breakpoints = no inversion, presence of a single breakpoint = inversion + deletion, and no data at both breakpoints = NA) (Supplementary Fig. 6a). These categories were then used to train a machine learning workflow to search for the presence or absence of 2,636,928 alignment patterns for 872 inversions across the 3K-RGP data set (Supplementary Fig. 6b, c and Supplementary Table 8). Of note, this analysis identified 241 inversions and 273 accessions with more than 30% missing inversion data and were thus filtered out, leaving a final data set of 631 inversions across 2751 accessions for downstream analysis (Supplementary Data 7 and Supplementary Note 4). These 631 inversions were then classified into genome-specific, subpopulation-specific, group-specific, and group-shared inversions based on their inversion frequencies (Supplementary Data 8). As shown in Fig. 4b, 94 genome-specific, 49 subpopulation-specific, and 37 group-specific inversions (including 30, 3, and 4 specific inversions to XI, cA, and GJ groups, respectively) were observed. We also observed that 451 inversions were shared among different groups, of which 386 (61.1% of total O. sativa specific inversions) were shared across the GJ, XI, cA, or cB groups, 58 were shared between the XI and cA groups, and seven were shared between the GJ and cB groups (Fig. 4b). In total, these results reveal that 91% of the 1426 cluster inversions tested were species-specific (i.e., O. punctata, O. rufipogon, and O. sativa) at the population scale, and may provide clues to their possible roles in speciation over evolutionary time.
Characterization of transposable element content within inversions and their breakpoints
Transposable elements (TEs) are known to be associated with inversions11,12. Thus, we analyzed the TE content across the inversion index and at their breakpoints. The total amount of TE related sequences present in the pan-genome inversion index was 63.4%, which is significantly higher (Student’s test, p = 0.0003) than the average TE content present in the 18-data genome data package at 51.3% (Supplementary Data 9). Furthermore, out of the complete set of 1769 inversions, 888 showed partial or total similarity to TEs at both breakpoint regions (+/−100 bp from the breakpoints), and another 389 showed partial or total similarity to TEs in at least one breakpoint. These results demonstrate that TEs are enriched within inversions and their breakpoints in Asian rice.
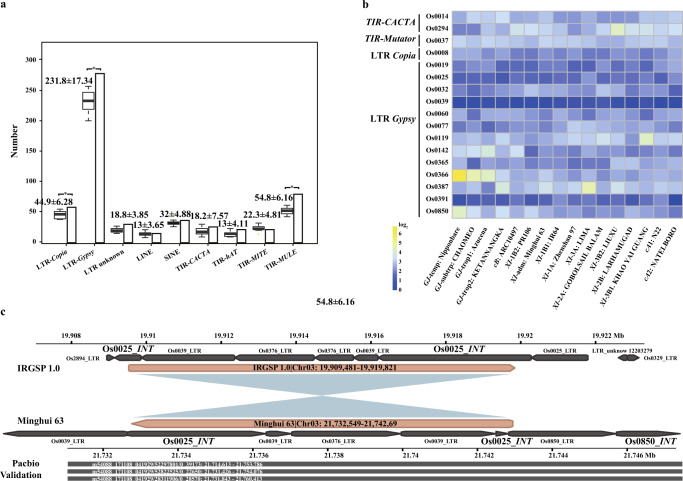
Analysis of these breakpoints revealed that both long terminal repeat retrotransposons (LTR-RTs, i.e., Ty3-Gypsy and Ty1-Copia) and DNA TE Mutator-like elements (MULEs) were significantly enriched (student’s test, p = 5.08E−11 to 0.027), when the frequency of their presence at the 3538 breakpoints was compared to 35,380 randomly selected genomic locations (i.e., ten replicates) (Fig. 5a). We further studied TEs at the breakpoint of each inversion that were shared across all Asian rice genomes. In doing so, we identified 17 TE families (i.e., 13 Ty3-Gypsy, 1 Ty1-Copia, 2 CACTA, and 1 Mutator) present at the breakpoints of more than 10 inversions (Fig. 5b and Supplementary Data 10). An example of an inversion enriched in TEs, including the internal and LTR portions of at least three different LTR-RTs, is shown in Fig. 5c.
Fig. 5. Transposable element analysis of the pan-genome inversions index of rice.
a The amount (y-axis) of different transposable element (TE) families (x-axis) show that three TE families (i.e., LTR-RT Ty1-copia, Ty3-gypsy and DNA-TE MULE) were observed in higher frequencies at the breakpoints of the pan-genome inversion index than the resampled control tests. Box- and bar-plots show the frequencies of TEs observed at the breakpoints of random resampled regions (n = 10 biologically independent resamples) and the pan-genome inversion index, respectively. Each boxplot presents the minimum, first quartile, median, third quartile, and maximum value, and along with mean ± SD (Standard Deviation) are shown. b Enrichment/depletion of 17 TEs present at the inversion breakpoints with more than 10 copies. c Details of Ty3-gypsy Os0025 presence at inversion breakpoints, with support from PacBio long reads. Os0025_LTR (long-terminal repeat), and Os0025_INT (integrase). Source data are provided as a Source Data file.
Since it is known that inverted repeats can trigger ectopic recombination, thereby leading to genome inversions40,41, we interrogated 100 randomly selected O. sativa inversions for direct or inverted repeats in close proximity to inversion breakpoints. This analysis revealed the presence of direct repeats (including microsatellites) at the breakpoints of both ends of 11 inversions, and inverted repeats at single breakpoints of 30 inversions (Supplementary Data 11). For the remaining 118 inversion breakpoints (i.e., 59 inversions), no clear evidence could be found for the presence of inverted repeats at their inversion breakpoints. Of note, our analysis only considered inverted repeats longer than 10 bp, even though shorter have been shown to trigger inversions as well42. For this reason, we caution that our estimate of the presence of inverted repeats at inversion breakpoints should be considered on the lower end of the spectrum.
Characterization of gene content within inversions and their breakpoints
Based on the pan-genome inversion index, we identified a total of ~971 annotated genes per genome within inversions or at their breakpoints (Supplementary Data 12). To investigate the possible effects of inversions on gene expression, we interrogated a set of transcriptome datasets derived from three O. sativa subpopulations (i.e., XI-adm: MH63, XI−1A: ZS97 and GJ-temp: Nipponbare (i.e., dataset#2 - see Methods). Based on a comparison of transcript abundance levels between Nipponbare and MH63 and ZS97 (across four tissue types - root, panicle, young leaf, and mature leaf) we detected that 5–12 genes from MH63 and 4–11 genes from ZS97 within inversions, 2–7 genes from MH63 and 2–4 genes from ZS97 in inverted flanking regions, and 19–42 genes from MH63 and 9–30 genes from ZS97 that were located in non-inverted randomly resampled 20 Kb regions, were differentially expressed (DEG, fold change >2, p value <0.01) (Supplementary Fig. 7).
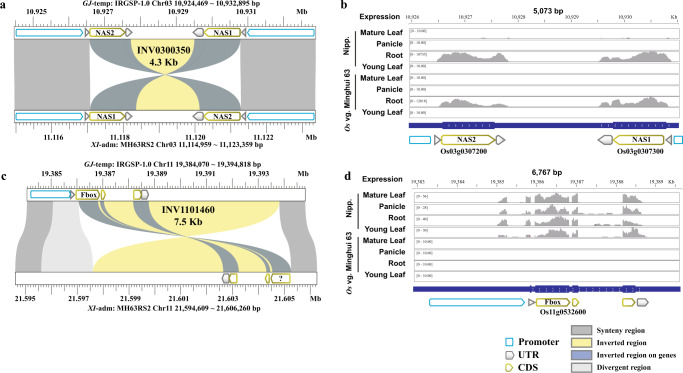
To investigate the effect of inversions on the transcription of genes located at inversion breakpoints - i.e., about 55 genes per genome (Supplementary Data 12), we interrogated both our baseline RNA-Seq datasets (dataset#1- see Methods) and dataset#2 for changes in transcript abundance. On average, transcript evidence for 28 of the 55 genes per genome could be detected in the tissues tested (Supplementary Data 12). Of these, transcript abundance of an average of 20 genes per genome did not change due to the presence of duplicated genes at both ends of their inversion breakpoints (Supplementary Data 12). An example of this observation is represented by two OsNAS genes (NAS1 and NAS2) located at the breakpoints of INV0300350 (~4.3 Kb) (Fig. 6a, b). The remaining ~8 genes per genome were single copy and were disrupted the inversion events, leading to the absence of transcript evidence (Supplementary Data 12). For example, transcripts of the Nipponbare Fbox gene (Os11g0532600) could be detected in the four tissues tested. However, the first exon of this gene was disrupted in MH63 by INV1101460, resulting in transcript ablation (Fig. 6c, d).
Fig. 6. Transcript abundance of genes located at inversion breakpoints.
a Two copies of the OsNAS gene lie at the ends of an inversion in the MH63 (XI-adm) genome. This inversion disrupted the 5’ UTR regions of two OsNAS genes (NAS1 and NAS2); b OsNAS transcript abundance in root tissue; c The coding sequence (CDS) of a Fbox gene was disrupted by an inversion in the MH63 (XI-adm) genome; d Fbox transcript abundance was suppressed in all tissues tested in the MH63 (XI-adm) genome.
Recombination rate and genomic inversions
To evaluate the effect of inversions on recombination frequency, a previously published recombinant inbred line (RIL-10) population of 210 inbred lines43 derived from a cross between O. sativa cv. XI-adm: MH63 and XI−1A: ZS97 was investigated. We detected 78 inversions between MH63 and ZS97, totaling 3.58 Mb and 3.51 Mb in size, based on the MH63 and ZS97 genome assemblies, respectively (Supplementary Data 13). The recombination rate along each chromosome was assessed by comparing genetic and physical distances between neighboring bins. The average recombination rate for each chromosome ranged from 5.95 (chromosome 6) to 9.92 (chromosome 12) cM/Mb, and varied from 0 to 153.93 cM/Mb across the genome with an average of 6.98 cM/Mb (Supplementary Fig. 8a). The average recombination rate over the 78 inverted regions was 4.00 cM/Mb (0–23.26 cM/Mb), which is significantly lower (Student’s t test, p = 0.0002) than that observed genome-wide (Supplementary Fig. 8b). These results indicate that a marked suppression of genetic recombination is associated with inversions.
Effect of large inversions on population SNP variation
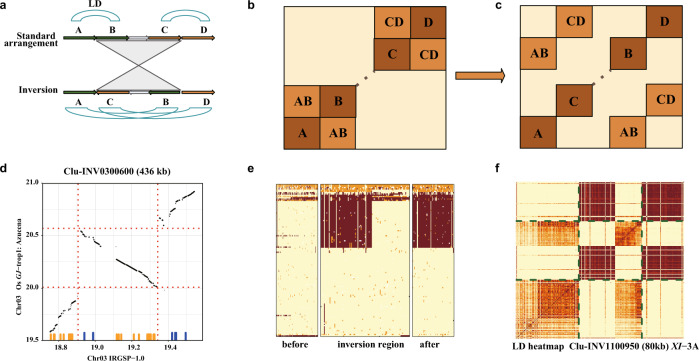
The occurrence of inversions can affect DNA polymorphism at the population level in several ways, including increased divergence in the inverted region and changes in linkage disequilibrium (LD) patterns40. The latter is particularly interesting as it can affect SNPs that are mapped to positions megabases apart, and can be a confounding factor in LD-based analyses. To determine whether large O. sativa inversions (>100 Kb) left a trace in patterns of LD along the IRGSP RefSeq, we used the 3K-RGP dataset to examine LD blocks near inverted regions (n = 88) (Supplementary Data 14). An inversion fixed in a population may lead to the disruption of LD blocks, in which some SNPs flanking the inversion on one side are in LD with SNPs on the distal part of the inversion, but not on the adjacent part (Fig. 7), due to the reversed order of SNPs inside the inverted region in samples that carry the inversion allele. By an LD block we mean only a set of SNPs in high LD (r2 > 0.8 in this analysis).
Fig. 7. Population linkage disequilibrium analysis of large inversions.
A schematic diagram of linkage disequilibrium (LD) block disruption arising from the presence of an inversion, as shown in A and B. a Cartoon view of an inversion with breakpoints disrupting two LD blocks; b Expected features of the corresponding LD heat map; c Example of SNP blocks in high LD that are disrupted by an inversion; d The panel shows alignments, with the inversion marked by dotted lines. Small vertical lines above the horizontal axis mark the location of SNPs constituting a disrupted LD block. Orange and blue colors delineate two LD blocks that are contiguous in the of GJ-trop1 population, but appear as split when aligned to the IRGSP RefSeq (GJ-temp). Disruption of Azucena (GJ-trop1) haplotype blocks along the IRGSP RefSeq in the region of INV030410, as shown in e and f; e Genotype heat map of the GJ-trop1 subpopulation (samples in rows, SNPs in columns; light yellow: reference call, orange: heterozygous, brown: homozygous variant); f LD heat map of the same subpopulation. Dotted lines show the inversion region. Darker colors show larger r2. Note that the scaling of X-axis in the genotype heat map is not uniform, allotting half of X-axis space to the inverted region. Source data are provided as a Source Data file.
Next, we examined the entire 3K-RGP variation data set and searched for LD blocks that connect the flanking regions of inversions, having no SNPs in the proximal parts of each inversion. Such blocks (Fig. 7) were found in nearly all large inversions (126 out of 147 [85.7%], or 74 out of 88 [84%] inversion clusters) (Supplementary Data 14) with exceptions falling into two categories: i.e., inversions in regions of complex chromosomal rearrangements (Chr04:14.1–15 Mb, Chr11:6.6–6.85 Mb, Chr11:9.4–9.7 Mb), and three putative recent inversions (INV0401400, INV0500310, INV1000040), each of which were found in single genomes and may lack sufficient frequencies in a population to contain traces of recombination. Some of the disrupted LD blocks contained a particularly large number of SNPs and were seen as a distinctive checkered pattern on LD heatmaps (Fig. 7). This comparatively large number of SNPs along with low haplotype diversity, despite the presence of recombination, could be a consequence of selective pressure.
Discussion
Inversions are an important class of structural variations that have been shown to play important roles in the suppression of recombination that can lead to the selection of adaptive traits, reproductive isolation and eventual speciation, and are quite common in plants31,40,41. For example, over the 50–60 MY history of the Poaceae, where gene order has been largely conserved, Ahn and Tanksley showed (using molecular genetic maps) that multiple inversions and translocations occurred during the evolution of maize and rice from a common ancestor42.
Here, we present a comprehensive analysis of the inversion landscape of Asian rice at the population structure level with the discovery of 1769 non-redundant inversions that range in cumulative size from 0.3 Mb to 15 Mb (Supplementary Data 3). This analysis allowed us to estimate inversion rates at multiples levels (i.e., genome type (AA vs. BB: 67.4 inversions per MY), AA species (O. sativa vs. O. rufipogon: 320 inversions per MY), and O. sativa populations (K = 15: 735–749 inversions per MY), all of which were 2.1–4.3×, 10.6–21.3×, and 16.6–50× higher than previously estimated40, respectively. Huang and Rieseberg40 predicted that these earlier inversion rate estimates for plants should be considered as underestimates (15–30 inversions per MY), as they were dependent on the quality of the genomes analyzed, and other factors, which bears true with our analyses. These inversion rate estimates over such a short time period may be reflective of high fixation rates of rearrangements in plants44, high chromosomal evolution rates in annual plants45–47, and intense human selection since the dawn of agriculture11,28.
Pan-genomes, first described in bacteria48, can be defined as a representation of the entire set of genes within a species, consisting of a core genome - containing sequences shared between all individuals of the species - and the ‘dispensable’ genome. To date, five pan-genomes have been published for Asian rice (summarized in Table 3), where three reported inversions, none of which were validated. To compare our data with the first reported inversion genome scan11, we extracted the inversion coordinates (i.e., 2402 INVs, average total length 43.3 Kb) from the same set of 15 accessions used to generate our Rice Population Reference Panel and found that only 200 could be validated with dot plots (a 91.7% false positive rate) (Supplementary Table 9), 196 of which overlapped with our inversion index. The 6 remaining contained 2 that overlapped, and only 4 that were not present in our inversion index (Supplementary Data 15). These analyses combined reveal the limitations of inversion callers with short read data and provide a cautionary note as to the validity of many of the inversions cataloged to date.
Table 3.
A comparison of public pan-genome research in Asian rice
| Pan-genome studies | #genomes | Species | Contig N50s | Assembly | Subpopulations (K) | Sequencing Technology | Sequence validation | Inversion callers | Validated | # INVs | Data access |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Wang et al.28 Fuentes et al.11 |
3K | O. sativa | 5.44 ± 2.87 Kb | Contigs/scaffolds | 9 | Illumina | NA | DELLY & LUMPY | NO | 210,301 (raw) | |
| Zhao et al.92 | 66 |
O. sativa, O. rufipogon |
40.5 ± 13 Kb | Contigs | 4 | Illumina | NA | NA | NA | NA | http://www.ncgr.ac.cn/RicePanGenome |
| Qin et al.12 | 33 |
O. sativa, O. glaberrima |
12.6 ± 4.8 Mb | Chromosome | 9 | PacBio |
BioNanao HiC |
Nucmer & SyRI | NO | 954 | http://www.ricerc.com/ |
| Zhang et al.93 | 111 |
O. sativa, O. rufipogon |
7.4 ± 2.1 Mb | Chromosome/scaffolds | 5 & 9 | PacBio & ONT | NA | NA | NA | NA | https://cgm.sjtu.edu.cn/TGSrice/index.html |
| Shang et al.94 | 251 |
O. sativa, O. rufipogon, O. glaberrima, O. barthii |
10.9 ± 3.7 Mb | Contigs | 2/15(174 accessions from 15 subpopulations of 3K-RGP, GJ-trop2 is missing) | ONT | HiC | Minimap2, NGMLR, and Sniffles | NO | 2784 (raw) | http://www.ricesuperpir.com/ |
| Our study | 75 |
O. sativa, O. rufipogon, O. punctata |
22.7 ± 8.2 Mb | Chromosome | 15 | PacBio & ONT | BioNanao | Nucmer & SyRI | YES | 1769 (validated) | https://yongzhou2019.github.io/Rice-Population-Reference-Panel/ |
More recently, Qin et al. identified 718 inversions using 29 PacBio genomes of Asian rice mapped to the IRGSP RefSeq, 557 of which overlapped with our analysis and the remaining 161 could not be validated. Although informative, a combination of genomes that bridge the K = 15 population structure of Asian rice and a minimum of 60 genomes are required for a comprehensive analysis of inversions in Asian rice, as demonstrated here (Fig. 1).
Several key factors led to our ability to generate a definitive pan-genome inversion index for cultivated Asian rice. The first was our use of a set 75 high-quality reference genomes that span the K = 15 population structure of Asian rice32 (Supplementary Data 2), plus 2 high-quality genomes from the wild ancestors of rice that served as phylogenetically anchored outgroup species (Supplementary Table 1). Secondly, we did not computationally collapse these 75 genomes into a pan-genome (e.g., genome graph), but maintained all 75 rice genomes in their native state. This was key to our ability to precisely compare all genomes one-by-one. Lastly, we interrogated the 3K-RGP data to estimate and validate the population genetics of each inversion. As sequencing costs continue to plummet, the ease at which ultra-high-quality genomes can be generated, and with computational power exceeding current limits49–51, we predict that there will no longer be a need to computationally generate pan-genomes to perform similar analyses as demonstrated here across much larger genomes, such as wheat (genome size = 15 Gb)52–54.
The Asian rice pan-genome inversion index is the first step on our quest to precisely discover all standing natural variation that exists in Asian rice and eventually the genus Oryza as a whole. The next step will be the generation of a digital genebank for Asian rice whereby resequencing data from >100,000 accessions will be mapped to our Rice Population Reference Panel. Preliminary data (unpublished) shows that we can now easily call SNPs with resequencing data from >3000 individuals in 5 days per genome or less using high-performance computational workflows optimized for GATK455 software. Such call rates will undoubtedly increase over the next year with a targeted rice digital genebank release date of January 1st, 2025.
Methods
Sequence and assembly
Seed and/or tissue from O. rufipogon (IRGC 106523) and O. punctata (IRGC 105690) species were obtained from the International Rice Research Institute (IRRI), Philippines. The O. rufipogon accession was selected as a true O. rufipogon representative based on its origin (Papua New Guinea, reproductively isolated from rice cultivation) and phenotype. The two genomes were sequenced to a minimum of 100× coverage using PacBio long-read technology (PacBio RSII), and were assembled and validated to a PSRefSeq quality level following the identical strategy as described in Supplementary Note 1. The Benchmarking Universal Single-Copy Orthologs (BUSCO v4.0) software package56 was employed to evaluate the gene space completeness of each assembly.
Genome annotation
Genome annotation used PacBio Iso-Seq and Illumina RNA-Seq data derived from RNA isolated root, panicle, and young leaf tissue from Nipponbare and 13 newly sequenced O. sativa accessions32 as baseline transcript evidence (i.e., RNA-Seq dataset#1). In addition, we collected deep RNA-Seq data from O. sativa cvs. Nipponbare, Minghui 63 and Zhenshan 97 (RNA-Seq dataset#257) for upgrading their annotations with the same pipeline and downstream transcriptome analyses. PacBio Iso-Seq data was deposited in NCBI under BioProject PRJNA760839. The RNA-Seq dataset#1 is deposited in NCBI under BioProject PRJNA659864. RNA-Seq dataset#2 is retrieved from NCBI BioProject PRJNA59707057.
Protein coding genes for the 16 O. sativa genomes above were predicted using MAKER-P (3.01.03)58 including expression evidence, homology and ab initio gene predictors FGENESH (v.8.0.0)59,60, SNAP (0.15.7)59,60. Repeat masking was performed with RepeatMasker (http://www.repeatmasker.org) using an Oryza specific repeat library31. Expression evidence included reference guided transcript assemblies generated using StringTie (v1.3.4a)61 and Cufflinks (v2.2.1)61,62. To generate assembled transcripts, quality inspected RNA-Seq reads from each library were mapped to their respective genomes using STAR (v2.5.3a)63 with an iterative 2-pass mapping approach in which splice junctions generated from the first round were used to refine alignments in the subsequent round. Mapped reads from each library were merged, sorted, and indexed using SAMTools (v1.9)64 to generate input for transcript assembly programs. All software packages were run with default options. High quality full length transcripts were clustered using CD-HIT (v4.6)65 for 95% sequence identity using parameters -c 0.95 -n 10 -d 0 -M 3000. The clustered transcripts were further filtered for intron retention events using SUPPA266. Additional transcript and homology evidence was used31 to run MAKER-P with keep_preds option set to 1. The gene structure of the predicted models was further improved using PASA (v2.4.1)67 using full length cDNA and EST’s downloaded from GenBank with the query “EST [Keyword] AND Oryza sativa [Organism]”. Functional domain identification was completed with InterProScan (v5.38-76.0)68. TRaCE (https://github.com/warelab/TRaCE)69 was used to assign canonical transcripts based on domain coverage, protein length, and similarity to transcripts assembled by Stringtie (v1.3.4a)61. Annotation quality was assessed with MAKER-P generated Annotation Edit Distance (AED) values70 and BUSCO, respectively. Only transcripts with AED scores <1 were retained. Finally, gene annotations were imported to Ensembl core databases, verified, and validated for translation using the Ensembl API71. All genome annotations are available at the Gramene Pan Oryza database (https://oryza.gramene.org/) and the Rice Population Reference Panel (RPRP, https://yongzhou2019.github.io/Rice-Population-Reference-Panel/data/).
Genome visualization
For alignment, analysis, visualization, and public availability, we uploaded the 18-genome data package into the Persephone® multi-genome browser (https://web.persephonesoft.com/). The types of data tracks visualized include gene models, marker locations, BLAST matches, RNA-Seq coverage and sequence tracks. As the genomes are closely related, the maps could be aligned by connecting short sequence tags (100 bp) derived from the IRGSP-1.0 RefSeq and mapped onto the other 17 genomes using BLASTN, as shown in Supplementary Fig. 2.
Transposable element annotation
All 16 cultivated O. sativa, plus the O. rufipogon and O. punctata genomes were re-annotated using the output of the latest version of the EDTA (v1.7.4)72 transposable element (TE) annotation pipeline. The entire output was loaded into RepeatMasker (v 4.0.8)73, with the exception of predicted helitron elements that were skipped because of a high false positive rate.
Identification of genomic inversions and assessments
To discover large inversions (>100 bp), we first tested four different analysis workflows (https://gitlab.kaust.edu.sa/zhouy0e/sv-for-o.sativa)74 on two genomes, i.e., GJ-temp: IRGSP-1.0 and XI-adm: MH63 (the genome of Minghui 63 accession).
Workflow 1: The MH63 genome assembly was split into overlapping reads of 50 Kb in length at 5 Kb step intervals, resulting in ~10× coverage. The reads were then mapped onto the IRGSP-1.0 genome sequence using the tool CoNvex Gap-cost alignMents for Long Reads (NGMLR, v0.2.7)75. Inversions were called with SVIM (v1.1.0)76, retaining only inversions with a depth greater > 6 that passed the caller’s filtration criteria.
Workflow 2: Steps were the same as in workflow 1, except that Sniffles (v1.0.7)75 was used to call inversions.
Workflow 3: The MH63 genome assembly was aligned to IRGSP-1.0 reference sequence using Minimap2 (v 2.15)77. This step was followed by filtration for identity greater than 90% and length longer than 100 bp. Inversions were called using SyRI (i.e., Synteny and Rearrangement Identifier, v1.4)78.
Workflow 4: Steps were the same as in workflow3, except the alignment tool used, i.e., Nucmer, included with the MUMmer (v4)79 software package.
To assess the accuracy of the four workflows, dot-plots of the syntenic regions including the putative inversions were generated to visually validate the inversions. Out of all workflows, workflow4 was selected based on our validation criteria. As a final validation check of workflow 4, PacBio raw reads were also applied for validation of a subset inversions. Raw reads of query genomes were mapped to the IRGSP RefSeq, and the assemblies of the query genomes themselves. If breakpoints could be observed in the reference but not in the assemblies of the query genomes, the breakpoints were called as ‘supported’ by the PacBio raw reads, and vice versa.
Subsequently, the sequences of all 74 genomes were aligned to the IRGSP RefSeq with the MUMmer79, filtering the alignments for a minimum identity of 90%, and minimum length of 100 bp, and the coordinates were retrieved using the function “show-coords” of MUMmer79. Finally, inversions were called using the SyRI tool (v1.4)78 with default parameters, which provided VCFs (v.3) with ID, start, end of reference and query genome coordinates that was leveraged for pan-genome comparisons downstream.
Pan-genome inversion index of 75 high-quality genomes
Seventy-four inversion vcf files derived from 74 genomes compared to the IRGSP RefSeq were generated. Briefly, vcf files were sorted according to chromosome ID, coordinates and strand, and were merged using SURVIVOR (v1.0.7)80, run under default parameters (https://github.com/fritzsedlazeck/SURVIVOR/wiki), except for the maximum allowed distance of 10 bp. In this case, inversions having start and end coordinates no more different than 10 bp were collapsed and considered as single inversions.
Genome-wide distribution of the pan-genome inversion index
To investigate the genome-wide distribution of the pan-genome inversion index the Kolmogorov–Smirnov (KS) test was performed on the coordinates of inversions81,82. For each chromosome, we performed 10,000 simulations of uniformly distributed positions of the same number as the inversions reported for each chromosome and calculated p values (the rate of uniform inversion distribution in simulations (p values > 0.05) based on the Monte Carlo method. In addition, inversions were further classified into different lengths (<1 Kb, 1–5 Kb, 5–10 Kb, and >10 Kb) and tested for uniform distribution independently. The p values were adjusted using the Benjamini–Hochberg procedure83,84 to reduce the false positive rate on a per-chromosome basis.
Inversion rate estimations
To estimate the inversion rate (IR) across the pan-genome of Asian rice, we considered pairs of populations or genomes with existing estimates of divergence times to a most recent common ancestor (MRCA), and divided the total number of inversions by twice the time to the MRCA (TMRCA, corresponding to the total branch length of the genealogy on two nodes), i.e. the estimate is calculated using the following equation:
| 1 |
Differential transcript abundance analysis within inversions
To investigate the effect of an inversion on gene expression, we searched for differences in transcript abundance within inversions in Asian rice based on two RNA-Seq datasets (dataset#1 and dataset#2). FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) values were identified following an accurate pipeline85, which included HISAT2 (version 2.2.1)86 for alignment, and StringTie (v1.3.4a)61 to obtain normalized FPKM values. Taking into account different transcript abundances between the two RNA-Seq datasets (2 Gb for dataset#1 vs 6 Gb for dataset#2), we used different minimum filtration of, i.e., FPKM > 0.1 in RNA-Seq dataset#1 and FPKM > 1 in RNA-Seq dataset#2. Differential Transcript Abundance (DTA) of dataset#2, was carried out by edgeR (3.1.0)87 with p value <0.01 and abundance change >2 cutoffs.
Genome recombination rate of inversions
To study the genome recombination rate of inversions, we used genetic data based on a RIL population derived from accessions O. sativa cv. XI-adm: Minghui 63 and XI−1A: Zhenshan 9788,89. Briefly, re-sequencing data was obtained from 210 RILs and a Bin Map containing 1619 bins was generated. The physical positions of each bin were derived from updated versions both the MH63 and ZS97 reference genomes89. Then, a genetic map based on the RILs panel of 1619 bins was constructed using the MSTMap algorithm90. By comparing genetic and physical distances between neighboring Bin Map markers, we estimated the relative changes of the recombination rate of the two genomes91. Then, we compared recombination rates in the bins that overlapped with inversions and genome-wide, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was supported by King Abdullah University of Science & Technology’s Baseline funding, and the University of Arizona’s Bud Antle Endowed Chair for Excellent in Agriculture to R.A.W.; Huazhong Agricultural University’s Start-up Fund, Fundamental Research Funds for the Central Universities (2662020SKPY010), and the Major Project of Hubei Hongshan Laboratory (2022HSZD031) to J.Z.; USDA ARS 8062- 21000-041-00D to D.W. The authors acknowledge support from the Shaheen Cray XC40 platform at KAUST Supercomputing Laboratory, the computing platform of the National Key Laboratory of Crop Genetic Improvement at HZAU, Peter VanBuren for systems support and the Elzar High Performance Computing facility (NIH S10 OD0286321-01) at Cold Spring Harbor Laboratory for providing computational resources, and help from the Persephone team that hosts our 18-genome data package on their portal for genome visualization.
Source data
Author contributions
A.Z., D.W., K.L.M., J.Z., and R.A.W. designed and conceived the research. K.M. and IRRI provided seed and/or tissue for all Oryza accessions. D.K., N.M., D.Ch., and M.L. performed DNA extractions and genome sequencing. Y.Z., Z.Y., and J.Z. performed sequence assembly, GPM edit and validation of 18 genome sequences. Y.Z., N.M., D.K. and V.L. carried out the optical map sequence and analysis. D.Ch., Y.Z., J.S. and K.L.M. performed population genetic analysis. K.C., Z.L., and D.W. performed the genome annotation and validation. Y.Z., Z.Y., J.Z. and A.Z. performed the gene expression analysis and TE annotation. Y.Z., A.Z., J.S., A.A., S.M., Z.Y., and J.Z. carried out the inversion identification and population level validation. M.Th., L.R. and Y.Z. performed the machine learning study for inversion detection. L.R., N.K., M.Tr., C.D.G., and K.C. managed the computing platforms. Y.Z., Z.Y., D.Co., K.C., N.A., A.F., A.Z., K.L.M., J.Z., and R.A.W. wrote and edited the paper. All authors read and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Nils Stein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All sequencing data has been submitted to NCBI. The assembly data of IRGSP-1.0/NIPPONBARE (GCA_001433935.1), CHAO MEO::IRGC 80273-1 (GCA_009831315.1), Azucena (GCA_009830595.1), KETAN NANGKA::IRGC 19961-2 (GCA_009831275.1), ARC 10497::IRGC 12485-1 (GCA_009831255.1), PR 106::IRGC 53418-1 (GCA_009831045.1), Minghui 63 (GCA_001623365.2), IR 64 (GCA_009914875.1), Zhenshan 97 (GCA_001623345.2), LIMA::IRGC 81487-1 (GCA_009829395.1), KHAO YAI GUANG::IRGC 65972-1 (GCA_009831295.1), GOBOL SAIL (BALAM)::IRGC 26624-2 (GCA_009831025.1), LIU XU::IRGC 109232-1 (GCA_009829375.1), LARHA MUGAD::IRGC 52339-1 (GCA_009831355.1), N22 (N 22::IRGC 19379-1) (GCA_001952365.2), NATEL BORO::IRGC 34749-1 (GCA_009831335.1), O. rufipogon PNG91-7::IRGC 106523-1 (GCA_023541355.1), and O. punctata:: IRGC 105690 (GCA_000573905.2) were deposited in GeneBank of NCBI. The raw sequencing data of O. rufipogon and O. punctata generated in this study were deposited in NCBI under BioProject PRJNA609053 and PRJNA13770, respectively. PacBio Iso-Seq data for all accessions in this study was deposited in NCBI under BioProject PRJNA760839. The RNA-Seq dataset#1 was deposited in NCBI under BioProject PRJNA659864. RNA-Seq dataset#2 was retrieved from NCBI BioProject PRJNA597070. The details are also listed in Table 1 and the Rice Population Reference Panel [https://yongzhou2019.github.io/Rice-Population-Reference-Panel/data/]. Source data are provided with this paper.
Code availability
Code for the different inversion calling workflows are available at the Rice Population Reference Panel [https://yongzhou2019.github.io/Rice-Population-Reference-Panel/software/]74.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yong Zhou, Zhichao Yu, Dmytro Chebotarov, Kapeel Chougule.
These authors jointly supervised this work: Andrea Zuccolo, Doreen Ware, Kenneth McNally, Jianwei Zhang, Rod A. Wing.
Contributor Information
Andrea Zuccolo, Email: andrea.zuccolo@kaust.edu.sa.
Doreen Ware, Email: Doreen.ware@usda.gov.
Kenneth McNally, Email: k.mcnally@irri.org.
Jianwei Zhang, Email: jzhang@mail.hzau.edu.cn.
Rod A. Wing, Email: rod.wing@kaust.edu.sa
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-37004-y.
References
- 1.Hossain M, Fischer K. Rice research for food security and sustainable agricultural development in Asia: achievements and future challenges. GeoJournal. 1995;35:286–298. doi: 10.1007/BF00989136. [DOI] [Google Scholar]
- 2.Wing RA, Purugganan MD, Zhang Q. The rice genome revolution: from an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018;19:505–517. doi: 10.1038/s41576-018-0024-z. [DOI] [PubMed] [Google Scholar]
- 3.Vollset SE, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285–1306. doi: 10.1016/S0140-6736(20)30677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturtevant AH. A case of rearrangement of genes in Drosophila. Proc. Natl Acad. Sci. USA. 1921;7:235. doi: 10.1073/pnas.7.8.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkert FC, Broach JR. Site-specific recombination promotes plasmid amplification in yeast. Cell. 1986;46:541–550. doi: 10.1016/0092-8674(86)90879-2. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, R. C. Bacterial site-specific DNA inversion systems. In Mobile DNA II 230-271 (American Society of Microbiology, 2002).
- 7.Zapata L, et al. Chromosome-level assembly of Arabidopsis thaliana Ler reveals the extent of translocation and inversion polymorphisms. Proc. Natl Acad. Sci. USA. 2016;113:E4052–E4060. doi: 10.1073/pnas.1607532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonierbale, M. W., Plaisted, R. L. & Tanksley, S.D. RFLP maps based on a common set of clones reveal modes of chromosomal evolution in potato and tomato. Genetics.120, 1095–1103 (1988). [DOI] [PMC free article] [PubMed]
- 9.Zhou, C. et al. Genome-scale analysis of homologous genes among subgenomes of bread wheat (Triticum aestivum L.). Int. J. Mol. Sci.21, 3015 (2020). [DOI] [PMC free article] [PubMed]
- 10.Crow T, et al. Gene regulatory effects of a large chromosomal inversion in highland maize. PLoS Genet. 2020;16:e1009213. doi: 10.1371/journal.pgen.1009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes RR, et al. Structural variants in 3000 rice genomes. Genome Res. 2019;29:870–880. doi: 10.1101/gr.241240.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin P, et al. Pan-genome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell. 2021;184:3542–3558. doi: 10.1016/j.cell.2021.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Hammer MF, Schimenti J, Silver LM. Evolution of mouse chromosome 17 and the origin of inversions associated with t haplotypes. Proc. Natl Acad. Sci. USA. 1989;86:3261–3265. doi: 10.1073/pnas.86.9.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores M, et al. Recurrent DNA inversion rearrangements in the human genome. Proc. Natl Acad. Sci. USA. 2007;104:6099–6106. doi: 10.1073/pnas.0701631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellen, E. H. Inversions and evolution of the human genome. eLS 1–6 (2015).
- 16.Hey J. Speciation and inversions: chimps and humans. Bioessays. 2003;25:825–828. doi: 10.1002/bies.10336. [DOI] [PubMed] [Google Scholar]
- 17.Levy-Sakin M, et al. Genome maps across 26 human populations reveal population-specific patterns of structural variation. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-08992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karageorgiou, C., Gámez-Visairas, V., Tarrío, R. & Rodríguez-Trelles, F. Long-read based assembly and synteny analysis of a reference Drosophila subobscura genome reveals signatures of structural evolution driven by inversions recombination-suppression effects. BMC Genomics20, 223 (2019). [DOI] [PMC free article] [PubMed]
- 19.Da Silva, V. H. et al. The genomic complexity of a large inversion in great tits. Genome Biol. Evol.11, 1870–1881 (2019). [DOI] [PMC free article] [PubMed]
- 20.Lakich, D., Kazazian, H. H., Antonarakis, S. E. & Gitschier, J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat. Genet.5, 236–241 (1993). [DOI] [PubMed]
- 21.Webb, A. et al. Role of the tau gene region chromosome inversion in progressive supranuclear palsy, corticobasal degeneration, and related disorders. Arch. Neurol.65, 1473–1478 (2008). [DOI] [PMC free article] [PubMed]
- 22.Salm MP, Horswell SD, Hutchison CE. The origin, global distribution, and functional impact of the human 8p23 inversion polymorphism[J] Genome Res. 2012;22:1144–1153. doi: 10.1101/gr.126037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannesson, M. et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 48, 624–633 (2016). [DOI] [PMC free article] [PubMed]
- 24.De Jong, S. et al. Common inversion polymorphism at 17q21. 31 affects expression of multiple genes in tissue-specific manner. BMC Genomics13, 458 (2012). [DOI] [PMC free article] [PubMed]
- 25.Huang, K., Andrew, R. L., Owens, G. L., Ostevik, K. L. & Rieseberg, L. H. Multiple chromosomal inversions contribute to adaptive divergence of a dune sunflower ecotype. Mol. Ecol.29, 2535–2549 (2020). [DOI] [PubMed]
- 26.Lee, C.-R. et al. Young inversion with multiple linked QTLs under selection in a hybrid zone. Nat. Ecol. Evol.1, 0119 (2017). [DOI] [PMC free article] [PubMed]
- 27.Guan, J. et al. Genome structure variation analyses of peach reveal population dynamics and a 1.67 Mb causal inversion for fruit shape. Genome Biol.22, 1–25 (2021). [DOI] [PMC free article] [PubMed]
- 28.Wang W, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018;557:43–49. doi: 10.1038/s41586-018-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, et al. The 3000 rice genomes project. Gigascience. 2014;3:7. doi: 10.1186/2047-217X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kou Y, et al. Evolutionary genomics of structural variation in Asian rice (Oryza sativa) domestication. Mol. Biol. Evol. 2020;37:3507–3524. doi: 10.1093/molbev/msaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JC, et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza. Nat. Genet. 2018;50:285–296. doi: 10.1038/s41588-018-0040-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, et al. A platinum standard pan-genome resource that represents the population structure of Asian rice. Sci. Data. 2020;7:113. doi: 10.1038/s41597-020-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, et al. Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc. Natl Acad. Sci. USA. 2016;113:E5163–E5171. doi: 10.1073/pnas.1611012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawahara Y, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, Q. & Ge, S. J. Phylogenetic relationships among A‐genome species of the genus Oryza revealed by intron sequences of four nuclear genes. New Phytol.167, 249–265 (2005). [DOI] [PubMed]
- 36.Kim H, et al. Comparative physical mapping between Oryza sativa (AA genome type) and O. punctata (BB genome type) Genetics. 2007;176:379–390. doi: 10.1534/genetics.106.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutaker, R. M. et al. Genomic history and ecology of the geographic spread of rice. Nat. Plants6, 492–502 (2020). [DOI] [PubMed]
- 38.Rosenberg NA, Feldman MW. The relationship between coalescence times and population divergence times. Mod. Dev. Theor. Popul. Genet. 2002;130:164. [Google Scholar]
- 39.Alexandrov, N. et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res.43, D1023-D1027 (2015). [DOI] [PMC free article] [PubMed]
- 40.Huang K, Rieseberg LH. Frequency, origins, and evolutionary role of chromosomal inversions in plants. Front. Plant Sci. 2020;11:296. doi: 10.3389/fpls.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClintock, B. Cytological observations of deficiencies involving known genes, translocations and an inversion in Zea mays, (University of Missouri, College of Agriculture, Agricultural Experiment Station, 1931).
- 42.Ahn S, Tanksley S. Comparative linkage maps of the rice and maize genomes. Proc. Natl Acad. Sci. USA. 1993;90:7980–7984. doi: 10.1073/pnas.90.17.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Longbiao, et al. Genetic Analysis and Utilization of the Important Agronomic Traits on Zhenshan 97* Minghui 63 Recombinant Inbred Lines (RIL) in Rice (Oryza sativa L.) Zuo Wu Xue Bao. 2002;28:644–649. [Google Scholar]
- 44.Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu. Rev. Ecol. Evol. Syst. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke JM, et al. Comparative mapping and rapid karyotypic evolution in the genus helianthus. Genetics. 2004;167:449–457. doi: 10.1534/genetics.167.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husband, B. Chromosomal variation in plant evolution (JSTOR, 2004).
- 47.Levin, D. A. & Donald, A. The role of chromosomal change in plant evolution (Oxford University Press, USA, 2002).
- 48.Tettelin, H. et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. 102, 13950–13955 (2005). [DOI] [PMC free article] [PubMed]
- 49.Gage JL, Monier B, Giri A, Buckler ES. Ten years of the maize nested association mapping population: impact, limitations, and future directions. Plant Cell. 2020;32:2083–2093. doi: 10.1105/tpc.19.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Computational Pan-Genomics Consortium. Computational pan-genomics: status, promises and challenges. Brief. Bioinform. 2018;19:118–135. doi: 10.1093/bib/bbw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernikos, G. S. A review of pangenome tools and recent studies. In The Pangenome: Diversity, Dynamics and Evolution of Genomes (eds. Tettelin, H. & Medini, D.) 89–112 (Cham (CH), 2020). [PubMed]
- 52.Athiyannan N, et al. Long-read genome sequencing of bread wheat facilitates disease resistance gene cloning. Nat. Genet. 2022;54:227–231. doi: 10.1038/s41588-022-01022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science361, eaar7191 (2018). [DOI] [PubMed]
- 54.International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science345, 1251788 (2014). [DOI] [PubMed]
- 55.Bathke J, Lühken G. OVarFlow: a resource optimized GATK 4 based Open source Variant calling workFlow. BMC Bioinforma. 2021;22:1–18. doi: 10.1186/s12859-021-04317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 57.Zhao L, et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 2020;11:2658. doi: 10.1038/s41467-020-16457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell MS, Holt C, Moore B, Yandell M. Genome annotation and curation using MAKER and MAKER‐P. Curr. Protoc. Bioinforma. 2014;48:4.11. 1–4.11. 39. doi: 10.1002/0471250953.bi0411s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salamov AA, Solovyev VV. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 2000;10:516–522. doi: 10.1101/gr.10.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korf I. Gene finding in novel genomes. BMC Bioinforma. 2004;5:59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 66.Trincado JL, et al. SUPPA2: fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 2018;19:40. doi: 10.1186/s13059-018-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haas BJ, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31:5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson, A. J. & Ware, D. Ranked choice voting for representative transcripts with TRaCE. Bioinformatics38, 261–264 (2021). [DOI] [PMC free article] [PubMed]
- 70.Campbell MS, et al. MAKER-P: a tool kit for the rapid creation, management, and quality control of plant genome annotations. Plant Physiol. 2014;164:513–524. doi: 10.1104/pp.113.230144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stabenau A, et al. The Ensembl core software libraries. Genome Res. 2004;14:929–933. doi: 10.1101/gr.1857204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ou S, et al. Benchmarking transposable element annotation methods for creation of a streamlined, comprehensive pipeline. Genome Biol. 2019;20:275. doi: 10.1186/s13059-019-1905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinforma. 2009;4:4–10. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 74.Zhou, Y. et al. Pan-genome inversion index reveals evolutionary insights into the subpopulation structure of Asian rice (Oryza sativa). Github10.5281/zenodo.7636314 (2022). [DOI] [PMC free article] [PubMed]
- 75.Sedlazeck FJ, et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heller D, Vingron M. SVIM: structural variant identification using mapped long reads. Bioinformatics. 2019;35:2907–2915. doi: 10.1093/bioinformatics/btz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goel M, Sun H, Jiao WB, Schneeberger K. SyRI: finding genomic rearrangements and local sequence differences from whole-genome assemblies. Genome Biol. 2019;20:277. doi: 10.1186/s13059-019-1911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delcher AL, Salzberg SL, Phillippy AM. Using MUMmer to identify similar regions in large sequence sets. Curr. Protoc. Bioinforma. 2003;00:10.3.1–10.3.18. doi: 10.1002/0471250953.bi1003s00. [DOI] [PubMed] [Google Scholar]
- 80.Jeffares DC, et al. Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nat. Commun. 2017;8:14061. doi: 10.1038/ncomms14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massey FJ., Jr The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951;46:68–78. doi: 10.1080/01621459.1951.10500769. [DOI] [Google Scholar]
- 82.Virtanen P, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B. 1995;57:289–300. [Google Scholar]
- 84.Seabold, S. & Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference Vol. 57 61 (Austin, TX, 2010).
- 85.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim D, Langmead B, Salzberg SJNM. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hua J, et al. Genetic dissection of an elite rice hybrid revealed that heterozygotes are not always advantageous for performance. Genetics. 2002;162:1885–1895. doi: 10.1093/genetics/162.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou G, et al. Genetic composition of yield heterosis in an elite rice hybrid. Proc. Natl Acad. Sci. USA. 2012;109:15847–15852. doi: 10.1073/pnas.1214141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, Bhat PR, Close TJ, Lonardi S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet. 2008;4:e1000212. doi: 10.1371/journal.pgen.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, et al. Physical maps and recombination frequency of six rice chromosomes. Plant J. 2003;36:720–730. doi: 10.1046/j.1365-313X.2003.01903.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Q, et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018;50:278–284. doi: 10.1038/s41588-018-0041-z. [DOI] [PubMed] [Google Scholar]
- 93.Zhang, F. et al. Long-read sequencing of 111 rice genomes reveals significantly larger pan-genomes. Genome Res.32, 853–863 (2022). [DOI] [PMC free article] [PubMed]
- 94.Shang, L. et al. A super pan-genomic landscape of rice. Cell Res.32, 878–896 (2022). [DOI] [PMC free article] [PubMed]
- 95.Sokal RRJSA. Numerical taxonomy. Scientific American. 1966;215:106–117. doi: 10.1038/scientificamerican1266-106. [DOI] [Google Scholar]
- 96.Kumar, S., Stecher, G., Tamura, K. J. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.33, 1870–1874 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All sequencing data has been submitted to NCBI. The assembly data of IRGSP-1.0/NIPPONBARE (GCA_001433935.1), CHAO MEO::IRGC 80273-1 (GCA_009831315.1), Azucena (GCA_009830595.1), KETAN NANGKA::IRGC 19961-2 (GCA_009831275.1), ARC 10497::IRGC 12485-1 (GCA_009831255.1), PR 106::IRGC 53418-1 (GCA_009831045.1), Minghui 63 (GCA_001623365.2), IR 64 (GCA_009914875.1), Zhenshan 97 (GCA_001623345.2), LIMA::IRGC 81487-1 (GCA_009829395.1), KHAO YAI GUANG::IRGC 65972-1 (GCA_009831295.1), GOBOL SAIL (BALAM)::IRGC 26624-2 (GCA_009831025.1), LIU XU::IRGC 109232-1 (GCA_009829375.1), LARHA MUGAD::IRGC 52339-1 (GCA_009831355.1), N22 (N 22::IRGC 19379-1) (GCA_001952365.2), NATEL BORO::IRGC 34749-1 (GCA_009831335.1), O. rufipogon PNG91-7::IRGC 106523-1 (GCA_023541355.1), and O. punctata:: IRGC 105690 (GCA_000573905.2) were deposited in GeneBank of NCBI. The raw sequencing data of O. rufipogon and O. punctata generated in this study were deposited in NCBI under BioProject PRJNA609053 and PRJNA13770, respectively. PacBio Iso-Seq data for all accessions in this study was deposited in NCBI under BioProject PRJNA760839. The RNA-Seq dataset#1 was deposited in NCBI under BioProject PRJNA659864. RNA-Seq dataset#2 was retrieved from NCBI BioProject PRJNA597070. The details are also listed in Table 1 and the Rice Population Reference Panel [https://yongzhou2019.github.io/Rice-Population-Reference-Panel/data/]. Source data are provided with this paper.
Code for the different inversion calling workflows are available at the Rice Population Reference Panel [https://yongzhou2019.github.io/Rice-Population-Reference-Panel/software/]74.