Abstract
Surgical site infection (SSI) is a common and serious complication of transforaminal lumbar interbody fusion (TLIF), and the occurrence of SSI usually leads to prolonged hospitalisation, increased medical costs, poor prognosis, and even death. The objectives of this study were to compare the incidence of SSI in patients with type 2 diabetes, investigate the correlation between perioperative glycemic variability and postoperative SSI, and develop a nomogram model to predict the risk of SSI. This study retrospectively analysed 339 patients with type 2 diabetes who underwent TLIF in the spinal surgery department of the Affiliated Zhongda Hospital of Southeast University from January 2018 to September 2021. The medical records of all patients were collected, and postoperative infection cases were determined according to the diagnostic criteria of surgical site infection. The risk factors for postoperative SSI were analysed by univariate and multivariate logistic regression. And Nomogram prediction model was established and validated. The nomogram incorporated seven independent predictors. Preoperative FPG‐CV was the most important independent risk predictor of SSI, followed by preoperative MFBG, duration of drain placement, postoperative FPG‐CV, preoperative blood glucose control scheme, duration of diabetes >5 years, and the number of fused vertebrae ≥2. The nomogram showed good diagnostic accuracy for the SS of both the training cohort and the validation cohort (AUC = 0.915 and AUC = 0.890). The calibration curves for the two cohorts both showed optimal agreement between nomogram prediction and actual observation. In conclusion, preoperative and postoperative glycemic variability is closely related to the occurrence of SSI. We developed and validated a nomogram to accurately predict the risk of SSI after TLIF surgery. It's helpful for spinal surgeons to formulate reasonable treatment plans and prevention strategies for type 2 diabetes patients.
Keywords: diabetes mellitus, glycemic variability, lumbar fusion, nomogram, surgical site infection
1. INTRODUCTION
According to statistics, the number of diabetes patients in the world is increasing year by year, and it is expected that one in 10 adults will have diabetes by 2040. 1 With age and changes in working styles, degenerative diseases of the lumbar disc have become one of the major causes of human dysfunction and economic burden worldwide. Lumbar disc degeneration gradually evolved, leading to lumbar disc herniation, lumbar spinal stenosis, and spondylolisthesis, which seriously affect the quality of life of patients. 2 , 3 With the increasing prevalence of degenerative diseases of the lumbar spine, more patients with diabetes and lumbar disc degenerative diseases will choose lumbar decompression and fusion in the future. The common kind of lumbar fusion is transforaminal lumbar interbody fusion (TLIF). Many studies have reported that diabetes is considered to be a risk of surgical site infection (SSI) after lumbar fusion. 4 , 5 Previous studies have found that the incidence of SSI after spinal fusion is 2% to 5%. The risk of SSI after spinal fusion increased from 2.4% to 8.5% if implants were added during surgery. 6 SSI not only lengthens hospital stay but also increases the risk of deformities that can lead to high medical costs and even death. Despite improvements in surgical techniques, postoperative care, and perioperative prophylactic use of antibiotics, SSI continues to influence outcomes in patients undergoing lumbar surgery. How to reduce the incidence of SSI after TLIF has always been a major concern of spinal surgeons.
Studies have shown that diabetes is a risk factor for SSI after TLIF, and poor control of perioperative glycemia can increase the risk of SSI. Controlling perioperative blood glucose has always been a concern for surgeons, and proper blood glucose control can effectively reduce postoperative complications. 7
Previous studies have focused more on the relationship between diabetes and postoperative surgical site infection, but less research has been done on the link between some diabetes characteristics and SSI, such as perioperative glycemic control, insulin use, diabetes duration, etc. Perioperative glycemic control indicators also included mean fasting blood glucose, glycated haemoglobin (HbA1c), and glycemic variability (GV). Glycemic variability accurately reflects the degree of blood glucose fluctuation, and studies have found that both the increase of blood glucose fluctuation level and the extension of fluctuation time will increase the risk of diabetes complications. 8 Glycemic variability, as an indicator of blood glucose fluctuation, can show the stability and fluctuation range of blood glucose in a period and is an important content for evaluating whether blood glucose in diabetic patients reaches the standard. A recent study has found a certain correlation between glycemic variability and the occurrence of postoperative SSI. 9 Diabetes patients are usually accompanied by hyperglycemia. Long‐term hyperglycemia can inhibit the function of inflammatory cells in the body, reduce immunity, and cause tissue damage. The increase of vascular fragility caused by hyperglycemia can lead to ischemia and hypoxia at the surgical site, delay the healing of the surgical incision, and thus increase the possibility of infection at the surgical site. 10 However, Bardia A found no relationship between the rate of major adverse events after surgery and postoperative glycemic variability. 11 At present, the relationship between glycemic variability and postoperative SSI is still controversial, and further research is needed to explore the relationship between them.
Although many studies have investigated the incidence and risk factors of SSI after TLIF, there is currently no model available to predict the risk of SSI after TLIF in type 2 diabetes patients. The purpose of this study was to explore the potential influencing factors of SSI after TLIF in type 2 diabetes patients and establish a nomogram model of postoperative SSI risk in the Chinese population, providing an effective method for early identification and timely intervention of SSI following TLIF in type 2 diabetes patients. In addition, this research intends to explore the correlation between perioperative glycemic variability and the occurrence of SSI after TLIF by including preoperative and postoperative glycemic variability and obtain more indicators for predicting SSI in diabetes. It can effectively improve our knowledge of risk factors of postoperative SSI in TLIF patients with type 2 diabetes mellitus and improve preoperative risk stratification, providing direction for early intervention.
2. MATERIAL AND METHODS
2.1. Patients
This is a retrospective study. The clinical data of 339 type 2 diabetes patients who received transforaminal lumbar interbody fusion at the Spine Surgery Department of Zhongda hospital from January 2018 to September 2021 were retrospectively analysed. Eligible patients were included according to inclusion and exclusion criteria, and each selected patient was rigorously evaluated for surgical indications. Two surgeons separately obtained all data from the hospital's medical record system, and any contested data were updated with the approval of the two physicians who retrieved the data. The surgery was performed using conventional TLIF, and the surgeons were all senior chief physicians in charge. The inclusion criteria: the clinical diagnosis was lumbar spinal stenosis, lumbar disc herniation, or lumbar spondylolisthesis; after 3 to 6 months of conventional conservative treatment, the symptoms were not relieved well, which seriously affected the patient's work and life; patients who underwent transforaminal lumbar interbody fusion; combined with type 2 diabetes. The exclusion criteria were as follows: suffer from lumbar infection, spinal trauma or tumours, scoliosis, lumbar tuberculosis; history of previous lumbar spine surgery; diabetic peripheral neuropathy; incomplete clinical data.
2.2. SSI diagnostic criteria
The diagnosis of SSI was based on the incision observation and imaging examination. This study used CDC criteria (Centres for Disease Control and Prevention) to define SSI. 12 In this study, SSI was specified as acute spinal infection within 30 days after TLIF. Surgical site infection includes superficial incision infection, deep incision infection, and organ space infection occurring within 30 days after surgery (or within 1 year of hardware implantation).
2.3. Variables
According to the inclusion and exclusion criteria, the inpatient medical records of all patients who met the inclusion criteria were reviewed. A total of 339 patients were included in this study. According to the SSI diagnostic criteria, the patients were divided into the infection group and the non‐infection group, including 56 cases in the infection group and 283 cases in the non‐infection group. The infection group included three cases of deep infection and 53 cases of superficial infection. The demographic variables include sex, age, body mass index (BMI), the duration of diabetes mellitus, history of cardiovascular disease, serum glucose on admission, HbA1c, preoperative blood glucose control scheme (subcutaneous injection of insulin, combined medication, oral hypoglycemic agent, diet therapy). Operative and postoperative variables were the number of fused vertebrae, operative time, intraoperative blood loss, intraoperative blood transfusion, length of the incision, and duration of drain placement. Blood glucose monitoring variables include Mean fasting blood glucose (MFBG) and variation coefficient of fasting plasma glucose (FPG‐CV) before and after TLIF. The GV was defined by the variation coefficient of fasting plasma glucose (FPG‐CV). The FPG‐CV was calculated as the ratio of standard deviation (SD) to mean glucose values, expressed as a percentage.
2.4. Statistics analysis
SPSS 21 software (SPSS Inc, Chicago, IL) and R 4.1.0 (https://www.r-project.org/) was used for statistical analysis. The quantitative variables were tested for normality and homogeneity of variance, shown as the mean ± standard deviation (SD), and Student's t‐test or Mann–Whitney U test was used for comparison between groups. Qualitative variables were described by frequency and percentage, which were compared by the Chi‐square test. Significance was set at P < .05 (two‐sided). Univariate analysis and multivariate logistic regression analysis were performed for each covariate to determine the predictors of surgical site infection after TLIF.
In order to maximise the predictive ability of the model, the linear prediction method was adopted, and the nomogram model was established by using the variables with P less than .05 in the single and multiple logistic regression. On the basis of the results of the multivariate logistic regression model, a nomogram was formulated by R 4.1.0 (http://www.r-project.org) with the rms package. The ROC analysis method was used to evaluate the reliability of the risk assessment model based on the total score. The area under the curve (AUC) was used to judge the model resolution and evaluate the model performance in predicting the risk of SSI. The higher the AUC, the better the discriminative ability.
3. RESULTS
3.1. Patient characteristics
Finally, 339 type 2 diabetes patients were enrolled in the study, and all of their clinical data were carefully gathered and arranged (Table 1), with 238 patients entering the training group and 101 cases being assigned to the validation cohort. There were no significant differences in the characteristics between the training and the validation cohorts, which justified their use as training and validation cohorts.
TABLE 1.
Patient characteristics of the training cohort and the validation cohort
| All cases | Training cohort | Validation cohort | p‐value | ||
|---|---|---|---|---|---|
| No. of patients | No. of patients (% of total) | 339 (100%) | 238 (70.2%) | 101 (29.8%) | |
| Sex | No. of patients (% of total) | .982 | |||
| Male | 149 (43.7) | 104 (43.7) | 44 (43.6) | ||
| Female | 191 (56.3) | 134 (56.3) | 57 (56.4) | ||
| Age | Years (median, IQR) | 69.00 (61.00, 74.00) | 68.00 (61.00, 74.25) | 69.00 (62.00, 73.00) | .534 |
| BMI | No. of patients (% of total) | .675 | |||
| <25 | 113 (33.3) | 81 (34.0) | 32 (31.7) | ||
| ≥25 | 226 (66.7) | 157 (66.0) | 69 (68.3) | ||
| Cardiovascular disease | No. of patients (% of total) | .904 | |||
| No | 113 (33.3) | 81 (34.0) | 32 (31.7) | ||
| Yes | 226 (66.7) | 157 (66.0) | 69 (68.3) | ||
| Number of fused vertebrae | No. of patients (% of total) | .646 | |||
| 1 | 118 (34.8) | 81 (34.0) | 37 (36.6) | ||
| ≥2 | 221 (65.2) | 157 (66.0) | 64 (63.4) | ||
| Operative time | minutes (median, IQR) | 170.00 (130.00, 200.00) | 170.00 (135.00, 201.25) | 160.00 (120.00, 197.50) | .334 |
| Intraoperative blood loss | ml (median, IQR) | 250.00 (150.00, 400.00) | 250.00 (150.00, 400.00) | 200.00 (150.00, 400.00) | .619 |
| Intraoperative blood transfusion | ml (median, IQR) | 200.00 (0.00, 300.00) | 200.00 (0.00, 300.00) | 200.00 (0.00, 400.00) | .188 |
| Length of the incision | cm (median, IQR) | 9.00 (8.00, 12.00) | 9.00 (8.00, 12.00) | 9.00 (9.00, 12.00) | .674 |
| Duration of drain placement | days (median, IQR) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | .961 |
| Duration of diabetes (yr) | No. of patients (% of total) | .320 | |||
| ≤5 | 138 (40.7) | 101 (42.4) | 37 (36.6) | ||
| >5 | 201 (59.3) | 137 (57.6) | 64 (63.4) | ||
| HbA1c | % (median, IQR) | 7.34 (6.80, 7.34) | 7.34 (6.72, 7.34) | 7.34 (7.06, 7.50) | .449 |
| Preoperative blood glucose control scheme | No. of patients (% of total) | .553 | |||
| Subcutaneous injection of insulin | 76 (22.4) | 50 (21.0) | 26 (25.7) | ||
| Combined medication | 65 (19.2) | 43 (18.1) | 22 (21.8) | ||
| Oral hypoglycemic agent | 96 (28.3) | 70 (29.4) | 26 (25.7) | ||
| Diet therapy | 102 (30.1) | 75 (31.5) | 27 (26.8) | ||
| Preoperative MFBG | mmol/l (median, IQR) | 7.13 (6.18, 8.20) | 7.06 (6.05, 8.12) | 7.23 (6.38, 8.56) | .148 |
| Preoperative FPG‐CV | % (median, IQR) | 9.38 (5.49, 9.38) | 9.39 (5.47, 14.53) | 9.38 (5.48, 14.57) | .868 |
| Postoperative MFBG | mmol/l (median, IQR) | 7.73 (6.68, 8.80) | 7.73 (6.58, 8.78) | 7.70 (6.97, 9.02) | .415 |
| Postoperative FPG‐CV | % (median, IQR) | 13.08 (8.22, 18.75) | 13.35 (8.21, 19.28) | 13.06 (8.28, 18.34) | .617 |
Note: Continuous variables are presented as median (interquartile range; IQR), while categorical variables are presented as patients (%).
Abbreviations: FPG‐CV: variation coefficient of fasting plasma glucose; MFBG: mean fasting blood glucose.
3.2. Baseline data of the infected and non‐infected groups in the training cohort
Table 2 shows the baseline data of 238 type 2 diabetes patients after TLIF who were randomly included in the training cohort using the R function “createDataPartition.” These baseline data were examined by univariate analysis. Among the surgical risk factors, the number of fused vertebrae ≥2 (P = .001), length of the incision (P = .038), and duration of drain placement (P = .004) were markedly related to the SSI. As diabetes‐related variables, duration of diabetes >5 years (P = .005), preoperative blood glucose control scheme (P = .012), preoperative MFBG (P = .000), preoperative FPG‐CV (P = .000), postoperative FPG‐CV (P = .001) were significant risk factors of SSI.
TABLE 2.
Baseline data on SSI of patients in the training cohort
| All cases of training cohort | SSI | Non‐SSI | p‐value | ||
|---|---|---|---|---|---|
| No. of patients | No. of patients (% of total) | 238 (100) | 34 (14.3) | 204 (85.7) | |
| Sex | No. of patients (% of total) | .957 | |||
| Male | 104 (43.7) | 15 (44.1) | 89 (43.6) | ||
| Female | 134 (56.3) | 19 (55.9) | 115 (56.4) | ||
| Age | Years (median, IQR) | 68.00 (61.00, 74.25) | 70.00 (62.00, 78.00) | 67.00 (62.00, 78.00) | .09 |
| BMI | No. of patients (% of total) | .525 | |||
| <25 | 114 (47.9) | 18 (52.9) | 96 (47.1) | ||
| ≥25 | 124 (52.1) | 16 (47.1) | 108 (52.9) | ||
| Cardiovascular disease | No. of patients (% of total) | .823 | |||
| No | 81 (34.0) | 11 (32.4) | 70 (34.3) | ||
| Yes | 157 (66.0) | 23 (67.6) | 134 (65.7) | ||
| Number of fused vertebrae | No. of patients (% of total) | .001* | |||
| 1 | 81 (34.0) | 3 (8.8) | 78 (38.2) | ||
| ≥2 | 157 (66.0) | 31 (91.2) | 126 (61.8) | ||
| Operative time | minutes (median, IQR) | 170.00 (130.00, 200.00) | 180.00 (137.50, 232.50) | 170.00 (135.00, 200.00) | .675 |
| Intraoperative blood loss | ml (median, IQR) | 250.00 (150.00, 400.00) | 300.00 (200.00, 500.00) | 250.00 (150.00, 350.00) | .280 |
| Intraoperative blood transfusion | ml (median, IQR) | 200.00 (0.00, 300.00) | 250.00 (0.00, 500.00) | 200.00 (0.00, 250.00) | .337 |
| Length of the incision | cm (median, IQR) | 9.00 (8.00, 12.00) | 9.50 (8.00, 12.00) | 9.00 (8.00, 12.00) | .038* |
| Duration of drain placement | days (median, IQR) | 4.00 (3.00, 4.00) | 4.00 (3.75, 5.00) | 4.00 (3.00, 4.00) | .004* |
| Duration of diabetes (yr) | No. of patients (% of total) | .005* | |||
| ≤5 | 101 (42.4) | 7 (20.6) | 94 (46.1) | ||
| >5 | 137 (57.6) | 27 (79.4) | 110 (53.9) | ||
| HbA1c | % (median, IQR) | 7.34 (6.72, 7.34) | 7.34 (6.98, 7.85) | 7.34 (6.70, 7.34) | .402 |
| Preoperative blood glucose control scheme | No. of patients (% of total) | .012* | |||
| Subcutaneous injection of insulin | 50 (21.0) | 2 (5.9) | 48 (23.5) | ||
| Combined medication | 43 (18.1) | 11 (32.4) | 32 (15.7) | ||
| Oral hypoglycemic agent | 70 (29.4) | 7 (20.6) | 63 (30.9) | ||
| Diet therapy | 75 (31.5) | 14 (41.1) | 61 (29.9) | ||
| Preoperative MFBG | mmol/l (median, IQR) | 7.06 (6.04, 8.12) | 7.06 (6.05, 8.12) | 7.23 (6.38, 8.56) | .000* |
| Preoperative FPG‐CV | % (median, IQR) | 9.39 (5.47, 14.53) | 9.39 (5.47, 14.53) | 9.38 (5.48, 14.57) | .000* |
| Postoperative MFBG | mmol/l (median, IQR) | 7.73 (6.57, 8.78) | 7.73 (6.58, 8.78) | 7.70 (6.97, 9.02) | .537 |
| Postoperative FPG‐CV | % (median, IQR) | 13.35 (8.21, 19.28) | 21.41 (10.48, 33.10) | 12.68 (8.15, 16.87) | .001* |
Note: Continuous variables are presented as median (interquartile range; IQR), while categorical variables are presented as patients (%).
Abbreviations: FPG‐CV: variation coefficient of fasting plasma glucose; MFBG: mean fasting blood glucose.
*Statistically significant.
3.3. Identification of independent risk predictors
In univariate regression analysis, the number of fused vertebrae, length of the incision, duration of drain placement, duration of diabetes, preoperative blood glucose control scheme, preoperative MFBG, preoperative FPG‐CV, and postoperative FPG‐CV showed statistically significant predictors. These factors were further included in the multivariate logistic regression analysis (Table 3). Finally, the number of fused vertebrae, length of the incision, duration of drain placement, duration of diabetes, preoperative blood glucose control scheme, preoperative MFBG, preoperative FPG‐CV, and postoperative FPG‐CV were identified as independent risk predictors of SSI.
TABLE 3.
Univariate and multivariate regression analysis for risk factors of SSI on training cohort
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | 95% CI | P value | OR | 95% CI | 95% CI | P value | |
| Sex | ||||||||
| Male | reference | |||||||
| Female | 0.980 | 0.472 | 2.037 | .957 | ||||
| Age | 1.035 | 0.994 | 1.077 | .092 | ||||
| BMI | ||||||||
| <25 | reference | |||||||
| ≥25 | 0.790 | 0.382 | 1.635 | .526 | ||||
| Cardiovascular disease | ||||||||
| No | reference | |||||||
| Yes | 1.092 | 0.503 | 2.370 | .823 | ||||
| Number of fused vertebrae | ||||||||
| 1 | reference | reference | ||||||
| ≥2 | 6.397 | 1.892 | 21.629 | .003* | 9.222 | 1.500 | 56.688 | .017* |
| Operative time | 1.001 | 0.998 | 1.003 | .680 | ||||
| Intraoperative blood loss | 1.001 | 0.999 | 1.002 | .285 | ||||
| Intraoperative blood transfusion | 1.001 | 0.999 | 1.002 | .338 | ||||
| Length of the incision | 1.145 | 1.006 | 1.303 | .040* | ||||
| Duration of drain placement | 1.529 | 1.122 | 2.082 | .007* | 1.629 | 1.066 | 2.489 | .024* |
| Duration of diabetes (yr) | ||||||||
| ≤5 | reference | reference | ||||||
| >5 | 3.296 | 1.373 | 7.912 | .008* | 6.226 | 1.655 | 23.423 | .007* |
| HbA1c | 1.151 | 0.829 | 1.598 | .401 | ||||
| Preoperative blood glucose control scheme | .023* | .012* | ||||||
| Subcutaneous injection of insulin | reference | reference | ||||||
| Combined medication | 8.250 | 1.714 | 39.720 | .008* | 3.822 | .193 | ||
| Oral hypoglycemic agent | 2.667 | 0.530 | 13.417 | .234 | 13.183 | .022* | ||
| Diet therapy | 5.508 | 1.194 | 25.415 | .029* | 26.442 | .002* | ||
| Preoperative MFBG | 1.591 | 1.265 | 2.001 | .000* | 1.618 | 1.098 | 2.386 | .015* |
| Preoperative FPG‐CV | 1.159 | 1.105 | 1.216 | .000* | 1.194 | 1.106 | 1.289 | .000* |
| Postoperative MFBG | 1.068 | 0.868 | 1.313 | .536 | ||||
| Postoperative FPG‐CV | 1.079 | 1.042 | 1.117 | .000* | 1.063 | 1.010 | 1.120 | .022* |
Note: OR odds ratio, 95% CI 95% confidence interval. *P < .05. *Statistically significant preoperative blood glucose control scheme is multi‐categorical variable, so covariate is generated for analysis.
Abbreviations: FPG‐CV: variation coefficient of fasting plasma glucose; MFBG: mean fasting blood glucose.
*Statistically significant preoperative blood glucose control scheme is multi‐categorical variable, so covariate is generated for analysis.
3.4. Construction of the predicting SSI nomogram
The establishment of a nomogram model that was used to predict the risk of SSI was based on all significant independent risk factors of postoperative SSI which was analysed by multivariate logistic regression. The nomogram showed that preoperative FPG‐CV contributed the most to the prediction, followed by preoperative MFBG, duration of drain placement, postoperative FPG‐CV, preoperative blood glucose control scheme, duration of diabetes >5 years, and the number of fused vertebrae ≥2. Each factor of the nomogram was assigned a weighted point, and the sum of the points for each patient is consistent with the risk prediction of SSI (Figure 1).
FIGURE 1.
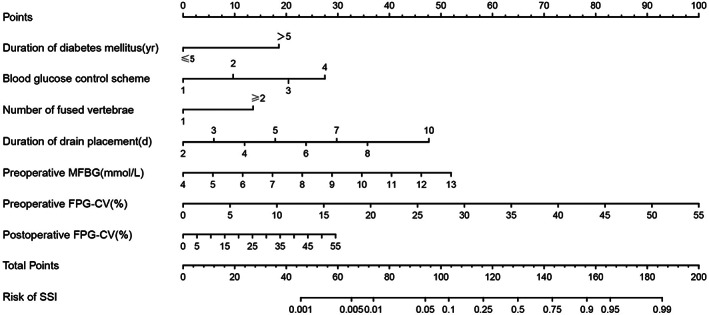
Nomogram for estimating the risk of SSI in patients. Nomogram for PCa patients to predict the risk of SSI. SSI, surgical site infection; FPG‐CV: variation coefficient of fasting plasma glucose; MFBG: mean fasting blood glucose. The method of using the nomogram is as follows: all variables (Duration of diabetes, Blood glucose control scheme, Number of fused vertebrae, Duration of drain placement, preoperative MFBG, preoperative FPG‐CV, postoperative FPG‐CV) of the patient can get a specific score on the Points‐axis of the nomogram. Add the corresponding scores of all variables to get the total points. The total score of each patient can be obtained through the corresponding relationship between the Total Points‐axis and the Risk of SSI‐axis to get the specific upgrade risk
3.5. The nomogram and its predictive performance
The model for estimation of SSI risk was constructed based on the regression co‐efficient from the logistic model, in which the standardised net benefit and high risk threshold were manifested in Figure 2.
FIGURE 2.
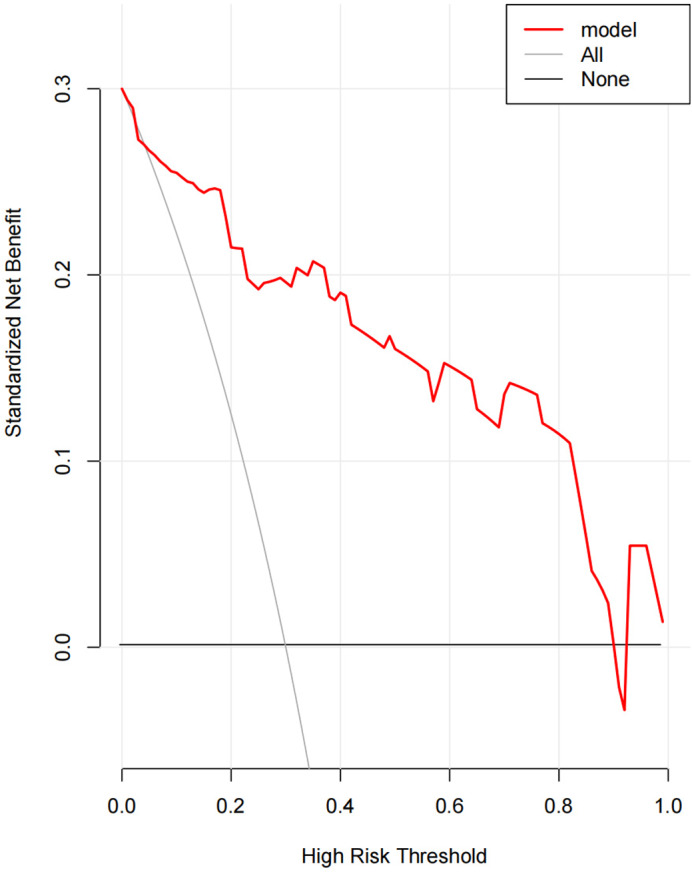
The standardised net benefit, high‐risk threshold
3.6. Calibration of the nomogram
Figures 3 and 4 presented calibration plots, in which the agreement between nomogram predictions and actual observations of SSI risk in both training and validation cohorts was excellent.
FIGURE 3.
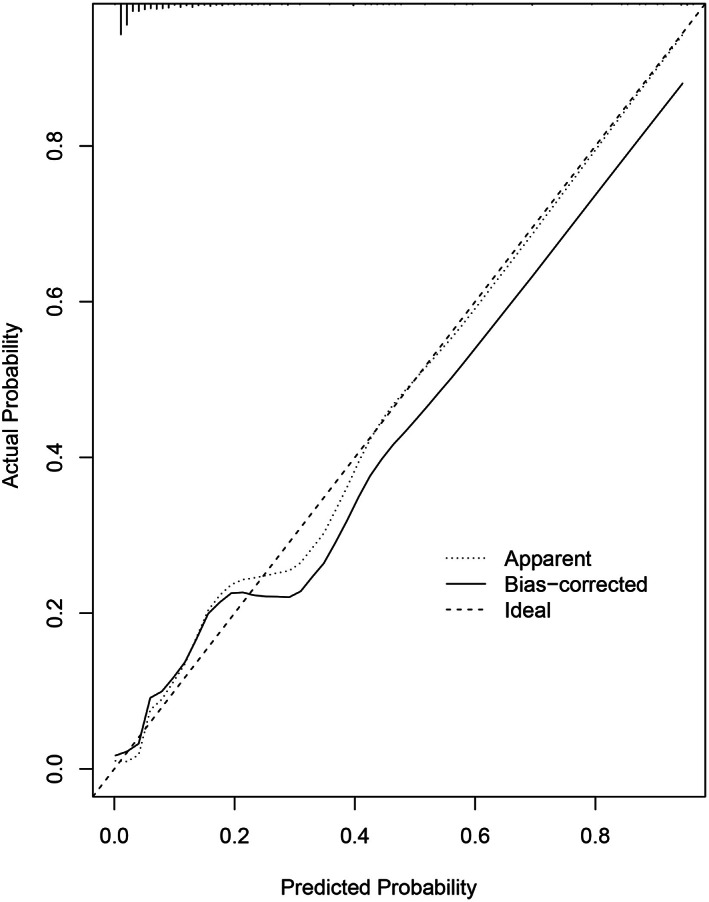
Calibration plots of nomogram models for predicting postoperative surgical site infection in the training cohort. Nomogram‐predicted probability of SSI is plotted on the x‐axis; the actual probability of SSI is plotted on the y‐axis. A plot along the 45° line would indicate a perfect calibration model in which the predicted probabilities are identical to the actual outcomes
FIGURE 4.
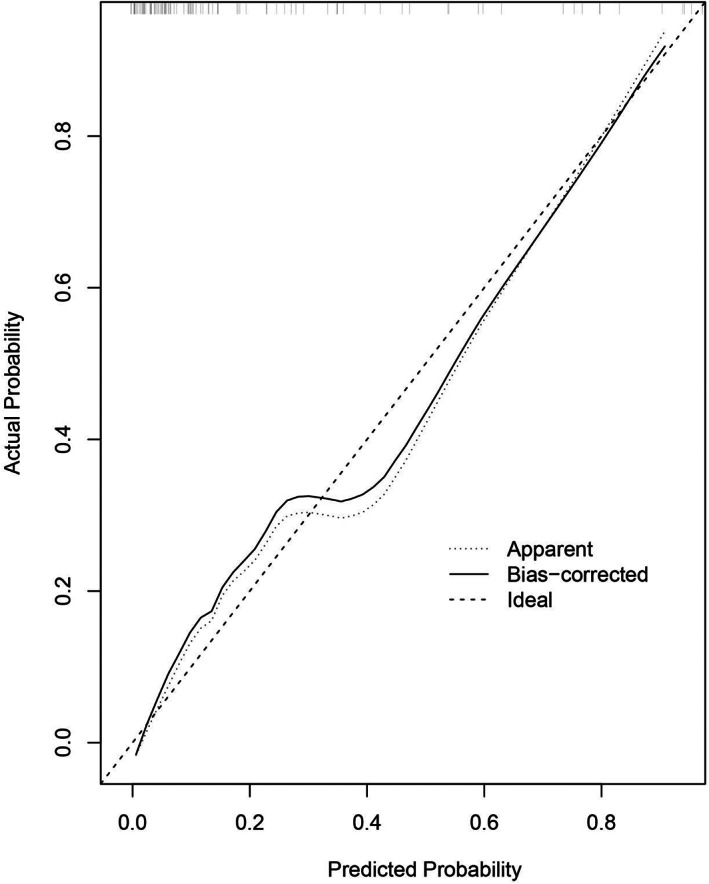
Calibration plots of nomogram models for predicting postoperative surgical site infection in the validation cohort. Nomogram‐predicted probability of SSI is plotted on the x‐axis; actual probability of SSI is plotted on the y‐axis. A plot along the 45° line would indicate a perfect calibration model in which the predicted probabilities are identical to the actual outcomes
3.7. Validation of the nomogram
Receiver operating characteristic (ROC) curves of a nomogram model for predicting surgical site infection after TLIF were shown in Figures 5 and 6, which demonstrated good discriminative abilities. The area under the curve (AUC) of the model with FPG‐CV, FPG‐CV, and the model without FPG‐CV in the training cohort was 0.915, 0.829, and 0.838, respectively, while the AUC of them in the validation cohort was 0.890, 0.801, and 0.822 respectively. Whether in the training cohort or the validation cohort, FPG‐CV shows great value to the nomogram.
FIGURE 5.
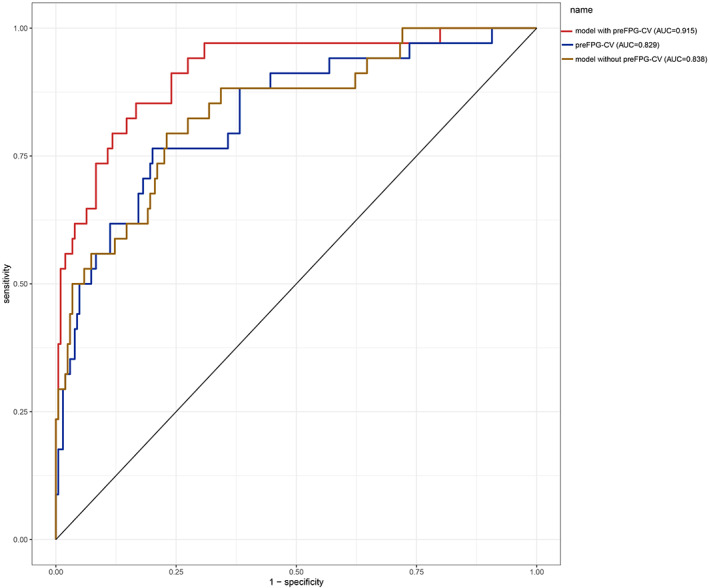
ROC curves of the training cohort. preFPG‐CV: preoperative FPG‐CV. ROC curves of the predicting SSI nomogram showed that the AUC of the model with preFPG‐CV, preFPG‐CV, the model without preFPG‐CV in the training cohort was 0.915, 0.829, and 0.838 respectively
FIGURE 6.
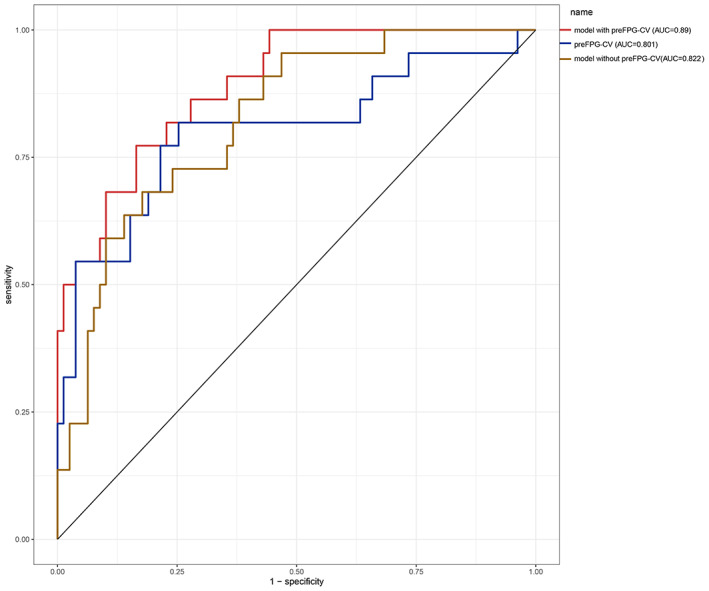
ROC curves of the validation cohort. preFPG‐CV: preoperative FPG‐CV. ROC curves of the predicting SSI nomogram showed that the AUC of the model with preFPG‐CV, preFPG‐CV, the model without preFPG‐CV in the validation cohort was 0.890, 0.811, and 0.822 respectively
4. DISCUSSION
SSI is one of the most common complications after spinal surgery, which not only leads to increased treatment costs and prolonged hospitalisation but also seriously affects the quality of life of patients, bringing huge burden to patients' families, and even society. 13 In this study, the retrospective data of spinal surgery in Zhongda Hospital in the past 3 years were used to establish a nomogram model including seven predictive factors, such as the number of fused vertebrae ≥2, duration of drain placement, duration of diabetes >5 years, preoperative blood glucose control scheme, preoperative MFBG, preoperative FPG‐CV (P = .000), postoperative FPG‐CV (P = .003), to predict the risk of spinal postoperative surgical site infection. The model has been verified in the training cohort and validation cohort and shows good performance in calibration and discriminative abilities. The construction of this model can further study the risk factors of SSI following TLIF in type 2 diabetes patients, so as to make beneficial perioperative treatment plans for diabetes patients. In addition, more indicators of diabetes predicting SSI can be obtained, and intervention can effectively reduce the risk and improve our postoperative SSI in patients with type 2 diabetes after TLIF by exploring the correlation between perioperative glycemic variability and the occurrence of SSI after TLIF. The understanding of risk factors can improve preoperative risk stratification, provide direction for early intervention, and improve the quality of life of patients after surgery.
We got seven risk factors of SSI by univariate analysis and subsequent multivariable regression analysis. Based on this, a nomogram was built, during which preoperative FPG‐CV was the most important independent risk predictor, followed by preoperative MFBG, duration of drain placement, postoperative FPG‐CV, preoperative blood glucose control scheme, duration of diabetes >5 years, and the number of fused vertebrae ≥2.
It was found that preoperative FPG‐CV was the most important independent risk predictor of SSI. FPG‐CV is one of the glycemic variability indicators. Glycemic variability (GV), also known as blood sugar volatility or blood sugar stability, refers to the unstable state in which the blood sugar level of the human body continuously fluctuates between peaks and valleys. Due to various factors, the monitoring value varies at different times. The coefficient of variation of fasting blood glucose (FPG‐CV) is used to evaluate the degree of dispersion of fasting blood glucose and can be used as an important evaluation parameter for day‐to‐day variability. Its advantages are that fasting blood sugar is less disturbed than postprandial blood sugar, has strong repeatability, and is relatively simple to calculate. Studies have confirmed that blood glucose instability can lead to the expression of inflammatory factors and the occurrence of oxidative stress, which is closely related to vascular endothelial injury, atherosclerosis, and impaired pancreatic β‐cell function. 14 More and more studies have found that GV is an independent risk factor for diabetes complications, and with the increase of variability, the incidence of complications will gradually increase. 15 A study on the relationship between glycemic variability and adverse outcomes after lumbar fusion found that although glycated haemoglobin is often used as the primary indicator to assess glycemic control, the results showed that CV was a strong predictor of adverse postoperative outcomes, patients with high CV have higher rates of postoperative infection and longer hospital stay. Our results are similar to this study, except that this study focused on postoperative glycemic variability, whereas our study focused on perioperative glycemic variability, including preoperative and postoperative glycemic coefficients of variation. Previous studies have compared the predictive value of glycemic variability and mean blood glucose for adverse outcomes after surgery and found that the higher variability increases the risk of postoperative infection, and the patients with normal mean blood glucose levels (preoperatively and postoperatively) still have high risks of SSI when stratified according to glycemic variability. 16 Blood glucose variability (GV) is associated with fluctuations in blood glucose levels and may differ in patients with similar mean blood glucose and glycated haemoglobin levels. 17 As a predictor, the glycemic variability is more valuable than mean fasting blood glucose in predicting postoperative infection, which is highly consistent with the results of our study. The reason may be that short‐term large fluctuations of blood glucose will activate pro‐inflammatory proteins and excessive oxidative stress, and the activation of oxidative stress is one of the main mechanisms leading to diabetes complications. Meanwhile, chronic hyperglycemia will not cause the above results. 18
Therefore, stabilising acute blood glucose fluctuations is helpful for the recovery of patients after TLIF, improving prognosis, reducing the risk of infection, and providing new indicators for further intervention in blood glucose control, not only limited to mean blood glucose and glycated haemoglobin. Similar findings have been found in some non‐orthopaedic literature. Many previous studies have linked glycemic variability with the length of stay and mortality, suggesting that higher glycemic variability will increase the length of stay and mortality. 19 High glycemic variability is associated with major adverse events after cardiothoracic surgery, including deep sternal infections. 20 Different from previous studies, perioperative glycemic variability was studied in this research. Through observation and analysis of glycemic variability throughout the perioperative period, it was further confirmed that glycemic variability was closely related to postoperative SSI. Reducing glycemic variability and maintaining blood glucose stability may be beneficial to reduce the incidence of postoperative SSI.
Other factors associated with diabetes in this study were the preoperative blood glucose control scheme and the duration of diabetes, both of which were statistically significant risk factors for the development of postoperative SSI. Previous studies on the correlation between preoperative blood glucose control scheme and postoperative SSI are scarce, and the preoperative blood glucose control scheme listed in this paper includes subcutaneous injection of insulin, combined medication, oral hypoglycemic agent, and diet therapy. This study found that the preoperative subcutaneous insulin injection scheme had the lowest infection rate of the four regimens. It is controversial whether the preoperative use of insulin has an impact on reducing the incidence of SSI in the postoperative period. In a previous study, the use of insulin during hospitalisation was found to reduce the occurrence of postoperative complications including SSI, 21 while others found that there was no significant difference between whether diabetic patients received preoperative insulin therapy or not on the occurrence of SSI after surgery. 22 There are fewer studies on the relationship between the choice of preoperative glucose‐lowering regimen and postoperative SSI, and future prospective studies could be selected to further explore this. Another controversial factor is the duration of diabetes mellitus. Our findings show that the duration of diabetes >5 years is an independent risk factor for postoperative infection. However, in an article on predictors of postoperative complications in diabetic patients, the duration of diabetes was divided into three groups (<5 years, 5–10 years, >10 years) and the results showed no predictive value for postoperative infections. 23 As the duration of diabetes increases, the patient's abnormal glucose metabolism becomes more severe, and thus the ability to resist infection is severely reduced, which, coupled with the fact that the spine surgery patient's own body will also experience a stress response, causing a rapid rise in blood sugar, will further increase the patient's risk of infection. The relationship between the duration of diabetes and postoperative complications needs to be explored in depth; the longer the duration of diabetes, the more comorbidities there may be, which may also have an impact on postoperative complications, so control of comorbidities is needed to analyse the relationship between duration of disease and postoperative complications.
This research focused on the correlation between glycemic variability and postoperative SSI after TLIF, but this study also found that surgery‐related factors were also correlated with postoperative SSI, with longer postoperative drainage time in the infected group than in the non‐infected group, and longer postoperative drainage time was an independent risk factor for the development of postoperative SSI after TLIF. The drainage tube is placed in the surgical incision and connected to the external environment, which increases the risk of infection at the surgical site. Good intraoperative haemostasis can help remove the drainage tube as soon as possible after surgery, and care for the surgical incision and the drainage tube should be strengthened during the placement of the drainage tube to minimise its contact with the outside world so that the drainage tube can be maintained in a relatively sterile state. 24 Rao et al found that prolonged drainage was a stronger independent risk factor for SSI after spinal fusion and recommended early removal of the drainage tube to reduce the rate of infection. 25 In addition to this, our study also found that the number of fused vertebrae ≥2 was also a risk factor for postoperative SSI, and other scholars also hold the same conclusion. 25 The number of fused vertebrae, which predisposes to the formation of a dead wound cavity, tissue necrosis, and inadequate vascular perfusion of the local wound, which in turn prevents the bactericidal effect of neutrophils and leads to SSI. Also, the high number of surgical segments leads to an increased risk of wound exposure, and the patient is hit harder by the surgery and has a reduced immunity, leading to an increased risk of infection.
5. CONCLUSION
In summary, further investigation of diabetes‐related factors can enrich the predictors of postoperative SSI. The nomograph model established based on the factors like duration of diabetes >5 years, preoperative blood glucose control scheme, the number of fused vertebrae ≥2, duration of drain placement, preoperative MFBG, preoperative FPG‐CV, postoperative FPG‐CV has potential clinical application value in predicting SSI in spinal surgery, but the reliability and applicability of the model need to be verified in other medical institutions in the future.
CONFLICT OF INTEREST
The authors declare no conflict of financial interest or benefit.
ACKNOWLEDGMENTS
We thank Qiang Hu and Lei Liu for their assistance and encouragement during the preparation of this manuscript.
Liu H, Zhang W, Hu Q, et al. A nomogram for accurately predicting the surgical site infection following transforaminal lumbar interbody fusion in type 2 diabetes patients, based on glycemic variability. Int Wound J. 2023;20(4):981‐994. doi: 10.1111/iwj.13948
Contributor Information
Genyang Jing, Email: genyang.101@163.com.
Yuntao Wang, Email: wangyttod@aliyun.com.
DATA AVAILABILITY STATEMENT
The article file contains all the datasets that support the conclusions of this study.
REFERENCES
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4‐14. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: asystematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moazzeni K, Kazemi KA, Khanmohammad R, Eslamian M, Rostami M, Faghih‐Jouibari M. Comparison of surgical outcome between diabetic versus nondiabetic patients after lumbar fusion. Int J Spine Surg. 2018;12(4):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El‐Kadi M, Donovan E, Kerr L, et al. Risk factors for postoperative spinal infection: a retrospective analysis of 5065 cases. Surg Neurol Int. 2019;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sono T, Fujibayashi S, Izeki M, et al. Decreased rate of surgical site infection after spinal surgery with instrumentation using bundled approach including surveillance and intrawound vancomycin application. Medicine (Baltimore). 2018;97(34):e12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannata F, Vadalà G, Ambrosio L, et al. Intervertebral disc degeneration: a focus on obesity and type 2 diabetes. Diabetes Metab Res Rev. 2020;36(1):e3224. [DOI] [PubMed] [Google Scholar]
- 8. Alpantaki K, Kampouroglou A, Koutserimpas C, Effraimidis G, Hadjipavlou A. Diabetes mellitus as a risk factor for intervertebral disc degeneration: a critical review. Eur Spine J. 2019;28(9):2129‐2144. [DOI] [PubMed] [Google Scholar]
- 9. Canseco JA, Chang M, Karamian BA, et al. Postoperative glycemic variability as a predictor of adverse outcomes following lumbar fusion. Spine. 2022;47(7):E304‐E311. [DOI] [PubMed] [Google Scholar]
- 10. Schleicher E, Friess U. Oxidative stress, age, and atherosclerosis. Kidney Int Suppl. 2007;106:S17‐S26. [DOI] [PubMed] [Google Scholar]
- 11. Bardia A, Khabbaz K, Mueller A, et al. The association between preoperative hemoglobin A1C and postoperative glycemic variability on 30‐day major adverse outcomes following isolated cardiac Valvular surgery. Anesth Analg. 2017;124(1):16‐22. [DOI] [PubMed] [Google Scholar]
- 12. Berríos‐Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784‐791. [DOI] [PubMed] [Google Scholar]
- 13. Imajo Y, Taguchi T, Neo M, et al. Complications of spinal surgery for elderly patients with lumbar spinal stenosis in a super‐aging country: an analysis of 8033 patients. J Orthop Sci. 2017;22(1):10‐15. [DOI] [PubMed] [Google Scholar]
- 14. Nalysnyk L, Hernandez‐Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12(4):288‐298. [DOI] [PubMed] [Google Scholar]
- 15. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, for the DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. 2008;57(4):995‐1001. [DOI] [PubMed] [Google Scholar]
- 16. Shohat N, Restrepo C, Allierezaie A, Tarabichi M, Goel R, Parvizi J. Increased postoperative glucose variability is associated with adverse outcomes following Total joint arthroplasty. J Bone Joint Surg Am. 2018;100(13):1110‐1117. [DOI] [PubMed] [Google Scholar]
- 17. Siegelaar SE, Holleman F, Hoekstra JBL, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171‐182. [DOI] [PubMed] [Google Scholar]
- 18. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681‐1687. [DOI] [PubMed] [Google Scholar]
- 19. Mendez CE, Mok KT, Ata A, Tanenberg RJ, Calles‐Escandon J, Umpierrez GE. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care. 2013;36(12):4091‐4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Subramaniam B, Lerner A, Novack V, et al. Increased glycemic variability in patients with elevated preoperative HbA1C predicts adverse outcomes following coronary artery bypass grafting surgery. Anesth Analg. 2014;118(2):277‐287. [DOI] [PubMed] [Google Scholar]
- 21. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hikata T, Iwanami A, Hosogane N, et al. High preoperative hemoglobin A1c is a risk factor for surgical site infection after posterior thoracic and lumbar spinal instrumentation surgery. J Orthop Sci. 2014;19(2):223‐238. [DOI] [PubMed] [Google Scholar]
- 23. Tao LS, Mackenzie CR, Charlson ME. Predictors of postoperative complications in the patient with diabetes mellitus. J Diabetes Complicat. 2008;22(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 24. Liu JM, Deng HL, Chen XY, et al. Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine. 2018;43(10):732‐737. [DOI] [PubMed] [Google Scholar]
- 25. Rao SB, Vasquez G, Harrop J, et al. Risk factors for surgical site infections following spinal fusion procedures: a case‐control study. Clin Infect Dis. 2011;53(7):686‐692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The article file contains all the datasets that support the conclusions of this study.


