Highlights
-
•
Ultrasound-assisted calcium lactate significantly altered the structure of collagen.
-
•
The high calcium lactate concentration could limit the impact of ultrasound processing.
-
•
At low calcium lactate concentration, ultrasound improved the gastric digestibility of collagen.
-
•
Ultrasound-assisted calcium lactate treatment is an efficient method for change collagen.
Keywords: Type I collagen, Ultrasound treatment, Digestibility, Calcium lactate, Rheological properties
Abstract
Type I collagen has a relatively stable quality while quite resistant to digestion because of the complex triple helix structure. This study was conducted to explore the acoustic conditions of ultrasound (UD)-assisted calcium lactate processing of collagen and control the processing process through its sono-physico-chemical effects. The findings demonstrated that UD might lower the average particle size of collagen and increase its zeta potential. In contrast, the rise in calcium lactate concentration could dramatically limit the impact of UD processing. This may be because of its low acoustic cavitation effect, as demonstrated by the phthalic acid method (the fluorescence value decreased from 81245.67 to 18243.67). Poor changes in tertiary and secondary structures confirmed the detrimental effect of calcium lactate concentration on UD-assisted processing. Although UD-assisted calcium lactate processing can significantly alter the structure of collagen, the integrity of the collagen is basically preserved. Furthermore, the addition of UD and a trace amount of calcium lactate (0.1%) increased the roughness of the fiber structure. At this relatively low calcium lactate concentration, ultrasound improved the gastric digestibility of collagen by nearly 20%.
1. Introduction
As a result of the people's desire for good health conditions, scientists have developed some foods for their specialized purposes [1]. For instance, the production of red wine-based beverages may both nourish the blood and stimulate the body's metabolism. The manufacturing of myofibrillar protein-based products (e.g., beverage and edible films) is advantageous to human health [2]. Moreover, moderate supplementation with collagen-based products might prevent the aging of the skin. Compared with other proteins, marketing collagen-based beverages may be simpler. First, consumers assume that the majority of collagen is of animal origin and that no allergy exists [3]. Second, the strong structural stability of collagen-based products contributes to their relatively steady quality due to its complex triple helix structure [4]. Finally, collagen possesses several exceptional functional qualities that help ameliorate skin issues caused by collagen deficiency. Reports indicated that there were several types of collagen [3], [5]. Type I collagen, which accounts for 65–80 percent of the total quantity, is better suited for development and use in associated beverage items. Unfortunately, the unique triple helix structure of type I collagen makes it very resistant to digestion [6]. Additionally, it is difficult to dissolve type I collagen in neutral solutions. These drawbacks restrict the development of type I collagen as a beverage for select individuals.
The addition of calcium lactate may facilitate the dissolution and digestion of type I collagen. Unfortunately, while the inclusion of excessive calcium lactate (0.5%, w/v) is advantageous for the production of type I collagen beverages, consuming high quantities of calcium lactate might produce constipation and nausea. Additionally, prolonged usage of calcium lactate may cause a rebound rise in stomach acid. Consequently, it is required to enhance the solubility and digestibility of type I collagen at low calcium lactate concentrations (0.1%-0.3%, w/v) by the use of some novel processing techniques.
The combination of ultrasound (UD) and calcium lactate treatment has been believed to be an innovative strategy for improving the unfavorable properties of type I collagen recently. Efficiency enhancement may be regarded as one of the most important benefits of UD-assisted modification during manufacture. Strong cavitation of UD may alter the compact structure of type I collagen, allowing it easier to be broken into polypeptides, oligopeptides, and free amino acids during digestion. UD can also modify the interaction of proteins with other dietary components [7]. The interaction between collagen and calcium lactate in a type I collagen-based food matrix may considerably impact the in vitro digestibility of the product. Notably, the presence of calcium lactate may also affect the sonophysical and sonochemical effects of UD. Chen et al. (2022) [2] showed that environmental conditions may impact the outcome of UD. As a result, it is critical to investigate the actual effect of UD at different calcium lactate concentrations. This is also essential for maximizing the benefits of sonochemical kinetics in a food system containing calcium lactate.
In this investigation, different calcium lactate concentrations (low concentration: 0.1%, moderate concentration: 0.3%, and high concentration: 0.5%) were selected for UD treatment. This research aims to investigate the acoustic conditions of UD-assisted calcium lactate processing of collagen and their influence on processing efficiency. Initially, the secondary structure, particle size, and zeta potential were evaluated. The system states and in vitro digestibility of various proteins were then compared. This research aids in maximizing the benefits of sonochemical dynamics in the presence of calcium lactate.
2. Materials and methods
2.1. Materials and reagents
The type I collagen (sourced from Bovine Achilles Tendon) and calcium lactate were provided by Solarbio Technology Co., Ltd. (Beijing, China). The insoluble components in type I collagen were extracted through centrifugation at 11, 000 g for 15 min (4 °C). Bovine serum albumin, sodium hydroxide, and copper sulfate were supplied by Maikelin Technology Co., Ltd. (Shanghai, China).
2.2. Preparation of collagen samples
Different samples of type I collagen were diluted to a concentration of 10 mg/mL, and then they were applied with 8 different treatments: Ca0: both calcium lactate and UD were not conducted; Ca1: low concentration (0.1%, w/v) of calcium lactate was added, but UD was not conducted; Ca3: moderate concentration (0.3%, w/v) of calcium lactate was added, but UD was not conducted; Ca5: high concentration (0.5%, w/v) of calcium lactate was added, but UD was not conducted; UCa0: calcium lactate was not added, but UD was conducted; UCa1: low concentration (0.1%, w/v) of calcium lactate was added, and UD was also conducted; UCa3: moderate concentration (0.3%, w/v) of calcium lactate was added, and UD was also conducted; UCa5: high concentration (0.5%, w/v) of calcium lactate was added, and UD was also conducted. For the UCa1-UCa5 groups, calcium lactate solutions of varying concentrations (30 ml, 0.2%, 0.6%, and 1.0%, w/v) were added to collagen samples (30 ml, 20 mg/mL) to form the mixture (60 ml) before the homogenization (4500 rpm, 25 s). In contrast, UD (power: ∼400 W/L, time: 10 min) was only performed in the Ca0-Ca5 groups. The samples were then refrigerated at 4°for the subsequent experiment.
2.3. Quantification of UD treatments
2.3.1. Confirmation of triiodide
UD treatments in Ca0-Ca5 and UCa0-UCa5 were quantified through triiodide according to the method of Chen et al. (2022) [8]. Briefly, 0.1 M of potassium iodide was evenly dissolved in diverse samples. Then, UD treatment was applied in different systems. The operation parameters were consistent with those in section 2.2. Finally, the absorbance of different groups (Ca0-Ca5 and UCa0-UCa5) at 355 nm was utilized to indicate the amount of triiodide in the systems.
2.3.2. Confirmation of 2-hydroxyterephthalic acid
UD treatments in Ca0-Ca5 and UCa0-UCa5 were also quantified through 2-hydroxyterephthalic acid based on the method of Chen et al. (2022) [2]. Briefly, 2 mmol/L of terephthalic acid was evenly dissolved in diverse samples. Then, 25 mmol/L of NaOH was added to adjust the pH of the samples to 8.0. UD treatment was also applied in different systems. The operation parameters were consistent with those in section 2.2. Finally, the fluorescence intensity of different groups (Ca0-Ca5 and UCa0-UCa5) from 400 to 500 nm (excitation wavelength: 310 nm) was utilized to indicate the amount of 2-hydroxyterephthalic acid in the systems.
2.4. Average sizes and zeta potential
The laser light scattering method was used to examine the particle sizes of the collagen samples (Ca0-Ca5 and UCa0-UCa5) following UD (Malvern Mastersizer 3000, England). Diverse collagen samples were diluted in deionized water about 20 times before being vibrated for 60 s to ensure homogeneous separation. 0.5 mg/ml is the final collagen concentration for this detection. The particle size was represented by the average mean of five measurements of collagen samples. The refractive and absorption indices were 1.440 and 0.001, respectively.
The electrophoretic diffusion of a diluted collagen solution (0.15%, w/v) was used to assess the surface charge characteristics of several collagen samples (Ca0-Ca5 and UCa0-UCa5) after UD, which was previously undertaken by Chen et al. (2022) [8]. Diverse collagens were vibrated for 60 s before the measurement of a Nano ZS instrument. The zeta potential values of collagen samples were directly shown via the Malvern instrument.
2.5. Intrinsic fluorescence and synchronous fluorescence
The intrinsic tryptophan fluorescence of the diluted collagen solution was evaluated through a Tecan microplate reader. The collagen solution produced in Section 2.2 was adjusted to a concentration of 0.02 mg/ml by homogenizing it for 40 s at a speed of 6500 rpm and a temperature of 4 °C. At an excitation wavelength of 280 nm, various fluorescence intensity values of collagen solution were measured. Meanwhile, spectra of emission were collected between 290 and 450 nm.
The synchronous fluorescence of the diluted collagen solution was examined via a Shimadzu spectrophotometer following the methodology of our previous investigation [3]. The collagen solution was also adjusted to a concentration of 0.02 mg/ml by homogenizing. The samples of diluted collagen were then put into the cuvette. The wavelength of excitation and the fluorescence shift was adjusted to 220 nm and 20 nm, respectively. Collagen solution fluorescence spectra were recorded from 250 to 350 nm.
2.6. Circular dichroism
The secondary structure of the diluted collagen solution was assessed through a circular dichroism (CD) spectrometer (J1500, Japan) [3]. The collagen solution was diluted to a concentration of 0.02 mg/ml by homogenizing it for 40 s at a speed of 6500 rpm and a temperature of 4 °C. About 1 ml of the diluted collagen was then put into the quartz cuvettes (length: 0.1 cm). Sample information of collagen solution was collected in a nitrogen environment from 250 to 190 nm.
2.7. Protein patterns of collagen
Protein patterns of the collagen samples (Ca0-Ca5 and UCa0-UCa5) following UD were determined through gel electrophoresis. The loading buffer (1% SDS/β-mercaptoethanol, 0.02% bromophenol blue, and 20% glycerol) and diluted collagen samples (2.5 mg/mL) were vortexed for 45 s at a set ratio of 4: 1 (v/v). Meanwhile, diluted collagen samples (2.5 mg/mL) were mixed with the same buffer that did not contain β-mercaptoethanol. Then, 6 L of different mixtures containing collagen (Ca0–Ca5 and UCa0–UCa5) were sampled onto 12% of the prepared polyacrylamide gels for the electrophoresis (90 V). After 180 min of incubation with a portable dye and decolor solution, the protein patterns were finally identified.
2.8. Raman spectra
The O-H stretching of the collagen samples (Ca0-Ca5 and UCa0-UCa5) following UD was evaluated through the Jobin Evolution spectrometer (Longjumeau, France). Collagen samples were dripped into the grooves of the glass sheet for further testing. The spectral information from 3000 to 3500 cm−1 was collected in the DPSS laser environment. Other parameters: exposure time of 55 s, speed of 100 cm−1/min, and temperature of 4 °C. The software named Labspec (Longjumeau, France) was applied for the analysis of Raman spectra.
2.9. Static shear behavior
The static shear behaviors of the collagen samples (Ca0-Ca5 and UCa0-UCa5) after UD were measured through a typical rotational rheometer (Anton Paar, Austria) [2]. To confirm the static shear behavior, a viscosity sweep was done in this experiment. For all collagen solutions, shear rate tests ranging from 0.009 to 1000 s−1 at room temperature were performed to acquire the rheological spectra.
2.10. Scanning electron microscope (SEM)
The micromorphology changes of the collagen samples (Ca0-Ca5 and UCa0-UCa5) after UD were also evaluated through a scanning electron microscope (SEM, Bruker Ltd., Japan). Different collagen samples were first dried in an oven (Fudanxi Ltd., China) at 25 °C. Approximately 0.2 g of the collagen sample was fixed on a smooth mica surface with gold-platinum. In SE mode (8 kV), images of the collagen microstructure were collected.
2.11. Atomic force microscope (AFM)
The micromorphology alterations of the collagen samples (Ca0-Ca5 and UCa0-UCa5) after UD were measured using an atomic force microscope (AFM, Bruker Ltd., USA). Approximately 0.2 ml of the collagen sample was placed on a smooth mica surface. The collagen solutions were then dried in the air for ten minutes. In tapping mode (0.5 N/m), images of the collagen microstructure were captured. NanoScope software was used for the picture analysis (Bruker Ltd., USA).
2.12. In vitro digestibility
The in vitro digestibility transformations of the collagen samples (Ca0-Ca5 and UCa0-UCa5) were evaluated based on the methods of Brodkorb et al. (2019) [9]. First, the protein concentration (W0) of the mixture containing modified collagen samples (3 ml, 10 mg/ml) and gastric fluid (3 ml) was determined. After maintaining these collagen mixtures at 37 °C, pepsin (500 U/mL, 25:1) was added. Activating the digestion of collagen mixes by changing the pH to 2.0. Finally, the gastric process was halted by neutralizing the acidic environment with a pH of 7.0. The protein content of the digestion products was reassessed and labeled W1. The following equation was used to determine the gastric digestibility of various collagen samples.
| (1) |
Afterward, the stomach digest (3 ml, pH 7.0) was mixed with the intestinal fluid (3 ml, pH 7.0) containing pancreatin (5 mg/mL). Eventually, the intestinal digestion process was halted by heating. The protein content of the digestion products was reassessed and labeled W2. The following equation was applied to evaluate the intestinal digestibility of different collagen samples.
| (2) |
2.13. Statistical analysis
The statistical significance (p <.05) of these data was determined by a two-way analysis of variance and SAS software. At this time, the sonication and calcium lactate concentrations were set as independent, and the obtained results were set as a dependent. The Origin 2020 program was then used to generate all the figures associated with the findings.
3. Results and discussion
3.1. Verification of sono-physico-chemical effects
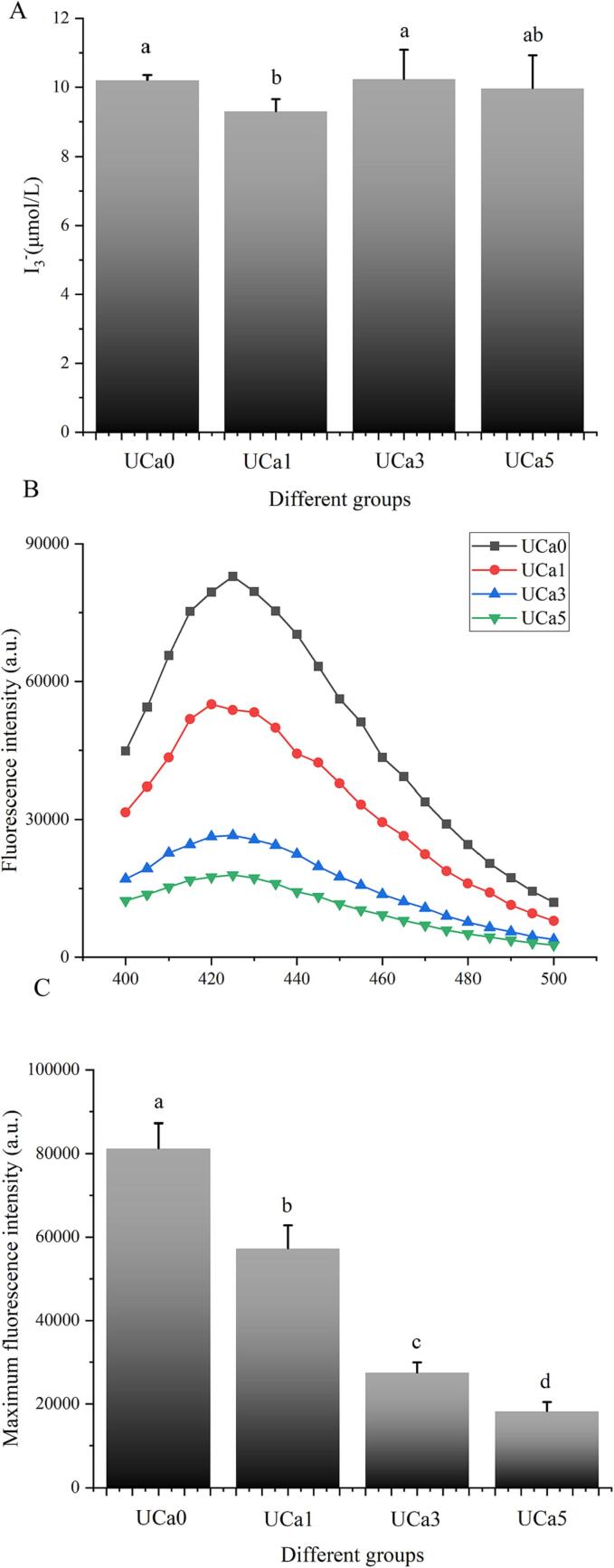
Determining acoustic cavitation in a system containing calcium lactate would be useful for working on UD-assisted processes and, thus, controlling the impact of sonication according to the proper application of collagen [2]. In this study, sonophysical and sonochemical effects of UD in different groups were measured through triiodide and 2-hydroxyterephthalic acid (Fig. 1). The results implied that the presence of calcium lactate in the food system did not increase the sonophysical and sonochemical effects, but decreased the generation of triiodide and 2-hydroxyterephthalic acid. Moreover, the rise in calcium lactate concentration is not favorable to UD treatment. Additionally, Hu et al. (2022) discovered that calcium lactate inhibited the generation of hydroxyl radicals [10]. Compared to the validation of the phthalic acid approach, the iodimetry method demonstrates worse anti-interference and increased variability. Previous research favored the phthalic acid approach over the iodometric method [2]. To measure the sonophysical and sonochemical effects of this investigation, 2-hydroxyterephthalic acid was also selected. It should be highlighted that the effect of calcium lactate in collagen samples on the generation of free radicals may have an impact on the actual effect of UD-assisted processing, which will be validated in the structure part of collagen.
Fig. 1.
The impact of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) on the free radical content of UD-assisted systems as measured by iodometry (A) and phenanthroline (B-C). a,b,c,d, denotes significant differences among different groups (p <.05).
In general, a greater UD-assisted processing impact can be achieved if a greater number of products resulting from the reaction with free radicals [2]. In this research, a high quantity of calcium lactate might inhibit UD-assisted processing. This was consistent with the variables restricting collagen formation since a high calcium lactate content in protein beverages might lead to constipation and nausea. In addition, the degree of sonochemical action is connected with the viscosity of the medium [11], which will be further explored in the section on rheological characteristics.
3.2. Average sizes
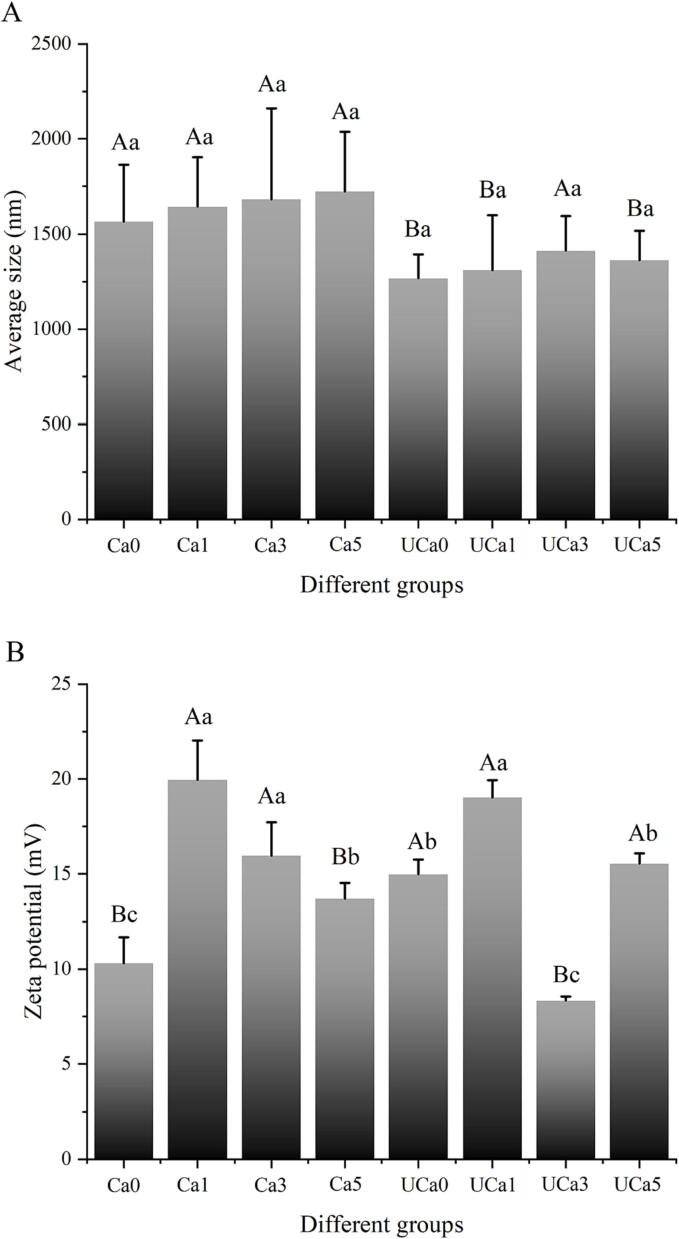
The average particle sizes of distinct groups (Ca0-Ca5 and UCa0-UCa5) correlate positively with the solubility and/or disaggregation of collagen [8]. As seen in Fig. 2A, UD treatment led to a considerable reduction in the average particle size. This might be attributable to the disaggregation characteristic of collagen samples. As reported by Jiang et al. (2022) [12], UD might break down noncovalent forces (such as electrostatic forces and hydrogen bonds) in collagen, enabling the aggregated molecules to be equally dissolved. In addition, the reduction in collagen particle size may be related to the physical strength and free radical oxidation of UD. Akram et al. (2020) [13] attributed the reduction in the average particle size of collagen after ultrasonography to the fragmentation of its surface. However, another study [14] suggested that UD had little effect on collagen aggregation and solubility. Raw materials and processing conditions may account for the disparity in outcomes. The steak was the subject of UD treatment in the investigation by Chang et al. (2015). The amount of collagen in the steak that UD may impact at this stage is quite modest. Interestingly, the rise in calcium lactate concentration further increases the average particle size of collagen, suggesting that the increase in calcium lactate concentration diminishes the actual impact of UD. Unfortunately, no significant difference was observed in the groups ranging from UCa0-UCa5.
Fig. 2.
The effects of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) and UD on the average sizes (A) and zeta potential (B) of the type I collagen system. A, B, denotes significant differences between UD treatment (p <.05). a, b, c, denotes significant differences among different calcium lactate concentrations (p <.05).
3.3. Zeta potential
The zeta potential study was performed to estimate the electrostatic forces at collagen's surface [8]. The UD treatment enhanced the zeta potential value of the UCa0 group when compared with the Ca0 control group. Increases in these zeta potential values indicated an enhancement in the electrostatic repulsion force between collagen molecules. The findings demonstrated that UD decreased the probability of collision between collagen molecules and enhanced the protein solution's stability. In a new investigation, Zou et al. (2017) [15] also found that ultrasonic may enhance the net positive charge on the surface of collagen, which they attributed to particle size change. According to Petcharat et al. (2020) [16], UD might alter the distribution of amino acids on the surface of collagen by a substantial mechanical force, which corresponded to a dramatic shift in zeta potential. Even though UD could cut collagen in different places through their acoustic cavitation, the treatment is usually very mild. As a consequence, UD-assisted processing is an effective technique for improving the quality of collagen.
Compared to the untreated control group (Ca0), the zeta potential values of the UCa0, UCa1, UCa3, and Uca5 groups increased by 45.24%, 84.54%, −19.10%, and 50.74%, respectively. Among all UD-assisted calcium lactate treatment groups, the low-concentration calcium lactate treatment group (UCa1) exhibited the highest zeta potential value (19.02 mV). This was consistent with the real impact of UD regarding sonophysical and sonochemical effects, as described in section 3.1. At a lower concentration of calcium lactate (0.1%), UD showed a greater impact, corresponding to a greater zeta potential in Fig. 2B. This might be related to the increased ultrasonic cavitation, which exposed more active areas and increased electrostatic repulsion between collagen molecules. Ma et al. (2020) [17] also demonstrated that more amino groups were exposed on the surface of the collagen if its size decreased, leading to an increase in its positive charge and zeta potential. Thus, the combination of UD with low concentrations of calcium lactate (0.1%) may facilitate the uniform dispersion of collagen and improve the performance of collagen-based products.
3.4. Intrinsic fluorescence and synchronous fluorescence
Typically, the intrinsic fluorescence of tyrosine and phenylalanine residues in collagen is considered a suitable probe for protein structural alteration [18]. To examine the structural properties of collagen in various settings, the intrinsic fluorescence of several collagen solutions was triggered in this study. Type I collagen, unlike other proteins, does not include tryptophan [3]. Consequently, the fluorescence intensity of tyrosine residues coincides with the peak of endogenous fluorescence at 310 nm (Fig. 3A). The variation in fluorescence intensity at 310 nm can be attributed to the hydrogen bond between collagen and calcium lactate. Once calcium lactate was introduced to the mixed solution, the fluorescence intensity of the collagen samples increased significantly. Calcium lactate in the solution is capable of colliding with collagen molecules and promoting a change in fluorescence intensity [19], [20]. After ultrasonography, the group's fluorescence intensity (UCa0) is significantly greater than that of the control group (Ca0). This may be because UD lowers the particle size of collagen molecules and further increases the likelihood of protein molecules colliding [19]. In contrast, the combination of UD and a low calcium lactate concentration (0.1%) resulted in the greatest change in fluorescence intensity (Fig. 3A). This is understandable given that the sonophysical and sonochemical effects of UD are higher at low concentrations of calcium lactate. The findings of endogenous fluorescence confirmed the superposition effect of UD and calcium lactate for the treatment of collagen.
Fig. 3.
The effects of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) and UD on the endogenous fluorescence spectra (A) and synchronous fluorescence spectra (B) of the type I collagen system.
Due to the simultaneous scanning of the excitation and emission wavelengths of distinct fluorescent groups, synchronous fluorescence is more sensitive than the classic endogenous fluorescence assessment approach [3], [21]. Hence, the synchronous fluorescence technique was used to investigate the structural changes of the collagen-calcium lactate combination (Fig. 3B). Similar to the results in the intrinsic fluorescence part, the structure of collagen molecules was altered by the combination of UD and calcium lactate. The effect of UD-assisted processing on the synchronous fluorescence intensity of collagen was still reduced in the order of UCa1, UCa0, UCa3, and UCa5. This was consistent with the ultrasonic cavitation effect, which indicated that a modest concentration of calcium lactate (0.1%) might boost UD-aided processing efficiency more than a high concentration. Also, the effect of these changes in fluorescence is clearer than the effect of heat on collagen seen in a previous test [3]. Intriguingly, the fluorescence intensity value of the UCa1 group is even greater than that of the UCa0 group, indicating that the combination of a low calcium lactate concentration (0.1%) and UD favorably promoted the structural modification of collagen.
3.5. Secondary structure
CD signal was reported to show a tight relationship with the secondary structure of the complex containing collagen and calcium lactate [3]. As depicted in Fig. 4A, all samples of type I collagen display two peaks, including negative and positive ones. The maximum, minimum, and crossing points of the CD spectra of the Ca0 group appear at 221, 198, and 209–215 nm, respectively, which are indicative of the triple helix conformation of collagen [22], [23]. In this case, the addition of calcium lactate increases the amplitude of the CD signal (Fig. 4A), and this change is more pronounced in the UD-assisted calcium lactate processing group. After being treated with UD and calcium lactate, the unique secondary structure of the type I collagen sample was shown to have been transformed into a random structure [3].
Fig. 4.
The effects of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) and UD on the circular dichroism spectra (A) and Rpn value (B) of the type I collagen system. A, B, denotes significant differences between UD treatment (p <.05). a, b, denotes significant differences among different calcium lactate concentrations (p <.05).
To objectively measure the influence of secondary structural modification in collagen, the absolute value of the positive and negative peak ratios (Rpn) of CD spectra was used in this study. According to a prior study [24], the Rpn value is often used to monitor the intermolecular tight contact in collagen mixture systems. As seen in Fig. 4B, the addition of calcium lactate induced a little change in the secondary structure of collagen, while UD caused a significant shift in Rpn. The findings indicated that the slight open chain structure of collagen molecules was detected in the calcium lactate system. Also, the structures of these collagen samples (UCa0-UCa5) collapsed following UD-assisted processing. This was consistent with the processing technique during the application. UD-assisted processing involved very powerful physical pressures and free radical oxidation in addition to the interaction between calcium lactate and collagen [2]. Together, these treatments induced a substantial alteration in the secondary structure of collagen, which corresponded to a substantial shift in Rpn value. It should be noted that the UCa1 group exhibited the least RPN among all UD-assisted machining groups. The UCa1 sample declined by 7.44% in comparison to the control group (Ca0). Results indicated that this processing method caused the greatest structural denaturation of the collagen. This demonstrated that the triple helix structure was nearly entirely shattered, which might be attributed to the tremendous energy density generated by cavitation and turbulence during the UD-assisted calcium lactate treatment [8].
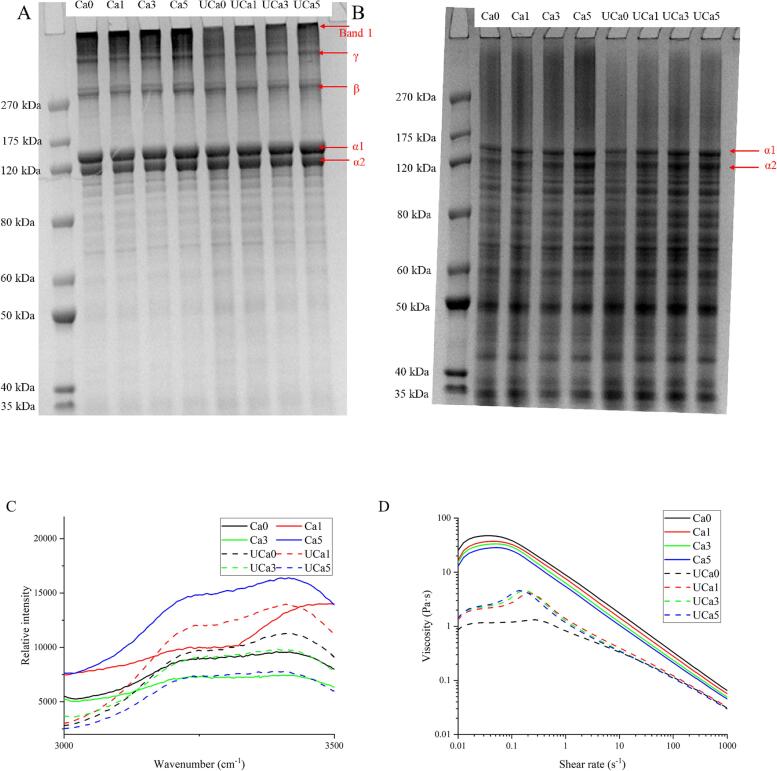
3.6. SDS-Page
Fig. 4A-B depicts the variations in the protein bands of several collagen samples under reduced and non-reduced settings. As demonstrated in Fig. 4A, all samples of collagen include α chain, β chain, and γ chain. Notably, β chain and γ chains are dimers and trimers of α chains, respectively [15]. All characteristic bands of collagen can be observed clearly, which demonstrated the mild nature of UD processing and calcium lactate treatment. Therefore, these treatments could not alter collagen's structural integrity. These typical bands have also been identified by other researchers in recent years [15], [25]. However, the collagen of soft-shelled turtles (Pelodiscus sinensis) showed a relatively lower molecular weight than that of this experiment. It could be ascribed to the origin of collagen. Before UD, varying calcium lactate concentrations (0.1%-0.5%) did not cause significant changes in band 1, indicating that calcium lactate concentrations showed very little influence on the protein band. In contrast, the degradation of band 1 after exposure to ultrasonic waves is relatively more apparent. Compared to the UCa5 group, band alterations in the UCa1 group were more pronounced. This supported the previous conclusion in section 3.1. That is to say, UD and low-concentration calcium lactate treatments accelerated the disintegration of high-molecular-weight collagen bands. The synergistic use of UD and other treatments may also result in the development of excessive free radicals. Excessive protein oxidation is not only detrimental to the breakdown of proteins with a high molecular weight, but it may also compromise the structure of proteins [26]. The results of this study showed that low concentrations of calcium lactate (0.1%) combined with UD might not change the excellent quality of collagen products negatively.
In the reducing gel pattern (Fig. 4B), a significant number of irregular bands with molecular weights larger than 175 kDa were seen in all calcium lactate treatment groups (Ca1-Ca5) but not in the calcium lactate processing group that was subjected to UD (UCa1-UCa5). These results suggested that UD-assisted processing is crucial for the improvement of collagen products.
3.7. Raman spectra
The OH stretching of the collagen samples (Ca0-Ca5 and UCa0-UCa5) was obtained by analyzing the Raman spectra between 3220 cm−1 and 3440 cm−1 (Fig. 5C), which corresponds to the intramolecular vibration of the hydrogen bond in the composite system of collagen and calcium lactate [27]. According to a previous publication [28], the intensity ratio of the Raman spectra at 3220 cm−1 and 3440 cm−1 is utilized to measure this structural change. UD treatment considerably decreased the ratio of 3220 cm−1 to 3440 cm−1 of control collagen samples (from 0.94 to 0.85), suggesting that UD enhanced the system's water permeability. UD might alter the collagen system's equilibrium based on Le Chatelier's principle. During the process of the system regaining balance, water molecules entered various collagen molecules [29], therefore promoting the expansion of protein molecules. This facilitated the structure changes of collagen. As reported by Xue et al. (2018) [29], the OH stretching vibration shift might be connected with the microstructure change. In the next part, the alterations in collagen microstructure will be further discussed.
Fig. 5.
SDS-PAGE patterns (A and B), Raman spectra (C), and static shear behavior (D) of collagen treated with different calcium lactate concentrations (0.1%, 0.3%, and 0.5%) and UD. SDS-PAGE patterns without (A) or with (B) β-mercapto-ethanol correspond to Fig. 5A and Fig. 5B, respectively.
For different collagen samples (UCa1, UCa3, and UCa5), the ratio of Raman peaks at 3220 cm−1 and 3440 cm−1 increased from 0.85 to 0.96 when the content of calcium lactate rose from 0.1% to 0.5%. This is comprehensible since a rise in calcium lactate concentration might diminish the sonophysical and sonochemical impacts of UD, which correlate to a poor OH stretching vibration state [29]. In UD-assisted calcium lactate processing, it is essential to take into account the influence of calcium lactate on the sonophysical and sonochemical effects of UD [2]. According to the results of this study, a relatively high calcium lactate concentration is not favorable to the processing of collagen.
3.8. Static shear behavior
Based on the findings of Chen et al. (2022) [2], the apparent viscosity changes of the collagen system (Ca0-Ca5 and UCa0-UCa5) were strongly related to the actual impact of UD (including sonophysical and sonochemical effects). Fig. 5D illustrates the shear characteristics at a steady state for all collagen samples. In response to an increase in shear rate, collagen's viscosity first climbed quickly and then subsequently reduced dramatically. These behaviors indicated that the system containing collagen and calcium lactate exhibited non-Newtonian properties, which were similar to those of the majority of proteins [2], [8]. The apparent viscosities of Ca0, Ca1, Ca3, Ca5, UCa0, UCa1, UCa3, and UCa5 at a shear rate of 0.01 s−1 are 24.60, 16.20, 14.90, 12.60, 0.85, 1.31, 1.44, and 1.41 Pa·s, respectively. UD decreased the apparent viscosity of the collagen system by its intense cavitation, which may be a result of the rearranging of collagen molecules during UD [30]. In addition, UD might change the system's apparent viscosity by altering the strength of the hydrogen bond interaction between collagen and calcium lactate.
In general, a system with low viscosity has a strong oscillation effect, which assists in the formation and action of cavitation bubbles during the UD process [11]. The addition of calcium lactate enhanced the viscosity of the system relative to UCa0, limiting the formation of cavitation bubbles. When the calcium lactate concentration reached 0.1%, the system's viscosity was at its lowest, corresponding to the greatest UD-assisted processing effect. This was in line with the findings of sonophysical and sonochemical effects in section 3.1. By decreasing the viscosity of the myofibrillar protein system, previous research also tried to control the impact of covalent modification [2]. Therefore, the viscosity properties of collagen with low quantities of calcium lactate increase the efficiency of UD processing.
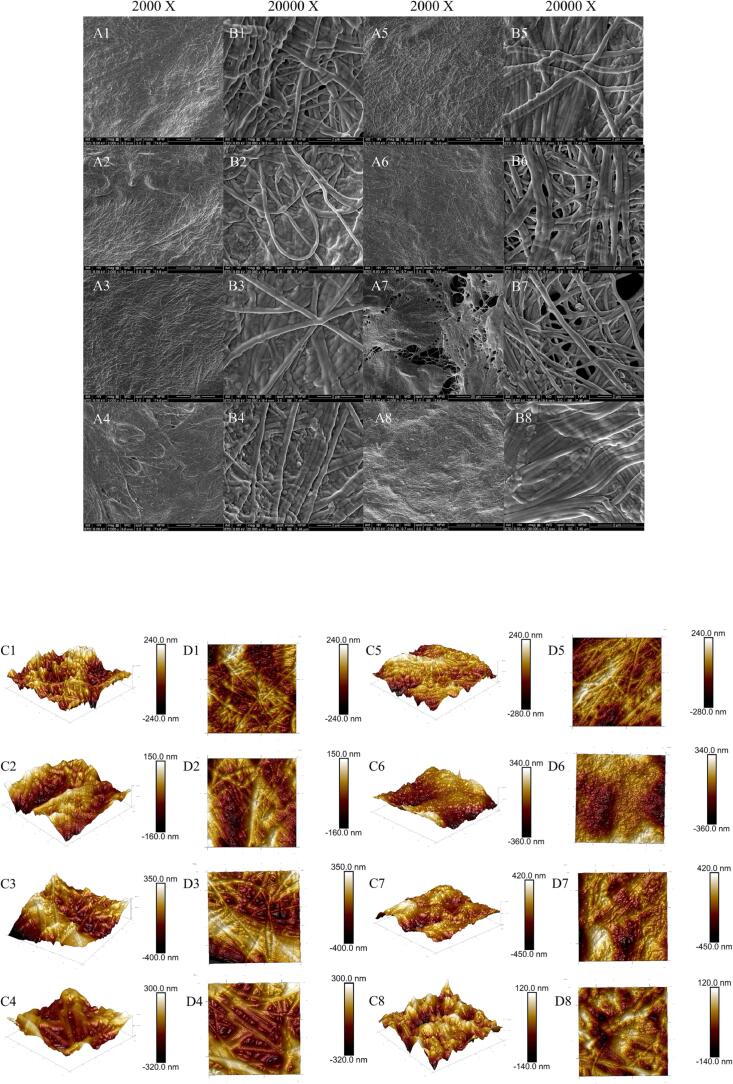
3.9. SEM images
Fig. 6 A-B presents the findings from observing the microstructure of collagen samples using SEM. Collagen is comparable to a soft, fibrous sponge. Due to the unavoidable requirement for dehydration in the SEM pretreatment [7], all collagen samples (Ca0-Ca5 and UCa0-UCa5) exhibited a multilayered and uneven structure (20000 X). At relatively low magnification (2000 X), all of the collagen samples (Ca0-Ca5 and UCa0-UCa5) showed a dense monolayer structure. Zou et al. (2017) [15] reported that collagen also includes uneven holes, which are mostly the consequence of fibrous collagen's random distribution. Intriguingly, fibrous collagen was substantially thicker in the UD-assisted calcium lactate processing groups (UCa1-UCa5) than in the control group (Ca0). The expansion of collagen may be a result of the processing-induced introduction of water molecules. In the process of re-establishing the system's equilibrium, water molecules enter diverse collagen molecules [29], accelerating the changes in the collagen microstructure. This was consistent with the results obtained in Raman spectroscopy. Due to the inherent limitations of SEM, AFM was used to lessen the detrimental alterations in collagen samples during pretreatment.
Fig. 6.
Scanning electron microscopy (A1-A8, B1-B8) of the type I collagen system after various treatments with different magnifications (2000 X, and 20,000 X). The effects of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) and UD on the 3D images (C1-C8), and 2D images (D1-D8) of the type I collagen system.
3.10. AFM images
The microscopic morphology of dietary proteins has been investigated using AFM imaging technology in recent years [31]. Fig. 6C-D displays the ribbon fiber structure of type I collagen, as determined by AFM. The collagen from the barracuda was found to have a comparable microstructure [32]. Furthermore, the nanostructure of collagen was unaffected by calcium lactate treatment at varying doses. It should be highlighted that the incorporation of calcium lactate increased the width of nanoribbons, which was proven by the thicker fiber structure (Fig. 6 C2–C4). Collagen morphological alterations were particularly evident in all groups (UCa1-UCa5) that underwent calcium lactate processing with UD assistance. The morphology of the UCa1, UCa3, and UCa5 groups was an amorphous structure, minor quantities of aggregates, and irregular aggregates, respectively. In UD-assisted processing, the shape of collagen fiber was dependent on the calcium lactate concentration. When the concentration of calcium lactate was high enough (∼0.5%), collagen fiber showed a fibrous appearance. This differs from the findings of previous research on the influence of UD and heat treatment on the ultrastructure of bovine tendon collagen fibers [33]. The important factor, according to the outcomes of Wan et al. (2021) [33], was heat treatment, not UD. In this investigation, various calcium lactate and UD treatments had minimal influence on the microstructure of collagen, but their combination treatment showed a substantial effect. Additionally, the combination of UD and low amounts of calcium lactate (0.1%) promoted the synthesis of amorphous collagen. The increase in fiber structure coarseness coincided with the expansion of the protein molecules, which contributes to the enhancement of collagen product quality. In conclusion, the findings of the microstructure investigation further verified the benefits of UD in conjunction with low calcium lactate concentrations (0.1%).
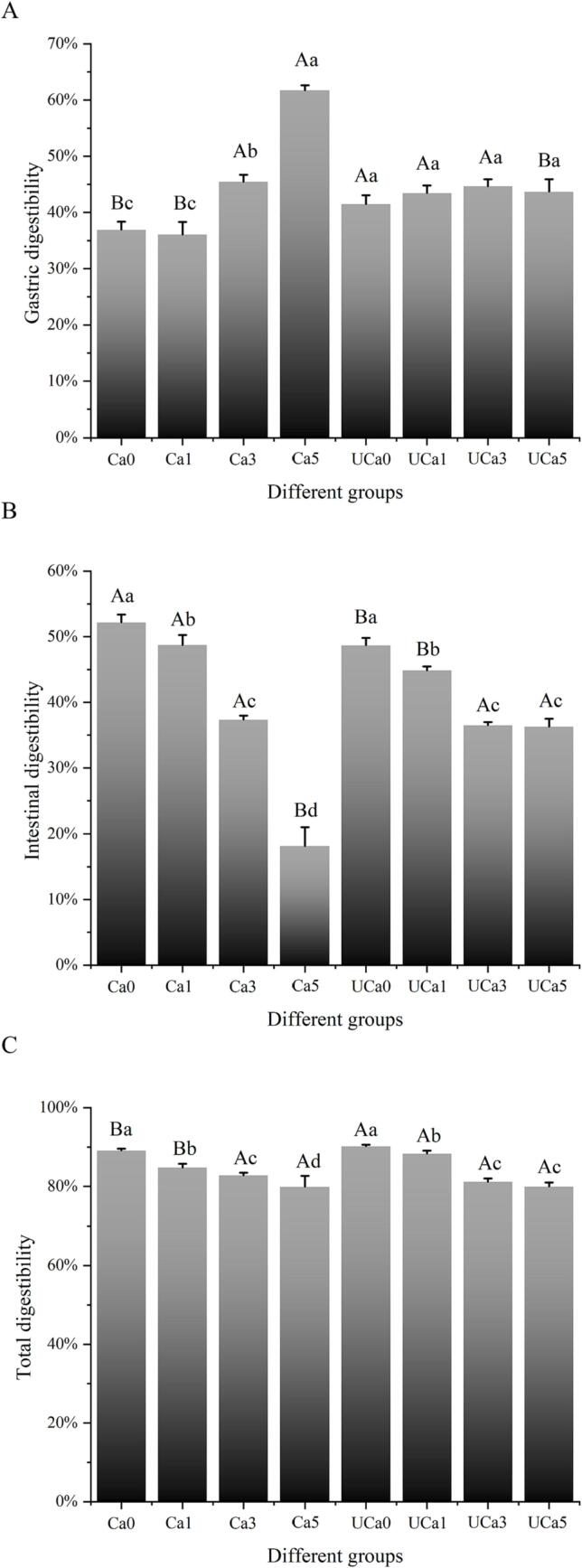
3.11. In vitro digestibility
High protein digestibility is an effective factor for attracting customers. Type I collagen displays poor digestion characteristics due to its unusual triple helix shape [3]. This drawback impedes the development of type I collagen as a personalized beverage. The impact of UD and calcium lactate on the digesting characteristics of collagen was illustrated in Fig. 7. The incorporation of various calcium lactates (0.1%-0.5%) considerably enhanced collagen's digestibility throughout the stomach's digestion phase (Fig. 7A). This is due to the influence of calcium lactate on the pH of the collagen solution [34]. Increased calcium lactate concentration (from 0.1% to 0.5%) might reduce the solution's pH, thereby enhancing the performance of pepsin. However, at relatively low concentrations of calcium lactate (0.1%, the Ca1 group), there were no significant alterations in stomach digestion when compared with the Ca0 group. This is because calcium lactate concentration with a relatively low concentration (0.1%) has a negligible impact on the pH of the collagen solution. Compared to the Ca1 group, the UD-assisted low calcium lactate concentration processing group (UCa1) dramatically improved gastric digestibility from 36.07% to 43.46%. This is connected with the impact of UD on the structure of collagen. According to a previous study by Zou et al. (2017) [15], ultrasonography increased the number of exposed cleavage sites. More so, UD could enhance the effectiveness of pepsin by modulating the interaction between calcium lactate and collagen.
Fig. 7.
The effects of various calcium lactate concentrations (0.1%, 0.3%, and 0.5%, w/v) and UD on the gastric, intestinal, and total digestibility of the type I collagen system. A, B, denotes significant differences between UD treatment (p <.05). a, b, c, d, denotes significant differences among different calcium lactate concentrations (p <.05).
The rate of intestinal digestion reduced from 48.73% to 18.16% when the concentration of calcium lactate increased from 0.1% to 0.5% during the intestinal digestion stage. The selectivity of pancreatic digestion is very robust [21]. The addition of calcium lactate might enhance steric hindrance and diminish the digestive action of pancreatin. Moreover, the stronger digestive action at the stomach stage limited the number of sites accessible for pancreatic digestion in the intestinal stage. As seen in Fig. 7B, UD-assisted calcium lactate processing enhanced the intestinal digestibility of collagen by a substantial amount. This was due to the transformation of collagen's secondary and tertiary structures [15], [35], which were induced by UD. In light of the distinct digesting outcomes of the stomach and small intestine, the overall digestion rate was also investigated in this work (Fig. 7C). Notably, UD improved the digestion of collagen at a lower calcium lactate concentration (0.1%). However, at relatively high concentrations of calcium lactate, UD showed no discernible impact. The actual sonophysical and sonochemical effects of UD are greatest at a low concentration (0.1%) of calcium lactate, according to these findings.
3.12. Potential mechanism and further discussion
UD-assisted calcium lactate treatment can be considered an efficient method for producing type I collagen as a personalized beverage. By comprehending the UD-assisted calcium lactate processing mechanism, the actual sonophysical and sonochemical effects can be further optimized [36]. In this study, the formation and impact of cavitation bubbles in the UD system can be affected by the concentration of calcium lactate. The combination of low-concentration calcium lactate (0.1%) with UD could decrease the system's viscosity and increase the cavitation effect. In this instance, the cavitation of UD exposed more active areas and enhanced electrostatic repulsion between collagen molecules. The UCa1 group exhibited more secondary and tertiary structural alterations as a result of the increased cavitation impact. These structural modifications facilitated the penetration of water molecules, resulting in considerable microstructural modifications [37]. Therefore, UD and low concentrations of calcium lactate (0.1%) showed the greatest favorable influence on the structure, rheology, and physicochemical characteristics of collagen.
4. Conclusions
This investigation confirmed that the acoustic circumstances of the collagen-calcium lactate composite system must be taken into account to maximize the benefits of sonochemical kinetics. The addition of calcium lactate showed a negative effect on the formation of acoustic cavitation bubbles and the impact of UD-assisted collagen processing. In the actual manufacturing procedure, a lower concentration of calcium lactate (0.1%) should be used. The mild structural alterations of collagen (zeta potential, endogenous fluorescence, positive and negative peak ratios of the CD spectrum) proved that relatively high concentrations of calcium lactate (0.5%) exhibited an adverse impact on the actual effect of UD. When the calcium lactate concentration approached 0.1%, the viscosity of the system was at its lowest (1.31 Pa·s), which corresponded to the highest UD-assisted processing impact. This study aids in determining the best method for processing collagen using calcium lactate and UD assistance, and provides a new insight into the associations between sono-physico-chemical effects and collagen nutrition.
CRediT authorship contribution statement
Miao Zhang: Conceptualization, Methodology, Data curation, Writing – original draft. Tingxuan Gao: Formal analysis, Data curation. Yu Han: Formal analysis, Data curation. Dejiang Xue: Investigation, Formal analysis. Shuai Jiang: Investigation, Formal analysis. Qian Li: Visualization. Chunbao Li: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Jiangsu Department of Education (Innovation Group of Meat Nutrition, Health and Biotechnology), the Ministry of Science and Technology of the People’s Republic of China (10000 Talent) and the Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB335).
Data availability
Data will be made available on request.
References
- 1.Hays N.P., Kim H., Wells A.M., Kajkenova O., Evans W.J. Effects of whey and fortified collagen hydrolysate protein supplements on nitrogen balance and body composition in older women. J. Am. Diet. Assoc. 2009;109:1082–1087. doi: 10.1016/j.jada.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Chen X., Zhou G., Xu X. New insights into the ultrasound impact on covalent reactions of myofibrillar protein. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M., Zhao D., Zhu S., Nian Y., Xu X., Zhou G., Li C. Overheating induced structural changes of type I collagen and impaired the protein digestibility. Food Res. Int. 2020;134 doi: 10.1016/j.foodres.2020.109225. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.M., Geng F., Wang Y., Cao J.X. Textural modification of Chinese traditional stewed pig trotter: Effect of acid or alkaline-induced degradation of collagen fibers. J Texture Stud. 2022 doi: 10.1111/jtxs.12735. [DOI] [PubMed] [Google Scholar]
- 5.Subhan F., Hussain Z., Tauseef I., Shehzad A., Wahid F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. 2021;61:1027–1037. doi: 10.1080/10408398.2020.1751585. [DOI] [PubMed] [Google Scholar]
- 6.Exposito J.Y., Valcourt U., Cluzel C., Lethias C. The fibrillar collagen family. Int. J. Mol. Sci. 2010;11:407–426. doi: 10.3390/ijms11020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Chen X., Zhou G., Xu X. Ultrasound: A reliable method for regulating food component interactions in protein-based food matrices. Trends Food Sci. Tech. 2022;128:316–330. [Google Scholar]
- 8.Chen J., Zhang X., Chen X., Bassey A.P., Zhou G., Xu X. Phenolic modification of myofibrillar protein enhanced by ultrasound: The structure of phenol matters. Food Chem. 2022;386 doi: 10.1016/j.foodchem.2022.132662. [DOI] [PubMed] [Google Scholar]
- 9.Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 10.Hu X., Chen Y., Wu X., Liu W., Jing X., Liu Y., Yan J., Liu S., Qin W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X. 2022;14 doi: 10.1016/j.fochx.2022.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan S., Ghani N.A., Aminuddin N.F., Quraishi K.S., Razafindramangarafara B.L., Baup S., Leveque J.M. Unexpected acceleration of Ultrasonic-Assisted iodide dosimetry in the catalytic presence of ionic liquids. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S., Zhang M., Liu H., Li Q., Xue D., Nian Y., Zhao D., Shan K., Dai C., Li C. Ultrasound treatment can increase digestibility of myofibrillar protein of pork with modified atmosphere packaging. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2021.131811. [DOI] [PubMed] [Google Scholar]
- 13.Akram A.N., Zhang C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105053. [DOI] [PubMed] [Google Scholar]
- 14.Chang H.J., Wang Q., Tang C.H., Zhou G.H. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J. Food Quality. 2015;38:256–267. [Google Scholar]
- 15.Zou Y., Xu P., Li P., Cai P., Zhang M., Sun Z., Sun C., Xu W., Wang D. Effect of ultrasound pre-treatment on the characterization and properties of collagen extracted from soft-shelled turtle (Pelodiscus sinensis) LWT-Food Sci. Technol. 2017;82:72–81. [Google Scholar]
- 16.Petcharat T., Benjakul S., Karnjanapratum S., Nalinanon S. Ultrasound-assisted extraction of collagen from clown featherback (Chitala ornata) skin: yield and molecular characteristics. J. Sci. Food Agr. 2021;101:648–658. doi: 10.1002/jsfa.10677. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Teng A., Zhao K., Zhang K., Zhao H., Duan S., Li S., Guo Y., Wang W. A top-down approach to improve collagen film’s performance: The comparisons of macro, micro and nano sized fibers. Food Chem. 2020;309 doi: 10.1016/j.foodchem.2019.125624. [DOI] [PubMed] [Google Scholar]
- 18.Wu K., Liu W., Li G. The aggregation behavior of native collagen in dilute solution studied by intrinsic fluorescence and external probing. Spectrochim. Acta A. 2013;102:186–193. doi: 10.1016/j.saa.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Xu S., Gu M., Wu K., Li G. Unraveling the interaction mechanism between collagen and alcohols with different chain lengths and hydroxyl positions. Colloid. Surface. B. 2021;199 doi: 10.1016/j.colsurfb.2021.111559. [DOI] [PubMed] [Google Scholar]
- 20.Huang L., Ding S., Chen T., Wu R., Wang X., Jia S. Structural and functional properties of collagen from tilapia scales pretreated by heat-assisted ionic liquids. J. Appl. Polym. Sci. 2022;139:51903. [Google Scholar]
- 21.Chen J., Gao Q., Zhang X., Bassey A.P., Zeng X., Zhou G., Xu X. A structural explanation for protein digestibility changes in different food matrices. Food Hydrocolloid. 2023;136 [Google Scholar]
- 22.Wang Y., Yang S., Zhang L., Yuan F., Mao L., Liu J., Gao Y. Effects of different mechanical processes on the structural and powdery properties of insoluble undenatured type II collagen. Food Chem. 2023;406 doi: 10.1016/j.foodchem.2022.135068. [DOI] [PubMed] [Google Scholar]
- 23.Liu D., Nikoo M., Boran G., Zhou P., Regenstein J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Tech. 2015;6:527–557. doi: 10.1146/annurev-food-031414-111800. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y., Liu L., Dan W., Dan N., Gu Z. Evaluation of 1-ethyl-3-methylimidazolium acetate based ionic liquid systems as a suitable solvent for collagen. J. Appl. Polym. Sci. 2013;130:2245–2256. [Google Scholar]
- 25.Nurubhasha R., Sampath Kumar N., Thirumalasetti S.K., Simhachalam G., Dirisala V.R. Extraction and characterization of collagen from the skin of Pterygoplichthys pardalis and its potential application in food industries. Food Sci. Biotechnol. 2019;28:1811–1817. doi: 10.1007/s10068-019-00601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-González I., Carmona P., Moreno P., Borderías J., Sanchez-Alonso I., Rodríguez-Casado A., Careche M. Protein and water structural changes in fish surimi during gelation as revealed by isotopic H/D exchange and Raman spectroscopy. Food Chem. 2008;106:56–64. [Google Scholar]
- 28.Maeda Y., Kitano H. The structure of water in polymer systems as revealed by Raman spectroscopy. Spectrochim. Acta A. 1995;51:2433–2446. [Google Scholar]
- 29.Xue S., Qian C., Kim Y.H.B., Xu X., Zhou G. High-pressure effects on myosin in relation to heat gelation: A micro-perspective study. Food Hydrocolloid. 2018;84:219–228. [Google Scholar]
- 30.Akram A.N., Zhang C. Effect of ultrasonication on the yield, functional and physicochemical characteristics of collagen-II from chicken sternal cartilage. Food Chem. 2020;307 doi: 10.1016/j.foodchem.2019.125544. [DOI] [PubMed] [Google Scholar]
- 31.Shi C., He Y., Ding M., Wang Y., Zhong J. Nanoimaging of food proteins by atomic force microscopy. Part II: Application for food proteins from different sources. Trends Food Sci. Tech. 2019;87:14–25. [Google Scholar]
- 32.Kozlowska J., Sionkowska A., Skopinska-Wisniewska J., Piechowicz K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015;81:220–227. doi: 10.1016/j.ijbiomac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y., Gao Y., Shao J., Tumarbekova A., Zhang D., Zhu J. Effects of ultrasound and thermal treatment on the ultrastructure of collagen fibers from bovine tendon using atomic force microscopy. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2020.128985. [DOI] [PubMed] [Google Scholar]
- 34.Guo H., Hong Z., Yi R. Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J. Food Sci. 2015;80:N1595–N1601. doi: 10.1111/1750-3841.12912. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y., Wang Y., Jin G., Duan J., Zhang Y., Cao J. The texture change of Chinese traditional pig trotter with soy sauce during stewing processing: based on a thermal degradation model of collagen fibers. Foods. 2022;11:1772. doi: 10.3390/foods11121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranjha M.M.A.N., Irfan S., Lorenzo J.M., Shafique B., Kanwal R., Pateiro M., Arshad R.N., Wang L.F., Nayik G.A., Roobab U., Aadil R.M. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes. 2021;9:1406. [Google Scholar]
- 37.Mukhtar K., Nabi B.G., Arshad R.N., Roobab U., Yaseen B., Ranjha M.M.A.N., Aadil R.M., Ibrahim S.A. Potential impact of ultrasound, pulsed electric field, high-pressure processing and microfludization against thermal treatments preservation regarding sugarcane juice (Saccharum officinarum) Ultrason Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.