Abstract
Viscum album L. (Santalaceae) is an important medicinal plant traditionally used to treat several diseases, including cancer therapy. This paper provides detailed morpho-anatomical characteristics of the leaves, stems and berries of Viscum album subsp. album growing as hemi-parasite on the branches of Malus domestica (Suckow) Borkh. (Rosaceae) to aid species identification and botanical characterization. Additionally, for the first time, microchemical analyses of all tissues and Energy Dispersive X-Ray Spectroscopy analyses of the calcium oxalate crystals are provided for the first time. The plant features leathery presents green leaves with parallel veins, small yellow unisexual flowers in 3-flowered cymes, and the dioecious inflorescences usually consist of three flowers, with female flowers generating white fleshy berries, in which a seed is embedded in the mucilaginous mesocarp, normally containing two embryos. Anatomically, the analyzed leaves were isobilateral and amphistomatic, and showed straight anticlinal epidermal cell walls, thick cuticles with epicuticular wax crystalloids, and paracytic stomata. The midrib is flat on both sides and has a single vascular bundle, whereas the strongly shortened petiole is concave-convex in shape and contains five bundles. The stems show a primary structure with a ring of nine vascular bundles enclosing the pith. Calcium oxalate druses and cubic and quadrangular prisms were observed in different plant parts. The results of this study provide new microscopy information that can help in the authentication of mistletoe raw materials.
Subject terms: Plant sciences, Microscopy, Electron microscopy
Introduction
The genus Viscum (Santalaceae) comprises about 110–141 species1,2. Nearly two-thirds of the species are found in Africa and Madagascar, and some in tropical Asia. A few species belonging to the Viscum album group have adapted to more temperate regions in Eurasia3, of which only two species, Viscum cruciatum ex Boiss. and Viscum album L., are found in Europe4. Viscum species, commonly called mistletoes, are shrubby hemi-parasites growing on the aerial parts of host trees and shrubs. They embed into the host branches with a haustorium to nourish and obtain water5. These haustoria can extend over more than 5 cm within the host6. Viscum album grows on more than 450 different species of hosts through its three subspecies, V. album subsp. album, V. album subsp. austriacum (Wiesb.) Vollm., and V. album subsp. abietis (Wiesb.) Abrom., with distinct host preferences and, to some extent, specific geographic distribution7.
Viscum album subsp. album is commonly known as European mistletoe, gui, Mistel, vischio, or muérdago4. Its berries consist of a whitish exocarp, a thick mucilaginous mesocarp, a thin endocarp, and a seed that normally contains two embryos8. The dispersal of mistletoe seeds depends on birds that feed from the berries during winter: mistle thrush (Turdus viscivorus) and waxwing (Bombicylla garrulus) gulp several fruits and after digestion excrete the seeds together with the exocarp and the sticky inner mesocarp. The blackcap (Sylvia atricapilla) digests only the exocarp and the outer mesocarp, after separating the seed and sticking it with the attached inner mesocarp on a thin branch. Separating seed and exocarp, the birds enable Viscum album embryos to germinate9.
In spring, the hypocotyl of the embryos elongates, turns to the bark of the host branch and flattens to a holdfast from which the primary haustorium grows with a tip meristem through the bark. When the meristem is embedded into the cambium of the host, it turns into an intercalary meristem, from which synchronously with the secondary thickness growth of the host’s xylem the formation of the primary sinker starts. Originating from the primary haustorium, the cortical strands grow longitudinally and circularly within the inner bark. Whenever their meristems encounter the cambium, they give rise to the formation of secondary sinkers5,10.
Viscum album is an important medicinal plant. Steiner and Wegman introduced its use in complementary oncological therapy in the early 1900s11; since then, many reports have been published describing its medicinal properties12,13. In addition to its use in carcinosis treatment, mistletoe is used to treat spleen diseases, menstruation problems, infertility, cardiovascular diseases, ulcers and epilepsy13–15. The plant is reported to contain several pharmacologically active compounds, such as amino acids, flavonoids, phenolic acids, polysaccharides, terpenoids, viscotoxins and mistletoe lectins16–21.
As only limited information on the morpho-anatomy, micromorphology or histochemistry of Viscum album subsp. album growing on Malus domestica (Suckow) Borkh. is available so far, the present study aimed to fill this gap. This study can also help in taxonomy, species identification, future comparisons with plants growing on other hosts, and quality control of the botanicals.
Results and discussion
Important morphological aspects of V. album subsp. album
Viscum album subsp. album is a hemi-parasite growing on the branches of deciduous trees such as Malus domestica. (Fig. 1a). It grows as dichasium with several articulated, glabrous and green stems (Fig. 1b,c). The leaves are simple, opposite and leathery with an obtuse apex, attenuated base and entire margins, with 3–5 parallel veins, green to yellowish-green. The leaves are highly variable in shape and size, ranging from oblanceolate to obovate-oblong and measuring 2–6 cm long and 0.3–2 cm wide (Fig. 1b,c). Shape and size of leaves may vary considerably, not only within an individual, but between different individuals of the same host tree or of different host trees22,23. The fruit is a pseudo berry, globular, whitish, translucent, sessile, 0.8–1 cm in diameter (Fig. 1d–f), crowned by the remains of the dried stigma and remains of the perigone leaves (Fig. 1e); they contain a seed coated by a skinny endocarp and embedded in a very viscous translucent mesocarp. The berries ripen during autumn before winter solstice, with the exocarp changing from green to white or yellowish-white when ripe. Berry color supports the differentiation of V. album (European mistletoe) from the closely related Viscum coloratum (Kom.) Nakai (Korean mistletoe, with red or yellow fruits) of Eastern Asia22.
Figure 1.
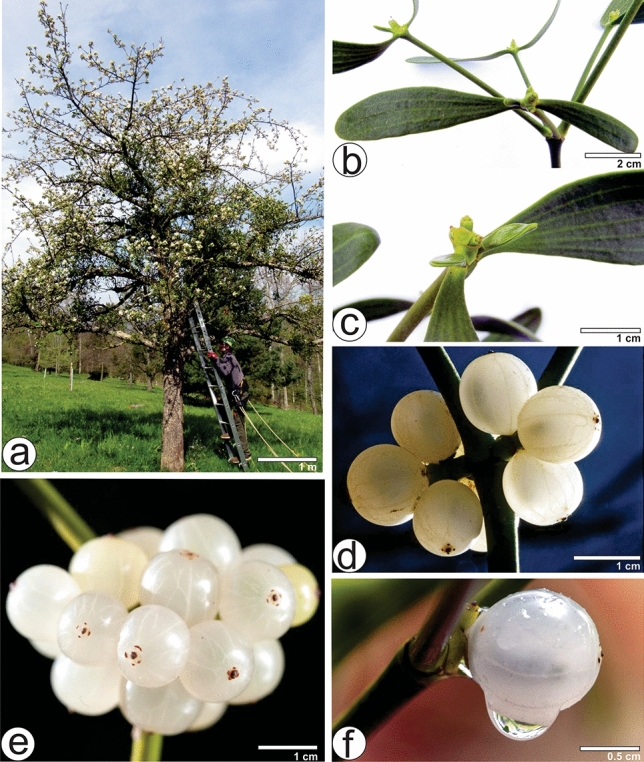
Morphology of Viscum album subsp. album. (a) Plants growing on host branches of Malus domestica. (b) Several shoots originate from the same node. (c) Stem with two opposite leaves. (d) Berries. (e) Infructescence with berries showing terminal ring of four lateral (tepalar) and one central (stigmal) scars. (f) Berry with a raindrop.
Anatomic features by microscopy tools
Leaf anatomy
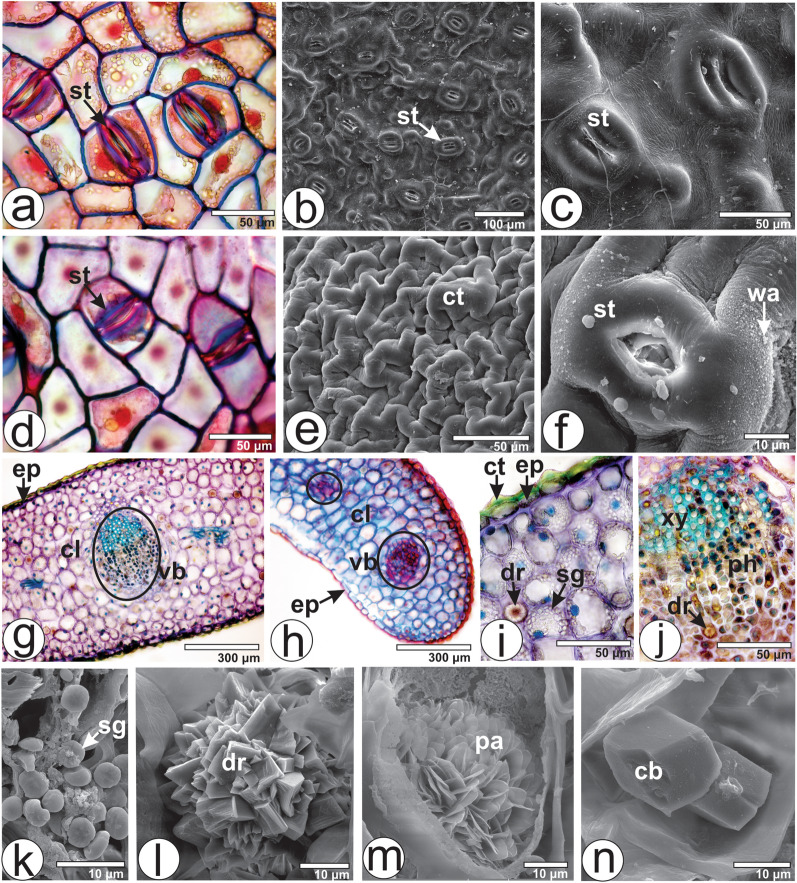
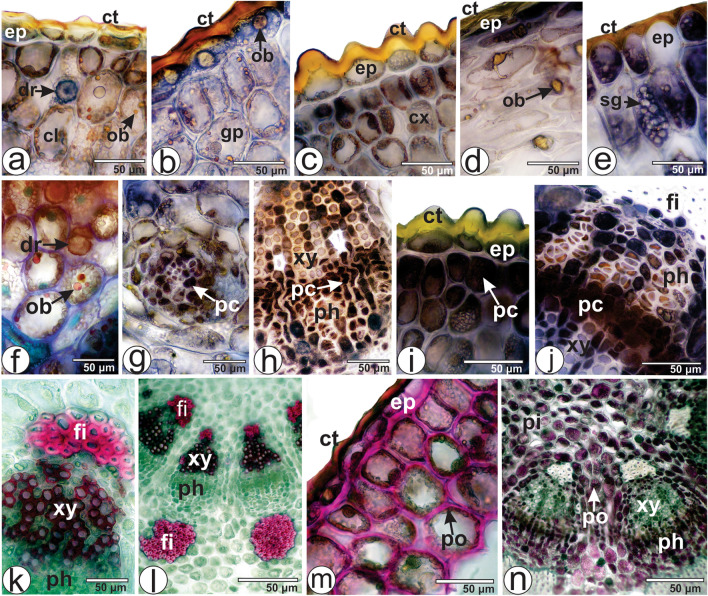
The surface view of the clarified leaf evidences straight and thin anticlinal cell walls on both adaxial and abaxial epidermises (Fig. 2a,d), covered by a thick cuticle (Fig. 2e). Paracytic stomata (Fig. 2a,d) are located at the same level as the adjacent epidermal cells, and are found on both sides of the lamina (Fig. 2a–d,f). Epicuticular wax crystalloids were observed on both epidermises (Fig. 2f).
Figure 2.
Anatomy of the leaves of Viscum album subsp. album: (a,d,g–j) light microscopy and (b,c,e,f,k–n) SEM. (a–c) Adaxial and (d–f) abaxial sides on the leaf. (a–f) Leaf in surface view. (g–n) Transverse sections. cb cubic-shaped crystal, cl chlorenchyma, ct cuticle, dr druse, ep epidermis, ph phloem, pa platy aggregation cluster crystal, sg starch grain, st stomata, vb vascular bundle, wa epicuticular wax, xy xylem.
Anatomical features such as epidermis with straight anticlinal cell walls, amphistomatic leaves, the presence of paracytic stomata, epicuticular wax crystalloids and thick cuticle, are commonly observed in Viscum species24–30. However, anomocytic stomata were evidenced in V. album subsp. austriacum. This subspecies also evidenced glandular trichomes on the adaxial side of the leaves44; this feature was not observed in the present study. The type of stomata and trichomes can be used as anatomical markers to distinguish between different subspecies of V. album subsp. austriacum.
In cross-section, the leaf epidermis is unilayered with strongly cutinized external cell walls on both sides (Fig. 2e,i). Although the leaves are photosynthetic, the mesophyll is undifferentiated and comprises polygonal cells (Fig. 2g,h). However, a dorsiventral mesophyll was found in V. album subsp. album collected from a host plant Tilia cordata Mill.27 and V. album subsp. austriacum growing on Pinus sp.30. Minor collateral vascular bundles are distributed in the mesophyll (Fig. 2h).
Metcalfe and Chalk24 stated that the mesophyll of the biennial leaves of V. album is formed by isodiametric cells during the first year, yet a single layer of palisade parenchyma develops towards both sides in the second year. From May until August the shoots of V. album have both types of leaves: in the first and in the second year of growth, as can be seen in Fig. 1c.
The mesophyll cells have several starch grains (Fig. 2i,k). They are small, rounded or elongated, and found solitarily or in groups. The presence of starch grains has been reported in various species and subspecies of Viscum30. Also, different morphotypes of crystals are distributed in the mesophyll (Fig. 2i) and in the vascular bundle (Fig. 2j). They are rectangular prisms, druses (Fig. 2l), platy aggregations of cluster crystals (Fig. 2m), and cubic-shaped crystals (Fig. 2n). Under light microscopy, the druses have a central thick and black region surrounded by many polygonal small crystals (Fig. 2i,j). While cubical, prismatic and druse crystals were observed in the mesophyll of V. album subsp. album in the present study, only druses were reported in V. album subsp. golestanicum29. The presence of crystals is commonly reported in various species and subspecies of Viscum27,28,31. However, no crystals were reported in a previous study of V. album subsp. album by Khan et al.28. Also, the presence of platy aggregation cluster crystals in V. album subsp. album is reported here for the first time. The grouping of more than one crystal morphotype can be present as the feature of the subspecies, species, section, subgenus or genus, giving support to the taxonomy32.
The midrib is flat on both sides (Fig. 2g). The vascular system is represented by a central collateral vascular bundle (Fig. 2g). Fibers are abutting the xylem and phloem (Fig. 2g,j). Bicollateral vascular bundles were found in V. album and in V. cruciatum Sieber ex Boiss. in a study by Khan et al.28.
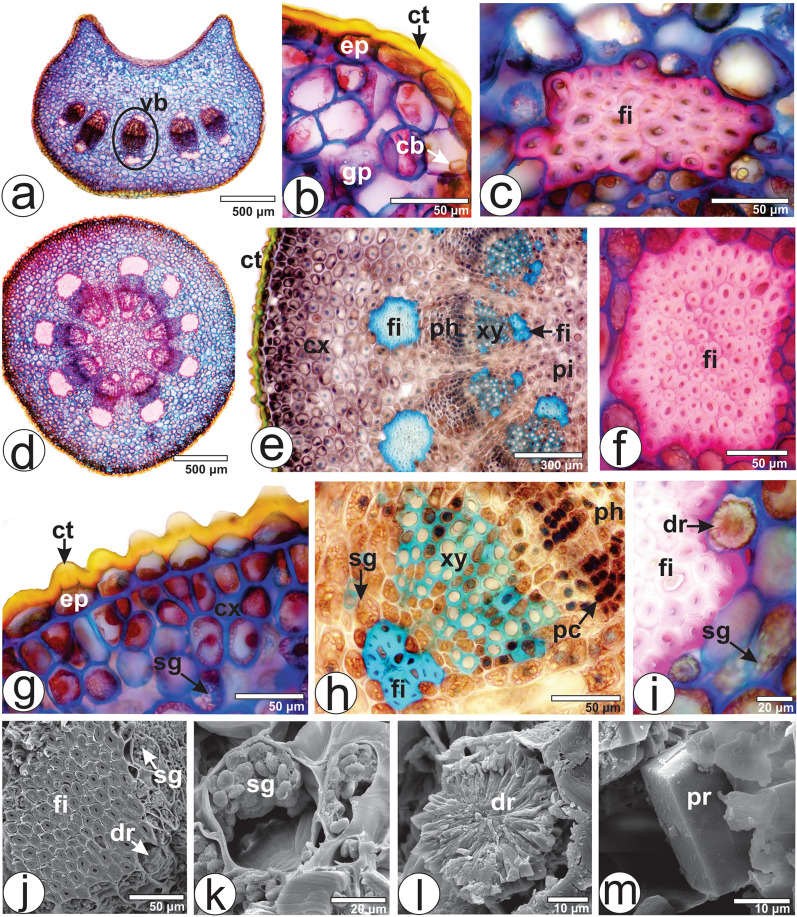
The strongly shortened and weakly differentiated petiole of V. album subsp. album, sectioned transversely at the medial portion, had a concave-convex shape with two wings on adaxial side (Fig. 3a). The epidermal cells are covered by a thick cuticle (Fig. 3b). The ground tissue is undifferentiated as in the lamina. The vascular system is collateral and was represented by five bundles organized in an open arc (Fig. 3a). A cap of perivascular fiber adjoins the xylem and phloem (Fig. 3c). The same crystal morphotypes previously described for lamina were observed in the ground parenchyma (Fig. 3b). However, Khan et al.28 have not reported any crystal in the petiole of V. album. Although the outline of the petiole is a general diagnostic feature for higher plants33, there are only few studies involving the anatomy of the petiole of Viscum species. The same features were found in V. album subsp. album and V. album subsp. golestanicum29. However, 6–7 vascular bundles were observed in V. album and V. cruciatum in the study by Khan et al.28.
Figure 3.
Leaf (petiole) and stem anatomy of Viscum album subsp. album in cross-section: (a–c) Petiole. (d–m) Stem. (a–i) light microscopy and (j–m) SEM. cb cubic-shaped crystal, ct cuticle, cx cortex, dr druse, ep epidermis, fi fiber, gp ground parenchyma, pc phenolic compounds, ph phloem, pi pith, pr quadrangular prismatic crystal, sg starch grain, vb vascular bundle, xy xylem.
Stem anatomy
The stem is circular in cross-section (Fig. 3d). The epidermis is unilayered with tangentially elongated cells (Fig. 3g). The external wall is particularly thick and fully cutinized (Fig. 3g). Over each external wall, in the middle of the cell, a projecting monticule or papilla, half-moon or elliptical shaped, could be observed (Fig. 3e,g). The cortex is discreetly collenchymatous and formed by several layers of parenchyma cells that increase in size towards the vascular system (Fig. 3e). The vascular system is represented by nine vascular bundles forming a ring and delimiting the pith (Fig. 3d). The row and number of cells in the xylem are higher than in the phloem (Fig. 3e). One cap of perivascular fibers is attached to the xylem and another to the phloem (Fig. 3d–f,i), the latter being more developed. The pith is at the center of the stem and is composed of parenchyma cells (Fig. 3d). Idioblasts containing brownish substances corresponding to phenolic compounds are present in the phloem (Fig. 3h). Cortical, vascular and medullary parenchyma are filled with starch grains (Fig. 3g–k) with the same features previously described for leaves; however, these are not present in the first layers of the cortex.
Druses (Fig. 3i,j,l), cubic and quadrangular prismatic (Fig. 3m) crystals are also spread in the stem tissues. Abundant crystals are present in the cortex and phloem of V. cruciatum, yet are absent in V. album28. Mehrvarz et al.29 have reported that the distribution and morphotype of the calcium oxalate crystals could provide valuable support in delimitating of subspecific taxa in V. album. The use of calcium oxalate crystals in solving taxonomic problems has been suggested in previous studies of other plant groups such as Baccharis32,34, Eucalyptus35 and Piper36.
Most characteristics observed in the present study have been described for Viscum species27–30. The vascular bundles showed poorly developed phloem but well-developed xylem. It means that they can resourcefully take up water and prepared inorganic and organic nutrients from the host plant37.
Berry anatomy
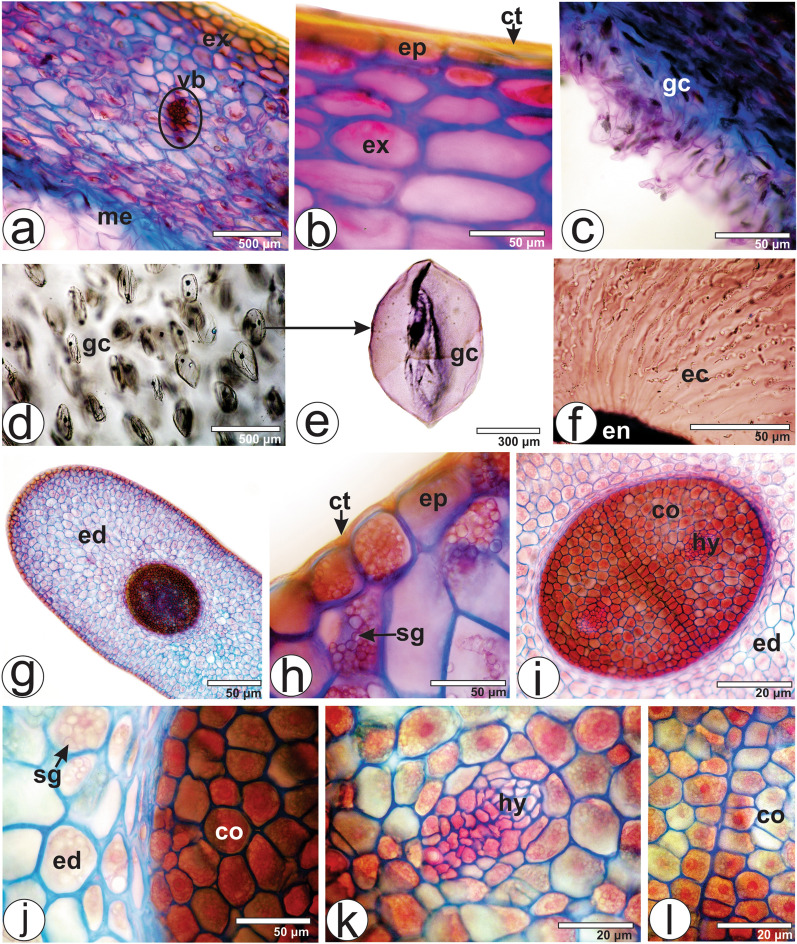
The berry, in frontal view, presents an epidermis with straight anticlinal cell walls. In cross-section, the exocarp is formed by an unilayered epidermis covered by a smooth cuticle and 3–4 layers of angular collenchyma (Fig. 4a,b). In the medial region, small collateral vascular bundles are present (Fig. 4a). The mesocarp, which surrounds the seed, contains an outer fleshy layer and an inner sticky viscin tissue (Fig. 4a,c). The fleshy layer is not sticky.
Figure 4.
Berry and seed anatomy of Viscum album subsp. album in cross-section. (a–f) Berry. (g–l) Seed. ct cuticle, co cotyledon, ec elongated cells with spiral thickening, ep epidermis, ed endosperm, en endocarp, ex exocarp, gc globular cells, hy hypocotyl, me mesocarp, sg starch grain, vb vascular bundle.
The viscin tissue is composed of strongly vacuolated globular cells (Fig. 4c–e) and highly viscous, sticky and extensible, long cells with spirally thickened walls located towards the endocarp (Fig. 4f). These cells contain druses and rectangular prism crystals, and a few starch grains. The endocarp is thin and encloses the seed. Grazi and Urech38 reported that there is no information on the extensible filaments between the seeds and the outer mesocarp in V. album subsp. abietis and V. album subsp. austriacum growing on conifers.
Seed anatomy
The seed is green and does not have a seed coat. Heide-Jørgensen39 stated that in Viscum genus no seed coat is formed since the integuments are lacking. In cross-section, the seed has an unilayered epidermis covered by a cuticle (Fig. 4h). The epidermal cells have several starch grains (Fig. 4h). The endosperm cells are large, parenchymatous and contain chlorophyll and large amounts of starch grains (Fig. 4g–j). The seed usually has two embryos, each with two cotyledons and a hypocotyl (Fig. 4i–l). The number of embryos per berry varies in Viscum album. Commonly, V. album subsp. album has two embryos, sometimes only one embryo, and rarely three or even four embryos, while the percentage of monoembryonal berries is higher in subsp. abietis and V. album subsp. austriacum8,26.
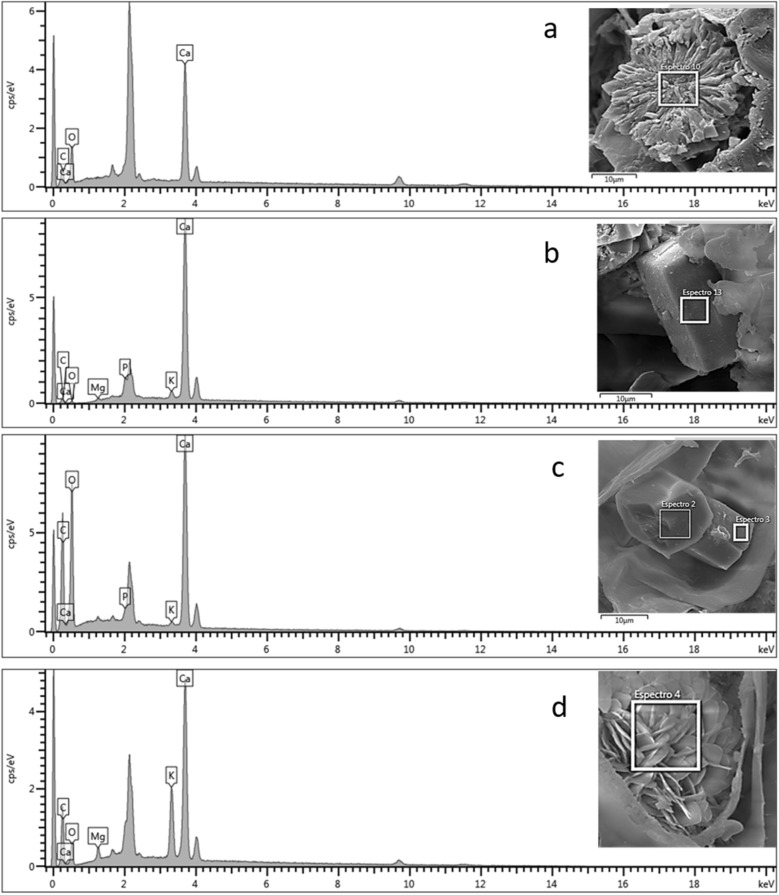
Elemental analysis of crystals using EDS
The chemical composition of the crystals occurring in plants can be identified using EDS40,41. In the present study, four morphotypes of calcium oxalate crystals were found in various tissues of V. album subsp. album (Figs. 2i,j,l–n, 3b,i,j,l,m). The EDS spectra of druses (Fig. 5a) exhibited prominent peaks for calcium (Ca), carbon (C), and oxygen (O). However, in addition, other elements such as magnesium (Mg), phosphorous (P), and potassium (K) were also found in minor concentrations in the cubic (Fig. 5c) and rectangular prisms (Fig. 5b), and platy aggregation crystals (Fig. 5d). The most common minerals formed by plants are calcium oxalate, calcium carbonate and silica42. This is the first study of the elemental chemical composition of crystals of Viscum species using EDS.
Figure 5.
SEM image and EDS spectra of the druse (a), rectangular prism (b), cubic prism (c) and platy aggregated (d) crystals from Viscum album subsp. album. The prominent unlabeled peak at 0 keV is the noise peak, and the peak near 2.1 keV is for gold (Au) used for sputter-coating the samples for SEM analysis.
Histochemical tests: a link between chemical compounds and its anatomical compartmentalization
Microchemical analyses using specific reagents and stains under light microscopy are helpful for the characterization of chemical compounds present in plant tissues. They can also be used to detect, within specific cells and tissues, the distribution and accumulation of the compounds or groups of secondary metabolites, such as lipophilic material, protein, mucilage, lignified elements, and phenolic compounds43–53. In the present study, an array of histochemical tests were conducted using several (Table 1). No histochemical tests were previously reported for V. album subsp. album.
Table 1.
Results of microchemical tests with V. album subsp. album.
| Microchemical reagents | Reaction | Occurrence in plant organs | |||||
|---|---|---|---|---|---|---|---|
| Leaf | Stem | Berry | Seed | ||||
| Lamina | Midrib | Petiole | |||||
| Sudan III | Staining lipids red or red–orange | Cuticle and oil bodies (epidermis and mesophyll) | Cuticle and oil bodies (epidermis and ground parenchyma) | Cuticle | Cuticle and oil bodies in the exocarp cells | Cuticle | |
| Sudan black | Staining lipids black | ||||||
| Nile blue | Stains neutral fats in red and fatty acids in blue | Oil bodies | Oil bodies | ND | Oil bodies | ND | |
| Potassium dichromate | Gives phenolics a brown or reddish-brown color | Minor vascular bundles | Vascular bundles | Cortex and vascular bundles | ND | ||
| Ferric chloride | Turns phenolics to dark brown | ||||||
| Vanillin/HCl | Gives rise to a bright red vanillin-tannin condensate | ND | |||||
| Phloroglucinol/HCl | Reveals lignified elements in pink to red color | Fibers and vessel elements | Fibers and vessel elements | Elongate cells | Endocarp cells | ||
| PAS (periodic acid-Schiff) | Neutral polysaccharides become magenta | Epidermis and mesophyll cells | Epidermis, ground parenchyma and phloem | Phloem and pith | Exocarp and mesocarp | All the cell walls, especially near the embryo | |
| Iodine solution | Stain starch in dark blue to black | Epidermis and mesophyll cells | Ground parenchyma | ND | Epidermis, cortex and pith | Mesocarp (globular cells region) | Endosperm |
| Xylidine Ponceau | Reveals protein bodies in red color | Protein globular corpuscles | Endosperm, hypocotyl and embryo | ||||
| Coomassie Brilliant Blue | Turns protein bodies blue | Protein globular corpuscles | |||||
| Ruthenium red | Reacts with pectins, mucilages and gums turning them pink to red | Epidermis, phloem and mesophyll cells | Epidermis, ground parenchyma and phloem | Epidermis and exocarp cells | |||
| Dragendorff, Ellram and Wagner | Gives alkaloids an orange to reddish-brown color | ND | |||||
ND not detected.
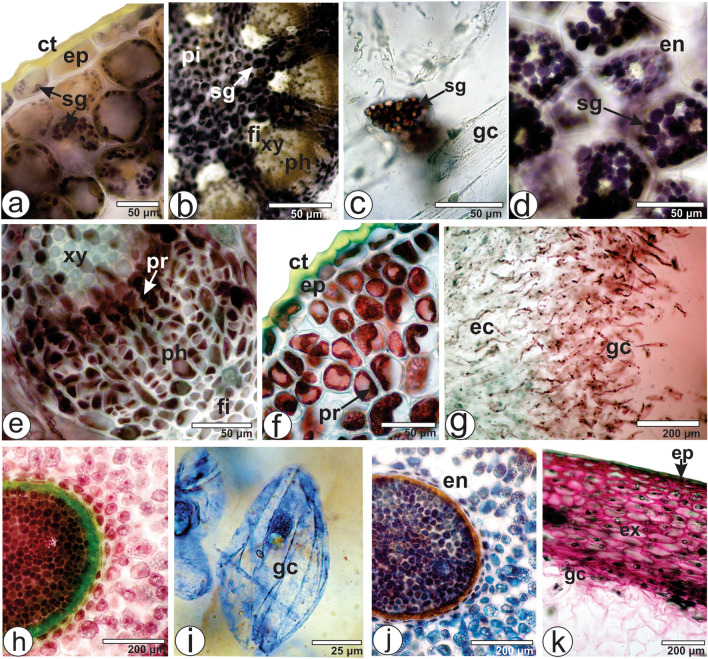
Viscum album subsp. album reacts positively with Sudan III and Sudan black, showing lipophilic compounds in cuticles of the leaves [lamina (Fig. 6a) and petiole (Fig. 6b)], stems (Fig. 6c), berries (Fig. 6d) and seeds (Fig. 6e). Oil bodies also react with this reagent32 and are found in the epidermis and mesophyll (Fig. 6a) of the lamina, in the epidermis (Fig. 6b) and ground parenchyma (Fig. 6b) of the petiole, and in the exocarp cells of berries (Fig. 6d). Oil bodies (Fig. 6f) stain with Nile blue as well, indicating that the bodies contain neutral fats. Nile blue is a basic dye in the oxazine group that stains neutral fats and fatty acids in red and blue, respectively54.
Figure 6.
Microchemical tests of Viscum album subsp. album in cross-section. (a,b,g,h,k,m) Leaf [(a,g) Lamina, (f,m,k) Midrib, (b,h) Petiole]. (c,i,j,l,n) Stem. (d) Berry. (e) Seed. Reagents: (a–e) Sudan III. (f) Nile blue. (g,h) Potassium dichromate. (i,j) Ferric chloride. (k,l) Phloroglucinol/HCl. (m,n) PAS (periodic acid-Schiff reagent). cl chlorenchyma, ct cuticle, cx cortex, dr druses, ep epidermis, fi fibers, gp ground parenchyma, hy hypocotyl, ob oil bodies, pc phenolic compounds, ph phloem, po neutral polysaccharides, sg starch grain, xy xylem.
Phenolic compounds can be evidenced using different reagents: ferric chloride solution, potassium dichromate solution and vanillin-hydrochloric. Viscum album subsp. album reacts positively with potassium dichromate (Fig. 6g,h) and ferric chloride (Fig. 6i,j) solutions, and the cells containing phenolic compounds were found in the vascular bundles of leaves and stems. However, no condensed tannins were found in this study.
Lignified elements can be detected using phloroglucinol/HCl. Lignin is a compound present in several or all the secondary wall layers that contribute to the lignification process resulting in the modification of cell wall properties35. In the present study, lignified elements were evidenced in fibers and vessel elements in the leaves and stems (Fig. 6k,l).
Plant polysaccharides are macromolecules comprised of several identical or different monosaccharides with α- or β-glycosidic bonds. In microchemical tests, the Schiff reagent is frequently used to distinguish certain mucins and other carbohydrates in a staining sequence called the PAS (periodic acid-Schiff) test. Polysaccharides that comprehend a pair of adjacent hydroxyl groups can be oxidized to aldehydes by periodic acid. The aldehydes react with colorless Schiff reagent and the positive tissue sites become magenta. Neutral polysaccharides are spread in the cell walls in the leaf epidermis, midrib ground parenchyma (Fig. 6m), and phloem and pith in the stem (Fig. 6n), exocarp and mesocarp of berry and all the cell walls in the seed, especially near the embryo.
Iodine solution is used to identify stain starch. Almost all other structures stain yellow, but this color has no specific meaning. Starch is one of the main ergastic substances of the protoplast and contains a long chain of polysaccharides grouped around a hilum and forming characteristic granules. Starch grains are found in epidermis and mesophyll cells (Fig. 7a) of the lamina and ground parenchyma of the midrib. They are widespread in the cortex, medullary rays, and pith of the stem (Fig. 7b), in some mesocarp cells in the berry (Fig. 7c), and in the endosperm of the seed (Fig. 7d). They are small, rounded and found in groups.
Figure 7.
Microchemical tests of Viscum album subsp. album (Cross-sections of (a) Lamina, (e) Midrib, (b,f) Stem, (c,g,i) Berry, and (d,j,k) Seed. Reagents: (a-d) Iodine solution. (e–h) Xylidine Ponceau. (i,j) Coomassie Brilliant blue. (k) Ruthenium red. ct cuticle, ec palisade-like elongated cells with spirally thickened walls, en endosperm, ep epidermis, ex exocarp, fi fiber, gc globular cells, ph phloem, pi pith, pr protein, sg starch grain, xy xylem.
Xylidine Ponceau and Coomassie Brilliant Blue react positively with V. album subsp. album. The presence of protein globular corpuscles is observed occasionally in the leaves (Fig. 7e), stems (Fig. 7f) and berries (Fig. 7g,i). However, they are commonly found in seeds, especially in the endosperm (Fig. 7h,j). The protein globular corpuscles are vacuolated structures that accumulate reserve protein in the seeds and are called protein bodies or aleurone grains.
Ruthenium red is a polycationic stain that reacts with pectic substances, mucilage, and gums56. These substances are detected in almost all cells in the leaves, stems, berries (Fig. 7k) and seeds, yet not in fibers and xylem elements. Azuma et al.57 analyzed the cellulose system of the viscous fibrous cellulosic polysaccharide (viscan) in the viscin tissue of V. album and reported that it is formed by cellulose and hemicellulose together with a minor amount of pectic substance. The viscin tissue assists in attaching the mistletoe berries to the host branches.
Dragendorff, Ellram and Wagner are reagents that detect alkaloids44,45. These secondary metabolites were not detected in any of the organs of the V. album subsp. album.
Materials and methods
Plant materials
The use of plants in the present study complies with international institutional guidelines. Viscum album subsp. album was harvested from Malus domestica growing on a cultivated natural site in Switzerland (belonging to the Society for Cancer Research-VfK). Therefore, no permission for harvesting was needed. The plants were identified by Dr. Marcelo Guerra Santes (Universidade Estadual do Rio de Janeiro, Brazil) and a voucher specimen (C.H. Quaresma 18.332) was deposited at the Herbarium of the Faculdade de Formação de Professores, Universidade Estadual do Rio de Janeiro, Brazil. Fresh specimens of the leaves, stems, berries and seeds of V. album subsp. album were collected for the first investigations in July 2016 from plants growing on the branches of Malus domestica from the Basel area (Rüti, Himmelried) in Switzerland. To develop the present study, the leaves and stems, as well as the berries and seeds of V. album subsp. album were collected in April 2021 and February 2022, respectively, on the same location in Switzerland (Rüti, Himmelried).
Preparation of samples for light microscopy
The mistletoe bush used in this study was about seven years old. The following samples were harvested from the same bush: six young leaves (1-year-old), four young stems (1st year), six berries, six matured leaves (2 years old), and four matured stems (2 years old). Only perfect and healthy organs, without diseases or infections, were collected following methodology previously standardized by our group18,19,21. Fresh samples of the stems, leaves, berries and seeds were collected from the plant and fixed in FAA 70 (formalin, acetic acid, 70% ethanol, 5:5:90 v/v/v) for three days. The samples were then washed with water, transferred into 70% ethanol, and free-hand sections were prepared using razor blades (thickness of cross-sections 15–30 μm). Selected sections were stained in toluidine blue58 and in a combination of Astra blue and basic fuchsin59. Then the sections were mounted in a drop of glycerin (50%)60 on glass slides, covered with a coverslip, and sealed with transparent nail polish. For the analysis of epidermal surfaces, small sections of the leaves were washed and then treated with sodium hypochlorite solution (50%) until translucent61. The materials were then washed with distilled water and neutralized in an acetic acid solution (5%). The sections were rewashed with distilled water, stained in safranin60 and mounted as described above.
Histochemical analyses
A series of multiple histochemical tests were conducted using different chemical reagents and stains (Table 1). Free-hand cross-sections of fresh material were exposed to phloroglucinol/HCl to detect traces of lignin43. Ellram, Wagner44 and Dragendorff45 reagents were used to detect alkaloids44,45. Potassium dichromate (10%) 46 and ferric chloride (5%) were used to detect the presence of phenolic substances47. Hydrochloric vanillin solution (0.5%) was applied to reveal condensed tannins48,55. Sudan III and Sudan black B were used to detect lipophilic compounds49. In addition, Nile blue sulfate was used to expose neutral and/or acidic lipids50; iodine solution (2%) to identify starch grains47; ruthenium red solution (0.002%) for pectins47; PAS (periodic acid-Schiff reagent) test for polysaccharides51; and Xylidine Ponceau52 and Coomassie Brilliant blue53 were used to evidence proteins. The reaction methods and results of these histochemical tests are summarized in Table 1. Appropriate controls were performed in parallel with the tests. Photomicrographs were captured using an Olympus CX 31 light microscope with the attached C-7070 control unit. The microscopic procedures were conducted in the Laboratory of Pharmacognosy at the State University of Ponta Grossa (UEPG, Brazil).
Preparation of samples for scanning electron microscopy (SEM)
The FAA-fixed samples were dehydrated by passing through increasing concentrations of ethanol in water (70%, 80%, 90%, and 100%). The samples were then dried in a Balzers CPD 030 critical point dryer (BAL-TEC AG, Balzers, Liechtenstein) supplied with liquid CO2 and then coated with gold using a Quorum (model SC7620) sputter coater. Photomicrographs were recorded using a Mira 3 field-emission scanning electron microscope (Tescan, Brno-Kohoutovice, Czech Republic).
Energy dispersive X-ray spectroscopy (EDS)
During the SEM procedure, EDS was performed to obtain the chemical composition spectra of the crystals. This analysis was made for the crystals as well as the cells devoid of crystals as a control, using an EDS detector on the same variable pressure microscope at 15 kV. SEM and EDS analyses were performed at the Multiuser Laboratory Complex (C-Labmu) of the State University of Ponta Grossa (UEPG, Brazil).
Conclusion
This study provides valuable anatomical, micromorphological and microchemical information about Viscum album subsp. album. The anatomy features of the leaf, stem, berry and seed are significant, and this study reveals several attributes of potential taxonomic importance at the genus and species levels. The present study will also provide a basis for future studies involving other taxa in the family for a better understanding of the morpho-anatomy, interaction with host plants, and phylogeny of this interesting group of hemi-parasitic plants.
Acknowledgements
We acknowledge the C-Labmu Center at the State University of Ponta Grossa (UEPG) for the SEM and EDS facilities. We are grateful to Jürg Buess for kindly supporting this publication with his photos. Konrad Urech was of great importance contributing with critical comments and inputs. This study was financed from institutional resources only, such as: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior of Brasil (CAPES; Finance Code 001; 88887.569108/2020-00), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; BPP 201.004/2022).
Author contributions
V.P.A. and I.T.M. performed the experiments. J.V.C.B. and M.G. collected and prepared the plant material conservation. Conceptualization and supervision were done by J.M. and C.H. The manuscript was written by V.P.A., J.V.C.B. and J.M. V.R., H.R., C.H. and S.B. revised and edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stephan Baumgartner, Email: stephan.baumgartner@unibe.ch.
Carla Holandino, Email: cholandino@gmail.com.
Jane Manfron, Email: janemanfron@hotmail.com.
References
- 1.POWO. Plants of The World Onlinehttps://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000304-2 (2022).
- 2.WFO. The World Flora Onlinehttp://www.worldfloraonline.org/taxon/wfo-4000040344 (2022).
- 3.Kirkup, D. W., Polhill, R. M. & Wiens, D. Viscum in the context of its family, Viscaceae, and its diversity in Africa. In Mistletoe: The Genus Viscum (ed. Büssing, A.) 7–29 (CRC Press, 2000).
- 4.Becker, H. European mistletoe: Taxonomy, host trees, parts used, physiology. In Mistletoe: The Genus Viscum (ed. Büssing, A.) 31–43 (CRC Press, 2000).
- 5.Sallé, G. Germination and establishment of Viscum album L. In The Biology of Mistletoes (eds. Calder, D. M. & Bernhardt, P.) 145–159 (Academic Press, 1983).
- 6.Mylo MD, et al. Advances on the visualization of the internal structures of the European Mistletoe: 3D reconstruction using microtomography. Front. Plant. Sci. 2021;12:715711. doi: 10.3389/fpls.2021.715711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barney CW, Hawksworth FG, Geils BW. Hosts of Viscum album. Eur. J. For. Pathol. 1998;28:187–208. doi: 10.1111/j.1439-0329.1998.tb01249.x. [DOI] [Google Scholar]
- 8.Ramm, H., Urech, K., Scheibler, M. & Grazi, G. Cultivation and development of Viscum album L. In Mistletoe: The Genus Viscum (ed. Büssing, A.) 75–94 (Taylor & Francis, 2004).
- 9.Melado A, Zamora R. Generalist birds govern the seed dispersal of a parasitic plant with strong recruitment constraints. Oecologia. 2014;176:139–147. doi: 10.1007/s00442-014-3013-8. [DOI] [PubMed] [Google Scholar]
- 10.Thoday D. The haustorial system of Viscum album. J. Exp. Bot. 1951;2:1–19. doi: 10.1093/jxb/2.1.1-a. [DOI] [Google Scholar]
- 11.Ramm, H. Mistletoe through cultural and medical history: The all-healing proves to be a cancer-specific remedy. In Mistletoe: From Mythology to Evidence-Based Medicine (eds. Zanker, K. S. & Kaveri, S. V.) 1–10 (Translational Research in Biomedicine, 2015).
- 12.Loef M, Walach H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020;20:227. doi: 10.1186/s12906-020-03013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostermann T, et al. A systematic review and meta-analysis on the survival of cancer patients treated with a fermented Viscum album L. extract (iscador): An update of findings. Complement. Med. Res. 2020;27:260–271. doi: 10.1159/000505202. [DOI] [PubMed] [Google Scholar]
- 14.Büssing, A. Mistletoe: The Genus Viscum (ed. Büssing, A.) 1–261. (CRC Press, 2000).
- 15.Poruthukaren KJ, Palatty PL, Baliga MS, Suresh S. Clinical evaluation of Viscum album mother tincture as an antihypertensive: A pilot study. J. Evid. Based Complement. Altern. Med. 2014;19:31–35. doi: 10.1177/2156587213507726. [DOI] [PubMed] [Google Scholar]
- 16.Schaller G, Urech K, Grazi G, Giannattasio M. Viscotoxin composition of the three European subspecies of Viscum album. Planta Med. 1998;64:677–678. doi: 10.1055/s-2006-957553. [DOI] [PubMed] [Google Scholar]
- 17.Urech K, Baumgartner S. Chemical constituents of Viscum album L.: Implications for the pharmaceutical preparation of mistletoe. Transl. Res. Biomed. 2015;4:11–23. [Google Scholar]
- 18.Melo MNO, et al. Viscum album mother tinctures: Harvest conditions and host trees influence the plant metabolome and the glycolytic pathway of breast cancer cells. Front. Pharmacol. 2022;13:1027931. doi: 10.3389/fphar.2022.1027931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holandino C, et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020;20:215–226. doi: 10.1186/s12906-020-02987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peñaloza E, et al. Comprehensive metabolome analysis of fermented aqueous extracts of Viscum album L. by liquid chromatography−High resolution tandem mass spectrometry. Molecules. 2020;25:4006–4020. doi: 10.3390/molecules25174006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäger T, et al. Metabolomics by UHPLC-Q-TOF reveals host tree-dependent phytochemical variation in Viscum album L. Plants. 2021;10:1726–1738. doi: 10.3390/plants10081726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuber D. Biological flora of Central Europe: Viscum album L. Flora Morphol. Distrib. Funct. Ecol. Plants. 2004;199:181–203. doi: 10.1078/0367-2530-00147. [DOI] [Google Scholar]
- 23.Bilgili E, Alperen Coşkuner KA, Öztürk M. Leaf area—sapwood area relationship in Scots pine (Pinus sylvestris L.) under mistletoe (Viscum album ssp. austriacum) infection. Dendrobiology. 2020;84:1–11. doi: 10.12657/denbio.084.001. [DOI] [Google Scholar]
- 24.Metcalfe, C. R. & Chalk, L. Anatomy of the Dicotyledons (Clarendon Press, 1979).
- 25.Haas K, Bauer M, Wollenweber E. Cuticular waxes and flavonol aglycones of mistletoes. Z. Naturforsch. C. 2003;58:464–470. doi: 10.1515/znc-2003-7-803. [DOI] [PubMed] [Google Scholar]
- 26.Becker, H. European Mistletoe: Taxonomy, host trees, parts used, physiology. In Mistletoe: The Genus Viscum (ed. Büssing, A.) 31–43 (Taylor & Francis, 2004).
- 27.Andronache A, Toma I, Toma C. The structure of vegetative organs in Viscum album and Loranthus europaeus. Analele Stiinţ. ale Univ. 2006;2:13–18. [Google Scholar]
- 28.Khan MA, Sharif T, Ahmad M, Zafar M, Tareen RB. Anatomical characterization of parasitic plants of Pakistan. Pak. J. Bot. 2009;41:2661–2669. [Google Scholar]
- 29.Mehrvarz SS, Shavvon RS, Golmohammadi N. Notes on the genus Viscum (Viscaceae) in Iran: A new combination based on morphological evidence. Afr. J. Agric. Res. 2012;7:1694–1702. doi: 10.5897/AJAR11.1382. [DOI] [Google Scholar]
- 30.Elkiran O. Comparative anatomical study of Viscum album subsp. album L. and Viscum album subsp. austriacum (Wiesb.) Vollman. Ejons Int. J. Math. Eng. Nat. Sci. 2022;21:12–17. doi: 10.38063/ejons.532. [DOI] [Google Scholar]
- 31.Mbagwu FN, Unamba CIN, Ezeibekwe KO. Leaf anatomical characteristics of five variants of the genus Viscum L. (Loranthaceae) Agric. J. 2009;4:161–163. [Google Scholar]
- 32.Almeida VP, et al. Microscopy and histochemistry of leaves and stems of Baccharis subgenus Coridifoliae (Asteraceae) through LM and SEM-EDS. Microsc. Microanal. 2021;27:1273–1289. doi: 10.1017/S1431927621012447. [DOI] [Google Scholar]
- 33.Almeida VP, et al. Comparative morphoanatomical analysis of Mikania species. Rev. Bras. Farmacogn. 2017;27:9–19. doi: 10.1016/j.bjp.2016.05.002. [DOI] [Google Scholar]
- 34.Manfron, J, Farago, P. V., Khan, I. A. & Raman, V. Morpho-anatomical characteristics of species of Baccharis. In Baccharis from Evolutionary and Ecological Aspects to Social Uses and Medicinal Applications (eds. Fernandes, G. W., Oki, Y. & Barbosa, M.) 217–237 (Springer Nature, 2021).
- 35.Brito PS, et al. Light and scanning electron microscopy, energy dispersive X-ray spectroscopy, and histochemistry of Eucalyptus tereticornis. Microsc. Microanal. 2021;27:1295–1303. doi: 10.1017/S1431927621012514. [DOI] [Google Scholar]
- 36.Santos VLP, et al. Review of Piper species growing in the Brazilian state of Paraná with emphasize on the vegetative anatomy and biological activities. Bot. Rev. 2021;87:23–54. doi: 10.1007/s12229-020-09239-7. [DOI] [Google Scholar]
- 37.Bokhari TZ, Younis U, Ummara U, Sharif T, Khan MA. Some parasitic plants of Pakistan—Anatomical and taxonomic attributes. Int. J. Sci. Eng. Res. 2013;4:963–968. [Google Scholar]
- 38.Grazi VG, Urech K. Einige morphologische merkmale der mistelheere (Viscum album L.) und deren taxonomische bedeutung. Beitr. Biol. Pflanzen. 1981;56:293–306. [Google Scholar]
- 39.Heide-Jørgensen, H. S. The Mistletoe Viscum albumhttps://viscum.dk/wp-content/uploads/2020/12/Viscum_2015_english_small.pdf (2015).
- 40.Pauzer MS, et al. Eucalyptus cinerea: microscopic profile, chemical composition of essential oil and its antioxidant, microbiological and cytotoxic activities. Braz. Arch. Biol. Technol. 2021;64:e21200772. doi: 10.1590/1678-4324-75years-2021200772. [DOI] [Google Scholar]
- 41.Formagio ASN, et al. Palicourea tomentosa (Aubl.) Borhidi: Microscopy, chemical composition and the analgesic, anti-inflammatory and anti-acetylcholinesterase potential. J. Ethnopharmacol. 2022;291:115050. doi: 10.1016/j.jep.2022.115050. [DOI] [PubMed] [Google Scholar]
- 42.Bouropoulos N, Weiner S, Addadi L. Calcium oxalate crystals in tomato and tobacco plants: Morphology and in vitro interactions of crystal-associated macromolecules. Chem. Eur. J. 2001;7:1881–1888. doi: 10.1002/1521-3765(20010504)7:9<1881::AID-CHEM1881>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira, F., Akisue, G. & Akisue, M. K. Farmacognosia: Identificação de Drogas Vegetais (Atheneu, 2014).
- 44.Furr M, Mahlberg PG. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J. Nat. Prod. 1981;44:153–159. doi: 10.1021/np50014a002. [DOI] [Google Scholar]
- 45.Yoder LR, Mahlberg PG. Reactions of alkaloid and histochemical indicators in laticifers and specialized parenchyma cells of Catharanthus roseus (Apocynaceae) Am. J. Bot. 1976;63:1167–1173. doi: 10.1002/j.1537-2197.1976.tb13202.x. [DOI] [Google Scholar]
- 46.Gabe, M. Techniques Histologiques (Masson & Cie, 1968).
- 47.Johansen, D.A. Plant Microtechnique (Mc Graw Hill Book, 1940).
- 48.Mace ME, Howell CR. Histochemistry and identification of condensed tannin precursor in roots of cotton seedlings. Can. J. Bot. 1974;52:2423–2426. doi: 10.1139/b74-314. [DOI] [Google Scholar]
- 49.Pearse, A. G. E. Histochemistry: Theoretical and Applied (The Williams & Wilkins Company, 1972).
- 50.Cain AJ. The use of Nile blue in the examination of lipids. J. Cell Sci. 1947;88:383–392. doi: 10.1242/jcs.s3-88.3.383. [DOI] [Google Scholar]
- 51.O’Brien, T. P. & McCully, M. E. The Study of Plant Structure: Principles and Selected Methods (Termarcarphi Pty Ltd, 1981).
- 52.Vidal BC. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Annales d’Histochimie. 1970;15:289–296. [PubMed] [Google Scholar]
- 53.Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- 54.Dunnigan MG. The use of nile blue sulphate in the histochemical identification of phospholipids. Stain Technol. 1968;43:249–256. doi: 10.3109/10520296809115077. [DOI] [PubMed] [Google Scholar]
- 55.Gardner RO. Vanillin-hydrochloric acid as a histochemical test for tannin. Stain Technol. 1975;50:315–317. doi: 10.3109/10520297509117081. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-de-Luque A, et al. Mucilage production during the incompatible interaction between Orobanche crenata and Vicia sativa. J. Exp. Bot. 2006;57:931–942. doi: 10.1093/jxb/erj078. [DOI] [PubMed] [Google Scholar]
- 57.Azuma JI, Kim NH, Heux L, Vuong R, Chanzy H. The cellulose system in viscin from mistletoe berries. Cellulose. 2000;7:3–19. doi: 10.1023/A:1009223730317. [DOI] [Google Scholar]
- 58.O’Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 1964;59:367–373. [Google Scholar]
- 59.Kraus JE, et al. Astra blue and basic fuchsin double staining of plant materials. Biotech. Histochem. 1998;73:235–243. doi: 10.3109/10520299809141117. [DOI] [PubMed] [Google Scholar]
- 60.Berlyn, G. P. & Miksche, J. P. Botanical Microtechnique and Cytochemistry (ed. Sass J. E.) 1–326 (Iowa State University Press, 1976).
- 61.Fuchs CH. Fuchsin staining with NaOH clearing for lignified elements of whole plants or plants organs. Stain Technol. 1963;38:141–144. doi: 10.3109/10520296309067156. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.