Abstract
The duration and protectiveness of antibodies against SARS-CoV-2 in infected subjects are still uncertain; nonetheless, anti-S-specific antibodies can contribute to protective immunity against new infections. It has been described that the level of antibodies produced in COVID-19 is related to the severity of symptoms, and the majority of the humoral response studies have been conducted in hospitalized patients who have been, then, followed over time. However, about 80% of SARS-CoV-2 infections in unvaccinated people are mild to asymptomatic, and this percentage reaches more than 95% in vaccinated individuals. Therefore, understanding the long-term dynamics of the antibody responses in this predominant part of the COVID-19-affected population is essential. In this study, we followed a cohort of individuals with mild COVID-19 who did not require hospitalization. We collected blood samples at sequential times after the SARS-CoV-2-positive qRT-PCR result. From 65 recruited patients, 50 had detectable antibodies at screening. Anti-SARS-CoV-2 IgM levels peaked around two weeks post-COVID-19 diagnostics, becoming undetectable after 65 days. IgG levels reached a peak in approximately one month and remained detectable for more than one year. In contrast to the levels of anti-SARS-CoV-2, antibody neutralization potency indexes persisted over time. In this study, humoral responses in mild COVID-19 patients persisted for more than one year. This is an important long-term follow-up study that includes responses from COVID-19 patients before and after vaccination, a scenery that has become increasingly difficult to evaluate due to the growing vaccination of the world human population.
Keywords: COVID-19, humoral responses to SARS-CoV-2, vaccination, IgM, IgG, neutralizing antibodies
Impact Statement
Here, we describe patterns of humoral responses to SARS-CoV-2 in a follow-up study of COVID-19 patients before vaccination. Then, part of the cohort was vaccinated, and part was not, as we continued to follow anti-SARS-CoV-2 antibodies dynamics. We detected a high degree of antibody response’s heterogeneity in naïve patients who were infected by SARS-CoV-2. We believe our results and conclusions are relevant and worth publishing in such a prestigious Journal as EBM for two other reasons: first, our cohort is composed of mild COVID-19 patients, who were not hospitalized during their illnesses. This cohort represents more than 80% of all infected people worldwide; nonetheless, most studies on humoral responses to SARS-CoV-2 were conducted in hospitalized patients presenting moderate to severe disease. Second, studies in SARS-CoV-2 naïve patients are nearly impossible nowadays, due to the high global attack rate of the virus, as well as the crescent vaccination levels.
Introduction
Since declared a pandemic public health emergency in March 2020, the coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 infections has caused approximately 6.4 million deaths in almost three years. 1 This disease can vary from severe illness to asymptomatic infection, 2 but most affected patients do not develop severe disease and do not need hospitalization. 3 In most cases, individuals with positive RT-qPCR diagnostic develop specific antibodies against the surface Spike (S) glycoprotein and nucleocapsid (N) within one to two weeks post infection, 4 and meanwhile, a percentage ranging between 10% and 20% show undetectable specific antibodies. 5 Understanding the dynamics of antibodies produced against SARS-CoV-2 proteins is important both to diagnose past infections in seroprevalence and/or surveillance studies and to verify protection against future infections.
The duration and neutralizing ability of antibodies are still subject to debate, especially after mild infections. It has been demonstrated that critically ill patients usually display hallmarks of extrafollicular B cell activation and produce high levels of low-potency neutralizing antibodies.6,7 Nonetheless, about 80% of all SARS-CoV-2 infections are mild to asymptomatic,3,8,9 and understanding the dynamics of anti-SARS-CoV-2 antibody responses of this dominant portion of COVID-19 affected population is extremely relevant to define public health strategies or even in terms of predictions about the future of COVID-19 amid us. Here, we investigated the antibody dynamics in mild COVID-19 patients over a period of one year after the onset of disease. The evaluated population included non-vaccinated and vaccinated individuals, and results showed important differences in these two subpopulations. Nonetheless, overall, our follow-up study indicates that anti-SARS-CoV-2 antibodies are long-lasting.
Material and methods
Ethics and recruitment
Sixty-five participants were recruited with the following inclusion criteria: positive qRT-PCR result for SARS-CoV-2 or inconclusive qRT-PCT result and a reagent result in the rapid DPP COVID-19 IgM/IgG Bio-Manguinhos test. Exclusion criteria were negative qRT-PCR result and no detectable antibodies until the fourth blood collection (see Figure 1). Demographic information, medical history, and COVID-19 symptoms were obtained by filling out electronic forms. This study included subjects who did not require hospitalization. The study was approved by the Ethics Committee of the Federal University of Minas Gerais (UFMG) (CAAE: 1686320.0.0000.5149). The subjects signed the free and informed consent form (TCLE) to enroll in the study.
Figure 1.
Study design and follow-up of participants. The blood sampling chronogram is divided into three stages: recruitment of COVID-19 mildly affected patients, screening within a follow-up time of three months, and the division of the cohort between vaccinated and unvaccinated individuals. Created with BioRender.com.
Sample collection methodology and chronogram
At first, individuals had blood samples collected at four sequential times using the RT-qPCR result positive as a set point: T1 (day 7), T2 (day 10), T3 (day 14) to T4 (day 29 if there were detectable specific antibodies if not, T4 took place one week after T3). Subjects with undetectable specific antibodies until T4 were unenrolled from the study. The others had two more blood collections: T5 with 60 days and T6 with 92 days. After that point the cohort was split into two groups: those who remained unvaccinated, with two more blood draws taking place, T7 and T8 (six months and one year after RT-qPCR result); and those who received either Coronavac (inactivated) or Pfizer (mRNA) vaccines. These individuals were subjected to five more collections: T7, T8, T9, T10, and T11 (two weeks after the first dose, three weeks, five and eight months after the second Coronavac vaccine dose) and one month after a Pfizer vaccine booster dose. The complete study design is shown in Figure 1.
DPP/ELISA
Serum samples were characterized by the rapid test DPP COVID-19 IgM/IgG Bio-Manguinhos (Lot 204EXVD01Z) following the manufacturer’s instructions and by enzyme-linked immunosorbent assay (ELISA) for detection of IgM-type antibodies using a combination of N (300 ng) and S (200 ng) proteins. For the detection of IgG-type antibodies, an ELISA containing N (400 ng) and S (400 ng) proteins was used as described. 10
Plaque reduction neutralization assay
Serum samples were tested at Biosafety level 3 facilities, in duplicate, days apart, as previously described. 11 The PRNT50 was defined as the highest sample dilution that showed 50% reduction in number of plaques formed compared with positive control, which consisted in the number of plaques in wells inoculated with SARS-CoV-2 alone.
Statistical analysis
We used standard descriptive statistics analysis to summarize data. The ELISA index was calculated as OD/cut-off. 12 For PRNT50 analysis, median for each sample was calculated as a mean of two duplicates. Sera dilutions were transformed in Log(X) and a nonlinear regression (curve fit) was made. The neutralizing potency index (NPI) was calculated as the PRNT50/ELISA index ratio where NPI < 100 are considered low neutralization potency index and >100 are considered high NPI as previously described. 13 Statistical significance between groups medians was determined by t-test. All data were analyzed using GraphPad Prism Software (version 8.0.1).
Results
Cohort description
Of the 65 subjects with SARS-CoV-2 infection confirmed by RT-qPCR, only 50 had antibody responses detected during the screening process. The cohort consisted of 58% women (29/50) and 42% men (21/50). The average age was 35.9 years (from 21 to 58). Ethnicity information was not collected on this cohort. Forty-two percent (21–50) were health care workers. Seventy-five percent had no declared comorbidities, and the others declared comorbidities that included, not exclusively, obesity, arterial hypertension, pulmonary disease, diabetes mellitus, thyroid changes, and cardiopathy. The most frequent COVID-19 reported symptoms were anosmia (80%), fatigue (77%), headache (61%), and running nose (52%). The duration of the symptoms varied considerably, but 8–14 days were the most frequent (36% of the cohort) (see Supplementary Material 1).
IgM and IgG levels
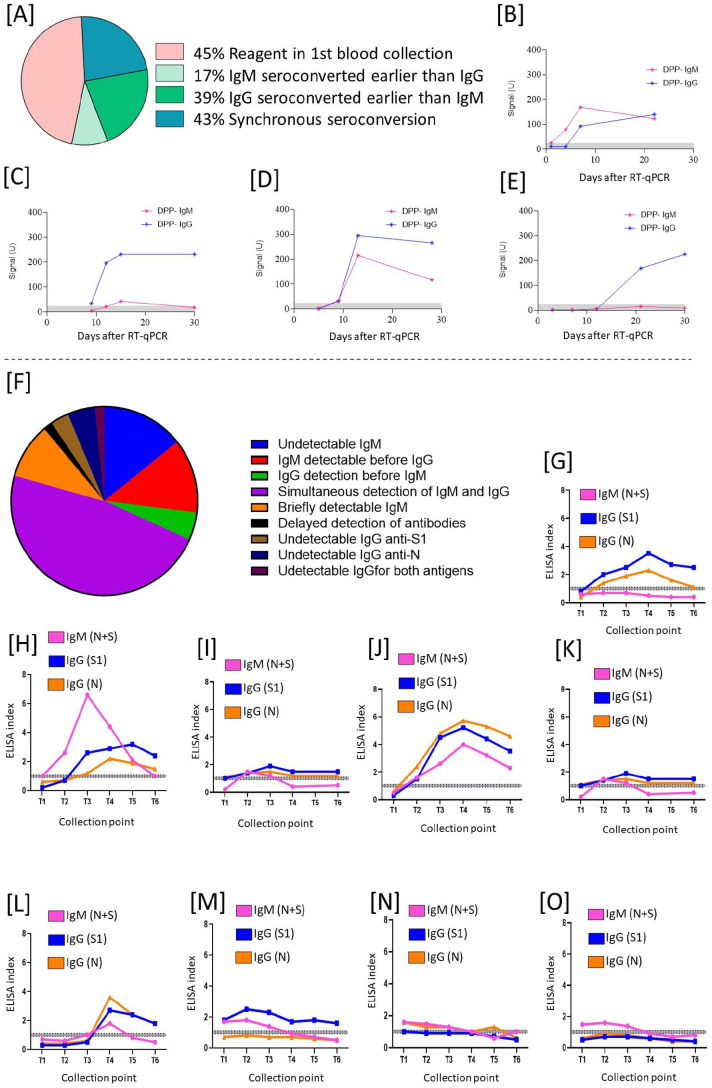
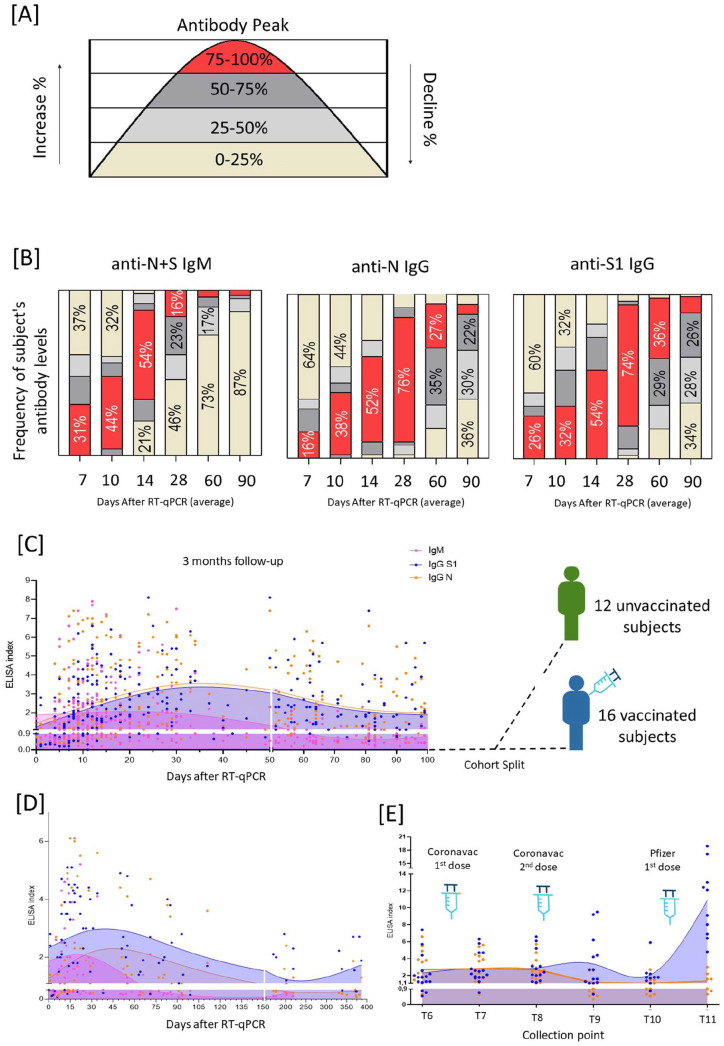
Initially, 77% (50/65) of the tested subjects had at least one of the specific antibodies detected by DPP or ELISA; meanwhile, 23% of the enrolled participants (15/65) were excluded from the study as they failed to produce antibodies, as measured by either method. The combination of DPP/ELISA testing showed nine different seroconversion patterns, four observed in DPP and ELISA, and five observed by ELISA only. Both methods indicated subjects with undetectable IgM (6% DPP/18% ELISA), IgM detectable before IgG (17% DPP/16% ELISA), IgG detectable earlier than IgM (39% DPP/6% ELISA), and simultaneous detection of IgM and IgG (43% DPP/60% ELISA) (Figure 2(A) to (J)). The patterns detected only in ELISA were IgM briefly detectable (12%), delayed detection of antibodies (2%), undetectable IgG anti-S1 (4%), undetectable IgG anti-N (6%), and undetectable IgG for both antigens (2%) (Figure 2(K) to (O)). To determine antibody kinetics during infection and convalescence periods, we first monitored anti-S1 and anti-N, IgG, and IgM for approximately 100 days (three months) in six serial collection time points (Figure 1). Most subjects reached maximum IgM detection in 14 days ACI (after confirmed infection) and dropped to undetectable levels near two months ACI. Anti-S1 and anti-N IgGs peaked between 30 and 40 days ACI and started to decrease, losing almost 50% of detection in the first three months of the follow-up. IgG seroconversion was similar for both antigens, although anti-S1 presented higher levels than anti-N in the follow-up period (Figure 3(B)). After three months, the cohort was divided into two groups: those who remained unvaccinated (n = 12) and those who were vaccinated (n = 16) with two doses of Coronavac or one dose of Pfizer (Figure 3(C)). For the unvaccinated group, after 100 days, the decrease rate of IgG became different for anti-N and anti-S1: anti-N IgG levels dropped below the ELISA cutoff between 140 and 150 days ACI, whereas IgG anti-S1 decrease slower, and remained detectable for up to 350–400 days ACI (Figure 3(D)). The vaccinated group, on the other hand, presented a different dynamic, as expected. The Coronavac first and second doses kept the ELISA indexes for IgG (anti-N and S1) similar to before vaccination antibody levels, although five months after the Coronavac second dose, an increase of IgG anti-S1 levels was observed whereas anti-N IgG levels dropped below the ELISA cutoff. Approximately eight months after Coronavac’s second dose, anti-S1 IgG dropped to the lowest levels, but antibody levels were quickly boosted after the Pfizer vaccine booster dose; anti-NS1 antibody levels raised from an average index of 2.1–10.9, representing a 519% increase. As expected, anti-N IgG did not show any increase after the vaccine booster dose (Figure 3(E)).
Figure 2.
Patterns of seroconversion for IgM and IgG anti-SARS-CoV-2 in mildly symptomatic, non-hospitalized COVID-19 patients. (A) Frequency of DPP seroconversions. (B to E) Four different patterns of seroconversion were observed using the DPP system. (F) Frequency of ELISA seroconversions. (G to O) Nine different seroconversion patterns were observed in ELISA for IgG anti-N, IgG anti-RBD, and IgM anti-N + S. The gray line represents the test borderline; below the gray line, samples were considered negative and above the gray line were considered positive.
Figure 3.
Antibody kinetics of anti-N IgG, anti-RBD IgG, and anti-N + S IgM. (A) The antibody curve was divided into areas (0–100% of antibodies) and each individual had normalized data in terms of its maximum peak. Then, it was possible to evaluate the frequency of subjects achieving different levels of antibody detection in terms of IgM (anti-N + S), IgG (anti-N), and IgG (anti-S). (B) Frequency of antibody levels detected 90 days cohort follow-up. (C) 100 Days follow-up, and cohort split into vaccinated and unvaccinated subjects. (D) Unvaccinated subject’s follow-up. (E) Vaccinated subject’s follow-up with vaccines given where point 1 represents the last sera sample before vaccination, point 2 represents two weeks after Coronavac first dose, point 3 represents three weeks after Coronavac second dose, point 4 represents five months after Coronavac second dose, point 5 represents sera day before Pfizer first dose, and point 6 represents one month after Pfizer booster dose. The curves were made by spline regression. The index cut-off for samples to be considered positive was ⩾1.1, and the borderline zone ranges from 0.8 to 1.1 (horizontal white bars).
PRNT and neutralization potency index (NPI)
We evaluated the serum neutralizing activity at different time points by PRNT50, for two SARS-CoV-2 lineages, B.1 and Gamma Variants. For unvaccinated individuals, we compared the ELISA antibody peak (approximately 28 days after ACI) to three months and one-year ACI, against the B.1 lineage. In those subjects, we observed that antibody peak had neutralizing titers of 1:275, whereas antibodies measured three months and one-year ACI presented approximately 2.6-fold less neutralizing power, with titers varying between 1:102 and 1:104 (P < 0.0001) (Figure 4(A)). For the vaccinated group, we compared the antibody peak ACI to three months ACI, and then to three weeks and five months after Coronavac’s second dose. The higher neutralizing activity was detected three weeks and five months after the Coronavac vaccination, with titers of 1:513 and 1:206, respectively. Samples obtained at the antibody peak and three months ACI had titers of 1:123 and 1:57, respectively (Figure 4(B)). Finally, samples obtained after the Pfizer vaccine booster dose generated neutralizing activities above the threshold limit of 1:5120 (data not shown).
Figure 4.
Serum neutralizing activity measured by PRNT50. In sera from COVID-19 patients in different time points. Plasma samples were incubated with Vero cells infected with SARS-CoV-2 lineages B.1 or Gamma to evaluate PRNT50 (defined as reciprocal plasma sample dilution that showed 50% protection against cytopathic effects). (A) Unvaccinated individuals, one-year follow-up against the B.1 isolate (n = 12). (B) Vaccinated individual’s follow-up against B.1 isolate (n = 16). (C) Sera from 28 days after confirmed infection (ACI) (antibody peak time point, measured by ELISA) against Gamma variant (n = 28). (D) Sera from unvaccinated subjects one-year AIC against Gamma variant (n = 12). (E) Sera from vaccinated individuals five months after the vaccine’s second dose, against Gamma variants (n = 16). Each data point represents the mean for each group at the dilution level, and error bars represent SEM made in a duplicate assay for each plasma sample; two independent assays were done for all groups. The curves were calculated as nonlinear regression (curve fit).
Concerning the measured antibody neutralizing capacity, all compared time points presented statistically significant differences. For instance, the antibody peak time point compared to five months after the Coronavac vaccine had a P-value of 0.0054; other comparisons had P-value < 0.0001 among them. When evaluated against the SARS-CoV-2 Gamma variant, all tested sera lost efficiency to neutralize the virus. In the ELISA antibody peak time point, we observed a 7.7-fold decrease in neutralizing titers, falling from 1:179 against the B.1 virus lineage to 1:23 for the Gamma variant (P ⩽ 0.0001) (Figure 4(C)). In samples obtained one-year ACI, we detected a two-fold decrease in neutralizing capacity, from 1:104 for B.1 lineage to 1:52 against the Gamma variant (P = 0.0002) (Figure 4(D)). Finally, serum obtained five months after Coronavac’s second dose presented a 2.8-fold decrease, from 1:206 against B.1 to 1:71 against the Gamma variant (P ⩽ 0.0001) (Figure 4(E)). In addition to determine the neutralization potency index (NPI) (PRNT50/ELISA index) of antibody samples, we first correlated the PRNT50 data with the ELISA indexes for both S and N antigens, tested to establish the best fit, and obtained an average correlation of 0.38 for PRNT50/IgG anti-N and 0.73 for PRNT50/IgG anti-S1. We estimated a low neutralizing potency index below 100 and high potency above 100 as described. 13 Comparing the cohort (n = 50) NPI during antibody peak and three-month ACI, we observed different NPI variations among subjects with a median potency of 57.7 in the antibody peak (approximately 28 days after ACI) against 47.5 measured three months ACI, with no significant differences (Figure 5(A)). In the unvaccinated group (n = 12), the timing follow-up showed median NPIs of 80.5, 45, and 101 at the antibody peak, three months, and one-year ACI, respectively, with no significant differences in potency among all time points (Figure 5(B)). Comparing the NPIs obtained for the vaccinated group (n = 12–16), we observed the lowest NPI during antibody peak and three months ACI (median NPI = 39.5 and 34). Nonetheless, after the Coronavac full vaccination, the median NPI rose to 210 – three weeks, and 126 – five months after vaccination, representing an increase of antibody neutralizing potency of 531% and 318% compared to the antibody peak ACI time point, respectively. Statistically, significant differences were observed when we compared the three weeks after vaccine second dose time point to sera obtained three months ACI (P ⩽ 0.0001); three months ACI compared to five months after Coronavac vaccine (P = 0.0078), and antibody peak time point to three weeks after Coronavac’s second dose (P = 0.0028) (Figure 5(C)). We did not calculate the NPI after Pfizer’s vaccine booster dose as the PRNT50 was above the measurable threshold.
Figure 5.
Neutralization Potency Indexes (NPI): (A) NPI comparison in three months ACI follow-up (n = 50). (B) NPI comparison in unvaccinated subject’s follow-up (n = 12). (C) NPI comparison in vaccinated subjects’ follow-up (n = 12–16#); P values are indicated as **P = 0.0028 for antibody peak versus two weeks after vaccine second dose and P = 0.0078 for three weeks after vaccine second dose versus five months after vaccine second dose; ***P ⩽ 0.0001. NPI was calculated as PRNT50/ELISA anti-RBD index. A non-parametric Kruskal–Wallis was performed for group comparisons. The gray area represents a low NPI (below 100). Antibody peak is 28 days ACI.
#Not all 16 vaccinated participants were available for blood collection in all time points.
Discussion
At the beginning of the Covid pandemic, there were many questions about how long the antibodies would last and what their neutralizing power would be. The first longitudinal studies were quite worrisome, as they indicated that detectable humoral responses could last as little as three months.14,15 Later cross-sectional cohort studies pointed to the detection of antibodies for a longer period. 16
The long follow-up over time in our study has given us a clearer view of the big picture concerning the anti-SARS-CoV-2 humoral responses after infection, both in terms of the most representative portion of the infected populations and the longevity and potency of the antibody responses. Indeed, we were able to detect the presence of antibodies with neutralizing capacity up to one year after COVID-19, even in non-vaccinated individuals. This is an important result because this is something increasingly difficult to assess today due to the growing vaccination of the world’s population.
Our initial screenings showed that not all subjects with SARS-CoV-2 infections confirmed by molecular or antigen tests present positive results in serological tests. Our analyses identified that 23% of the patients had no detectable levels of either anti-S1 or anti-N SARS-CoV-2-specific antibodies, which was similar to the rate found in a previous study. 8 Among the other participants of the study, we identified different patterns of antibody seroconversion, which is also consistent with other published works.4,17 –19
Our data provide evidence that although the detection of antibodies declines over time, the remaining antibodies are efficient in neutralizing the virus, one could speculate that even with more neutralizing capacity. This happens because there are fewer detectable antibodies over time, but at the same time, there is no significant difference in antibody neutralization potency index in the different time points evaluated. Although it is not possible to state that these subjects did not have contact with SARS-CoV-2 again in the period between collections – which could have boosted the humoral responses – it is known that the immune system evolves and adapts over time, 20 improving its efficiency in fighting pathogens. Of course, the appearance of variants with the ability to evade previous immune responses jeopardizes the protective effect of these antibodies.
In addition, it was possible to observe that vaccination changes the profile of antibody-mediated immunity. We observed that two doses of the Coronavac vaccine did not significantly increase the antibody index in ELISA, but it significantly increased the potency of these antibodies, providing a higher neutralization capacity than that acquired by the natural infection. Previous studies 21 have suggested that the use of heterologous vaccination protocols greatly improves the immune response. In this study, we show that after the Pfizer booster dose, the antibody index in ELISA increased significantly when compared to post-Coronavac vaccine levels, and even at higher levels than those obtained after natural infection, exceeding neutralizing levels of 1:5120. Unfortunately, it was not possible to calculate the neutralization power of these post-dose booster antibodies due to the method’s detection limit threshold (1:5120). We also examined the trends of antibody neutralization against the Gamma Variant as a way to infer how the appearance of SARS-CoV-2 mutants can impact the protective power of specific antibodies, and indeed antibodies were less protective, as already described and published in numerous studies. 11 It is important to mention that our cohort was recruited between June and November 2020 when this variant had not yet been detected. 22
These findings corroborate the fact that even though the vast majority of COVID-19 survivors can develop specific antibodies, and these antibodies can maintain their neutralizing potency index for one year or more, being vaccinated even after SARS-CoV-2 infection is still the best strategy to develop and maintain a robust neutralizing response against SARS-CoV-2.
Supplemental Material
Supplemental material, sj-jpg-1-ebm-10.1177_15353702231157941 for Heterogeneity of humoral response patterns in mildly symptomatic, non-hospitalized COVID-19 patients: A one-year longitudinal study by Luis AF Andrade, Flávia F Bagno, Sarah AR Sérgio, Pierina L Parise, Daniel A Toledo-Teixeira, Ana PSM Fernandes, Santuza MR Teixeira, Fabiana Granja, José Luiz Proenca-Modena and Flavio G da Fonseca in Experimental Biology and Medicine
Acknowledgments
The authors would like to thank all cohort participants’ patients. Many thanks to the Transportation Department at the Federal University of Minas Gerais (UFMG) for helping with the logistics; We also thank L. Benevenuto for her help in collecting blood samples. We finally thank the teams at CTVacinas/UFMG and Proença-Modena’s lab, at UNICAMP, for discussions and technical advice.
Footnotes
Authors’ Contributions: Conceptualization: FGdF, LAFA, and FFB; methodology: LAFA, FFB, SARS, PLP, DAT-T, and FG; validation: LAFA and FFB; formal analysis: LAFA and FFB; investigation: LAFA and FFB; data curation: LAFA and FFB; writing – original draft preparation: LAFA and FFB; writing – review and editing: LAFA and FFB; visualization: FGdF; supervision: FGdF, APSMF, SMRT, and JLP-M; project administration: FGdF; funding acquisition: FGdF, APSMF, and SMRT. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of the Federal University of Minas Gerais (UFMG) (CAAE: 1686320.0.0000.5149).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Brazilian Ministry of Science, Technology and Innovation (MCTI) through the “Rede Virus” initiative and its many individual projects (Rede Diagnóstico, Rede Corona-ômica and Laboratórios de Campanha). Further fundings were provided by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), Finep (Financiadora de Estudos e Projetos), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; grant no. 305628/2020-8). This study was also supported by grants from the São Paulo Research Foundation (FAPESP, grant nos 2016/00194-8 and 2020/04558-0) and the Fundo de Apoio ao Ensino, Pesquisa e Extensão of UNICAMP (FAEPEX-UNICAMP, grant no. 2266/20). Support was also provided by INCTV. APMF, SMRT, and JLPM are current CNPq fellowship recipients.
ORCID iDs: Sarah AR Sérgio  https://orcid.org/0000-0002-6101-660X
https://orcid.org/0000-0002-6101-660X
José Luiz Proenca-Modena  https://orcid.org/0000-0002-4996-3153
https://orcid.org/0000-0002-4996-3153
Flavio G da Fonseca  https://orcid.org/0000-0002-1416-8694
https://orcid.org/0000-0002-1416-8694
Supplemental Material: Supplemental material for this article is available online.
References
- 1. WHO coronavirus (COVID-19) dashboard, https://covid19.who.int/?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQjwtMCKBhDAARIsAG-2Eu8E9ckYUV9H8nOpgN-bLE76sN3_gbEDx3ggK-B8Ta0ZHG8l1M3LbuMaAmJmEALw_wcB (accessed 27 September 2021).
- 2. Swadling L, Diniz MO, Schmidt NM, Chandran A, Shaw E, Pade C, Gibbons JM, Bert NL, Tan AT, Jeffery-Smith A, Tan CCS, Tham CYL, Kucykowicz S, Aidoo-Micah G, Rosenheim J, Davies J, Johnson M, Jensen MP, Joy G, McCoy L, Valdes AM, Chain BM, Goldblatt D, Altmann DM, Boyton RJ, Manisty C, Treibel TA, Moon J C, Drop LV, Balloux F, Mcknight Á, Noursadeghi M, Bertoletti A, Maini M. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2 infection. Nature 2022;601:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2022;19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, Hemmings O, O’Byrne A, Kouphou N, Galao RP, Betancor G, Wilson H D, Signell AW, Winstone H, Kerridge C, Huettner I, Jimenez-Guardeño JM, Lista MJ, Temperton N, Snell LB, Bisnauthsing K, Moore A, Green A, Martinez L, Stokes B, Honey J, Izquierdo-Barras A, Arbane G, Patel A, Tan MKI, O’Connell L, O’Hara G, MacMahon E, Douthwaite S, Nebbia G, Batra R, Martinez-Nunez R, Shankar-Hari M, Edgeworth JD, Neil SJD, Malim MH, Doores KJ. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Yuan Q, Wang HM, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020;71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, Ley AM, Kyu S, Howell JC, Ozturk T. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 2020;21:1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bates TA, McBride SK, Leier HC, Guzman G, Lyski ZL, Schoen D, Winders B, Lee JY, Lee DX, Messer WB. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol 2022;7:eabn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masiá M, Telenti G, Fernández M, García JA, Agulló V, Padilla S, García-Abellán J, Guillén L, Mascarell P, Asenjo JC, Gutiérrez F. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infect Dis 2021;8:ofab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nehme M, Braillard O, Alcoba G, Aebischer-Perone S, Courvoisier D, Chappuis F, Guessous I. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med 2021;174: 723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagno FF, Sérgio SAR, Figueiredo MM, Godoi LC, Andrade LAF, Salazar NC, Soares CP, Aguiar A, Almeida FJ, da Silva ED, Ferreira AGP, Durigon EL, Gazzinelli RT, Teixeira SMR, Fernandes APSM, da Fonseca FG. Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19. J Clin Virol Plus 2022;2:100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Souza WM, Amorim MR, Sesti-Costa R, Coimbra LD, Brunetti NS, Toledo-Teixeira DA, de Souza GF, Muraro SP, Parise PL, Barbosa PP, Bispo-dos-Santos K, Mofatto LS, Simeoni CL, Claro IM, Duarte ASS, Coletti TM, Zangirolami AB, Costa-Lima C, Gomes ABSP, Buscaratti LI, Sales FC, Costa VA, Franco LAM, Candido DS, Pybus OG, de Jesus JG, Silva CAM, Ramundo MS, Ferreira GM, Pinho MC, Souza LM, Rocha EC, Andrade PS, Crispim MAE, Maktura GC, Manuli ER, Santos MNN, Camilo CC, Angerami RN, Moretti ML, Spilki FR, Arns CW, Addas-Carvalho M, Benites BD, Vinolo MAR, Mori MAS, Gaburo N, Dye C, Marques-Souza H, Marques RE, Farias AS, Diamond MS, Faria NR, Sabino EC, Granja F, Proença-Módena JL. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. Lancet Microbe 2021;2:e527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frey A, Di-Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 1998;221:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021;184: 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang K, Long Q-X, Deng H-J, Hu J, Gao Q-Z, Zhang G-J, He C-L, Huang L-Y, Hu J-L, Chen J, Tang N, Huang A-L. Longitudinal dynamics of the neutralizing antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Clin Infect Dis 2020; 73:e531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long Q-X, Tang X-J, Shi Q-L, Li Q, Deng H-J, Yuan J, Hu J-L, Xu W, Zhang Y, Lv F-J, Su K, Zhang F, Gong J, Wu B, Liu X-M, Li J-J, Qiu J-F, Chen J, Huang A-L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200–4. [DOI] [PubMed] [Google Scholar]
- 16. Choe PG, Kim K-H, Kang CK, Suh HJ, Kang E, Lee SY, Kim NJ, Yi J, Park W B, Oh M. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis 2021;27:928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 18. Zhang G, Nie S, Zhang Z, Zhang Z. Longitudinal change of SARS-Cov2 antibodies in patients with COVID-19. J Infect Dis 2020;222:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imai K, Kitagawa Y, Tabata S, Kubota K, Nagura-Ikeda M, Matsuoka M, Miyoshi K, Sakai J, Ishibashi N, Tarumoto N, Takeuchi S, Ito T, Maesaki S, Tamura K, Maeda T. Antibody response patterns in COVID-19 patients with different levels of disease severity in Japan. J Med Virol 2021;93:3211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O’Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 NCoV-19 and MRNA prime-boost vaccination against symptomatic covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health 2021;11:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujino T, Nomoto H, Kutsuna S, Ujiie M, Suzuki T, Sato R, Fujimoto T, Kuroda M, Wakita T, Ohmagari N. Novel SARS-CoV-2 variant in travelers from Brazil to Japan. Emerg Infect Dis 2021;27:1243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-ebm-10.1177_15353702231157941 for Heterogeneity of humoral response patterns in mildly symptomatic, non-hospitalized COVID-19 patients: A one-year longitudinal study by Luis AF Andrade, Flávia F Bagno, Sarah AR Sérgio, Pierina L Parise, Daniel A Toledo-Teixeira, Ana PSM Fernandes, Santuza MR Teixeira, Fabiana Granja, José Luiz Proenca-Modena and Flavio G da Fonseca in Experimental Biology and Medicine