Abstract
Chronic wounds affect millions globally and are a huge financial burden. Whilst there are many wound dressings commercially available to manage these wounds, the complexity of the repair process makes it difficult to select the right dressing for the right wound at the right time. Thus, in this narrative review, we have examined reasons why wounds fail to heal, summarised the pathophysiology of the chronic wound environment and provided an evidence‐based, clinically‐relevant compilation of the published literature relevant to dressing design and evaluation. This has highlighted the need for a deeper understanding of wound exudates, how exudates change throughout the healing process, and how they are impacted by different dressing materials. Studies assessing biochemical and biophysical changes in exudates throughout the healing process are extremely valuable in this regard, enhancing both our understanding of the wound healing process and the ability to assess dressing performance. In addition, this knowledge allows us to replicate various wound conditions in the laboratory, and develop clinically‐relevant models for testing current and new dressings, therefore providing a more comprehensive understanding of how and when they should be used. This approach makes the use of dressings more effective, thereby improving outcomes, and reducing the economic burden of chronic wounds.
Keywords: chronic wounds, clinical outcomes, dressing design and evaluation, exudate, wound healing
1. INTRODUCTION
Chronic wounds affect millions of people globally and are a financial burden on healthcare systems, costing tens of billions of dollars annually. 1 The prevalence of chronic wounds is relatively high and similar to that of heart failure, affecting 6.5 million people in the United States, which are 2% of the US population. 2 In addition, chronic wounds account for 3% to 6% of the total healthcare expenditure in developed countries, and conservative estimates for the US point to an associated cost of $28 billion per year to the American Medicare system. 3 The delayed healing observed in chronic wounds is multifactorial, and is further complicated by differing wound types and patient comorbidities. The wound repair process involves multiple cell types and activities, thousands of molecules and numerous coordinated biochemical processes are required for healing. Furthermore, given the number and type of molecules that have been reported to be elevated and/or reduced in non‐healing wounds, it is unlikely that a single therapy to heal all wounds exists. This complexity helps explains why so many clinical trials using topical application of individual molecules have had limited success. Instead, it is more likely that therapies or combinations thereof that address a number of the issues that occur in chronic wounds ought to have a higher likelihood of clinical success and provide improved quality of life for patients.
In this review, we will look at the wound healing process and what is required in order to achieve healing. We will also examine why some wounds fail to heal in a timely manner and the importance of the inflammatory response. Understanding the issues of non‐healing will allow us to assess how and when to use current wound dressings to manage the wound and improve clinical and economic outcomes. Ultimately, if we are to use wound therapies appropriately, and indeed develop improved wound treatments, we must understand the symptoms of the wound and its pathophysiology. In addition, we need to have clinically relevant laboratory test systems and their associated measures of success to evaluate effectively the different mechanisms of action. By understanding and considering both the physical and biochemical needs of the wound, we are much more likely to provide an effective and efficacious treatment. Accordingly, this narrative literature review provides an evidence‐based, clinically‐relevant compilation of the published biological and physiological knowledge relevant to dressing design and evaluation.
2. THE WOUND HEALING PROCESS AND WHY WOUNDS MAY FAIL TO HEAL
Wound healing follows a series of complex overlapping processes that ultimately result in the closure of the wound and restoration of the epithelial layer or skin. These processes include haemostasis, inflammation, angiogenesis, extracellular matrix formation, epithelialisation, and remodelling; all of which involve a wide range of cell types, regulators such as growth factors, cytokines, proteases and protease inhibitors, and the extracellular matrix. 4 It is the concerted and timely, orchestrated action of all these processes that are required for wound healing to occur; therefore, a defect in any process can delay healing, resulting in a stalled or chronic wound. The most prevalent classifications of chronic wounds are venous, pressure, arterial and diabetic foot ulcers. However, there are other wound types that exhibit delayed healing with similar underlying pathophysiology to chronic wounds, such as non‐healing surgical wounds, which require similar interventions for healing to occur.
Healing of acute wounds generally occurs within two to four weeks 5 while chronic wounds may persist for months or years, or for the rest of the patient's lifetime. 6 , 7 Therefore, it is generally accepted and guidelines typically describe a non‐healing or chronic wound as one that has not achieved complete wound closure within 4 weeks. 8 Of note, the 4‐week time is not definitive and some authors refer to a period of 6 weeks and up to 3 months to define the chronicity of a wound. 9 , 10 , 11
There are many reasons why chronic wounds fail to heal within the above timeframes, which can be linked to patient, wound and /or environmental factors, or a combination of these. Patient factors include medical history, wound aetiology and comorbidities such as diabetes, obesity, ischaemia, venous insufficiency, auto‐immune conditions such as rheumatoid arthritis, renal disease, traumatic injuries to the central nervous system, cerebrovascular accidents or malnutrition. 12 , 13 , 14 Wound‐related factors include exposure to bacterial or fungal bioburden, excess oedema, inappropriate management of the wound exudate, unsuitable or inadequate offloading, or pressure redistribution to relieve the affected anatomical area. Environmental factors are ambient conditions such as humidity or desiccation, or lifestyle choices the patient has made or has been exposed to, including smoking, alcohol, poor nutrition, UV light exposure, psychological stress, pollutants etc. 15 It is now acknowledged that exposure to these factors, their level and duration determines the overall health status and, in particular, the inflammatory response to stimuli of the individual. Thus, when we consider a patient with a non‐healing wound, the approach should be holistic and multi‐factorial issues should be considered, such as a patient with a poor health status who is predisposed to chronic inflammatory conditions. For such a patient, it is hardly surprising that their non‐healing wound would become trapped in the inflammatory phase of healing, and fail to progress to closure in a timely manner.
Correspondingly, studies examining the pathophysiology of chronic wounds have shown that fundamental biochemical differences exist between healing and non‐healing wounds and that non‐healing or chronic wounds are stuck in a prolonged and heightened inflammatory process that prevents healing because of its harmful effect on the wound and peri‐wound tissues. 16 , 17 This persistent inflammatory environment is characterised by increased exudate production, increased wound and peri‐wound temperature and production of a more alkaline wound environment with non‐healing wounds typically found to have a pH range of 7.15 to 8.9 18 , 19 (Figure 1). As the pH level affects the solubility, activity and physical properties of tissue components such as proteins, changes in pH will alter the biochemical, cellular and physical properties of the wound and peri‐wound environment. Indeed, several comparison studies between acute and chronic wounds or healing and non‐healing wounds have reported significant biochemical differences. Chronic or non‐healing wound fluid and tissue had increased pro‐inflammatory cytokines (eg, tumour necrosis factor‐alpha (TNF‐α), and the interleukin (IL) types IL‐1 beta, IL‐6 and IL‐8), free radicals or reactive oxygen species (eg, hydrogen peroxide) and proteases (eg, the matrix metalloproteinases [MMPs] and human neutrophil elastase) and less total protein and albumin. 16 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Cell physiology further changes under an altered pH environment, for example, the motility and migratory potential of fibroblasts is compromised. 29 Overall, these factors create a hostile biochemical environment that negatively impacts the healing and perpetuates chronicity, which must be corrected if healing is to occur. 30 , 31
FIGURE 1.
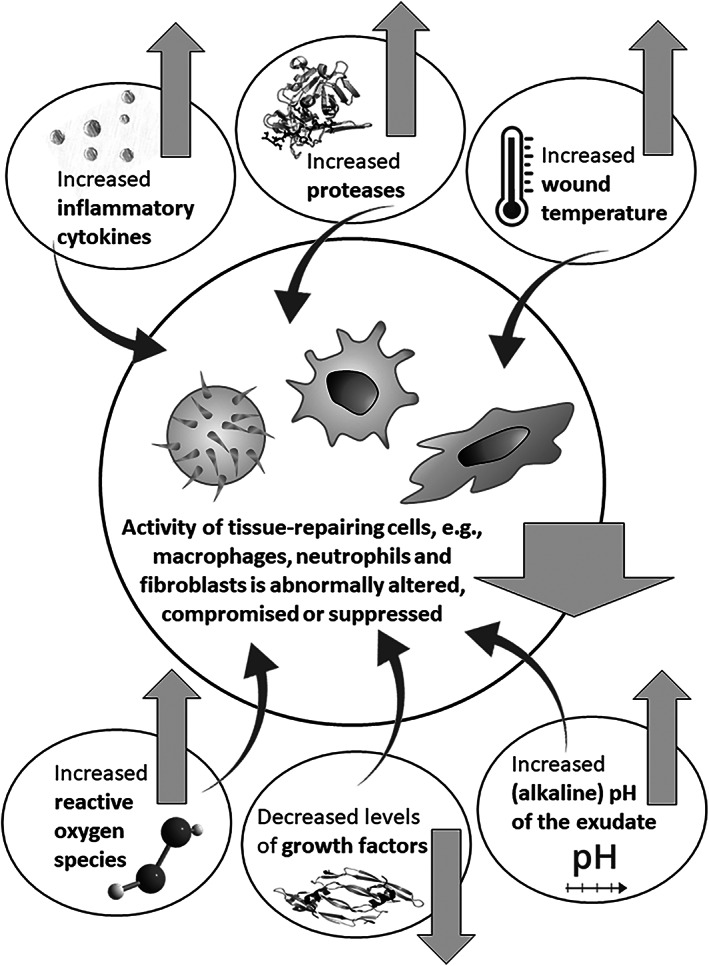
Important changes in the wound environment which are associated with wound chronicity and may abnormally alter, compromise or suppress the function of the tissue‐repairing cell types which are the most critical for wound healing, such as macrophages, neutrophils, and fibroblasts
Published literature over the last 30 years has demonstrated that inflammatory cytokines are elevated in chronic non‐healing wounds and the resultant pro‐inflammatory environment causes degradation of growth factors and extracellular matrix proteins. 32 , 33 These publications have studied various aspects of inflammation, comparing levels and activity of biochemical molecules in acute and chronic non‐healing wounds. 34 Studies conducted by Harris et al. have shown that wound fluid derived from venous leg ulcers was rich in inflammatory cytokines such as TNF‐α and IL‐1β and subsequently, had reduced levels of growth factors such as platelet‐derived growth factor. 20 The levels of these cytokines decreased as the wound healing progressed, indicating a significant relationship between non‐healing wounds and the increased levels of pro‐inflammatory cytokines. 27 Furthermore, chronic wound fluid containing elevated levels of these cytokines has been shown to inhibit the growth, and induce morphological changes in normal skin fibroblasts. 35 Moreover, macrophage activation, essential for initiating the proliferative phase of wound healing, was found to be suppressed in chronic venous leg ulcers, leading to an impaired inflammatory response, 36 which is likely associated with increased levels of TNF‐α in these wounds. 37 Likewise, Mirza and colleagues demonstrated that sustained IL‐1β expression in wounds of diabetic humans and mice was associated with a proinflammatory macrophage phenotype and that inhibiting the IL‐1β pathway in the wounds of diabetic mice induced a switch from proinflammatory to healing‐associated macrophage phenotypes, and improved the healing of these wounds. 38 Related clinical results published by Streit et al. (2006) showed that therapy‐resistant leg ulcers responded well to repeated topical administration of a solution or a gel containing the TNF‐alpha antibody infliximab which binds and neutralises TNF‐α. 39 In addition, white blood cells (polymorphonuclear leucocytes) from patients with chronic wounds have been shown to produce more reactive oxygen species than healthy controls. 40 Taken together, the above literature indicates that the elevated and sustained presence of inflammatory cytokines in the wound environment alters, compromises or suppresses the function of the cell types which are the most critical for wound healing such as macrophages, neutrophils and fibroblasts. 41
Reactive oxygen species, or ROS, is the term given to a family of molecules which are typically generated by neutrophils and contain oxygen but have been reduced to become highly reactive radicals. The family of ROS molecules, including superoxide anion, peroxide, hydrogen peroxide, hydroxyl radicals, and hydroxyl ions, have a pivotal role in the normal inflammatory response, maintaining cell function and early host defence against infections through phagocytes and reactive oxygen burst. 42 Published evidence has clearly demonstrated the role of low levels of ROS in cellular homeostasis, however, it is important to note that excessive and sustained ROS production leads to oxidative stress, which has detrimental effects on wound healing. 43 The precise balance between low and high levels of ROS is critical for normal wound healing to occur. 44 , 45
Many investigators have also studied the role of proteases in wound repair and have reported that many proteases are present in excess in chronic, non‐healing wounds, causing destruction of the wound and peri‐wound tissues through proteolysis of the extracellular matrix. While these proteases are beneficial in debriding the wound of damaged tissues at an initial stage, their excess and sustained presence extracellularly after the natural autolysis continues to degrade proteins and tissues that are required for healing and closure. Studies have measured various serine and matrix metalloproteases (MMP) comparing levels and activity in both acute and chronic wounds. Total MMP activity in chronic wound fluid has been found to be approximately 30 times higher than that in acute wound fluid. 16 More specifically, the activities of MMP‐2, MMP‐8, MMP‐9, and human neutrophil elastase (HNE) have been found to be considerably higher in chronic wound fluid than in fluid from normally healing wounds. 22 , 23 , 32 , 46 , 47 , 48 These proteases are predominantly produced by inflammatory cells, namely neutrophils when stimulated by inflammatory cytokines. 41 In normally healing wounds, neutrophils recruited from the circulation will eventually undergo apoptosis and be engulfed by macrophages, initiating a resolution process that terminates the inflammatory response, however, in non‐healing wounds, these inflammatory cells often continue to be recruited and activated, leading to persistent inflammation and continued protease production. 41 A non‐healing wound can thereby be shifted to a harmful cycle whereby the damage because of neutrophil‐derived proteases causes even more inflammation, which in turn results in additional tissue damage because of excess proteolytic activity, leading to the recruitment of more neutrophils and further escalation. The resultant effect of excess inflammatory cytokines is therefore an increase in inflammatory‐based proteases in the wound and peri‐wound area. To demonstrate the detrimental effect of inflammatory proteases on the wound environment, studies have shown that chronic wound fluid high in proteases, specifically HNE, degrade peptide growth factors, 32 and that the addition of protease inhibitors to chronic wound fluid can protect growth factor activity. 49 This is further supported by the fact that chronic wounds have been found to have lower levels of growth factors 16 and inhibitors such as alpha‐1‐antitrypsin, and tissue inhibitors of MMPs. 32 , 50 , 51 Similarly, studies have demonstrated that the degradation of extracellular matrix proteins such as fibronectin is dependent on the relative levels of proteases and their inhibitors in the wound fluid. 33 Investigators concluded that the proteolytic activity, and the resultant fibronectin fragmentation observed, may be related to retarded epithelisation and lack of healing. 52 Breakdown of growth factors and extracellular matrix proteins by proteases and the sustained release of inflammatory cytokines are likely contributors to these findings. 16 , 28
To summarise the above information, studies on non‐healing wounds concluded that the hostile pro‐inflammatory wound environment causes excessive tissue damage and degradation of key functional molecules. This result in further inflammation, an increase in degradative processes, and a subsequent decrease in the constructive processes required for wound healing. 32 A vicious circle of non‐healing ensues, in which inflammation is perpetuated, leading to the ongoing degradation of growth factors and extracellular matrix by elevated protease activity, which causes the release of additional inflammatory mediators and stimulates further protease production (Figure 2). This cycle can potentially continue indefinitely, which is why chronic wounds can persist for months and years.
FIGURE 2.
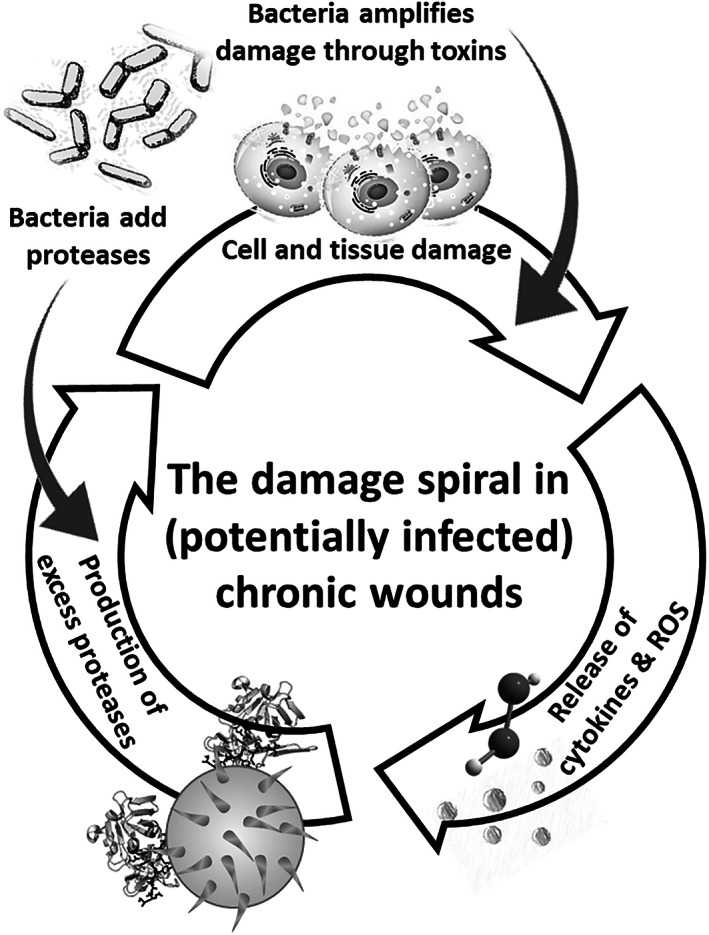
The damage spiral in (potentially infected) chronic wounds, where bacterial infections both intensify the direct cell and tissue damage by bacterial toxins and produce additional proteases which degrade extracellular matrix and inhibit healing (built upon the Cullen's circle of delayed wound healing depicted in Gibson et al., 2009). ROS, reactive oxygen species
The presence of bacteria can exacerbate the wound conditions, not only because of bacterial toxins released from living or lysed bacteria 53 , 54 , 55 but also, by intensifying the pro‐inflammatory environment, eliciting more inflammation, and producing virulence factors such as specific bacterial proteases (eg, Pseudomonas aeruginosa elastase B) that intensify the proteolytic milieu (Figure 2). 30 , 31 , 56 , 57 , 58 Bacteria are known to exploit chronic wounds as they provide a favourable environment for the survival and growth of bacterial colonies and biofilms; not only is the damaged sloughy tissue a good nutritional source but the alkaline pH associated with non‐healing wounds (Figure 1) provides an environment conducive for further bacterial growth. For healing, a mildly acidic environment, that is, a pH of approximately 6, is favourable, inducing fibroblast proliferation, promoting epithelisation and angiogenesis, while also facilitating the release of oxygen from haemoglobin. 59 For example, a shift of the wound pH value down to 6 entails a 40% to 90% decrease in the activity of proteolytic enzymes. 60 , 61 In contrast, an alkaline pH has an adverse effect on wound tissues, depriving the wound of oxygen and providing favourable conditions for bacteria. 62 Indeed, many studies have reported that increased alkalinity is indicative of local wound infection, 63 and that reducing the wound pH has been concomitant with improved outcomes. 64 This is why intact healthy skin is naturally acidic, ranging from pH 4 to 6, preserving resident skin flora, maintaining the skin barrier function and minimising bacterial contamination. 61 , 65 , * This physiological pH range results from the amino acids and fatty acids produced and secreted by the keratinocyte layer and the skin appendages in the intact skin. 61
Other noteworthy patient‐related factors that may delay wound healing or cause non‐healing are the use of certain drugs, for example, those that affect blood clotting or platelet function, as well as corticosteroids and chemotherapeutics that induce cell cycle arrest and thereby, reduce the rate of cell proliferation which is required for wound healing. 15 In addition, micro‐traumas such as repetitive loading of existing pressure ulcers/injuries at weight‐bearing body regions may mechanically damage epithelization and neovascularization or compromise existing perfusion and therefore, lead to delayed/non‐healing. 15
The extensive research evidence published over the last 40 years supports the hypothesis that in order to heal chronic wounds, it is necessary to break the vicious cycle of non‐healing by correcting the pro‐inflammatory environment, thereby, allowing the wound to progress to the next stages of healing. 30 , 66 , 67 However, correcting the underlying pathophysiology alone is not sufficient to mitigate the complexity of chronic wounds. It is also necessary to provide wound treatments which concurrently perform a number of important physical functions in order to optimise wound closure. This principle was first described as moist wound healing by George Winter in 1962, 68 and is now generally referred to as the standard of care. Today, this standard integrates moist wound healing principles with compression therapy for venous leg ulcers, off‐loading for diabetic foot ulcers and re‐positioning for pressure ulcers/injuries, and importantly, use of advanced wound dressings to maintain the optimal conditions for healing, as discussed further below.
3. THE POTENTIAL ROLES OF CONTEMPORARY WOUND DRESSINGS IN INFLUENCING THE WOUND ENVIRONMENT AND THE PROCESS OF CHOOSING DRESSINGS TO OPTIMISE TREATMENT
Historically, traditional wound care treatments such as gauze were used, which are passive in nature and function merely as a wound covering structure that also absorbs wound exudate to some extent. In contrast, moist wound healing has been shown to accelerate the rate at which wounds heal when compared with using these traditional gauze dressings where the wound is exposed to the air and allowed to dry. This has led to many advances in wound dressings and wound care devices over the years, and has seen the development of a multitude of sophisticated moist wound healing treatments which are highly effective in dealing with the symptoms of chronic or non‐healing wounds. It is now generally accepted that a moist wound healing dressing must perform a number of important roles to manage the wound and provide comfort for the patient. These include not only providing a moist wound healing environment but also keeping the wound clean and free of contamination; absorbing inflowing exudate without leakage or causing maceration to the peri‐wound skin; conveniently conforming to the body contours at the wound site; and minimising discomfort, pain and injury upon dressing removals while also being able to stay in place between dressing changes for the intended period of use. In addition to these clinical requirements, dressings must be suitable for a wide range of wound types, locations and sizes, exudate secretion rates and volume levels, and be safe, sterile, non‐sensitising, easy to apply and remove, compatible with other therapies such as compression therapy and offloading devices, and ideally be conformable and comfortable for the patient to wear routinely, thus allowing mobility, showering and overall normal life to continue. These design requirements are not only numerous but are often conflicting, making dressing development and their consistent function across wound and patient conditions extremely difficult and challenging. It may also explain why no one wound dressing product satisfies all these requirements to the full extent, but dressing combinations have been used together to achieve superior outcomes over a single dressing brand. An effective therapeutic approach, therefore, needs to provide a dressing that incorporates as many of these desirable characteristics as possible, whilst being compatible with an additional therapy that deals with the underlying pathophysiology of the non‐healing wound. This has led to a plethora of wound dressings to choose from, and becasue of the limited availability of standardised clinically‐relevant test systems in which to compare these dressings, it is often the cheapest dressing product that is selected in practice. Typically, this results in sub‐optimal clinical outcomes and ultimately, adds costs to the healthcare system.
Alternatively, we could simplify our classification of dressings by categorising them into one of 3 groups, with the selection of wound therapies depending on the need of the wound at that time. The first category is the largest as it incorporates all dressings and devices that affect or manage the symptoms of the wound including the exudate, pain, smell, fragile peri‐wound skin, and friable, fibrinous or sloughy wound tissues. The second group, for wounds suspected to be infected, helps to reduce the colonisation of excess bacteria and fungi by providing a physical barrier to keep the wound clean, in addition to delivering antimicrobial agents to kill multiple strains of bacteria present both planktonically and in biofilms, and/or using other antibacterial and antifungal strategies to affect the bioburden of the wound. The third and most diverse dressing group covers those therapies which help to actively change the wound environment in order to facilitate healing. These may include therapies that reduce negative factors such as sustained or excess inflammation, proteases and bacteria by releasing medications, or those that function through the advancement of positive processes such as promoting cell proliferation, angiogenesis and stimulation of new tissue deposition, for example, by delivering growth factors. Because in clinical practice, there is limited ability to diagnose the exact biochemical imbalance that exists in each specific non‐healing wound, a successful treatment which accelerates wound healing incorporates as many of these modes of action as possible.
Clinically, when a wound is assessed, it is possible to determine what are the physical requirements of the wound based on size, exudate level, and tissue type in the individual. Once this is decided, an appropriate category 1 dressing can be chosen. It is then necessary to determine if the wound is non‐healing because of excess bacteria and/or because of an underlying pro‐inflammatory environment. This helps to determine an appropriate category 2 or 3 therapy to prescribe. Considering both the physical and biochemical needs of the wound in this way would allow a clinician to deal with the underlying issues of the wound while also providing a treatment which mitigates the symptoms of the wound. This in turn will provide the patient with a comfortable, effective, and efficacious solution.
For a dressing to have a positive impact on the wound and improve the healing outcomes, it is necessary to understand not only the cellular and tissue components of the wound and peri‐wound but also the wound fluid which comprises the extracellular environment. The wound fluid or exudate reflects what is happening in the wound, and its composition contains negative and positive factors depending on the phase of healing, the status of the wound and the level of pathogens present in the wound environment. Consequently, it is important that we replicate the key features of wound fluids when testing wound dressings in a laboratory setting so that the tests are clinically‐relevant and correctly represent the various conditions observed in different wound types.
4. WOUND FLUID COMPOSITION AND IMPLICATIONS ON THE DESIGN OF SIMULATED WOUND FLUIDS
Despite the advances made in the development of moist wound healing dressings, our knowledge of wound fluid is still limited, with only a basic understanding of wound fluid composition and how this may change as the wound heals or deteriorates. We have yet to fully comprehend how the rate and volume of production, biochemical composition, and physical properties of wound fluid vary during different phases of wound repair, with each wound type or aetiology and more importantly how much exudate is optimal to facilitate moist wound healing. Without answers to these basic questions, it is not surprising that laboratory testing conditions used in the development of wound dressings are rudimentary and typically, do not reflect the complexity of real‐life clinical situations.
What is known is that exudate production is a normal feature of the repair process, however, when exudate is excessive, insufficient, or of the wrong composition, it is usually indicative of problems and healing is delayed. The main purpose of wound exudate is to support healing, facilitating the diffusion of key factors such as growth factors, oxygen and nutrients to the cells in the wound bed, and provide a moist wound environment which promotes cell proliferation, migration and differentiation, and aids autolysis of necrotic or damaged tissues. 69 Typically, exudate is clear, pale amber and of a watery consistency with levels usually reducing as the wound progresses to healing. 70 Its composition is complex, composed mainly of water containing electrolytes, nutrients, proteins, inflammatory mediators, proteases, growth factors and waste products, as well as various types of cells, for example, neutrophils, macrophages and platelets and extracellular matrix fragments. 71 Furthermore, because wounds are not sterile, the exudate frequently contains micro‐organisms, however, this does not necessarily mean the wound is infected unless concurrent with other signs or symptoms such as odour or purulent exudate.
Even though a moist wound environment is necessary for optimal healing, over‐ or under‐production of exudate may adversely affect healing. Generally, chronic wounds are associated with higher levels of exudate production, which is thought to impede healing as it slows down or even prevents cell proliferation, interferes with growth factor availability and contains elevated levels of inflammatory mediators and proteases. 28 It is thought that the prolonged inflammatory response observed in non‐healing wounds is responsible for the higher levels of exudate production, and for the change in its composition, producing a wound fluid which is detrimental and creates a hostile wound environment. In earlier studies, Trengove and colleagues examined how the composition of wound fluid compared with human serum. 72 Because wound fluids are always derived from plasma it is hardly surprising that the composition of both fluids ‐ exudate and plasma ‐ is similar in terms of the basic components as regards physiological electrolytes, minerals and organic compounds, for example, sodium, magnesium, phosphate, urea and creatinine. However, wound exudate was found to contain approximately half the total protein content of serum, with albumin accounting for the majority of the protein present in both fluids. 25 , 72 It was also noted that wound fluid from healing wounds contained higher albumin, total protein and glucose levels than for non‐healing wounds. 25 , 73 This may be because of increased fluid production in the prolonged inflammatory phase associated with non‐healing wounds, or truly reflect a reduced protein and nutrient bioavailability in the non‐healing state. Nonetheless, we can conclude from these studies that chronic wound fluid is a physiological solution, typically containing ~30 mg/mL of total protein, of which ~20 mg/mL is albumin. In addition, glucose is present at a concentration of ~2.2 mmol/L. Clearly, it should also be noted that these concentration values are averages as wound fluid composition is not consistent between patients albeit the inter‐patient wound characteristics may be comparable, or even within the same wound when it is observed at different healing or post‐treatment stages. 25 , 72 , 73
In addition to these biochemical changes in wound exudates associated with non‐healing, there is often an interrelated physical change in exudate colour, viscosity, volume, and pH which can exacerbate the problems in managing wounds. 74 As the pH level affects the solubility, activity, and physical properties of proteins in a solution, changes in wound fluid pH are likely to augment changes in exudate viscosity and appearance. 75 Ultimately, the consistency of exudate can vary from thick and viscous to thin and watery and is dependent on many variables related to both the patient and the wound. 76 Patient factors include the amount of fluid being produced by the host which is affected by their hydration status, the level of peri‐wound oedema, the location of the wound and the movement of the patient. Exudate viscosity is also affected by the presence of glycoproteins derived from cell debris, the number of white cells, and the level of bacteria in the wound. 74 Given the number of different variables affecting the wound fluid viscosity it is understandable why viscosity of wound exudate differs so much not only between wounds but also at different phases of the healing process.
The amount of exudate in a wound can also be difficult to assess, and the exudate volume can vary according to the size of the wound, the stage of healing and the wound's aetiology; for example, venous leg ulcers and burns can produce large amounts of exudate when compared with arterial ulcers. 77 Wounds stuck in the inflammatory phase of healing produce significantly more exudate because of increased cellular activity than at the latter stages of granulation tissue formation and reepithelization. 78 Because the volume of exudate is also related to the surface area of the wound, large wounds such as burns, venous leg ulcers, and skin donor sites typically produces higher volumes of exudate. Wounds which produce high levels of exudate are often labelled as more difficult to manage. However, with the wide range of moist wound healing dressings available, it should be possible to select an appropriate dressing to manage any level of exudate. The challenge for the clinician is to understand what factors are changing the volume and consistency of the exudate and to select a dressing appropriately to restore a satisfactory moist (but not excessively wet) wound environment that will support healing.
It is this dynamic nature of wound fluid which makes it difficult to replicate in laboratory testing. However, following a few basic principles, it should be possible to represent specific wound conditions and therefore, test wound dressings under more clinically relevant conditions. All wound fluids are physiological solutions containing proteins, glucose, and various other components. We should therefore consider the most basic version of a wound fluid for fluid handling‐related laboratory tests as physiological saline containing albumin. Glucose may be a potential additive, for example, in microbiology and cell biology testing but is not necessary for laboratory tests that evaluate the physical aspects of fluid handling of wound dressings. Specifically, if one prepares a test fluid for physical fluid handling tests, it is unlikely that the addition of glucose will change the fluid handling performance metrics of the tested dressings, but it can promote bacterial growth which is irrelevant for the intended testing. Moreover, other than being potentially hazardous for the testers, a simulated wound fluid contaminated with bacteria feeding on the glucose in the fluid may have undesirable and uncontrolled altered viscosity, surface tension or other important biophysical characteristics of the exudate substitute, as it is well known that bacterial colonies interact and may also change their biophysical environment. 79 , 80
More advanced exudate substitute fluid compositions would require the addition of other appropriate components, depending on the specific nature of the laboratory testing. For example, if testing the effects of a dressing on protease activity, then a combination of MMPs and human neutrophil elastase should be added at clinically‐relevant concentrations. If, however, we are optimising a dressing to manage exudate then it is necessary to not only look at the total volume of exudate a dressing can handle but also in detail evaluate the spreading and overall management of exudate in the dressing over time and when exposed to different flow rates. Additionally, investigating if a dressing can handle high/low viscosity fluid and high/low protein content could further aid in understanding its function for different types of wounds. Only then is it possible to determine how to use this dressing appropriately and provide correct usage guidance to clinicians. To further optimise testing, however, it is necessary to understand the changes in wound fluid compositions, the rate of exudate production and how these changes occur for each of the major wound aetiologies, in both acute and chronic settings. Therefore, studies assessing biochemical and biophysical changes in wound exudates are extremely valuable; aiding both our basic scientific understanding of the wound healing process and our ability to assess wound dressing performance using robust, reproducible, and clinically‐relevant laboratory test methods.
Today, many dressings are assessed based on whether the wound improves or deteriorates in clinical studies, but this is a multifaceted measure affected by many factors including but not exclusively the specific wound dressing type used, which therefore makes the clinical trend in the wound status an outcome measure that is not always appropriate. Other, more clinically‐relevant measures should include specific physical, biological, and healing parameters. For example, a measure of success for a moist wound healing dressing should relate to physical parameters such as the incidence of maceration, management of wound fluids, dressing leakage, and dressing adherence (stays in place or perhaps sticks to wound tissues or peri‐wound skin causing damage upon removal). A wound dressing designed to promote healing should have different measures of success, typically including biological and physiological parameters such as reduction in proteases or inflammatory factors present in the wound environment, reduction in skin inflammatory markers collected at the peri‐wound to indicate a reduced inflammatory status of the peri‐wound, reduced bacterial and/or fungal load, temperature of the wound and peri‐wound (eg, monitored using infrared thermography), oedema of the peri‐wound (eg, monitored using the SEM Scanner), and pH of the wound. These physical and biological parameters should be assessed in addition to the healing outcomes to achieve a more comprehensive understanding of the wound, its current status, and the wound dressing performance. By taking this approach, we are much more likely to deliver effective and efficacious treatment, improving outcomes and reducing the economic burden of chronic wounds.
5. CONCLUSIONS
Chronic wounds are clinically characterised by their inability to heal within an expected time frame, and have emerged as an increasingly important medical problem over the past several decades because of their increasing incidence and greater recognition of associated morbidity, mortality, and socio‐economic burden. 81 , 82 Certain patient populations suffer from complex conditions that cause their wounds to be particularly challenging to treat, for example, those using chemotherapeutic agents (either administered alone or in combination with surgery and radiation) which have detrimental effects on wounds owing to the inhibiting influence of chemotherapy on the cell proliferation needed for the wound healing. 83 , 84 Likewise, in patients with immune dysfunction, diabetes or circulatory pathologies, bacteria may overwhelm the immune system and any antimicrobial treatments, which makes infected chronic wounds in these patients also highly difficult to treat. 85 Treatments of difficult‐to‐heal wounds including in patients with comorbidities are established in daily clinical practice, 86 but the majority of therapeutic interventions, particularly with regards to the usage of wound dressings, lacks robust and rigorous data concerning efficacy, which is vital for determining for whom and when specifically, each treatment and product type should be used. 87 , 88 The healthcare costs associated with this sub‐optimal treatment of wounds, in the absence of this critical information, is vast. “Cost‐of‐illness” studies which consider, for example, the direct (treatment) and indirect (eg, litigation, insurance) expenditures incurred by health services and related care‐providing agencies, patients and families, losses to economic production, premature death, disability and impacts on health‐related quality of life, clearly point to a trend of congestion of the healthcare resources dedicated to wound care, which may lead to loss of quality of care. 89 , 90 , 91 , 92 Specifically, works recently reviewed by Bosanquet & Harding 88 showed that the current rate of wound healing must increase by at least 1% per year to slow the rise of prevalent chronic wound cases. The potential of achieving this goal (or beyond) by using an appropriate wound dressing for the right person and wound and in the right time, is huge. Accordingly, in this review and in the above context, we have examined the impact of non‐healing wounds, why some wounds fail to heal (Figures 1, 2), and what is required in order to achieve improved clinical outcomes. It is clear that a deeper understanding of the issues of non‐healing is necessary as this will facilitate more appropriate use of current wound dressings, while also supporting the development of new treatments to optimise wound management and improve clinical and economic outcomes.
Published literature over the last 50 years has provided evidence to support the principles of moist wound healing, demonstrated the impact of inflammation and excess proteases in delaying healing, and established the need to control or reduce bacterial and fungal bioburdens. It has also highlighted the importance of advanced wound dressings and the need to understand when and how to use them to achieve clinical success. This can be facilitated by increasing our knowledge of the wound environment, and most importantly, the wound exudate, as it can provide a vast amount of information about the wound and its healing status. However, further studies are required to assess the biochemical and physical changes in wound exudate composition of acute and chronic non‐healing wounds of different aetiologies throughout the various phases of healing. These studies will not only improve our understanding of the wound healing process, but will also provide insights into how wound dressings should be evaluated in biomedical and bioengineering laboratory settings, and how they may impact healing. Accordingly, this will help develop more effective and efficacious wound dressings in the future.
ACKNOWLEDGEMENTS
The current literature review was conducted in the framework of the International Wound Dressing Technology Expert Panel work and was supported by an educational grant from Mölnlycke Health Care (Gothenburg, Sweden).
Cullen B, Gefen A. The biological and physiological impact of the performance of wound dressings. Int Wound J. 2023;20(4):1292‐1303. doi: 10.1111/iwj.13960
Funding information Mölnlycke Health Care
Endnote
Of note, the pH of intact skin may increase for several hours (from a basal, “natural” level of approximately 4.7) after using soaps or tap water, which are also alkaline with a pH of 8 in Europe (Lambers et al., 2006).
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10(12):e045253. doi: 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US out‐patient wound centers: data from the US wound registry. Wounds. 2012;24:10‐17. [PubMed] [Google Scholar]
- 3. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 4. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514‐525. [DOI] [PubMed] [Google Scholar]
- 5. Wolcott RD, Rhoads DD. A study of biofilm‐based wound management in subjects with critical limb ischaemia. J Wound Care. 2004;17(4):145‐155. [DOI] [PubMed] [Google Scholar]
- 6. Lindholm C, Bergsten A, Berglund E. Chronic wounds and nursing care. J Wound Care. 1999;8(1):5‐10. [DOI] [PubMed] [Google Scholar]
- 7. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283‐289. [DOI] [PubMed] [Google Scholar]
- 8. International consensus . The Role of Proteases in Wound Diagnostics. An Expert Working Group Review. London: Wounds international; 2011. [Google Scholar]
- 9. Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J. 2008;5:361‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho SK, Mattke S, Gordon H, Sheridan M, Ennis W. Development of a model to predict healing of chronic wounds within 12 weeks. Adv Wound Care (New Rochelle). 2020;9(9):516‐524. doi: 10.1089/wound.2019.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ. 2002;324:160‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil H, Cullen M, Chambers H, Carroll M, Walker J. Elements affecting wound healing time: an evidence based analysis. Wound Repair Regen. 2015;23(4):550‐556. [DOI] [PubMed] [Google Scholar]
- 14. Marbourg JM, Bratasz A, Mo X, Popovich PG. Spinal cord injury suppresses cutaneous inflammation: implications for peripheral wound healing. J Neurotrauma. 2017;34(6):1149‐1155. doi: 10.1089/neu.2016.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stacey M. Why don't wounds heal? Wounds Int. 2016;7(1):16‐21. [Google Scholar]
- 16. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321‐325. [DOI] [PubMed] [Google Scholar]
- 17. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003; focus on infection and inflammation. Ostomy Wound Manage. 2003;49(11):24‐51. [PubMed] [Google Scholar]
- 18. Romanelli M, Dini V, Barbarera S, et al. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polyhexanide for wound irrigation. Skin Pharmacol Physiol. 2010;23:41‐44. doi: 10.1159/000318266 [DOI] [PubMed] [Google Scholar]
- 19. Fierheller M, Sibbald RG. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv Skin Wound Care. 2010;23(8):369, quiz 380‐1‐379. [DOI] [PubMed] [Google Scholar]
- 20. Harris IR, Yee KC, Walters CE, et al. Cytokine and protease levels in healing and non‐healing chronic venous leg ulcers. Exper Dermatol. 1995;4:342‐349. [DOI] [PubMed] [Google Scholar]
- 21. Salim AS. The role of oxygen‐derived free radicals in the management of venous (varicose) ulceration: a new approach. World J Surg. 1991;15(2):264‐269. [DOI] [PubMed] [Google Scholar]
- 22. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol. 1993;101:64‐68. [DOI] [PubMed] [Google Scholar]
- 23. Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7(6):433‐441. [DOI] [PubMed] [Google Scholar]
- 24. Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81(2):189‐195. [DOI] [PubMed] [Google Scholar]
- 25. James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen. 2000;8(4):264‐269. doi: 10.1046/j.1524-475x.2000.00264.x [DOI] [PubMed] [Google Scholar]
- 26. Quatresooz P, Henry F, Paquet P, Pierard‐Franchimont C, Harding K, Pierard G. Deciphering the impaired cytokine cascades in chronic leg ulcers (review). Int J Mol Med. 2003;11(4):411‐418. [PubMed] [Google Scholar]
- 27. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13‐25. [DOI] [PubMed] [Google Scholar]
- 28. Trengove NJ, Stacey MC, Macauley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442‐452. [DOI] [PubMed] [Google Scholar]
- 29. Topman G, Lin FH, Gefen A. The influence of ischemic factors on the migration rates of cell types involved in cutaneous and subcutaneous pressure ulcers. Ann Biomed Eng. 2012. Sep;40(9):1929‐1939. [DOI] [PubMed] [Google Scholar]
- 30. Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMPs made easy. Wounds Int. 2009;1(1):1‐6. http://www.woundsinternational.com [Google Scholar]
- 31. Cullen B, Martinez J. Underlying biochemistry in non‐healing wounds perpetuates chronicity. Wounds Int. 2016;7(4):10‐15. [Google Scholar]
- 32. Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23‐32. [DOI] [PubMed] [Google Scholar]
- 33. Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on relative levels of elastase, alpha‐1 proteinase inhibitor and alpha‐2 macroglobulin. J Invest Dermatol. 1996;106:335‐341. [DOI] [PubMed] [Google Scholar]
- 34. Falanga V, Grinnell F, Gilchrest B, Maddox YT, Moshell A. Workshop on the pathogenesis of chronic wounds. J Invest Dermatol. 1994;102:125‐127. [DOI] [PubMed] [Google Scholar]
- 35. Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: a potential mechanism for senescence in venous ulcers. J Vasc Surg. 1999;30(4):734‐743. [DOI] [PubMed] [Google Scholar]
- 36. Moore K, Ruge F, Harding KG. T lymphocytes and the lack of activated macrophages in wound margin biopsies from chronic leg ulcers. Br J Dermatol. 1997;137(2):188‐194. [DOI] [PubMed] [Google Scholar]
- 37. Charles CA, Romanelli P, Martinez ZB, Ma F, Roberts B, Kirsner RS. Tumor necrosis factor‐alfa in nonhealing venous leg ulcers. J Am Acad Dermatol. 2009. Jun;60(6):951‐955. doi: 10.1016/j.jaad.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 38. Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin‐1β induces a healing‐associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579‐2587. doi: 10.2337/db12-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor‐alpha antibody infliximab improves healing of chronic wounds. Int Wound J. 2006;3(3):171‐179. doi: 10.1111/j.1742-481X.2006.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whiston RJ, Hallett MB, Davies EV, Harding KG, Lane IF. Inappropriate neutrophil activation in venous disease. Br J Surg. 1994;81(5):695‐698. [DOI] [PubMed] [Google Scholar]
- 41. Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (New Rochelle). 2013;2(7):379‐388. doi: 10.1089/wound.2012.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS) – a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:249‐265. [DOI] [PubMed] [Google Scholar]
- 43. Wlaschek M, Singh K, Sindrilaru A, Crisan D, Scharffetter‐Kochanek K. Iron and iron‐dependent reactive oxygen species in the regulation of macrophages and fibroblasts in non‐healing chronic wounds. Free Radic Biol Med. 2019;133:262‐275. [DOI] [PubMed] [Google Scholar]
- 44. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165‐171. [DOI] [PubMed] [Google Scholar]
- 45. Dunnill C, Patton T, Brennan J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS‐modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix for treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16‐25. [DOI] [PubMed] [Google Scholar]
- 47. Serena TE, Cullen BM, Bayliff SW, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of non‐healing for targeting protease‐modulating treatment. Wound Repair Regen. 2016;24:589‐595. doi: 10.1111/wrr.12431 [DOI] [PubMed] [Google Scholar]
- 48. Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase‐9 (MMP‐9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951‐961. [DOI] [PubMed] [Google Scholar]
- 49. Wlaschek M, Pees D, Achterberg V, Meyer‐Ingold W, Scharfetter‐Kochanek K. Protease inhibitors protect growth factor activity in chronic wounds. Br J Dermatol. 1997;137:646‐647. [DOI] [PubMed] [Google Scholar]
- 50. Cullen B et al. A clinical study investigating the temporal changes in proteolytic activity in wounds treated with PROMOGRAN. WHS. 2005;13(2):A4‐076. [Google Scholar]
- 51. Bullen EC, Longaker MT, Updike DL, et al. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995;104(2):236‐240. [DOI] [PubMed] [Google Scholar]
- 52. Palolahti M, Lauharanta L, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Exp Dermatol. 1993;2:29‐37. [DOI] [PubMed] [Google Scholar]
- 53. Ovington L. Bacterial toxins and wound healing. Ostomy Wound Manage. 2003;49(7A Suppl):8‐12. [PubMed] [Google Scholar]
- 54. Lindsay S, Oates A, Bourdillon K. The detrimental impact of extracellular bacterial proteases on wound healing. Int Wound J. 2017;14(6):1237‐1247. doi: 10.1111/iwj.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rippon MG, Westgate S, Rogers AA. Implications of endotoxins in wound healing: a narrative review. J Wound Care. 2022;31(5):380‐392. [DOI] [PubMed] [Google Scholar]
- 56. Suleman L. Extracellular bacterial proteases in chronic wounds: a potential therapeutic target? Adv Wound Care (New Rochelle). 2016;5(10):455‐463. doi: 10.1089/wound.2015.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davies CE, Wilson MJ, Hill KE, et al. Use of molecular techniques to study microbial diversity in the skin. Chronic Wounds Re‐Evaluated WRR. 2001;9:332‐409. [DOI] [PubMed] [Google Scholar]
- 58. Schmidtchen A, Holst E, Tapper H, Björck L. Elastase‐producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb Pathog. 2003;34:47‐55. [DOI] [PubMed] [Google Scholar]
- 59. Liu Y, Kalen A, Risto O, Wahlstrom O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. WRR. 2002;10(5):336‐340. [DOI] [PubMed] [Google Scholar]
- 60. Greener B, Hughes AA, Bannister NP. Douglass J proteases and pH in chronic wounds. J Wound Care. 2005;14:59‐61. [DOI] [PubMed] [Google Scholar]
- 61. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound‐healing: a new perspective for wound‐therapy? Arch Dermatol Res. 2007;298(9):413‐420. doi: 10.1007/s00403-006-0713-x [DOI] [PubMed] [Google Scholar]
- 62. Jones EM, Cochrane CA, Percival SL. The effect of pH on extracellular matrix and biofilms. Adv Wound Care. 2015;4(7):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ono S, Imai R, Ida Y, Shibata D, Komiya T, Matsumura H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns. 2015;41(4):820‐824. [DOI] [PubMed] [Google Scholar]
- 64. Derwin R, Patton D, Avsar P, Strapp H, Moore Z. The impact of topical agents and dressings on pH and temperature on wound healing: a systematic narrative review. Int Wound J. 2021;1‐12. doi: 10.1111/iwj.13733. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lambers H, Pressens S, Bloem A, et al. Natural skin surface pH is on average below 5 which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359‐370. [DOI] [PubMed] [Google Scholar]
- 66. Hart J. Inflammation 2: its role in the healing of chronic wounds. J Wound Care. 2002;11(7):245‐249. [DOI] [PubMed] [Google Scholar]
- 67. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. doi: 10.3390/ijms17122085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Winter GD. Formation of a scab and rate of epithelialisation of superficial wounds in the skin of young domestic pig. Nature. 1962;193:293‐294. [DOI] [PubMed] [Google Scholar]
- 69. Thomas S. Assessment and management of wound exudate. J Wound Care. 1997;6(7):327‐330. [DOI] [PubMed] [Google Scholar]
- 70. Vowden K, Vowden P. Understanding exudate management and the role of exudate in the healing process. Br J Community Nurs. 2003;8(11 Suppl):4‐13. [DOI] [PubMed] [Google Scholar]
- 71. Cutting KF. Exudate: composition and functions. In: White R, ed. Trends in Wound Care: Volume III. Salisbury: Quay Books, MA Healthcare Ltd.; 2004:41‐49. [Google Scholar]
- 72. Trengove NJ, Langton SR, Stacey WC. Biochemical analysis of wound fluid from nonhealing and healing chronic ulcers. Wound Repair Regen. 1996;4(2):234‐239. [DOI] [PubMed] [Google Scholar]
- 73. Iizaka S, Sanada H, Minematsu T, et al. Do nutritional markers in wound fluid reflect pressure ulcer status? Wound Repair Regen. 2010;18:31‐37. [DOI] [PubMed] [Google Scholar]
- 74. Romanelli M, Vowden K, Weir D. Exudate management made easy. Wounds Int. 2010;1(2):1‐6. http://www.woundsinternational.com [Google Scholar]
- 75. Shukla VK, Shukla D, Tiwary SK. Evaluation of pH measurement as a method of wound assessment. J Wound Care. 2007;16(7):291‐294. [DOI] [PubMed] [Google Scholar]
- 76. Lustig A, Alves P, Call E, Santamaria N, Gefen A. The sorptivity and durability of gelling fibre dressings tested in a simulated sacral pressure ulcer system. Int Wound J. 2021. Apr;18(2):194‐208. doi: 10.1111/iwj.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rolstad BJ, Ovington LG. Principles of wound management. In: Bryant J, Nix DP, eds. Acute and Chronic Wounds: Current Management Concepts. 3rd ed. St Louis: Mosby; 2007. [Google Scholar]
- 78. White RJ, Cutting K Modern Exudate Management: A Review of Wound Treatments. 2006. www.worldwidewounds.com/2006/september/White/Modern-Exudate-Mgt.html
- 79. Fletcher M, Pringle JH. The effect of surface free energy and medium surface tension on bacterial attachment to solid surfaces. J Colloid Interface Sci. 1985;104(1):5‐14. doi: 10.1016/0021-9797(85)90004-9 [DOI] [Google Scholar]
- 80. Tamargo A, Dolores Álvarez CCM, Herranz B, Bartolomé B, Moreno‐Arribas MV, Laguna L. Influence of viscosity on the growth of human gut microbiota. Food Hydrocoll. 2018;77:163‐167. doi: 10.1016/j.foodhyd.2017.09.031 [DOI] [Google Scholar]
- 81. Lindholm C, Styche TJ, Horton HE. Diagnosis and treatment impacts on wound care efficiency drivers: real‐world analysis. J Wound Care. 2021;30(7):534‐542. [DOI] [PubMed] [Google Scholar]
- 82. Falanga V, Isseroff RR, Soulika AM, et al. Chronic wounds. Nat Rev Dis Primers. 2022;8(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty. 2008. Jan;11(8):e9. [PMC free article] [PubMed] [Google Scholar]
- 84. Deptuła M, Zieliński J, Wardowska A, Pikuła M. Wound healing complications in oncological patients: perspectives for cellular therapy. Postepy Dermatol Alergol. 2019;36(2):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Malone M, Schultz G. Challenges in the diagnosis and management of wound infection. Br J Dermatol. 2022;187(2):159‐166. [DOI] [PubMed] [Google Scholar]
- 86. Dissemond J, Romanelli M. Inflammatory skin diseases and wounds. Br J Dermatol. 2022;187(2):167‐177. [DOI] [PubMed] [Google Scholar]
- 87. Harding KG. Chronic wounds: a clinical problem requiring ownership and coordination. Br J Dermatol. 2022;187(2):133‐134. [DOI] [PubMed] [Google Scholar]
- 88. Bosanquet DC, Harding KG. Wound healing: potential therapeutic options. Br J Dermatol. 2022;187(2):149‐158. [DOI] [PubMed] [Google Scholar]
- 89. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 90. Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care (New Rochelle). 2021;10(5):281‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Graves N, Phillips CJ, Harding K. A narrative review of the epidemiology and economics of chronic wounds. Br J Dermatol. 2022;187(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 92. Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med. 2022;27(1):63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


