Abstract
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) is a glycoprotein and is derived from both hemopoietic and nonhemopoietic sources which exert immunomodulatory properties. Various theories have been proposed to explain why some wounds become chronic and non‐healing. Generalized suppression of inflammation locally or systemically may impede the body's physiological healing response by crippling the activity of reparative cells within the wound ecosystem. Thus, highlighting the importance of promoting host‐directed therapeutics with immunomodulatory properties. The temporal and spatial expression of GM‐CSF and GM‐CSF receptors in the integumentary system suggests that epithelial‐derived GM‐CSF functions in an autocrine/paracrine manner. This may positively affect wound healing physiology via local inflammatory regulation promoting macrophage survival. Although diabetes negatively affects multiple aspects of wound healing GM‐CSF activation is particularly impacted. Compared to acute/healthy wounds diabetic foot ulcers (DFU) only partially activate GM‐CSF activity. There is a deleterious chain of events associated with this unfortunate sequala. DFUs also have a high proportion of monocytes and an absence of activated macrophages which results in an impaired inflammatory response. This may potentially serve as a vital point for GM‐CSF to act as a companion diagnostic/theragnostic modality to help modulate the inflammatory response in wound healing. Correcting macrophage immune dysfunction with exogenous GM‐CSF may help restore the immune balance in the wound ecosystem and jumpstart the wound healing cascade. Thus, the recognized beneficial role of GM‐CSF in immune regulation across many studies provides a rationale for the initiation of the ongoing randomized controlled trials using GM‐CSF.
Keywords: granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), wound care, wound healing
1. INTRODUCTION
A wound is a disruption of normal anatomic structure and function that is usually inclusive of the skin. Wound healing is a series of well‐orchestrated biochemical events that ultimately lead to tissue regeneration and epithelial contraction which should result in the restoration of anatomical and functional integrity. 1 , 2 , 3 The wound healing process consists of key overlapping phases that include haemostasis, dynamic inflammation, cellular migration/proliferation, protein synthesis and tissue contraction/remodeling. 4 However, on the macro level, there are numerous factors that impact the precise timeline of complete healing which include existing comorbidities, anatomic location, body mass index (obesity), 1 and infection among others. Patients with diabetes are at risk of a plethora of complications including foot ulcers. 5 Diabetic neuropathic ulcers are generally attributed to shear force vectors, unstable lower extremity pathomechanics, and immunologic dysfunction leading to foot ulcerations. 5 , 6 After a trial of standard‐of‐care (SOC), wounds that do not heal in a timely fashion may indicate the need to implement advanced wound healing technologies into the treatment algorithm. Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) is a glycoprotein and is derived from both hemopoietic and nonhemopoietic sources which exert immunomodulatory properties. 1 , 7
2. CURRENT ROUTES OF ADMINISTRATION
Appropriate dose, route and schedules for recombinant human (rhu) GM‐CSF in various clinical settings have been defined via, intravenous infusion and subcutaneous injections. 8 In animal models of respiratory infections, the intranasal administration of GM‐CSF increased the proliferation of alveolar macrophages and improved outcomes. 9 Given the pleiotropic potential of GM‐CSF, host defense and inflammation, care should be taken with respect to dose, route and timing of administration for each therapeutic approach.
3. THE IMMUNOLOGY OF CHRONIC DIABETIC FOOT ULCERS AND THE COMPLEX RELATIONSHIP BETWEEN MACROPHAGES AND GM‐CSF
Various theories have been proposed to explain why some wounds become chronic and non‐healing. Despite the etiological differences in chronic wounds, they often share common pathophysiologic features which involve interactions of several cell types, extracellular matrix components and regulatory immunologic factors. In chronic wounds, there is markedly reduced cellular division which ultimately impedes cellular growth and proliferation. 10 Transcriptomic RNA‐seq data of diabetic foot ulcer (DFU) edges has demonstrated a deregulated immune response with a downregulation of forkhead box protein M1 (FOXM1) FOXM1 and GM‐CSF resulting in poor immune cell proliferation and survival. 11 It is important to note that in a chronic wound environment the inflammatory phase of wound healing may either be stalled or prolonged. 1 , 6 Extended inflammation can be attributed to elevated levels of pro inflammatory cytokines such as TNF‐α, IL‐6, and IL‐1β. 2 , 3 Vital cells that promote healing such as fibroblast and keratinocytes are markedly reduced secondary to a proinflammatory state. 4 , 6 Fibroblasts are critical cells that play a role in the formation of collagen, fibronectin, and other key matrix proteins. 4 Excessive degradation of the extracellular matrix would deprive cells of attachment sites and signals required for migration, differentiation, and proliferation. 12 The resulting wound bed, lacking attachment sites for migration, is “unfriendly” to keratinocytes leading to slow or absent wound closure. 1 , 13 Many of the biochemical alterations noted in chronic wounds may be responses to lack of adhesion to (or detachment from) an extracellular matrix (ECM) of specific structure and composition at the right time in the wound healing sequence. 7 , 12 Cellular senescence within the chronic wound ecosystem can be reversed by modulating the variables that help promote cellular activity. 14
Although there is at times a stigma with the term ‘inflammation’, it is important to keep in mind the inflammatory phase is an essential component of the wound healing cascade (Figure 1). Therefore, therapeutic agents should promote immunomodulatory effects rather than simply aim to reduce or eliminate inflammation. As it pertains to pulmonary homeostasis, GM‐CSF serves as immunomodulatory function under inflammatory conditions that include infection. 9 Pulmonary alveolar proteinosis (PAP) is a rare syndrome of alveolar surfactant accumulation, resulting in hypoxemic respiratory failure, and increased infection risk commonly categorised as primary, secondary or congenital PAP. 15 , 16 Primary PAP accounts for the majority of cases and is caused by disruption of GM‐CSF signalling, either by GM‐CSF autoantibodies (autoimmune PAP) 16 or genetic mutations involving the GM‐CSF receptor (ie, hereditary PAP).
FIGURE 1.
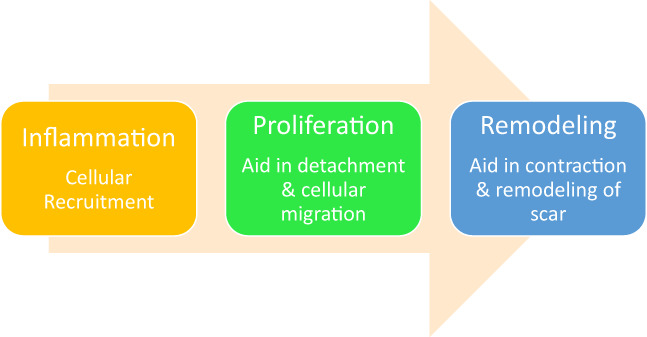
Wound healing cascade
Generalised suppression of inflammation locally or systemically may impede the bodies physiological healing response by crippling the activity of reparative cells within the wound ecosystem (Figure 2). Thus, highlighting the importance of promoting therapeutics with immunomodulation rather than complete anti‐inflammatory effects. Upon tissue injury, keratinocytes secrete pro‐inflammatory cytokines, such as IL‐1, TNF‐α, and (GM‐CSF). 17 GM‐CSF mRNA accumulates in keratinocytes early during the course of skin injury. 18 An early and essential effect of GM‐CSF is to stimulate the proliferation and migration of keratinocytes and endothelial cells. 18 , 19 , 20 Keratinocytes (in addition to haematopoietic cells) have also been identified as a source and target for GM‐CSF. 20 Recruited monocytes are terminally differentiated into macrophages by the local presence of GM‐CSF. 21 These early wound healing phase macrophages recognise pathogens and engulf them as well as synthesising metalloproteinases to digest the extracellular matrix and the thrombus in‐order to facilitate cellular migration. 4 Macrophages require GM‐CSF for viability and the bioenergetic activity of their mitochondria essential to their host defence functions and homeostatic activities. 22 Animal studies have demonstrated that GM‐CSF overexpression in mice has led to increased re‐epithelization and wound closure, whereas GM‐CSF depletion impaired wound healing significantly. 7 Keratinocyte‐derived cytokines, chemokines, extracellular vesicles, and antimicrobial peptides (AMPs) mediate the dynamic interactions between haematopoietic immune cells and keratinocytes. 23 After the inflammatory phase, the wound enters the growth phase of healing. During this phase, macrophages are transitioning into a pro‐healing phenotype. 4 This phenotype begins the process of efferocytosis, the engulfing of apoptotic cells.
FIGURE 2.
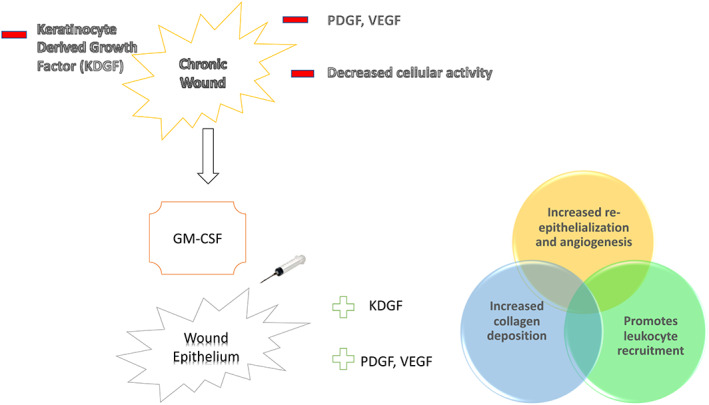
Granulocyte‐macrophage colony‐stimulating factor's role in wound healing
Neutrophils play important roles in influencing the behaviour and function of neighbouring cell types during inflammation. 24 Macrophages are programmed by their tissue environment to silently clear apoptotic cells. 25 Apoptotic cells secrete ‘find me’ signals that induce phagocytic recognition. The apoptotic cell is engulfed by the macrophage this process termed efferocytosis may lead to the successful resolution of inflammation. 26 During this process, the macrophage is filled with a metabolite load almost equal to the size of the cell itself elevating macrophage fatty acid and oxygen consumption. 27 Fatty acid oxidation is essential to macrophage efferocytosis. 27 For this GM‐CSF signalling is required. 22 Fatty acid β‐oxidation promotes catabolism within the mitochondrial ecosystem. 27
The dynamic balance between M1‐M2 (macrophages) is critical for proper wound healing. 28 In chronic non‐healing wounds, failure of macrophages to transition from the pro‐inflammatory M1 to a pro‐healing reparative M2 phenotype can lead to (Figure 3) 11 , 28
prolonged inflammation
reduced growth factors
reduced granulation tissue
impaired overall healing
FIGURE 3.
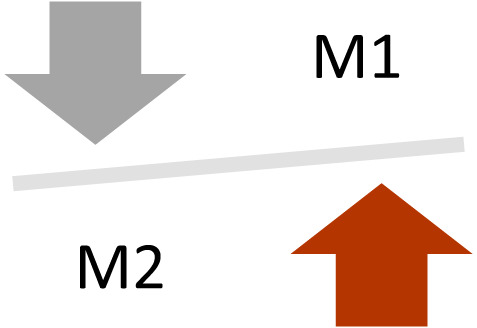
Chronic wound M1 and M2 environment
Studies have revealed that GM‐CSF induces peroxisome proliferator‐activated receptors (PPARs) gamma expression. This transcription factor is responsible for catalysing a series of biochemical events that help facilitate the transition of proinflammatory M1 to pro‐healing M2 macrophages 29 (Figure 4). GM‐CSF fosters adipose MSC regeneration capacity to promote cellular differentiation and tissue repair. 30 Additionally, GM‐CSF signals these elevated MSC populations that can be utilised to recruited to sites of tissue injury to promote angiogenesis and eventually differentiate into keratinocytes. 31
FIGURE 4.

Granulocyte‐macrophage colony‐stimulating factor M1, M2 regulation
Cianfarani et al demonstrated that GM‐CSF injections to non‐healing venous leg ulcers stimulated VEGF transcription in the wound bed, primarily within macrophages. 32 VEGF is an important cytokine that is vital in recruiting endothelial cells to the site of injury thereby promoting angiogenesis. 33 Neovascularization of the wound bed is critical in order to receive and optimise essential nutrients for tissue repair. 33 The consequence of a hyperglycemic microenvironment leads to endothelial cell dysfunction, reduced nitric oxide production, increased platelet aggregation, and decreased GM‐CSF production. 34 , 35 The reduced concentration of GM‐CSF also contributes to a delay in wound closure. Keratinocytes and fibroblasts are directly affected by GM‐CSF levels. As a result of lower levels of GM‐CSF, keratinocytes and fibroblasts may not proliferate and migrate towards the site of injury to assist in wound closure. There is a deleterious chain of events associated with this. Key transcription factors such as STAT3 and FOXM1 are only partially activated in DFUs. 11 Phosphorylation of STAT3 is dependent on GM‐CSF stimulation. 36 DFUs also have a high proportion of monocytes and an absence of activated macrophages which results in an impaired inflammatory response. 11
The temporal and spatial expression of GM‐CSF and GM‐CSF receptors in the integumentary system suggests that intrinsically‐derived GM‐CSF functions in an autocrine/paracrine manner. 7 This may positively affect wound healing physiology via local inflammatory regulation promoting macrophage survival. Correcting macrophage immune dysfunction with exogenous GM‐CSF may help restore the immune balance in the wound ecosystem and jumpstart the wound healing cascade. Thus, the recognising pathogenic role of GM‐CSF in immune overactivation across many studies provides a rationale for the initiation of the ongoing randomised controlled trials using GM‐CSF. Understanding the clinical and physiologic cues of wound healing helps pave the way for companion diagnostics/theragnostics. This will help clinicians make critical patient‐centered decisions.
CONFLICT OF INTEREST
Dr David Armstrong is a consultant for Partner Therapeutics, Inc.
ACKNOWLEDGEMENTS
We would like to thank Dr. Luis Leal for sharing his expertise of the biology of GM‐CSF. Partner Therapeutics, Inc. funded the submission of this manuscript. Dr. Leal is an employee of and has stock options for Partner Therapeutics, Inc.
Ead JK, Armstrong DG. Granulocyte‐macrophage colony‐stimulating factor: Conductor of the wound healing orchestra? Int Wound J. 2023;20(4):1229‐1234. doi: 10.1111/iwj.13919
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Snyder RJ, Driver V, Fife CE, et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage. 2011;57:36‐46. [PubMed] [Google Scholar]
- 2. Lazaro JL, Izzo V, Meaume S, Davies AH, Lobmann R, Uccioli L. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J Wound Care. 2016;25(5):277‐287. [DOI] [PubMed] [Google Scholar]
- 3. Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immunol. 2016;7:160. doi: 10.3389/fimmu.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz GS, Chin GA, Moldawer L, et al. Principles of wound healing. In: Fitridge R, Thompson M, eds. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Adelaide: University of Adelaide Press; 2011:23. [PubMed] [Google Scholar]
- 5. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 6. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665‐706. doi: 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang Y, Gong SJ, Xu YH, Hambly BD, Bao S. Impaired cutaneous wound healing in granulocyte‐macrophage colony‐stimulating factor knockout mice. Br J Dermatol. 2007;157(3):458‐465. doi: 10.1111/j.1365-2133.2007.07979.x [DOI] [PubMed] [Google Scholar]
- 8. Leukine®(Sargramostim) for Injection, for Subcuteous or Intravenous Use Prescribing Information. Partner Therapeutics, Inc; 2022. [Google Scholar]
- 9. McCormick TS, Hejal RB, Leal LO, Ghannoum MA. GM‐CSF: orchestrating the pulmonary response to infection. Front Pharmacol. 2022;17(12):735443. doi: 10.3389/fphar.2021.735443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. doi: 10.3390/ijms17122085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020;11:4678. doi: 10.1038/s41467-020-18276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xue MJC. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119‐136. doi: 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falanga V. Growth factors and wound healing. Dermatol Clin. 1993;11(4):667‐675. [PubMed] [Google Scholar]
- 14. Wei X, Li M, Zheng Z, et al. Senescence in chronic wounds and potential targeted therapies. Burns Trauma. 2022;10:tkab045. doi: 10.1093/burnst/tkab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215‐235. [DOI] [PubMed] [Google Scholar]
- 16. Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5:16. [DOI] [PubMed] [Google Scholar]
- 17. Coondoo A. The role of cytokines in the pathomechanism of cutaneous disorders. Indian J Dermatol. 2012;57(2):90‐96. doi: 10.4103/0019-5154.94272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson FM, Bijur GN, Oberyszyn AS, et al. Granulocyte‐macrophage colony stimulating factor gene expression and function during tumor promotion. Carcinogenesis. 1994;15:1017‐1029. [DOI] [PubMed] [Google Scholar]
- 19. Bussolino F, Ziche M, Wang JM, et al. In vitro and in vivo activation of endothelial cells by colony‐stimulating factors. J Clin Invest. 1991;87(3):986‐995. doi: 10.1172/JCI115107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte‐Derived Granulocyte‐Macrophage Colony Stimulating Factor Accelerates Wound Healing: Stimulation of Keratinocyte Proliferation, Granulation Tissue Formation, and Vascularization. J Invest Dermatol. 2001;117(6):1382‐1390. doi: 10.1046/j.0022-202x.2001.01600.x [DOI] [PubMed] [Google Scholar]
- 21. Burgess AW, Metcalf D. The nature and action of granulocyte‐macrophage colony stimulating factors. Blood. 1980;56(6):947‐958. [PubMed] [Google Scholar]
- 22. Wessendarp M, Watanabe‐Chailland M, Liu S, et al. Role of GM‐CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation. Mitochondrion. 2022;62:85‐101. doi: 10.1016/j.mito.2021.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piipponen M, Li D, Landén NX. The immune functions of keratinocytes in skin wound healing. Int J Mol Sci. 2020;21(22):8790. doi: 10.3390/ijms21228790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang F, Feng C, Zhang X, Lu J, Zhao Y. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation. 2017;40:311‐323. doi: 10.1007/s10753-016-0458-4 [DOI] [PubMed] [Google Scholar]
- 25. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue‐resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity. 2017;47:913.e‐927.e. doi: 10.1016/j.immuni.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5(3):e9539. doi: 10.1371/journal.pone.0009539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang S, Weinberg S, Deberge M, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29(2):443‐56.e5. doi: 10.1016/j.cmet.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18(7):1545. doi: 10.3390/ijms18071545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirza RE, Fang MM, Novak ML, et al. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol. 2015;236(4):433‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park SR, Cho A, Kim JW, Lee HY, Hong IS. A novel endogenous damage signal, Csf‐2, activates multiple beneficial functions of adipose tissue‐derived mesenchymal stem cells. Mol Ther. 2019;27(6):1087‐1100. doi: 10.1016/j.ymthe.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y, RCH Z, Tredget EE. Concise review: bone marrow‐derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28(5):905‐915. doi: 10.1002/stem.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cianfarani F, Tommasi R, Failla CM, et al. Granulocyte/macrophage colony‐stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol. 2006;154(1):34‐41. doi: 10.1111/j.1365-2133.2005.06925.x [DOI] [PubMed] [Google Scholar]
- 33. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647‐661. doi: 10.1089/wound.2013.0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3(6):853‐876. [PMC free article] [PubMed] [Google Scholar]
- 35. Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S. Granulocyte‐macrophage colony‐stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol. 2010;162(3):478‐486. doi: 10.1111/j.1365-2133.2009.09528.x [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi T, Mukasa T, Uchida E, Kanayasu‐Toyoda T, Hayakawa T. The role of Stat3 in granulocyte colony‐stimulating factor‐induced enhancement of neutrophilic differentiation of Me2so‐treated Hl‐60 cells. GM‐CSF inhibits the nuclear translocation of tyrosine‐phosphorylated Stat3. J Biol Chem. 1999;274(22):15575‐15581. doi: 10.1074/jbc.274.22.15575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


